Highlights
-
•
A patient developed encephalopathy two months after a mild case of COVID-19.
-
•
MRI Brain demonstrated extensive white matter lesions juxtacortically and subcortically.
-
•
Workup for infectious, paraneoplastic, toxic process was negative.
-
•
MBP was elevated, prompting diagnosis of post-infectious ADEM following COVID-19.
-
•
COVID-19 patients without hypoxia may have post-infectious leukoencephalopathy.
Keywords: COVID-19, Leukoencephalopathy, Acute disseminated encephalomyelitis
To the Editor
A 35-year-old African American female with history of gastric bypass surgery and iron deficiency anemia had a positive nasopharyngeal swab for SARS-CoV-2 PCR in April 2020 in the setting of anosmia and ageusia. Per her sister's report, she did not have confusion, dyspnea, or cough. She did not receive any treatment for COVID-19. Bloodwork in May 2020 demonstrated positive serum SARS-CoV-2 antibodies.
In early June 2020, she presented to an outside emergency department with six days of gait instability. Per documentation, her neurologic evaluation revealed symmetric distal neuropathy. MRI brain without contrast was read as “hemispheric white matter signal appears somewhat elevated…with sparing of juxtacortical regions...extend[ing] to anteromedial temporal lobes…less conspicuous in posterior fossa.” Vitamin and mineral levels were sent to evaluate her neuropathy; zinc, copper, and vitamins B1, B6 and B12, were normal, but vitamin E was low, so she was started on supplemental tocopherol. Additionally, due to her history of COVID-19, erythrocyte sedimentation rate (ESR) and D-dimer were checked; both were elevated [ESR was 105 mm/h (normal 0–22 mm/h) and D-dimer was 308 ng/mL (normal <230 ng/mL)]. She was discharged from the emergency room with a plan for outpatient follow up with neurology.
Seven days later, she presented to a different emergency room due to persistent gait instability, decreased appetite and generalized weakness. Per documentation, she was drowsy and slow to respond. Per report, non-contrast head CT, urine toxicology, C-reactive protein, HIV screen, basic metabolic panel and complete blood cell count were within normal limits. Nasopharyngeal swab for SARS-COV-2 PCR was negative. On hospital day 2, she became more lethargic, so a lumbar puncture was performed; it demonstrated 1 WBC, 0 RBC, protein of 22 mg/dL, glucose 76 mg/dL, negative meningitis-encephalitis panel (E. coli, H. flu, Listeria, N. Meningitis, S. Agalactaia, S. Pneumo, CMV, Enterovirus, HSV1, HSV2, HHV6, VZV, and cryptococcus), and negative culture. Transvaginal ultrasound did not reveal a teratoma. By hospital day 4, she was unarousable to noxious stimuli. MRI brain with and without contrast was repeated and, while it was severely motion degraded, it showed symmetric periventricular white matter FLAIR hyperintensities involving bilateral cerebral peduncles with mild diffusion restriction. Although she had been hemodynamically stable since admission and did not require supplemental oxygen, she was upgraded to the ICU for close monitoring. On hospital day 7, she was unable to clear her secretions and desaturated to 70%, prompting intubation. She had a CT chest which demonstrated “sequela of post-infectious chronic bronchiolitis.”
At this time, she was transferred to our tertiary hospital for further care. On initial examination, while on propofol, she was unarousable to noxious stimulation, had intact brainstem reflexes, extended her upper extremities, and triple flexed her lower extremities to deep nailbed pressure. Repeat MRI brain demonstrated extensive diffuse confluent periventricular, temporal, subcortical and midbrain hyperintensities overall mildly progressed since prior MRI (see Fig. 1, Fig. 2 ) with mild patchy diffusion restriction, no contrast enhancement, and no evidence of microhemorrhages on SWI. MRA head showed normal vessels. MRI of the total spine did not show any evidence of cord pathology. Electroencephalogram demonstrated mild to moderate slowing with no epileptiform activity.
Fig. 1.
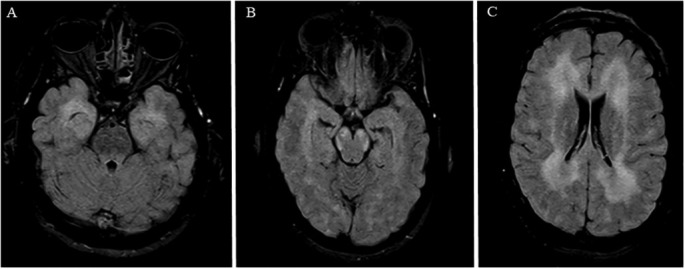
MRI Brain FLAIR axial cuts demonstrating diffuse confluent T2/FLAIR hyperintensity involving A) anteromedial temporal lobes bilaterally, B) cerebral peduncles bilaterally, and C) bilateral hemispheres.
Fig. 2.
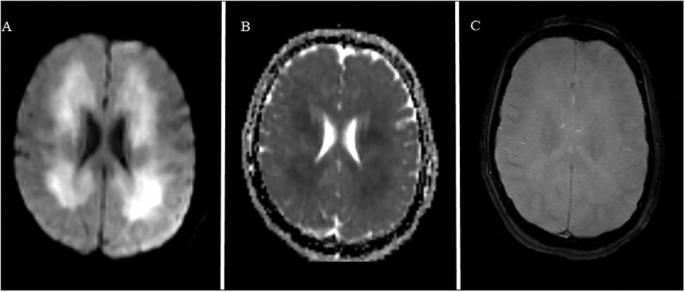
MRI Brain DWI, ADC and HEMOFLASH sequences demonstrating A) patchy diffusion restriction in the regions of leukoencephalopathy juxtacortically and subcortically with B) ADC correlate and C) no evidence of microhemorrhage.
A second lumbar puncture was performed and demonstrated 2 WBC, 51 RBC, protein 19 mg/dL, glucose 70 mg/dL and negative: culture, meningitis-encephalitis panel (same as afore mentioned panel), JC virus, West Nile PCR, EBV PCR, HSV1/2 PCR, and oligoclonal bands. Myelin Basic Protein (MBP) was elevated at 8.4 μg/L (normal 2.0–4.0 μg/L). Autoimmune encephalitis panel was normal. Serum aquaporin-4 antibody and myelin oligodendrocyte glycoprotein antibody were negative. Both nasopharyngeal swab for SARS-CoV-2 PCR and serum SARS-CoV-2 IgG were negative twice. We were unable to send SARS-CoV-2 PCR or antibody in the cerebrospinal fluid. Additional tests to assess for a rheumatologic process including anti-nuclear antibodies, anti-AMA, anti-JO-1, anti-LKM, anti-phospholipid antibodies and IgG levels all returned within normal limits. Serum ceruloplasmin, lyme serologies, and vitamin A were also within normal limits.
Based on these results, her condition was attributed to post-infectious acute disseminated encephalomyelitis (ADEM) related to her prior COVID-19 infection. She was treated with 1 mg/kg methylprednisolone for 5 days and 2 g/kg of intravenous immunoglobulin (IVIG) divided over 3 days. As she did not improve, we subsequently administered 5 days of plasma exchange (PLEX) therapy. Unfortunately, as of hospital day 48, she had not improved, and was transferred to a long term care facility.
There are many etiologies for white matter changes on an MRI brain including, but not limited to, ADEM, multiple sclerosis, inherited white matter diseases, lymphoma, progressive multifocal leukoencephalopathy, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, toxic metabolic changes, vasculitis, sarcoidosis, age, radiation, and lyme disease [1]. Recently, there have been multiple reports of leukoencephalopathy in patients with COVID-19, all of whom were intubated for acute respiratory failure due to COVID-19 [[2], [3], [4], [5], [6]]. Since our patient did not have neuroimaging prior to June 2020, we cannot be certain that her white matter changes developed after onset of COVID-19. She did not have neurologic complaints until after she had COVID-19, and our extensive workup did not reveal another etiology for these changes, so we believe they are related to COVID-19.
A number of mechanisms have been proposed to explain leukoencephalopathy in the setting of acute COVID-19 including endothelitis with thrombotic microangiopathy, cytokine release syndrome, hypoxia, toxic or metabolic changes, sepsis, posterior reversible leukoencephalopathy and demyelination [[2], [3], [4], [5], [6]]. However, many of these mechanisms could not account for our patient's leukoencephalopathy as she did not have severe COVID-19 and there was a lengthy delay between her initial symptoms of COVID-19 and the onset of her neurologic symptoms. While she did not complain of respiratory symptoms when she was diagnosed with COVID-19, it is worth noting that we cannot completely eliminate the possibility that she had mild hypoxia at this time, as some patients with COVID-19 have “silent hypoxia” without dyspnea [7]. Although SARS-CoV-2 has the potential to be neurotropic, white matter changes have not been attributed to direct invasion of the central nervous system [[2], [3], [4], [5], [6],8]. In fact, the neuropathology from a patient who died two weeks after development of multiorgan failure due to COVID-19 demonstrated hemorrhagic white matter changes surrounded by macrophages and axonal injury similar to changes seen in patients with ADEM [[9], [10]]. Accordingly, as our workup did not identify another etiology for her white matter changes, we concluded that our patient's diffuse leukoencephalopathy was due to delayed demyelination from ADEM following infection with COVID-19. Of note, it is unclear if it is significant that she initially had antibodies to SARS-CoV-2 a month prior to presentation then did not have them when she was admitted; it has been reported that patients with COVID-19 can have diminished antibody levels over time, particularly when symptoms were mild [11].
Although prognosis of ADEM is generally favorable, it can be fatal [12]. Based on this report and others in the literature, leukoencephalopathy due to COVID-19 appears to be a harbinger of poor outcome [[2], [3], [4], [5], [6]]. However, we are unsure if she would have had a better outcome if steroids, IVIG, and/or PLEX were administered earlier, when her white matter lesions were first identified and she only had gait instability.
Larger studies are needed to understand the frequency of both acute and delayed leukoencephalopathy in patients with COVID-19, their histopathology, ideal treatment and outcome.
Funding
None.
Disclosures
All authors report no disclosures.
References
- 1.Kanekar S., Devgun P. A pattern approach to focal white matter hyperintensities on magnetic resonance imaging. Radiol. Clin. N. Am. 2014;52:241–261. doi: 10.1016/j.rcl.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Radmanesh A., Derman A., Ishida K. COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J. Neurol. Sci. 2020;415 doi: 10.1016/j.jns.2020.116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs J.R., Gibbs K.W., Swor D.E., Sweeney A.P., Williams D.W., Burdette J.H. COVID-19-associated Leukoencephalopathy. Radiology. 2020 doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang M., Buch K., Li M.D., Mehan W.A., Jr., Lang A.L., Leslie-Mazwi T.M. Leukoencephalopathy associated with severe COVID-19 infection: sequela of hypoxemia? AJNR Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yachou Y., El Idrissi A., Belapasov V., Neuroinvasion Ait Benali S. Neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020:1–13. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelaziz O.S., Waffa Z. Neuropathogenic human coronaviruses: a review. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favresse J., Eucher C., Elsen M., Laffineur K., Dogne J.M., Douxfils J. Response of anti-SARS-CoV-2 total antibodies to nucleocapsid antigen in COVID-19 patients: a longitudinal study. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0962. [DOI] [PubMed] [Google Scholar]
- 12.Steiner I., Kennedy P.G. Acute disseminated encephalomyelitis: current knowledge and open questions. J. Neurovirol. 2015;21:473–479. doi: 10.1007/s13365-015-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


