Abstract
Songbirds often sing at higher frequency (pitch) in urban, noise-polluted areas, which reduces acoustic masking by low-frequency anthropogenic noise. Such frequency shifts, however, are less efficient at overcoming background noise than simply singing louder. Therefore, it was suggested that high-frequency singing might not be a functional adjustment to noise, but a physiological consequence of singing louder (also known as the Lombard effect). We tested for the first time the main tenet of this hypothesis, for birdsong whether increasing sound amplitude has a concomitant effect on song frequency, using a representative species with higher urban minimum frequency, the dark-eyed junco, Junco hyemalis. The frequency bandwidth of songs and syllables increased with amplitude, involving lower minimum frequency in louder songs and syllables. Therefore, louder singing does not explain the higher minimum frequency of urban dark-eyed juncos. Amplitude and peak frequency were weakly positively related across but not within songs, suggesting that increased frequency is not an obligatory outcome of singing louder. Instead, birds may adjust both amplitude and frequency in response to changing noise or motivation across songs. Our results suggest that adjustments in song frequency and amplitude are largely independent and, thus, can be complementary rather than alternative vocal adjustments to noise. We discuss oscine vocal physiology and details of the behaviour of urban birds, both of which we argue are consistent with the increased frequency of urban birdsong generally being a functional adjustment to noise, rather than a consequence of singing louder.
Keywords: birdsong, communication, dark-eyed junco, frequency, Junco hyemalis, Lombard effect, noise pollution, sound amplitude, urban ecology
Several oscines (songbirds) sing with higher sound frequency (pitch) in cities and other noisy environments (reviewed in Slabbekoorn & Ripmeester 2008). This has been interpreted as an adjustment to reduce acoustic masking by low-frequency anthropogenic noise (Brumm & Slabbekoorn 2005; Slabbekoorn & Ripmeester 2008). Songbirds can also change the sound amplitude of their songs, and some species have been shown to sing louder (i.e. with higher amplitude) when exposed to noise (e.g. Cynx et al. 1998; Brumm 2004; Brumm et al. 2009).
Increasing the amplitude of vocalizations as a reaction to background noise, known as the Lombard effect, has long been known in humans and more recently found in other animals (reviewed in Brumm & Slabbekoorn 2005). The Lombard effect may also comprise other adjustments, such as increased sound frequency. Recently, Nemeth & Brumm (2010) suggested that in birds the increased sound frequency might not be a functional adjustment to anthropogenic noise, since, for realistic values of frequency and amplitude adjustments, increasing song frequency is less efficient at reducing acoustic masking by anthropogenic noise than increasing sound amplitude. Rather, the higher frequency observed in urban birdsong could be a consequence of the Lombard effect as a physiological side-effect of singing louder (Nemeth & Brumm 2010; Verzijden et al. 2010). In some mammals and anurans, for example, the amplitude and frequency of vocalizations are coupled to some extent (e.g. Lopez et al. 1988; Lienard & Di Benedetto 1999). In nonoscine birds these may also be coupled, because the fundamental frequency of vocalizations is determined mostly by airsac pressure (e.g. ring doves, Streptopelia risoria, a nonpasserine, Beckers et al. 2003; and great kiskadees, Pitangus sulphuratus, a suboscine passerine, Amador et al. 2009), and airsac pressure should influence airflow, and therefore sound amplitude. This contrasts with the physiology of songbirds, in which sound frequency is determined by specialized syringeal muscles, allowing precise and independent control of amplitude and frequency over a wide frequency range (Suthers et al. 1999). Therefore, it is not clear whether amplitude-mediated changes in frequency are to be expected in oscine song and, as Nemeth & Brumm (2010) pointed out, this has not been tested for birdsong. Although not yet tested for birdsong, this has been examined for the calls of an oscine (the eastern towhee, Pipilo erythrophthalmus), and call frequency was found to be strongly and positively related to amplitude (Nelson 2000). This result suggests that a coupling of amplitude and frequency might be important in oscines too, but other observations suggest the contrary (e.g. some oscines sing the same songs loudly or softly as long- or short-range signals, with no apparent differences in frequency; Titus 1998; Anderson et al. 2008), and further tests are needed to resolve this question for birdsong.
We tested whether louder oscine songs are sung at higher frequency, which is a central tenet of the hypothesis that higher song frequency of urban songbirds is a consequence of singing louder. We used natural sound amplitude variation within- and among-songs of dark-eyed juncos, Junco hyemalis thurberi (Emberizidae), whose song frequency differs between urban and nonurban environments (Slabbekoorn et al. 2007; Cardoso & Atwell 2011). We also considered oscine vocal physiology and the vocal behaviour of urban birds, to evaluate whether the observed differences in urban birdsong frequency are more likely to be explained as by-products of increased amplitude, or as functional adjustments in response to noise.
METHODS
We used an existing database of over 1000 recordings of 151 individually marked dark-eyed junco males in southern California (Cardoso & Atwell 2011). The study sites are described in Yeh (2004), and information on capture and marking methods are described in Cardoso et al. (2007).
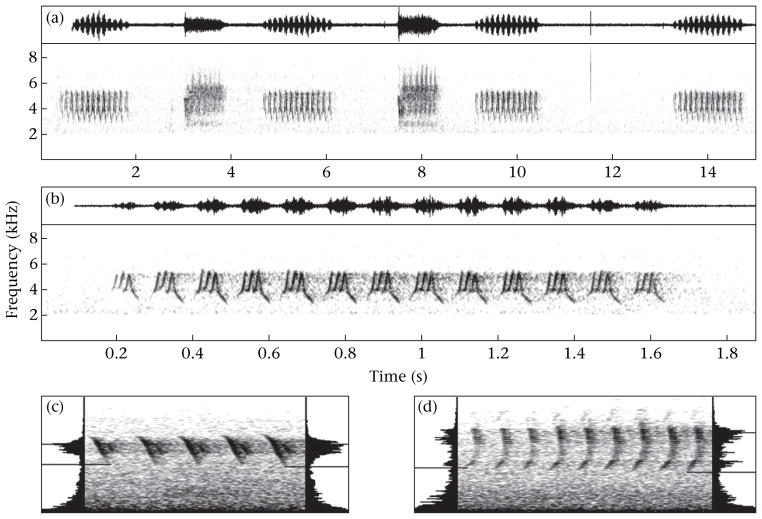
Most long-range songs (or broadcast songs; hereafter, simply songs) of dark-eyed juncos consist of a single trilled syllable (Fig. 1a, b), whereas a minority consist of two or more trilled syllables (‘multisyllable’ songs; Konishi 1964; Newman et al. 2008). The first and last few syllables of each song are usually softer, forming a ‘fade-in’ and ‘fade-out’ at the beginning and end of songs, respectively (Fig. 1b). Juncos generally perch and sing song bouts of the same song type, switching song types only occasionally (Williams & MacRoberts 1977; Newman et al. 2008).
Figure 1.
Waveform and spectrogram of (a) part of a dark-eyed junco song bout (in this case four songs interspersed with two spotted towhee songs at 3 and 8 s) and (b) the last song, showing slight differences in amplitude between songs and the softer initial and final syllables of each song compared to the central syllables. (c) and (d) Spectrograms of two songs (beginning to middle of song; spectrogram axes as in (b)) recorded amid urban noise with power spectra in the end panels for the two outer syllables represented, illustrating that minimum frequency can be measured visually on spectrograms (horizontal lines) even when embedded in noise of similar amplitude.
We tested for a relation between the amplitude and frequency of songs using natural variation at two different levels. First, we compared the frequency of the loudest and softest songs in a song bout. Second, we compared the frequency of the softer initial and final syllables with the louder remaining syllables of the same song.
Recordings of song bouts were made without changing the gain level or using gain limiters, and with the microphone in a static position, so that the distance to the bird remained the same during the bout and the relative sound amplitude of songs in the recording could be compared (e.g. Beebee 2004; Christie et al. 2004; Cardoso et al. 2007; Cardoso & Mota 2009). All our analyses used paired within-recording comparisons, so that variation in amplitude due to sound transmission or recording conditions is unlikely. However, head movements of the birds, or rapid changes in atmospheric conditions, can introduce some error in the measurements of relative amplitude even within recordings of perched birds. Such putative error is random regarding the hypothesis being tested, because wind or head movements away from or towards the microphone are not related to changes in song frequency. Therefore, this does not introduce bias into the tests, but it could make them conservative. We addressed whether this effect is relevant in our data by contrasting the results of among- and within-song comparisons. Since junco songs are short (average duration is 1.5 s, Newman et al. 2008), within-song comparisons are less prone to such interference to measurements than among-song comparisons within bouts. Therefore, all else being equal, if measurement error is relevant we predict more conservative results in among- than within-songs comparisons.
We refer to Cardoso et al. (2007, 2008) for details of recording and measurement methods, and here we summarize the relevant information. Measurements of this data set predate the recent papers suggesting an amplitude-mediated change in frequency for urban songs (Nemeth & Brumm 2010; Verzijden et al. 2010) and thus were made blindly relative to this hypothesis. We used Avisoft SASLAB version 4.34 (Avisoft Bioacoustics, Berlin, Germany) and recordings with a sampling rate of 22.05 kHz to create spectrograms with FFT length of 512 points, corresponding to a frequency bandwidth of 56 Hz. From each recording we selected a sequence of five songs (sometimes less, in short recordings or with excessive noise for measurements), and from those we measured the peak frequency, the minimum and maximum frequency, and the relative amplitude of each syllable. Peak frequency, the loudest frequency in the syllable, was measured as the peak in the amplitude spectrum of each syllable. Following most studies of birdsong in urban noise (e.g. Slabbekoorn & den Boer-Visser 2006; Wood & Yezerinac 2006), we measured minimum frequency as the lowest sound frequency on spectrograms (see Verzijden et al. 2010 for an experimental validation of this method), having adjusted the dynamic range (greyscale) of each recording’s spectrogram (up to 70 dB) to visualize the entire frequency range of syllables. This allowed us to detect soft elements that automatic measurements would confuse with background noise (Fig. 1c, d), especially in urban recordings, making these measurements of minimum frequency resilient to changes in the amplitude of syllables. Maximum frequency was similarly measured as the highest sound frequency on spectrograms. Relative sound amplitude was measured as the peak amplitude of each syllable in Volts, after applying a lower cutoff filter at 2 kHz to eliminate low-frequency noise, and expressed in a decibel scale relative to the maximum in the recording (an arbitrary reference). Decibels are logarithmic ratios between quantities that express their relative difference; this makes the measured amplitude differences in each recording independent from the absolute amplitude in the recording (and thus independent from the distance to the bird and other recording conditions).
Among-Songs Comparison
As in previous work on junco song (Cardoso et al. 2007), we did not use the measurements of the initial and final 10% of syllables in each song (rounded up to the nearest integer), which are often softer and not representative of the amplitude of the entire song (Fig. 1b). The measurements of the remaining syllables in each song were averaged to obtain values of frequency and relative amplitude for each song in the song bout.
We excluded recordings that contained more than one song type or syllable type, resulting in a sample of 141 different males. Therefore, all the paired comparisons below use songs of the same type. For each of these 141 males, we selected the recording with the largest difference in amplitude between songs, and from that recording selected the loudest and the softest song for comparison. Comparing the songs with the largest difference in amplitude maximizes our ability to detect associated differences in frequency, and using a single pair of songs from each male makes our pairs of data points independent.
Of these within-bout amplitude differences, 95% (134 males) fell in the range between 0.9 and 14 dB, and this subset of data did not deviate significantly from a normal distribution (Kolmogorov–Smirnov test: Z = 0.764, N = 134, P = 0.604). We analysed these data, and excluded the additional seven data points larger than 14 dB, which formed a skewed right-hand tail (range 14–48 dB). We chose 14 dB as an upper cutoff representative of the range of amplitude changes in noise, because this was the maximum value for amplitude differences found among individuals in another species (the nightingale, Luscinia megarhynchos, Brumm 2004), and because it roughly corresponds to the natural range of within-individual amplitude variation in broadcast songs of the only emberizid species studied to date (the song sparrow, Melospiza melodia, Anderson et al. 2008). Larger differences in sound amplitude are generally observed only when also considering soft or short-range song, which is a different class of song used for communication at close range (Titus 1998; Anderson et al. 2008). Paired differences in the three frequency measures were not normally distributed (all Z > 1.7, all P < 0.005) owing to kurtosis, which is difficult to correct, and therefore we used nonparametric tests.
We compared the minimum, peak and maximum frequencies of the loudest and softest song in each recording with paired Wilcoxon tests. We also correlated the differences in amplitude with the differences in frequency using Spearman correlations, to test whether larger amplitude differences resulted in larger frequency differences.
Within-Songs Comparison
Using the same 141 recordings as above, we selected for each male the song with the largest difference in relative amplitude between the initial and final 10% of syllables (average of those syllables) and the remaining syllables (average of the remaining syllables). As above, selecting the larger differences in amplitude maximizes the detection of changes in frequency, and all pairs of data are independent.
Of these within-song amplitude differences, 98% (138 males) fell in the range between 0.8 and 14 dB, and this subset of data did not deviate significantly from normality (Kolmogorov–Smirnov test: Z = 1.020, N = 138, P = 0.249). As above, we excluded the amplitude differences above 14 dB (range 14–26 dB). Again, paired differences in frequency were not normally distributed (all Z > 1.4, all P < 0.025), and we used nonparametric tests.
We compared the minimum, peak and maximum frequencies of the initial and final 10% of syllables (average of those syllables) and the remaining syllables (average of the remaining syllables) with paired Wilcoxon tests. As above, we also related the differences in amplitude with the differences in frequency using Spearman correlations. All tests were run using the software SPSS version 13 (SPSS Inc., Chicago, IL, U.S.A.).
RESULTS
Among-Songs Comparison
Louder songs had significantly lower minimum frequency and higher peak and maximum frequencies than their paired softer songs (paired Wilcoxon tests: minimum: Z = 2.852, P = 0.004; peak: Z = 3.225, P = 0.001; maximum: Z = 7.006, P < 0.001; all N = 134; all paired frequency differences in Fig. 2a, b, c).
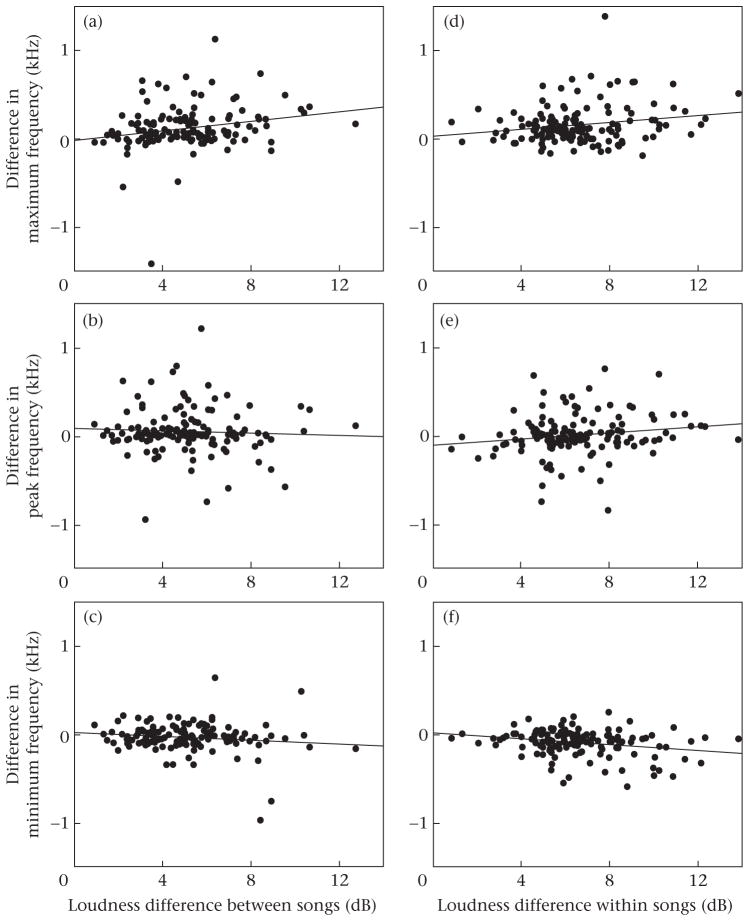
Figure 2.
Relation between amplitude differences between pairs of songs in a song bout (a, b, c), or between syllables within a song (d, e, f), and differences in maximum, peak and minimum frequency (louder minus softer). Regression lines are for illustration purposes only, as statistical tests were nonparametric.
Differences in minimum and peak frequencies did not correlate with differences in amplitude between songs (minimum: rS = −0.093, P = 0.286; peak: rS = −0.060, P = 0.489; both N = 134; Fig. 2b, c). The difference in maximum frequency correlated positively with the difference in amplitude (rS = 0.208, N = 134, P = 0.016; Fig. 2a).
Within-Songs Comparison
The louder central syllables of songs had significantly lower minimum frequency and higher maximum frequency than the softer outer syllables (paired Wilcoxon tests: minimum: Z = 6.814; maximum: Z = 7.946; both P < 0.001; both N = 138; paired differences in Fig. 2f, d, respectively). Peak frequency did not differ between central and outer syllables (paired Wilcoxon tests: Z = 0.546, N = 138, P = 0.585; paired differences in Fig. 2e).
Differences in minimum frequency correlated negatively with differences in amplitude between syllables (rS = −0.226, N = 138, P = 0.008; Fig. 2f;). Difference in peak and maximum frequencies correlated positively but nonsignificantly with differences in amplitude (peak: rS = 0.167, P = 0.051; maximum: rS = 0.135, P = 0.138; both N = 138; Fig. 2e, d, respectively).
Contrasting Among- and Within-Song Results
Among-song comparisons are more prone to random error in the measurements of relative amplitude owing to changes in recording conditions. This might augment the dispersion of the paired differences but, for large data sets, would not change the average or modal differences. Therefore, if measurement error affects these tests, results of among-song comparisons should be weaker than within-song comparisons. Below we note whether our results conform to this prediction, taking into account the magnitude of the differences in amplitude and frequency.
The differences in amplitude between and within songs were broadly similar (data in the horizontal axes of Fig. 2), although slightly smaller between songs (modal differences: 5.2 and 6.9 dB, respectively). This should contribute to make among-song results statistically weaker, as would also a possible effect of measurement error in amplitude. In contrast, and despite the modal difference for maximum frequency also being smaller between than within songs (0.073 and 0.110 kHz, respectively), the results of the paired comparisons of maximum frequency among and within songs were similar (among songs: Z = 7.006; within songs: Z = 7.946; see above). This is opposite to that expected if inaccuracy in measuring amplitude were important, indicating that it does not affect the results appreciably.
For minimum and peak frequency the modal differences between and within songs were also dissimilar (minimum frequency: −0.029 and −0.064 kHz, respectively; peak frequency: 0.024 and −0.005 kHz, respectively). The results of the corresponding among-and within-song comparisons (see above) match these modal differences, and should therefore be real, and not attributable to measurement error.
DISCUSSION
We tested for a relation between frequency and sound amplitude in birdsong, which is predicted by the hypothesis that the higher song frequency observed in urban songbirds could be a consequence of singing louder.
Opposite to the above prediction, the minimum frequency of dark-eyed junco songs was lower, not higher, in louder songs and syllables, and within songs this effect was more pronounced for larger differences in amplitude. The predicted relation between minimum frequency and amplitude could presumably exist only in extreme conditions, when the birds sing at their loudest. But our data set comprises a wide range of differences in amplitude, and we found no evidence that minimum frequency increases for larger differences in amplitude; on the contrary, the opposite was true (Fig. 2c, f). Therefore, for this species, putative louder singing does not explain the observed higher minimum frequency of urban birds (Slabbekoorn et al. 2007; Cardoso & Atwell 2011).
In line with the above prediction, we found that maximum song frequency was higher in louder songs and syllables, and that among songs this effect was stronger for larger differences in amplitude. Peak frequency was also higher in louder songs, but not demonstrably so in louder syllables within songs. In summary, frequency bandwidth increased with amplitude (owing to decreasing minimum and increasing maximum frequencies) and, among songs, peak frequency also increased.
Importantly, these associations with amplitude explain a very small part of the variation in song frequency (see Fig. 2), indicating that frequency and amplitude vary largely independently. Physiologically this is expected, because oscines control amplitude and frequency by distinct mechanisms: amplitude is controlled by the contraction of respiratory muscles that set air pressure and dorsal syrinx muscles that regulate syringeal aperture and airflow, while frequency is largely determined by the action of other specialized syrinx muscles that regulate tension in the sound-producing structures (Goller & Suthers 1996; Suthers et al. 1999). Therefore, even if a mechanistic link between amplitude and frequency exists in oscine physiology, as suggested by the correlation of these traits in towhee calls (Nelson 2000), birds may still be able to maintain stable frequency actively under varying amplitudes. Accordingly, we observed an association between song peak frequency and relative amplitude across songs but not within songs, indicating that increased peak frequency is not an obligatory outcome of singing louder. Instead, juncos may adjust both amplitude and peak frequency across songs in response to changing noise levels or to other motivational factors.
The hypothesis that higher song frequency in urban birds is a consequence of singing louder, as per the Lombard effect, also predicts that the frequency of the entire urban song should increase. But it is often reported that urban birds have higher minimum song frequency (reviewed in Slabbekoorn & Ripmeester 2008), not higher frequency of the whole song, which is consistent with this being a functional adjustment to reduce masking by low-frequency anthropogenic noise.
Urban juncos, for example, sing songs with higher minimum frequency than nonurban juncos (Slabbekoorn et al. 2007; Cardoso & Atwell 2011), but not with higher maximum frequency (Slabbekoorn et al. 2007). Analysing peak frequency with the same data set and methods as Cardoso & Atwell (2011), we found that urban juncos also have higher peak frequency (t = 2.69, N = 151 males, P = 0.008,), but with an average urban versus nonurban difference of 0.144 kHz, which is only about one-fourth of the difference in minimum frequency. Therefore, changes in urban junco songs were driven mostly by increasing minimum frequency. In other species, several studies reported increased urban minimum frequency but not all analysed both minimum frequency and other frequency parameters. The existing results, however, indicate that minimum frequency is the most common change: urban blackbirds, Turdus merula, do have higher minimum and peak frequencies (Nemeth & Brumm 2009; Ripmeester et al. 2010), but song sparrows (Wood & Yezerinac 2006), great tits, Parus major (Slabbekoorn & den Boer-Visser 2006; Mockford & Marshall 2009; Salaberria & Gil 2010), silvereyes, Zosterops lateralis (Potvin et al. 2011), and five of 12 common urban species studied by Hu & Cardoso (2010) were found to have significantly increased minimum frequency of songs or long-range calls, while only two of these had increased peak frequency (in one of them, the bell miner, Manorina melanophrys, calls are non-modulated, bell-like pure tones, and minimum and peak frequencies are therefore closely related).
Amplitude adjustments due to the Lombard effect are short term, and therefore amplitude-mediated effects on frequency should also be short term (Nemeth & Brumm 2010). Accordingly, several species can change song frequency in response to rapid natural or experimental increases in noise (Bermúdez-Cuamatzin et al. 2009, 2010; Halfwerk & Slabbekoorn 2009; Gross et al. 2010; Verzijden et al. 2010). In most cases, this is consistent with both the amplitude-mediated hypothesis and the usual interpretation that these are functional frequency adjustments to reduce masking. However, great tits do this not by changing the frequency of the same song type, but by switching to higher-frequency types (Halfwerk & Slabbekoorn 2009), which is not consistent with the amplitude-mediated hypothesis (and they also tend to switch to lower-frequency song types if experimentally played back noise of high, rather than low, frequencies; Halfwerk & Slabbekoorn 2009). There is also no evidence that higher-frequency song types are louder. On the contrary, in juncos the frequency of different song types is not related to amplitude (Cardoso et al. 2007), and existing studies in other species suggest that, if at all, high-frequency songs may generally be sung less loudly (Christie et al. 2004; Cardoso 2010). Also, other long-term differences in the minimum frequency of birdsong are known, such as seasonal differences (Slabbekoorn & Ripmeester 2008; Gross et al. 2010), suggesting mechanisms of frequency adjustment that are independent of sound amplitude.
The hypothesis that louder singing in cities increases the song frequency may be applicable for some species. But the above results and observations do not support this putative physiological coupling as a general explanation for the higher frequencies of urban birdsongs. In most cases they point to a more specific and uncoupled adjustment of minimum song frequency, consistent with the idea that singing with higher frequency is directly related to masking by low-frequency anthropogenic noise. The finding that this is less efficient than increasing sound amplitude (Nemeth & Brumm 2010) need not pose a paradox as to why it is common: while the benefit of increased song frequency is lower, its cost is also likely to be smaller. In oscines, increasing frequency requires mostly the contraction of specialized syrinx muscles (Goller & Suthers 1996; Suthers et al. 1999), while increasing amplitude requires syringeal action to regulate airflow and also increasing the contraction of the much larger expiratory muscles to generate the necessary higher air pressure (Suthers et al. 1999). Because of the difference in muscle masses involved, we hypothesize that the former should be less physiologically costly, especially if, as usually observed, birds only increase the minimum frequency of songs rather than the entire song.
In conclusion, considering the mechanisms by which oscines regulate sound amplitude and frequency, as well as the uncoupling of these two traits in our results suggests that the higher song frequency of urban birds is generally not a collateral effect of singing louder. Instead, singing louder and with higher frequency may be independent vocal adjustments to reduce masking by anthropogenic noise, and need not be viewed as alternatives. This is consistent with details of how urban birds increase song frequency (see above), and with a growing number of studies indicating that species with higher-frequency vocalizations are less impacted by anthropogenic noise (Rheindt 2003; Francis et al. 2009; Hu & Cardoso 2009; Goodwin & Shriver 2011).
Acknowledgments
We thank Ellen Ketterson and Trevor Price for discussions and ideas during the writing of this paper, and Russell Lande, Hopi Hoekstra, and Karen Marchetti for logistic support during fieldwork. This study was supported by the Fundação para a Ciência e a Tecnologia grant SFRH/BPD/21509/2005 to G.C.C. and a National Science Foundation grant (DEB-0808284) and graduate research fellowship to J.W.A.
References
- Amador A, Goller F, Mindlin GB. Frequency modulation during song in a suboscine does not require vocal muscles. Journal of Neurophysiology. 2009;99:2383–2389. doi: 10.1152/jn.01002.2007. [DOI] [PubMed] [Google Scholar]
- Anderson RC, Searcy WA, Peters S, Nowicki S. Soft song in song sparrows: acoustic structure and implications for signal function. Ethology. 2008;114:662–676. [Google Scholar]
- Beckers GJL, Suthers RA, ten Cate C. Mechanisms of frequency and amplitude modulation in ring dove song. Journal of Experimental Biology. 2003;206:1833–1843. doi: 10.1242/jeb.00364. [DOI] [PubMed] [Google Scholar]
- Beebee MD. Variation in vocal performance in the songs of a wood-warbler: evidence for the function of distinct singing modes. Ethology. 2004;110:531–542. [Google Scholar]
- Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM. Strategies of song adaptation to urban noise in the house finch: syllable pitch plasticity or differential syllable use? Behaviour. 2009;146:1269–1286. [Google Scholar]
- Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biology Letters. 2010;146:1269–1286. doi: 10.1098/rsbl.2010.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. Journal of Animal Ecology. 2004;73:434–440. [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Advances in the Study of Behavior. 2005;35:151–209. [Google Scholar]
- Brumm H, Schmidt R, Schrader L. Noise-dependent vocal plasticity in domestic fowl. Animal Behaviour. 2009;78:741–746. [Google Scholar]
- Cardoso GC. Loudness of birdsong is related to the body size, syntax and phonology of passerine species. Journal of Evolutionary Biology. 2010;23:212–219. doi: 10.1111/j.1420-9101.2009.01883.x. [DOI] [PubMed] [Google Scholar]
- Cardoso GC, Atwell JW. Directional cultural change by modification and replacement of memes. Evolution. 2011;65:295–300. doi: 10.1111/j.1558-5646.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso GC, Mota PG. Loudness of syllables is related to syntax and phonology in the songs of canaries and seedeaters. Behaviour. 2009;146:1649–1663. [Google Scholar]
- Cardoso GC, Atwell JW, Ketterson ED, Price TD. Inferring performance in the songs of dark-eyed juncos (Junco hyemalis) Behavioral Ecology. 2007;18:1051–1057. [Google Scholar]
- Cardoso GC, Mamede AT, Atwell JW, Mota PG, Ketterson ED, Price TD. Song frequency does not reflect differences in body size among males in two oscine species. Ethology. 2008;114:1084–1093. [Google Scholar]
- Christie PJ, Mennill DJ, Ratcliffe LM. Pitch shifts and song structure indicate male quality in the dawn chorus of black-capped chickadees. Behavioral Ecology and Sociobiology. 2004;55:341–348. [Google Scholar]
- Francis CD, Ortega CP, Cruz A. Noise pollution changes avian communities and species interactions. Current Biology. 2009;19:1–5. doi: 10.1016/j.cub.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Cynx J, Lewis R, Tavel B, Tse H. Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Animal Behaviour. 1998;56:107–113. doi: 10.1006/anbe.1998.0746. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. Journal of Neurophysiology. 1996;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goodwin SE, Shriver WG. Effects of traffic noise on occupancy patterns of forest birds. Conservation Biology. 2011;25:406–411. doi: 10.1111/j.1523-1739.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- Gross K, Pasinelli G, Kunc HP. Behavioral plasticity allows short-term adjustment to a novel environment. American Naturalist. 2010;176:456–464. doi: 10.1086/655428. [DOI] [PubMed] [Google Scholar]
- Halfwerk W, Slabbekoorn H. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Animal Behaviour. 2009;78:1301–1307. [Google Scholar]
- Hu Y, Cardoso GC. Are bird species that vocalize at higher frequencies preadapted to inhabit noisy urban areas? Behavioral Ecology. 2009;20:1268–1273. [Google Scholar]
- Hu Y, Cardoso GC. Which birds adjust the frequency of vocalizations in urban noise? Animal Behaviour. 2010;79:863–867. [Google Scholar]
- Konishi M. Song variation in a population of Oregon juncos. Condor. 1964;66:423–436. [Google Scholar]
- Lienard JS, Di Benedetto MG. Effect of vocal effort on spectral properties of vowels. Journal of the Acoustical Society of America. 1999;106:411–422. doi: 10.1121/1.428140. [DOI] [PubMed] [Google Scholar]
- Lopez PT, Narins PM, Lewis ER, Moore SW. Acoustically induced call modification in the white-lipped frog. Animal Behaviour. 1988;36:1295–1308. [Google Scholar]
- Mockford EJ, Marshall RC. Effects of urban noise on song and response behaviour in great tits. Proceedings of the Royal Society B. 2009;276:2979–2985. doi: 10.1098/rspb.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BS. Avian dependence on sound pressure level as an auditory distance cue. Animal Behaviour. 2000;59:57–67. doi: 10.1006/anbe.1999.1278. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Brumm H. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Animal Behaviour. 2009;78:637–641. [Google Scholar]
- Nemeth E, Brumm H. Birds and anthropogenic noise: are urban songs adaptive? American Naturalist. 2010;176:465–475. doi: 10.1086/656275. [DOI] [PubMed] [Google Scholar]
- Newman MM, Yeh PJ, Price TD. Song variation in a recently founded population of the dark-eyed junco (Junco hyemalis) Ethology. 2008;114:164–173. [Google Scholar]
- Potvin DA, Parris KM, Mulder RA. Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis) Proceedings of the Royal Society B. 2011;278:2464–2469. doi: 10.1098/rspb.2010.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheindt FE. The impact of roads on birds: does song frequency play a role in determining susceptibility to noise pollution? Journal für Ornithologie. 2003;144:295–306. [Google Scholar]
- Ripmeester EAP, Kok JS, van Rijssel JC, Slabbekoorn H. Habitat-related birdsong divergence: a multi-level study on the influence of territory density and ambient noise in European blackbirds. Behavioral Ecology and Sociobiology. 2010;64:409–418. doi: 10.1007/s00265-009-0857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaberria C, Gil D. Increase in song frequency in response to urban noise in the great tit Parus major as shown by data from the Madrid (Spain) city noise map. Ardeola. 2010;57:3–11. [Google Scholar]
- Slabbekoorn H, den Boer-Visser A. Cities change the songs of birds. Current Biology. 2006;16:2326–2331. doi: 10.1016/j.cub.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Ripmeester EAP. Birdsong and anthropogenic noise: implications and applications for conservation. Molecular Ecology. 2008;17:72–83. doi: 10.1111/j.1365-294X.2007.03487.x. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Yeh P, Hunt K. Sound transmission and song divergence: a comparison of urban and forest acoustics. Condor. 2007;109:67–78. [Google Scholar]
- Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Philosophical Transactions of the Royal Society B. 1999;354:927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RC. Short-range and long-range songs: use of two acoustically distinct song classes by dark-eyed juncos. Auk. 1998;115:386–393. [Google Scholar]
- Verzijden MN, Ripmeester EAP, Ohms VR, Snelderwaard P, Slabbekoorn H. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. Journal of Experimental Biology. 2010;213:2575–2581. doi: 10.1242/jeb.038299. [DOI] [PubMed] [Google Scholar]
- Williams L, MacRoberts MH. Individual variation in songs of dark-eyed juncos. Condor. 1977;79:106–112. [Google Scholar]
- Wood WE, Yezerinac SM. Song sparrow (Melospiza melodia) song varies with urban noise. Auk. 2006;123:650–659. [Google Scholar]
- Yeh PJ. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution. 2004;58:166–174. doi: 10.1111/j.0014-3820.2004.tb01583.x. [DOI] [PubMed] [Google Scholar]