Abstract
The very recent Covid-19 pandemic has made the need to understand biocompatible polymers as support material in drug delivery systems and controlled release clearer, especially for organo-hydrogels. This study aims to synthesize various new polymeric materials called gels, hydrogels, and organo-hydrogels according to the monomer used and to investigate their use as drug release systems. The agar-glycerol (AG) pair was used to synthesize the polymers, N, N, methylene bisacrylamide (MBA, m) and glutaraldehyde (GA, g) were used as cross-linkers and peppermint oil (PmO) was included to obtain the organo-hydrogels. Therefore, one AG gel and two p (AG-m) and p (GA-g) hydrogels were synthesized within the scope of the study. Six different organo-hydrogels based on p(AG-m-PmO) or p (AG-g-PmO) were also synthesized by varying the amount of peppermint oil. Paracetamol and carboplatin were selected as the sample drugs. Synthesized gels, hydrogels and organo-hydrogels were characterized by FTIR and SEM analysis. Additionally, swelling behaviors of the synthesized gels were investigated in different media (ID water, tap water, ethanol, acetone, ethanol/ID water (1:1), acetone/ID water (1:1) and gasoline) and at different pHs. Moreover, it was determined that organo-hydrogels were blood compatible and had antioxidant properties based on hemolysis, blood clotting and antioxidant analysis. Therefore, the release of paracetamol (a known antipyretic-painkiller, recommended and used in the treatment of Covid-19) and carboplatin (widely used in cancer treatment) were studied. Evidently, as the amount of PMO oil increases, the -OH groups in organo-hydrogels will increase and the chemical and physical bonding rates will increase; therefore it was observed that increasing peppermint oil in the organo-hydrogels structure to 0.3 mL stimulated the release of the drugs. For instance, maximum paracetamol release amount from p(AG-g-PmO) and p(AG-m-PmO) organo-hydrogels was calculated to be 72.3% at pH 7.4 and 69.8% at pH 2.0, respectively. The maximum carboplatin release amount from p(AG-g-PmO) and p(AG-m-PmO) organo-hydrogels was calculated to be 99.7% at pH 7.4 and 100% at pH 7.4, respectively. It was concluded that the synthesized organo-hydrogels might easily be used as drug carrier and controlled drug release materials.
Keywords: Agar, Carboplatin, Controlled release, Organo-hydrogel, Paracetamol, Peppermint oil
Graphical abstract
Highlights
-
•
Novel organo-hydrogels were synthesized using agar, glycerol and peppermint oil for drug carrier and controlled release.
-
•
Biocompatibility and antioxidant properties of organo-hydrogels were investigated.
-
•
Covid-19 and cancer sensitive drugs (Paracetamol and Carboplatin) were accomplished.
-
•
The superior properties of the synthesized organo-hydrogels make them useful in biomedical, pharmaceutical and drug delivery systems applications.
1. Introduction
Controlled release systems have been used in many fields, especially in recent years. The main areas are the pharmaceutical industry, medicine, cosmetics and environment. These release systems enable the release of the active substance at the preferred conditions and speed and at same time can be used with natural or synthetic polymers, which are selected according to the application place and purpose Especially, embedding, physical absorption, hydrogen bonding and electrostatic interactions play an active role between the active substance to be released and the support material [1]. Therefore the interaction of the active substances to be selected with the appropriate polymers, and the release of these substances from organo-hydrogels, which are polymeric material, are among popular topics recently [[2], [3], [4]]. Organo-hydrogels consist of low molecular weight materials or polymeric materials that are classified as physical and chemical organo-hydrogels. Chemical polymeric organo-hydrogels are three-dimensional macromolecular structures that absorb water and organic solvents which are more suitable for use in a variety of research including chemistry, biotechnology, medicine and pharmaceuticals [3,[5], [6], [7], [8]].
The peppermint (Mentha species) plant is included in the Lamiaceae family and contains essential oils such as peppermint essential oil, menthol, pulegon, p-menthon, isopulegon, verbenone, p-pinene, limonene, a-terpineol, b-bourbonen and menthyl acetate [9]. Medically, peppermint contains active ingredients which affect spasm and degassing, asthma, flu, bronchitis and cough blocker, migraine, insomnia and dizziness, as well as vitamin C, vitamin A and manganese and acts as an antimicrobial and diuretic. Vitamin A is an antioxidant and is important for manganese, fat and protein metabolism while helping prevent cancer, while Vitamin C helps reduce the damage from free radicals in the body. Different mint types have been used both as a folk remedy and in the pharmaceutical, food, perfumery, cosmetics and industry since ancient times because of various physiological effects in people, such as antimicrobial, antispasmodic, choleretic, and carminative properties [[9], [10], [11], [12]]. Medicines containing active ingredients from peppermint include colpermin (187 mg Menthol), Salonpas (3% menthol), Camfolin (10%), and Otrivine menthol (0.10%) [13,14]. Essential oils, which are among the various active ingredients in peppermint species, are used in the polymer and industrial fields [[15], [16], [17], [18], [19], [20], [21]].
Paracetamol (acetaminophen) is a drug active substance that is used as an alternative drug in aspirin-sensitive patients and has antipyretic effect. Although its analgesic effect is mild compared to new generation analgesics, it has almost no side effects in the gastrointestinal tract, is reliability and is safe for use in pregnant women, which ensures that paracetamol is always at the forefront as a classic analgesic [22]. Many publications recommend and apply antipyretic therapy such as paracetamol for first-line treatment of fever caused by COVID-19. Prolonged release of paracetamol may be an alternative drug for first-line treatment of fever caused by COVID-19 [[23], [24], [25]].
Carboplatin cis-Diammine (1,1-cyclobutanedicarboxylato) platinum (II) is a 2nd generation platinum compound. It is designed to stop the growth of cancer cells and kill them. It is effective on carboplatin DNA. Adenine and guanine are linked to the DNA from the N-7 position by a covalent ligament. As a result of the formation of DNA addition products, DNA synthesis and transcription are inhibited and the cell cannot divide. Binding to DNA and cytoplasmic proteins can result in cytotoxic effects. Broad-spectrum carboplatin is a frequently used chemotherapeutic agent for the treatment of childhood cancers. It is used for the treatment of osteosarcoma, hepatoblastoma, neuroblastoma, germ cell tumors, central nervous system tumors, head and neck cancers [26,27].
In fact, the main purpose of this research is to synthesize organo-hydrogels in a novel form that has the properties of both organogels and hydrogels. At the same time, the swelling behavior of these organo-hydrogels in different solvent environments, biocompatibility properties, and their effects on the release of the drug active substance were examined and enhanced. In this study, organo-hydrogels containing agar-based peppermint oil were synthesized using two different crosslinkers, MBA and GA. Characterization of synthesized gel, hydrogels and organo-hydrogels was carried out with FTIR and SEM. The swelling properties were examined in DI water, tap water, ethanol, acetone, ethanol/water, acetone/water, and gasoline and the usability of polymers as an effective sorbent was investigated. Additionally, the fundamental purpose of the study to investigate the ability of the synthesized gel, hydrogels and organo-hydrogels as antioxidants with blood clotting and hemolysis properties and their potential use as paracetamol and carboplatin carrier/delivery systems.
2. Materials and methods
2.1. Reagents
Glycerol (G), agar (99%), N, N, methylenebisacrylamide (MBA, 99%), glutaraldehyde (GA, 25% v/v), ethanol, acetone, calcium chloride (CaCl2), sodium hydroxide (NaOH) and hydrochloric acid (HCl) (36.5–38% v/v) were purchased from Sigma; ammonium persulfate (APS) (98%) was purchased from Merck. In terms of analytical grade, all reagents were of the highest cleanliness available, and they were used without additional purification. Peppermint oil (PmO), gasoline, paracetamol and carboplatin were procured from local suppliers. Distilled water (DI, 18.2 MΩ cm; Human I) was also employed from the beginning to the end of this study.
2.2. Experimental procedures
2.2.1. Agar-Glycerol-based gels, hydrogels and organo-hydrogels synthesis
Agar-Glycerol-based gel and hydrogels were synthesized via free radical polymerization in emulsion according to the preparation method given in Table 1 [8]. Briefly, gel and hydrogel were synthesized as follows with batch polymerization reactions were conducted in 20 mL flasks. Agar (2% w/v) solution was prepared by dissolving agar in water at 80 °C. Firstly, 2 mL of agar solution and 0.04 mL of glycerol were added to the 20 mL flask and homogenized by vigorous mixing (at 2500 rpm and 15 min). Secondly, MBA (0.1%) or glutaraldehyde reagent (0.5 mL of glutaraldehyde (25% v/v) + 0.5 mL of ethanol +0.1 mL of hydrochloric acid) was added as crosslinker and further homogenized. In this study, the polymer with no cross-linker used during synthesis was named gel and polymer synthesized using crosslinker was named hydrogel [28]. Gel and hydrogel compositions are given in Table 2 . Finally, the polymerization reaction was initiated by the addition of the initiator solution APS (0.1 mol% in proportion to total monomer amount) in 100 μL DI water. Reaction temperatures were maintained at 25 °C with a temperature-controlled hot plate. Immediately afterwards, the solution was transferred into a plastic petri with 35 mm diameter and was allowed to polymerize for 4 h. Obtained AG gels were stored at 25 °C for further uses.
Table 1.
Codes of different organo-hydrogel.
| Organo-hydrogel | Code |
|---|---|
| Agar-glycerol | AG |
| Poly (agar-glycerol)/MBA | p(AG-m) |
| Poly (agar-glycerol)/GA | p(AG-g) |
| Poly (agar-co-glycerol-co-peppermintoil)/MBA-1 | p(AG-m-PmO)1 |
| Poly (agar-co-glycerol-co-peppermintoil)/MBA-2 | p(AG-m-PmO)2 |
| Poly (agar-co-glycerol-co-peppermintoil)/MBA-3 | p(AG-m-PmO)3 |
| Poly (agar-co-glycerol-co-peppermintoil)/GA-1 | p(AG-g-PmO)1 |
| Poly (agar-co-glycerol-co-peppermintoil)/GA-2 | p(AG-g-PmO)2 |
| Poly (agar-co-glycerol-co-peppermintoil)/GA-3 | p(AG-g-PmO)3 |
Table 2.
Compositions and codes of different organo-hydrogel.
| Agar | Glycerol | PmO | Crosslinker | Code |
|---|---|---|---|---|
| 2% 2 mL | 0.04 mL | – | – | AG |
| 2% 2 mL | 0.04 mL | – | MBA | p(AG-m) |
| 2% 2 mL | 0.04 mL | – | GA | p(AG-g) |
| 2% 2 mL | 0.04 mL | 0.1 mL | MBA | p(AG-m-PmO)1 |
| 2% 2 mL | 0.04 mL | 0.2 mL | MBA | p(AG-m-PmO)2 |
| 2% 2 mL | 0.04 mL | 0.3 mL | MBA | p(AG-m-PmO)3 |
| 2% 2 mL | 0.04 mL | 0.1 mL | GA | p(AG-g-PmO)1 |
| 2% 2 mL | 0.04 mL | 0.2 mL | GA | p(AG-g-PmO)2 |
| 2% 2 mL | 0.04 mL | 0.3 mL | GA | p(AG-g-PmO)3 |
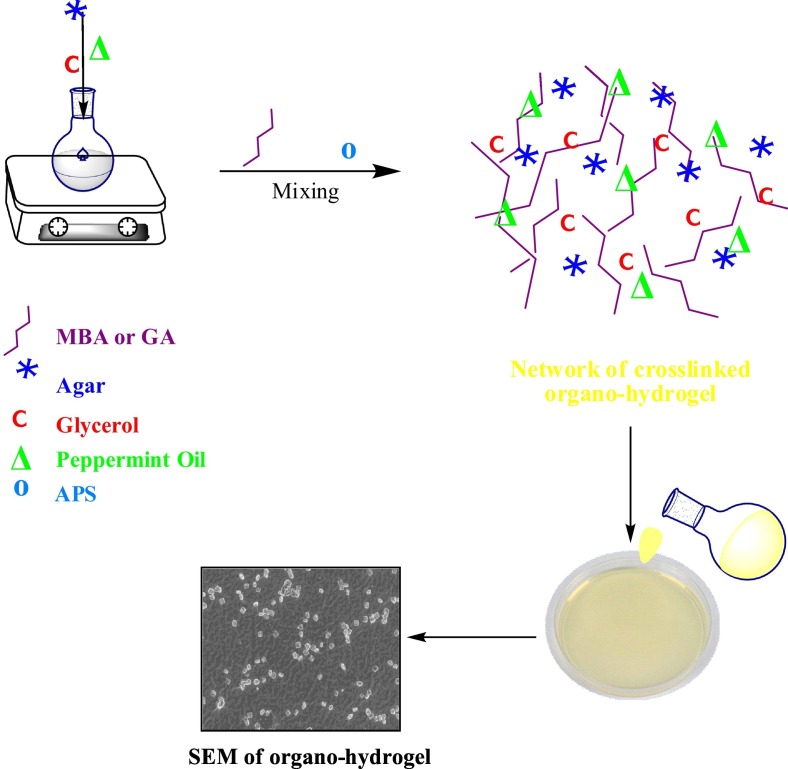
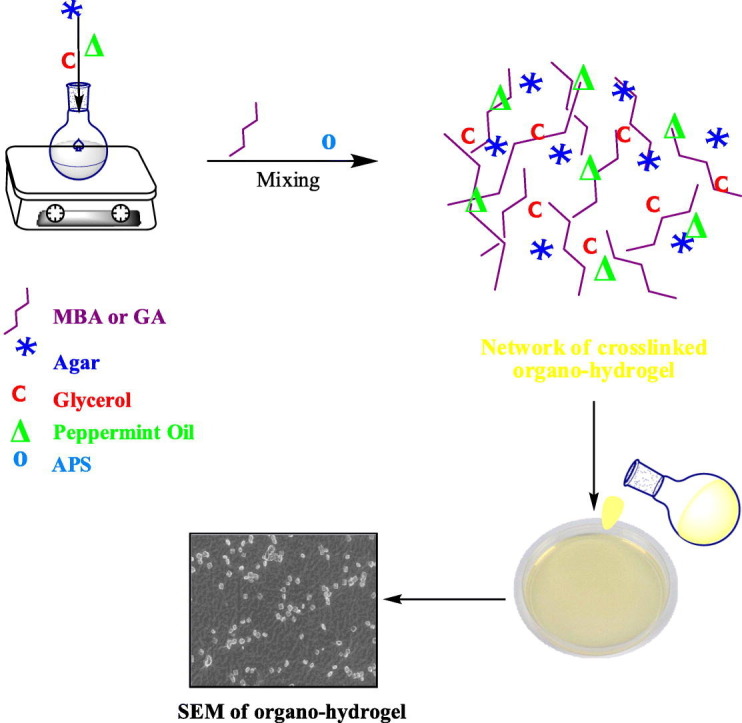
For the preparation of organo-hydrogels, the above method was used (Table 1). Briefly, firstly, 2 mL of agar solution and 0.04 mL of glycerol were added to a 20 mL flask and vigorously mixed (at 2500 rpm) to obtain a homogeneous solution. Secondly, different amounts of peppermint oil (0.1, 0.2 and 0.3 mL) were added in the reaction mixture, and stirred at 800 rpm for 15 min until a homogeneous mixture was formed. Thirdly, MBA (0.1%) or glutaraldehyde reagent was added as crosslinker and further homogenized. Organo-hydrogel compositions are given in Table 2. Finally, the polymerization reaction was initiated with the addition of the initiator solution APS in 100 μL DI water. Reaction temperatures were maintained at 25 °C with a temperature-controlled hot plate. Then, the solution was transferred to a plastic petri with 35 mm diameter and was allowed to polymerize. These preparation steps are schematically shown in Fig. 1 . The gel, hydrogels and organo-hydrogels were kept in DI water and washing water was renewed every 2 h for 8 h to eliminate unreactive monomers. The synthesized gel, hydrogels, p(AG-m-PmO) and p(AG-g-PmO) organo-hydrogels were dried in the oven at 40 °C until a constant weight was achieved and stored at 4 °C for further use.
Fig. 1.
Synthesis and schematic presentation of organo-hydrogels.
2.2.2. Organo-hydrogel synthesis containing paracetamol and carboplatin
Paracetamol and carboplatin drugs were added directly into the organo-hydrogels during their synthesis [29]. The synthesis of drug-loaded organo-hydrogels was the same as the synthesis procedure for the organo-hydrogels described above. In addition to the reaction mixture mentioned above, 50 ppm 1 mL paracetamol/carboplatin drug was added. Thus, drugs from the -OH group in the structure of paracetamol and the -NH3 group of carboplatin are physically bound to organo-hydrogels and drug-loaded organo-hydrogels were synthesized.
2.2.3. Swelling analysis
Swelling assays were carried out with certain amounts of dried gel, hydrogels, and organo-hydrogels placed in water, ethanol, acetone, ethanol/ID water (1:1), acetone/ID water (1:1) and gasoline and at different pHs (2.0–12.0) for 24 h. Swelling tests were performed at room temperature of 25 °C [7,30].
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR) analysis
Fourier Transform Infrared Spectroscopy (FTIR) (Thermo, model Nicolet iS10 Spectrometer, USA) was used for FTIR analysis. The analysis was performed to investigate the functional groups and possible interactions between all chemicals utilized to synthesize the gel, hydrogels and organo-hydrogels. The gel, hydrogels, and organo-hydrogels were crushed to obtain a powder then placed on the ATR sample plate. The spectral range was investigated from 4000 to 650 cm−1 with a resolution of 4 cm−1.
2.2.5. Scanning Electron Microscopy (SEM) analysis
SEM Ultra Field emission-Energy Dispersive Spectroscopy X-ray (ZEISS, model FESEM-EDX, Germany) was used to monitor the morphological properties of the gel, hydrogels and organo-hydrogels. The samples were scanned using a voltage of 15 and 20 kV. The surfaces of the gel, hydrogels, and organo-hydrogels were coated with gold before analysis.
2.2.6. Blood clotting and hemolysis analysis
Firstly, organo-hydrogels, AG, p(AG-m), and p(AG-g) were separately added to flat bottom falcon tubes and placed in a shaking water bath (swb) at 37.5 °C. Then, freshly prepared 0.24 mL of 0.2 M calcium chloride solution and 3 mL blood was added to each falcon tube. The solution containing 0.27 mL calcium chloride blood mix was added dropwise to the surface of the gel, hydrogels, and organo-hydrogels. Next the samples were incubated for 10 min in a swb at 37.5 °C. Afterwards, 10 mL of ID water was slowly added to the samples and diluted with water. Then the samples were centrifuged at 545 rpm for a minute and the supernatant portion of the samples was carefully separated. The supernatant was then diluted by adding 40 mL of ID water. As the control group, 0.27 mL of calcium chloride containing blood was added into 50 mL of ID water. The prepared solutions were incubated for 1 h in a swb at 37.5 °C. At the end of the period, the absorbance values of the samples were measured at 542 nm using a UV–Vis spectrophotometer (Thermo Genesys 10S). The blood clothing index (BCI) of the samples was calculated using Eq. (1).
| (1) |
where Asamples shows the absorbance value of organo-hydrogels in contact with blood samples and Actrl shows the absorbance value of blood samples diluted in 50 mL ID water.
In hemolysis experiments, firstly, gel, hydrogels, and organo-hydrogels weighing 50 mg were added to 9.8 mL of salt solution (0.9%) and incubated in a swb at 37.5 °C. Then 2 mL of blood was added to 2.5 mL of saline solution (0.9%). Later, 0.2 mL blood solution was intently added to 9.8 mL saline solution (0.9%) containing gel, hydrogels and organo-hydrogel. The samples were then incubated for 1 h in a swb at 37.5 °C. In control groups 9.8 mL saline and 9.8 mL ID water were used, respectively. Then, the samples were centrifuged at 9000 rpm for 5 min, the healthy red blood cells were precipitated and the supernatant portions of the samples were separated. Finally, the absorbance values of the samples at 542 nm wavelength were measured to determine the rate of hemoglobin released from hemolysis of erythrocytes. The degree of hemolysis of gel, hydrogels and organo-hydrogels was calculated according to Eq. (2).
| (2) |
that Asample is the absorbance value of organo-hydrogels in contact with blood samples, Apositive control is the absorbance value measured using 0.2 mL blood sample and distilled water and Anegative control is the absorbance value measured using 0.2 mL blood sample and saline [[31], [32], [33]].
2.2.7. Antioxidant analysis
To evaluate the antioxidant activity, Folin-Ciocalteu [[34], [35], [36]] and ABTS [35,37] methods were applied as explained in the literature.
2.2.8. Paracetamol and Carboplatin release studies
Synthesized drug-loaded gel, hydrogels and organo-hydrogels were used as controlled release systems for paracetamol (painkiller) and carboplatin (an anti-cancer agent), which are frequently used in the medical field. The gel, hydrogels and organo-hydrogels loaded with a certain amount (50 ppm) of paracetamol, were used in 50 mL at four different pH values (2.0, 5.5 7.4 and 8.0 pH) for paracetamol release. Carboplatin release [38] was performed in 50 mL 7.4 pH solution media. Released paracetamol and carboplatin quantities were calculated on calibration curves prepared at 244 nm and 210 nm wavelength with a UV–Visible region spectrophotometer, respectively. Each measurement was performed with 3 replicates and averaged with standard deviation values. The most common models, which are zero order (ZoM) (Eq. (3)) [39,40], first order (FoM) (Eq. (4)) [[39], [40], [41]], Higuci (HM) (Eq. (5)) [42] and Korsmeyer-Peppas (KPM) (the power law) (Eq. (6)) [39], were used to identify the release kinetics. These equations are given in Table 3 .
Table 3.
Mathematical models for drug release.
| Model | Mathematical equation | Release mechanism | Codes |
|---|---|---|---|
| Zero order kinetic model | Cr = C0 − k0. t | Diffusion Mechanism | ZoM |
| First order kinetic model | lnCr = ln C0 − k1. t | Fick's first law, diffusion mechanism | FoM |
| Higuchi Model | Diffusion medium based mechanism in Fick's first law | HM | |
| Korsmeyer-Peppas Model | Semi empirical model, diffusion-based mechanism | KPM |
Cr is concentration of urea release in time t (mg/L); C0 is the initial concentration of urea in the solution (most times, C0 = 0) (mg/L); k0 is the zero order release constant expressed in units of concentration/time (mg/(L.min)); t is time (min); k1 is the first order release constant (1/min); C∞ is concentration of fertilizer release in equilibrium (mg/L); kH is Higuchi release rate constant (1/); kKP is Korsmeyer-Peppas release rate constant; n is release exponent which is indicative of the transport mechanism (Mt/M∞ < 0.6 should only be used.
3. Results and discussion
In the synthesis of AG gel, the agar solution and glycerol were bonded to each other with weak hydrogen bonds without any crosslinker [28]. The structure obtained by hydrogen-strong bridge bonds between the –NH and -OH groups on the agar molecule and the -OH groups on the glycerol was called gel. In the synthesis of p(AG-m) and p(AG-g) hydrogels, the structure obtained by adding crosslinking agent to the agar solution and glycerol medium was called hydrogel [43,44]. In the synthesis of p(AG-m-PmO) and p(AG-g-PmO) organo-hydrogels, strong hydrogen bonds were formed between the -OH groups on peppermint and glycerol molecules and the -OH groups in the agar structure. At the same time, the crosslinking agent was added to the reaction medium and crosslinking was provided between peppermint oil, agar and glycerol [[45], [46], [47]]. Thus, the structure including both hydrogen bonds and cross-linked was called organo-hydrogel. By increasing the amount of peppermint oil added to the structure, both physical and chemical bonding was strengthened. In the synthesis of drug-loaded organo-hydrogels, drugs were added to the reaction medium in the final stage of the synthesis. These drugs were physically attached or trapped in the structure of organo-hydrogels. These gel, hydrogels and organo-hydrogels were subjected to the following analyses.
3.1. Swelling properties
The swelling ability of a polymeric gel is determined by the interaction between the functional groups in its structure and the solvent used. Push and pull between polymer chains is affected by non-covalent electrostatic, hydrophobic, Van der Waals, and hydrogen bonding. Hydrophobic interactions are physical crosslinker interactions and this affects the swelling behavior of the gel [48].
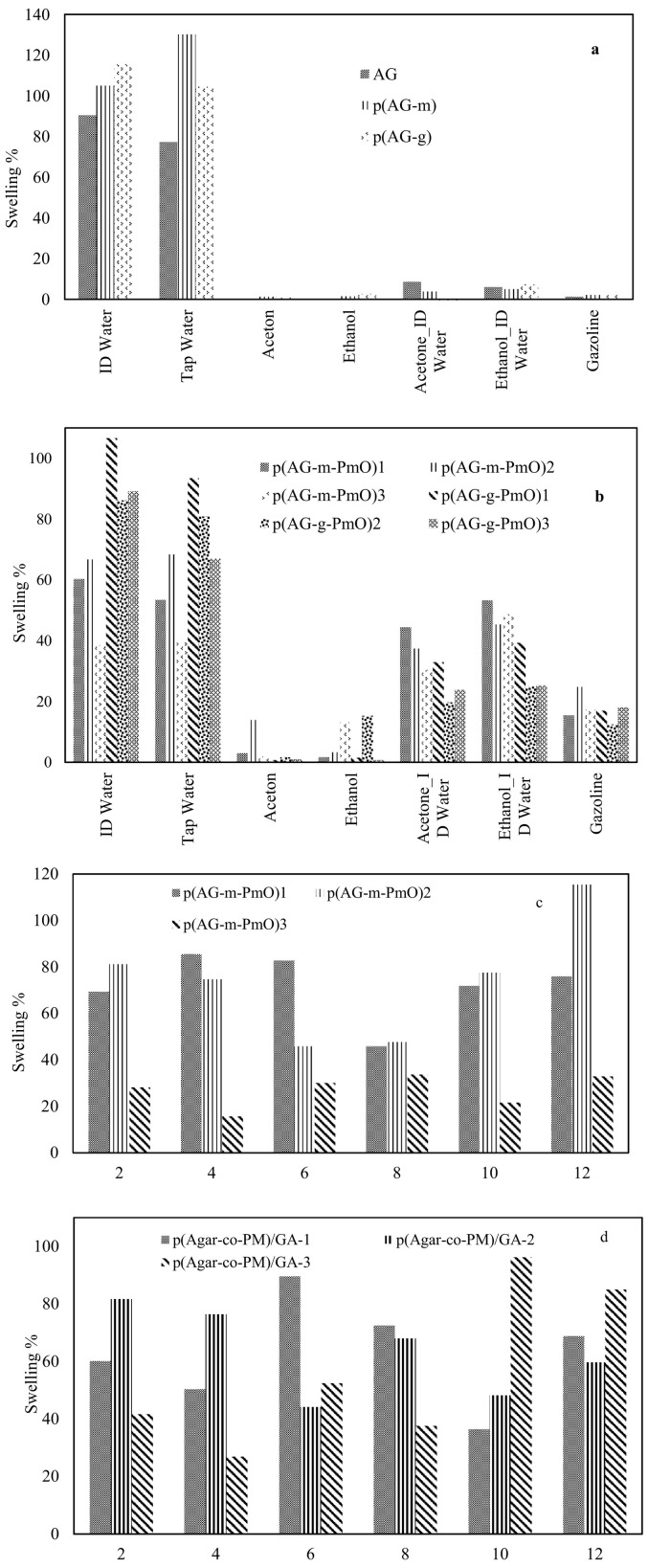
Swelling of gel, hydrogels and organo-hydrogels in different media such as water-organic solvent mixtures can be controlled by the solvent composition as well as crosslinker dosage. The change in percent swelling of gel, hydrogels and organo-hydrogels as a function of solvent concentration in water-organic solvent mixtures are shown in Fig. 2 . After the AG gel was crosslinked, the ID water absorption capacity increased by 16% for p(AG-m) and 27% for p(AG-g), and tap water absorption capacity increased by 35% for p(AG-g) and 68% for p(AG-m). When organo-hydrogels were compared among themselves, p(AG-g-PmO)1 organo-hydrogel has higher water absorption capacity than other organo-hydrogels and has 106.6% swelling ratio in ID water. It was observed that the S% of organo-hydrogels decreased with the increase in essential oil ratio in the organo-hydrogel composition. These observations are consistent with the literature[49,50].
Fig. 2.
Percent swelling degree of the (a) AG, p(AG-m), p(AG-g) and (b) organo-hydrogels with time in ID water, tap water, ethanol, acetone, ethanol/ID water (1:1), acetone/ID water (1:1) and gasoline. Swelling % of the (c) AG, p(AG-m), p(AG-g) and (d) organo-hydrogel as a function of 2.0–12.0 pH (pH was adjusted by the addition of 0.1 M HCl, 0.1 M NaOH).
A small amount of swelling was observed in acetone and ethanol media. This is attributed to the hydrophobic character in acetone and ethanol and the number of alkyl groups in the molecular structure. The hydrophobic property can be enhanced by increasing the alkyl groups in the organic molecule [51]. A small amount of swelling was observed in acetone and ethanol media. This is attributed to the hydrophobic character of p(AG-g-PmO) in acetone and ethanol and the number of alkyl groups in the molecular structure. The hydrophobic properties of synthesized organo-hydrogels can be enhanced by increasing the alkyl groups in the organic phase. Therefore, swelling values decreased compared to the swelling value in ID water with increasing acetone and ethanol concentrations in acetone - acetone/water (1:1) and ethanol - ethanol/water (1:1) solvents. This means that if the polarization of acetone and ethanol is lower than water, ionization in the environment decreases, and therefore the smaller swelling percent of organo-hydrogels can be explained.
When organo-hydrogels were evaluated in terms of cross-linkers, although GA cross-linked organo-hydrogels had higher swelling value in DI water and tap water than MBA cross-linked organo-hydrogels, the swelling values in ethanol, acetone, gasoline, water-acetone and water-ethanol media were low. When the swelling values in the solvents were evaluated according to the amount of essential oil contained in organo-hydrogels, the swelling values changed as the amount of essential oil increased.
The change in swelling properties in various media is due to different ion mobility in the medium of interest. At acidic and basic pH, the increase in ion concentrations causes high ionic strength, while increasing the ionic strength of the solution decreases the osmotic pressure difference between the organo-hydrogel and the medium [52,53]. It is not sensitive to pH since it does not contain an ionizable group in AG gel structure. It was observed that AG gel became sensitive to changes in pH values after being synthesized with MBA and GA crosslinkers. When PmO was added to the structure of the organo-hydrogels in different proportions, the number of ionizable groups in the structure of the organo-hydrogels increased and became sensitive to changes in different pH values. In other words, the anionic and cationic properties of the ionizable groups on organo-hydrogels increased and these organo-hydrogels showed different swelling behaviors at different pH values.
3.2. FTIR analysis
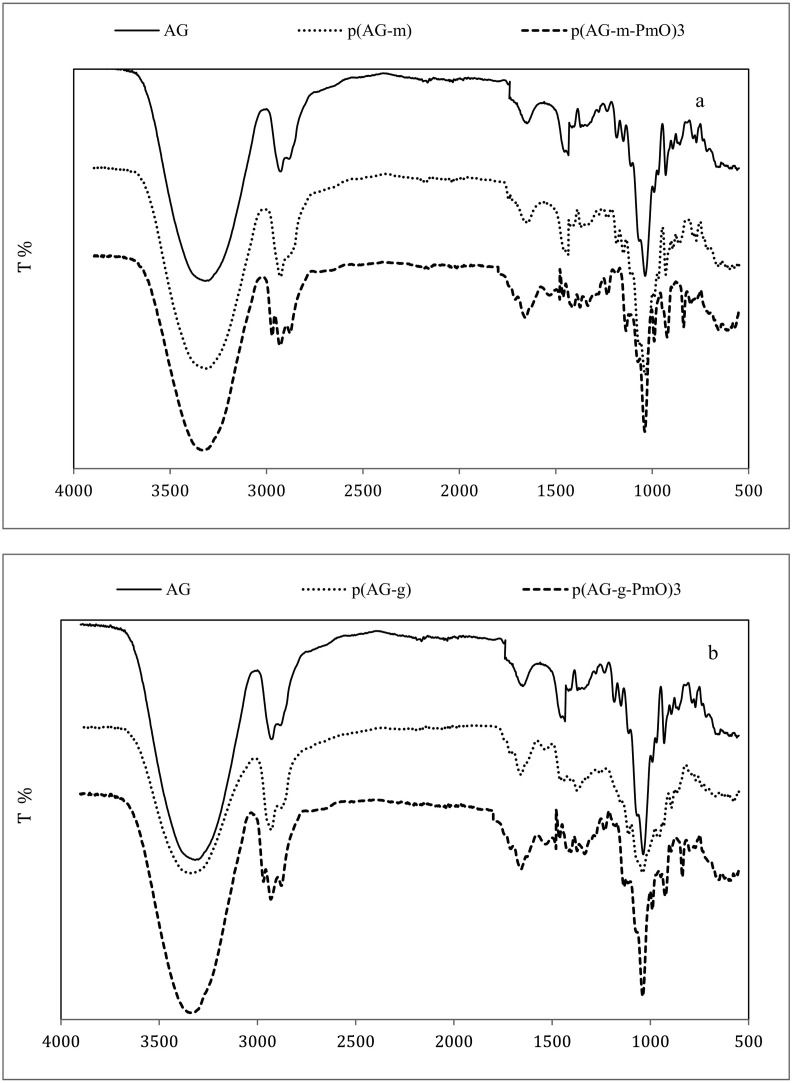
Organo-hydrogels, AG, p(AG-m), and p(AG-g) were prepared by free radical polymerization in emulsion media and the FTIR spectra are shown in Fig. 3 . Peaks which formed at 3400 cm−1, 2900 cm−1, 1643 cm−1, 1370 cm−1 and 1070 cm−1- 930 cm−1 wavelengths belonged to agar and these peaks are associated with -OH, methoxyl group, -NH, ester sulfate and anhydrous-galactose bonds, respectively [54,55]. Glycerol peaks formed at 3600–3000 cm−1, 2930 cm−1, 1637 cm−1, 1397 cm−1 and 1032 cm−1 wavelengths and these peaks belong to -OH, -C-H, -C-C, CH and C—O bonds, respectively [56]. Peppermint oil had a band peak at 3600–3000 cm−1 belonging to the vibrations of -OH groups, while bands at 1742 cm−1 and 1660 cm−1 represented C O vibrations. The peaks at 2922 cm−1 and 2853 cm−1 belong to the -CH3 and -CH2 groups and the peak at 1453 cm−1 belongs to the C H bands. The new bonds and structural diversity in organo-hydrogels demonstrated the existence of hydrogen-bond interactions. After the PmO was included in the structure of the organo-hydrogel, the incoming bands from characteristic aromatic compounds (such as 2922 cm−1 -2853 cm−1 and 1660 cm−1) [57] exhibited high density, and the peaks appeared to deepen or expand. Considering the peaks in the organo-hydrogel, the peaks at 2922 cm−1 -2853 cm−1 and 1653 cm−1 expanded and deepened, and the peak depth at 1037 cm−1 increased. The changes in these peaks indicate that PmO entered the structure of the organo-hydrogel.
Fig. 3.
FT-IR spectra of (a) AG, p(AG-m), and p(AG-m-PmO)3, (b) AG, p(AG-g) and p(AG-g-PmO)3 organo-hydrogels.
3.3. SEM analysis
The surface morphologies of the synthesized materials were monitored by SEM and are shown in Fig. 4a and b. Fig. 4a shows the surface image of p(AG-g-PmO) organo-hydrogels. As clearly seen, the intertwined mesh surface can be seen after polymerization was completed. Fig. 4b shows that as the peppermint oil content in p(AG-g-PmO) organo-hydrogel increased, the mesh structure opened and the oil globules penetrated better on and into the surface. The p(AG-g-PmO)3 organo-hydrogels surface image is intertwined on the surface and vice versa, the p(AG-m-PmO)3 organo-hydrogels surface is flatter with a background of fat globules.
Fig. 4.
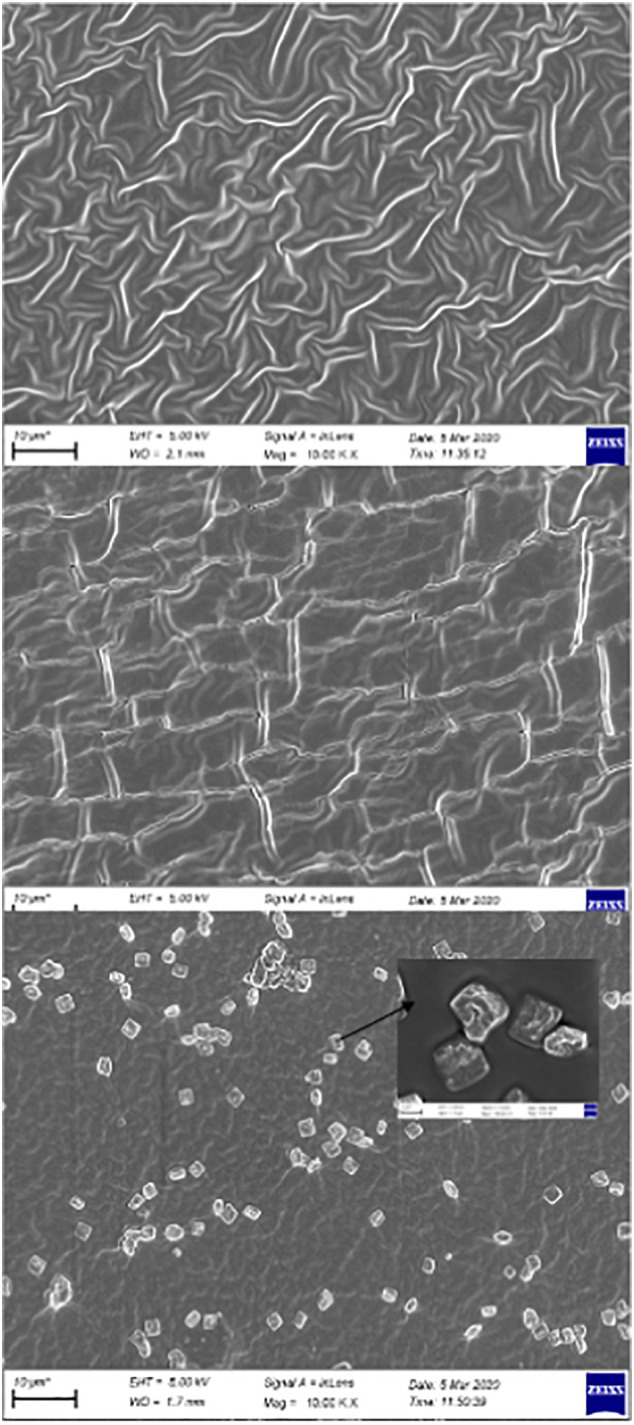
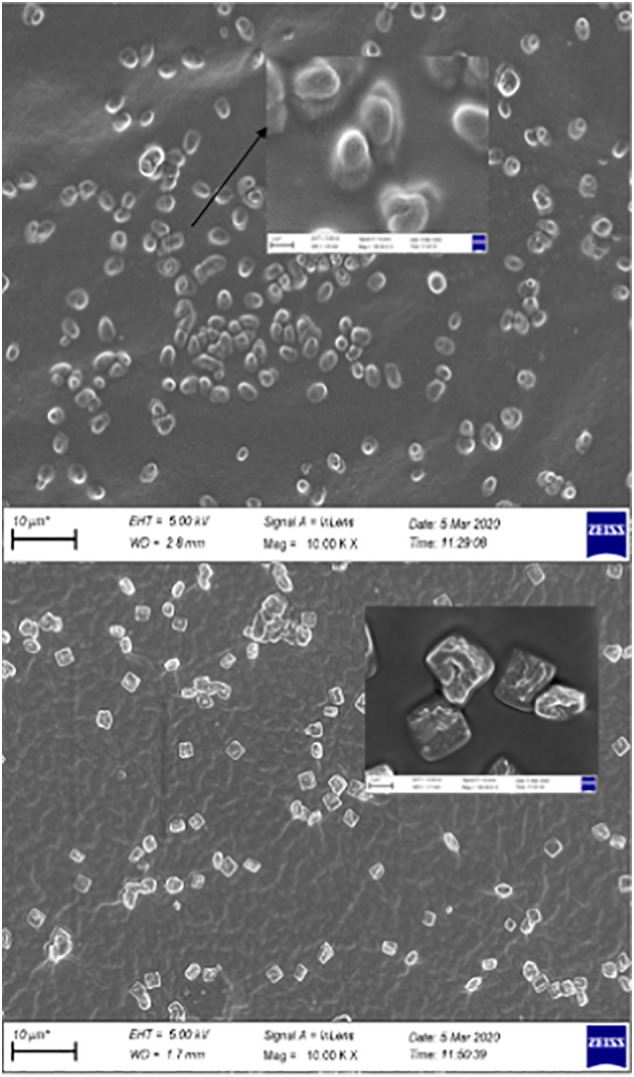
SEM images of (a) p(AG-g-PmO)1, p(AG-m-PmO)2, p(AG-m-PmO)3 and (b) (a) p(AG-m-PmO)3 and p(AG-g-PmO)3 organo-hydrogels.
3.4. Blood clotting and hemolysis analysis
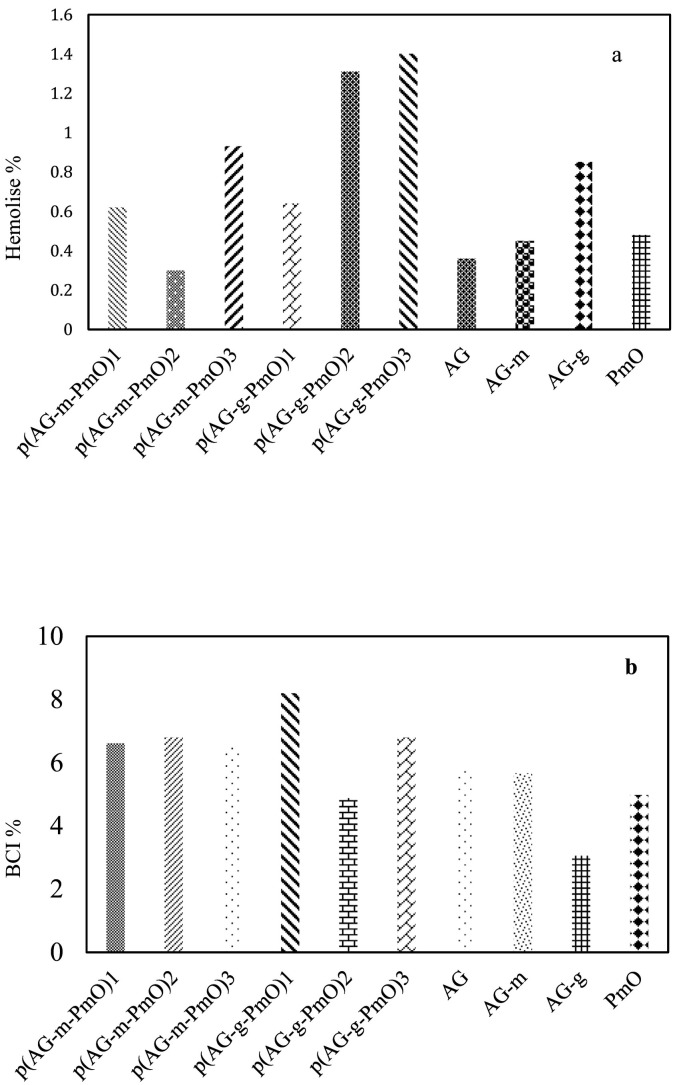
Biocompatibility means there is no reaction in tissue with the presence of the material. That is to say chemical interactions of materials and body fluids and whether the physiological consequences of this interaction harm the body. One focus of our study was how to eliminate and minimize unwanted interactions. In fact, it is desired and ideal that this property of organo-hydrogels can be determined by in vitro tests. For this reason, blood compatibility tests are important. In this study, hemolysis and blood clotting tests were performed to determine blood compatibility of organo-hydrogel [58,59]. Hemolysis was calculated for AG, p(AG-m), p(AG-g), PmO and organo-hydrogels at a concentration of 5 mg/mL and is given Fig. 5a. It is stated that the hemolysis rate is not hemolytic up to 5% [58,59] Therefore, it was concluded that organo-hydrogels were blood compatible at this rate. As organo-hydrogels were synthesized with peppermint oil, there was a slight increase in % hemolysis for organo-hydrogel, which increased from 0.3% to 1.4%, meaning organo-hydrogels containing essential oil did not have a negative effect on hemolysis.
Fig. 5.
Blood compatibility of AG, p(AG-m), p(AG-g) and organo-hydrogels (a) hemolysis (b) blood clotting.
Another method of evaluating the blood compatibility of organo-hydrogels is to determine the organo-hydrogel Blood Clotting Index (BCI). Therefore, the BCI's of organo-hydrogels are shown in Fig. 5b. In Fig. 5b, 5 mg/mL organo-hydrogel blood clotting indices were between 4.8% and 8.2%. For blood contact applications with these organo-hydrogel, the gel amount should be less than 5 mg/mL.
3.5. Antioxidant analysis
The antioxidant activity of PmO, p (AG-m), p(AG-g) and organo-hydrogels is given in Table 4 as gallic acid equivalent value. The PmO, p(AG-m), p(AG-g) and organo-hydrogels reduction capacity can be used to determine the antioxidant activity. When Table 4 is analyzed, as the concentration of the substance increases, the reduction power also increases due to the absorbents. When these values are considered, organo-hydrogels show higher antioxidant activity than other gels [9,10].
Table 4.
Total phenol content values.
| Total phenol values (mg) | |
|---|---|
| Substance | |
| p(AG-m-PmO)1 | 83 |
| p(AG-m-PmO)2 | 90 |
| p(AG-m-PmO)3 | 104 |
| p(AG-g-PmO)1 | 1575 |
| p(AG-g-PmO)2 | 1597 |
| p(AG-g-PmO)3 | 1626 |
| Oil | |
| Peppermint oil | 5818 |
3.6. Paracetamol and Carboplatin release studies
Environmentally sensitive systems release drugs in response to a change in the environmental conditions such as solvent, temperature and pH. Recently, multi-responsive polymers named smart polymers, have played a key role in such applications [3,60,61]. To perform drug release of paracetamol- and carboplatin-loaded organo-hydrogels, gastric, intestinal, oral blood and skin media were imitated in vitro. Thus, release analyses were conducted in different pH environments. All organo-hydrogels did not show the same behavior in every pH environment. For example, some organo-hydrogels shrink at the pH of the stomach, while they swell at intestinal pH and release the drug. For some organo-hydrogels, the opposite is true. Some organo-hydrogels release drugs at a pH of 5.5 on the skin surface, while others release drugs in body fluids at a pH of 7.4 [62].
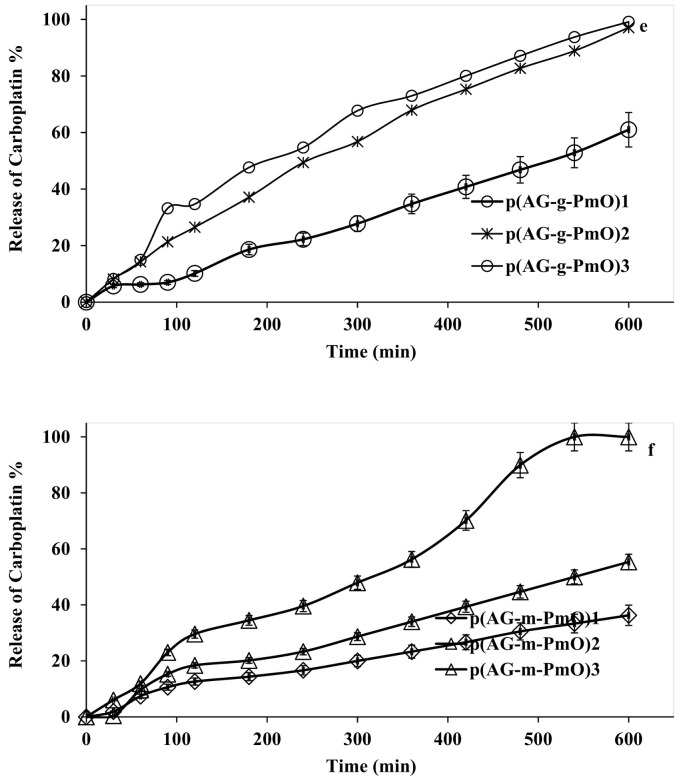
Another focus of our study was to investigate the paracetamol and carboplatin release capacities of the new biocompatible gel, hydrogels and organo-hydrogels. Paracetamol and carboplatin loading amounts in gel, hydrogels and organo-hydrogels were determined as 50 ppm. Fig. 6 shows the drug release behaviors of gel, hydrogels and organo-hydrogels at 37.5 °C. Due to the difference in concentration of the release event, while the solvent enters the gel, hydrogels and organo-hydrogel structure, the loaded drug switches to the solvent medium. The release analyses of paracetamol loaded on gel, hydrogels and organo-hydrogels were conducted separately at body temperature (37.5 °C), at different pH values (2.0, 5.5, 7.4, 8.0) and release results are given in Fig. 6. As seen in Fig. 6 (a-c), all organo-hydrogels released paracetamol slowly. Moreover, AG, p(AG-m) and p(AG-g) had maximum paracetamol release of 8.0% at pH 8.0, 7.8% at pH 2.0 and 8.2% at pH 2.0, respectively. The amount of paracetamol released from the p(AG-m-PmO) organo-hydrogel system is given in Table 5 . The highest paracetamol release amounts occurred at pH 2.0. When paracetamol release was compared between p(AG-m-PmO) organo-hydrogels, it appeared that the minimum release was from the p(AG-m-PmO)1 organo-hydrogel. The amounts of paracetamol released from the p(AG-g-PmO) organo-hydrogel system are given in Table 5. The highest paracetamol release rates occurred at pH 7.4 and pH 8.0. When paracetamol release was compared between p(AG-g-PmO) organo-hydrogels, it was shown that the minimum release was from p(AG-g-PmO)1 organo-hydrogel. The highest cumulative paracetamol release from organo-hydrogels was observed in organo-hydrogels synthesized with GA crosslinker. Paracetamol release can be controlled by crosslinker type and changing the amount of peppermint oil in the organo-hydrogels.
Fig. 6.
Release behavior for paracetamol at (a) 2.0 pH (b) 5.5 pH (c) 7.4 pH (d) 8.0 pH from organo-hydrogels and carboplatin (e) p(AG-m-PmO) 7.4 pH and (f) p(AG-g-PmO) organo-hydrogels (The first 600 min of paracetamol and carboplatin release are given in the graph).
Table 5.
Paracetamol and carboplatin % release values.
| Paracetamol |
Carboplatin |
|||||
|---|---|---|---|---|---|---|
| pH 2.0 |
pH 5.5 |
pH 7.4 |
pH 8.0 |
pH 7.4 |
||
| Release % | Release % | |||||
| AG | 2.3 | 3.5 | 3.1 | 8.1 | AG | 1.8 |
| p(AG-m) | 7.8 | 4.4 | 3.4 | 3.9 | p(AG-m) | 1.6 |
| p(AG-g) | 8.2 | 6.3 | 5.2 | 6.8 | p(AG-g) | 2.5 |
| p(AG-m-PmO)1 | 14.3 | 23.8 | 17.5 | 28.6 | p(AG-m-PmO)1 | 54.8 |
| p(AG-m-PmO)2 | 25.0 | 26.7 | 33.3 | 27.8 | p(AG-m-PmO)2 | 97.8 |
| p(AG-m-PmO)3 | 69.8 | 50.0 | 37.5 | 47.9 | p(AG-m-PmO)3 | 100.0 |
| p(AG-g-PmO)1 | 37.0 | 38.9 | 34.1 | 53.7 | p(AG-g-PmO)1 | 99.4 |
| p(AG-g-PmO)2 | 46.9 | 29.6 | 53.2 | 67.9 | p(AG-g-PmO)2 | 98.8 |
| p(AG-g-PmO)3 | 58.3 | 60.3 | 72.3 | 57.1 | p(AG-g-PmO)3 | 93.8 |
According to Fig. 6 and Table 5, the highest carboplatin release between gels, hydrogels and organo-hydrogels was observed from organo-hydrogels. The results showed that there was a high rate of carboplatin release from organo-hydrogels, both synthesized by MBA and synthesized by GA agent. AG, p(AG-m) and p(AG-g) maximum carboplatin release was 1.7%, 1.6%, and 2.5% at pH 7.4, respectively. The maximum carboplatin release was found to be 100% for p(AG-m-PMO)3 and 99.4% for p(AG-g-PMO)1 (Table 5). When the release results were evaluated according to the amount of peppermint oil, it was clearly observed that the amount of release increased as the amount of peppermint oil increased in organo-hydrogels cross-linked with MBA. It was determined that the increase in the amount of peppermint did not affect the release amount from organo-hydrogels connected with GA reagent. It was evident that the p(AG-g-PmO)3 (paracetamol) and p(AG-m-PmO)3 (carboplatin) organo-hydrogels had higher release capacity than the other organo-hydrogels. Moreover some of the other reported material in the literature include HC50SA50Ca0.5 (40% paracetamol), HC50SA50Zn0.5 (30% paracetamol), HC50SA50Cu0.5 (38% paracetamol), HC75SA25 (80% paracetamol), HC50SA50 (90% paracetamol) and HC25SA75 (100% paracetamol) [63], carboxylated lignin (70% paracetamol), lignin tablet (70% paracetamol) and non-lignin tablet (70% paracetamol) [64], and pure drug (100% Carboplatin), CP-loaded PEGylated MWCNTs (95% carboplatin) and enteric-coated PEGylated MWCNTs (95% carboplatin) [26] so on.
The release kinetics of the synthesized organo-hydrogels were investigated to obtain the most appropriate release model for the data obtained. Mathematical models play an important role in predicting the release mechanism in organo-hydrogels and provide more general information to improve their mechanisms. Common kinetic model equations were applied to determine the release kinetics of the drug from organo-hydrogels (Table 6, Table 7 ). The drug release data from organo-hydrogels were processed with these kinetic models to investigate release kinetics and systems. Given the best R-square values, the most suitable model was chosen to symbolize drug release behavior. The paracetamol release from organo-hydrogels revealed a very high R-square with HM and KPM. The n values calculated with the KPM method to determine the paracetamol release mechanisms of organo-hydrogels are given in Table 6. When the n values of paracetamol release were examined, it was determined that other organo-hydrogels excluding p(AG-m-PMO)3 organo-hydrogel released in accordance with the Fick type release law. In the Fick type release mechanism, n values are less than 0.45 and release is controlled by the diffusion rate of water into the organo-hydrogel. Carboplatin release from organo-hydrogels revealed a very high R-square with the ZoM, HM and KPM. The n values calculated with KPM for organo-hydrogels are shown in Table 7. When n values of carboplatin release are examined, it was determined that all organo-hydrogels (excluding p(AG-g-PMO)1 organo-hydrogel) released according to the non-Fick type release law. In the non-Fick release mechanism, n values range from 0.45 to 0.89, and release is controlled by both diffusion and relaxation of the organo-hydrogel. The calculated n value for p(AG-g-PMO)1 organo-hydrogel was greater than 1 and release is controlled by the relaxation of the organo-hydrogel.
Table 6.
Release kinetic and mechanism of Paracetamol release.
| p(AG-m-PmO)1 | 2.0 | 5.5 | 7.4 | 8.0 | p(AG-g-PmO)1 | 2.0 | 5.4 | 7.4 | 8.0 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZoM | Co | 1.692 | 1.470 | 1.512 | 1.856 | ZoM | Co | 1.651 | 1.651 | 2.113 | 2.552 |
| ko | −0.003 | −0.002 | −0.006 | −0.006 | ko | −0.008 | −0.007 | −0.007 | −0.013 | ||
| R2 | 0.887 | 0.822 | 0.713 | 0.751 | R2 | 0.822 | 0.846 | 0.658 | 0.800 | ||
| FoM | Co | 5.208 | 3.662 | 1.640 | 1.571 | FoM | Co | 0.686 | 0.316 | 2.004 | 2.203 |
| k1 | −0.009 | −0.009 | −0.018 | −0.002 | k1 | −0.002 | −0.002 | −0.008 | −0.007 | ||
| R2 | 1.650 | 1.298 | 0.784 | 0.568 | R2 | 0.704 | 0.810 | 0.718 | 0.913 | ||
| HM | kh | 0.034 | 0.028 | 0.008 | 0.028 | HM | kh | 0.225 | 0.911 | 0.036 | 0.037 |
| R2 | 0.997 | 0.959 | 0.955 | 0.959 | R2 | 0.990 | 0.031 | 0.991 | 0.993 | ||
| KPM | n | 0.238 | 0.261 | 0.392 | 0.271 | KPM | n | 0.391 | 0.472 | 0.313 | 0.422 |
| kkp | 0.158 | 0.157 | 0.091 | 0.146 | kkp | 0.083 | 0.044 | 0.105 | 0.056 | ||
| R2 | 0.983 | 0.891 | 0.841 | 0.935 | R2 | 0.844 | 0.889 | 0.872 | 0.934 | ||
| p(AG-m-PmO)2 | 2.0 | 5.5 | 7.4 | 8.0 | p(AG-g-PmO)2 | 2.0 | 5.5 | 7.4 | 8.0 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZoM | Co | 1.452 | 1.893 | 1.494 | 1.448 | ZoM | Co | 4.419 | 1.229 | 2.143 | 2.374 |
| ko | −0.004 | −0.002 | −0.003 | −0.005 | ko | −0.005 | −0.004 | −0.004 | −0.009 | ||
| R2 | 0.752 | 0.705 | 0.728 | 0.726 | R2 | 0.793 | 0.895 | 0.831 | 2.552 | ||
| FoM | Co | 5.576 | 10.318 | 1.975 | 2.299 | FoM | Co | 1.488 | 0.347 | 2.143 | 2.851 |
| k1 | −0.007 | −0.007 | −0.001 | −0.001 | k1 | −0.001 | −0.002 | −0.004 | −0.007 | ||
| R2 | 0.935 | 0.946 | 0.885 | 0.618 | R2 | 0.704 | 0.803 | 0.831 | 0.962 | ||
| HM | kh | 0.039 | 0.012 | 0.024 | 0.028 | HM | kh | 0.017 | 0.905 | 0.038 | 0.038 |
| R2 | 0.996 | 0.986 | 0.952 | 0.959 | R2 | 0.997 | 0.025 | 0.992 | 0.992 | ||
| KPM | n | 0.143 | 0.207 | 0.378 | 0.431 | KPM | n | 0.170 | 0.473 | 0.238 | 0.333 |
| kkp | 0.349 | 0.139 | 0.071 | 0.057 | kkp | 0.301 | 0.047 | 0.160 | 0.092 | ||
| R2 | 0.925 | 0.897 | 0.929 | 0.968 | R2 | 0.964 | 0.955 | 0.969 | 0.964 | ||
| p(AG-m-PmO)3 | 2.0 | 5.5 | 7.4 | 8.0 | p(AG-g-PmO)3 | 2.0 | 5.5 | 7.4 | 8.0 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZoM | Co | 0.421 | 1.004 | 0.842 | 0.932 | ZoM | Co | 1.498 | 0.291 | 0.496 | 0.754 |
| ko | −0.005 | −0.003 | −0.006 | −0.004 | ko | −0.006 | −0.007 | −0.006 | −0.013 | ||
| R2 | 0.928 | 0.759 | 0.839 | 0.639 | R2 | 0.699 | 0.983 | 0.953 | 0.951 | ||
| FoM | Co | 1.626 | 1.020 | 3.453 | 1.141 | FoM | Co | 1.384 | 0.717 | 3.437 | 2.897 |
| k1 | −0.003 | −0.002 | −0.009 | −0.011 | k1 | −0.001 | −0.002 | −0.003 | −0.005 | ||
| R2 | 0.676 | 0.888 | 0.945 | 0.984 | R2 | 0.792 | 0.850 | 0.884 | 0.963 | ||
| HM | kh | 0.025 | 0.017 | 0.025 | 0.025 | HM | kh | −0.036 | 0.977 | 0.036 | 0.036 |
| R2 | 0.944 | 0.969 | 0.953 | 0.953 | R2 | 0.915 | 0.163 | 0.994 | 0.994 | ||
| KPM | n | 0.524 | 0.289 | 0.371 | 0.371 | KPM | n | 0.203 | 0.492 | 0.199 | 0.316 |
| kkp | 0.022 | 1.000 | 0.057 | 0.109 | kkp | 0.270 | 0.041 | 0.224 | 0.106 | ||
| R2 | 0.933 | 0.957 | 0.954 | 0.852 | R2 | 0.948 | 0.952 | 0.905 | 0.951 | ||
Fickian diffusion mechanism n ≤ 0.45, non-Fickian (anomalous) diffusion mechanism.0.45 < n < 0.89.
Table 7.
Release kinetic and mechanism of Carboplatin release.
| p(AG-m-PmO)1 | 7.4 | p(AG-g-PmO)1 | 7.4 | ||
|---|---|---|---|---|---|
| ZoM | Co | 1.637 | ZoM | Co | 0.035 |
| ko | −0.003 | ko | −0.035 | ||
| R2 | 0.983 | R2 | 0.994 | ||
| FoM | Co | 2.083 | FoM | Co | 2.672 |
| k1 | −0.004 | k1 | −0.006 | ||
| R2 | 0.708 | R2 | 0.895 | ||
| HM | kh | 0.034 | HM | kh | −0.225 |
| R2 | 0.997 | R2 | 0.990 | ||
| KPM | n | 0.605 | KPM | n | 1.415 |
| kkp | 0.012 | kkp | 0.247 | ||
| R2 | 0.982 | R2 | 0.904 | ||
| p(AG-m-PmO)2 | 7.4 | p(AG-g-PmO)2 | 7.4 | ||
|---|---|---|---|---|---|
| ZoM | Co | 1.231 | ZoM | Co | 2.456 |
| ko | −0.025 | ko | −0.041 | ||
| R2 | 0.969 | R2 | 0.991 | ||
| FoM | Co | 3.402 | FoM | Co | 1.565 |
| k1 | −0.003 | k1 | −0.003 | ||
| R2 | 0.943 | R2 | 0.897 | ||
| HM | kh | 0.039 | HM | kh | −0.175 |
| R2 | 0.996 | R2 | 0.841 | ||
| KPM | n | 0.731 | KPM | n | 0.808 |
| kkp | 204.302 | kkp | 0.006 | ||
| R2 | 0.957 | R2 | 0.998 | ||
| p(AG-m-PmO)3 | 7.4 | p(AG-g-PmO)3 | 7.4 | ||
|---|---|---|---|---|---|
| ZoM | Co | 0.332 | ZoM | Co | 1.783 |
| ko | −0.028 | ko | −0.025 | ||
| R2 | 0.977 | R2 | 0.959 | ||
| FoM | Co | 1.939 | FoM | Co | 1.584 |
| k1 | −0.004 | k1 | −0.002 | ||
| R2 | 0.846 | R2 | 0.939 | ||
| HM | kh | 0.025 | HM | kh | −0.036 |
| R2 | 0.944 | R2 | 0.915 | ||
| KPM | n | 0.800 | KPM | n | 0.613 |
| kkp | 0.006 | kkp | 0.020 | ||
| R2 | 0.971 | R2 | 0.990 | ||
Fickian diffusion mechanism n ≤ 0.45, non-Fickian (anomalous) diffusion mechanism.0.45 < n < 0.89.
4. Conclusion
In the present study, the superior properties of synthesized novel organo-hydrogels were investigated as drug carrier/release. The organo-hydrogels contained agar as a natural polymer while glycerol and peppermint oil were used as the organic phases. The resistance of the organo-hydrogels was improved by crosslinkers. The organo-hydrogels were synthesized via free radical polymerization in emulsion in one step without toxic by-products. The additional biocompatible and antioxidant properties of the organo-gels make them useful for model drugs. Paracetamol (antipyretic-painkiller, recommended and used in the treatment of Covid-19) and carboplatin (widely used in cancer treatments) were used as model drugs. Moreover, organo-hydrogels release kinetics exhibited very high correlation with the ZoM, HM and KPM semi-empirical release kinetics. Therefore, either antioxidant and blood compatible properties or controlled release abilities for paracetamol and carboplatin of the organo-hydrogels synthesized with agar, glycerol and peppermint oil make them useful novel materials. Moreover, those superior properties make organo-hydrogels containing peppermint oil potential materials for biomedical, pharmaceutical and drug delivery system applications.
CRediT authorship contribution statement
Duygu Alpaslan: Formal analysis, Validation resources, Investigation, Writing - Review. Tuba Erşen Dudu: Visualization,Writing. Nahit Aktaş: Supervision and Editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Pooresmaeil M., Namazi H. Mater. Chem. Phys. 2018;218:62–69. [Google Scholar]
- 2.Pan A., Roy S.G., Haldar U., Mahapatra R.D., Harper G.R., Low W.L., De P., Hardy J.G. Gels. 2019;5:1–17. doi: 10.3390/gels5040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vintiloiu A., Leroux J.C. J. Control. Release. 2008;125:179–192. doi: 10.1016/j.jconrel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Hu X., Wang Y., Zhang L., Xu M., Zhang J., Dong W. Int. J. Biol. Macromol. 2018;108:558–567. doi: 10.1016/j.ijbiomac.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Penzes T., Csoka I., Eros I. Rheol. Acta. 2004;43:457–463. [Google Scholar]
- 6.Yang Y., Wang S., Xu H., Sun C., Li X., Zheng J. Asian Journal of Pharmaceutical Sciences. 2008;3:175–183. [Google Scholar]
- 7.Alpaslan D. Bull. Mater. Sci. 2019;42:1–11. [Google Scholar]
- 8.Boral S., Bohidar H.B. J. Phys. Chem. B. 2012;116:7113–7121. doi: 10.1021/jp3022024. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaztekin M., Levic S., Kalusevic A., Cam M., Bugarski B., Rakic V., Pavlovic V., Nedovic V. J. Microencapsul. 2019;36:109–119. doi: 10.1080/02652048.2019.1607596. [DOI] [PubMed] [Google Scholar]
- 10.McKay D.L., Blumberg J.B. Phytother. Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 11.Riachi L., Abi-Zaid I., Moreira R., Maria C.D. Arch. Latinoam. Nutr. 2012;64:389–392. [PubMed] [Google Scholar]
- 12.Grigoleit H.G., Grigoleit P. Phytomedicine. 2005;12:601–607. doi: 10.1016/j.phymed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food & Drug, 2020.
- 14.Turkish Medicines and Medical Devices Agency, 2020.
- 15.Hamontree C., Rangsimawong W., Wattanasri P., Akkaramongkolporn P., Tonglairoum P., Ngawhirunpat T., Opanasopit P. MATEC Web of Conferences. 2018;192 doi: 10.1208/s12249-018-1003-6. [DOI] [PubMed] [Google Scholar]
- 16.Saha S., Shivarajakumar R., Karri V.V.S.R. Int. J. Pharm. Sci. Res. 2018;9:1000–1010. [Google Scholar]
- 17.Yogev S., Mizrahi B. ACS Applied Polymer Materials. 2020;2:2070–2076. [Google Scholar]
- 18.Charoensumran P., Ajiro H. React. Funct. Polym. 2020;148:104460–104469. [Google Scholar]
- 19.Murphy R.D., Bobbi E., Oliveira F.C.S., Cryan S.A., Heise A. J. Polym. Sci. A Polym. Chem. 2019;57:1209–1215. [Google Scholar]
- 20.Knoll K., Leyendecker M., Thiele C.M. Eur. J. Org. Chem. 2019;2019:720–727. [Google Scholar]
- 21.Tubtimsri S., Limmatvapirat C., Limsirichaikul S., Akkaramongkolporn P., Inoue Y., Limmatvapirat S. Asian J Pharm Sci. 2018;13:425–437. doi: 10.1016/j.ajps.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham G.G., Scott K.F. Inammopharmacology. 2003;11:401–413. doi: 10.1163/156856003322699573. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S.C., Chang Y.C., Fan Chiang Y.L., Chien Y.C., Cheng M., Yang C.H., Huang C.H., Hsu Y.N. J. Formos. Med. Assoc. 2020;119:747–751. doi: 10.1016/j.jfma.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo C., Garcia Lopez-Hortelano M., de Carlos Vicente J.C., Vazquez Martinez J.L. An Pediatr (Barc) 2020;92 (241.e242-e211) [Google Scholar]
- 25.Van Cuong L., Giang H.T.N., Linh L.K., Shah J., Van Sy L., Hung T.H., Reda A., Truong L.N., Tien D.X., Huy N.T. Lancet Infect. Dis. 2020;20:408–409. doi: 10.1016/S1473-3099(20)30111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S., Naskar S., Kuotsu K. Carbon Letters. 2019:1–13. [Google Scholar]
- 27.Wagstaff A.J., Ward A., Benfield P., Heel R.C. Drugs. 1989;37:162–190. doi: 10.2165/00003495-198937020-00005. [DOI] [PubMed] [Google Scholar]
- 28.Akkaya H., Saloğlu D. Sinop Uni J Nat Sci. 2016;1:11. [Google Scholar]
- 29.Zhang J., Muirhead B., Dodd M., Liu L., Xu F., Mangiacotte N., Hoare T., Sheardown H. Biomacromolecules. 2016;17:3648–3658. doi: 10.1021/acs.biomac.6b01148. [DOI] [PubMed] [Google Scholar]
- 30.Alpaslan D., Dudu T.E., Sahiner N., Aktasa N. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;108:110339. doi: 10.1016/j.msec.2019.110339. [DOI] [PubMed] [Google Scholar]
- 31.Cheng X., Shao Z., Li C., Yu L., Raja M.A., Liu C. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanthini G.M., Martin C.A., Sakthivel N., Veerla S.C., Elayaraja K., Lakshmi B.S., Asokan K., Kanjilal D., Kalkura S.N. Appl. Surf. Sci. 2015;329:116–126. [Google Scholar]
- 33.Wang Y., Yin M., Li Z., Liu Y., Ren X., Huang T.S. Colloids Surf B Biointerfaces. 2018;165:199–206. doi: 10.1016/j.colsurfb.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 34.Singleton R.J.A.J., VL American Journal of Enology and Viticulture. 1965;16:12. [Google Scholar]
- 35.Alpaslan D., Dudu T.E., Aktaş N. MANAS Journal of Engineering. 2018;6:15–33. [Google Scholar]
- 36.Alpaslan D., Dudu T.E., Aktas N. Soft Materials. 2020:1–14. [Google Scholar]
- 37.Obert W.G.B., Childs E. Biochem. J. 1975;145:10. [Google Scholar]
- 38.Pasqua A.J.D., Goodisman J., Kerwood D.J., Toms B.B., Dubowy R.L., Dabrowiak J.C. Chem. Res. Toxicol. 2006;19:139–149. doi: 10.1021/tx050261s. [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer Richard W., Peppas N.A. J. Control. Release. 1984;1:10. [Google Scholar]
- 40.Varelas C.G., Dixon D.G., Steiner C.A. Journal of Controlled Release. 1995;34:7. [Google Scholar]
- 41.Paulo Costa J.M.S.L. European Journal of Pharmaceutical Sciences. 2001;13 [Google Scholar]
- 42.Higuchi T. J. Pharm. Sci. 1963;52:5. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y., Wang L., Guo B., P X.M. J. Mater. Chem. B. 2014;2:3674–3685. doi: 10.1039/c3tb21716g. [DOI] [PubMed] [Google Scholar]
- 44.Rossi F., Perale G., Masi M. Chem. Pap. 2010;64:573–578. [Google Scholar]
- 45.Singh V.K., Pramanik K., Ray S.S., Pal K. AAPS PharmSciTech. 2015;16:293–305. doi: 10.1208/s12249-014-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y., Larock R.C. Macromol. Rapid Commun. 2011;32:1331–1337. doi: 10.1002/marc.201100203. [DOI] [PubMed] [Google Scholar]
- 47.Kim H.-M., Kim H.-R., Hou C.T., Kim B.S. J. Am. Oil Chem. Soc. 2010;87:1451–1459. [Google Scholar]
- 48.Peniche C., Cohen M.E., Vazquez B., Roman J.S. Polymer. 1996;38:5977–5983. [Google Scholar]
- 49.Roy S.G., De P. Polymer. 2014;55:5425–5434. [Google Scholar]
- 50.Zohuriaan-Mehr M.J., Kabiri K., Kheirabadi M. J. Appl. Polym. Sci. 2010;117:1127–1136. [Google Scholar]
- 51.Üzüm Ö.B., Karadağ E. Polym.-Plast. Technol. Eng. 2010;49:609–616. [Google Scholar]
- 52.Yin Z.C., Wang Y.L., Wang K. Journal of Drug Delivery Science and Technology. 2018;43:12–18. [Google Scholar]
- 53.Orakdogen N., Okay O. Polymer. 2006;47:561–568. [Google Scholar]
- 54.Samadi N., Sabzi M., Babaahmadi M. Int. J. Biol. Macromol. 2018;107:2291–2297. doi: 10.1016/j.ijbiomac.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 55.Shankar S., Rhim J.W. Carbohydr. Polym. 2016;135:18–26. doi: 10.1016/j.carbpol.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 56.Danish M., Fakhar M.W.M.M., Rashid U. Chiang Mai J. Sci. 2016;43:1–13. [Google Scholar]
- 57.Liu C., Li M., Ji N., Liu J., Xiong L., Sun Q. J. Agric. Food Chem. 2017;65:8363–8373. doi: 10.1021/acs.jafc.7b02938. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Cai D., Yang J., Wang Y., Zhang X., Yin S. Int. J. Clin. Exp. Med. 2014;7:1233–1244. [PMC free article] [PubMed] [Google Scholar]
- 59.Belanger M.C., Marois Y. Journal of Biomedical Materials. 2001;58:467–477. doi: 10.1002/jbm.1043. [DOI] [PubMed] [Google Scholar]
- 60.Sahoo S., Kumar N., Bhattacharya C., Sagiri S.S., Jain K., Pal K., Ray S.S., Nayak B. Designed Monomers and Polymers. 2012;14:95–108. [Google Scholar]
- 61.Campbell S.B., Hoare T. Current Opinion in Chemical Engineering. 2014;4:1–10. [Google Scholar]
- 62.Patel I.R., Amiji M.M. Pharm. Res. 1996;13:588–593. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 63.Treenate P., Monvisade P. Int. J. Biol. Macromol. 2017;99:71–78. doi: 10.1016/j.ijbiomac.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 64.Pishnamazi M., Hafizi H., Shirazian S., Culebras M., Walker G.M., Collins M.N. Polymers (Basel) 2019;11:1059–1069. doi: 10.3390/polym11061059. [DOI] [PMC free article] [PubMed] [Google Scholar]