Abstract
In the vestibular peripheral organs, type I and type II hair cells (HCs) transmit incoming signals via glutamatergic quantal transmission onto afferent nerve fibers. Additionally, type I HCs transmit via “non-quantal” transmission to calyx afferent fibers, by accumulation of glutamate and potassium in the synaptic cleft. Vestibular efferent inputs originating in the brainstem contact type II HCs and vestibular afferents. Here, synaptic inputs to type II HCs were characterized by using electrical and optogenetic stimulation of efferent fibers combined with in vitro whole cell patch-clamp recording from type II HCs in the rodent vestibular crista. Properties of efferent synaptic currents in type II HCs were similar to those found in cochlear HCs and mediated by activation of α9-containing nicotinic acetylcholine receptors (nAChRs) and small-conductance calcium-activated potassium (SK) channels. While efferents showed a low probability of release at low frequencies of stimulation, repetitive stimulation resulted in facilitation and increased probability of release. Notably, the membrane potential of type II HCs during optogenetic stimulation of efferents showed a strong hyperpolarization in response to single pulses and was further enhanced by repetitive stimulation. Such efferent-mediated inhibition of type II HCs can provide a mechanism to adjust the contribution of signals from type I and type II HCs to vestibular nerve fibers, with a shift of the response to be more like that of calyx-only afferents with faster non-quantal responses.
NEW & NOTEWORTHY Type II vestibular hair cells (HCs) receive inputs from efferent neurons in the brain stem. We used in vitro optogenetic and electrical stimulation of vestibular efferent fibers to study their synaptic inputs to type II HCs. Stimulation of efferents inhibited type II HCs, similar to efferent effects on cochlear HCs. We propose that efferent inputs adjust the contribution of signals from type I and II HCs to vestibular nerve fibers.
Keywords: α9-ACh receptor, efferent, hair cell, synaptic transmission, vestibular
INTRODUCTION
The peripheral vestibular end organs (saccule, utricle, and 3 semicircular canals) convey signals about head position (re gravity) and head motion to the brain. The peripheral afferent pathway consists of vestibular nerve fibers that innervate hair cells (HCs) via calyx-type terminals that ensheath type I HCs and bouton endings that innervate type II HCs (see schematics, Fig. 1, C and D). Most vestibular afferent fibers receive inputs from both type I and type II HCs (so-called dimorphic afferents) (Goldberg 2000), whereby the input from type II HCs can come via synapses with bouton endings or with the outer wall of calyx afferent terminals (Lysakowski and Goldberg 1997, 2008) (see schematic, Fig. 8).
Fig. 1.
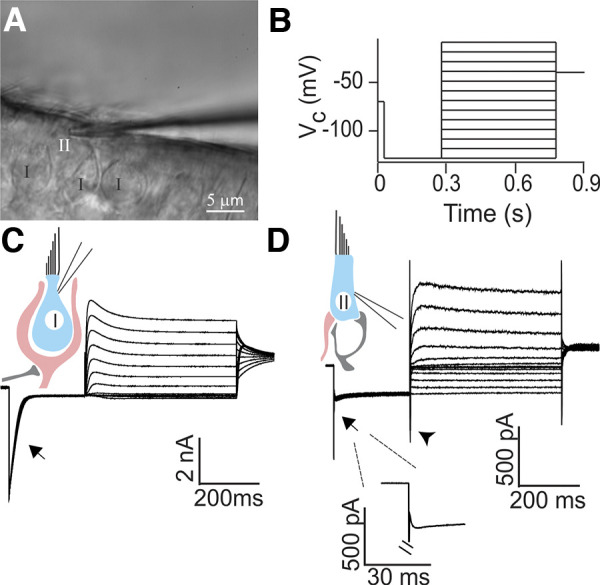
Whole cell patch-clamp recording from morphologically and physiologically identified rat type II hair cells (HCs). A: in a whole mount preparation of the rat crista, putative type II HCs were recognized based on the lack of a surrounding calyx using differential interference contrast (DIC) optics. Electrode is shown approaching the HC from the right. B: characteristic conductances of HCs were examined by a voltage-clamp protocol consisting of a 250-ms hyperpolarization step from −70 mV to −130 mV (held for 250 ms) followed by a family of 500-ms depolarization steps with an increment of 10 mV up to a holding potential of −10 mV. A final potential of −40 mV was held for 100 ms. Vc, voltage command. C: type I HCs showed a large, slowly inactivating inward current (IK,L; arrow) in response to a negative voltage step. D: type II HCs lacked the large IK,L current found in type I HCs (arrow, and enlarged in inset). An inward current (likely a hyperpolarization-activated current, Ih) could be seen in response to a depolarizing voltage step in type II HCs (arrowhead) as described previously (Brichta et al. 2002; Meredith et al. 2012; Rüsch et al. 1998).
Fig. 8.
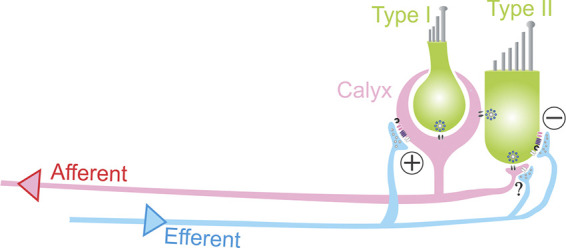
Afferent and efferent innervation in the vestibular periphery. The peripheral vestibular afferent pathway consists of vestibular nerve fibers that innervate hair cells (HCs) via calyx-type terminals that ensheath type I HCs and bouton endings that innervate type II HCs. Most vestibular afferent fibers receive inputs from both type I and type II HCs (so-called dimorphic afferents). Type II HCs can form synapses with bouton endings or with the outer wall of calyx afferent terminals. Additionally (not shown), some afferent fibers receive input from type I HCs via “calyx-only” synapses, and others receive input from type II HCs via “bouton-only” synapses. Efferent fibers originating in the brain stem form synapses onto type II HCs as well as onto calyx and bouton afferent endings. Both hair cell types use glutamatergic quantal transmission via ribbon synapses, and type I HCs also mediate signals by nonquantal transmission via potassium and glutamate accumulation in the synaptic cleft. Inhibition of type II HCs by efferents provides a mechanism for type I HC inputs and calyx terminal activity to become the dominant afferent signal.
A group of neurons in the brain stem innervate vestibular end organs bilaterally (Gacek and Lyon 1974; Goldberg and Fernández 1980; Leijon and Magnusson 2014; Mathews et al. 2015; Warr 1975). Despite their low numbers, efferent fibers branch extensively to form synapses onto type II HCs as well as onto calyx-type and bouton afferent endings (Lysakowski and Goldberg 1997, 2008). In vivo efferent stimulation results in distinct effects on afferent firing rate in different species. In amphibians and reptiles, afferent fibers can exhibit excitation, inhibition, or a mixture of excitation-inhibition in response to electrical stimulation of efferents (Brichta and Goldberg 1996; Holt et al. 2006; Rossi et al. 1980). In fish and mammals, efferent modulation of afferent resting discharge is almost exclusively excitatory (Boyle and Highstein 1990; Goldberg and Fernández 1980; Highstein and Baker 1985; Marlinski et al. 2004; Plotnik et al. 2002; Sadeghi et al. 2009).
Several studies have contributed to elucidating the functional roles of efferent inputs to the vestibular periphery. Efferent stimulation has been shown to decrease the rotational sensitivity of afferents in squirrel monkey (Goldberg and Fernández 1980) and toadfish (Boyle and Highstein 1990). In toadfish, electrical stimulation of efferents leads to an increase in the afferent resting discharge rate and to inhibition of hair cells, which is thought to result in the reduced gain of the afferent response to canal stimulation (Boyle et al. 2009). Furthermore, it has been shown that efferent stimulation decreases bundle movements and mechanotransduction in vestibular hair cells of frogs (Castellano-Muñoz et al. 2010; Lin and Bozovic 2020) and suppresses amplification by hair cells in response to low-velocity rotations in toadfish (Rabbitt et al. 2010), suggesting that these are possible mechanisms involved in the decrease in afferent sensitivity observed with efferent stimulation. Although such dynamic gain adjustments can theoretically be used to adjust the dynamic range of neurons [e.g., during fast self-generated head movements by toadfish (Highstein and Baker 1985)], evidence for this phenomenon has not been seen in primates (Sadeghi et al. 2007). Here, increased efferent activity may play a role in adjusting the relative input from type I and type II hair cells (HCs) to dimorphic afferents and changes in afferent terminal properties. Additionally, efferent modulation of afferent response properties may underlie compensatory mechanisms after acute lesions or during aging (Sadeghi et al. 2007).
A prominent efferent neurotransmitter in the inner ear is acetylcholine (ACh), although other transmitters also exist in the efferent system (reviewed in Holt et al. 2011). Efferent synapses onto calyx endings produce excitatory responses most likely operating through α4β2-containing nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs) (as shown in turtle) and produce excitatory responses in afferent fibers (Holt et al. 2011, 2015, 2017; Jordan et al. 2013, 2015; Pérez et al. 2009). In the turtle, it has also been suggested that efferent inputs have an excitatory effect on afferent bouton terminals (Holt et al. 2006). Finally, vestibular efferent synapses with type II HCs share features with the well-characterized inhibitory efferent synapses formed with cochlear HCs. In the cochlea, the inhibitory effect is due to HC hyperpolarization through the combined action of α9-containing nicotinic acetylcholine receptors (nAChRs) and calcium-dependent large-conductance (BK) or small-conductance potassium (SK) channels (Elgoyhen et al. 1994; Glowatzki and Fuchs 2000; Oliver et al. 2000; Wersinger et al. 2010; Yuhas and Fuchs 1999). Cochlear efferent synapses have postsynaptic specializations, the “subsynaptic cisterns” (Saito 1980) that can contribute to the HC efferent response (Lioudyno et al. 2004). Similarly, vestibular type II HCs express α9-containing nAChRs and BK and SK channels (Parks et al. 2017; Poppi et al. 2018) and have cisterns postsynaptic to their efferent inputs (Lysakowski and Goldberg 1997). It was recently shown that ACh application has an inhibitory effect on type II vestibular HCs in mice (Poppi et al. 2018). This is an intriguing result considering that efferent stimulation results in an increase in the resting discharge of afferents in mammals. However, to understand efferent-mediated effects on afferent resting discharges and sensitivities during stimulation, the combined efferent inputs onto calyx afferents, bouton afferents, and type II HCs have to be considered (Boyle et al. 2009).
The study here aimed to characterize the properties and effects of efferent synaptic inputs specifically to type II HCs. Efferent fibers in the excised rat or mouse crista were electrically or optogenetically stimulated and efferent synaptic activity was recorded in type II HCs. Efferent synaptic currents in type II HCs display similar properties as found in cochlear HCs and are mediated by nAChRs and SK channels. Low-frequency efferent stimulation results in a low probability of release, whereas during repetitive stimulation the response is greatly potentiated. Optogenetic stimulation of efferents allowed voltage changes to be measured in type II HCs and showed strong inhibition of type II HCs with single pulse stimulation, which was further enhanced and prolonged with repetitive stimulation. Efferent inputs may therefore shift the relative contribution of inputs toward type I HCs in dimorphic afferents.
MATERIALS AND METHODS
Animals.
All animals were handled in accordance with animal protocols approved by The Johns Hopkins University Animal Care and Use Committee. Experiments were performed in 13- to 28-day-old rats and mice of either sex. The rat strain was Sprague-Dawley (Charles River Laboratories).
To specifically drive gene expression in vestibular efferents, ChAT-Cre mice [B6;129S6-Chattm2(cre)Lowl/J; JAX no. 006410] were used. By crossing the Ai32 [B6;129S-Gt(Rosa)26Sortm32(CAG-COP4*H134R/EYFP)/Hze/J] mouse line and the ChAT-Cre [B6;129S-Chattm2(cre)Lowl/J] mouse line, the light-gated ion channel channelrhodopsin2 (ChR2) was expressed under the control of the choline acetyltransferase (ChAT) promoter. Stable yellow fluorescent protein (YFP) signals were detected in efferents in ChAT-Cre; Ai32 mice at postnatal day 21 (P21) and older.
Tissue preparation.
Preparation of the vestibular crista for electrophysiological recordings has been described before (Sadeghi et al. 2014). Mice or rats were deeply anesthetized by isoflurane inhalation and decapitated. The inner ear tissue was removed from the temporal bone and placed into extracellular solution. The bony labyrinth was opened, and part of the membranous labyrinth was dissected, including ampullae of the horizontal and superior canals, and Scarpa’s ganglion. The membranous labyrinth was then opened above the cristae, and remaining cupulae located on top of HCs were removed to expose the neuroepithelia.
Note that the efferent fibers in this inner ear preparation were separated from their somata located in the brain stem. Type II HCs have been shown to acquire mature morphology and ion conductances by P8–P10 in the mouse utricle (Rüsch et al. 1998), and efferent innervation has been reported to be fully established around P12 in the rat vestibular periphery (Demêmes and Broca 1998). Therefore, to target relatively mature HCs and efferent fibers, the study was confined to an age range between postnatal days 13 and 28 (P13–P28).
Electrophysiology recordings.
The preparation was secured on a coverslip under a pin, transferred to the recording chamber, and perfused with extracellular solution at a rate of 1.5−3 mL/min. The extracellular solution contained (in mM) 5.8 KCl, 144 NaCl, 0.9 MgCl2, 1.3 CaCl2, 0.7 NaH2PO4, 5.6 glucose, and 10 HEPES, 300 mosM, pH 7.4 (NaOH). In some experiments, to increase the extracellular K+ concentration to 40 mM, equimolar NaCl was replaced with KCl. To perform patch-clamp recordings, tissue was visualized with a ×40 water-immersion objective under differential interference contrast (DIC) optics (Axioskop2 microscope, Zeiss) and viewed on a monitor via a video camera (Dage MTI LSC 70 or IR1000).
Patch-clamp recording pipettes were fabricated from borosilicate glass (1-mm inner diameter; WPI). Pipettes were pulled with a multistep horizontal puller (Sutter), coated with Sylgard (Dow Corning), and fire polished. Pipette resistances were 5–8 MΩ. The intracellular solution for HCs contained (in mM) 20 KCl, 110 K-methanesulfonate, 0.1 CaCl2, 5 EGTA, 5 HEPES, 5 Na2 phosphocreatine, 4 MgATP, and 0.3 Tris-GTP, 290 mosM, pH 7.2 (KOH). All measurements were acquired using pCLAMP10.2 software in conjunction with a Multiclamp 700B amplifier (Molecular Devices), digitized at 50 kHz with a Digidata 1440A, and filtered at 10 kHz. Both current- and voltage-clamp recordings were corrected for the measured liquid junction potentials of 9 mV. In voltage clamp, a 10-mV hyperpolarization step from −75 or −80 mV (50 ms in duration) was applied to examine membrane resistance (Rm) and series resistance (Rs). Only recordings that had Rs < 25 MΩ were included in data analysis. Series resistance Rs was not compensated for.
Putative type II HCs were distinguished from putative type I HCs by the absence of calyx-terminal surroundings, a structure that could be visualized as a thickened double layer under DIC optics. Type I HCs were exposed for recordings by separating the surrounding calyx terminals with positive pressure.
Drug solution was either applied to the bath directly or to the tissue focally. Focal application of solutions was performed using a gravity-driven flow pipette (~100 μm in diameter) placed near the recorded type II HC, connected with a VC-6 channel valve controller (Warner Instruments, Hamden, CT). With focal application, final concentrations were reached within 10–15 s. Iberiotoxin (IBTX), apamin, and 4-aminopyridine (4-AP) were purchased from Tocris Bioscience. Acetylcholine chloride and strychnine hydrochloride were purchased from Sigma. α-RgIA was synthesized as previously described (Ellison et al. 2006).
Electrical stimulation of vestibular efferents.
Monopolar electrical stimulation of efferents was delivered through a glass micropipette (the same size as the patch pipette) filled with extracellular solution. It approached the targeted HC from the opposite side of the recording electrode’s approach and was placed into the tissue ~100 μm beneath the HC. An electrically isolated constant current source (model DS3; Digitimer Ltd, Welwyn Garden City, UK) was triggered via the data acquisition computer to generate pulses of 8–50 mA, 20–40 μs long to activate efferents. The position of the stimulation pipette was slowly adjusted so that the electrical pulses could evoke synaptic events with minimal contamination of artifacts. To ensure that the stimulation strength was sufficient to reliably trigger synaptic events, release probability was monitored by delivering 100–200 pulses at 2 Hz at each testing amplitude. The stimulation amplitude was set at a value where no further increase of release probability was detected with a twofold increase in stimulation amplitude.
Optogenetic stimulation.
Blue light pulses were delivered through an optical fiber (diameter 910 μm; Thorlab) coupled to an LED light source (XLamp; 485 nm; Cree). The optical fiber tip was positioned next to the objective so that the light beam reached the whole tissue preparation. The intensity and timing of light pulses were controlled through an LED driver (Mightex) that operated under the command of the data acquisition computer. The maximum output light intensity reached by this system is ~80–100 mW/mm2. For stimulating axons, brief light pulses of 3–5 ms were applied. Temperature-mediated changes in the vestibular system (Rabbitt et al. 2016; Raghu et al. 2019) are not a concern for the optogenetic stimulation parameters used here since such changes depend on the rate and/or peak temperatures attained and significant temperature changes with blue LED sources require >1,000 ms (Raghu et al. 2019) rather than the 3–5 ms used in the present study.
Immunohistochemistry and imaging.
Freshly excised temporal bone tissue with bony labyrinth opened or tissue after electrophysiology recording was dropped into 4% paraformaldehyde (PFA) for fixation of 1 h to 24 h at 4°C and then rinsed in PBS solution. For antibody-based immunolabeling, samples were first incubated in blocking buffer (PBS with 10% normal donkey serum, 0.3% Triton X-100) for 1 h. Samples were then incubated in primary antibody diluted in blocking buffer for 24–48 h at 4°C. Samples were rinsed in PBS before incubation in secondary antibody diluted 1:500–1:750 in blocking buffer for 2 h. After being rinsed in PBS three to five times, samples were mounted on glass slides in FluorSave reagent medium (EMD Millipore). Primary antibodies used in this work included the following: goat anti-green fluorescent protein (GFP; 1:5000; SicGen) (Wu et al. 2020), mouse anti-β-tubulin III (TUJ1; 1:250; BioLegend) (Macova et al. 2019), rabbit anti-myosin VIIa (1:250; Sigma) (Kros et al. 2002), and mouse IgG1anti-synaptic vesicle glucoprotein 2A (SV2; 1:400; Developmental Studies Hybridoma Bank, The University of Iowa, Department of Biology, Iowa City, IA) (Buckley and Kelly 1985). Secondary antibodies used in this work included the following: Alexa Fluor (488) conjugated donkey anti-goat IgG heavy/light chain (H+L) (Barlow-Anacker et al. 2017), Alexa Fluor (568) conjugated donkey anti-rabbit IgG(H+L) (Wang et al. 2016), and Alexa Fluor (568, 647) conjugated donkey anti-mouse IgG(H+L) (ThermoFisher) (Anderson et al. 2017; Wang et al. 2016).
Fluorescence images were acquired using a laser scanning confocal microscope (LSM700; Zeiss) under the software control of ZEN. Imaged Z stacks were collected under near-saturating laser intensities for each channel.
Data analysis.
Electrophysiology data were analyzed using Clampfit (Molecular Devices) and MiniAnalysis (Synaptosoft). Synaptic events were detected using a routine in MiniAnalysis, with a threshold set at three times the value of the root mean square of the baseline noise. In addition, search parameters (e.g., baseline, period to search for a decay time, threshold of the area of events) were set for individual recordings to mark events, and marked events were verified by eye. At least five points were averaged for a peak value. The decay of synaptic events was fit with a single exponential, and only fitting results with coefficient of determination (R2) > 0.85 were considered as a good fit and included for further analysis. For those well-fitted events, 90–10% decay time constants (τdecay) were calculated. For averaging, synaptic events were aligned by their rise time. Igor Pro (WaveMetrics) was used for making plots. An Igor Pro script written by Dr. Juan Goutman was used to read pClamp files. Data are reported as means ± SE and compared using t tests (paired or unpaired), with α set at 0.05 for significance.
Probability of release (Pr) was calculated as the number of successes divided by the number of stimuli. For identifying if a success had occurred, the response to a stimulus had to be larger than three times the standard deviation of the baseline preceding the stimulus. For pulsed stimulation, the last 5 ms of the preceding response were used as the baseline of the following response. Successes were confirmed by eye.
RESULTS
Recordings from type II HCs in the crista of the semicircular canals.
To investigate the properties of efferent synaptic inputs to vestibular type II HC, whole cell patch-clamp recordings were performed in the central zone of acutely isolated whole mount tissue of the rat crista, from either the horizontal or superior canal (see materials and methods; Sadeghi et al. 2014). To target relatively mature HCs and efferent fibers, the study was confined to ages between P13 and P28.
Type I HCs and type II HCs are intermingled in the crista. Type I HCs are surrounded by calyx endings that appear as a double layer of membranes under DIC optics. To preselect putative type II HCs for recordings, HCs were chosen that lack a surrounding calyx (Fig. 1A). However, the final distinction between type I HCs and type II HCs was made based on their response to a voltage-step protocol (Fig. 1B). In type I HCs, a hyperpolarizing voltage step from −70 mV to −130 mV triggered large, slowly inactivating currents in the nanoampere range resembling IK,L, a delayed rectifier potassium channel (Fig. 1C, arrow) previously described in type I HCs in mouse utricle (Rüsch et al. 1998) as well as the cristae of gerbil (Rennie et al. 1996) and pigeon (Ricci et al. 1996). In contrast, in putative type II HCs, the same hyperpolarizing step activated noninactivating inward currents of a few hundred picoamperes (−418.0 ± 31.9 pA, n = 10; Fig. 1D, arrow and inset). These currents were likely mediated by inward rectifier currents (Horwitz et al. 2011; Levin and Holt 2012) and by Ih, a hyperpolarization-activated current (Rüsch et al. 1998). Only HCs that lacked the large and slowly inactivating IK,L were considered as type II HCs for the subsequent study. Resting membrane potentials in type II HCs were −69.5 ± 2.3 mV (n = 25, range: −40 to −88 mV), slightly more depolarized than −77 ± 3.7 mV for type I HCs as shown previously in rats (Rüsch et al. 1998). As reported by previous studies, the membrane resistance (Rm) of type II HCs (Hurley et al. 2006; Rabbitt et al. 2016) was an order of magnitude larger than Rm in type I HCs (Chen and Eatock 2000; Hurley et al. 2006; Li et al. 2010) [type II HCs: 528.6 ± 62.0 MΩ, n = 25; type I HCs: 50.0 ± 12.7 MΩ, n = 3; holding potential (Vh) = −80 mV].
Properties of spontaneous and evoked efferent synaptic currents in type II HCs.
Both spontaneous and electrically evoked efferent synaptic events in type II HCs were recorded and analyzed. Initially during recordings, spontaneous synaptic events were only seen rarely. However, after efferent stimulation, a subset of type II HCs (5/23) exhibited randomly occurring synaptic events that are described as “spontaneous” excitatory postsynaptic currents (sEPSCs) here (Fig. 2, A1 and A2). An example sEPSC amplitude distribution is shown in Fig. 2A3. The mean sEPSC amplitude was 12.96 ± 0.21 pA, the peak of the distribution was at 10.67 pA, and the median was 12.21 pA. For the population of recorded HCs, the sEPSC amplitude was 21.7 ± 4.3 pA (range: 7.3–72.0 pA), the 10–90% rise time was 6.4 ± 0.8 ms, and a time constant of decay was 36.4 ± 2.9 ms (n = 5 HCs; 529 events).
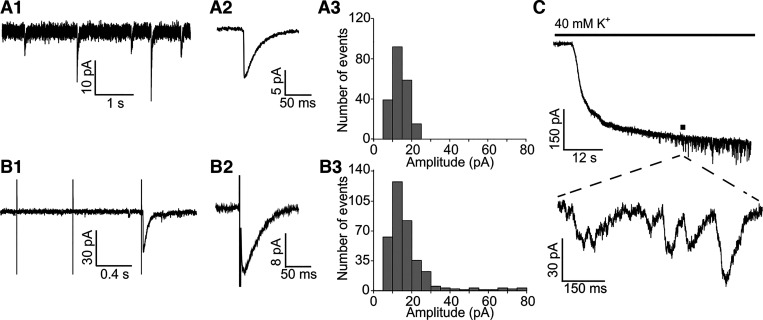
Fig. 2.
Spontaneous and evoked efferent synaptic events in rat type II hair cells (HCs). A1: spontaneous efferent excitatory postsynaptic currents (sEPSCs) in type II HCs. A2: average of sEPSC waveform from a single HC (40 events averaged). A3: sEPSC amplitude distribution from an individual type II HC recording (n = 216 events). B1: electrically evoked EPSCs (eEPSCs) in type II HCs. The first two electrical pulses only resulted in transient artifacts, while the third pulse triggered a synaptic response. B2: average eEPSC waveform. B3: eEPSC amplitude distribution for the population of type II HC recordings (n = 515 events from 12 HCs). C: application of 40 mM external solution triggered efferent synaptic events in type II HCs due to depolarization of efferent terminals. The delay in response is due to the delay of 40 mM potassium reaching the tissue after switching to this solution. All recordings were performed at a holding potential Vh = −90 mV.
Electrical stimulation of efferent fibers was delivered by a monopolar glass electrode that was placed into the tissue ~100 μm beneath the recorded HC (see materials and methods; Goutman et al. 2005). The number of efferent synapses were counted in the chinchilla to be approximately three or four on each type II HC (Lysakowski and Goldberg 1997). Electrical stimulation reaches many fibers, and the stimulation amplitude was set at a value where no further increase of release probability (Pr) was detected with a twofold increase in stimulation amplitude. Therefore, it is assumed here that most, if not all, fibers innervating a type II HC are stimulated by this approach. This needs to be taken into account when comparing properties like probability of release of this “multiefferent fiber unit per HC” with other systems where a different number of efferents innervate a single HC.
At a holding potential of −90 mV, stimulation artifacts were brief, allowing for the EPSC waveform to be assessed (Fig. 2, B1 and B2). Electrical stimulation induced synaptic events in almost all type II HCs tested (22/23). At a stimulation rate of 1–2 Hz, electrical shocks evoked EPSCs with a low success rate [probability of release (Pr) = number of successes/number of stimuli] of ~0.06 (Pr = 0.056 ± 0.015, n = 8). The waveform of electrically evoked EPSCs (eEPSCs) was similar to those of sEPSCs. At a holding potential (Vh) of −90 mV, the mean eEPSC amplitude was 22.0 ± 3.0 pA (range: 5.5–76.3 pA), the median was 15.3 pA, and the peak of the eEPSC amplitude distributions was at 15 pA (n = 12 HCs; 515 events analyzed; Fig. 2B3). eEPSCs had a 10–90% rise time of 7.1 ± 1.1 ms and a time constant of decay of 35.0 ± 3.4 ms (n = 7 HCs; 156 events).
Efferent synaptic currents can also be elicited in type II HCs by applying extracellular solution with an elevated potassium concentration (40 mM K+ used here) and thereby depolarizing efferent fibers (Fig. 2C). The first synaptic event following 40 mM K+ application appeared after a long latency of 38.0 ± 7.4 s (n = 11 cells), consistent with an initially low probability of release. The change in extracellular potassium, however, shifts the K+ equilibrium potential to a more positive value, resulting in a larger component of synaptic events carried by potassium currents at the holding potential of −90 mV (see Fig. 3) and thereby in a slower waveform. Further analysis in Figs. 3–5 is therefore limited to synaptic events evoked by electrical stimulation of efferents.
Fig. 3.
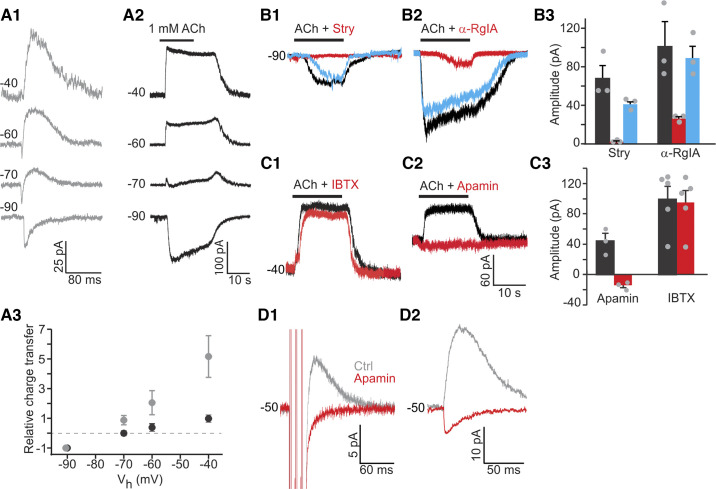
Efferent synaptic responses in rat type II hair cells (HCs) are mediated through α9-containing nicotinic acetylcholine receptors (nAChRs) and small-conductance potassium (SK) channels. A1: spontaneous excitatory postsynaptic currents (sEPSCs) were recorded in type II HCs at different holding potentials (Vh), as indicated at each trace. Biphasic responses at −60 mV and −70 mV are consistent with influx of cations (including calcium ions) through nAChRs followed by an outward current through calcium-activated K+ channels. A2: application of 1 mM ACh induced currents that showed a change from inward to outward currents at more positive Vh. A3: relative total charge transfer (normalized to charge transfer at −90 mV) at different Vh for synaptic currents (gray) and ACh-induced currents (black). B1 and B2: example recordings showing blockade of responses to application of 1 mM ACh by antagonists of α9-containing nAChRs [10 μM strychnine (Stry) or 600 nM α-RgIA]. Black, red, and blue traces show control (Ctrl), drug application, and post-wash conditions. B3: average effects of strychnine or α-RgIA (n = 3 type II HCs for each drug). C1 and C2: example recordings showing blockade of outward potassium currents in the presence of SK channel antagonist apamin (300 nM). Antagonist of large-conductance potassium (BK) channels [100 nM iberiotoxin (IBTX)] did not have any effect. C3: average effects of apamin (n = 3 type II HCs) and IBTX (n = 5 type II HCs). D1: 300 nM apamin blocked outward evoked synaptic currents (eEPSCs) at −50 mV. Three pulses at 100 Hz were used to trigger responses. Traces were averaged from 200 trials. D2: 300 nM apamin reversed the polarity of spontaneous synaptic currents. Traces were averaged from 15–20 events.
Fig. 5.
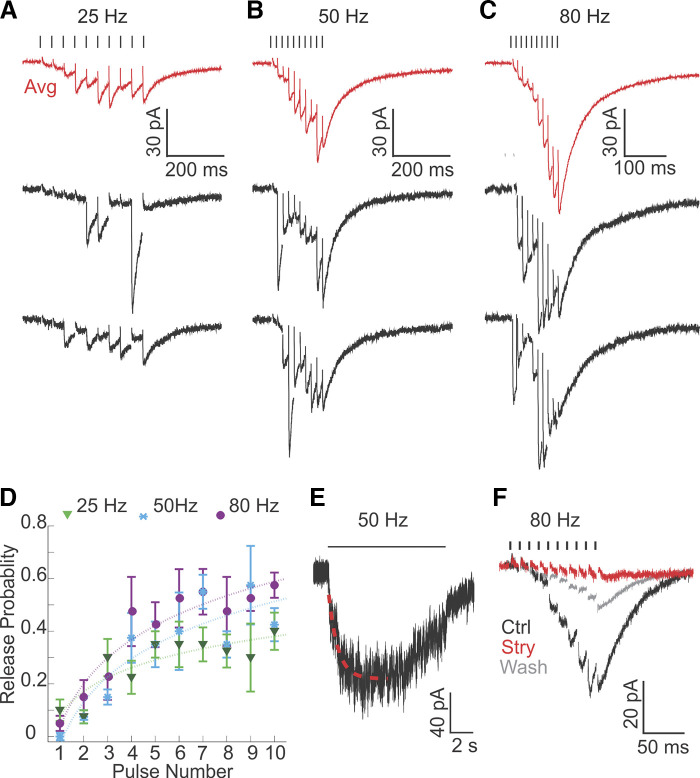
Efferent inputs to rat type II hair cells (HCs) are potentiated by high-frequency train stimulation. A–C: synaptic responses from an individual recording evoked by 10-pulse train stimulation at frequencies of 25 Hz (A), 50 Hz (B), and 80 Hz (C) every 15 s. Note different scale bars. Data are averaged responses across 10 trials (red traces) and examples of single responses (black traces). D: release probability continuously increased during train stimulations for each frequency of stimulation (n = 4 HCs). E: example synaptic response evoked by 10 s of stimulation at 50 Hz (note the timescale) showing maintained synaptic release. The rise of the response was fitted with an exponential function (red dashed line). F: 200 nM strychnine (Stry) almost completely abolished the synaptic response evoked by 80-Hz train stimulation (averaged response across 10 trials). Ctrl, control.
In summary, almost all type II HCs received efferent synaptic inputs. However, these inputs showed a low probability of release at low stimulation rates (1–2 Hz), raising the question of how big the impact of efferent inputs on type II HC activity at low efferent firing rates can be.
Efferent synaptic responses in type II hair cells are mediated by α9-containing nAChRs and calcium-activated SK2 potassium channels.
To identify the ion channels mediating efferent synaptic currents in type II HCs, as a first step, spontaneous synaptic events were recorded at different holding potentials (Vh). Synaptic currents were inward at −90 mV, biphasic at −70 and −60 mV, with an inward current followed by an outward current, and outward at −50 mV and above (Fig. 3A1). The relative total charge transfer of synaptic currents was measured as the area of the current deviating from baseline and normalized to the value measured at the holding potential of −90 mV. It reversed between −90 and −70 mV, close to the potassium equilibrium potential (EK = −83 mV; Fig. 3A3), suggesting that a potassium conductance dominated the response. The biphasic waveform and negative reversal potential of the efferent synaptic events in type II HCs resembled the properties of efferent events observed in cochlear HCs (Goutman et al. 2005). For cochlear HCs, it has been demonstrated that efferent synaptic events are mediated by Ca2+-permeable α9-containing nAChRs and subsequent activation of Ca2+-activated (SK or BK) potassium channels (reviewed in Katz et al. 2011). The biphasic nature of synaptic currents was explained by an initial influx of cations including Ca2+ ions through α9-containing nAChRs and subsequent efflux of K+ through Ca2+-activated potassium channels. Second, for type II HCs in the mouse crista, it has been shown that ACh also elicits a response mediated by α9-containing nAChRs and SK channel activation (Poppi et al. 2018, 2020).
To test whether a similar postsynaptic mechanism might account for efferent responses in type II rat vestibular HCs, application of 1 mM ACh was used to mimic efferent inputs, as the majority of efferents are likely to be cholinergic (reviewed in: Holt et al. 2011). ACh (1 mM) was used based on the experience of recording ACh responses in cochlear HCs, where sometimes 100 μM ACh did not elicit a response in a HC but 1 mM ACh did (Roux et al. 2016). Some but not all recordings showed a decline in the ACh-evoked current during ACh application, suggesting some level of desensitization (Elgoyhen et al. 2001) that might affect the precision of the amplitude measurements. All type II HCs tested responded to ACh with inward currents of −103.2 ± 10.6 pA at Vh of −90 mV (n = 22). At Vh positive to −70 mV, outward currents started to appear and became dominant at more positive holding potentials (Fig. 3, A2 and A3), similar to the findings for synaptic responses. The relative total charge transfer of the ACh response reversed at −70 mV (Fig. 3A3), again, close to EK, although slightly more positive compared with the synaptic response.
To test if α9-containing nAChRs mediate the inward ACh response in type II HCs, specific blockers were applied. Strychnine (10 μM), an antagonist of α7- and α9-containing nAChRs (Elgoyhen et al. 1994), almost completely and reversibly blocked the ACh response [n = 3; voltage command (Vc) = −81 mV; with strychnine: −2.3 ± 0.8 pA; control: −69.0 ± 13.8 pA; P = 0.04, paired t test; Fig. 3, B1 and B3). α-RgIA (600 nM), a specific antagonist of α9-containing nAChRs (Ellison et al. 2006), reversibly blocked ACh by ~74% (n = 3; with α-RgIA: −26.6 ± 2.2 pA; control: −102.8 ± 27.0 pA; P = 0.06, paired t test; Fig. 3, B2 and B3). The strychnine and α-RgIA block suggested that ACh responses were mediated by α9-containing nAChRs.
To test whether calcium-dependent potassium channels were involved in the outward ACh response in rat type II HCs, blockers for small (SK)- and big-conductance (BK) potassium channels were applied. Both BK and SK channel blockers have been found to affect ACh responses and efferent synaptic currents in rat cochlear HCs, with different effects depending on the apical/basal location of the HCs along the cochlea (Wersinger et al. 2010). Moreover, it has been suggested that BK channels in combination with muscarinic AChRs might contribute to ACh responses in isolated guinea pig utricular HCs (Kong et al. 2005). However, SK channels might be involved in ACh responses in type II HCs, as apamin, an SK channel blocker, removed efferent-induced inhibition in afferents and blocked ACh-activated outward currents in type II HCs in turtles (Holt et al. 2006; Parks et al. 2017), mice (Poppi et al. 2018, 2020), and guinea pigs (Zhou et al. 2013).
In the rat crista, IBTX (100 nM), a BK channel blocker, did not significantly affect ACh induced outward currents at Vh of −40 mV in type II HCs (with IBTX: 95.0 ± 17.4 pA; control: 99.4 ± 15.9 pA; n = 5, P = 0.3107, paired t test; Fig. 3, C1 and C3). However, 300 nM apamin, an SK channel blocker, completely abolished outward currents at −40 mV (with apamin: 44.6 ± 10.0 pA; control: −13.8 ± 3.4 pA; n = 3, P = 0.0316, paired t test; Fig. 3, C2 and C3), suggesting that SK channels were coupled to α9-containing nAChRs and accounted for the outward component of ACh responses. The small inward current uncovered by apamin most likely represents the nonselective cation current through the AChR alone (Fig. 3C2). To test if outward synaptic currents are also mediated by SK channels, efferent stimulation consisting of three pulses at 100 Hz was used to trigger synaptic responses reliably. Outward synaptic currents at −50 mV were completely abolished by 300 nM apamin (n = 3; Fig. 3D1). In one recording with spontaneous synaptic activity, after apamin application, outward synaptic currents at −50 mV changed into inward currents with shorter duration and smaller amplitude (Fig. 3D2), again representing the synaptic current through the AChR alone. Together, these results suggest that SK channels mediate the outward inhibitory component of efferent inputs to type II HCs in the rat crista.
Efferent inputs to type II HCs exhibit short-term facilitation.
Ample studies have indicated that synaptic efficacy is directly regulated by the history of synaptic activity (reviewed in Blitz et al. 2004). To investigate if efferent synaptic strength at type II HCs depends on the efferent activity level, paired-pulse experiments were performed to probe for short-term plasticity. Efferents were electrically stimulated by paired pulses with interstimulus intervals (ISIs) ranging from 20 to 500 ms (Fig. 4, A1–A4). Per ISI, 200 trials were applied, one every second, and average amplitudes for S1 responses (to the first stimulus) and S2 responses (to the second stimulus) were measured (see materials and methods) and their relative size S2/S1 was calculated. Average amplitudes included “success” and “failure” trials.
Fig. 4.
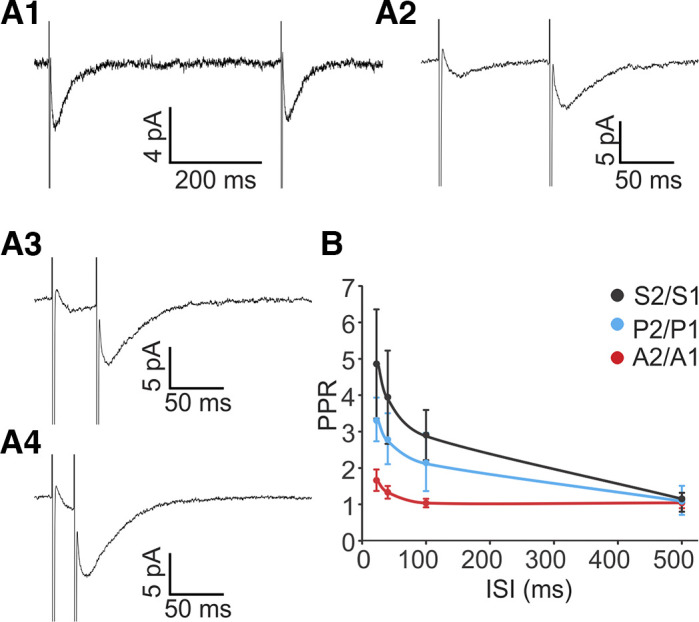
Efferent synapses at rat type II hair cells (HCs) exhibit paired-pulse facilitation. A: averaged paired-pulse responses with interstimulus intervals (ISIs) of 500 ms (A1), 100 ms (A2), 40 ms (A3), and 20 ms (A4) averaged across 200 trials for each ISI. B: paired-pulse ratio (PPR) is inversely correlated with ISIs. Ratios of overall responses (S2/S1) and release probability (P2/P1) were enhanced at shorter intervals (20 ms: P2 = 0.30 ± 0.09, P1 = 0.09 ± 0.02; 40 ms: P2 = 0.25 ± 0.06, P1 = 0.09 ± 0.01; 100 ms: P2 = 0.20 ± 0.04, P1 = 0.09 ± 0.01; 500 ms: P2 = 0.11 ± 0.04, P1 = 0.10 ± 0.04; n = 4, paired t test: P < 0.05 for 20, 40, and 100 ms, P = 0.3 for 500 ms), while the ratio of amplitudes (A2/A1) was unaltered (20 ms: A2 = 16.6 ± 4.7, A1 = 11.7 ± 5.0; 40 ms: A2 = 18.0 ± 5.6, A1 = 13.0 ± 3.2; 100 ms: A2 = 19.0 ± 5.0, A1 = 19.2 ± 6.7; 500 ms: A2 = 14.5 ± 3.1, A1 = 14.1 ± 3.1; n = 4, paired t test: P > 0.05 for all).
At 500-ms interstimulus intervals, no statistical difference was detected between S2 and S1 average amplitudes (S2/S1 = 1.2 ± 0.3; n = 5, P = 0.98, paired t test; Fig. 4, A1 and B). However, paired-pulse facilitation occurred at shorter interstimulus intervals (100 ms and shorter), with S2 being significantly larger than S1 (Fig. 4, A2–A4 and B). The degree of facilitation was inversely correlated with interstimulus interval duration. At 20-, 40-, and 100-ms intervals, the S2 response was approximately three to five times larger than the S1 response (20 ms: S2 = 5.9 ± 1.2, S1 = 1.7 ± 0.6; 40 ms: S2 = 4.5 ± 1.1, S1 = 1.5 ± 0.5; 100 ms: S2 = 3.5 ± 0.8, S1 = 1.4 ± 0.5; n = 5, paired t test, P < 0.05 for all; Fig. 4B).
As successes and failures were included in the analysis, the S1 and S2 average amplitudes are affected by the probability of release as well as by the size of the individual EPSCs. To determine if the observed facilitation was mediated through an increase of release probability Pr and/or through an increase of EPSC amplitude A (successes only), these parameters were analyzed separately (Fig. 4B). P2/P1 increased significantly as the stimulus interval became shorter. In contrast, no significant change of A2/A1 was detected across all tested ISIs, even at the ISI of 20 ms that had the largest A2/A1 value. These results suggest that efferent paired-pulse facilitation is largely due to enhanced release probability, perhaps caused by a buildup of calcium in the presynaptic terminal.
Efferent inputs to type II HCs are potentiated by high frequency train stimulation.
To provide a more physiological stimulation pattern, trains of shocks were used in the next set of experiments. In paralyzed alert toadfish, efferents displayed spontaneous firing rates of 4–5 spikes/s, and when the animals were activated behaviorally, firing rates of 80–100 spikes/s could be reached (Highstein and Baker 1985). Recordings from putative efferent neurons identified by antidromic activation in decerebrate, decerebellate guinea pigs showed that they fired spontaneously at 10–50 spikes/s and increased their firing rates up to 150 spikes/s in response to vestibular or somatosensory stimulation (Marlinsky 1995). However, firing rates of efferent neurons have not been investigated in further detail in mammals. Vestibular efferent neurons in mouse brain stem slices have spontaneous resting discharges of up to 5 spikes/s (Leijon and Magnusson 2014; Mathews et al. 2015, 2017). For efferent stimulation, high-frequency stimulation trains have been applied (50 to 333 Hz) in vivo and have been shown to effectively modulate afferent firing rates (Goldberg and Fernández 1980; Marlinski et al. 2004).
To assess synaptic responses in type II HCs during high-level efferent activity, 10-pulse train stimulation at frequencies of 25, 50, and 80 Hz was used (every 15 s), mimicking expected physiological firing rates of efferents based on the in vitro studies listed above (Fig. 5, A–C). For every data point, 10 trials of stimulation were performed and responses were averaged. Average peak amplitudes were −28.4 ± 11.0 pA, −36.2 ± 12.2 pA and −56.5 ± 17.5 pA, at 25 Hz, 50 Hz and 80 Hz stimulation, respectively (Vh = −90 mV, n = 4 HCs), and significantly larger at 50 Hz vs. 25 Hz (P = 0.007, paired t test) and at 80 Hz vs. 50 Hz (P = 0.031). Both summation and facilitation contributed to the increase of peak amplitudes at higher stimulation rates. However, to only measure the effect of facilitation, the last 5 ms of each response were considered as the baseline for the subsequent stimulation (see materials and methods). At all stimulation frequencies tested, Pr increased with the number of pulses in the train (Fig. 5D). For example, Pr of the tenth pulse in the 80-Hz train stimulation had increased ~10-fold (to ~0.6) compared with baseline Pr. Pr rose more steeply at higher stimulation frequencies. For all frequencies, the increase of Pr slowed down with consecutive pulses, suggesting that either facilitation was reaching saturation or that, additionally, synaptic depression increased. After stimulation ended, the decay time constant of synaptic responses was slow (τdecay = 35.0 ± 3.4 ms), as effects had summated during stimulation.
In in vivo studies in mammals, high-frequency efferent stimulation was often applied over seconds, raising the issue that depletion of synaptic release could have occurred during such prolonged stimulation (Goldberg and Fernández 1980; Marlinski et al. 2004; McCue and Guinan 1994). To test this possibility, efferent synaptic currents were measured in response to 10-s-long train stimulations at 50 Hz (Fig. 5E). A plateau in the response amplitude (−115.0 ± 28.9 pA) was reached with a time constant τrise of 1.07 ± 0.20 s (fitted with an exponential function; n = 6). Synaptic release was maintained throughout the 10-s stimulation; however, it showed some reduction in amplitude after ~5 s in half of the recordings.
Other synaptic transmitters besides ACh have been suggested to also function at vestibular efferents (reviewed in Holt et al. 2011). For example the neuropeptide calcitonin gene-related peptide (CGRP) has been localized in efferent fibers (Demêmes and Broca 1998; Jordan et al. 2015; Luebke et al. 2014; Mathews et al. 2015) and shown to suppress HC responses to mechanical stimulation in the lateral line organ of Xenopus (Bailey and Sewell 2000). Neuropeptides are preferentially released during high-frequency firing (Dreifuss et al. 1971; Gainer et al. 1986; Muschol and Salzberg 2000). To test if the efferent response to train stimulation in type II HCs includes effects of other transmitters than ACh, responses to 10-pulse train stimulations at 80 Hz were compared, with and without blocking the ACh response. Strychnine (200 nM) reversibly blocked the efferent response by 85% (with strychnine: −7.6 ± 1.4 pA, control: −48.3 ± 11.8 pA; n = 3 HCs, P = 0.04, paired t test; Fig. 5F), indicating that under the recording conditions here, even for potentiated responses at high-frequency stimulation, efferent synaptic responses were mainly mediated by ACh.
Comparison of optogenetic and electrical stimulation of efferent inputs to type II HCs.
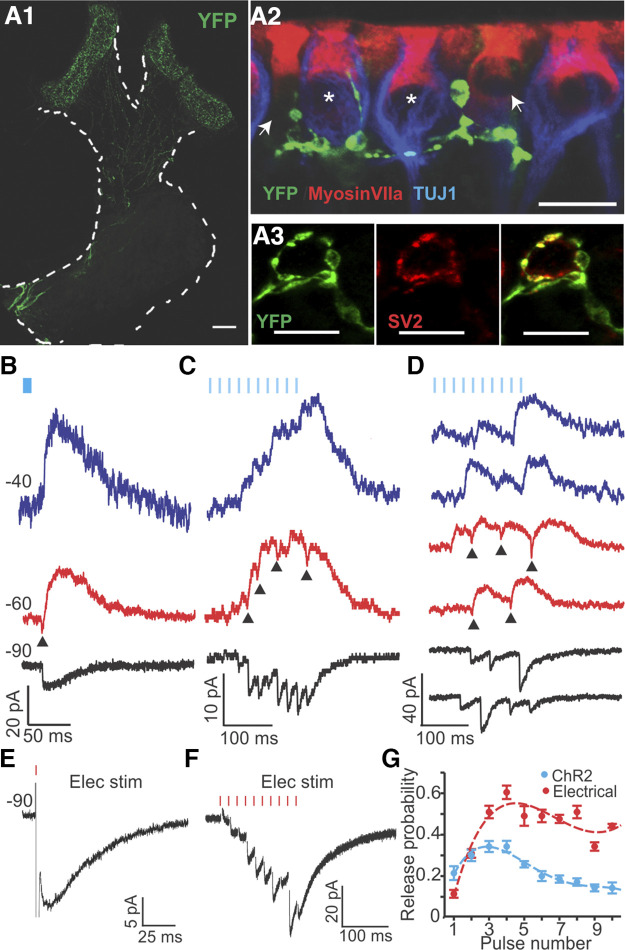
The membrane potential (Vm) of type II HCs sets the level of transmission at the afferent synapses between type II HCs and vestibular nerve fibers. Understanding how efferent inputs affect Vm in type II HCs will provide insights into how efferents may modulate vestibular nerve activity. Experiments with electrical stimulation of efferents have provided valuable descriptions of synaptic currents in voltage clamp; however, when Vm was monitored in current clamp, electrical stimulation caused artifacts that overwhelmed the synaptic responses, most likely due to activation of voltage-dependent conductances in HCs. Therefore, an optogenetic approach was implemented to stimulate efferents. Channelrhodopsin2 (ChR2) was expressed under the control of the choline acetyltransferase (ChAT) promoter in ChAT-Cre; Ai32 mice. In these mice, fluorescently labeled, thin ChR2-YFP-positive fibers projected into to crista, similar to the efferent innervation pattern (Fig. 6A1). Vestibular afferent neurons did not show any fluorescence (labeled by TUJ), suggesting that the expression of ChR2 was confined to efferents. In the hair cell region, ChR2-YFP-positive fibers branched extensively and formed contacts with type II HCs and calyces (Jordan et al. 2015) (Fig. 6A2). Moreover, ChR2-YFP-positive terminals largely overlapped with efferent endings at the base of HCs, identified by immunolabeling for the synaptic vesicle protein SV2 (Fig. 6A3).
To stimulate efferent fibers optically, 5-ms blue light pulses were delivered through an optical fiber. In 19 of 20 type II HCs recorded from 20 ChAT-Cre; Ai32 mice, light pulses successfully triggered efferent synaptic currents (Fig. 6, B–D). At single-pulse stimulation at a 2-Hz rate, light-evoked EPSCs (l-EPSCs) had similar characteristics to sEPSCs and eEPSCs recorded in rat type II HCs (Figs. 2 and 3A1). Individual l-EPSCs recorded at Vh of −90 mV were inward, at −60 mV biphasic, and at −40 mV outward currents (n = 7; Fig. 6B). At −90 mV, l-EPSCs had an amplitude of −17.1 ± 0.9 pA (n = 8 HCs; 100 trials averaged per recording), rise time of 4.2 ± 0.7 ms, decay time constant of 31.0 ± 3.5 ms, and Pr of 0.105 ± 0.018 (n = 11).
Fig. 6.
Comparison of optogenetic and electrical stimulation of efferent inputs to type II hair cells (HCs) in ChAT-Cre; Ai32 mice. A1: channelrhodopsin 2 conjugated to enhanced yellow fluorescent protein (ChR2-EYFP; green) was expressed in cholinergic fibers by crossing ChAT-Cre and Ai32 lines. ChR2 expression was visualized by anti-YFP immunolabeling in the cristae of the horizontal (left) and anterior semicircular canals (right). Scale bar, 100 μm. A2: terminals of YFP-positive fibers (green) were located close to afferent terminals (anti-TUJ1 labeling; blue) and type II HCs (anti-myosin VII labeling (red), identified as type II as they had no calyces (no anti-TUJ1 labeling) surrounding them. Scale bar, 10 μm. A3: terminals of ChR2-EYFP-positive fibers colocalized with synaptic vesicle protein SV2 (red), marking them as presynaptic terminals. Scale bars, 10 μm. B–D: synaptic responses induced by blue light stimulation (5-ms pulses; blue markers) in ChAT-Cre; Ai32 mice recorded at different holding potentials (Vh: blue = −40 mV, red = −60 mV, black = −90 mV). Arrowheads mark biphasic responses at −60 mV. B: light-induced excitatory postsynaptic currents (l-EPSCs) activated by single light pulses. C: average of synaptic responses to 10-pulse stimulation at 50 Hz over 10 trials. D: two examples of individual synaptic responses for every holding potential to 10-pulse light stimulation at 50 Hz. E and F: synaptic responses induced by electrical stimulation (Elec Stim; red markers) in ChAT-Cre; Ai32 mice at Vh = −90 mV. E: individual response to a single pulse of electrical stimulation (eEPSC). F: average of responses to 10-pulse electrical stimulation at 50 Hz over 10 trials. G: both optogenetic (n = 6 HCs) and electrical stimulation (n = 4 HCs) revealed an initial increase and a later reduction in release probability during a 10-pulse train at 50 Hz. Note that from pulse 4 on, release probability was about two times larger for electrical vs. optical stimulation.
Electrical stimulation experiments were less successful in mice because their cristae are smaller and more delicate than those of rats (Desai et al. 2005a), causing a larger degree of damage by the close positioning of the stimulation electrodes. However, for direct comparison, a small set of experiments was performed in mice. Both eEPSCs in response to single-pulse electrical stimulation (n = 2 of the cells used also for optical stimulation) and sEPSCs (n = 1) in ChAT-ChR2 mice showed similar properties compared with l-EPSCs, with an amplitude of −17.0 ± 3.7 pA, rise time of 5.3 ± 0.8 ms, decay time constant of 33.4 ± 3.4 ms (n = 3; eEPSC and sEPSC recordings pooled), and Pr of 0.08 and 0.26 for the two eEPSC recordings.
It has been reported that the optogenetic method can affect synaptic short-term plasticity, depending on cell type (Jackman et al. 2014). Therefore, light-stimulated facilitated efferent responses were compared with electrically stimulated responses. Light pulses were applied in 10-pulse trains at 50 Hz. Individual response traces shown in Fig. 6D show inward currents at −90 mV, biphasic currents at −60 mV (arrowheads), and outward currents at −40 mV, again assuring that the response was due to efferent activity. Average responses for the same experiment are shown in Fig. 6C. Efferent responses to optical (Fig. 6, C and D) and electrical stimulation (Fig. 6F) in mice both showed facilitation and summation, similarly to electrical stimulation responses in rats. For both methods, Pr rose sharply during the first several pulses and declined again through later pulses (Fig. 6G), unlike in experiments in rats, where Pr grew monotonically during the electrical 10-pulse train stimulation (Fig. 5B). The additional slow depression component observed in mice possibly reflects a species difference. Also, there were differences between optogenetic (n = 6 HCs) and electrical stimulation (n = 4 HCs; different animals than used for optogenetic stimulation) for 10-pulse train stimulation in mice (Fig. 6, C, D, and G). The onset of light-evoked synaptic responses had a relatively long delay of 9.0 ± 1.6 ms (calculated from stimulus onset; n = 6 HCs), while the electrically evoked responses followed the stimulus instantly (n = 4 HCs). Electrical stimulation resulted in stronger facilitation, and from pulse 4 on, Pr was about twice as large for electrical compared with optical stimulation (Fig. 6G). The peak amplitude for responses to electrical stimulation was −56.7 ± 36.5 pA (average: −36.2 ± 12.2 pA, n = 4 HCs), whereas for optical stimulation (holding potential of −90 mV) it was −48.1 ± 22.2 pA (average: −35.4 ± 18.8, n = 6 HCs). Increasing the optical 10-pulse stimulation rate from 25 Hz to 50 Hz resulted in higher maximum Pr; however, stimulation at 80 Hz did not further increase Pr (data not shown for 25 and 80 Hz). Therefore, in the next set of experiments, 50 Hz was used as the protocol for testing efferent effects on Vm.
Efferent inputs strongly hyperpolarize type II HCs in response to single-pulse and train stimulation.
To examine how efferent inputs modulate Vm in type II HCs, optical stimulation was applied using either a single pulse (5 ms) applied every 500 ms (Fig. 7A1) or a 10-pulse train at 50 Hz, applied every 15 s (Fig. 7B1). Before stimulation, Vm was preset to different membrane potentials by current injection (−80, −60, and −40 mV), covering a range from hyperpolarized to depolarized HC membrane potentials. Note that although a resting membrane potential of −69.5 mV was reported here for type II HC recordings in vitro, it is likely that their resting membrane potential is more depolarized in vivo, as has been shown for cochlear HCs (Fettiplace 2017). Both pulse protocols induced qualitatively similar responses. At −80 mV, efferent inputs induced a depolarization, followed by a smaller hyperpolarization. HCs depolarized by 1.3 ± 0.5 mV in response to single pulses (n = 4 HCs) and by 2.40 ± 0.50 mV in response to train stimulation (n = 6 HCs). The biphasic response can be explained by an initial dominance of a depolarizing ACh-mediated cation current that is followed by a delayed increase of a hyperpolarizing calcium-dependent SK current. At −60 mV and −40 mV, efferent stimulation induced strong hyperpolarization, with larger effects in response to train stimulation compared with single pulses. For single pulses, HCs hyperpolarized by 8.2 ± 0.8 mV at −60 mV (range: 7.3 to 10.8 mV) and by 5.3 ± 1.4 mV at −40 mV (range: 4.2 to 10.6 mV) (only successes analyzed; n = 4, P = 0.11, paired t test). For train stimulation, HCs hyperpolarized by 10.47 ± 1.70 mV (range: 4.26 to 15.06 mV) at −60 mV and by 10.60 ± 3.18 mV (range: 3.82 to 22.74 mV) at −40 mV (n = 6) (Fig. 7A2, B2). Compared with single pulses, this constitutes a 28% increase in inhibition at −60 mV and a 100% increase at −40 mV. However, even single-pulse stimulation of efferents has a surprisingly large inhibitory effect on type II HC.
For both pulse protocols, decay time constants of the efferent responses varied depending on the initially set membrane potential. For the 10-pulse protocol, the decay time constant of the efferent response (measured after stimulation ended) was short at −80 mV (31.98 ± 5.63 ms; n = 6) compared with the inhibitory responses at −60 and −40 mV (see below), suggesting that at −60 to −40 mV, efferent input has longer lasting impact compared with that at −80 mV. Moreover, the decay time constant at −60 mV was more than twice as long as at −40 mV (−60 mV: 239.99 ± 55.77 ms; −40 mV: 108.18 ± 30.17 ms; n = 6, P = 0.019, paired t test; Fig. 7C), suggesting a longer lasting impact of the efferent input at −60 mV compared with −40 mV. In comparison, for the single-pulse protocol, decay time constants were shorter at both −60 and −40 mV; however, also in this setting, the decay time constant at −60 mV was more than twice as long as at −40 mV (−60 mV: 68.03 ± 5.31 ms; −40 mV: 32.02 ± 3.93 ms;, n = 4 cells, P = 0.016, paired t test). Therefore, the main impact of train stimulation (vs. single pulses) is a longer duration of inhibition.
Fig. 7.
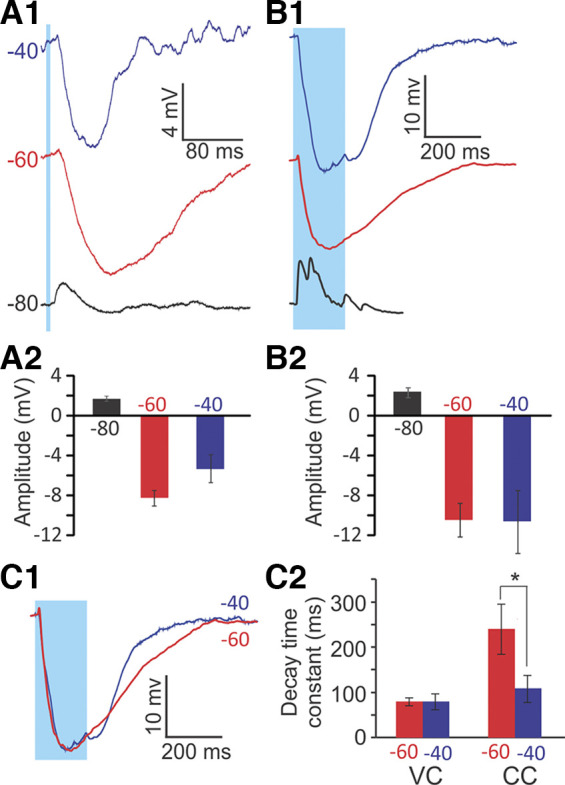
Optogenetic stimulation of efferents results in a robust hyperpolarization of type II hair cells (HCs) in ChAT-Cre; Ai32 mice. A1: an example type II HC response to single-pulse (2-Hz rate) optogenetic stimulation in the crista of ChAT-Cre; Ai32 mice. Traces were averaged across 40–80 trials. Before stimulation, the membrane potential was preset by current injection to different voltage levels, as indicated on each trace. A2: mean (±SE) amplitudes of voltage changes in response to single pulses (n = 4 HCs). B1: response of an example type II HC to 10-pulse trains (50-Hz rate, every 15 s) of optogenetic stimulation, averaged across 10 trials. B2: mean (±SE) amplitudes of voltage changes in response to 10-pulse trains (n = 6 HCs). For both stimulation protocols, HCs showed strong hyperpolarization in response to efferent stimulation at −60 mV and −40 mV, and a small depolarization followed by hyperpolarization at −80 mV. C1: overlaid normalized traces of responses to a 10-pulse protocol at −60 mV (red) and −40 mV (blue). Same data as in B1. C2: mean (±SE) 90–10% decay time constants of HC responses to optogenetic efferent stimulation with a 10-pulse protocol at −60 mV (red) and −40 mV (blue). In current clamp (CC), the 90–10% decay time constants of efferent-induced inhibition were significantly longer at −60 mV compared with −40 mV (*P = 0.019, paired t test, n = 6). In voltage clamp (VC), no significant difference was found between the two potentials.
In voltage clamp, decay time constants did not differ significantly. For the 10-pulse protocol, decay time constants were 79.32 ± 8.90 ms at −60 mV and 80.32 ± 17.43 ms at −40 mV (P = 0.92, paired t test, n = 4 cells; Fig. 7C2), and for the single-pulse protocol, decay time constants were 51.67 ± 8.28 ms at −60 mV and 54.60 ± 5.19 ms at −40 mV (P = 0.69, paired t test, n = 7 cells). That a difference in decay time constants between −60 and −40 mV is found in current clamp but not in voltage clamp suggests a voltage-dependent mechanisms. As a result, efferent inhibition may have the most impact on type II HCs near the resting membrane potential.
DISCUSSION
Conserved efferent mechanisms in the vestibular and auditory peripheries.
Properties of efferent synaptic activity onto HCs seem to be highly conserved. In the cochlea, α9-containing nAChRs associated with calcium-activated potassium channels mediate inhibition (Elgoyhen et al. 1994; Glowatzki and Fuchs 2000). Similarly, ACh responses of type II HCs in the crista are mediated by α9-containing nAChRs associated with SK channels, and ACh application induces inhibition in HCs (Hiel et al. 1996; Holt et al. 2006; Luebke et al. 2005; Parks et al. 2017; Poppi et al. 2018). Our study confirms this mechanism for rat efferent input to type II HCs. In outer HCs of the cochlea, α9-containing nAChRs operate with SK and/or BK channels (Rohmann et al. 2015; Wersinger et al. 2010). In this study, no BK channel contribution was detected in the ACh induced responses in type II HCs. However, recordings were limited to the central zone, and it remains possible that BK channels are involved in other subsets of type II HCs (Kong et al. 2005). EPSC waveforms, including EPSC amplitudes and decay time constants, were also found to be comparable between cochlear and type II vestibular HCs. Changing HC holding potentials from −90 to −60 to −40 mV resulted in the typical waveform changes from inward to biphasic to outward currents, as have been described in mammalian and nonmammalian studies (Art et al. 1982, 1984; Glowatzki and Fuchs 2000), and suggests a similar relative contribution of nAChRs and SK channels to the efferent response as found before. Muscarinic AChR effects have been found in vestibular HCs also (Jordan et al. 2013; Li et al. 2007; Poppi et al. 2020; Zhou et al. 2013); however, in the study here the recorded HC synaptic activity is clearly mediated by α9-containing nAChRs, as even the potentiated response to a pulse train could be mostly inhibited with strychnine, a blocker for α9-containing nAChRs. This does not exclude that additional mAChR effects on type II HCs exist.
Presynaptic properties such as a low basal release probability, short-term facilitation, and summation in response to paired-pulse protocols and pulse trains observed in this study also resemble closely the presynaptic properties demonstrated for efferent inputs to rodent cochlear inner and outer HCs. Notably, the basal efferent release probability recorded in type II HCs (Pr type II HC: 0.06; 3–4 endings/type II HC) is even lower compared with that of cochlear HCs [Pr outer HC (OHC): 0.25; 1–3 endings/OHC; Pr inner HC (IHC): 0.65; 16 (endings/IHC)] (Ballestero et al. 2011; Goutman et al. 2005; Roux et al. 2011; Vetter et al. 1999).
Efferent hyperpolarization of the type II HC membrane potential.
Optogenetic stimulation of cholinergic efferent fibers allowed us to investigate the effects of efferent activity on the type II HC membrane potential. A surprising result was that even a single optical pulse could hyperpolarize type II HCs substantially, by ~8 mV at −60 mV. Furthermore, the membrane potential stayed hyperpolarized after the end of the pulse and slowly returned to resting values with a decay time constant of ~70 ms at −60 mV. This shows that even at a low probability of release, efferents may be able to impose a significant inhibitory effect on type II HCs. The decay time constant in response to a single pulse may mainly reflect postsynaptic calcium accumulation in the HC and the properties of SK channel activation/deactivation (Kong et al. 2008; Oliver et al. 2000). In current clamp, the decay time constant was larger (i.e., slower) at −60 mV, near the resting membrane potential, and was smaller at a more depolarized potential (−40 mV) (Fig. 7, A1 and C2), suggesting that efferent stimulation may have a larger impact on HC activity close to its resting state. The shortening of the decay time constant at −40 mV in current clamp is due to voltage-dependent mechanisms, as it was not found in voltage clamp (Fig. 7C2).
Repeated stimulation of efferents resulted in facilitation and summation, and thereby in an increase in the amplitude of the hyperpolarization compared with single-pulse stimulation (compare Fig. 7, A2 and B2). Presynaptic calcium accumulation during facilitation may underlie the longer time constants of decay observed after repeated stimulation (Fig. 5, A–D, and Fig. 7, B1 and C2). Similar to single-pulse stimulation, the decay time constant was larger near the resting membrane potential.
Measurements relating to type II HC exocytosis, like the amplitude of calcium currents and membrane capacitance, have shown that these measures most dynamically change within the HC membrane potential range of −70 to −20 mV (Bao et al. 2003; Dulon et al. 2009). Therefore, the amount of efferent inhibition reported here can effectively reduce or even shut down the output of type II HCs to afferent neurons.
Regardless of the underlying mechanism, the results show that stimulation of efferents will result in a strong and prolonged inhibitory effect on type II HCs, both near the resting membrane potential as well as at depolarized membrane potentials that may either reduce or even eliminate transmitter release.
Efferent modulation of the relative inputs of type I and type II HCs to afferent activity.
Efferent inhibition of type II HCs is likely to affect the relative inputs of type I and type II HCs on afferent activity. Type II HCs comprise about half of HCs in vestibular end organs of rodents (Desai et al. 2005a, 2005b), and most afferent fibers have dimorphic terminals that receive inputs from both type I and type II HCs (Fernández et al. 1988; Lysakowski et al. 1995) (Fig. 8). Each type II HC can form ~10–20 ribbon synapses with bouton afferents as well as with the outer wall of calyx terminals (Lysakowski and Goldberg 1997), mediating “quantal” transmission resulting in glutamatergic excitatory postsynaptic currents (EPSPs) in afferent endings. Type I HCs transmit signals through quantal EPSPs via ribbon synapses as well as by “nonquantal” transmission due to accumulation of glutamate, K+, and probably H+ in the closed synaptic cleft between type I HC and its calyx terminal (Contini et al. 2017, 2020; Eatock 2018; Highstein et al. 2014; Meredith and Rennie 2016; Sadeghi et al. 2014; Songer and Eatock 2013). Regarding activation of glutamate release at ribbon synapses, type II HCs are likely to be more sensitive and respond to smaller stimuli than type I HCs, due to their ~10-fold larger input resistance (Holt et al. 1999; Rüsch et al. 1998). It has been shown that nonquantal transmission is faster than the glutamatergic quantal transmission (Contini et al. 2020; Eatock 2018; Songer and Eatock 2013), and it has been suggested that these two types of HCs likely channel distinct vestibular information to the afferent neurons (reviewed in Eatock and Songer 2011). As a result, inhibition of type II HCs by efferents can be a mechanism for decreasing the contribution of bouton terminals and emphasizing the faster dynamics of nonquantal transmission between type I HC–calyx terminals in dimorphic afferents. In line with the effect of α9/α10 nAChRs, there is evidence that mAChRs are also present in type II HCs and have an inhibitory effect through activation of BK channels (Guo et al. 2012; Zhou et al. 2013). To further push the balance of response dynamics toward type I HC–calyx transmission, cholinergic inputs on calyx terminals exert an excitatory effect through muscarinic and α4β2 nAChRs (Holt et al. 2015, 2017).
Thus overall efferent inputs will most likely decrease the strength of the type II HC-mediated pathway and thereby increase the contribution of the type I HC–calyx pathway in dimorphic afferents. Such a change can result in dimorphic afferents responding more like calyx-only afferents with lower sensitivities and a shift toward faster (nonquantal) and more phasic response properties in the crista central zone (or less tonic in the peripheral zone). Such inhibition of type II HCs by efferents will also affect bouton-only afferents that receive inputs only from type II HCs. However, this should be considered together with the additional (most likely) excitatory effect of efferents directly on bouton terminals (Holt et al. 2006). The combined inhibitory and excitatory inputs may explain the small effect of efferent stimulation on the most regular afferent fibers (e.g., Plotnik et al. 2002), which most likely only innervate type II HCs (Fernández et al. 1988).
Reconciling the type II HC efferent inhibitory effects with the in vivo excitatory effects on afferent activity.
Considering the purely excitatory effect of in vivo stimulation of efferents on resting discharges of afferents in mammals (Goldberg and Fernández 1980; Marlinski et al. 2004; Sadeghi et al. 2009) and toadfish (Boyle and Highstein 1990; Boyle et al. 2009; Highstein and Baker 1985), the inhibitory efferent input to type II HCs of mice and rats seems surprising at first. Although efferent inputs to HCs can be excitatory at membrane potentials below approximately −60 mV, little vesicular release is expected at this negative potential. However, recent studies in turtle have shown that cholinergic stimulation has excitatory effects on the calyx terminal in the turtle through α4β2-nAChR (Holt et al. 2015) and most likely mAChR (Holt et al. 2017), mediating fast and slow efferent-mediated effects, respectively. Since most afferents are dimorphic, the effect of efferents will be the sum of their effects on type II HCs (inhibitory, both nAChR and mAChR), calyces (excitatory, both nAChR and mAChR), and bouton afferent terminals (most likely excitatory). Irregular afferents have more calyx terminals and fewer boutons/type II HC contacts compared with regular afferents (Holmes et al. 2017), and as a result, the net effect of type II HC inhibition and calyx excitation can be excitatory. In contrast, for regular afferents that have more type II HC/boutons, the net effect can be null or inhibitory. This simplified explanation does not consider possible differences between properties of cells in the central zone (i.e., where irregular afferents innervate) and peripheral zone (i.e., where regular afferents innervate). However, it can explain why irregular afferents with calyx-only terminals, that receive little or no inputs from type II HCs (Baird et al. 1988), show the largest increase in firing rate during efferent stimulation (Goldberg and Fernández 1980; Marlinski et al. 2004). These combined results can also explain that efferent-mediated effects on resting discharges are related to the irregularity of discharge. As a result, one can speculate that the excitatory effect of efferents on calyx terminals outweigh inhibitory effects on type II HCs in terms of determining the resting discharges of afferents.
In vivo studies have also shown that stimulation of efferents results in a decrease in the sensitivity of afferents to stimulation of canals in squirrel monkeys (Goldberg and Fernández 1980) and toadfish (Boyle and Highstein 1990). This change fits well with the observed efferent inhibition of type II HCs shown in the present study and emphasizes the important contribution of type II HC inputs to afferents (boutons and calyx terminals) during stimulation.
Optogenetic versus electrical stimulation of efferent fibers in the vestibular periphery.
Here, we used optogenetic stimulation of channelrhodopsin to activate cholinergic efferent neurons in the vestibular periphery in mice. Optogenetic stimulation provides the means for targeted stimulation of cholinergic fibers, while electrical stimulation, besides stimulation of cholinergic fibers, may result in additional activation of noncholinergic efferent fibers that contact type II HCs.
We found that individual optogenetic or electrical stimulation resulted in efferent-mediated synaptic events with similar amplitudes, rise times, and decay time constants and biphasic responses typical for the combined α9-containing nAChR/SK channel-mediated responses. The similarities of responses to the two types of stimuli, together with the inhibition of synaptic events by α9-nAChR and SK channel antagonists, even those activated by a pulse train, suggest that both stimulation protocols activated cholinergic efferent inputs to type II HCs. Additional protocols and analysis of the type II HC response in current clamp and on different timescales will be needed to test if other transmitter systems [e.g., CGRP (Jones et al. 2018; Luebke et al. 2014; Matsuda 1996], GABA (Kong et al. 1998a, 1998b, 2002; Matsubara et al. 1995; Usami et al. 1987), or nitric oxide (Chen and Eatock 2000; Lv et al. 2010; Lysakowski and Singer 2000)] are also activated by efferent stimulation and if such activation differentiates between transmitter systems for electrical vs. optogenetic stimulation of Chat-Cre-Ai32 mice. These other neurotransmitters can be expressed separately or in conjunction with acetylcholine (CGRP in vestibular efferents: Jordan et al. 2013; GABA in other brain areas: Saunders et al. 2015; Tritsch et al. 2016).
Electrical train stimulation at 50 Hz (10 pulses) was more effective compared with optogenetic train stimulation. Both methods resulted in an increase in the probability of release for the first three to four pulses. However, for later pulses, the probability of release either reached a plateau or slightly decreased with electrical stimulation, whereas it dropped to about half the maximum value by the end of optogenetic stimulation. Depression in responses to ChR2 stimulation has previously been reported in other brain areas and has been attributed to possible intrinsic kinetics of ChR2 (Jackman et al. 2014; Zhang and Oertner 2007). However, one cannot rule out that differences between electrical and optogenetic stimulation are due to activation of different subsets of efferent fibers. Regardless of the mechanism, as optogenetic stimulation provided a smaller effect compared with electrical stimulation, one can extrapolate that the measured efferent inhibition induced by optogenetic stimulation may be underestimating the effect of cholinergic efferents on type II HCs under physiological conditions.
GRANTS
This work was supported by National Institutes of Health (NIH) grants DC006476 and R01DC012957 (to E.G.), R03DC015091 (to S.G.S.), 1F31DC014910-01 (to Z.Y.), P30DC005211 (to the Center for Hearing and Balance Core Grant at Johns Hopkins), and GM103801 and GM48677 (to J.M.M.). The mouse IgG1 anti-synaptic vesicle glucoprotein 2A (SV2) antibody deposited by K. M. Buckley, Harvard Medical School, Boston MA, was obtained from the Developmental Studies Hybridoma Bank, created by NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.Y. and E.G. conceived and designed research; Z.Y. performed experiments; Z.Y. and S.G.S. analyzed data; Z.Y., S.G.S., and E.G. interpreted results of experiments; Z.Y. and S.G.S. prepared figures; Z.Y., S.G.S., and E.G. drafted manuscript; J.M.M., S.G.S., and E.G. edited and revised manuscript; Z.Y., J.M.M., S.G.S., and E.G. approved final version of manuscript.
REFERENCES
- Anderson PJ, Lynch TJ, Engelhardt JF. Multipotent myoepithelial progenitor cells are born early during airway submucosal gland development. Am J Respir Cell Mol Biol 56: 716–726, 2017. doi: 10.1165/rcmb.2016-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci 216: 377–384, 1982. doi: 10.1098/rspb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R, Fuchs PA. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol 356: 525–550, 1984. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GP, Sewell WF. Calcitonin gene-related peptide suppresses hair cell responses to mechanical stimulation in the Xenopus lateral line organ. J Neurosci 20: 5163–5169, 2000. doi: 10.1523/JNEUROSCI.20-13-05163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Ballestero J, Zorrilla de San Martín J, Goutman J, Elgoyhen AB, Fuchs PA, Katz E. Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells. J Neurosci 31: 14763–14774, 2011. doi: 10.1523/JNEUROSCI.6788-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol 90: 155–164, 2003. doi: 10.1152/jn.00244.2003. [DOI] [PubMed] [Google Scholar]
- Barlow-Anacker AJ, Fu M, Erickson CS, Bertocchini F, Gosain A. Neural crest cells contribute an astrocyte-like glial population to the spleen. Sci Rep 7: 45645, 2017. doi: 10.1038/srep45645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Foster KA, Regehr WG. Short-term synaptic plasticity: a comparison of two synapses. Nat Rev Neurosci 5: 630–640, 2004. doi: 10.1038/nrn1475. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci 10: 1570–1582, 1990. doi: 10.1523/JNEUROSCI.10-05-01570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Rabbitt RD, Highstein SM. Efferent control of hair cell and afferent responses in the semicircular canals. J Neurophysiol 102: 1513–1525, 2009. doi: 10.1152/jn.91367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Aubert A, Eatock RA, Goldberg JM. Regional analysis of whole cell currents from hair cells of the turtle posterior crista. J Neurophysiol 88: 3259–3278, 2002. doi: 10.1152/jn.00770.2001. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Afferent and efferent responses from morphological fiber classes in the turtle posterior crista. Ann N Y Acad Sci 781: 183–195, 1996. doi: 10.1111/j.1749-6632.1996.tb15701.x. [DOI] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 100: 1284–1294, 1985. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Muñoz M, Israel SH, Hudspeth AJ. Efferent control of the electrical and mechanical properties of hair cells in the bullfrog’s sacculus. PLoS One 5: e13777, 2010. doi: 10.1371/journal.pone.0013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Eatock RA. Major potassium conductance in type I hair cells from rat semicircular canals: characterization and modulation by nitric oxide. J Neurophysiol 84: 139–151, 2000. doi: 10.1152/jn.2000.84.1.139. [DOI] [PubMed] [Google Scholar]
- Contini D, Holstein GR, Art JJ. Synaptic cleft microenvironment influences potassium permeation and synaptic transmission in hair cells surrounded by calyx afferents in the turtle. J Physiol 598: 853–889, 2020. doi: 10.1113/JP278680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D, Price SD, Art JJ. Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. J Physiol 595: 777–803, 2017. doi: 10.1113/JP273060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demêmes D, Broca C. Calcitonin gene-related peptide immunoreactivity in the rat efferent vestibular system during development. Brain Res Dev Brain Res 108: 59–67, 1998. doi: 10.1016/S0165-3806(98)00030-3. [DOI] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol 93: 267–280, 2005a. doi: 10.1152/jn.00747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol 93: 251–266, 2005b. doi: 10.1152/jn.00746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss JJ, Kalnins I, Kelly JS, Ruf KB. Action potentials and release of neurohypophysial hormones in vitro. J Physiol 215: 805–817, 1971. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci 29: 10474–10487, 2009. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA. Specializations for fast signaling in the amniote vestibular inner ear. Integr Comp Biol 58: 341–350, 2018. doi: 10.1093/icb/icy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011. doi: 10.1146/annurev-neuro-061010-113710. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79: 705–715, 1994. doi: 10.1016/0092-8674(94)90555-X. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98: 3501–3506, 2001. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M, Haberlandt C, Gomez-Casati ME, Watkins M, Elgoyhen AB, McIntosh JM, Olivera BM. Alpha-RgIA: a novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry 45: 1511–1517, 2006. doi: 10.1021/bi0520129. [DOI] [PubMed] [Google Scholar]
- Fernández C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol 60: 167–181, 1988. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Fettiplace R. Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Compr Physiol 7: 1197–1227, 2017. doi: 10.1002/cphy.c160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol 77: 92–101, 1974. doi: 10.3109/00016487409124603. [DOI] [PubMed] [Google Scholar]
- Gainer H, Wolfe SA Jr, Obaid AL, Salzberg BM. Action potentials and frequency-dependent secretion in the mouse neurohypophysis. Neuroendocrinology 43: 557–563, 1986. doi: 10.1159/000124582. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288: 2366–2368, 2000. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43: 986–1025, 1980. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E. Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol 566: 49–59, 2005. doi: 10.1113/jphysiol.2005.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CK, Wang Y, Zhou T, Yu H, Zhang WJ, Kong WJ. M2 muscarinic ACh receptors sensitive BK channels mediate cholinergic inhibition of type II vestibular hair cells. Hear Res 285: 13–19, 2012. doi: 10.1016/j.heares.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Hiel H, Elgoyhen AB, Drescher DG, Morley BJ. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res 738: 347–352, 1996. doi: 10.1016/S0006-8993(96)01046-3. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol 54: 370–384, 1985. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR, Mann MA, Rabbitt RD. Evidence that protons act as neurotransmitters at vestibular hair cell-calyx afferent synapses. Proc Natl Acad Sci USA 111: 5421–5426, 2014. doi: 10.1073/pnas.1319561111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WR, Huwe JA, Williams B, Rowe MH, Peterson EH. Models of utricular bouton afferents: role of afferent-hair cell connectivity in determining spike train regularity. J Neurophysiol 117: 1969–1986, 2017. doi: 10.1152/jn.00895.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Jordan PM, Lysakowski A, Shah A, Barsz K, Contini D. Muscarinic acetylcholine receptors and M-currents underlie efferent-mediated slow excitation in calyx-bearing vestibular afferents. J Neurosci 37: 1873–1887, 2017. doi: 10.1523/JNEUROSCI.2322-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Kewin K, Jordan PM, Cameron P, Klapczynski M, McIntosh JM, Crooks PA, Dwoskin LP, Lysakowski A. Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents. J Neurosci 35: 3625–3643, 2015. doi: 10.1523/JNEUROSCI.3388-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Lysakowski A, Goldberg JM. Mechanisms of efferent-mediated responses in the turtle posterior crista. J Neurosci 26: 13180–13193, 2006. doi: 10.1523/JNEUROSCI.3539-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Lysakowski A, Goldberg JM. The efferent vestibular system. In: Auditory and Vestibular Efferents, edited by Ryugo DK, Fay RR, Popper AN. New York: Springer, 2011, p. 135–186. doi: 10.1007/978-1-4419-7070-1. [DOI] [Google Scholar]
- Holt JR, Vollrath MA, Eatock RA. Stimulus processing by type II hair cells in the mouse utricle. Ann N Y Acad Sci 871: 15–26, 1999. doi: 10.1111/j.1749-6632.1999.tb09172.x. [DOI] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Jones SM, Holt JR. HCN channels expressed in the inner ear are necessary for normal balance function. J Neurosci 31: 16814–16825, 2011. doi: 10.1523/JNEUROSCI.3064-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26: 10253–10269, 2006. doi: 10.1523/JNEUROSCI.2596-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR, Regehr WG. Achieving high-frequency optical control of synaptic transmission. J Neurosci 34: 7704–7714, 2014. doi: 10.1523/JNEUROSCI.4694-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Vijayakumar S, Dow SA, Holt JC, Jordan PM, Luebke AE. Loss of α-calcitonin gene-related peptide (αCGRP) reduces otolith activation timing dynamics and impairs balance. Front Mol Neurosci 11: 289, 2018. doi: 10.3389/fnmol.2018.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Fettis M, Holt JC. Efferent innervation of turtle semicircular canal cristae: comparisons with bird and mouse. J Comp Neurol 523: 1258–1280, 2015. doi: 10.1002/cne.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Parks XX, Contini D, Holt JC. A review of synaptic mechanisms of vestibular efferent signaling in turtles: extrapolation to efferent actions in mammals. J Vestib Res 23: 161–175, 2013. doi: 10.3233/VES-130492. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Fuchs PA. Cholinergic inhibition of hair cells. In: Auditory and Vestibular Efferents, edited by Ryugo DK, Fay RR, Popper AN. New York: Springer, 2011, p. 103–133. doi: 10.1007/978-1-4419-7070-1. [DOI] [Google Scholar]
- Kong JH, Adelman JP, Fuchs PA. Expression of the SK2 calcium-activated potassium channel is required for cholinergic function in mouse cochlear hair cells. J Physiol 586: 5471–5485, 2008. doi: 10.1113/jphysiol.2008.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WJ, Guo CK, Zhang S, Hao J, Wang YJ, Li ZW. The properties of ACh-induced BK currents in guinea pig type II vestibular hair cells. Hear Res 209: 1–9, 2005. doi: 10.1016/j.heares.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the human utricular macula. Hear Res 119: 104–112, 1998a. doi: 10.1016/S0378-5955(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the vestibular periphery of the rat. Acta Otolaryngol 118: 90–95, 1998b. doi: 10.1080/00016489850155198. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Scholtz AW, Hussl B, Kammen-Jolly K, Schrott-Fischer A. Localization of efferent neurotransmitters in the inner ear of the homozygous Bronx waltzer mutant mouse. Hear Res 167: 136–155, 2002. doi: 10.1016/S0378-5955(02)00382-9. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci 5: 41–47, 2002. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Leijon S, Magnusson AK. Physiological characterization of vestibular efferent brainstem neurons using a transgenic mouse model. PLoS One 9: e98277, 2014. doi: 10.1371/journal.pone.0098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ME, Holt JR. The function and molecular identity of inward rectifier channels in vestibular hair cells of the mouse inner ear. J Neurophysiol 108: 175–186, 2012. doi: 10.1152/jn.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Kevetter GA, Leonard RB, Prusak DJ, Wood TG, Correia MJ. Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neuroscience 146: 384–402, 2007. doi: 10.1016/j.neuroscience.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Meredith FL, Rennie KJ. Development of K+ and Na+ conductances in rodent postnatal semicircular canal type I hair cells. Am J Physiol Regul Integr Comp Physiol 298: R351–R358, 2010. doi: 10.1152/ajpregu.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Bozovic D. Effects of efferent activity on hair bundle mechanics. J Neurosci 40: 2390–2402, 2020. doi: 10.1523/JNEUROSCI.1312-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci 24: 11160–11164, 2004. doi: 10.1523/JNEUROSCI.3674-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke AE, Holt JC, Jordan PM, Wong YS, Caldwell JS, Cullen KE. Loss of α-calcitonin gene-related peptide (αCGRP) reduces the efficacy of the Vestibulo-ocular Reflex (VOR). J Neurosci 34: 10453–10458, 2014. doi: 10.1523/JNEUROSCI.3336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke AE, Maroni PD, Guth SM, Lysakowski A. Alpha-9 nicotinic acetylcholine receptor immunoreactivity in the rodent vestibular labyrinth. J Comp Neurol 492: 323–333, 2005. doi: 10.1002/cne.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P, Rodriguez-Contreras A, Kim HJ, Zhu J, Wei D, Choong-Ryoul S, Eastwood E, Mu K, Levic S, Song H, Yevgeniy PY, Smith PJ, Yamoah EN. Release and elementary mechanisms of nitric oxide in hair cells. J Neurophysiol 103: 2494–2505, 2010. doi: 10.1152/jn.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol 389: 419–443, 1997. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511: 47–64, 2008. doi: 10.1002/cne.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernández C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Singer M. Nitric oxide synthase localized in a subpopulation of vestibular efferents with NADPH diaphorase histochemistry and nitric oxide synthase immunohistochemistry. J Comp Neurol 427: 508–521, 2000. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macova I, Pysanenko K, Chumak T, Dvorakova M, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J Neurosci 39: 984–1004, 2019. doi: 10.1523/JNEUROSCI.2557-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Plotnik M, Goldberg JM. Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol 5: 126–143, 2004. doi: 10.1007/s10162-003-4029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinsky VV. The effect of somatosensory stimulation on second-order and efferent vestibular neurons in the decerebrate decerebellate guinea-pig. Neuroscience 69: 661–669, 1995. doi: 10.1016/0306-4522(95)00231-7. [DOI] [PubMed] [Google Scholar]
- Mathews MA, Camp AJ, Murray AJ. Reviewing the role of the efferent vestibular system in motor and vestibular circuits. Front Physiol 8: 552, 2017. [Erratum in Front Physiol 9: 687, 2018]. doi: 10.3389/fphys.2017.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MA, Murray A, Wijesinghe R, Cullen K, Tung VW, Camp AJ. Efferent vestibular neurons show homogenous discharge output but heterogeneous synaptic input profile in vitro. PLoS One 10: e0139548, 2015. doi: 10.1371/journal.pone.0139548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Usami S, Fujita S, Shinkawa H. Expression of substance P, CGRP, and GABA in the vestibular periphery, with special reference to species differences. Acta Otolaryngol Suppl 115, Suppl 519: 248–252, 1995. doi: 10.3109/00016489509121916. [DOI] [PubMed] [Google Scholar]
- Matsuda Y. Localization of choline acetyltransferase and calcitonin gene-related peptide immunoreactivities in the vestibular end-organs of the guinea pig. Osaka City Med J 42: 61–76, 1996. [PubMed] [Google Scholar]
- McCue MP, Guinan JJ Jr. Influence of efferent stimulation on acoustically responsive vestibular afferents in the cat. J Neurosci 14: 6071–6083, 1994. doi: 10.1523/JNEUROSCI.14-10-06071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Benke TA, Rennie KJ. Hyperpolarization-activated current (Ih) in vestibular calyx terminals: characterization and role in shaping postsynaptic events. J Assoc Res Otolaryngol 13: 745–758, 2012. doi: 10.1007/s10162-012-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Rennie KJ. Channeling your inner ear potassium: K+ channels in vestibular hair cells. Hear Res 338: 40–51, 2016. doi: 10.1016/j.heares.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Muschol M, Salzberg BM. Dependence of transient and residual calcium dynamics on action-potential patterning during neuropeptide secretion. J Neurosci 20: 6773–6780, 2000. doi: 10.1523/JNEUROSCI.20-18-06773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Klöcker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26: 595–601, 2000. doi: 10.1016/S0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Parks XX, Contini D, Jordan PM, Holt JC. Confirming a role for α9nAChRs and SK potassium channels in type II hair cells of the turtle posterior crista. Front Cell Neurosci 11: 356, 2017. doi: 10.3389/fncel.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C, Limón A, Vega R, Soto E. The muscarinic inhibition of the potassium M-current modulates the action-potential discharge in the vestibular primary-afferent neurons of the rat. Neuroscience 158: 1662–1674, 2009. doi: 10.1016/j.neuroscience.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol 88: 1234–1244, 2002. doi: 10.1152/jn.2002.88.3.1234. [DOI] [PubMed] [Google Scholar]
- Poppi LA, Holt JC, Lim R, Brichta AM. A review of efferent cholinergic synaptic transmission in the vestibular periphery and its functional implications. J Neurophysiol 123: 608–629, 2020. doi: 10.1152/jn.00053.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Tabatabaee H, Drury HR, Jobling P, Callister RJ, Migliaccio AA, Jordan PM, Holt JC, Rabbitt RD, Lim R, Brichta AM. ACh-induced hyperpolarization and decreased resistance in mammalian type II vestibular hair cells. J Neurophysiol 119: 312–325, 2018. doi: 10.1152/jn.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt RD, Boyle R, Highstein SM. Mechanical amplification by hair cells in the semicircular canals. Proc Natl Acad Sci USA 107: 3864–3869, 2010. doi: 10.1073/pnas.0906765107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt RD, Brichta AM, Tabatabaee H, Boutros PJ, Ahn J, Della Santina CC, Poppi LA, Lim R. Heat pulse excitability of vestibular hair cells and afferent neurons. J Neurophysiol 116: 825–843, 2016. doi: 10.1152/jn.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu V, Salvi R, Sadeghi SG. Efferent inputs are required for normal function of vestibular nerve afferents. J Neurosci 39: 6922–6935, 2019. doi: 10.1523/JNEUROSCI.0237-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie KJ, Ricci AJ, Correia MJ. Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol 75: 2117–2123, 1996. doi: 10.1152/jn.1996.75.5.2117. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Rennie KJ, Correia MJ. The delayed rectifier, Ikh, is the major conductance in type I vestibular hair cells across vestibular end organs. Pflugers Arch 432: 34–42, 1996. doi: 10.1007/s004240050102. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Wersinger E, Braude JP, Pyott SJ, Fuchs PA. Activation of BK and SK channels by efferent synapses on outer hair cells in high-frequency regions of the rodent cochlea. J Neurosci 35: 1821–1830, 2015. doi: 10.1523/JNEUROSCI.2790-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi ML, Prigioni I, Valli P, Casella C. Activation of the efferent system in the isolated frog labyrinth: effects on the afferent EPSPs and spike discharge recorded from single fibres of the posterior nerve. Brain Res 185: 125–137, 1980. doi: 10.1016/0006-8993(80)90677-0. [DOI] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci 31: 15092–15101, 2011. doi: 10.1523/JNEUROSCI.2743-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Wu JS, McIntosh JM, Glowatzki E. Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea. J Neurophysiol 116: 479–492, 2016. doi: 10.1152/jn.01038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J Neurophysiol 101: 988–1001, 2009. doi: 10.1152/jn.91112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E. Glutamatergic signaling at the vestibular hair cell calyx synapse. J Neurosci 34: 14536–14550, 2014. doi: 10.1523/JNEUROSCI.0369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of the guinea pig organ of Corti: afferent and efferent synapses of hair cells. J Ultrastruct Res 71: 222–232, 1980. doi: 10.1016/S0022-5320(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife 4: e06412, 2015. doi: 10.7554/eLife.06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. doi: 10.1523/JNEUROSCI.4067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat Rev Neurosci 17: 139–145, 2016. doi: 10.1038/nrn.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Igarashi M, Thompson GC. GABA-like immunoreactivity in the squirrel monkey vestibular endorgans. Brain Res 417: 367–370, 1987. doi: 10.1016/0006-8993(87)90466-5. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93–103, 1999. doi: 10.1016/S0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Hou S, Han YG. Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat Neurosci 19: 888–896, 2016. doi: 10.1038/nn.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brain stem: their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol 161: 159–181, 1975. doi: 10.1002/cne.901610203. [DOI] [PubMed] [Google Scholar]
- Wersinger E, McLean WJ, Fuchs PA, Pyott SJ. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PLoS One 5: e13836, 2010. doi: 10.1371/journal.pone.0013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Yi E, Manca M, Javaid H, Lauer AM, Glowatzki E. Sound exposure dynamically induces dopamine synthesis in cholinergic LOC efferents for feedback to auditory nerve fibers. eLife 9: e52419, 2020. doi: 10.7554/eLife.52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas WA, Fuchs PA. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 185: 455–462, 1999. doi: 10.1007/s003590050406. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods 4: 139–141, 2007. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- Zhou T, Wang Y, Guo CK, Zhang WJ, Yu H, Zhang K, Kong WJ. Two distinct channels mediated by m2mAChR and α9nAChR co-exist in type II vestibular hair cells of guinea pig. Int J Mol Sci 14: 8818–8831, 2013. doi: 10.3390/ijms14058818. [DOI] [PMC free article] [PubMed] [Google Scholar]