Abstract
Herbal medicines have accumulated valuable clinical experience in thousands of years of applications in traditional Chinese medicine (TCM) or ethnomedicine. The unique multi-target efficacy on complex diseases made herbal medicines gained a global popularity in recent years. However, the characteristic of multi-component acting on multi-target poses a dilemma for the evaluation of therapeutic efficacy of herbal medicines. Advances in metabolomics enable efficient identification of the various changes in biological systems exposed to different treatments or conditions. The use of serum pharmacochemistry of TCM has significant implications for tackling the major issue in herbal medicines development—pharmacodynamic material basis. Chinmedomics integrates metabolomics and serum pharmacochemistry of TCM to investigate the pharmacodynamic material basis and effective mechanisms of herbal medicines on the basis of TCM syndromes and holds the promise of explaining therapeutic efficacy of herbal medicines in scientific language. In this review, the historical development of chinmedomics from concept formation to successful applications was discussed. We also took the systematic research of Yin Chen Hao Tang (YCHT) as an example to show the research strategy of chinmedomics.
Keywords: Herbal medicines, Chinmedomics, Pharmacodynamic material basis, TCM syndrome, Quality control of herbal medicines
Abbreviations: AChE, Acetylcholinesterase; AD, Alzheimer's disease; APL, Acute promyelocytic leukemia; ASL, Acanthopanax senticosus leaf; COVID-19, Corona Virus Disease 2019; CW, Caowu; DHJS, Dampness-heat jaundice syndrome; ECB, Endometriosis of cold coagulation and blood stasis; EGFR, Epidermal growth factor receptor; FGFR-4, Fibroblast growth factor receptor-4; GFW, Guizhi Fuling Wan; GR, Glucocorticoid receptor; HQD, Heart-qi deficiency; ISBA, Isovaleroylbinankadsurin A; KXS, Kai Xin San; KYDS, Kidney-yang deficiency syndrome; LW, Liu Wei Di Huang Wan; NOL, Nanshi Oral Liquid; PAC, Phellodendri amurensis cortex; PCMS, Plotting of correlation between marker metabolites and serum constituents; PDL, Pudilan; PQ, Panax quinquefolius; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SF, Shuanghuanglian formula; SMS, Sheng Mai San; SQW, Shen Qi Wan; SZRD, Suan Zao Ren Decoction; TCM, Traditional Chinese medicine; TQC, Tian Qi Jiang Tang capsule; WXF, Wen Xin Fang; YCHT, Yin Chen Hao Tang; YHS, Yanghuang syndrome; YNBY, Yunnan Baiyao
1. Introduction
To cope with the challenges from natural environments, plants have greatly expanded their metabolic systems to produce a dazzling array of secondary metabolites with a broad range of pharmacological properties (Joyner & Cichewicz, 2011; Weng, Philippe, & Noel, 2012; Zálešák, Bon, & Pospíšil, 2019). Herbal medicines mainly come from plants, and have accumulated long-time and large-scale clinical experience on the interventions of complex diseases in some ancient countries (Buyel, 2018; Willcox & Bodeker, 2004). In the West, it is a prevailing view that a drug must be either a highly purified or synthetic agent. However, herbal medicines emphasize multi-component synergy rather than a single compound. This holistic nature of multi-component presents a difficulty for the assessment of which components contribute to therapeutic effects and how they synergistically work (Xu, 2011). The active chemical constituents contained in herbal medicines that can express clinical efficacy of the drug are defined as pharmacodynamic material basis of herbal medicines (Wang, 2015). Elucidating the pharmacodynamic material basis is necessary for the evaluation of therapeutic efficacy of herbal medicines. For example, to explore the pharmacodynamic material basis of Phellodendri amurensis cortex (PAC) for treating prostate cancer, a total of 54 compounds of PAC were identified and 38 constituents that could be absorbed into blood after oral administration of PAC were characterised. Among the 38 constituents characterised in vivo, 10 absorbed chemical constituents were remarkably correlated with the therapeutic markers and therefore selected as the active chemical constituents of PAC against prostate cancer. The research of action mechanisms showed that these 10 active chemical constituents were closely related to 8 protein targets and 5 pathways, which suggested that 10 active chemical constituents might be the main pharmacodynamic material basis of PAC against prostate cancer. The accuracy of this conclusion will be further verified by pharmacological studies (Li et al., 2017). Multiple methods were developed for deciphering the pharmacodynamic material basis and action mechanisms of herbal medicines and a series of fruitful results have been achieved (Gu & Lai, 2020; Liu et al., 2014; Liu et al., 2016). However, a systematic methodology that could be adopted for the investigations of therapeutic efficacy of herbal medicines is still urgently needed.
Chinmedomics is a new discipline emerging in recent years. It integrates the theories and technologies across system biology and serum pharmacochemistry of traditional Chinese medicine (TCM) to form a systematic methodology for identifying biomarkers of syndromes, evaluating the effectiveness of herbal formulas, and discovering the pharmacodynamic material basis of herbal medicines/herbal formulas (Wang, Zhang, & Sun, 2012). Here, we reviewed the developing progress of chinmedomics, namely concept formation of chinmedomics, theoretical connotations of chinmedomics, key technological platforms in chinmedomics and major applications of chinmedomics in the past ten years. We also discussed several methods that applied to interpreting the therapeutic efficacy of herbal medicines.
2. Opportunities and challenges of herbal medicines
Plants first appeared on land around 500 million years ago, and have been identified and classified for nearly 360,000 species to date (The Plant List, n.d.). Plant based dietary pattern from palaeolithic period awakened people's awareness to use plants as therapeutic agents (Hardy et al., 2012; Lietava, 1992). Numerous preserved classical medical text documents such as Egyptian Papyrus Ebers and Shennong's Herbal Classic of Materia Medica recorded the special roles of herbal medicines on health maintenance for human beings over the last thousands of years. During the past decades, millions of patients around the world use herbal medicines or a related practice indicated that herbal medicines have gained a global popularity (Cheung, 2011).
Advances in health sciences have revealed accumulating evidences of the limitations of single target drug discovery, thus multiple drugs with multiple targets are emerging as the next paradigm in drug discovery (Csermely, Agoston, & Pongor, 2005; Medina-Franco, Giulianotti, Welmaker, & Houghten, 2013; Sams-Dodd, 2005). It is postulated that pharmacological efficacy of herbal medicines comes from simultaneous binding of multiple chemicals to multiple targeting sites, and/or synergistic actions on a single site (Liang, Ruan, Ouyang, & Lai, 2016; Liu et al., 2014). Herbal medicines have attracted increasing attentions due to its unique multi-component and multi-target effects. For instance, Compound Danshen Dripping Pills (Dantonic®), a well-known Chinese prescription used for the treatment of myocardial ischemic disease, has completed a Phase III clinical trial to evaluate the safety and efficacy on treating chronic stable angina pectoris (ClinicalTrials.gov Identifier: NCT01659580) (Liao et al., 2019).
Different from modern western medicine, the use of herbal medicines is mainly based on practice and theories rooted in ancient philosophy. In the era of evidence-based medicine, the scientific evaluation of herbal medicines with modern medical practice approaches is critical for the development of herbal medicines (Shan et al., 2020). However, due to the diversity of chemical constituents and the complexity of the interactions between herbal medicines and the human body, information is limited available on the pharmacokinetics, pharmacodynamics, efficacy, and safety of herbal medicines, among which lack of scientific evidences for therapeutic efficacy has become a central concern for both health authorities and the public (Tachjian, Maria, & Jahangir, 2010).
3. Efforts on understanding the therapeutic efficacy of herbal medicines
The combination of chromatographic separation techniques and spectroscopic methods greatly improved the isolated and identified efficiency of natural products, which made bioassay-guided fractionation prevalent in the discovery of active principles from herbal medicines in the nineteenth and twentieth centuries (Phillipson, 2001). The most successful story was that of artemisinin (qinghaosu), an active compound isolated from Qinghao (the Chinese name of Artemisia annua L.). Artemisinin has been identified as a bioactive component of qinghao extraction with antimalarial activity, and further developed into an antimalarial drug which played a key role in malaria related mortality (Bhatt et al., 2015; Tu, 2011). One of the inventors, Youyou Tu, was awarded the 2015 Nobel Prize in Physiology or Medicine for her tremendous contributions to the discovery of artemisinin (Su & Miller, 2015).
Along with the development of modern biotechnology, high-throughput activity screening was introduced into the investigations of bioactive components of herbal medicines and offered a strong arm in the process of the studies (Qv, Jiang, & Piao, 2010). Integration of cell membrane or biomacromolecules with liquid chromatography implemented the idea of dynamically separating the constituents and observing the interactions between target components and receptors (Chen et al., 2013; Sun, Ma, Guo, Hu, & He, 2013). For example, an improved rat basophilic leukemia-2H3 cell membrane chromatography was successfully applied to screening and identifying two potential allergenic components in huangqi injection (Bu, Hu, Xu, Xie, & Wang, 2018). Additionally, a 2D epidermal growth factor receptor (EGFR) and fibroblast growth factor receptor-4 (FGFR-4) dual-mixed cell membrane chromatography was employed to screen the active components from Salviae miltiorrhizae radix. As a result, salvianolic acid C, tanshinone I, tanshinone IIA, and cryptotanshinone were identified as bioactive components with EGFR and FGFR-4 activities (Fu, Lv, Jia, Lin, & Han, 2019).
Computational methods, mainly including pharmacophore-based virtual screening and molecular docking, are well-established tools that help to select plant materials with a high possibility of biological activity in drug discovery. As an example, a virtual screening analysis from TCM database, Druglike database, and MiniMaybridge database was performed on an established pharmacophore model for the acquisition of top 10 Acetylcholinesterase (AChE) inhibitors. Accordingly, three potential AChE inhibitor candidates were finally got by assessing 8 different scoring functions (Jiang & Gao, 2018). In addition, computational methods could predict the positioning of a ligand within a protein binding pocket and estimate the strength of the binding with a docking score, which may provide the means to elucidate the mechanisms of actions. Zuo et al. used molecular docking to clarify the possible mechanisms of isovaleroylbinankadsurin A (ISBA) ameliorating cardiac ischemia/reperfusion injury (Zuo et al., 2020). Molecular modeling data demonstrated that ISBA has tight interactions with the active state of the glucocorticoid receptor (GR) and may perform its cardio-protective effects through targeting and activating GR.
Network pharmacology is first proposed by Andrew L. Hopkins and is an approach to drug design that integrates network biology and polypharmacology (Hopkins, 2007, Hopkins, 2008). Network pharmacology has emerged as a powerful means to analyze pharmacological mechanisms of herbal medicines from a systems perspective and at the molecular level (Fang et al., 2017; Lyu et al., 2017). One recent example is about the investigation of molecular mechanisms of Pudilan (PDL) against Corona Virus Disease 2019 (COVID-19). PDL is a four-herb formula with therapeutic potentials for COVID-19 while the underlying mechanisms remain to be clarified. Bioinformatics and network pharmacology integrated analysis showed PDL might inhibit the cytokine storm by regulating and targeting many cytokines and chemokines, prevent the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into cells, as well as moderate the immune system to shorten the course of COVID-19 (Kong et al., 2020).
4. The emergence of chinmedomics and its research strategy
Within East Asia, notably China, an herbal formula is comprised of more than one herb medicine following the principles of compatibility to exert the synergistic therapeutic effects (Kiyohara, Matsumoto, & Yamada, 2004; Zhang, Zhu, Fan, & Zhang, 2015). In clinical practice, TCM practitioners prescribe herbal formulas as clinical drugs according to comprehensive analysis of clinical information obtained from observation, listening, questioning, and pulse analyses, which is known as syndrome differentiation (Jiang et al., 2012). Syndrome differentiation is the basic principle that guides the prescribing of herbal formulas (Xu, Cui, Kong, Tang, & Dong, 2018). Systematic and integrated interpretation of the therapeutic effects of herbal formulas on basis of their adaptive syndromes is an essential part in TCM research. However, the ambiguity of TCM syndromes and the complexity of herbal formulas greatly limit the confirmation of the pharmacodynamic material basis and the evaluation of therapeutic efficacy of herbal medicines. Moreover, current limited pathological and clinical chemical indicators are difficult to reflect the overall role of herbal formulas in treating complex diseases (Wang, Zhang, Sun, & Yan, 2017).
4.1. The theoretical connotations of chinmedomics
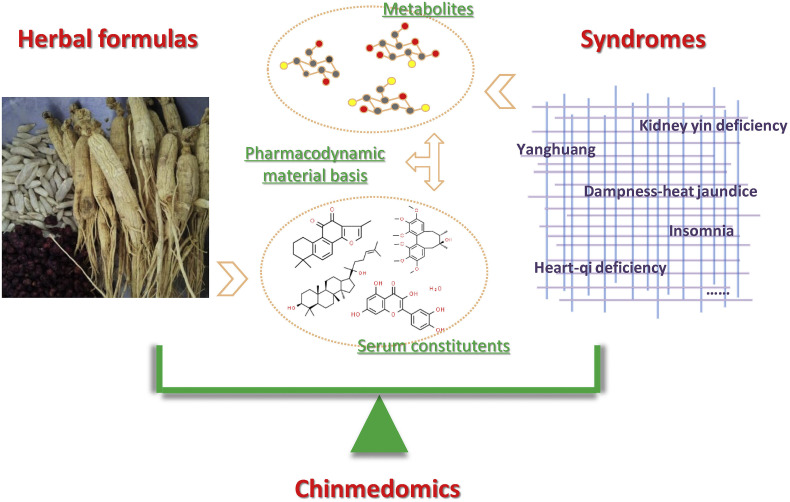
The concept of chinmedomics was proposed in 2012, aiming at exploring the scientific mechanisms of herbal medicines from a holistic perspective of syndrome-formula correlations (Wang, Zhang, & Sun, 2012). Chinmedomics is defined as a systematic methodology for the investigations of pharmacodynamic material basis of herbal medicines, that is, taking syndromes as the entry point and herbal formulas as the research objects, chinmedomics employs metabolomics to characterize the metabolic profiles and identify the biomarkers of syndromes, thus these metabolic perturbations are used as indicators to accurately evaluate the clinical therapeutic effects of herbal formulas; after confirming the curative effects, bioactive components of herbal formulas in vivo are detected using serum pharmacochemistry of TCM; correlation analysis between endogenous metabolites and exogenous active components is introduced to identify the pharmacodynamic material basis of herbal medicines, furthermore, mechanisms of drug actions will be revealed with the biological verification of pharmacodynamic material basis (Fig. 1 ). The emergence of chinmedomics will contribute to the discovery of quality markers of herbal medicines that related to pharmacodynamic material basis, the manufacture and creation of new drugs from herbal medicines, and the explanation of compatibility rules and effective mechanisms of herbal formulas.
Fig. 1.
The systematic methodology of Chinmedomics.
According to the theoretical connotations, chinmedomics focuses on solving the following key scientific problems including: 1) Establishment of biological system for efficacy evaluation of herbal medicines—discovering the biomarkers of syndromes/diseases, which contributes to comprehensively understanding the essence of syndromes/diseases and provides basic premise for precisely diagnosing of syndromes and evaluating therapeutic effects of herbal medicines. 2) The holographic analysis of active components in vivo under the acting state with serum pharmacochemistry of TCM—different from serum chemistry which exhibits the changes of chemical indicators or clinical chemistry in human body influenced by drugs, serum pharmacochemistry of TCM gives emphasis to human body's selective absorption of drugs and proposes that the constituents of drugs which could be absorbed into blood after oral administration of herbal medicines are more likely to exert a therapeutic effect. Therefore, following a confirmed result of the effectiveness of treatment, constituents of herbal medicines that could be absorbed into blood after oral administration will be measured simultaneously with high accuracy and sensitivity from only minimal amounts of serum sample. This is the basic step in the research of pharmacodynamic material basis. 3) Revelation of pharmacodynamic material basis and effective mechanisms of herbal medicines—the relationships between biomarkers and active components in vivo are revealed to clarify which active components interfered with which marker trajectories to express clinical efficacy, and thus the active components that are remarkably correlated with the changes of biomarker trajectories are recommended as the potential pharmacodynamic material basis. Furthermore, mechanisms of drug actions will be revealed with the biological verification of pharmacodynamic material basis.
4.2. Key technological platforms in chinmedomics strategy
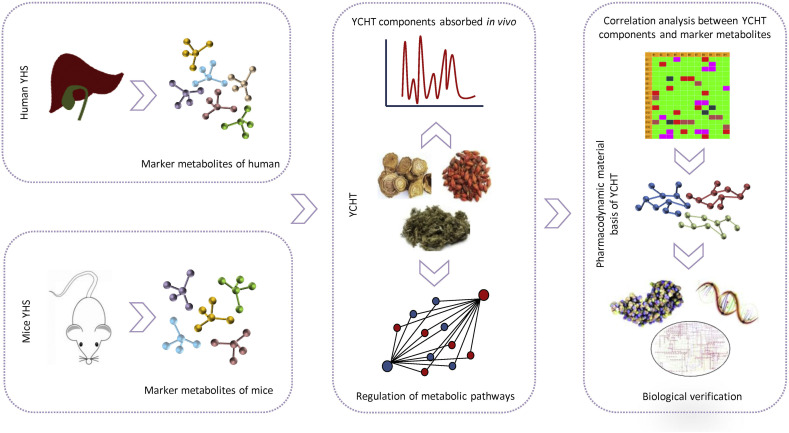
Here, we take the systematic research of Yin Chen Hao Tang (YCHT) as an example to show the research strategy of chinmedomics. YCHT is a famous herbal formula used for treating Yanghuang syndrome (YHS) which closely related with jaundice, and it was firstly recorded in “Shang Han Lun” written by Zhang Zhongjing. Taking YHS as an entry point, and taking YCHT as the research object, the metabolic profile and biomarkers of YHS were firstly described and further used as indicators to evaluate the clinical efficacy of YCHT in the treatment of YHS. Meanwhile, an integrated study was carried out to identify the components absorbed into blood using serum pharmacochemistry of TCM under the status of YCHT expressing clinical efficacy. The components with high correlations to the changes of biomarker trajectories in vivo were selected as potential pharmacodynamic material basis. Finally, the pharmacodynamic material basis and the biological mechanisms of YCHT in the treatment of YHS were elucidated with biological verification of potential pharmacodynamic material basis (Fang et al., 2016; Liu et al., 2018; Wang et al., 2008; Wang et al., 2012; Zhang et al., 2016; Zhang, Sun, Qiu, & Wang, 2013) (Fig. 2 ). This strategy has been systematically applied to about a dozen syndromes and their corresponding herbal formulas/herbal medicines (Table 1 , Table 2 ), thereby forming a systematic methodology for investigations of the pharmacodynamic material basis of herbal medicines.
Fig. 2.
The systematic research of Yin Chen Hao Tang (YCHT) treating Yanghuang syndrome (YHS) using chinmedomics research strategy.
Table 1.
Chinmedomics applications in the interpretation of therapeutic effects of herbal formulas/herbal medicines.
| Herbal formulas/Herbal medicines | Syndromes/Diseases | Syndromes/Disease biomarkers | Therapeutic efficacy of herbal formulas/herbal medicines | References |
|---|---|---|---|---|
| Yin Chen Hao Tang (YCHT) | Dampness-heat jaundice syndrome (DHJS) | A total of 22 serum biomarkers may be involved in the potential biological chemistry mechanisms of DHJS occurrence. | YCHT could call back most of the DHJS biomarkers. | (Sun et al., 2018) |
| Liu Wei Di Huang Wan (LW) | Kidney yin deficiency | A total of 20 potential biomarkers such as creatinine, estrone, cytidine, and glucosamine were found in kidney yin deficiency syndrome. | LW-treated rats had the most similar metabolic profile to that of the control rats, indicating the greatest efficiency of the formula LW on kidney yin deficiency syndrome. | (Wang et al., 2010) |
| Tian Qi Jiang Tang capsule (TQC) | Type 2 diabetes | A total of 12 differentiating metabolites were identified in type 2 diabetes. | TQC exhibited pharmacological effects on type 2 diabetes through regulating starch and sucrose metabolism and pentose and glucuronate interconversions. | (Wang et al., 2013) |
| Suan Zao Ren Decoction (SZRD) | Insomnia | A total of 20 differentiating metabolites were detected in insomnia. | SZRD exerts the therapeutic effects on insomnia by mediating the serotonergic activation. | (Yang et al., 2012) |
| Wen Xin Fang (WXF) | Heart-qi deficiency (HQD) | A total of 17 biomarkers involved in glycolysis or gluconeogenesis metabolism, biosynthesis of unsaturated fatty acids metabolism, fatty acid biosynthesis, and purine metabolism were acutely perturbed in HQD syndrome. | WXF could regulate multiple perturbed pathways in HQD syndrome to normal state and thus exhibited potential pharmacological effects in treating HQD syndrome. | (Wang et al., 2013) |
| Sheng Mai San (SMS) | Alzheimer's disease (AD) | A total of 37 potential biomarkers were identified in serum samples of AD. | SMS intervention could call back 72.32% proportion of AD serum potential biomarkers. The mechanisms of SMS treating AD were mainly related to lipid peroxidation, such as inhibiting the generation of linoleic acid hydroperoxides (13-HPODE, 9-HPODE, and 9-OxoODE). | (Lu et al., 2017) |
| Kai Xin San (KXS) | AD | A total of 16 lipid-related biomarkers were found associated with AD. | KXS could call back 8 lipid biomarkers that mainly involved in linoleic acid metabolism, arachidonic acid metabolism, and steroid hormone biosynthesis. | (Gao et al., 2018) |
| Nanshi Oral Liquid (NOL) | Kidney-yang deficiency syndrome (KYDS) | A total of 30 potential biomarkers were identified in the urine samples of KYDS. | NOL could completely reverse a total of 22 biomarkers of KYDS. | (Zhang et al., 2016) |
| ShenQi pill | KYDS | A total of 27 ions were observed changed in the serum samples of KYDS. | ShenQi pill could restore a total of 17 changed ions in KYDS. | (Nan et al., 2016) |
| Shen Qi Wan (SQW) | KYDS | A total of 23 differential metabolites were found as the potential biomarkers of KYDS through 4 repeated tests in the serial studies on SQW treating KYDS. | SQW led to significant restoration of abnormal metabolism such as tyrosine metabolism, tryptophan metabolism, and steroid hormone biosynthesis in KYDS. | (Zhou et al., 2016) |
| Guizhi Fuling Wan (GFW) | Endometriosis of cold coagulation and blood stasis (ECB) | A total of 20 differential biomarkers were involved in the disturbed metabolic networks in ECB. | GFW showed therapeutic effects on ECB through regulating the disturbed metabolic pathways involved in ECB. | (Wu et al., 2018) |
| Phellodendri amurensis cortex (PAC) | Prostate cancer | A total of 34 metabolic biomarkers were related to prostate cancer. | PAC could completely reverse 24 biomarkers of prostate cancer to normal levels. | (Li, Zhang, et al., 2017) |
| Acanthopanax senticosus leaf (ASL) | Acute promyelocytic leukemia (APL) | A total of 13 biomarkers were found greatly contributing to the pathology of APL. | ASL showed potential therapeutic effects on APL by adjusting the disorders of some metabolic pathway nodes including citric acid, lactic acid, l-glutamine, inosine, and isoleucine. | (Han et al., 2018) |
Table 2.
Chinmedomics applications in the revelation of the pharmacodynamic material basis of herbal formulas/herbal medicines.
| Herbal formulas/Herbal medicines | Pharmacodynamic material basis of herbal formulas/herbal medicines | References |
|---|---|---|
| Yin Chen Hao Tang (YCHT) | 6,7-dimethylesculetin, geniposide, and rhein were selected as the active ingredients in YCHT. | (Zhang et al., 2012, 2013) |
| Suan Zao Ren Decoction (SZRD) | Jujuboside A and Jujuboside B may be the pharmacodynamic material basis of SZRD in the treatment of insomnia. | (Wang, Yang, Sun, & Zhang, 2012, Wang, Yang, Zhang, Sun, & Yan, 2012) |
| Wen Xin Fang (WXF) | A total of 32 components including 26 prototype components and 6 metabolites from WXF may be the potential bioactive components. | (Cao et al., 2014) |
| Sheng Mai San (SMS) | A total of 8 constituents were recommended as potential quality markers of SMS, including schisandrin, isoschisandrin, angeloylgomisin Q, gomisin D, angeloylgomisin H, gomisin M2, ginsenoside F1, 20(R)-ginsenoside Rg3. | (Zhang et al., 2018) |
| Da-Bu-Yin-Wan | 38 constituents consisted of 22 prototypes and 16 metabolites in Da-Bu-Yin-Wan were detected and identified in vivo. | (Li et al., 2017) |
| Kai Xin San (KXS) | Ginsenoside Rf, ginsenoside F1, 20-O-glucopyranosyl ginsenoside Rf, dehydropachymic acid, and E-3, 4, 5-trimethoxycinnamic acid were proposed as the pharmacodynamic material basis of KXS. | (Wang et al., 2019) |
| Zi Shen Wan | A total of 33 components including 22 prototype components and 11 metabolites in Zi Shen Wan were described in vivo. | (Li et al., 2016) |
| Shen Qi Wan (SQW) | A total of 20 compositions had a highly correlated relationship with marker metabolites of therapeutic effects, which might play a key role in the therapeutic effects of SQW. | (Wang et al., 2016) |
| Shuanghuanglian formula (SF) | A total of 68 ions of interest (39 prototype components and 29 metabolites of SF) were extracted and identified from blood samples. | (Yan et al., 2013) |
| Phellodendri amurensis cortex (PAC) | A total of 10 chemical compounds were determined as being potential pharmacodynamic material basis of PAC against prostatic cancer. | (Li, Zhang, et al., 2017) |
| Acanthopanax senticosus leaf (ASL) | A total of 21 metabolites were identified after oral administration of ASL. | (Zhang et al., 2016) |
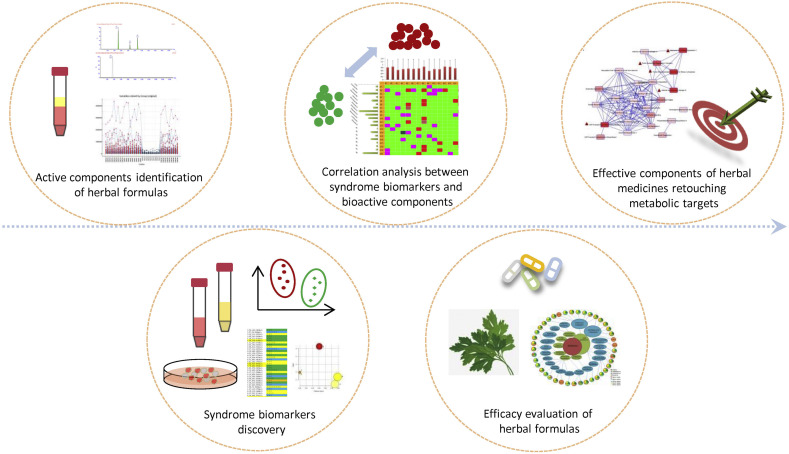
Chinmedomcis research strategy covers five innovative key technological platforms (Fig. 3 ). 1) Metabolomics-based technological platform for the discovery of syndrome biomarkers. 2) Syndrome biomarkers-based technological platform for the evaluation of clinical efficacy of herbal formulas. 3) Serum pharmacochemistry of TCM-based technological platform for the identification of active components in herbal formulas. 4) Technological platform for the correlation analysis between endogenous syndrome biomarkers and exogenous bioactive components. 5) Technological platform for the identification of effective components of herbal medicines retouching metabolic targets. These five key technological platforms all have got the Computer Software Copyright Registration Certificates issued by National Copyright Administration of the People's Republic of China.
Fig. 3.
Key technological platforms in chinmedomics strategy.
5. Chinmedomics applications in herbal medicines research
After nearly a decade of development, chinmedomics has been successfully applied to the studies of scores of herbal formulas/herbal medicines. These successful practices have accumulated a wealth of valuable data for the scientific interpretation of key issues in the development of herbal medicines, such as the clarification of therapeutic effects, the exploration of pharmacodynamic material basis and the establishment of quality standards.
5.1. Interpretation of the therapeutic effects
Elucidation of the mechanisms of actions is the key to evaluating the efficacy of herbal medicines (WHO, 2000). With the applications of chinmedomics, the therapeutic targets of numerous herbal formulas/herbal medicines have been demonstrated (Table 1). In a recent research, chinmedomics method was applied to assessing the therapeutic efficacy of an herbal prescription named AS1350 on Kidney-Yang Deficiency Syndrome (KYDS) (Liu et al., 2016). Serum pharmacochemistry of TCM analysis identified a total of 47 compounds in vivo from the constituents of AS1350. Metabolic profiling results showed that 48 marker metabolites associated with fatty acid metabolism, lipid metabolism, steroid hormone biosynthesis, and amino acid metabolism were involved in the pathological process of KYDS. The active ingredients of AS1350 against KYDS including betaine, scoparone, clovene, stepharine, longipedunin C, gomisin S, schizandrin, auxin A, and 1, 11-undecanedicarboxylicacid were confirmed by PCMS software. Further results of potential targets prediction manifested that the components in AS1350 could rebalance metabolic disorders in KYDS through multi-targets.
5.2. Revelation of the pharmacodynamic material basis
Numerous studies have displayed the utility of chinmedomics to discover the pharmacodynamic material basis of herbal medicines/herbal formulas (Table 2). One prospective study is about the classic herbal formula Sheng Mai San (SMS). The effectiveness of SMS in the treatment of Alzheimer's disease (AD) was firstly evaluated by serum metabolomics. A 72.32% call-back proportion of AD potential biomarkers intervened by SMS indicated the benefits of SMS in treating AD. The mechanisms were mainly related to lipid peroxidation, such as inhibiting the generation of linoleic acid hydroperoxides (13-HPODE, 9-HPODE, and 9-OxoODE). Correlation analysis suggested 8 constituents including schisandrin, isoschisandrin, angeloylgomisin Q, gomisin D, angeloylgomisin H, gomisin M2, ginsenoside F1, and 20(R)-ginsenoside Rg3 might be the candidate leading compounds of SMS against AD (Lu et al., 2017; Zhang et al., 2018).
5.3. Quality control of herbal medicines
Quality control is one of the hot issues in the modernization of herbal medicines (Zhang et al., 2015). The fundamental purpose of quality control is to control the effectiveness of herbal medicines (Liu et al., 2016). Pharmacodynamic material basis-guided quality markers discovery cuts to the heart of quality control and provides the foundation of standardization studies of herbal medicines and establishment of quality standards (Sun et al., 2019; Wang et al., 2019; Zhang et al., 2018; Zhao et al., 2019). Panax quinquefolius (PQ) is a famous herbal medicine with the efficacy of outstanding replenishing qi, nourishing yin, clearing heat and generating fluid (Yu et al., 2014). With the aim of offering basic data for the establishment of PQ quality standard, chinmedomics technological platforms were introduced to investigate pharmacodynamic material basis-based quality markers of PQ in the background of Compound Zaofan Pill (Xiong et al., 2020). Correlation analysis of 41 blood and urine marker metabolites with 14 serum constituents showed pseudoginsenoside F11 and ginsenoside Rd were highly correlated with the therapeutic effects of PQ on qi-blood deficiency syndrome. Pseudoginsenoside F11 and Ginsenoside Rd were finally selected as the potential quality markers of PQ.
5.4. Toxicity and safety assessment of herbal medicines
With the prevalence of herbal medicines use in modern medicinal systems, herbal medicines are still not subject to the same surveillance and regulation as conventional drugs for the reason that they are viewed as being natural and therefore safe (Shaito et al., 2020). Nonetheless, the safety of herbal medicines yet remains to be properly validated since some herbal medicines may have adverse outcomes and induce possible herb-drug interactions (Li & Wang, 2020). Besides applications in efficacy evaluation of herbal medicines, chinmedomics has been used to the assessment of toxicity of herbal medicines or formulas. Yunnan Baiyao (YNBY) is a famous herbal prescription that has been used for traumatology worldwide (Ness et al., 2017; Ren et al., 2017). However, the sales of YNBY were once banned in Hong Kong on account of its controversial content of Caowu (Aconiti Kusnezoffii Radix, CW). In order to investigate the safe use of YNBY, the study of potential biochemical mechanisms of CW toxicity was performed and a total of 13 phenotypic toxicity biomarkers of CW were obtained (Yan et al., 2017). YNBY administration within a treatment cycle exhibited no obvious toxicity and could regulate 5 core toxicity biomarkers of CW to normal condition. These findings indicated that the toxicity of CW was attenuated in the compatibility of YNBY (Ren et al., 2020). Moreover, following the confirmed therapeutic effects of YNBY on blood stasis syndrome, 7 alkaloids components from CW were proposed as potential pharmacodynamic material basis of YNBY for activating blood circulation and removing blood stasis (Yang et al., 2019).
6. Conclusion and perspective
Chinmedomics is an approach to effective evaluation of herbal medicines that encompasses systems biology, serum pharmacochemistry of TCM and correlation analysis. Chinmedomics offers a way of interpreting herbal medicines that simultaneously embraces the syndrome and the herbal formula—two of the most important elements in clinical applications of TCM. A variety of studies have demonstrated the power of chinmedomics in understanding the efficacy of herbal medicines. Furthermore, recent applications of chinmedomics in the field of pharmacodynamic material basis-guided quality markers discovery facilitated quality control of herbal medicines—the other important reason restricting the modernization of herbal medicines.
The event that Youyou Tu won the Nobel Prize has re-introduced herbal medicines into worldwide attentions. The repertoire of herbal medicines could offer rich pickings for modern drug developers, but researchers must first explain and decipher herbal medicines with modern language. The innovations of analytical technologies and bioinformatic methodologies enable the identifications of chemical constituents and endogenous metabolites with high accuracy and sensitivity. One big concern comes from the validation of potential biomarkers of syndromes. External validation is critical in ensuring the utility of metabolomics findings. The marker metabolites selected in untargeted metabolomics studies are suggested to be validated by targeted quantitative determination to demonstrate the reliability of being diagnostic syndrome biomarkers. Another challenge is explaining the mechanisms of drug actions in-depth. Current chinmedomics strategy focuses on evaluating the efficacy of herbal medicines from a system level. More efforts should be made in the years ahead to take a closer look at therapeutic mechanisms point-to-point. For example, marker metabolites should be traced back to the relevant enzymes or RNAs, therefore, the interactions of pharmacodynamic material basis with these enzymes or RNAs could be explained using pharmacological tools in order to better understand the therapeutic mechanisms of herbal medicines. Given the increasing globalization of herbal medicines, chinmedomcis offers a new framework for thinking about how to innovate drug discovery of herbal medicines based on clinical effectiveness, and thus it is an idea whose time has come.
Declaration of Competing Interest
Ying Han, Hui Sun, Aihua Zhang, Guangli Yan, and Xi-jun Wang declare that there are no conflicts of interest.
Acknowledgments
This work was supported by Program of Natural Science Foundation of State (Grant nos. 81830110, 81430093), State Administration of Traditional Chinese Medicine of the People's Republic of China (Grant no. 2015468004-2), University Nursing Program for Young Scholars with Creative Talents of Heilongjiang Province (UNPYSCT-2016212), Program of Natural Science Foundation of Heilongjiang Province (QC2018117), Program of Returned Overseas Scholars Foundation of Heilongjiang Province (2017QD0025), Nursing Program for Young Scholars with Creative Talents of Heilongjiang University of Chinese Medicine (2018RCQ13), Heilongjiang Touyan Innovation Team Program.
References
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. The effect of malaria control on plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Y., Hu Q., Xu K., Xie X., Wang S. Improved cell membrane bioaffinity sample pretreatment technique with enhanced stability for screening of potential allergenic components from traditional Chinese medicine injections. Journal of Materials Chemistry B. 2018;6:624–633. doi: 10.1039/c7tb02768k. [DOI] [PubMed] [Google Scholar]
- Buyel J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnology Advances. 2018;36:506–520. doi: 10.1016/j.biotechadv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Cao H., Zhang A., Zhang F.M., Wang Q.Q., Zhang H., Song Y.H., et al. Ultra-performance liquid chromatography tandem mass spectrometry combined with automated MetaboLynx analysis approach to screen the bioactive components and their metabolites in Wen-Xin-Formula. Biomedical Chromatography. 2014;28:1774–1781. doi: 10.1002/bmc.3220. [DOI] [PubMed] [Google Scholar]
- Chen C., Yang F.Q., Zuo H.L., Song Y.L., Xia Z.N., Xiao W. Applications of biochromatography in the screening of bioactive natural products. Journal of Chromatographic Science. 2013;51:780–790. doi: 10.1093/chromsci/bmt002. [DOI] [PubMed] [Google Scholar]
- Cheung F. TCM: Made in China. Nature. 2011;480:S82–S83. doi: 10.1038/480S82a. [DOI] [PubMed] [Google Scholar]
- Csermely P., Agoston V., Pongor S. The efficiency of multi-target drugs: The network approach might help drug design. Trends in Pharmacological Sciences. 2005;26:178–182. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Fang H., Zhang A., Yu J., Wang L., Liu C., Zhou X., et al. Insight into the metabolic mechanism of scoparone on biomarkers for inhibiting Yanghuang syndrome. Scientific Reports. 2016;6:37519. doi: 10.1038/srep37519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H.Y., Zeng H.W., Lin L.M., Chen X., Shen X.N., Fu P., et al. A network-based method for mechanistic investigation of Shexiang Baoxin Pill's treatment of cardiovascular diseases. Scientific Reports. 2017;7:43632. doi: 10.1038/srep43632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Lv Y., Jia Q., Lin Y., Han S. Dual-mixed/CMC model for screening target components from traditional Chinese medicines simultaneously acting on EGFR & FGFR4 receptors. Talanta. 2019;192:248–254. doi: 10.1016/j.talanta.2018.09.053. [DOI] [PubMed] [Google Scholar]
- Gao H.L., Zhang A.H., Yu J.B., Sun H., Kong L., Wang X.Q., et al. High-throughput lipidomics characterize key lipid molecules as potential therapeutic targets of Kaixinsan protects against Alzheimer's disease in APP/PS1 transgenic mice. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2018;1092:286–295. doi: 10.1016/j.jchromb.2018.06.032. [DOI] [PubMed] [Google Scholar]
- Gu S., Lai L.H. Associating 197 Chinese herbal medicine with drug targets and diseases using the similarity ensemble approach. Acta Pharmacologica Sinica. 2020;41:432–438. doi: 10.1038/s41401-019-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Zhang A., Zhang Y., Sun H., Meng X., Wang X. Chemical metabolomics for investigating the protective effectiveness of Acanthopanax senticosus Harms leaf against acute promyelocytic leukemia. RSC Advances. 2018;8:11983–11990. doi: 10.1039/c8ra01029c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K., Buckley S., Collins M.J., Estalrrich A., Brothwell D., Copeland L., et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften. 2012;99:617–626. doi: 10.1007/s00114-012-0942-0. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology. Nature Biotechnology. 2007;25:1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology: The next paradigm in drug discovery. Nature Chemical Biology. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Jiang M., Lu C., Zhang C., Yang J., Tan Y., Lu A., et al. Syndrome differentiation in modern research of traditional Chinese medicine. Journal of Ethnopharmacology. 2012;140:634–642. doi: 10.1016/j.jep.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Gao H. Pharmacophore-based drug design for potential AChE inhibitors from traditional Chinese medicine database. Bioorganic Chemistry. 2018;76:400–414. doi: 10.1016/j.bioorg.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Joyner P.M., Cichewicz R.H. Bringing natural products into the fold - exploring the therapeutic lead potential of secondary metabolites for the treatment of protein-misfolding-related neurodegenerative diseases. Natural Product Reports. 2011;28:26–47. doi: 10.1039/c0np00017e. [DOI] [PubMed] [Google Scholar]
- Kiyohara H., Matsumoto T., Yamada H. Combination effects of herbs in a multi-herbal formula: Expression of Juzen-taiho-to's Immuno-modulatory activity on the intestinal immune system. Evidence-based Complementary and Alternative Medicine. 2004;1:83–91. doi: 10.1093/ecam/neh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Wu Y., Gu Y., Lv Q., Qi F., Gong S., et al. Analysis of the molecular mechanism of Pudilan (PDL) treatment for COVID-19 by network pharmacology tools. Biomedicine & Pharmacotherapy. 2020;128:110316. doi: 10.1016/j.biopha.2020.110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang C. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: A review. Journal of Ethnopharmacology. 2020:113231. doi: 10.1016/j.jep.2020.113231. [DOI] [PubMed] [Google Scholar]
- Li X., Sun H., Zhang A., Liu Z., Zou D., Song Y., et al. High-throughput LC-MS method for the rapid characterization of multiple chemical constituents and metabolites of Da-Bu-Yin-Wan. Journal of Separation Science. 2017;40:4102–4112. doi: 10.1002/jssc.201700568. [DOI] [PubMed] [Google Scholar]
- Li X.N., Zhang A., Sun H., Song Y., Zou D., Wang X. Rapid discovery of absorbed constituents and metabolites in rat plasma after the oral administration of Zi Shen Wan using high-throughput UHPLC-MS with a multivariate analysis approach. Journal of Separation Science. 2016;39:4700–4711. doi: 10.1002/jssc.201600812. [DOI] [PubMed] [Google Scholar]
- Li X.N., Zhang A., Wang M., Sun H., Liu Z., Qiu S., et al. Screening the active compounds of Phellodendri Amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Scientific Reports. 2017;7:46234. doi: 10.1038/srep46234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Ruan H., Ouyang Q., Lai L. Herb-target interaction network analysis helps to disclose molecular mechanism of traditional Chinese medicine. Scientific Reports. 2016;6:36767. doi: 10.1038/srep36767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Ma X., Li J., Li X., Guo Z., Zhou S., et al. A review of the mechanism of action of Dantonic® for the treatment of chronic stable angina. Biomedicine & Pharmacotherapy. 2019;109:690–700. doi: 10.1016/j.biopha.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Lietava J. Medicinal plants in a middle Paleolithic grave Shanidar IV. Journal of Ethnopharmacology. 1992;35:263–266. doi: 10.1016/0378-8741(92)90023-k. [DOI] [PubMed] [Google Scholar]
- Liu C., Chen S., Xiao X., Zhang T., Hou W., Liao M. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chinese Traditional and Herbal Drugs. 2016;47:1443–1457. [Google Scholar]
- Liu P., Yang H., Long F., Hao H.P., Xu X., Liu Y., et al. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharmaceutical Research. 2014;31:1788–1800. doi: 10.1007/s11095-013-1283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang A., Wang L., Yan G., Zhao H., Sun H., et al. High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Scientific Reports. 2016;6:38437. doi: 10.1038/srep38437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang A., Fang H., Li M., Song Q., Su J., et al. Serum metabolomics strategy for understanding the therapeutic effects of yin-Chen-Hao-Tang against Yang Huang syndrome. RSC Advances. 2018;8:7403–7413. doi: 10.1039/c7ra11048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H., et al. BATMAN-TCM: A bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Scientific Reports. 2016;6:21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Han Y., Chu H., Kong L., Zhang A., Yan G., et al. Characterizing serum metabolic alterations of Alzheimer's disease and intervention of Shengmai-san by ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Food & Function. 2017;8:1660–1671. doi: 10.1039/c7fo00154a. [DOI] [PubMed] [Google Scholar]
- Lyu M., Yan C.L., Liu H.X., Wang T.Y., Shi X.H., Liu J.P., et al. Network pharmacology exploration reveals endothelial inflammation as a common mechanism for stroke and coronary artery disease treatment of Danhong injection. Scientific Reports. 2017;7:15427. doi: 10.1038/s41598-017-14692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Franco J.L., Giulianotti M.A., Welmaker G.S., Houghten R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discovery Today. 2013;18:495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Y., Zhou X., Liu Q., Zhang A., Guan Y., Lin S., et al. Serum metabolomics strategy for understanding pharmacological effects of ShenQi pill acting on kidney yang deficiency syndrome. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2016;1026:217–226. doi: 10.1016/j.jchromb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ness S.L., Frye A.H., Divers T.J., Rishniw M., Erb H.N., Brooks M.B. Randomized placebo-controlled study of the effects of Yunnan Baiyao on hemostasis in horses. American Journal of Veterinary Research. 2017;78:969–976. doi: 10.2460/ajvr.78.8.969. [DOI] [PubMed] [Google Scholar]
- Phillipson J.D. Phytochemistry and medicinal plants. Phytochemistry. 2001;56:237–243. doi: 10.1016/s0031-9422(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Qv X.Y., Jiang J.G., Piao J.H. Pharmacodynamic studies of Chinese medicine at levels of whole animal, cell and molecular models. Current Medicinal Chemistry. 2010;17:4521–4537. doi: 10.2174/092986710794182926. [DOI] [PubMed] [Google Scholar]
- Ren J.L., Dong H., Han Y., Yang L., Zhang A.H., Sun H., et al. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine. 2020;77:153266. doi: 10.1016/j.phymed.2020.153266. [DOI] [PubMed] [Google Scholar]
- Ren X., Zhang M., Chen L., Zhang W., Huang Y., Luo H., et al. The anti-inflammatory effects of Yunnan Baiyao are involved in regulation of the phospholipase A2/arachidonic acid metabolites pathways in acute inflammation rat model. Molecular Medicine Reports. 2017;16:4045–4053. doi: 10.3892/mmr.2017.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams-Dodd F. Target-based drug discovery: Is something wrong. Drug Discovery Today. 2005;10:139–147. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- Shaito A., Thuan D., Phu H.T., Nguyen T., Hasan H., Halabi S., et al. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Frontiers in Pharmacology. 2020;11:422. doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q.Y., Sang X.N., Hui H., Shou Q.Y., Fu H.Y., Hao M., et al. Processing and Polyherbal Formulation of Tetradium ruticarpum (A. Juss.) Hartley: Phytochemistry, Pharmacokinetics, and Toxicity. Frontiers in Pharmacology. 2020;11:133. doi: 10.3389/fphar.2020.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.Z., Miller L.H. The discovery of artemisinin and the Nobel prize in physiology or medicine. Science China. Life Sciences. 2015;58:1175–1179. doi: 10.1007/s11427-015-4948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Yang L., Li M.X., Fang H., Zhang A.H., Song Q., et al. UPLC-G2Si-HDMS untargeted metabolomics for identification of metabolic targets of Yin-Chen-Hao-Tang used as a therapeutic agent of dampness-heat jaundice syndrome. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2018;1081-1082:41–50. doi: 10.1016/j.jchromb.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Sun H., Zhang A.H., Yang L., Li M.X., Fang H., Xie J., et al. High-throughput chinmedomics strategy for discovering the quality-markers and potential targets for Yinchenhao decoction. Phytomedicine. 2019;54:328–338. doi: 10.1016/j.phymed.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Sun M., Ma W.N., Guo Y., Hu Z.G., He L.C. Simultaneous screening of four epidermal growth factor receptor antagonists from Curcuma longa via cell membrane chromatography online coupled with HPLC-MS. Journal of Separation Science. 2013;36:2096–2103. doi: 10.1002/jssc.201200961. [DOI] [PubMed] [Google Scholar]
- Tachjian A., Maria V., Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. Journal of the American College of Cardiology. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Plant List (n.d.) (accessed 23 May 2020) http://www.theplantlist.org/.
- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nature Medicine. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Wang P., Sun H., Lv H., Sun W., Yuan Y., Han Y., et al. Thyroxine and reserpine-induced changes in metabolic profiles of rat urine and the therapeutic effect of Liu Wei Di Huang Wan detected by UPLC-HDMS. Journal of Pharmaceutical and Biomedical Analysis. 2010;53:631–645. doi: 10.1016/j.jpba.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Wang X. Methodology for systematic analysis of in vivo efficacy material base of traditional Chinese medicine—Chinmedomics. China journal of Chinese materia medica. 2015;40:13–17. [PubMed] [Google Scholar]
- Wang X., Lv H., Sun H., Liu L., Yang B., Sun W., et al. Metabolic urinary profiling of alcohol hepatotoxicity and intervention effects of yin Chen Hao Tang in rats using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:1161–1168. doi: 10.1016/j.jpba.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Q., Zhang A., Zhang F., Zhang H., Sun H., et al. Metabolomics study of intervention effects of Wen-Xin-Formula using ultra high-performance liquid chromatography/mass spectrometry coupled with pattern recognition approach. Journal of Pharmaceutical and Biomedical Analysis. 2013;74:22–30. doi: 10.1016/j.jpba.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang B., Sun H., Zhang A. Pattern recognition approaches and computational systems tools for ultra performance liquid chromatography-mass spectrometry-based comprehensive metabolomic profiling and pathways analysis of biological data sets. Analytical Chemistry. 2012;84:428–439. doi: 10.1021/ac202828r. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang B., Zhang A., Sun H., Yan G. Potential drug targets on insomnia and intervention effects of Jujuboside A through metabolic pathway analysis as revealed by UPLC/ESI-SYNAPT-HDMS coupled with pattern recognition approach. Journal of Proteomics. 2012;75:1411–1427. doi: 10.1016/j.jprot.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang A., Han Y., Wang P., Sun H., Song G., et al. Urine metabolomics analysis for biomarker discovery and detection of jaundice syndrome in patients with liver disease. Molecular & Cellular Proteomics. 2012;11:370–380. doi: 10.1074/mcp.M111.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang A., Sun H. Future perspectives of Chinese medical formulae: Chinmedomics as an effector. OMICS. 2012;16:414–421. doi: 10.1089/omi.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang A., Sun H., Yan G. Precision diagnosis of Chinese medicine syndrome and evaluation of prescription efficacy based on Chinmedomics. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2017;19:30–34. [Google Scholar]
- Wang X., Zhang A., Zhou X., Liu Q., Nan Y., Guan Y., et al. An integrated chinmedomics strategy for discovery of effective constituents from traditional herbal medicine. Scientific Reports. 2016;6:18997. doi: 10.1038/srep18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang S., Zhang A., Yan G., Wu X., Han Y., et al. Metabolomics study of type 2 diabetes and therapeutic effects of Tianqijiangtang-capsule using ultra performance liquid chromatography/electrospray ionization quadruple timeof-flight mass spectrometry. Analytical Methods. 2013;5:2218–2226. [Google Scholar]
- Wang X.J., Zhang A.H., Kong L., Yu J.B., Gao H.L., Liu Z.D., et al. Rapid discovery of quality-markers from Kaixin san using chinmedomics analysis approach. Phytomedicine. 2019;54:371–381. doi: 10.1016/j.phymed.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Weng J.K., Philippe R.N., Noel J.P. The rise of chemodiversity in plants. Science. 2012;336:1667–1670. doi: 10.1126/science.1217411. [DOI] [PubMed] [Google Scholar]
- WHO . 2000. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. [Google Scholar]
- Willcox M.L., Bodeker G. Traditional herbal medicines for malaria. BMJ. 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhao C., Zhang A., Zhang J., Wang X., Sun X., et al. High-throughput metabolomics used to identify potential therapeutic targets of Guizhi Fuling Wan against endometriosis of cold coagulation and blood stasis. RSC Advances. 2018;8:19238–19250. doi: 10.1039/c8ra00978c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Zhang A.H., Zhao Q.Q., Yan G.L., Sun H., Wang X.J. Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine. 2020;74:152928. doi: 10.1016/j.phymed.2019.152928. [DOI] [PubMed] [Google Scholar]
- Xu F., Cui W., Kong Q., Tang Z., Dong J. A real-world evidence study for distribution of traditional Chinese medicine syndrome and its elements on respiratory disease. Evidence-based Complementary and Alternative Medicine. 2018;2018:8305892. doi: 10.1155/2018/8305892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Modernization: One step at a time. Nature. 2011;480:S90–S92. doi: 10.1038/480S90a. [DOI] [PubMed] [Google Scholar]
- Yan G.L., Zhang A.H., Sun H., Han Y., Shi H., Zhou Y., et al. An effective method for determining the ingredients of Shuanghuanglian formula in blood samples using high-resolution LC-MS coupled with background subtraction and a multiple data processing approach. Journal of Separation Science. 2013;36:3191–3199. doi: 10.1002/jssc.201300529. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhang A., Dong H., Yan G., Sun H., Wu X., et al. Toxicity and detoxification effects of herbal Caowu via ultra performance liquid chromatography/mass spectrometry metabolomics analyzed using pattern recognition method. Pharmacognosy Magazine. 2017;13:683–692. doi: 10.4103/pm.pm_475_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Han Y., Zhang Q.Y., Dong H., Sun H., Wang X.J. Study on absorbed components of Aconitum kusnezoffii under Yunnan Baiyao compatibility in effect of activating blood circulation and removing blood stasis. Zhongguo Zhong Yao Za Zhi. 2019;44:3349–3357. doi: 10.19540/j.cnki.cjcmm.20190711.202. [DOI] [PubMed] [Google Scholar]
- Yang B., Zhang A., Sun H., Dong W., Yan G., Li T., et al. Metabolomic study of insomnia and intervention effects of Suanzaoren decoction using ultraperformance liquid–chromatography/electrospray-ionization synapt highdefinition mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2012;58:113–124. doi: 10.1016/j.jpba.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Yu C., Wang C.Z., Zhou C.J., Wang B., Han L., Zhang C.F., et al. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. Journal of Pharmaceutical and Biomedical Analysis. 2014;99:8–15. doi: 10.1016/j.jpba.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zálešák F., Bon D., Pospíšil J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacological Research. 2019;146:104284. doi: 10.1016/j.phrs.2019.104284. [DOI] [PubMed] [Google Scholar]
- Zhang A., Liu Q., Zhao H., Zhou X., Sun H., Nan Y., et al. Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Scientific Reports. 2016;6:19333. doi: 10.1038/srep19333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Sun H., Qiu S., Wang X. Advancing drug discovery and development from active constituents of yinchenhao tang, a famous traditional chinese medicine formula. Evidence-based Complementary and Alternative Medicine. 2013;2013:257909. doi: 10.1155/2013/257909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Sun H., Wang X., Jiao G., Yuan Y., Sun W. Simultaneous in vivo RP-HPLC-DAD quantification of multiple-component and drug-drug interaction by pharmacokinetics, using 6,7-dimethylesculetin, geniposide and rhein as examples. Biomedical Chromatography. 2012;26:844–850. doi: 10.1002/bmc.1739. [DOI] [PubMed] [Google Scholar]
- Zhang A., Yan G., Zhou X., Wang Y., Han Y., Guan Y., et al. High resolution metabolomics technology reveals widespread pathway changes of alcoholic liver disease. Molecular BioSystems. 2016;12:262–273. doi: 10.1039/c5mb00603a. [DOI] [PubMed] [Google Scholar]
- Zhang A.H., Yu J.B., Sun H., Kong L., Wang X.Q., Zhang Q.Y., et al. Identifying quality-markers from Shengmai san protects against transgenic mouse model of Alzheimer's disease using chinmedomics approach. Phytomedicine. 2018;45:84–92. doi: 10.1016/j.phymed.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang J.H., Zhu Y., Fan X.H., Zhang B.L. Efficacy-oriented compatibility for component-based Chinese medicine. Acta Pharmacologica Sinica. 2015;36:654–658. doi: 10.1038/aps.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang A., Zhang Y., Sun H., Meng X., Yan G., et al. Application of Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanax senticosus Harms Leaf. Pharmacognosy Magazine. 2016;12:145–152. doi: 10.4103/0973-1296.177902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Gao X., Yan G., Zhang A., Sun H., Han Y., et al. Chinmedomics facilitated quality-marker discovery of Sijunzi decoction to treat spleen qi deficiency syndrome. Frontiers in Medicine. 2019;14:335–356. doi: 10.1007/s11684-019-0705-9. [DOI] [PubMed] [Google Scholar]
- Zhou X.H., Zhang A.H., Wang L., Tan Y.L., Guan Y., Han Y., et al. Novel chinmedomics strategy for discovering effective constituents from ShenQiWan acting on ShenYangXu syndrome. Chinese Journal of Natural Medicines. 2016;14:561–581. doi: 10.1016/S1875-5364(16)30067-X. [DOI] [PubMed] [Google Scholar]
- Zuo Y.H., Liu Y.B., Cheng C.S., Yang Y.P., Xie Y., Luo P., et al. Isovaleroylbinankadsurin A ameliorates cardiac ischemia/reperfusion injury through activating GR dependent RISK signaling. Pharmacological Research. 2020;158:104897. doi: 10.1016/j.phrs.2020.104897. [DOI] [PubMed] [Google Scholar]