Keywords: differentiation, epigenetic control, stem cells, transcription factors, transcriptional regulation
Abstract
To fulfill the lifelong need to supply diverse epithelial cells, intestinal stem cells (ISCs) rely on executing accurate transcriptional programs. This review addresses the mechanisms that control those programs. Genes that define cell behaviors and identities are regulated principally through thousands of dispersed enhancers, each individually <1 kb long and positioned from a few to hundreds of kilobases away from transcription start sites, upstream or downstream from coding genes or within introns. Wnt, Notch, and other epithelial control signals feed into these cis-regulatory DNA elements, which are also common loci of polymorphisms and mutations that confer disease risk. Cell-specific gene activity requires promoters to interact with the correct combination of signal-responsive enhancers. We review the current state of knowledge in ISCs regarding active enhancers, the nucleosome modifications that may enable appropriate and hinder inappropriate enhancer-promoter contacts, and the roles of lineage-restricted transcription factors.
Intestinal stem cells (ISCs) renew the epithelial lining continuously and throughout life, producing progeny that differentiate into absorptive enterocytes or various secretory cell types. The diversity of cells emanating from ISCs requires cessation of ISC-specific transcription and the initiation of discrete new transcriptional programs. Nature tackles the “one genome, many outcomes” problem through compartmental organization of chromatin, restricted access to transcription factors (TFs), and targeted nucleosome modifications. Cell-specific gene activity requires promoters to interact with the correct combination of signal-responsive enhancers. Here, we review the transcriptional basis of ISC differentiation through the lens of cis-regulatory element dynamics.
GENOME ORGANIZATION FOR CONTROLLED, CELL TYPE-SPECIFIC GENE ACTIVITY
Chromatin interaction and imaging assays reveal that each interphase chromosome occupies a unique domain (75), so genes interact with their cognate enhancers predominantly in cis and rarely, if ever, in trans. Chromosomes subdivide grossly into inactive heterochromatin and transcriptionally active euchromatin, with specific covalent histone modifications enriched in each compartment (Fig. 1). For example, heterochromatin may be methylated on lysine 9 (K9) and lysine 27 (K27) in the NH2-terminal tails of histone H3 (H3K9me and H3K27me3), whereas active cis-elements are typically enriched for methyl (me) and acetyl (ac) groups on the same histone’s K4 and K27 residues, respectively (68). In the next known unit of chromosome order, short- (across a few kb) and long- (>1 Mb) range DNA interactions occur largely within topological associating domains (TADs) (52). Promoters, thereby constrained to interact with the enhancers available within their respective TADs, do so through diverse transcription-dependent subdomain contacts (29). Nucleosomes, the smallest units of chromosome order, carry 146 bp of DNA wrapped around histone octamers, and TF activities require these octamers to be displaced, in part, by ATP-dependent chromatin remodeling enzymes (9). Individual enhancers generally span the distance corresponding to one or two nucleosomes and are enriched for sequence motifs that bind selected TFs (Fig. 1). Among the 1 million to 2 million potential enhancers encoded in mammalian genomes, tens of thousands are nucleosome-free in any cell type. TF actions that occur synchronously at subsets of these cis-elements confer tissue-specific identities and functions.
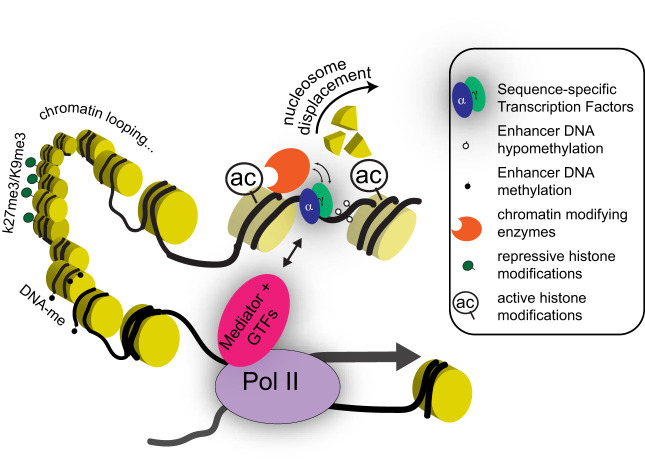
Fig. 1.
Epigenetic regulatory mechanisms discussed in this review. Gene transcription is influenced by multiple regulatory inputs coordinated by sequence-specific transcription factors (TFs) at distal cis-regulatory elements (enhancers). Chromatin accessibility is established at these genomic regions by pioneer TFs and remodeling enzymes, which together displace nucleosomes. Other TF-recruited enzymes endow neighboring nucleosomes with covalent posttranslational modifications associated with gene activity (e.g., H3K27 acetylation shown here as “ac”) or silencing (e.g., H3K27me3 and H3K9me3). In addition to carrying specific TF-binding motifs, enhancer DNA is hypomethylated at CpG dinucleotides, compared with inactive promoters or the genome background. Enhancers are thought to modulate transcription by forming three-dimensional loops that bring them close to RNA polymerase 2 (Pol II) and basal transcription machinery at target gene promoters. The protein complexes that bridge these loops contain Mediator and other general transcription factors (GTFs). Table 1 summarizes the current knowledge of these regulatory factors in the intestinal epithelium.
TFs deploy their DNA sequence specificity to compete with nucleosomes, bringing along chromatin-, histone- and DNA-modifying enzymes that contribute toward cis-regulatory activity (Fig. 1). The resulting activities enable identification and characterization of the full repertoire of enhancers active in a cell population. Nucleosome-depleted regions, synonymous with accessible or “open” chromatin, are sensitive to cleavage by DNase I or by transposase Tn5 in the assay for transposase-accessible chromatin (ATAC-seq) (11, 62), while those that immediately flank active enhancers usually carry covalent histone marks such as H3K4me1, H3K4me2, and H3K27ac (1). Chromatin immunoprecipitation and sequencing of the associated DNA fragments (ChIP-seq) identifies the sites that carry these canonical marks and bind specific TFs and ancillary proteins (4). Additionally, whereas most CpG dinucleotides in mammalian genomes are methylated (meCpG), those present in many active enhancers are not methylated to the same degree across cell populations, and whole-genome bisulfite sequencing (WGBS) identifies the cell type-restricted complement of hypomethylated enhancer DNA (60).
ATAC-seq, TF, or histone ChIP-seq, and WGBS data from ISCs, crypt progenitors, and mature villus cells capture transitions of active and inactive cis-elements that accompany, and may underlie, differentiation. These findings, which we review here, collectively inform current understanding of gene regulation during ISC differentiation and dedifferentiation. Of note, these approaches map putative enhancers, whose distant target genes and contributions toward their expression have not been validated experimentally. In general, however, enhancers so identified in intestinal and other cell populations lie near genes expressed in those cells (26, 37, 40, 69); even if some tissue-restricted enhancers are assigned incorrectly, aggregate statements are generally robust and accurate.
EPIGENETIC TRANSITIONS IN INTESTINAL CRYPT-VILLUS DIFFERENTIATION
Current challenges with ISC enhancers include understanding how they are chosen and maintained, and how they integrate external and cell-intrinsic signals to execute the ISC-specific transcriptional program and cell-specific functions. Molecular marker Lgr5 allows isolation of murine ISCs to high purity (3), but cell yields are currently sufficient only to reveal open chromatin by ATAC-seq, hypomethylated DNA using WGBS, and selected histone modifications by ChIP-seq. Cell numbers remain limiting for robust ChIP-seq analysis of many TFs and for proximity-based DNA ligation methods to impute 3D genome organization. Accordingly, detailed understanding of ISC enhancers lags behind that of the sites active in mature villus and proliferative crypt cells (20). Cell numbers are also low in intestinal organoid cultures, and because organoid differentiation occurs to an unknown extent under nonphysiological conditions, here, we limit discussion to in vivo findings (Table 1).
Table 1.
Summary of epigenetic regulatory changes in the intestinal epithelium
| Epigenomic Feature | Intestinal Epithelial Process | References |
|---|---|---|
| 3D chromatin looping | TADs are thought to be stable across cell types | |
| Genome-wide 3D chromatin interactions have not been mapped in the intestine | ||
| Chromatin accessiblity | Generally similar across intestinal epithelial cell populations | 37, 40 |
| Secretory cells have specific accessible regions bound to secretory cell-specific TFs | 32, 49 | |
| These regions lose chromatin accessiblity when secretory cells dedifferentiate into ISCs | 32 | |
| Histone Modifications | ||
| H3K4me1/2, H3K27ac | Overall similar across intestinal cell lineages | 37, 40 |
| Differences correlate with dynamic gene expression | 69 | |
| H3K27me3 | Associated with promoters of silent genes and largely stable across cell types. About 200 ISC-specific genes show increases H3K27me3 levels in differentiated cells | 31 |
| Loss of H3K27 methyltransferase activity permits reexpression of developmentally silenced genes, but ISC-specific genes are not reactivated in mature cells. | 31 | |
| DNA methylation | Majority of enhancers have similar methylation profiles across intestinal epithelial cell types | 30, 35 |
| Small differences occur at a subset of cell type-specific genes | 58 |
ISC, intestinal stem cells; TADS, topological associating domains; TFs, transcription factors; 3D, three-dimensional.
Human Caco-2 cells partially mimic intestinal epithelial turnover, with replicating subconfluent cells resembling the ISC/progenitor pool and postconfluent cultures resembling differentiated cells. The histone mark H3K4me2 identifies some sites that are common to both cell states and additional thousands that are highly enriched in one or the other state; different DNA sequence motifs are enriched in the two groups of state-specific sites, suggesting that ISC and mature cell functions reflect combinatorial TF actions at distinct enhancers (69). Differences in enhancer states are less stark between mouse small intestine ISCs, crypt progenitors, and villus epithelial cells, where H3K4me1/2, H3K27ac, and open chromatin profiles are strikingly similar, especially between ISCs and the enterocyte lineage (37, 40). These findings imply that in vivo the cis-element repertoire for differentiated cell functions is largely established in Lgr5+ ISCs and that differentiation into postmitotic enterocytes is dominated by activation of these existing sites, with a smaller contribution from TFs generating access to new enhancers.
Adding to the evidence for limited enhancer dynamics, meCpG profiles defined by WGBS differ little between adult ISCs and mature villus enterocytes (30, 35) or colonocytes (73). Statistical comparisons of DNA hypomethylation are, however, less robust in areas of low CpG density, and another study found discernible meCpG differences at enhancers near 10% to 15% of ISC- and enterocyte-selective genes (58); thus, many intestinal enhancers are, indeed, dynamic. When the methyltransferase DNMT1 is absent from the intestine, meDNA is globally reduced, crypt cell proliferation is increased (22), and differentiation is notably impaired (73) in young mice. Additional loss of another methyltransferase, DNMT3B, results in lethality (21), a phenotype that is not established to reflect defective enhancer function. In contrast to the single-base resolution of WGBS, immunoprecipitation of hydroxymethylated DNA returns data at lower, ~150-bp resolution. The latter method identifies thousands of differences between crypt stem/progenitor and villus cell populations in hydroxymethylated DNA at coding regions, largely correlated with gene activity (39, 65). These changes during differentiation are mediated by the same enzymes that demethylate DNA, but only a minority localize at distant enhancers, where the role in gene regulation remains unclear.
Gene expression evolves rapidly during development, requiring enhancers to be recruited and decommissioned in quick succession. Enhancers active during development subsequently lose accessible chromatin, TF occupancy, and H3K27ac marks, but may retain hypomethylated DNA and trace H3K4me1 over scores to hundreds of cell divisions, well into adulthood (28). As a result, the complement of hypomethylated sites in adult intestinal cells encompasses not only active enhancers but also a majority of those used during organogenesis (30). Genomic regions that carry H3K4me1 but lack H3K27ac are regarded as “poised” for future activity (18). However, their numbers in short-lived intestinal epithelium exceed those of active H3K27ac+ enhancers, and rather than poised cis-elements, this large pool of H3K4me1+ H3K27ac− sites with hypomethylated DNA appears to be a vestige of fetal cis-regulatory activity (18).
ATAC-seq analysis of purified adult enteroendocrine and goblet cell precursors subsequently revealed thousands of genomic regions that are selectively open in these secretory cells (i.e., inaccessible in ISCs or enterocytes), lie near lineage-restricted genes, and carry DNA sequence motifs that bind secretory-cell TFs (32). A recent study verified the broad base of equally open chromatin among mouse intestinal epithelial cells, with additional sites detected in secretory cells (49). As a group, these putative enhancers lack H3K27ac and have little H3K4me1, so their apparent activity in the secretory lineage (32) challenges the idea that those histone marks are essential for cis-element function. Active enhancers lacking canonical histone marks have also been detected in embryonic stem cells (47) and experiments in other cells and species suggest that H3K4 methylation is dispensable for gene activity in vivo (19, 50). One provocative possibility is that the purpose of “active” histone modifications is not to influence cis-element activity per se but to flag them during cell division (“mitotic bookmarking”) (36). In principle, postmitotic secretory cells may, therefore, dispense with certain histone marks, while retaining the open chromatin necessary for TF binding and enhancer activity.
TFS IN INTESTINAL CRYPT-VILLUS DIFFERENTIATION
ISCs must express TFs that recognize DNA sequences in the desired genomic sites and bear domains that interact with crucial signals from the Wnt, Notch, and other pathways. These TFs’ dynamic interactions, with external signals on one hand and specific cis-regulatory elements on the other, collectively determine ISC responses such as cell division, remaining in or leaving the ISC pool, and differentiation into enterocyte or secretory progenitors (Fig. 2). TFs that uniquely support ISC functions are not as yet fully characterized. The Krüppel-like zinc-finger protein KLF5 is one of few crypt-restricted TFs (38), and the basic-helix-loop-helix factor ASCL2 is the only known TF tightly restricted to Lgr5+ ISCs (66). The sequence motif for Krüppel-like TFs is particularly enriched among enhancers marked in ISCs, and depletion of KLF5 results in ISC failure, with precocious differentiation (38). Early findings suggested that Ascl2 is similarly indispensable for ISC survival (66), but subsequent studies revealed only a subtle role in ensuring long-term integrity of the ISC compartment and a more overt requirement in allowing progenitors to dedifferentiate when ISCs are deficient (43, 57). Crucial transcriptional targets of KLF5 and ASCL2 have been hard to define, highlighting the technical challenges of ChIP-seq in ISC, and available data indicate that ASCL2 facilitates the intestine-specific response to Wnt signaling at many loci (57).
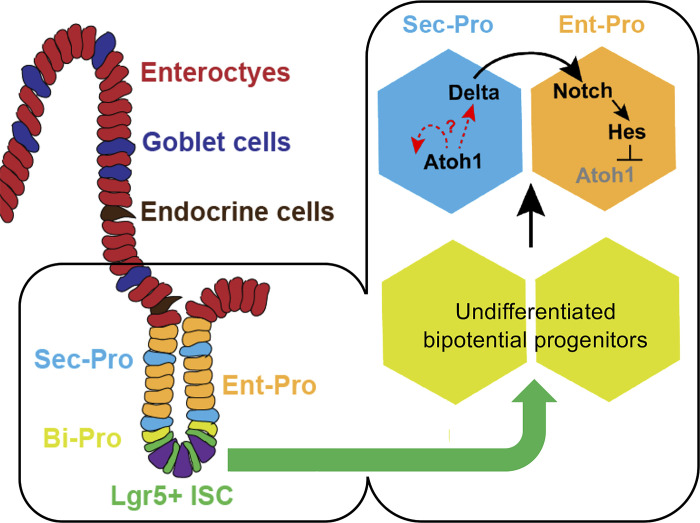
Fig. 2.
Crucial cell fate decisions in intestinal epithelium. The choice between absorptive enterocytes (Ent) and secretory (Sec) cells is enacted in transient bipotential progenitor (Bi-Pro) cells very soon after intestinal stem cells (ISCs) leave the stem-cell compartment. This cell fate decision depends on signaling from cell-bound ligands of the Delta family to adjoining cells that carry Notch-family receptors. Notch signaling activates the transcriptional repressor Hes1, which tightly represses expression of the transcription factor (TF) gene Atoh1. Delta-positive cells increase Atoh1 expression and adopt the Sec fate, while Atoh1-negative cells become enterocytes. [Adapted with permission from T.-H. Kim et al., Nature 506: 511–515, 2014.] Examples of TFs enriched in ISCs and different epithelial progenitors (Pro) are listed in Table 2.
Enterocyte-active regions are most enriched for sequence motifs that bind AP-1, HNF4, CDX, KLF, and GATA-family TFs, and the epithelium expresses certain members of each family. Notable examples, such as the intestine-specifying homeodomain protein CDX2, its functional partners HNF4A and HNF4G, and region-specifying zinc-finger proteins GATA4 and GATA6, are expressed in most or all epithelial cells, including ISCs (5–7, 15, 54, 55, 69). CDX2 is present throughout the epithelium, but at least some target genes are different in ISCs and enterocytes (55). Absence of CDX2 profoundly impairs ISC replication, presumably acting through ISC-selective enhancers (55); additional absence of its homolog CDX1 blocks replication of both ISC and transit-amplifying crypt cells (69, 70). In contrast, TFs such as ATOH1, GFI1, SPDEF, NEUROG3, and NEUROD1 are restricted to single or all Sec cell types (25, 33, 40, 44, 59, 72) and likely interact selectively with Sec-restricted enhancers.
In summary, a handful of intestinal TFs (some restricted to ISCs, others expressed broadly) interact with available enhancers to fulfill ISC- and enterocyte-specific functions. Separately, ATOH1-mediated secretory differentiation activates thousands of lineage-restricted enhancers that presumably engage secretory cell-specific TFs to drive goblet, enteroendocrine, Paneth, and tuft cell identities and functions (Table 2). Additional ChIP, chromosomal conformation, and functional data are necessary to determine whether the panoply of intestinal TFs controls overlapping or distinct groups of enhancers and genes.
Table 2.
Exemplary intestinal epithelial lineage-restricted transcription factors
| Transcription Factor |
|---|
| Expressed in all or most epithelial cells |
| Hnf4a |
| Hnf4g |
| Cdx2 |
| Gata4 |
| Gata6 |
| Enriched in resting and regenerating ISCs |
| Ascl2 |
| Enriched in enterocytes |
| Hes1 |
| Klf5 (enriched in crypt cells, including ISCs) |
| Enriched in secretory-fated cells |
| Atoh1 |
| Spdef |
| Gfi1 |
| Neurog3(enriched in endocrine cells) |
| Neurod1(enriched in endocrine cells) |
| Klf4 (enriched in goblet cells) |
ISC, intestinal stem cells.
ROLES FOR GENE SILENCING
Gene regulation involves both activation and controlled repression. One histone modification classically associated with gene silencing, H3K27me3, is found predominantly near transcription start sites and is largely absent from distant enhancers (27). Its erasure reactivates certain developmentally silenced genes in many tissues and in embryonic stem cells (8, 31, 45). At least in the latter, chemical interference with RNA transcription causes H3K27me3 to appear at experimentally silenced promoters (51), raising the prospect that H3K27me3 does not “cause” transcriptional repression per se. Although thousands of transcripts are modulated during the crypt-villus transition in wild-type mice, genome-wide H3K27me3 distributions are substantively similar in ISCs and postmitotic villus cells, and gene activity correlates little with promoter H3K27me3 dynamics (31). Thus, the H3K27me3 profile is largely specific to the tissue and not to its constituent cell states, although ~200 ISC-selective genes, including Lgr5 and Ascl2, acquire H3K27me3 locus-wide when cells exit the ISC compartment. Mouse intestines with epithelium-specific absence of the H3K27 methyltransferase complex PRC2 derepress hundreds of developmentally silenced genes within 3 or 4 days, but do not express H3K27me3-marked ISC genes in mature cells (31). Thus, when ISCs differentiate, PRC2-catalyzed H3K27me3 signifies, but does not cause, state-specific transcriptional silencing of canonical ISC genes.
An unexpected finding after many days of PRC2 deficiency in adult intestines is extensive recovery of the archival memory encapsulated in hypomethylated developmental enhancers. Decommissioned fetal enhancers, which usually bear traces of H3K4me1 and ATAC sensitivity, reactivate sooner than embryonic enhancers, where reduced meCpG is the only known residual feature (30). Linked genes become expressed as a result of this developmental cis-element reactivation, but PRC2 activity being largely absent from adult or embryonic enhancers, this striking consequence of its absence must be indirect. Developmental TFs activated early as a direct result of promoter H3K27me3 erasure likely later reactivate the dormant tissue-specific fetal and embryonic enhancers (30).
AN EPIGENETIC BASIS FOR CRYPT CELL DEDIFFERENTIATION?
Intestinal crypts’ ability to restore ablated Lgr5-positive ISCs is well recognized. “Reserve” stem cells located in the 4th to 6th crypt tiers (+4 cells) were regarded as a principal source of ISC recovery (46, 56), but the evidence now favors various crypt progenitor cells—recent progeny of Lgr5-positive ISC that survived ISC ablation—as the predominant source. Both secretory (10, 32, 34, 56, 63, 67, 71, 74) and absorptive (61) cells harbor the latent capacity to revert into ISCs, raising the question of what chromatin barriers these cells overcome to accommodate the altered state. An additional, intriguing possibility is that initial ISC differentiation is inherently unstable, with crypt base cells shuttling between ISC and post-ISC states until one stabilizes. As noted above, chromatin states signified by H3K4me1/2, H3K27ac, and H3K27me3 marks are substantively similar along the ISC-enterocyte continuum. This similarity may reflect a low chromatin barrier for toggling between cell states and, thus, explain the remarkable ability of specified progenitors to revert quickly into Lgr5+ ISC; thus, both ISC differentiation and dedifferentiation may reflect TFs’ alternative interactions with a broadly permissive chromatin landscape. The appeal of this idea notwithstanding, when Sec cells dedifferentiate in response to ISC ablation, the chromatin at thousands of putative Sec-restricted enhancers becomes inaccessible within 36 h to 48 h (32). Thus, these areas of open, unmarked chromatin in differentiated cells do not represent a barrier that cells find difficult to breach. Adoption of a “different” tissue’s chromatin landscape is rare (e.g., gastric intestinal metaplasia), but chromatin transitions that occur naturally within the adult intestinal epithelium seem to reverse readily.
DELINATION OF INTESTINAL ENHANCERS AND THEIR INTERACTIONS WITH TFS
ISC functions depend on well-known signaling pathways, including Notch, Hippo, and, most famously, Wnt. However, the same pathways control diverse processes in nearly every organ, with vastly different outcomes arising through the same transcriptional effectors: TCF/β-catenin, RBPJK, and TEAD protein complexes for Wnt, Notch, and Hippo signaling, respectively. How do cells craft unique transcriptional responses to the same signal? One solution lies in the repertoire of enhancers available to the global effector complexes: chromatin is accessible at different sites in intestinal than in other cells. Consistent with this idea, one ChIP study of the Wnt-effector protein TCF7L2 in cell lines from different tissues found that only 1.6% of 116,000 binding sites were present in all six lines (23). Therefore, adult tissue responses to external signals are dictated in large part by the stable, tissue-specific enhancer repertoires established during development. The intestinal chromatin landscape is initiated, in part, through the actions of CDX2 in the early embryo (2, 42), then reshaped late in gestation toward a nearly mature chromatin landscape by concerted actions of CDX2 and HNF4 factors (14). BMP signaling is an important determinant of adult crypt-villus differentiation, suppressing expression of ISC-specific genes in crypts (48) and cooperating in the villus with HNF4 factors, via the BMP-effector SMAD4, to promote enterocyte differentiation (15).
FRONTIERS OF EPIGENETIC REGULATION IN ISCS
Much remains to be learned in biochemical terms about how cis-elements assimilate diverse cues to implement crypt- and villus-specific signal-responsive gene expression. For example, do the TFs that direct Wnt and BMP responses, such as CDX2 (55), ASCL2 (43, 57), and HNF4 (15), stabilize binding of the corresponding signal effectors at cognate enhancers? Are discrete enhancers strengthened as a result, and is that modulation manifested in the amplitude or the frequency of transcriptional bursts? Mapping of the intestinal enhancer repertoire paves the way to characterize chromatin dynamics in response to specific physiologic and pathologic signals.
The intestinal epithelium responds to changes in diet, age, microbes, and immune activation. In most cases, it is unknown whether these responses occur in mature or progenitor cells and whether they involve epigenetic reprogramming or TF interactions with a fixed epigenome. Chromatin accessibility is largely similar in the epithelium of conventionally raised and germ-free mice (12), although responses to specific microbes have not been investigated in detail, and another group identified limited differences in methylation of selected CpG residues in germ-free mice (73). Dietary perturbations produce dynamic metabolic changes that may alter the epigenome. For example, ketone bodies that accumulate during fasting may inhibit histone deacetylases and, thereby, promote ISC renewal (16). However, dietary and metabolic influences on the intestinal epigenome remain largely unexplored, as do the effects from acute inflammation, compromised barrier function, and chronic immune conditions. These questions merit mechanistic investigation layered upon the growing understanding of interactions between canonical intestinal TFs and cell-specific epigenome states summarized in this article. Finally, noncoding RNAs are believed to regulate and mediate certain epigenome changes (13, 41). The intestinal epithelium expresses hundreds of microRNAs, some very selectively compared with other endoderm-derived tissues (24), and their biological functions have been reviewed previously (53). Examples such as miR-31 are necessary for ISC activity and regeneration (64), but it remains unclear if those requirements represent direct regulation of target transcripts or some epigenome process. Further investigation in vivo or in organoid models will shed light on these questions and, more broadly, on diverse epigenetic bases for human intestinal disorders.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.P.V. and R.A.S. drafted manuscript; M.P.V. and R.A.S. edited and revised manuscript; M.P.V. and R.A.S. approved final version of manuscript.
REFERENCES
- 1.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet 17: 487–500, 2016. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee KK, Saxena M, Kumar N, Chen L, Cavazza A, Toke NH, O’Neill NK, Madha S, Jadhav U, Verzi MP, Shivdasani RA. Enhancer, transcriptional, and cell fate plasticity precedes intestinal determination during endoderm development. Genes Dev 32: 1430–1442, 2018. doi: 10.1101/gad.318832.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 4.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837, 2007. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Battle MA, Bondow BJ, Iverson MA, Adams SJ, Jandacek RJ, Tso P, Duncan SA. GATA4 is essential for jejunal function in mice. Gastroenterology 135: 1676–1686.e1, 2008. doi: 10.1053/j.gastro.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuling E, Baffour-Awuah NY, Stapleton KA, Aronson BE, Noah TK, Shroyer NF, Duncan SA, Fleet JC, Krasinski SD. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology 140: 1219–1229.e2, 2011. doi: 10.1053/j.gastro.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1α, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem 277: 31909–31917, 2002. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 8.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441: 349–353, 2006. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 9.Brahma S, Henikoff S. Epigenome regulation by dynamic nucleosome unwrapping. Trends Biochem Sci 45: 13–26, 2020. doi: 10.1016/j.tibs.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 11.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10: 1213–1218, 2013. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camp JG, Frank CL, Lickwar CR, Guturu H, Rube T, Wenger AM, Chen J, Bejerano G, Crawford GE, Rawls JF. Microbiota modulate transcription in the intestinal epithelium without remodeling the accessible chromatin landscape. Genome Res 24: 1504–1516, 2014. doi: 10.1101/gr.165845.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157: 77–94, 2014. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Toke NH, Luo S, Vasoya RP, Aita R, Parthasarathy A, Tsai YH, Spence JR, Verzi MP. HNF4 factors control chromatin accessibility and are redundantly required for maturation of the fetal intestine. Development 146: dev179432, 2019. doi: 10.1242/dev.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Toke NH, Luo S, Vasoya RP, Fullem RL, Parthasarathy A, Perekatt AO, Verzi MP. A reinforcing HNF4-SMAD4 feed-forward module stabilizes enterocyte identity. Nat Genet 51: 777–785, 2019. doi: 10.1038/s41588-019-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, Moreno-Serrano M, Iqbal AM, Bauer-Rowe KE, Imada S, Ulutas MS, Mylonas C, Whary MT, Levine SS, Basbinar Y, Hynes RO, Mino-Kenudson M, Deshpande V, Boyer LA, Fox JG, Terranova C, Rai K, Piwnica-Worms H, Mihaylova MM, Regev A, Yilmaz OH. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 178: 1115–1131.e15, 2019. doi: 10.1016/j.cell.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA 107: 21931–21936, 2010. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorighi KM, Swigut T, Henriques T, Bhanu NV, Scruggs BS, Nady N, Still CD II, Garcia BA, Adelman K, Wysocka J. Mll3 and Mll4 facilitate enhancer RNA synthesis and transcription from promoters independently of H3K4 monomethylation. Mol Cell 66: 568–576.e4, 2017. doi: 10.1016/j.molcel.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott EN, Kaestner KH. Epigenetic regulation of the intestinal epithelium. Cell Mol Life Sci 72: 4139–4156, 2015. doi: 10.1007/s00018-015-1997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott EN, Sheaffer KL, Kaestner KH. The ‘de novo’ DNA methyltransferase Dnmt3b compensates the Dnmt1-deficient intestinal epithelium. eLife 5: e12975, 2016. doi: 10.7554/eLife.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott EN, Sheaffer KL, Schug J, Stappenbeck TS, Kaestner KH. Dnmt1 is essential to maintain progenitors in the perinatal intestinal epithelium. Development 142: 2163–2172, 2015. doi: 10.1242/dev.117341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frietze S, Wang R, Yao L, Tak YG, Ye Z, Gaddis M, Witt H, Farnham PJ, Jin VX. Cell type-specific binding patterns reveal that TCF7L2 can be tethered to the genome by association with GATA3. Genome Biol 13: R52, 2012. doi: 10.1186/gb-2012-13-9-r52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Schug J, McKenna LB, Le Lay J, Kaestner KH, Greenbaum LE. Tissue-specific regulation of mouse microRNA genes in endoderm-derived tissues. Nucleic Acids Res 39: 454–463, 2011. doi: 10.1093/nar/gkq782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137: 1333–1345.e3, 2009. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459: 108–112, 2009. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39: 311–318, 2007. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 28.Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD, Ren B. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet 45: 1198–1206, 2013. doi: 10.1038/ng.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh TS, Cattoglio C, Slobodyanyuk E, Hansen AS, Rando OJ, Tjian R, Darzacq X. Resolving the 3D landscape of transcription-linked mammalian chromatin folding. Mol Cell 78: 539–553.e8, 2020. doi: 10.1016/j.molcel.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadhav U, Cavazza A, Banerjee KK, Xie H, O’Neill NK, Saenz-Vash V, Herbert Z, Madha S, Orkin SH, Zhai H, Shivdasani RA. Extensive recovery of embryonic enhancer and gene memory stored in hypomethylated enhancer DNA. Mol Cell 74: 542–554.e5, 2019. doi: 10.1016/j.molcel.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadhav U, Nalapareddy K, Saxena M, O’Neill NK, Pinello L, Yuan GC, Orkin SH, Shivdasani RA. Acquired tissue-specific promoter bivalency is a basis for PRC2 necessity in adult cells. Cell 165: 1389–1400, 2016. doi: 10.1016/j.cell.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 21: 65–77.e5, 2017. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21: 6338–6347, 2002. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones JC, Brindley CD, Elder NH, Myers MG Jr, Rajala MW, Dekaney CM, McNamee EN, Frey MR, Shroyer NF, Dempsey PJ. Cellular plasticity of Defa4(Cre)-expressing Paneth cells in response to Notch activation and intestinal injury. Cell Mol Gastroenterol Hepatol 7: 533–554, 2019. doi: 10.1016/j.jcmgh.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaaij LT, van de Wetering M, Fang F, Decato B, Molaro A, van de Werken HJ, van Es JH, Schuijers J, de Wit E, de Laat W, Hannon GJ, Clevers HC, Smith AD, Ketting RF. DNA methylation dynamics during intestinal stem cell differentiation reveals enhancers driving gene expression in the villus. Genome Biol 14: R50, 2013. doi: 10.1186/gb-2013-14-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics Chromatin 6: 6, 2013. doi: 10.1186/1756-8935-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazakevych J, Sayols S, Messner B, Krienke C, Soshnikova N. Dynamic changes in chromatin states during specification and differentiation of adult intestinal stem cells. Nucleic Acids Res 45: 5770–5784, 2017. doi: 10.1093/nar/gkx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim CK, Saxena M, Maharjan K, Song JJ, Shroyer KR, Bialkowska AB, Shivdasani RA, Yang VW. Kruppel-like Factor 5 regulates stemness, lineage specification, and regeneration of intestinal epithelial stem cells. Cell Mol Gastroenterol Hepatol 9: 587–609, 2020. doi: 10.1016/j.jcmgh.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim R, Sheaffer KL, Choi I, Won KJ, Kaestner KH. Epigenetic regulation of intestinal stem cells by Tet1-mediated DNA hydroxymethylation. Genes Dev 30: 2433–2442, 2016. doi: 10.1101/gad.288035.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature 506: 511–515, 2014. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 172: 393–407, 2018. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar N, Tsai YH, Chen L, Zhou A, Banerjee KK, Saxena M, Huang S, Toke NH, Xing J, Shivdasani RA, Spence JR, Verzi MP. The lineage-specific transcription factor CDX2 navigates dynamic chromatin to control distinct stages of intestine development. Development 146: dev172189, 2019. doi: 10.1242/dev.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata K, Jadhav U, Madha S, van Es J, Dean J, Cavazza A, Wucherpfennig K, Michor F, Clevers H, Shivdasani RA. Ascl2-dependent cell dedifferentiation drives regeneration of ablated intestinal stem cells. Cell Stem Cell 26: 377–390.e6, 2020. doi: 10.1016/j.stem.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutoh H, Fung BP, Naya FJ, Tsai MJ, Nishitani J, Leiter AB. The basic helix-loop-helix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc Natl Acad Sci USA 94: 3560–3564, 1997. doi: 10.1073/pnas.94.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol 27: 3769–3779, 2007. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Pradeepa MM, Grimes GR, Kumar Y, Olley G, Taylor GC, Schneider R, Bickmore WA. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet 48: 681–686, 2016. doi: 10.1038/ng.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, Li B, Han JJ, Chen YG. BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat Commun 8: 13824, 2017. doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raab JR, Tulasi DY, Wager KE, Morowitz JM, Magness ST, Gracz AD. Quantitative classification of chromatin dynamics reveals regulators of intestinal stem cell differentiation. Development 147: dev181966, 2020. doi: 10.1242/dev.181966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rickels R, Herz HM, Sze CC, Cao K, Morgan MA, Collings CK, Gause M, Takahashi YH, Wang L, Rendleman EJ, Marshall SA, Krueger A, Bartom ET, Piunti A, Smith ER, Abshiru NA, Kelleher NL, Dorsett D, Shilatifard A. Histone H3K4 monomethylation catalyzed by Trr and mammalian COMPASS-like proteins at enhancers is dispensable for development and viability. Nat Genet 49: 1647–1653, 2017. doi: 10.1038/ng.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riising EM, Comet I, Leblanc B, Wu X, Johansen JV, Helin K. Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol Cell 55: 347–360, 2014. doi: 10.1016/j.molcel.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Rowley MJ, Corces VG. Organizational principles of 3D genome architecture. Nat Rev Genet 19: 789–800, 2018. doi: 10.1038/s41576-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Runtsch MC, Round JL, O’Connell RM. MicroRNAs and the regulation of intestinal homeostasis. Front Genet 5: 347, 2014. doi: 10.3389/fgene.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.San Roman AK, Aronson BE, Krasinski SD, Shivdasani RA, Verzi MP. Transcription factors GATA4 and HNF4A control distinct aspects of intestinal homeostasis in conjunction with transcription factor CDX2. J Biol Chem 290: 1850–1860, 2015. doi: 10.1074/jbc.M114.620211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.San Roman AK, Tovaglieri A, Breault DT, Shivdasani RA. Distinct processes and transcriptional targets underlie CDX2 requirements in intestinal stem cells and differentiated villus cells. Stem Cell Reports 5: 673–681, 2015. doi: 10.1016/j.stemcr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V, van der Flier LG, Cuppen E, van Oudenaarden A, Clevers H. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16: 158–170, 2015. doi: 10.1016/j.stem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, Schübeler D, Kaestner KH. DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28: 652–664, 2014. doi: 10.1101/gad.230318.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev 19: 2412–2417, 2005. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stadler MB, Murr R, Burger L, Ivanek R, Lienert F, Schöler A, van Nimwegen E, Wirbelauer C, Oakeley EJ, Gaidatzis D, Tiwari VK, Schübeler D. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature 480: 490–495, 2011. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 61.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 18: 203–213, 2016. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA. The accessible chromatin landscape of the human genome. Nature 489: 75–82, 2012. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011. [Erratum in Nature 482: 120, 2012.] doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian Y, Ma X, Lv C, Sheng X, Li X, Zhao R, Song Y, Andl T, Plikus MV, Sun J, Ren F, Shuai J, Lengner CJ, Cui W, Yu Z. Stress responsive miR-31 is a major modulator of mouse intestinal stem cells during regeneration and tumorigenesis. eLife 6: e29538, 2017. doi: 10.7554/eLife.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uribe-Lewis S, Carroll T, Menon S, Nicholson A, Manasterski PJ, Winton DJ, Buczacki SJA, Murrell A. 5-Hydroxymethylcytosine and gene activity in mouse intestinal differentiation. Sci Rep 10: 546, 2020. doi: 10.1038/s41598-019-57214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136: 903–912, 2009. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 67.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14: 1099–1104, 2012. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Steensel B, Belmont AS. Lamina-associated domains: Links with chromosome architecture, heterochromatin, and gene repression. Cell 169: 780–791, 2017. doi: 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verzi MP, Shin H, He HH, Sulahian R, Meyer CA, Montgomery RK, Fleet JC, Brown M, Liu XS, Shivdasani RA. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell 19: 713–726, 2010. [Erratum in Dev Cell 31: 801, 2014.] doi: 10.1016/j.devcel.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verzi MP, Shin H, Ho LL, Liu XS, Shivdasani RA. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol 31: 2026–2039, 2011. doi: 10.1128/MCB.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158, 2001. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 73.Yu DH, Gadkari M, Zhou Q, Yu S, Gao N, Guan Y, Schady D, Roshan TN, Chen MH, Laritsky E, Ge Z, Wang H, Chen R, Westwater C, Bry L, Waterland RA, Moriarty C, Hwang C, Swennes AG, Moore SR, Shen L. Postnatal epigenetic regulation of intestinal stem cells requires DNA methylation and is guided by the microbiome. Genome Biol 16: 211, 2015. doi: 10.1186/s13059-015-0763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N. Paneth cell multipotency induced by Notch activation following injury. Cell Stem Cell 23: 46–59.e5, 2018. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng H, Xie W. The role of 3D genome organization in development and cell differentiation. Nat Rev Mol Cell Biol 20: 535–550, 2019. doi: 10.1038/s41580-019-0132-4. [DOI] [PubMed] [Google Scholar]