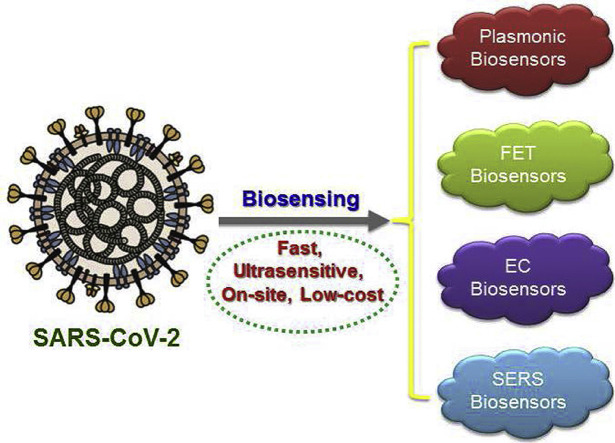
Abstract
Herein, we have summarized and argued about biomarkers and indicators used for the detection of severe acute respiratory syndrome coronavirus 2. Antibody detection methods are not considered suitable to screen individuals at early stages and asymptomatic cases. The diagnosis of coronavirus disease 2019 using biomarkers and indicators at point-of-care level is much crucial. Therefore, it is urgently needed to develop rapid and sensitive detection methods which can target antigens. We have critically elaborated key role of biosensors to cope the outbreak situation. In this review, the importance of biosensors including electrochemical, surface enhanced Raman scattering, field-effect transistor, and surface plasmon resonance biosensors in the detection of severe acute respiratory syndrome coronavirus 2 has been underscored. Finally, we have outlined pros and cons of diagnostic approaches and future directions.
Keywords: SARS-CoV-2, Diagnostics, Plasmonic biosensors, Electrochemical biosensors, Field-effect transistor biosensors
Graphical abstract
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared as pandemic on 13 March 2020. It has not only become the leading cause of mortalities around the globe but has also become the unexpected socioeconomic burden [1]. Since the COVID-19 outbreak reported in early December 2019 in Wuhan, millions of people have been infected, thousands of them died, and even the economy of many countries has halted [2]. The transmission of virus may occur through breathing, aerosols particles, and direct touching of abiotic surfaces. There are also evidences of virus transmission through fecal because the SARS-CoV-2 has been found in feces samples. The transfer of virus through asymptomatic patients has also been observed in many cases [3,4]. In this regards, World Health Organization has urged the scientific community to carry out huge amount of diagnostic tests to curb the spread of virus because testing is an important tool to understand the epidemiology of the outbreak. Furthermore, fast diagnostic testing is very crucial in making prompt decisions to treat and isolate the infected patients which can ultimately slow down the transmission of infectious disease.
The testing platforms together with the risk management and the health-care system are vital responses in all outbreaks. In this outbreak, three different types of diagnosis tests are being used including (i) chest computed tomography (CT) scan along with clinical indications, (ii) RNA detection using reverse transcription-polymerase chain reaction (RT-PCR) assay, and (iii) lateral flow assays, full automatic chemiluminescence method, enzyme-linked immunosorbent assay for the determination of antibodies [5]. Nevertheless, there are some drawbacks related to CT scan method such as the use of CT scan diagnosis is limited to big hospitals, rural hospitals do not have the facility of CT scan, well-trained radiologists are required to analyze the images of CT scan, and CT scan method cannot distinguish whether the infection is caused by SARS-CoV-2 or any other virus. On contrary, RT-PCR is a time consuming assay that may take 4 h to execute one test and possesses great possibility of false-negative results. The patients with initial false-negative test results can transmit the virus to healthy individuals while preventing the proper control of infection. In the meantime, antibody response appears at about 10th day after the onset of symptoms so all the assays that can target antibodies cannot be reliable in case of early diagnosis and identification of asymptomatic individuals. The false-positive results are also most likely owing to the interference caused by other proteins that are present in serological samples.
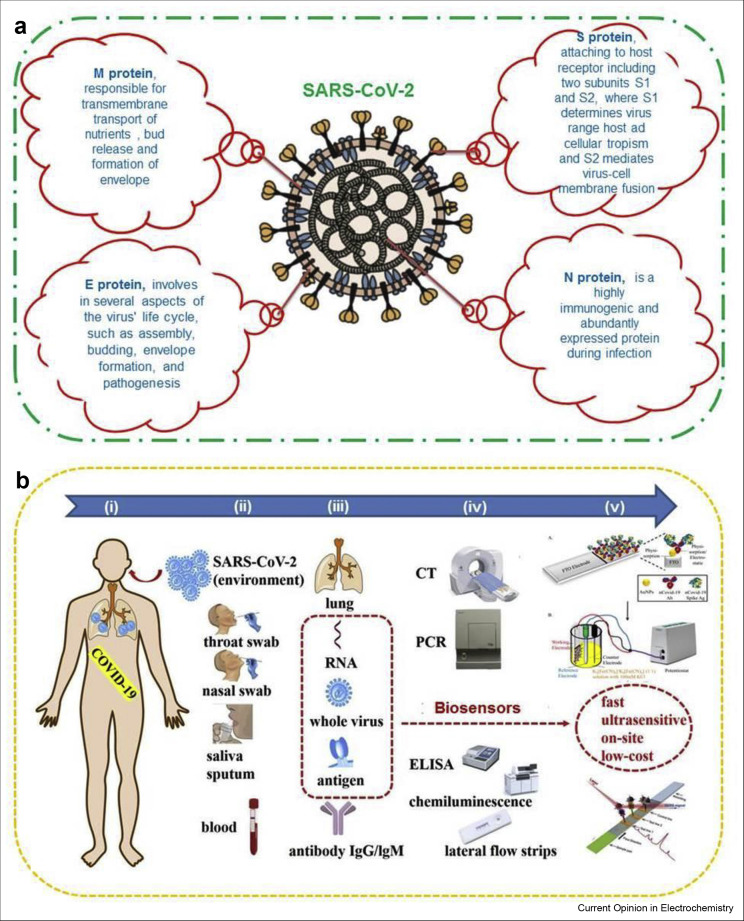
The more precise and targeted detection of the virus can be carried out using biosensor-based approaches. The technology that exists behind the testing is biosensing platforms that apply the strategy of biorecognition elements or binding target molecules in a particular way for the detection of biological analytes [6,7]. This type of binding acts as transducers which create measurable signals either directly through impedance measurements, surface plasmon resonance, or labeling the molecules such as enzymes/optical compounds [8,9]. In this review, we have summarized the biosensor-based technologies which are able to detect SARS-CoV-2 effectively. Fig. 1 shows the ideal schematic illustration of SARS-CoV-2. The possible biomarkers, biochemical indicators, and samples have been discussed in details. Here we have emphasized the highly sensitive biosensors for diagnosing COVID-19. In addition, electrochemical (EC) biosensors are the superb diagnostic podiums which support sample preparation-free sensing in different biological environments, pathogen present on surface of object and detection possibilities via wireless actuation. The innovation in biosensing technologies can escalate the possibilities of early diagnosis and therapeutic window of COVID-19 worldwide.
Figure 1.
Structure of SARS-CoV-2 and detection approaches. (a) Schematic illustration of SARS-CoV-2 structure and four structural proteins. (b) Schematic illustration of currently used diagnostic techniques and possible biosensing platforms for COVID-19. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Current biomarkers and indicators for SARS-CoV-2
There are several biomarkers and indicators that can be used for the detection of SARS-CoV-2.
Single-stranded RNA
Single-stranded RNA is very crucial biomarker which is used to detect SARS-CoV-2. Generally, conserved or fully expressed genes are the desired targets for RT-PCR assays [5,10]. There are some special primers that can specifically target these genes with high sensitivity in detecting SARS-CoV-2 while rule out the detection of other similar types of viruses including Middle East respiratory syndrome (MERS), (OC43 and 229 E) and influenza [11]. Three different kinds of novel RT-PCR assays were developed that targeted N and S genes of SARS-CoV-2 and also compared with each other [12]. The excellent sensitivity was achieved while using nasopharyngeal samples and no cross-reactivity with other coronaviruses was observed.
Antigen
The diagnosis of COVID-19 may also be carried out by using the structural proteins such as S, M, E, and N proteins (Figure 1A) as antigens. Expectedly, SARS-CoV-2 has 28 different types of proteins [13]. It has been reported in several studies that M and E proteins are very crucial in assembling the virus structure [14]. The S protein is of much importance that combines with host cells and receptor-binding domain of S protein interacts with ACE2 receptors. It is more likely that S and N proteins could be imperative antigen biomarkers which might be used for the detection of SARS-CoV-2 because the same proteins have also been used previously in various methods for the detection of SARS-CoV [15,16].
Recently, Jiang et al. [17] fabricated the proteome microarray using 18 of 28 proteins and used it to monitor antibody responses. The patients in recovering stage show full antibodies response to proteome particularly to protein N, S1 but not S2. Moreover, detection technique based on viral proteins has also been investigated using lateral flow assays for the diagnosis of COVID-19.
Antibody
The diagnosis of COVID-19 can also be carried out by detecting specific antibodies to SARS-CoV-2 in serological samples of patients. It is well documented that IgM responses early to the viral infection, whereas IgG responses late to the infection or long-term immunity [18]. A study executed by Zhang at al. [19] showed that the detection of IgM and IgG could be carried out in the patients after five days of appearance of symptoms. Another study conducted for the detection of IgM and IgG in nucleic confirmed patients revealed different percentages of antibodies at different stages of COVID-19 infection, for instance, 11.1% at early stage up to 7th day, 92.9% at medium stage up to 14th day, and 96.8% at later stage after 15th day of the onset of symptoms [20]. Till this end, the diagnosis of COVID-19 using antibodies detection assays is not reliable. The antibody detection can be helpful for the patients at recovering phase and much crucial for the design of vaccine because its exact level correlates with virus neutralization titer [18].
Artificial intelligence–based detection of other biomarkers
There are some other biomarkers as well that can be monitored for the diagnosis of COVID-19 including blood and urine samples, infection index, hemagglutination level, blood gas index, and cytokine levels. Table 1 presents the possible biomarkers and indicators used for the diagnosis of COVID-19 pneumonia. These biomarkers and indicators are very essential where RT-PCR method is not enough to confirm the patient with onset symptoms. The artificial intelligence technology is very effective in reducing the man-made decision which may ultimately enhance the diagnosis and prediction [21]. The severe COVID-19–infected people have high level of lymphopenia and a proinflammatory cytokine storm compared with mild infection persons.
Table 1.
Emerging diagnosis biomarkers and indicators which are being used for COVID-19 diagnostics and prognosis.
| Biomarker/indicators | Mode of action | Sample | Remarks | POC |
|---|---|---|---|---|
| Lungs | Clinically observed symptoms | CT scan of chest | Only available in urban hospitals | N |
| RNA, whole virus, and antigen | Virus genome and protein | Throat/pharyngeal/oropharyngeal swab, BALF, urine, aerosols | High rate of false-negative outcomes, required highly sensitive approches | N |
| Antibody | Response of immunity | Human blood | Possibility of false-positive results | Y |
| Other biomarkers (cytokine) | Cytokine storm in human body | Human blood | Ability to prognose severity of COVID-19 | N |
POC, Point-of-care.
Current biosensor candidates for SARS-CoV-2 detection
A biosensor is defined as an analytical tool consisting of a transducer portion and a biological element. Besides the clinically used approaches for the diagnostics purposes in hospitals, various biosensor-based technologies are being developed, and some have already been established for the diagnosis of COVID-19 pneumonia. Figure 1B shows the schematic illustration of currently used diagnostic techniques and possible biosensing platforms for COVID-19, (i) COVID-19 patient, (ii) sampling ways, (iii) biomarkers and indicators, (iv) diagnostic methods, (v) promising biosensors. Biosensors being capable for continuous monitoring of biomarkers would be potential candidates for diagnosing patients affected with COVID-19 with mild to critical conditions and evaluating the success rate of anti-inflammation therapies [22]. Although the nucleic acid testing and antibody detection using RT-PCR and enzyme-linked immunosorbent assay, respectively, have been well developed, these approaches still suffer from some practical limitations. Therefore, biosensors are the ideal alternative tools which show rapid response, high accuracy, enhanced sensitivity with early detection possibilities, particularly the probability of being driven with smartphones [23]. There are various types of biosensors such as fluorescence-based biosensor, colorimetric, localized surface plasmon resonance (LSPR), surface-enhanced Raman scattering (SERS), quartz crystal microbalance, field-effect transistor (FET)-based and EC biosensors that are being developed or used for the diagnosis of COVID-19 [24, 25, 26]. However, the most frequently used biosensors in this COVID-19 pandemic include LSPR, FET, EC and SERS biosensors.
Plasmonic biosensors
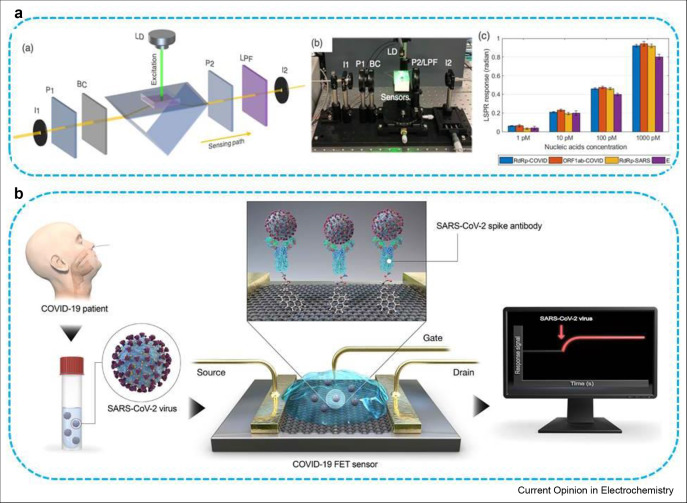
The surface plasmonic resonance (SPR) biosensors are now essential tools and have obtained the key role in characterizing and quantifying bio-analytical targets both in life science and pharmaceutical research. These biosensors are label-free, highly sensitive and can be applied to different types of clinically interested target analytes. The SPR biosensors have also been used for the detection of antibody of SARS-CoV using a protein which was created by genetically fusing gold binding polypeptides to a SARS coronaviral surface antigen [27]. Recently, Masson's research group has reported the use of human serum sample without dilution for the detection of nucleocapsid antibodies which are specific against the SARS-CoV-2 using SPR biosensing technology [28]. The peptide monolayer was successfully coated on SPR biosensor and further functionalized with virus nucleocapsid protein which was finally able to detect SARS-CoV-2 antibodies at nanomolar level. The portable SPR instrument was used to carry out the bioassay. The working mechanism is that when the sensor is exposed to SARS-CoV-2, the immune system gives response by expressing antibodies at levels which can be detected and monitored to find out the patients immunized against SARS-CoV-2 and support the efforts for vaccine development strategically. By exactly detecting the antibodies, we can assist the vaccine development and evaluation of individuals that have become immune to SARS-CoV-2. Moreover, Wang's research group has demonstrated that the dual-functional plasmonic biosensor constructed up using combined effects of plasmonic photothermal (PPT) and LSPR have provided encouraging COVID-19 diagnosis capabilities [29]. Two-dimensional gold nanoislands (AuNIs) combined with complementary DNA receptors can detect selective sequence from SARS-CoV-2 by hybridizing nucleic acid as shown in Figure 2 A. The detection abilities were further enhanced through the generation of plasmonic heat on the same surface of AuNIs when they started to illuminate at their plasmonic resonance frequency. The locally generated PPT heat has the ability to increase in situ hybridization temperature which in turn enables differentiation of two same gene sequences. This dual-functionalized LSPR biosensor has presented superb sensing performance in the detection of selective SARS-CoV-2 sequence with low detection limit of 0.22 pM as well as allowed accurate determination of particular target in a multigene mixture.
Figure 2.
Plasmonic photothermal biosensors in SARS-CoV-2 detection. (a) Scheme and (b) experimental setup of the dual-functional PPT-enhanced LSPR biosensor, (c) concentrations of several viral oligos measured using LSPR biosensor, (b) Schematic representation of FET-based biosensor for COVID-19 diagnosis [31]. FET, field-effect transistor; PPT, plasmonic photothermal; LSPR, localized surface plasmon resonance. For figure 2A: Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J: Dualfunctional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14:5268–5277. Note: further permissions related to the material excerpted should be directed to the ACS: https://pubs.acs.org/doi/10.1021/acsnano.0c02439 (for the article DOI: 10.1021/acsnano.0c02439). For figure 2B: Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor (ACS Nano 2020, 14, 4, 5135–5142). Note: further permissions related to the material excerpted should be directed to the ACS: https://pubs.acs.org/doi/10.1021/acsnano.0c02823 (for the article DOI: 10.1021/acsnano.0c02823).
FET-based biosensing
Considering the availability of current diagnostic approaches, FET-based biosensing platforms have many promising benefits such as capability to be very sensitive and to detect small volume of target analyte instantaneously. These biosensors have potential use in clinical analysis, point-of-care tests, and on-site diagnostics [30]. Graphene with the hexagonal carbon atoms exposed on its surface, being electronically conductive, having high charge mobility and specific surface area, has proved to be ultrasensitive in sensing systems owing to its capability to detect nearby variations on their surface and to provide an ideal sensing platform. Therefore, graphene-based FET biosensors are very important to carry out the immunological diagnosis with high sensitivity. In this regard, Seo and co-workers have successfully fabricated a device based on FET technology for the detection of SARS-CoV-2 in clinical specimens as shown in Figure 2B [31]. The graphene sheets of the FET were conjugated with specific antibodies against SARS-CoV-2 spike protein in order to construct the biosensor.
The sensing aptitude of the biosensor was evaluated using antigen protein, self-cultured virus, and nasopharyngeal swab samples taken from people infected with COVID-19 pneumonia. The FET biosensor was able to detect SARS-CoV-2 spike protein 1 fg/mL in phosphate-buffer saline and 100 fg/mL clinical transport medium. In addition, FET biosensor performed very well in detection of SARCoV-2 in self-cultured medium and nasopharyngeal swab samples with detection limits of 1.6 × 101 plaque-forming units/mL (pfu/mL) and 2.42 × 102 copies/mL. Interestingly, the fabricated biosensing device showed no any quantifiable cross-reactivity with MERS-CoV antigen.
EC biosensors
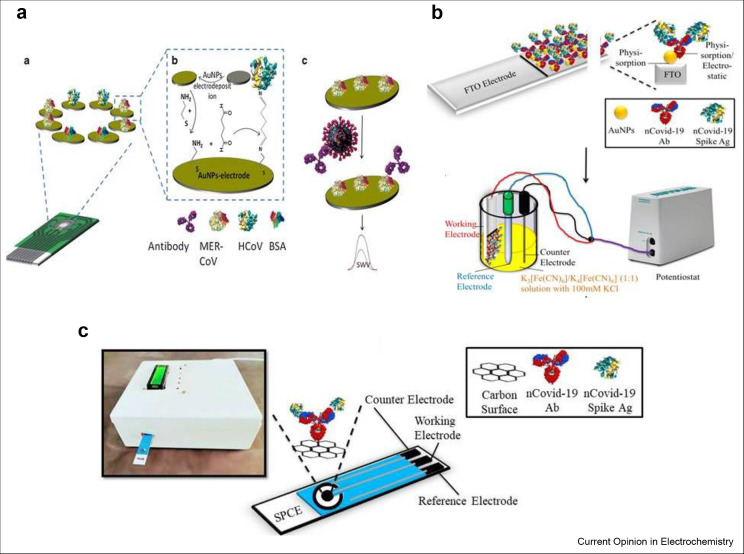
EC biosensors have attained much attention of the analytical researchers because of their simplicity, low cost, ease in miniaturization and bulk fabrication. They have also point-of-care usability at homes or at clinics [32, 33, 34]. Due to the absence of any vaccine or specific drugs available for the treatment of COVID-19 infection, early diagnostics are the only way to manage and combat with this virus. The MERS-CoV and other human CoV have been detected by Eissa's research group using recombinant spike protein S1 which acted as a biomarker for MERS-CoV and the design of multiplexed electrode array has been presented in Figure 3 A. The bioassay with turnout of 20 min achieved low detection limits of 0.4 pg/mL and 1 pg/mL for human CoV and MERS-CoV, respectively [35]. The fabricated EC immunosensor was fruitfully employed in spiked nasal samples and no any obvious interference was measured in the presence of influenza A and B. Another simple, economical, and easy-to-use EC genosensor was constructed on gold films by Abad-Valle et al. [36] for the detection of SARS-CoV gene. The genosensor achieved a detection limit of 6 pM for this DNA sequence applying square wave voltammetry.
Figure 3.
Electrochemical biosensors in SARS-CoV-2 detection. (a) Chip array, immunosensor fabrication process, and detection of viruses [35]. (b) The modification process of FTO electrode and its working in three electrodes system. (c) Schematic representation of in-house built EC eCovSens device [37]. FTO, fluorine-doped tin oxide electrode; EC, electrochemical.
Recently, Gandhi's research group fabricated an in-house built biosensor device (eCovSens) and compared it with commercially available potentiostatic device for the diagnosis of COVID-19 spike antigen protein (COVID-19 Ag). The performance was also evaluated in spiked saliva specimens. To fabricate potentiostatic biosensor, fluorine-doped tin oxide electrode (FTO) was decorated with AuNPs and immobilized with COVID-19 monoclonal antibody (COVID-19 Ab) to determine the variation in electrical conductivity as shown in Figure 3B. Similarly, to construct eCovSens, screen-printed carbon electrode was immobilized with COVID-19 Ab to determine the variation in electrical conductivity as presented by Figure 3C [37]. Both the FTO-based immunosensor and screen-printed carbon electrode–based biosensor showed superb efficiency in detecting COVID-19 Ag in the range of 1 fM to 1 μM. The eCovSens and potentiostatic devices displayed detection limits of 90 fM and 120 fM, respectively, in spiked saliva specimens. The as-fabricated eCovSens device is able to rapidly detect COVID-19 Ag within 10–30 s. Therefore, this platform can be used as an alternate diagnostic device to detect traces of COVID-19 Ag in saliva samples of infected persons with enhanced selectivity and specificity. The eCovSens device is very economical, portable, and uses very less voltage 1.3–3 V and even can be operated with battery, therefore possesses the ability to be used as point-of-care diagnostics.
SERS-based biosensors
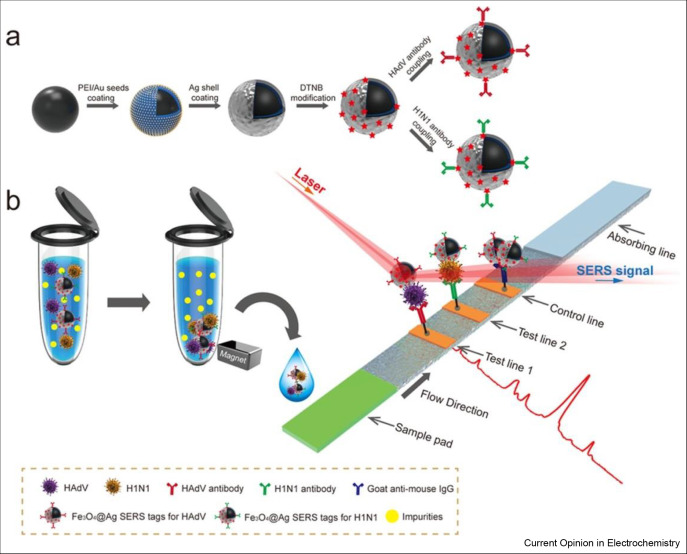
The SERS-based biosensors have fascinated immense attention of the researchers because of highly sensitive and quantitative determination of analyte using SERS-encoded nanoparticles (SERS tags) as an alternative of colloidal gold to report signal. There are three basic parts of SERS tags; Au/Ag nanoparticle as Raman enhanced substrate, adsorbed Raman reporter dyes to produce characteristic SERS signals, and specific antibodies to bind targets. Wang et al. [38] used Fe3O4@Ag nanoparticles as magnetic SERS nanotags to fabricate SERS-based biosensor to simultaneously detect two respiratory track viruses such as influenza A (H1N1) and human adenovirus as shown in Figure 4 . The lowest limits of detections measured with SERS-based biosensor were 50 pfu/mL and 10 pfu/mL for H1N1 and human adenovirus, respectively. The sensitivity of biosensor was 2000 times higher than sensitivity of commonly used colloidal gold strip method. The proposed biosensor is able to use directly for the analysis of biological samples without any pretreatment. Moreover, SERS-based biosensor was constructed using porous carbon substrate coated with Ag nanoparticles for the detection of three different kinds of viruses including porcine circovirus type 2, porcine parvovirus, and porcine pseudorabies virus [39]. The low possible concentration detected with this biosensor was 1 × 107 copies/mL for these three viruses. The SERS spectra enabled the discrimination of viruses.
Figure 4.
SERS biosensors in the detection of respiratory viruses. (a) Preparation of antibody-modified Fe3O4@Ag nanotags. (b) Mechanistic representation of magnetic SERS-based biosensor for the detection of respiratory viruses [38].
Comparison among various diagnostic approaches
The different approaches used for the diagnosis of COVID-19 have their own pros and cons considering various parameters. In Table 2 , we have compared all the possible diagnostic techniques.
Table 2.
Comparison among all the possible diagnostic techniques for COVID-19.
| Technique | Apparatus | Time required | Sensitivity | Limit of detection | Reference |
|---|---|---|---|---|---|
| Chest CT scan | CT machine | – | 97%, 25%, | – | [40] |
| RT-PCR | PCR apparatus | 4 h | 71% | – | [41] |
| MNPs with RT-PCR | PCR apparatus | Around 30 min | – | 10 copies | [42] |
| Colorimetric assay | Water bath | 30 min | 97.6% | – | [43] |
| ELISA reder | ELISA machine | 2 h | 87.3% | – | [44] |
| LFICS Au-NPs colloid (IgM + IgG) | POC strip | ≤15 min | (11.1%, 92.9%, 96.8%)A | – | [45] |
| Chemiluminescence (total Ab) | Automatic analyzer | – | 86.9%, 99.2% | – | [46] |
| Plasmonic biosensor | Dual-functional LSPR System | – | 3.7 RNA copies | 0.22 pM | [29] |
| Electrochemical biosensor | Electrochemical work station | Few min | – | 90 fM | [37] |
| Field-effect transistor–based biosensor | Semiconductor analyzer | – | – | 2.42 × 102 copies/mL | [31] |
ELISA, enzyme-linked immunosorbent assay; POC, point-of-care.
In the previous table, “A” describes the different stages, for example, sensitivity was 11.1%, during the early stage of patient from 1to 7 days after onset of symptoms, 92.9% during the intermediate stage of patient from 8 to 14 days after onset of symptoms and 96.8% at late stage of patient.
Biosensor-oriented treatments
The previous treatment of patients affected with SARS-CoV-1 and MERS with corticosteroids gave disparate results that is why World Health Organization has discouraged the usage of corticosteroids for COVID-19 treatment. However some reports show good results if they are administered at cautious doses [47,48]. Kinetic profile of cytokine-like IL-6 can convey important data which further can guide the onset of corticosteroids therapy and regulate the doses to lower the inflammation while keeping side effects at minimum level [49]. The severely COVID-19–infected people have high level of lymphopenia and a proinflammatory cytokine storm compared with mild infection persons. Table 3 shows some other anti-inflammatory therapies that can take advantages from biosensor-based guide for administration of hydroxychloroquine [50], immunoglobulins, azithromycin [51], and convalescent plasma treatments [52]. Other drugs such as tocilizumab [53] or anakinra [54] have also anti-inflammatory effects for COVID-19. The mechanism of these treatments is to halt the particular proinflammatory signaling pathways. These drugs can be administrated using some analogous techniques ‘companion diagnostics’ which are used for cancer care. The exact monitoring of specific proinflammatory factors would guide how to administrate these drugs. For instance, the mechanism of tocilizumab is to bind with receptor IL-6 and hinder the interaction with membrane binding that consequently stops the stimulation of downstream Janus kinase accountable for signal cascading [55]. These blockers of IL6-mediated inflammatory response including tocilizumab and sarilumab must be directed by measuring IL-6 [56,57]. It is well documented that antibodies are crucial in the treatment of unwanted cytokine excrete conditions in immune anticancer treatments. Moreover, measuring serial IL-6 shows that after appropriately administrating tocilizumab there is small upsurge in IL-6 after the decrease in time [56]. The aforementioned studies show that the improvement of inflammatory infections can be monitored by executing kinetic measurements of biomarkers.
Table 3.
The key immunomodulators that have been proposed to manage COVID-19 inflammation.
| Immunomodulators | Target | Mode of action |
|---|---|---|
| Hydroxy chloroquine | Not specific | It suppresses the activation of T cells in the body |
| Corticosteroids | Not specific | It binds to receptor to increase or decrease the transcription of inflammation genes |
| Immunoglobulins | Not specific | It binds to Fc receptors |
| Convalescent plasma | Not specific | Unknown |
| Azithromycin | Not specific | It suppresses proinflammatory response |
| Baricitinib | JAK | It inhibits the Janus kinase (JAK) |
| Anakinra | IL-1 | Antagonist of the IL-1 receptor |
| Tocilizumab | IL-6 | It binds to IL-6 receptor which ultimately block the interaction with gp130 |
Conclusions and future outlooks
In conclusion, early detection and diagnosis techniques are mandatory to enhance the control on infection, treatment, and vaccine research. Recently, chest CT scan method, RT-PCR, and lateral flow assays are being used for the diagnosis of COVID-19 pneumonia. Ominously, numerous suspected individuals may not be confirmed and isolated timely owing to overloaded work in hospitals at severe outbreak zones. Therefore, extra trustworthy, quick response, economical, and broadly accessible analytical devices or diagnostic approaches are crucially required. We have critically reviewed and argued the biomarkers and indicators used for COVID-19 diagnostics or SARS-CoV-2 detection. In this regards, biosensors are powerful tools in early diagnosis of COVID-19 infection via targeting virus antigens to assess the clinical progress and offer awareness on severity and critical trends of infection. Specifically, the importance of EC biosensors, SERS-based biosensors, FET-based biosensors, and SPR-based biosensors in the diagnosis of COVID-19 is emphasized.
The multiplex biosensors could be the alternate approaches to improve the accurateness of virus detection if the combined detection of various biomarkers is carried out. The reliability and reproducibility of biosensors should be further enhanced by developing the platforms which enable machine learning–based signal processing and direct readout of results. For asymptomatic cases, in-house built biosensor devices should be readily available for everybody to confirm the presence or absence of SARS-CoV-2 in individuals anywhere and everywhere. The biosensors have capability of being on-site, rapid and highly sensitive to target virus antigen which can ultimately pave the way for early diagnosis of COVID-19. They can screen the people at hospitals, airports, and other most crowded areas. The usage of nanoparticles combined with EC diagnostic methods is promising in detection of viruses. There should be development in nanomaterials and nanotechnologies to advance biosensors with excellent sensitivity and selectivity for detecting virus antigens. Technologically advanced smartphone-based biosensing devices and calorimetric strips to target antibodies or antigens should be developed immediately to control quickly spreading SARS-CoV-2–associated COVID-19 pandemic. The wearable biosensors are able to monitor the patients continuously, a much-desired feature of biosensors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2018YFF0215002), The Foundation of Hubei Key Laboratory of Material Chemistry and Service Failure (2017), Key Laboratory of Material Chemistry for Energy Conversion and Storage, Ministry of Education (2018).
This review comes from a themed issue on Sensors and Biosensors
Edited by Zbigniew Stojek
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santiago I. Trends and innovations in biosensors for COVID-19 mass testing. Chembiochem : Eur J Chem Biol. 2020;21:1–11. doi: 10.1002/cbic.202000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., et al. Evidence of SARS-CoV-2 infection in returning travelers from wuhan, China. N Engl J Med. 2020 Mar 26;382(13):1278–1280. doi: 10.1056/NEJMc2001899. Epub 2020 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu P., Zhu J., Zhang Z., Han A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221:1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asif M., Haitao W., Shuang D., Aziz A., Zhang G., Xiao F., et al. Metal oxide intercalated layered double hydroxide nanosphere: with enhanced electrocatalyic activity towards H2O2 for biological applications. Sensor Actuator B Chem. 2017;239:243–252. [Google Scholar]
- 7.Asif M., Liu H., Aziz A., Wang H., Wang Z., Ajmal M., et al. Core-shell iron oxide-layered double hydroxide: high electrochemical sensing performance of H2O2 biomarker in live cancer cells with plasma therapeutics. Biosens Bioelectron. 2017;97:352–359. doi: 10.1016/j.bios.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Dincer C., Bruch R., Costa-Rama E., Fernández-Abedul M.T., Merkoçi A., Manz A., et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv Mater. 2019;31:1806739. doi: 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]; Importance of disposable sensors in food and environmental monitoring
- 9.Asif M., Aziz A., Wang Z., Ashraf G., Wang J., Luo H., et al. Hierarchical CNTs@CuMn layered double hydroxide nanohybrid with enhanced electrochemical performance in H2S detection from live cells. Anal Chem. 2019;91:3912–3920. doi: 10.1021/acs.analchem.8b04685. [DOI] [PubMed] [Google Scholar]
- 10.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:1560–7917. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Won J., Lee S., Park M., Kim T.Y., Park M.G., Choi B.Y., et al. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the coronavirus disease 2019 (COVID-19) Exp Neurobiol. 2020;29:107–119. doi: 10.5607/en20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan J.F., Yip C.C., To K.K., Tang T.H., Wong S.C., Leung K.H., et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58:310–320. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo P.C.Y., Lau S.K.P., Wong B.H.L., Tsoi H.W., Fung A.M.Y., Kao R.Y.T., et al. Differential sensitivities of severe acute respiratory syndrome (SARS) coronavirus spike polypeptide enzyme-linked mmunosorbent assay (ELISA) and SARS coronavirus nucleocapsid protein ELISA for serodiagnosis of SARS coronavirus pneumonia. J Clin Microbiol. 2005;43:3054–3058. doi: 10.1128/JCM.43.7.3054-3058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H.W., Li Y., Zhang H.N., Wang W., Men D., Yang X., et al. Global profiling of SARS-CoV-2 specific IgG/IgM responses of convalescents using a proteome microarray. MedRxiv. 2020 doi: 10.1038/s41467-020-17488-8. 2020.03.20.20039495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.W., Yip C.C.Y., Lai C.Y.W., Wong C.K.H., Ho D.T.Y., Pang P.K.P., et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]; A good article comparing the detection rate of respiratory viruses between saliva and nasopharyngea aspirate among adult hospitalized patients using Xpert® Xpress Flu/RSV
- 19.Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020;81:28–32. doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Yang J., Shi Q., Ying L., Zhu H., Zhu G., et al. 2020. Artificial intelligence application in COVID-19 diagnosis and prediction. [Google Scholar]; This is a novel and accurate method to quickly achieve COVID-19 diagnosis association indexes to improve confirmed diagnosis rate for clinical use
- 22.Aziz A., Asif M., Azeem M., Ashraf G., Wang Z., Xiao F., et al. Self-stacking of exfoliated charged nanosheets of LDHs and graphene as biosensor with real-time tracking of dopamine from live cells. Anal Chim Acta. 2019;1047:197–207. doi: 10.1016/j.aca.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Huang X., Xu D., Chen J., Liu J., Li Y., Song J., et al. Smartphone-based analytical biosensors. Analyst. 2018;143:5339–5351. doi: 10.1039/c8an01269e. [DOI] [PubMed] [Google Scholar]; This paper reviews the recent development of four types of smartphone based analytical biosensory systems at the POC
- 24.Kaya S.I., Karadurmus L., Ozcelikay G., Bakirhan N.K., Ozkan S.A. In: Nanosens smart cities. Han B., Tomer V.K., Nguyen T.A., Farmani A., Kumar Singh P., editors. Elsevier; 2020. Chapter 18 - electrochemical virus detections with nanobiosensors; pp. 303–326. [Google Scholar]
- 25.Asif M., Aziz A., Azeem M., Wang Z., Ashraf G., Xiao F., et al. A review on electrochemical biosensing platform based on layered double hydroxides for small molecule biomarkers determination. Adv Colloid Interface Sci. 2018;262:21–38. doi: 10.1016/j.cis.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Aziz A., Asif M., Ashraf G., Azeem M., Majeed I., Ajmal M., et al. Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides. A review. Microchim Acta. 2019;186:671. doi: 10.1007/s00604-019-3776-z. [DOI] [PubMed] [Google Scholar]
- 27.Park T.J., Hyun M.S., Lee H.J., Lee S.Y., Ko S. A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome. Talanta. 2009;79:295–301. doi: 10.1016/j.talanta.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelhadi D., Benjamin C., Maryam Hojjat J., Vincent T., Julien C., Keisean S., et al. 2020. A rapid and quantitative serum test for SARS-CoV-2 antibodies with portable surface plasmon resonance sensing. [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]; Development of dual functional plasmonic photothermal (PPT) effect and localized surface plasmon resonance (LSPR) biosensor for the detection of SARS-CoV-2
- 30.Liu J., Chen X., Wang Q., Xiao M., Zhong D., Sun W., et al. Ultrasensitive monolayer MoS2 field-effect transistor based DNA sensors for screening of down syndrome. Nano Lett. 2019;19:1437–1444. doi: 10.1021/acs.nanolett.8b03818. [DOI] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]; The development of FET-based biosensor for SARS-CoV-2 detection
- 32.Asif M., Aziz A., Wang H., Wang Z., Wang W., Ajmal M., et al. Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchim Acta. 2019;186:61. doi: 10.1007/s00604-018-3158-y. [DOI] [PubMed] [Google Scholar]
- 33.Asif M., Aziz A., Dao A.Q., Hakeem A., Wang H., Dong S., et al. Real-time tracking of hydrogen peroxide secreted by live cells using MnO2 nanoparticles intercalated layered doubled hydroxide nanohybrids. Anal Cchim Acta. 2015;898:34–41. doi: 10.1016/j.aca.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 34.Asif M., Aziz A., Ashraf G., Wang Z., Wang J., Azeem M., et al. Facet-inspired core-shell gold nanoislands on metal oxide octadecahedral Heterostructures: high sensing performance toward sulfide in biotic fluids. ACS Appl Mater Interfaces. 2018;10:36675–36685. doi: 10.1021/acsami.8b12186. [DOI] [PubMed] [Google Scholar]
- 35.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim Acta. 2019;186:19–3345. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abad-Valle P., Fernandez-Abedul M.T., Costa-Garcia A. Genosensor on gold films with enzymatic electrochemical detection of a SARS virus sequence. Biosens Bioelectron. 2005;20:2251–2260. doi: 10.1016/j.bios.2004.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19. bioRxiv. 2020 [Google Scholar]; In-house built electrochemical biosensor to detect SARS-CoV-2 as POC
- 38.Wang C., Wang C., Wang X., Wang K., Zhu Y., Rong Z., et al. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses. ACS Appl Mater Interfaces. 2019;11:19495–19505. doi: 10.1021/acsami.9b03920. [DOI] [PubMed] [Google Scholar]
- 39.Luo Z., Chen L., Liang C., Wei Q., Chen Y., Wang J. Porous carbon films decorated with silver nanoparticles as a sensitive SERS substrate, and their application to virus identification. Microchim Acta. 2017;184:3505–3511. [Google Scholar]
- 40.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;26 doi: 10.1148/radiol.2020200642. 2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent comparison between chest CT scan method and RT-PCR method for COVID-19 diagnosis
- 42.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020:961268. 2020.02.22. [Google Scholar]
- 43.Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V., et al. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., et al. Evaluation of enzyme-linked immunoassay and colloidal gold-immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) medRxiv. 2020 [Google Scholar]
- 45.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia N., Wang G., Gong W. 2020. Serological test is an efficient supplement of RNA detection for confirmation of SARS-CoV-2 infection. [Google Scholar]
- 47.Zha L., Li S., Pan L., Tefsen B., Li Y., French N., et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng C., Wang J., Guo H., Lu Z., Ma Y., Zhu Y., et al. Risk-adapted treatment strategy for COVID-19 patients. Int J Infect Dis. 2020;94:74–77. doi: 10.1016/j.ijid.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci Unit States Am. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumat. 2020;2:276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:1–5. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]