Abstract
Pulmonary surfactant is a heterogeneous active surface complex made up of lipids and proteins. The major glycoprotein in surfactant is surfactant protein A (SP-A), which is released into the alveolar lumen from cytoplasmic lamellar bodies in type II alveolar epithelial cells. SP-A is involved in phospholipid absorption. SP-A together with other surfactant proteins and phospholipids prevent alveolar collapse during respiration by decreasing the surface tension of the air-liquid interface. Additionally, SP-A interacts with pathogens to prevent their propagation and regulate host immune responses. Studies in human and animal models have shown that deficiencies or mutations in surfactant components result in various lung or kidney pathologies, suggesting a role for SP-A in the development of lung and kidney diseases. In this mini-review, we discuss the current understanding of SP-A functions, recent findings of its dysfunction in specific lung and kidney pathologies, and how SP-A has been used as a biomarker to detect the outcome of lung diseases.
Keywords: immune responses, kidney, pulmonary host defense, SP-A, surfactant
INTRODUCTION
During the respiratory cycle, it is necessary for the alveoli to remain in an inflated state for optimal gas exchange. This occurs due to the surface-active function of surfactants, which reduces the surface forces acting on the air-liquid interface in the alveolar lumen (8). The stability of the alveolar structure is attained partially by pulmonary surfactants, which reduce the surface tension level as the alveolar surface decreases. Lung alveolar surface is formed by two types of epithelial cells, types I and II alveolar epithelial cells. Alveolar epithelial type II cells (AE2Cs) secrete and produce a mixture of phospholipids and surfactant proteins that are stored in cytoplasmic lamellar bodies and released into the alveoli (5). The metabolic cycle of surfactants begins when surfactants are secreted into the alveolar lumen and ends once it is cleared from the air space by AE2Cs or alveolar macrophages (48). During gas exchange, surfactants stabilize the lung volume by preventing collapse or fluid flooding of the alveoli and support the clearance of pathogens (47).
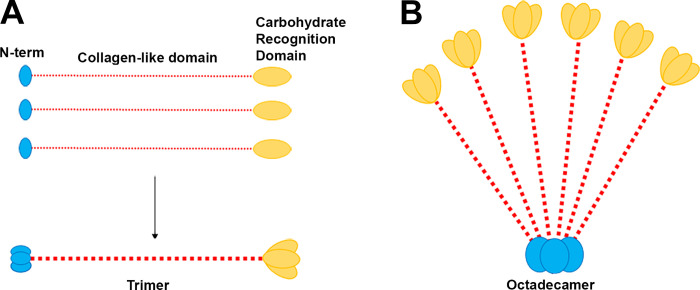
The main components in pulmonary surfactant complex are lipids and proteins. Lipids, especially phospholipids such as dipalmitoylphosphatidylcholine (DPPC), make up ~90% of the complex (5). The remaining 10% of the surfactant is made up of proteins, including hydrophobic surfactant proteins (SP) B and C and hydrophilic surfactant proteins SP-A and SP-D. SP-A and SP-D are oligomeric large proteins belonging to the collectin family that are characterized by a NH2-terminal cysteine-rich domain, a collagen-like domain, and a COOH-terminal carbohydrate recognition domain (CRD) (Fig. 1) (5). SP-D makes up ~0.5% of the total mass of surfactant. However, SP-A is the most abundant glycoprotein in surfactant, making up about 3–4% of the total surfactant mass (5).
Fig. 1.
The structural components and organization of surfactant protein A (SP-A). A: SP-A belongs to the collectin family and consists of a collagen domain (red), a COOH-terminal carbohydrate recognition domain (CRD; yellow), and a cysteine-rich NH2-terminal domain (N-term; blue). Three monomer subunits of SP-A form a trimer. B: 6 trimers assemble into the mature SP-A protein, shown as an octadecamer or “flower bouquet.”
The synthesis of surfactant occurs mainly in the lungs. However, SP-A mRNA and protein expression is also found in nonpulmonary sites, such as brain, kidney, and female reproductive tract. SP-A regulates the immune homeostasis in these organs (15, 20, 23, 36, 37). AE2Cs synthesize the four surfactant proteins along with surfactant lipids in the lamellar body. After stimulation, like birth or a deep breath, the contents in the lamellar body are secreted into the thin, hypophase liquid that covers the epithelium of the alveoli. The formation of a surface film containing phosphatidylcholine reduces the surface tension, enabling the lungs to expand. In addition, airway cells, such as Clara cells and submucosal cells, also synthesize SP-A, SP-B, and SP-D (44). The functions of surfactant proteins secreted by the airway cells are not well understood. Since the airway secretory products lack lipids, they may not participate in the regulation of the surface tension (44).
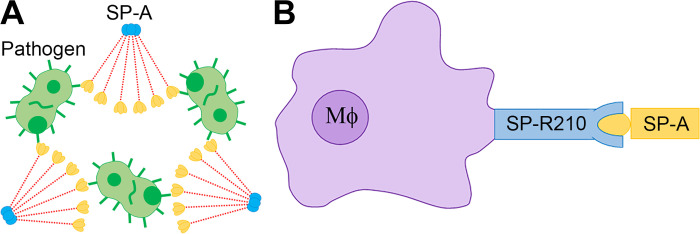
SP-A not only functions as a structural protein to reduce the surface tension in the lungs, but also serves as an innate immune protein of the lungs. Studies in vivo and in vitro demonstrate that SP-A facilitates pathogen clearance via opsonization and phagocytosis (24, 39, 46). SP-A pathogen opsonization is achieved by the collectin CRD binding to the pathogen surface (Fig. 2A) (24). The interaction between the collectin and immune cell improves the phagocytosis of the pathogen. SP-A enhances the opsonic and nonopsonic phagocytosis via binding to SP-A receptor SP-R210 in macrophages (Fig. 2B) (39).
Fig. 2.
The roles of surfactant protein A (SP-A) in pathogen clearance. A: the collectin carbohydrate recognition domain of SP-A binds to the pathogen surface for opsonization. B: SP-A opsonizes pathogens by directly interacting with macrophages (Mφ). SP-A binds to the SP-R210 receptor and enhances opsonic and nonopsonic phagocytosis of the pathogen.
The binding of SP-A, along with surfactant membranes, to phospholipid DPPC contributes to the active phospholipid absorption at the air-liquid interface. This binding enables the surfactant vesicle turnover by AE2C via the p63 receptor (4). Additionally, the binding of SP-A to lipids allows SP-A to act as an initial barrier for protection against inhaled pathogens and toxins (5). In this mini-review, we discuss SP-A functions and the consequences of SP-A genetic mutations or pulmonary surfactant deficiencies, with a specific emphasis on the recent progress in the association of SP-A with pathological changes due to lung and kidney injury. We also highlight the recent studies on how SP-A is used as a biomarker to detect the outcome of lung pathologies.
GENETIC VARIATIONS OF SP-A
In humans and baboons, SP-A is composed of two functional gene products, SP-A1, encoded by SFTPA1, and SP-A2, encoded by SFTPA2 (32). Both genes are expressed in AE2Cs and epithelial cells within the large and small intestines of rats. However, SP-A2 is additionally expressed in tracheal and bronchial submucosal gland cells (12, 44). Structurally, the main differences between SP-A1 and SP-A2 are variants at four core amino acids within the collagen-like domain (12). Although humans and baboons need two functional genes, one SP-A gene is efficient to exercise its function in other vertebrates (32).
Human SP-A variants SP-A1 and SP-A2 have been shown to differ in their biochemical and functional properties. For instance, the variants modulate macrophage function differently in idiopathic interstitial pneumonia, fibrosis, and cancer (25, 26, 30). In a recent mouse study, SP-A1 and SP-A2 were found to distinctively bind to and regulate neonatal and adult alveolar macrophages (41). In addition, alveolar macrophages from transgenic mice expressing human SP-A1 and SP-A2 display differential expression of cell surface proteins (41). Furthermore, the proteomes of SP-A knockout alveolar macrophages show sex differences after treatment with SP-A1 or SP-A2 proteins. Male mice have more changes in oxidative stress, protease/chaperones, and inflammation-related genes in response to treatment with SP-A2. However, female mice display more changes in actin cytoskeleton and oxidative stress genes in response to SP-A1 treatment. The proteomic changes in SP-A1 and SP-A2 treatments suggest that females are more responsive to SP-A1, whereas males are more responsive to SP-A2 (31). These findings are further supported by a recent study showing that SP-A2 contributes to the microRNA-mediated sex-specific differences in response to oxidative stress. These differences include changes in inflammatory, antiapoptotic, and antioxidant pathways specifically in males (27). Together, these studies suggest that SP-A1 and SP-A2 function differently in regulating macrophage activities. Therefore, specific SP-A variants need to be considered in case the exogenous SP-A1 or SP-A2 is used to manipulate macrophage functions.
SP-A genetic variants are also associated with lung diseases. For example, genetic mutations of SP-A are associated with a higher prevalence of idiopathic pulmonary fibrosis (IPF), high-altitude pulmonary edema, acute respiratory distress syndrome, lung carcinoma, and bronchopulmonary dysplasia (18, 29, 34, 37, 38). Moreover, a rare SFTPA2 missense mutation is correlated with the development of familial IPF and lung cancer (45). Recently, a genetic variation in SP-A2, i.e., a glutamine to a lysine amino acid substitution in the lectin domain at position 223, was found to modify SP-A capacity to prevent eosinophil degranulation in patients with asthma (10). Another study suggests that patients after lung transplantations with donor SP-A2 gene variants of 1A0 or 1A01A0 have a greater survival rate 1 yr after the transplantation. However, patients receiving donor lungs with SP-A2 variant 1A1 display a reduced percentage of survival (9). Additionally, patients with cystic fibrosis (CF), a rare autosomal recessive disease, show a genetic association with surfactant protein gene variants. It appears that surfactant protein gene single-nucleotide polymorphism (SNP)-SNP interactions contribute to pulmonary disease progression in patients with CF. Moreover, surfactant protein genetic variations are associated with disease severity. A single SFTPB SNP variant is associated with mild CF, whereas several intergenic interactions among SFTPs (A–D) are associated with either mild or moderate/severe CF (19). These results indicate that surfactant proteins may serve as modifiers for the progression or severity of pulmonary disease with CF (19).
NEW MECHANISMS OF SP-A IN LUNG DISEASES AND INFLAMMATION
Besides genetic variants, SP-A has been shown to be directly involved in lung disease. The pathophysiological role of surfactant protein was first identified in premature infants with respiratory distress syndrome (RDS) (14). During the embryonic development, an increase in pulmonary surfactant synthesis initiates toward the end of gestation in the fetal lung. Defective surfactant synthesis in the lungs of premature infants results in the RDS. Clinical trials show that replacing surfactant exogenously can treat this condition (14).
In addition to the effects caused by defective synthesis, SP-A is involved in inflammatory responses. For example, lung injury is often a result of lipopolysaccharide (LPS)-induced inflammation due to pulmonary neutrophil infiltration. CD26/DPP4 is a membrane-bound protein influencing inflammatory processes. Asthmatic CD26/DPP4-deficient rats with a transport block in rough endoplasmic reticulum due to a point mutation show a decrease in pulmonary inflammation (50). These rats have a low level of SP-A expression along with a reduced influx of alveolar macrophages and neutrophils (50). These studies suggest that alteration of SP-A expression may be involved in CD26/DPP4-regulated lung inflammation.
SP-A regulates inflammatory response also by inhibiting the expression of inflammatory cytokines (6). SP-A binds to Toll-like receptors (TLRs) 2 and 4 to regulate inflammatory responses initiated by infectious challenges (33). Activation of TLRs through SP-A modulates the production of cytokines and inflammatory mediators such as tumor necrosis factor (TNF) and interleukin-1β (IL-1β) (16). SP-A also inhibits interleukin-8 (IL-8) production in eosinophils (6). IL-8 produced by macrophages and epithelial cells induces acute lung injury (ALI) through SP-A. Recent studies identify a negative correlation between IL-8 and SP-A in human lung epithelial A549 cells (49). Silencing of IL-8 increases SP-A and B‐cell leukemia/lymphoma‐2 (Bcl-2) expression but decreases various apoptosis-related proteins and immune cell regulators, leading to reduced apoptosis and increased cell viability. On the other hand, silencing of SP-A enhances the expression of IL-8, apoptotic proteins, and immune cell regulators while decreasing Bcl-2. Furthermore, IL-8 interacts with SP-A to enhance the response of inflammatory cells, eventually leading to ALI (49). Together, these studies suggest that IL-8 may exacerbate damages to alveolar epithelial cells by inhibiting SP-A expression, which could promote lung inflammation.
Other than in ALI, SP-A is considered as one of the first lines of immune defense against lung infection (5). Recently, Schicke et al. (35) showed that extracellular SP-A levels are influenced by Staphylococcus aureus infection. In a human alveolus-on-a-chip model, SP-A expression is not affected by virus or bacteria but significantly downregulated by TNF-α, a cytokine produced by phagocytes in response to bacterial infections, suggesting that the downregulation of SP-A is significantly dependent on macrophages. Importantly, S. aureus also decreases SP-A in vivo in a murine model of pneumonia (35). These observations suggest that epithelial-immune cell interactions are essential for downregulating SP-A in the lung, which may contribute to a severe outcome of bacterial pneumonia.
SP-A IN KIDNEY DISEASES AND INFLAMMATION
Similar to the lung, kidneys are formed from the endoderm and may be exposed to external pathogens, which raises the question of whether SP-A is expressed in kidneys. Indeed, SP-A is observed in human renal tubular epithelial cells in both the proximal and distal convoluted tubules. In addition, LPS time- and dose-dependently increases the expression of both SP-A1 and SP-A2 in cultured renal tubular epithelial cells (22). These findings suggest that SP-A may play roles in inflammatory modulation of the kidneys (22). Our studies demonstrate that SP-A regulates macrophage infiltration in fibrotic kidneys. Unilateral ureter obstruction induces SP-A expression in mouse kidney epithelium while causing renal fibrosis. SP-A deficiency exacerbates kidney interstitial inflammation and fibrosis (42). SP-A appears to protect mouse kidneys by attenuating high-mobility group box 1 (HMGB1) function in the induction of transforming growth factor-β expression and fibroblast activation. Although how SP-A regulates macrophage function remains to be determined, our earlier study shows that HMGB1 is an essential contributor in classic macrophage activation at the early stage of obstructive injury, an important event leading to renal fibrosis (43).
Besides damage-associated molecular patterns, SP-A is also involved in pathogen-associated molecular patterns in mouse kidneys. SP-A and SP-D levels are attenuated in urine from female patients with recurrent urinary tract infection (UTI), suggesting that SP-A and SP-D are involved in the host defense against UTI (21). Indeed, in a murine model of uropathogenic Escherichia coli-induced UTI, Hu et al. (17) show that knockout of SP-A and SP-D increases bacterial loads and neutrophil infiltration in the kidneys, indicating that SP-A and SP-D may attenuate kidney infection by inhibiting bacterial growth and modulating renal inflammation.
SP-A is expressed not only in normal tissues, but also in cancer cells. A recent study shows that SP-A is expressed in renal cell carcinoma (RCC; Ref. 3). Strong SP-A immunoreactivity is detected in the tumor tissues. Besides, there is a significant correlation between the SP-A level and tumor size (3). This interesting study suggests that increased SP-A expression in RCC is associated with favorable prognosis.
EXOGENOUS TREATMENT WITH SP-A
Surfactant replacement therapy is a standard therapeutic option in respiratory distress. Exogenous surfactants have been extensively studied in animal models and clinical trials (11). Surfactant administration in preterm infants with established respiratory distress syndrome reduces mortality, decreases the incidence of pulmonary air leak, and lowers the risk of chronic lung diseases or death at 28 days of age (13). A recent study suggests that SP-A may be used to treat type II inflammation in asthma (13). IL-13 is a type II cytokine important for asthma phenotypes, such as goblet cell hyperplasia, increased airway hyperactivity, and tissue remodeling. SP-A deficiency increases IL-13 activities in a mouse model, including increased recruitment of neutrophils and eosinophils, mucin production, and asthma-associated cytokines in bronchioalveolar lavage fluid. However, exogenous SP-A treatment diminishes IL-13 challenge-induced production of asthma-associated factors in asthmatic human airway epithelial cells (13). Mechanistically, SP-A inhibits IL-13-induced IL-6/signal transducer and activator of transcription 3 (STAT3) signaling (13).
Since interactions between SP-A and TLR-4 play critical roles in host defense, SP-A peptide (SPA4) has been used to control Pseudomonas aeruginosa lung infection (2). SPA4 peptide treatment causes the uptake and localization of bacteria in the phagolysosomes of immune cells. More importantly, the therapeutic administration of SPA4 peptide reduces bacterial burden, inflammatory cytokine and chemokine production, lactate levels, lung edema, and tissue damage in P. aeruginosa-infected mice (2). Interestingly, in contrast to its role in reducing bacterial infection, a recombinant truncated human SP-A peptide promotes the influenza A virus (IAV) replication, as shown by an upregulation of M1 protein, an important determinant of viral replication, in lung epithelial cell line A549 when challenged with pH1N1 and H3N2 IAV subtypes (1). However, human full-length native SP-A downregulates the M1 level in A549 cells challenged with IAV subtypes (1), suggesting that a complete SP-A molecule is required for protection against IAV (2).
SP-A AS A DISEASE BIOMARKER
SP-A has been widely proven as a serum biomarker for diagnosing lung diseases (7, 40). SP-A is also related to the progression of IPF and the associated mortality (7, 40). A recent clinical study indicates that SP-A may also be used as a biomarker for outcomes of antifibrotic drug therapy in patients with IPF (50). After the patients with IPF were treated with antifibrotic drugs pirfenidone and nintedanib, their serum SP-A levels were negatively correlated with the change in forced vital capacity, the amount of air that can be forcibly exhaled from lungs after a deep breath, as measured by spirometry. These results indicate that serum SP-A levels reflect the efficacy of antifibrotic drug therapy. Thus serum SP-A may be used as a potential biomarker for therapeutic outcomes for antifibrotic drugs.
Besides IPF, SP-A may also be used as a biomarker for the outcome of chronic obstructive pulmonary disease (COPD). In a recent exploratory study, Papaioannou and colleagues (28) indicate that although not related to disease severity and pulmonary function in patients with COPD, serum SP-A levels are significantly lower in patients with early relapse compared with those with late or no relapse. Thus low SP-A levels could be predictors of early relapse. The decreased SP-A levels, associated with decreased immunity, might be related to the increased susceptibility of COPD exacerbation.
CONCLUDING REMARKS
Pulmonary surfactant is known to decrease the surface tension of the alveoli during respiration. SP-A has both surfactant regulatory properties and immunoregulatory functions. Emerging evidence supports that SP-A functions are not exclusive to the lungs but also important for other organs such as kidney. Most previous studies focused on lung development and functions. However, the research interest of SP-A biology in other organs is likely to increase in the future. Given that exogeneous treatment with SP-A is beneficial in certain animal models and that SP-A has become a biomarker for lung diseases, there is a potential to develop SP-A as an alternative means to prevent or treat surfactant protein-related diseases in other systems once critical research data are generated. Moreover, SP-A appears to be a very promising therapeutic agent for treating bacteria and/or viral infections.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL123302, HL119053, HL135854, and HL147313 awarded to S.-Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.D.K. prepared figures; S.D.K. and S.-Y.C. drafted manuscript; S.D.K. and S.-Y.C. edited and revised manuscript; S.D.K. and S.-Y.C. approved final version of manuscript.
REFERENCES
- 1.Al-Qahtani AA, Murugaiah V, Bashir HA, Pathan AA, Abozaid SM, Makarov E, Nal-Rogier B, Kishore U, Al-Ahdal MN. Full-length human surfactant protein A inhibits influenza A virus infection of A549 lung epithelial cells: a recombinant form containing neck and lectin domains promotes infectivity. Immunobiology 224: 408–418, 2019. doi: 10.1016/j.imbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Awasthi S, Singh B, Ramani V, Xie J, Kosanke S. TLR4-interacting SPA4 peptide improves host defense and alleviates tissue injury in a mouse model of Pseudomonas aeruginosa lung infection. PLoS One 14: e0210979, 2019. doi: 10.1371/journal.pone.0210979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassorgun CI, Sayar EC, Baykara M, Kankavi O. Alteration of surfactant protein A expression in renal cell carcinoma. Biotech Histochem 93: 519–525, 2018. doi: 10.1080/10520295.2018.1472296. [DOI] [PubMed] [Google Scholar]
- 4.Bates SR. P63 (CKAP4) as an SP-A receptor: implications for surfactant turnover. Cell Physiol Biochem 25: 41–54, 2010. doi: 10.1159/000272062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casals C. Role of surfactant protein A (SP-A)/lipid interactions for SP-A functions in the lung. Pediatr Pathol Mol Med 20: 249–268, 2001. doi: 10.1080/15513810109168821. [DOI] [PubMed] [Google Scholar]
- 6.Cheng G, Ueda T, Nakajima H, Nakajima A, Arima M, Kinjyo S, Fukuda T. Surfactant protein A exhibits inhibitory effect on eosinophils IL-8 production. Biochem Biophys Res Commun 270: 831–835, 2000. doi: 10.1006/bbrc.2000.2515. [DOI] [PubMed] [Google Scholar]
- 7.Chiba H, Otsuka M, Takahashi H. Significance of molecular biomarkers in idiopathic pulmonary fibrosis: a mini review. Respir Investig 56: 384–391, 2018. doi: 10.1016/j.resinv.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Clements JA, Hustead RF, Johnson RP, Gribetz I. Pulmonary surface tension and alveolar stability. J Appl Physiol 16: 444–450, 1961. doi: 10.1152/jappl.1961.16.3.444. [DOI] [PubMed] [Google Scholar]
- 9.D’Ovidio F, Floros J, Aramini B, Lederer D, DiAngelo SL, Arcasoy S, Sonett JR, Robbins H, Shah L, Costa J, Urso A. Donor surfactant protein A2 polymorphism and lung transplant survival. Eur Respir J 55: 1900618, 2020. doi: 10.1183/13993003.00618-2019. [DOI] [PubMed] [Google Scholar]
- 10.Dy ABC, Arif MZ, Addison KJ, Que LG, Boitano S, Kraft M, Ledford JG. Genetic variation in surfactant protein-A2 delays resolution of eosinophilia in asthma. J Immunol 203: 1122–1130, 2019. doi: 10.4049/jimmunol.1900546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Gendy N, Kaviratna A, Berkland C, Dhar P. Delivery and performance of surfactant replacement therapies to treat pulmonary disorders. Ther Deliv 4: 951–980, 2013. doi: 10.4155/tde.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floros J, Hoover RR. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta 1408: 312–322, 1998. doi: 10.1016/S0925-4439(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 13.Francisco D, Wang Y, Conway M, Hurbon AN, Dy ABC, Addison KJ, Chu HW, Voelker DR, Ledford JG, Kraft M. Surfactant protein-A protects against IL-13-induced inflammation in asthma. J Immunol 204: 2829–2839, 2020. doi: 10.4049/jimmunol.1901227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara T, Chida S, Watabe Y, Maeta H, Morita T, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet 315: 55–59, 1980. doi: 10.1016/S0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Verdugo I, Tanfin Z, Dallot E, Leroy M-J, Breuiller-Fouché M. Surfactant protein A signaling pathways in human uterine smooth muscle cells. Biol Reprod 79: 348–355, 2008. doi: 10.1095/biolreprod.108.068338. [DOI] [PubMed] [Google Scholar]
- 16.Hickling TP, Sim RB, Malhotra R. Induction of TNF-α release from human buffy coat cells by Pseudomonas aeruginosa is reduced by lung surfactant protein A. FEBS Lett 437: 65–69, 1998. doi: 10.1016/S0014-5793(98)01200-9. [DOI] [PubMed] [Google Scholar]
- 17.Hu F, Ding G, Zhang Z, Gatto LA, Hawgood S, Poulain FR, Cooney RN, Wang G. Innate immunity of surfactant proteins A and D in urinary tract infection with uropathogenic Escherichia coli. Innate Immun 22: 9–20, 2016. doi: 10.1177/1753425915609973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, Pison U, Floros J. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet 58: 181–191, 2000. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Thorenoor N, Wu R, DiAngelo SL, Ye M, Thomas NJ, Liao X, Lin TR, Warren S, Floros J. Genetic association of pulmonary surfactant protein genes, SFTPA1, SFTPA2, SFTPB, SFTPC, and SFTPD with cystic fibrosis. Front Immunol 9: 2256, 2018. doi: 10.3389/fimmu.2018.02256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Abdel-Razek O, Liu Z, Hu F, Zhou Q, Cooney RN, Wang G. Role of surfactant proteins A and D in sepsis-induced acute kidney injury. Shock 43: 31–38, 2015. doi: 10.1097/SHK.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Hu F, Liang W, Wang G, Singhal PC, Ding G. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in chinese women. Tohoku J Exp Med 221: 35–42, 2010. doi: 10.1620/tjem.221.35. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Hu F, Wang G, Zhou Q, Ding G. Lipopolysaccharide-induced expression of surfactant proteins A1 and A2 in human renal tubular epithelial cells. J Inflamm (Lond) 10: 2, 2013. doi: 10.1186/1476-9255-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology 111: 91–99, 2004. doi: 10.1111/j.1365-2567.2004.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack FX, Festa AL, Andrews RP, Linke M, Walzer PD. The carbohydrate recognition domain of surfactant protein A mediates binding to the major surface glycoprotein of Pneumocystis carinii. Biochemistry 36: 8092–8099, 1997. doi: 10.1021/bi970313f. [DOI] [PubMed] [Google Scholar]
- 25.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, Floros J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect Immun 75: 1403–1412, 2007. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan N, Giraud V, Picard C, Nunes H, Dastot-Le Moal F, Copin B, Galeron L, De Ligniville A, Kuziner N, Reynaud-Gaubert M, Valeyre D, Couderc LJ, Chinet T, Borie R, Crestani B, Simansour M, Nau V, Tissier S, Duquesnoy P, Mansour-Hendili L, Legendre M, Kannengiesser C, Coulomb-L’Hermine A, Gouya L, Amselem S, Clement A. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet 25: 1457–1467, 2016. doi: 10.1093/hmg/ddw014. [DOI] [PubMed] [Google Scholar]
- 27.Noutsios GT, Thorenoor N, Zhang X, Phelps DS, Umstead TM, Durrani F, Floros J. Major effect of oxidative stress on the male, but not female, SP-A1 type II cell miRNome. Front Immunol 10: 1514, 2019. doi: 10.3389/fimmu.2019.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaioannou AI, Konstantelou E, Papaporfyriou A, Bartziokas K, Spathis A, Bakakos P, Loukides S, Koulouris N, Papiris S, Kostikas K. Serum surfactant protein levels in patients admitted to the hospital with acute COPD exacerbation. Hai 196: 201–205, 2018. doi: 10.1007/s00408-018-0099-5. [DOI] [PubMed] [Google Scholar]
- 29.Pavlovic J, Papagaroufalis C, Xanthou M, Liu W, Fan R, Thomas NJ, Apostolidou I, Papathoma E, Megaloyianni E, DiAngelo S, Floros J. Genetic variants of surfactant proteins A, B, C, and D in bronchopulmonary dysplasia. Dis Markers 22: 277–291, 2006. doi: 10.1155/2006/817805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps DS, Umstead TM, Floros J. Sex differences in the acute in vivo effects of different human SP-A variants on the mouse alveolar macrophage proteome. J Proteomics 108: 427–444, 2014. doi: 10.1016/j.jprot.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phelps DS, Umstead TM, Silveyra P, Hu S, Wang G, Floros J. Differences in the alveolar macrophage proteome in transgenic mice expressing human SP-A1 and SP-A2. J Proteom Genom Res 1: 2–26, 2013. doi: 10.14302/issn.2326-0793.jpgr-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Barbero F, Rivas G, Steinhilber W, Casals C. Structural and functional differences among human surfactant proteins SP-A1, SP-A2 and co-expressed SP-A1/SP-A2: role of supratrimeric oligomerization. Biochem J 406: 479–489, 2007. doi: 10.1042/BJ20070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J Immunol 171: 417–425, 2003. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 34.Saxena S, Kumar R, Madan T, Gupta V, Muralidhar K, Sarma PU. Association of polymorphisms in pulmonary surfactant protein A1 and A2 genes with high-altitude pulmonary edema. Chest 128: 1611–1619, 2005. doi: 10.1378/chest.128.3.1611. [DOI] [PubMed] [Google Scholar]
- 35.Schicke E, Cseresnyés Z, Rennert K, Vau V, Haupt KF, Hornung F, Nietzsche S, Swiczak F, Schmidtke M, Glück B, Koch M, Schacke M, Heller R, Mosig AS, Figge MT, Ehrhardt C, Löffler B, Deinhardt-Emmer S. Staphylococcus aureus lung infection results in down-regulation of surfactant protein-A mainly caused by pro-inflammatory macrophages. Microorganisms 8: 577, 2020. doi: 10.3390/microorganisms8040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schob S, Dieckow J, Fehrenbach M, Peukert N, Weiss A, Kluth D, Thome U, Quäschling U, Lacher M, Preuß M. Occurrence and colocalization of surfactant proteins A, B, C and D in the developing and adult rat brain. Ann Anat 210: 121–127, 2017. doi: 10.1016/j.aanat.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Seifart C, Lin HM, Seifart U, Plagens A, DiAngelo S, von Wichert P, Floros J. Rare SP-A alleles and the SP-A1-6A4 allele associate with risk for lung carcinoma. Clin Genet 68: 128–136, 2005. doi: 10.1111/j.1399-0004.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 38.Selman M, Lin HM, Montaño M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo SL, Guo X, Umstead TM, Lang CM, Pardo A, Phelps DS, Floros J. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum Genet 113: 542–550, 2003. doi: 10.1007/s00439-003-1015-4. [DOI] [PubMed] [Google Scholar]
- 39.Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, Herrmann M, Hussain M, Geisbrecht BV, Kobzik L, Chroneos ZC. Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A. J Biol Chem 286: 4854–4870, 2011. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi H, Sano H, Chiba H, Kuroki Y. Pulmonary surfactant proteins A and D: innate immune functions and biomarkers for lung diseases. Curr Pharm Des 12: 589–598, 2006. doi: 10.2174/138161206775474387. [DOI] [PubMed] [Google Scholar]
- 41.Thorenoor N, Umstead TM, Zhang X, Phelps DS, Floros J. Survival of surfactant protein-A1 and SP-A2 transgenic mice after Klebsiella pneumoniae infection, exhibits sex-, gene-, and variant specific differences; treatment with surfactant protein improves survival. Front Immunol 9: 2404, 2018. doi: 10.3389/fimmu.2018.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian S, Li C, Ran R, Chen SY. Surfactant protein A deficiency exacerbates renal interstitial fibrosis following obstructive injury in mice. Biochim Biophys Acta Mol Basis Dis 1863: 509–517, 2017. doi: 10.1016/j.bbadis.2016.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol 308: F69–F75, 2015. doi: 10.1152/ajprenal.00484.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker SR, Williams MC, Benson B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 34: 1137–1148, 1986. doi: 10.1177/34.9.2426341. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84: 52–59, 2009. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weikert LF, Lopez JP, Abdolrasulnia R, Chroneos ZC, Shepherd VL. Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol 279: L216–L223, 2000. doi: 10.1152/ajplung.2000.279.2.L216. [DOI] [PubMed] [Google Scholar]
- 47.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 48.Wright JR, Hawgood S. Pulmonary surfactant metabolism. Clin Chest Med 10: 83–93, 1989. [PubMed] [Google Scholar]
- 49.Yang Y, Li Q, Tan F, Zhang J, Zhu W. Mechanism of IL-8-induced acute lung injury through pulmonary surfactant proteins A and B. Exp Ther Med 19: 287–293, 2020. doi: 10.3892/etm.2019.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zientara A, Stephan M, von Hörsten S, Schmiedl A. Differential severity of LPS-induced lung injury in CD26/DPP4 positive and deficient F344 rats. Histol Histopathol 34: 1151–1171, 2019. doi: 10.14670/HH-18-117. [DOI] [PubMed] [Google Scholar]