Abstract
We stabilize the dynamic visual world on our retina by moving our eyes in response to motion signals. Coordinated movements between the two eyes are characterized as version when both eyes move in the same direction and vergence when the two eyes move in opposite directions. Vergence eye movements are necessary to track objects in three dimensions. In primates they can be elicited by intraocular differences in either spatial signals (disparity) or velocity, requiring the integration of left and right eye inputs. Whether mice are capable of similar behaviors is not known. To address this issue, we measured vergence eye movements in mice using a stereoscopic stimulus known to elicit vergence eye movements in primates. We found that mice also exhibit vergence eye movements, although at a low gain and that the primary driver of these vergence eye movements is interocular motion. Spatial disparity cues alone are ineffective. We also found that the vergence eye movements we observed in mice were robust to silencing visual cortex and to manipulations that disrupt the normal development of binocularity in visual cortex. A sublinear combination of motor commands driven by monocular signals is sufficient to account for our results.
NEW & NOTEWORTHY The visual system integrates signals from the left and right eye to generate a representation of the world in depth. The binocular integration of signals may be observed from the coordinated vergence eye movements elicited by object motion in depth. We explored the circuits and signals responsible for these vergence eye movements in rodent and find these vergence eye movements are generated by a comparison of the motion and not spatial visual signals.
Keywords: binocularity, disparity, interocular velocity, parvalbumin, primary visual cortex
INTRODUCTION
The sensory system integrates multiple signals coming from the environment so the organism can behave appropriately in its surroundings. One example of this process is the integration of visual inputs from the two eyes. Eyes are offset horizontally, providing distinct perspectives on our visual environment. Even though the images from left and right eyes are distinct, humans integrate these perspectives generating a single perception of the visual world, also known as the cyclopean view. The integration of left and right eye signals provides cues for an object depth in the world and aids in stabilizing gaze on objects in depth.
Mammals use a number of different eye movements to stabilize objects on their retina. One of these stabilizing eye movements, the optokinetic reflex (OKR), is triggered by global motion in the environment. Importantly this eye movement is not only sensitive to motion in the plane of fixation but to changes in depth. In primates, global shifts of the visual scene in depth cause the two eyes to converge or diverge, depending on whether the scene shifts toward or away from the animal. Previous studies have shown that sensory structures, the frontal eye fields and frontal cortex (Gamlin and Yoon 2000), the cerebellum (Nitta et al. 2008), and the midbrain pretectal and tectal areas (Mays 1984) all play a role in generating OKR eye movements although it is not yet clear which circuits are responsible for coordinating the binocular eye movements that reflect shifts in scene depth.
Vergence responses to visual motion in depth not been examined in rodents thus far. Rodents do exhibit OKR movements for two-dimensional motion (Cahill and Nathans 2008; Tabata et al. 2010; Liu et al. 2016; Samonds et al. 2018) but it is unclear if mice coordinate their eyes to generate the vergence eye movements required to stabilize changes in scene depth. Because their eyes are laterally placed on the head, their binocular field of view is limited and they may not require coordination (Wallace et al. 2013), although rats do generate vergence in response to forward-backward vestibular stimulation (Hess and Dieringer 1991). Here, using stereoscopic random dot stimuli, we demonstrate that mice produce vergence eye movements in response to visual signals normally associated with a stimulus moving toward or away from the animal. We demonstrate that these vergence eye movements are driven by motion signals rather than disparity signals and that they could result from a sublinear combination of the motor signals induced by each monocular input independently. Finally, we show that activity in the visual cortex has little impact on these vergence eye movements. Neither monocular deprivation, which disrupts binocular integration in the visual cortex, nor optogenetically silencing visual cortex has an impact on these vergence eye movements. These disruptions of visual cortical circuitry without impact on these vergence eye movements suggest that other brain regions, subcortical structures, or cortical areas outside of visual cortex, are primarily responsible for the gaze stabilizing vergence eye movements observed in rodents.
METHODS
Experimental Procedures
All procedures were approved by The University of Texas at Austin Institutional Animal Care and Use Committee and conformed to National Institutes of Health standards.
Animal Preparation and Surgery
Eleven male and female mice (C57/Bl6, Jackson Laboratories) were used in these experiments. To generate experimental animals for which visual cortex could be inactivated, parvalbumin (PV)-Cre knockin mice (Scholl et al. 2015) were crossed to a Cre-dependent channelrhodopsin-2 (ChR2)-EYFP strain (Madisen et al. 2012). These progenies selectively expressed ChR2 in PV+ interneurons.
To immobilize the head during training and our measurements, a titanium bar was placed on the skull and secured with dental acrylic under isoflurane anesthesia (Kuhlman et al. 2011). Craniotomies were made over the visual cortex in both hemispheres and covered with glass windows. Light penetration was blocked by occluding the window during periods in which no inactivation was used.
Awake Eye Movement Recording
The animals were initially acclimated to the training apparatus for 3 days before the experiment. Animals walked and stopped freely on a floating Styrofoam ball while they were head fixed (Dombeck et al. 2007). Polarizing lenses were mounted in front of each eye of the animals. After 3 days of acclimatization, we began to record eye movements in response to stimuli presented dichoptically.
Eye tracking.
We used two infrared cameras, one mounted in front of each eye. We positioned the cameras so that they were perpendicular to the orbital axis of each eye (50° from fronto-parallel and pointed slightly downward). The cameras occluded portions of both peripheral monocular visual fields but not the binocular field of view. The cameras collected 800 × 600-pixel images at 30 frames per second. We saved cropped images of 250 × 250 pixels centered over the eye for all experiments and used infrared lighting to minimize shadows. Custom Matlab software was used to track the centers and sizes of the pupils and corneal reflections offline. The horizontal and vertical positions of the pupil centers were calibrated to degrees of visual angle by placing a 3.25-mm diameter artificial eyeball in the same location as the mouse eyeball, rotating it ± 60°, and measuring how the tracking position varied systematically. We examined the feasibility of using the same sized eyeball for calibration for all mice by normalizing the distributions of saccades sizes for each mouse to have either the same mean or same median. In all cases, this only reduced the width of the overall recombined distribution by less than 5% compared with the original distribution, suggesting that there was minimal variability between eyeball sizes for our mice (Samonds et al. 2018). Few vertical eye movements were made during our experiments, and we focus our analysis on the horizontal eye movements made in response to our visual stimulus.
Visual Stimulus
We used a DepthQ HDs3D2 projector (DepthQ/Lightspeed Design, Inc.) with a refresh rate of 120 Hz at full HD resolution (1,920 × 1,080), operating in gray-scale mode (mean luminance = 59.75 cd/m2). Stimuli were either rear projected onto a polarization-preserving screen (Da-Lite 3D virtual black rear screen fabric, model no. 35929) or front-projected onto a silver polarization-preserving screen (Severtson, SeVision 3D GX, 2.2 Silver). The left and right images were modulated by a circular polarization alternator in front of the optics of the projector. One pixel subtended 0.1° at a viewing distance of 22 cm. Black and white dot motion stimuli moving in a sinusoidal motion (±12.4°) were generated using MATLAB (Mathworks, Natick, MA) and Psychtoolbox (Brainard 1997). Each of 400 dots of 5° in diameter was displayed within a 108° square aperture in front of the mouse, and the stimulus lasted for 20 s followed by 15 s blank period. The dots moved ±12.4° in the 20 s, following a sinusoidal trajectory with a peak speed of 3.90°/s for our standard paradigm. The direction of motion of the dots varied between toward first condition (left eye: rightward motion first; right eye: leftward motion first) and away condition (left eye: leftward motion first; right eye: rightward motion first) and was randomly repeated during the trial.
In the control stimulation motion and disparity cues were generated by presenting the same random dot stimulus (RDS) to both eyes and moving the dots in opposite directions in the two eyes (depending on toward first or away first conditions). The interocular velocity difference (IOVD)-only stimulus was generated by presenting distinct patterns of random dots to the two eyes. The dots in the two monocular images moved in opposite directions. Finally, the disparity-only stimulus was created by presenting identical RDS to the two eyes but changing the images every frame or every six frames. At each change, the disparity between the two monocular RDS changed (disparity becoming smaller for toward first condition and disparity becoming bigger for away first condition).
Inactivation
Six male and female mice (2–6 mo old) were used in the cortical inactivation experiment. We used a 470-nm fiber-coupled LED light (Thor laboratories) to activate ChR2. The light covered the entire 3-mm cranial window on both hemispheres, and the intensity of the light was between 1 and 1.3 mW, measured by an optical power meter (ThorLabs, S130VC). The light was turned on 500ms before the stimulus and for the entire stimulus duration (20.5 s). Extracellular recordings were made from animals using 2 MΩ tungsten-in-glass electrodes (Alpha-Omega, Israel) while presenting visual stimuli (Scholl et al. 2013).
Monocular Deprivation
Five male and female mice [postnatal day (P23–36)] were used for monocular deprivation experiment. We sutured shut the right eyelid of each animal at age P23–24 under 1.5% isoflurane anesthesia and checked daily that the sutures were intact. For three animals the monocular deprivation was successful for the period of 10 days and we examined the eye movements in those animals. At P30, we attached the head plate (see animal preparation and surgery). At P33–34, we took the sutures out under 1.5% isoflurane anesthesia and artificial tear was applied to the opened eye. We waited 1 or 2 days following eye opening before conducting our eye movement measures.
Data Analysis
Vergence was calculated by subtracting horizontal left eye position from horizontal right eye position (Right – Left). We define rightward changes in eye position using positive values and leftward changes using negative values. We eliminated eye movement traces that had more than five saccades (saccade was defined by motion within 3 frames larger than 5°) in a given trial. Because the motion in depth stimulus was sinusoidal, the amplitude and phase of vergence eye movements were computed using the Fourier transform. We report twice the magnitude of the F1 amplitude as a measure of the peak to trough amplitude.
Statistical analysis was conducted by comparing the mean of all animals and conducting t test between each condition groups. For control, IOVD-only, change in disparity (CD)-only, and monocular conditions, six animals were used to generate a group mean for the condition group while CD-only slow refresh, CD-only low amplitude, monocular deprivation (MD), and optogenetics (ChR) conditions used three animals, and the mean for the group was computed to conduct t test for analysis.
RESULTS
Vergence Eye Movements in Mice
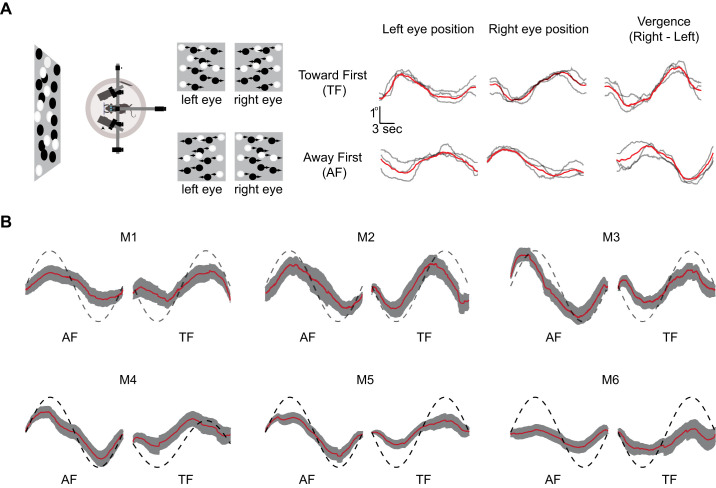
We set out to determine whether changes in scene depth would evoke vergence eye movements in rodents. We are particularly interested in situations in which the left and right eyes move in the same direction (version) relative to cases in which the two eyes move in opposite directions (vergence) that can be evoked by changes in scene depth. We placed awake mice on an air-floated styrofoam ball and measured eye movements while random dot stereograms were presented to the animals in which the right and left eye viewed dots moving in opposite directions (Fig. 1A). We measured the eye movements in six animals while these binocular motion stimuli were being presented. In response to this visual stimulation, mice had smooth eye movements in the direction of the motion of dots presented to each eye (Fig. 1A, right). To compute the vergence eye movement elicited by this stimulus, we subtracted the left eye positions from the right eye position traces. These vergence eye movement traces followed the motion in depth signals that are present in this stimulus. We quantified the vergence eye movement by computing the amplitude of the sinusoidal vergence at the frequency of the motion oscillation and found slightly larger vergence amplitude for the away first condition (AF) compared with toward first condition (TF) (P = 0.038, paired t test), although these stimuli induced vergence movements at the opposite temporal phase (AF: mean phase = −72 ± 5.6°; TF: mean phase = 101 ± 6.9°, Fig. 1B). Despite their small binocular visual field mice make vergence eye movements when presented with this stereoscopic stimulus.
Fig. 1.
Vergence eye movements in mice. A: diagram of the experimental setup and the stimulus motion presented to each eye is shown on the left. Individual eye movement traces for the left eye, right eye, and vergence are shown on the right for stimuli in which the stimulus moved toward first (TF; top) or away first (AF; bottom). The vergence eye movement was calculated by subtracting the left eye position from the right eye position. Red lines indicate the mean across all trials. B: average vergence eye movement for 6 animals [mouse 1 to 6 (M1–M6)] for TF and AF conditions. Red lines indicate mean of all trials, and the gray shaded area is means ± SE. The dashed sinusoidal line is the motion in depth, but note that the actual stimulus motion was max −12 to 12°.
Motion Signals Drive Vergence Eye Movement in Mice
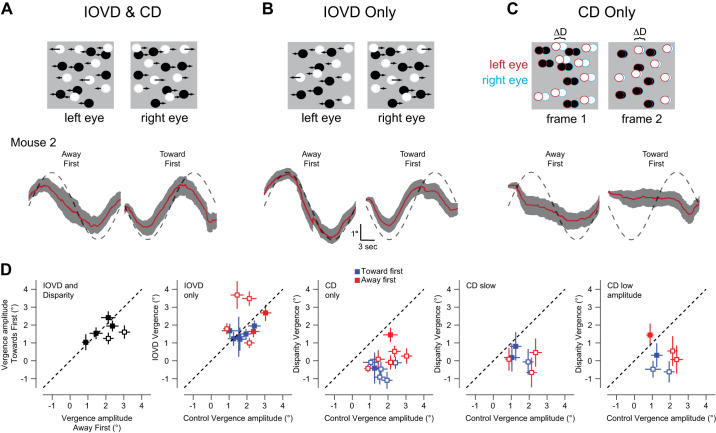
There are two major cues primates are known to use for depth perception: interocular velocity difference (IOVD) and change in disparity (CD) signals (Shioiri et al. 2000). Studies have shown vergence eye movement driven by CD signals (Masson et al. 1997; Rambold and Miles 2008) and IOVD signals (Erkelens and Collewijn 1985a, 1985b; Sheliga et al. 2016) in both humans and nonhuman primates. We initially used a stimulus that included both disparity and motion signals (Fig. 2A, as naturally occurs in our environment and then we dissected those cues into interocular velocity cues (Fig. 2B) or disparity cues (Fig. 2C). Presenting both IOVD and disparity cues elicited vergence eye movements (AF = 1.99 ± 0.33, TF = 1.63 ± 0.22, Fig. 2D, IOVD and Disparity). The gain of the response relative to the motion of the stimulus was thus 0.04 for AF and 0.033 for TF, much smaller than that found in humans with similar stimuli (~0.9; Erkelens and Collewijn 1985b). The difference in the amplitude of the vergence response to AF and TF stimuli was statistically significant in only two of six animals (Fig. 2D, IOVD and Disparity, open symbols). Note that while these stimuli generated consistent vergence eye movements, version eye movements did not vary significantly over the period of visual stimulation.
Fig. 2.
Segregating signals that drive vergence eye movements. A: the interocular velocity difference (IOVD) and change in disparity (CD) condition present both motion signals and disparity signals that may generate vergence eye movements. The stimulus contains correlated dots that move in opposite directions (top). Average vergence eye movements for toward first (TF) and away first (AF) condition of mouse 2 (M2) during control condition are plotted at the bottom. Red lines indicate mean of all trials, and the gray shaded area is means ± SE. B: as in A, but for the IOVD-only condition in which the dots were uncorrelated to eliminate disparity signals (see methods). C: as in A, but for a stimulus in which the two eyes view correlated dots with disparity signals but in every frame, the dots are repositioned to eliminate motion signals (see methods). D: vergence amplitude in each stimulus condition during toward first (TF) and away first (AF) condition for control, IOVD-only, change in disparity (CD)-only, change in disparity-only condition where the refresh rate of the dots was reduced to 6 Hz (CD only Slow Refresh), and change in disparity amplitude was reduced to max ± 6° (CD only Low Amp) for both TF and AF conditions. Each point represents an individual animal. Error bars indicate the means ± SE. Open symbols in the IOVD and Disparity panels indicate animals that exhibited a significant difference between AF and TF conditions (paired t test, P < 0.05). Open symbols in the IOVD panel in the IOVD-only condition indicates animals that exhibited a significantly different response to IOVD only and the IOVD and CD stimulus (paired t test, P < 0.05). Open symbols in the other panels indicate that vergence amplitude did not differ from 0 (t test, P < 0.05).
To determine whether IOVD or CD signals are responsible for these vergence eye movements we generated stimuli that isolate these signals. We generated an IOVD-only stimulus by presenting different patterns of dots to each eye, but each pattern had the same velocity profile as in our control condition. The vergence eye movements elicited by this stimulus closely matched the control condition (AF: 2.37 ± 0.48, TF = 1.48 ± 0.13, Fig. 2D, IOVD only). Differences in evoked vergence were not statistically significant from the control [AF: control vs. IOVD only: P = 0.51 (paired t test); TF: control vs. IOVD only P = 0.44 (paired t test)]. It therefore appears that the IOVD signal is sufficient to elicit for these vergence eye movements. To present a signal that contained only CD signals, we presented matched dots to each eye in which a consistent disparity was enforced, but we generated a novel random dot stimulus on a frame by frame basis. The disparity increased or decreased over time as in our control condition, but because the dots were randomly replotted on each frame, there was no overall motion signal for each eye (Sanada and DeAngelis 2014). This condition induced a dramatic decline in the vergence amplitude for both toward first and away first stimuli and failed to elicit a significant vergence eye movements with the same timing as the control condition except for in a single animal in the away first condition only (AF CD only: 0.31 ± 0.29, P = 30; TF CD only: −0.50 ± 0.17, P = 0.04, Fig. 2D, CD only).
One potential problem with the CD isolating stimulus is that the rapid update of the dots may make generating appropriate disparity signals difficult for the mouse visual system. An additional problem is that the disparities we have employed may be outside the range that may be encoded by the mouse visual system (La Chioma et al. 2019; Samonds et al. 2019). We therefore first altered the rate at which dots were redrawn (from every frame to every 5th frame). Even when we slowed the refresh rates of the dots, we still did not observe significant vergence eye movements with the same timing as the control condition (AF: −0.03 ± 0.40, P = 0.93; TF: 0.31 ± 0.32, P = 0.35, Fig. 2D, CD slow). Next, we decreased the disparity amplitude to ± 6° in our stimulus to see if this smaller range of disparity cues could elicit vergence eye movement. This condition also failed to elicit significant vergence eye movements in any individual animals (Fig. 2D, CD only, low amplitude AF: 0.69 ± 0.49, P = 0.23; TF: −0.26 ± 0.35, P = 0.46). Mouse vergence OKR, therefore, seems to be dominated by motion signals instead of disparity signals.
Sublinear Combination in Vergence Eye Movement in Mice
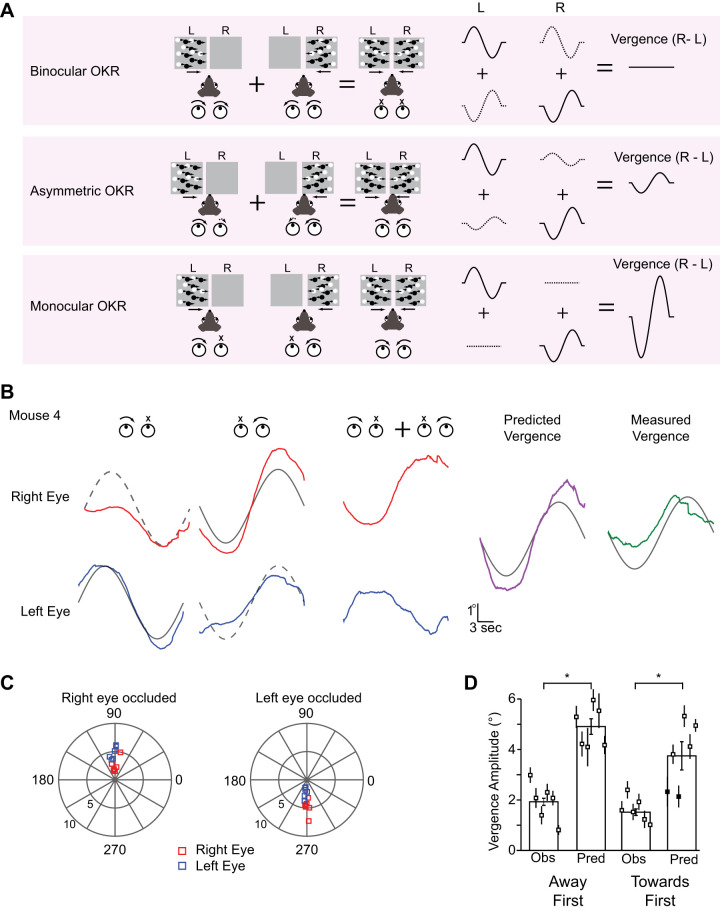
We have demonstrated that IOVD signals can elicit vergence eye movements in mice. Monocular responses can give us clues about the mechanism underlying these IOVD-driven eye movements. If monocular stimuli induce conjugate (i.e., identical) movements of the two eyes (Fig. 3A, top), as for example found in humans for the open-loop phase of ocular following (Quaia et al. 2018), IOVD-induced vergence eye movements must rely on the presence of IOVD detectors in the brain (i.e., neurons that compare the motion signals received by the 2 eyes), like those found in primate area MT (Czuba et al. 2014). If instead monocular stimuli induce movements that are significantly stronger (Fig. 3A, middle) in the viewing eye, or at the extreme only drive the viewing eye (Fig. 3A, bottom), a simple combination of the eye movements induced independently by monocular motion signals might be sufficient to explain our results, without needing to invoke the presence of neural IOVD detectors.
Fig. 3.
Sublinear combination generates vergence eye movement in mice. A: 3 eye movement potential models for vergence optokinetic reflex (OKR). In the first model both eyes make an equal motion during monocular viewing, and direct summation of left and right eye movement will generate cancelation of vergence eye movement (top). In the second model, the eyes make unequal eye movements during monocular viewing and summation of monocular eye movements will generate a vergence eye movement (middle). In the third model, the eye presented with the stimulus moves and the nonstimulated eye does not make an eye movement (bottom). This condition will generate bigger vergence eye movement than the asymmetric condition. B: left and right eye movements during monocular stimulation of either the left eye (top) or right eye (bottom) for mouse 4 (M4) (purple) for a toward first stimulus. Left and right eye predicted traces were generated by combining signals from monocular conditions and the resulting predicted vergence eye movement was computed by subtracting the predicted left eye from the predicted left eye (right). The observed (red) traces are from the control TF condition from M4. C: comparison between the left and right eye during right eye closed (top right) and left eye closed condition in polar plots (bottom right). Each point represents an individual animal. Red squares represent right eyes and blue squares represent left eye. D: quantification between observed (Obs) vergence amplitude and observed vergence amplitude. Each point in the group is an individual animal. Filled points indicate that the predicted (Pred) vergence amplitude was not significantly different from the measured vergence amplitude. The 95% confidence intervals were computed via bootstrapping (Sokal and Rohlf 1995).
To constrain these models for vergence eye movements, we measured eye movements for the away first or toward first conditions but with one eye occluded. As before, we presented sinusoidally moving dot patterns moving either rightward or leftward moving first stimulus to one eye while occluding the other. We then tested whether the vergence response to our binocular stimuli could be predicted from a linear combination of these responses to monocular stimulation.
We first examined the eye movements elicited by motion signals that exist in the toward first condition. We observed that when only one eye was presented with a motion signal, both eyes moved in the same direction as the motion pattern. Importantly, however, the amplitude of the eye movements was distinct: the occluded eye moved less than the eye that was presented the motion stimulus but had a similar time course (Fig. 3B). We assessed eye movement amplitude and timing of both eyes by calculating the Fourier phase and amplitude at the frequency of the stimulation for six mice (Fig. 3C, TF Left eye occluded (LEO): Right eye: 4.83 ± 0.56, Left eye: 2.23 ± 0.39. Right eye occluded (REO): Right eye: 2.54 ± 0.52, Left eye: 4.79 ± 0.45; AF LEO: Right eye: 1.64 ± 0.40, Left eye: 0.77 ± 0.21. REO: Right eye: 0.76 ± 0.22, Left eye: 1.88 ± 0.30). In all cases the eye that received visual stimulation moved more than the eye that was occluded, indicating that the asymmetry is not caused by an overall dominance of one eye over the other. Using the eye movements elicited by monocular motion cues, we then made linear predictions about the vergence eye movements that would be evoked for the away first condition (Fig. 3B, right). We combined left and right eye movements evoked by left eye stimulation with the left and right eye movements evoked by right eye stimulation. Note that the motion trajectories in each condition are the opposite, as is the case for the IOVD stimulus. We then subtracted the predicted right and left eye positions to generate the predicted vergence eye movement (Fig. 3B, purple). The linear predicted vergence eye movement had a larger amplitude than the observed vergence eye movement (green) for both the away first condition and in the toward first condition (TF: 3.73 ± 0.56, AF: 4.91 ± 0.31; t test, TF: P = 0.009; AF: P = 9.25e-7, Fig. 3D), indicating a sublinear combination between left and right eye. It is important to note that the asymmetric interaction of the two eyes is required to account for vergence eye movements (Fig. 3A, middle) and the eye movements do not simply reflect the visual motion that each eye receives (Fig. 3A, bottom). The vergence eye movements we observe could therefore emerge from a sublinear combination of the eye movements evoked by monocular stimulation alone.
Circuits for OKR Vergence Eye Movement in Mice
Previous studies in primates have implicated circuits in the sensory cortex (Takemura et al. 2007), frontal cortex (Gamlin and Yoon 2000), cerebellum (Nitta et al. 2008), and subcortical regions (Freedman and Sparks 1997; Mays 1984) in generating OKR vergence eye movements. Left and right eye visual signals are known to converge along the primate thalomocortical visual pathway: V1 neurons are known to be disparity selective (Pettigrew et al. 1968; Poggio and Fischer 1977), and MT neurons are selective for motion-in-depth (Czuba et al. 2014). In mice, binocular responses have been observed in the thalamus (Howarth et al. 2014) and disparity selectivity has been measured in visual cortex (La Chioma et al. 2019; Samonds et al. 2019; Scholl et al. 2013). We hypothesized that the integration of left and right eye motion signals to generate vergence eye movements could stem from interactions that rely on activity in the visual cortex.
It is well known that disrupting visual input to one or both eyes in a young animal has an impact on how the binocular visual system develops throughout adulthood. Monocular deprivation in an animal during the critical period is known to shift the ocular dominance, alter visual acuity in the deprived eye, and alter the disparity selectivity in V1 neurons throughout the adulthood of the animal (Gordon and Stryker 1996; Scholl et al. 2017; Shatz and Stryker 1978; Wiesel and Hubel 1963). To see if altering binocularity of neurons in the visual cortex could alter OKR vergence eye movement, we performed monocular deprivation (MD) in young mice during the developmental critical period. All animals went through MD for 10 days (P23–P33), and we measured the eye movements to motion in depth signals 1–2 days after we opened the deprived eye (Fig. 4A). We observed large vergence eye movements and found no changes in vergence eye movement in MD animals when compared with that of the control animals for the TF condition (MDTF: 1.77 ± 0.16. t test, P = 0.44; Fig. 4B) although vergence eye movement for the AF condition were smaller (MDAF: 1.29 ± 0.14, t test P = 0.005). The evoked vergence eye movements were due to the movement of both the nondeprived and deprived eye. In sum these data suggest that the disruption of binocular signals that follows monocular deprivation has little effect on vergence eye movements.
Fig. 4.
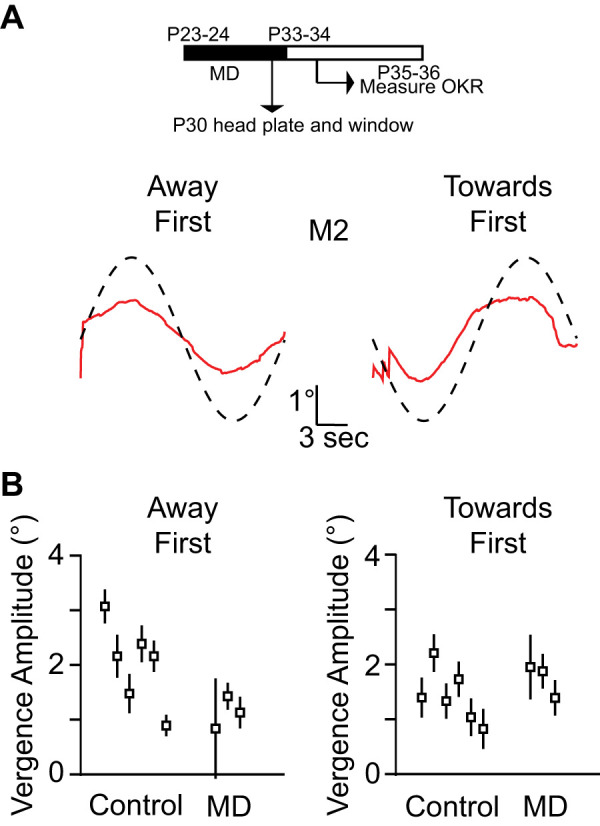
Monocular deprivation (MD) and vergence eye movements. A: timeline of monocular deprivation (top). Mean vergence eye movement of animal [mouse 2 (M2)] after MD for the toward first and the away first conditions (bottom). B: vergence amplitude comparison between control animals and monocular-deprived animals for toward first and away first stimuli. Error bars indicate means ± SE of the individual animals. OKR, optokinetic reflex; P, postnatal day.
Next, we tested whether a dramatic alteration of cortical signals can alter vergence eye movements in mice. We inactivated the visual cortex using PV-ChR2 transgenic mice, optogenetically silencing the cortex while the animals were viewing the moving dot stereogram (Fig. 5A) (Keller et al. 2017; Liu et al. 2016; Lien and Scanziani 2018; Madisen et al. 2012; Pafundo et al. 2016; Yang et al. 2017). The area where the cranial window was placed was 3 mm in diameter above the visual cortex, which covered binocular, monocular, and some extrastriate area of the visual cortex. Both hemispheres of the visual cortex were covered with cranial windows. We found that inactivation of visual cortex had little impact on vergence eye movements. The amplitude of the vergence eye movement was not difference for both TF and AF conditions (AF: 2.34 ± 0.18, t test P = 0.06; TF: 1.14 ± 0.15, t test P = 0.07, Fig. 5B). This finding buttresses the MD results indicating that activity in visual cortex has little influence on the OKR vergence eye movement.
Fig. 5.
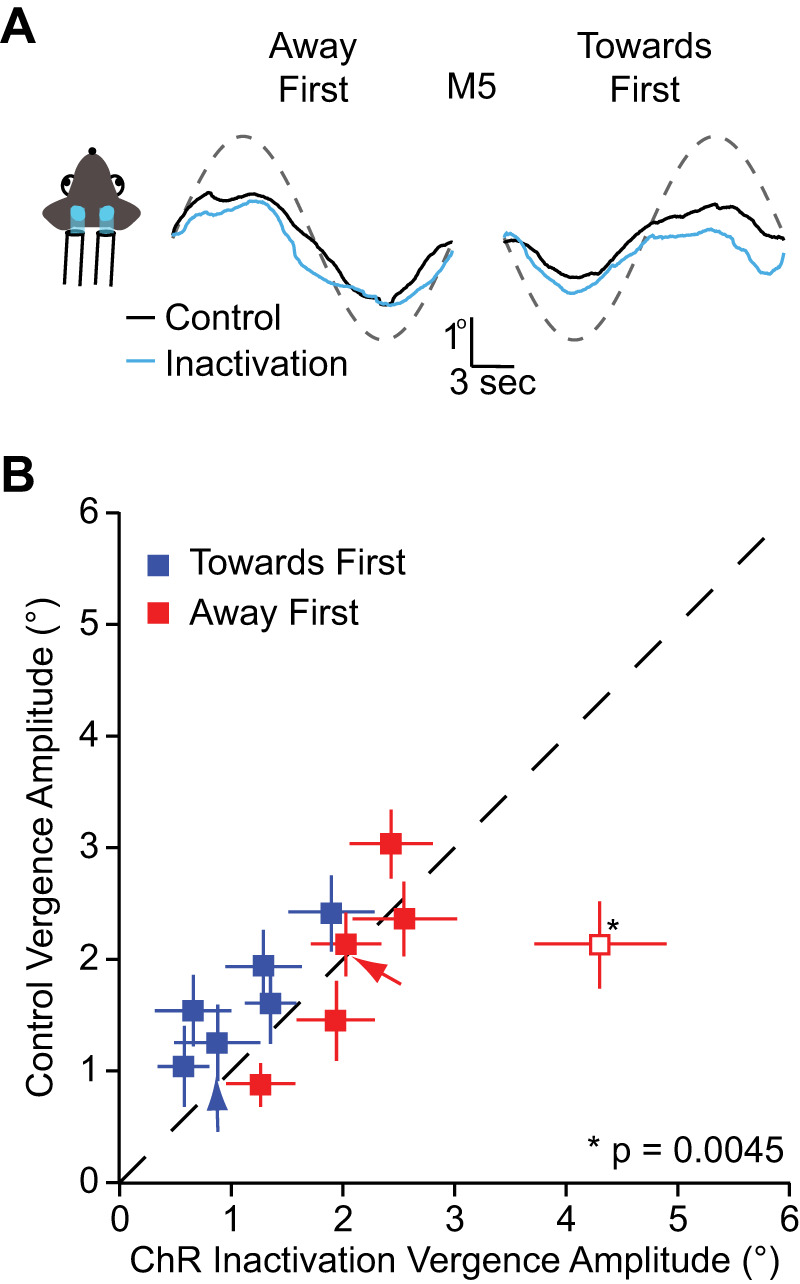
Visual cortex inactivation and vergence eye movements A: mean vergence eye movements of animal [mouse 5 (M5)] during visual cortex inactivation for the toward first (left) and the away first conditions (right). Red line indicates the mean vergence eye movements during control condition while the cyan line indicates vergence during inactivation. B: vergence amplitude comparison between control condition and channelrhodopsin-2 (ChR2) condition for toward first and away first stimulus across animals. Error bars indicate means ± SE of the individual animals. One animal had a statistically different vergence amplitude during inactivation (P = 0.0045). For that animal, vergence was larger during inactivation than in control conditions.
One concern with our inactivation experiment is that the LED light used to activate PV+ neurons may not be sufficiently strong to silence visual cortex. To test if the PV neurons in these transgenic animals are being activated by the LED light, and thus silencing other cortical cells, we recorded neuronal activity from an awake animal using tungsten electrodes. Without the optogenetic silencing, there is a clear deflection in the LFP at the onset and offset of the visual stimulus (Fig. 6A, black arrows). In 26 LFP recordings, this visually evoked response was eliminated when visual cortex was inactivated by the increased activity of PV+ neurons. Instead of observing responses to the visual stimulus, there is a clear response to the onset and offset of the LED light that was used to activate PV+ neurons (Fig. 6A, bottom, blue arrows). The response to both optogenetic and visual stimulus matches that observed to the LED light alone (Fig. 6B, top). There was no significant difference between local field potential (LFP) amplitude during optogenetic activation in conditions with and without visual stimulation (Onset LFP amplitude, P = 0.999, Offset LFP amplitude, P = 0.994, offset, Fig. 6, A and B). We not only recorded the LFP responses, but we also wished to demonstrate that the blue light evoked responses in V1. We therefore measured the multiunit responses optogenetic stimulation (Fig. 6B, bottom). When the blue light was turned on, barrages of action potentials were observed from neurons with narrow spike waveforms. Because of the high evoked firing rate of these neurons, it was difficult to isolate single units, although in one recording site we were able to record a single unit (Fig. 6B, inset) with a narrow spike width, consistent with a PV+ neuron. On average, the LED induced an increase in multiunit activity to 73.8 ± 6.7 spikes/s from 11.3 ± 1.7 spikes/s (means ± SE). These records demonstrate that light was able to activate neurons. We next conducted whole cell intracellular recordings in vitro using one of our animals to confirm that light activation caused suppression in pyramidal neurons. A recorded excitatory pyramidal neuron fired action potentials when depolarizing current was injected, but the blue LED light suppressed these responses (Fig. 6C). These records demonstrate that LED light evoked responses in neurons and that those evoked responses led to suppression of pyramidal cell activity in the visual cortex. That suppression, however, may be incomplete in vivo, and it was difficult from our extracellular records to separate the effects of the LED light on excitatory and inhibitory responses. We therefore performed whole cell recordings in vivo from 23 pyramidal neurons to assay the degree to which LED light was able to suppress visually evoked responses in mouse V1. We observed complete suppression in all pyramidal neurons in V1. In all cells, optogenetic stimulation reduced the spike rate of neurons to zero (Fig. 6D). The effect was significant in all cells measured, as no cell elicited an action potential while the LED was on. In sum, we demonstrate that light elicited strong responses from neurons with narrow spiking and silence the spiking activity of recorded pyramidal cells. Therefore, our inactivation experiments successfully silenced the visual cortex despite the absence of an effect on the vergence eye movements induced by the stereo motion signals.
Fig. 6.
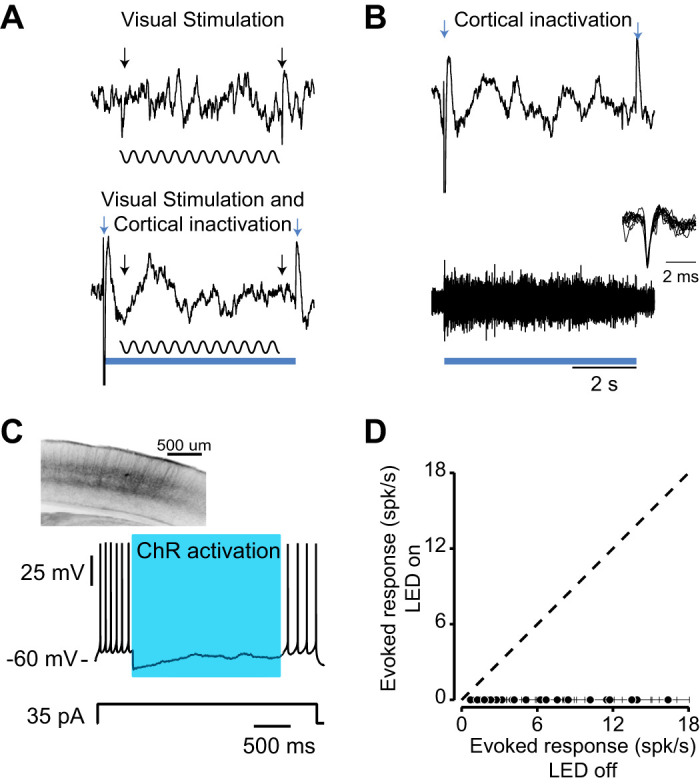
Extracellular recording during optogenetic stimulation. A: local field potential (LFP) recordings during visual stimulation alone (top) and visual stimulation with optogenetic stimulation (bottom). The blue square indicates the duration of optogenetic activation and the sinusoidal wave indicates the visual stimulus. Sinusoidal grating (with duration of 5 s) with 100% contrast with 45° orientation was used to stimulate the neurons in the visual cortex. The onset and offset LFP changes are indicated by black arrows (top). The optogenetic blue light (470 nm) came on 500 ms before the visual stimulation and lasted until 500 ms after the visual stimulation. Black and blue arrows indicate the onset and offset of LFP responses to visual and optogenetic stimulation respectively (bottom). B: LFP and multiunit responses during optogenetic stimulation only. Blue arrows indicate the onset and offset LFP response to optogenetic stimulation (top). Multiunit activity during optogenetic stimulation (bottom) and isolated single unit with a narrow spike width, consistent with a parvalbumin+ neuron (inset). C: in vitro recording of pyramidal neuron during optogenetic activation. Brain slice shows the PV neurons tagged with channelrhodopsin-2 (ChR2)-YFP (top). The blue square indicates the duration of optogenetic activation. In vitro whole cell recording of excitatory neurons when injecting 35-pA current and LED light stimulation (bottom). D: in vivo whole cell recordings from V1 neurons (n = 23) during optogenetic stimulation in current-clamp configuration. The spiking responses of neurons were recorded when LED light is on and when LED light is off while being presented their preferred visual stimulus. Each symbol indicates the spike rate of a neuron during visual stimulation with or without LED light (black dots). Error bars indicate the SE of the spike rate of each individual neuron.
DISCUSSION
In response to motion in three-dimensional space, the eyes move in opposite directions. These vergence eye movements have been studied in primates, which are elicited by both disparity cues (Erkelens and Collewijn 1985a, 1985b; Masson et al. 1997) and motion cues (Sheliga et al. 2016, although see Giesel et al. 2019). To better understand the signals and circuitry that guide smooth vergence eye movements, we developed a paradigm to measure these eye movements in mice where the visual pathways may be dissected.
We found that mice make smooth vergence eye movements when viewing a stereoscopic stimulus that is primarily driven by motion signals (IOVD). We also demonstrate that these vergence OKR eye movements may be predicted by a sublinear combination of the responses elicited by monocular stimuli. While both eyes move in response to a monocularly presented stimulus, the eye that receives the motion signals moves more than the other eye. Similar eye movement asymmetries have also been observed to frontoparallel optokinetic stimulation in binocular versus monocular conditions in rats (De’sperati et al. 1994). This asymmetry predicts the direction of the vergence eye movements but overestimates the amplitude of those elicited by binocular stimuli. It is important to note that we have examined a specific vergence behavior, one induced by slow motion signals to each eye. It is known that the perception of motion in depth in primates depends critically on the specific parameters of the experimental paradigm (Czuba et al. 2010; Shioiri et al. 2000), shifting from IOVD to disparity signals with spatial frequency.
Responses in the neocortex have been implicated in the generation of vergence eye movements (Gamlin and Yoon 2000; Takemura et al. 2007). While we found that disparity signals had little influence on vergence eye movements, the visual cortex may nonetheless be an essential node in the visual pathway for these vergence eye movements. We therefore used two techniques to determine whether visual cortex plays a role in generating vergence OKR. First, we disrupted the development of binocular circuitry in visual cortex by performing monocular deprivation during the critical period. This manipulation had little impact on the vergence OKR. Second, we used optogenetics to inactivate visual cortex while the animal viewed the stereoscopic stimulus. This manipulation also did not impact vergence OKR, similar to the absence of impact on the OKR driven by frontoparallel motion (Harvey et al. 1997). Surprisingly we found a slight increase in the vergence OKR by this manipulation, which may suggest that disparity suppress rather than enhance these eye movements. Both of these experiments demonstrate that processing along the geniculocortical pathway in mice has little impact on the generation of vergence OKR. While the visual cortex in rodents may not be the primary site to generate the vergence OKR, it may nonetheless regulate the gain of these eye movements (Liu et al. 2016; Tusa et al. 1989). In primates, the visual cortex may be acting as a gate to vergence eye movements based on the binocular correlation of two input patterns. In humans, opposite direction motion elicits weak vergence eye movements, perhaps because this gate is not open (Masson et al. 2002). Other cortical areas, beyond visual cortex, may be involved in these motions evoked vergence eye movements such as the frontal cortex or prefrontal cortex (primate: Gamlin and Yoon 2000; cat: Tusa et al. 1989).
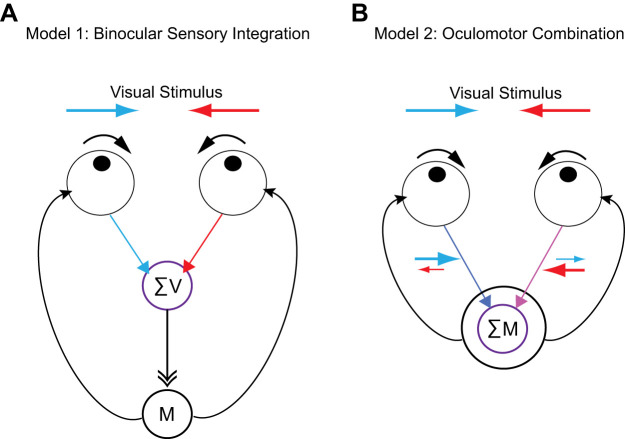
At least two potential models could explain the vergence eye movements we observe. First, these vergence eye movements could arise from a binocular integration of sensory information from left and right eyes, which is then relayed to appropriate motor areas and converted into eye movements (Fig. 7A). Alternatively, the integration necessary for the execution of vergence eye movements may not require sensory integration, but instead be the product of the integration of motor signals resulting from sensory stimulation to each of the eyes alone (Fig. 7B). In this case, the visual sensory signals between the left and right eye are not integrated, instead it is the motor signals from the left eye and right eye that are integrated to elicit eye movements. Our monocular experiment, in which we restricted visual signals to one eye, demonstrates that sublinear combination of left and right eye motor signals, instead of sensory signals, can account for OKR vergence eye movements (Fig. 3). The monocular visual stimulation experiment demonstrates both eyes move in the same direction but to a different degree. The integration of these unequal signals leads to predictions of vergence eye movements that match the direction of the vergence eye movements elicited by binocular stimulation. While this monocular experiment is consistent with a locus for the integration of signals in the motor system, there are still unknown variables influencing the gain of eye movements, as our predicted eye movements from the monocular condition has significantly larger vergence amplitudes than those measured in binocular conditions.
Fig. 7.
Models for optokinetic reflex (OKR) vergence eye movement. A: binocular sensory integration model. The first model illustrates a vergence mechanism in which sensory signals (red and blue) are first integrated and motor signals (black) are generated following that integration. B: oculomotor combination model. The second model illustrates a mechanism in which vergence eye movement is generated by combination of motor signals from the two eyes. The visual signals drive asymmetric motor signals (purple), which are then summed to generate the vergence eye movement (black). Red: leftward motion in right eye; blue: rightward motion in left eye. The motor signal from the two eyes are combined to generate vergence eye movement (black).
It is known that motion signals necessary for frontoparallel OKR eye movements emanate from retinal processing in rodents. Eliminating starburst amacrine neurons in the mouse retina abolishes OKR in mice (Yoshida et al. 2001). The subcortical nature of the circuitry for vergence eye movements we have uncovered may reflect this difference in where direction selectivity is extracted. For rodents this initially occurs in the retina, whereas in primates, direction selectivity is ascribed to processing in the visual cortex. Several subcortical structures are known to receive binocular projections and may be key to coordinating these vergence eye movements. The nucleus of the optic tract (NOT) is known to play an important role in OKR eye movement (Yakushin et al. 2000) and contains binocular neurons (Cynader and Hoffmann 1981). The superior colliculus is another subcortical structure that receives binocular input and sends out projections to the ocular premotor neurons. The superior colliculus receives direct retinal input that projects, which innervates distinct collicular layers with matched in retinotopy (Dräger and Hubel 1976). The superficial layer contains binocular neurons (Economides et al. 2018) and deep layer contains ocular motor neurons that generate saccades and smooth eye movements that can have vergence components (Robinson 1972; Schiller and Stryker 1972; Van Horn et al. 2013; Wurtz and Albano 1980). A bilateral lesion of the rostral superior colliculus can result in convergence palsy in humans (Ohtsuka et al. 2002), indicating that the superior colliculus may be essential node for the control of vergence eye movements. The binocular integration required for the OKR vergence eye movements we have measured likely relies on these subcortical structures.
In summary, we examined the signals underlying OKR vergence eye movements in mice and show that a motion signal (IOVD) is the primary driving signal behind this behavior. We also found the computation behind this vergence eye movement is a sublinear integration between left and right eye movements and surprisingly limited ongoing involvement of the visual cortex for this behavior.
GRANTS
This work was supported by National Institutes of Health Grant EY-025102 and Human Frontiers Science Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.C. and N.J.P. conceived and designed research; V.C. performed experiments; V.C. and N.J.P. analyzed data; V.C. and N.J.P. interpreted results of experiments; V.C. and N.J.P. prepared figures; V.C. and N.J.P. drafted manuscript; V.C. and N.J.P. edited and revised manuscript; V.C. and N.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Jason Samonds and Jessica Hanover for helpful discussions and comments. We are also grateful to Jagruti Pattadkal for assistance with data collection.
REFERENCES
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: application to genetic and drug-induced variation. PLoS One 3: e2055, 2008. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Hoffmann KP. Strabismus disrupts binocular convergence in cat nucleus of the optic tract. Brain Res 227: 132–136, 1981. doi: 10.1016/0165-3806(81)90100-0. [DOI] [PubMed] [Google Scholar]
- Czuba TB, Huk AC, Cormack LK, Kohn A. Area MT encodes three-dimensional motion. J Neurosci 34: 15522–15533, 2014. doi: 10.1523/JNEUROSCI.1081-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuba TB, Rokers B, Huk AC, Cormack LK. Speed and eccentricity tuning reveal a central role for the velocity-based cue to 3D visual motion. J Neurophysiol 104: 2886–2899, 2010. doi: 10.1152/jn.00585.2009. [DOI] [PubMed] [Google Scholar]
- De’sperati C, Tempia F, Harvey R, Strata P. Vergence compensation during binocularly- and monocularly-evoked horizontal optokinetic nystagmus in the pigmented rat. Vision Res 34: 3335–3345, 1994. doi: 10.1016/0042-6989(94)90068-X. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger UC, Hubel DH. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol 39: 91–101, 1976. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- Economides JR, Rapone BC, Adams DL, Horton JC. Normal topography and binocularity of the superior colliculus in strabismus. J Neurosci 38: 173–182, 2018. 10.1523/JNEUROSCI.2589-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkelens CJ, Collewijn H. Motion perception during dichoptic viewing of moving random-dot stereograms. Vision Res 25: 583–588, 1985a. doi: 10.1016/0042-6989(85)90164-6. [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Collewijn H. Eye movements and stereopsis during dichoptic viewing of moving random-dot stereograms. Vision Res 25: 1689–1700, 1985b. doi: 10.1016/0042-6989(85)90141-5. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K. An area for vergence eye movement in primate frontal cortex. Nature 407: 1003–1007, 2000. doi: 10.1038/35039506. [DOI] [PubMed] [Google Scholar]
- Giesel M, Yakovleva A, Bloj M, Wade AR, Norcia AM, Harris JM. Relative contributions to vergence eye movements of two binocular cues for motion-in-depth. Sci Rep 9: 17412, 2019. doi: 10.1038/s41598-019-53902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274–3286, 1996. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, De’Sperati C, Strata P. The early phase of horizontal optokinetic responses in the pigmented rat and the effects of lesions of the visual cortex. Vision Res 37: 1615–1625, 1997. doi: 10.1016/S0042-6989(96)00292-1. [DOI] [PubMed] [Google Scholar]
- Hess BJ, Dieringer N. Spatial organization of linear vestibuloocular reflexes of the rat: responses during horizontal and vertical linear acceleration. J Neurophysiol 66: 1805–1818, 1991. doi: 10.1152/jn.1991.66.6.1805. [DOI] [PubMed] [Google Scholar]
- Howarth M, Walmsley L, Brown TM. Binocular integration in the mouse lateral geniculate nuclei. Curr Biol 24: 1241–1247, 2014. doi: 10.1016/j.cub.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AJ, Houlton R, Kampa BM, Lesica NA, Mrsic-Flogel TD, Keller GB, Helmchen F. Stimulus relevance modulates contrast adaptation in visual cortex. eLife 6: e21589, 2017. doi: 10.7554/eLife.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Tring E, Trachtenberg JT. Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat Neurosci 14: 1121–1123, 2011. doi: 10.1038/nn.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Chioma A, Bonhoeffer T, Hübener M. Area-specific mapping of binocular disparity across mouse visual cortex. Curr Biol 29: 2954–2960.e5, 2019. doi: 10.1016/j.cub.2019.07.037. [DOI] [PubMed] [Google Scholar]
- Lien AD, Scanziani M. Cortical direction selectivity emerges at convergence of thalamic synapses. Nature 558: 80–86, 2018. doi: 10.1038/s41586-018-0148-5. [DOI] [PubMed] [Google Scholar]
- Liu BH, Huberman AD, Scanziani M. Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour. Nature 538: 383–387, 2016. doi: 10.1038/nature19818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Miles FA. Vergence eye movements in response to binocular disparity without depth perception. Nature 389: 283–286, 1997. doi: 10.1038/38496. [DOI] [PubMed] [Google Scholar]
- Masson GS, Yang DS, Miles FA. Version and vergence eye movements in humans: open-loop dynamics determined by monocular rather than binocular image speed. Vision Res 42: 2853–2867, 2002. doi: 10.1016/s0042-6989(02)00334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol 51: 1091–1108, 1984. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- Nitta T, Akao T, Kurkin S, Fukushima K. Vergence eye movement signals in the cerebellar dorsal vermis. Prog Brain Res 171: 173–176, 2008. doi: 10.1016/S0079-6123(08)00623-7. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Maeda S, Oguri N. Accommodation and convergence palsy caused by lesions in the bilateral rostral superior colliculus. Am J Ophthalmol 133: 425–427, 2002. doi: 10.1016/S0002-9394(01)01356-3. [DOI] [PubMed] [Google Scholar]
- Pafundo DE, Nicholas MA, Zhang R, Kuhlman SJ. Top-down-mediated facilitation in the visual cortex is gated by subcortical neuromodulation. J Neurosci 36: 2904–2914, 2016. doi: 10.1523/JNEUROSCI.2909-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD, Nikara T, Bishop PO. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res 6: 391–410, 1968. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Poggio GF, Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol 40: 1392–1405, 1977. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- Quaia C, Optican LM, Cumming BG. Binocular summation for reflexive eye movements. J Vis 18: 7, 2018. doi: 10.1167/18.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold HA, Miles FA. Human vergence eye movements to oblique disparity stimuli: evidence for an anisotropy favoring horizontal disparities. Vision Res 48: 2006–2019, 2008. doi: 10.1016/j.visres.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808, 1972. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Samonds JM, Choi V, Priebe NJ. Mice discriminate stereoscopic surfaces without fixating in depth. J Neurosci 39: 8024–8037, 2019. doi: 10.1523/JNEUROSCI.0895-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds JM, Geisler WS, Priebe NJ. Natural image and receptive field statistics predict saccade sizes. Nat Neurosci 21: 1591–1599, 2018. doi: 10.1038/s41593-018-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada TM, DeAngelis GC. Neural representation of motion-in-depth in area MT. J Neurosci 34: 15508–15521, 2014. doi: 10.1523/JNEUROSCI.1072-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924, 1972. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J, Priebe NJ. Binocular integration and disparity selectivity in mouse primary visual cortex. J Neurophysiol 109: 3013–3024, 2013. doi: 10.1152/jn.01021.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Pattadkal JJ, Dilly GA, Priebe NJ, Zemelman BV. Local integration accounts for weak selectivity of mouse neocortical parvalbumin interneurons. Neuron 87: 424–436, 2015. doi: 10.1016/j.neuron.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Pattadkal JJ, Priebe NJ. Binocular Disparity Selectivity Weakened after Monocular Deprivation in Mouse V1. J Neurosci 37: 6517–6526, 2017. doi: 10.1523/JNEUROSCI.1193-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol 281: 267–283, 1978. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Quaia C, FitzGibbon EJ, Cumming BG. Human short-latency ocular vergence responses produced by interocular velocity differences. J Vis 16: 11, 2016. doi: 10.1167/16.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioiri S, Saisho H, Yaguchi H. Motion in depth based on inter-ocular velocity differences. Vision Res 40: 2565–2572, 2000. doi: 10.1016/S0042-6989(00)00130-9. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Sciences. New York: W.H. Freeman Company, 1995. [Google Scholar]
- Tabata H, Shimizu N, Wada Y, Miura K, Kawano K. Initiation of the optokinetic response (OKR) in mice. J Vis 10: 1–17, 2010. doi: 10.1167/10.1.13. [DOI] [PubMed] [Google Scholar]
- Takemura A, Murata Y, Kawano K, Miles FA. Deficits in short-latency tracking eye movements after chemical lesions in monkey cortical areas MT and MST. J Neurosci 27: 529–541, 2007. doi: 10.1523/JNEUROSCI.3455-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusa RJ, Demer JL, Herdman SJ. Cortical areas involved in OKN and VOR in cats: cortical lesions. J Neurosci 9: 1163–1178, 1989. doi: 10.1523/JNEUROSCI.09-04-01163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn MR, Waitzman DM, Cullen KE. Vergence neurons identified in the rostral superior colliculus code smooth eye movements in 3D space. J Neurosci 33: 7274–7284, 2013. doi: 10.1523/JNEUROSCI.2268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JND. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498: 65–69, 2013. doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26: 1003–1017, 1963. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3: 189–226, 1980. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Gizzi M, Reisine H, Raphan T, Büttner-Ennever J, Cohen B. Functions of the nucleus of the optic tract (NOT). II. Control of ocular pursuit. Exp Brain Res 131: 433–447, 2000. doi: 10.1007/s002219900302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Prouvot PH, Reyes-Puerta V, Stüttgen MC, Stroh A, Luhmann HJ. Optogenetic modulation of a minor fraction of parvalbumin-positive interneurons specifically affects spatiotemporal dynamics of spontaneous and sensory-evoked activity in mouse somatosensory cortex in vivo. Cereb Cortex 27: 5784–5803, 2017. doi: 10.1093/cercor/bhx261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30: 771–780, 2001. doi: 10.1016/S0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]