Abstract
Land plants are exposed to not only biotic stresses such as pathogen infection and herbivore wounding, but abiotic stresses such as cold, heat, drought, and salt. Elaborate strategies have been developed to avoid or abide the adverse effects, with unsaturated fatty acids (UFAs) emerging as general defenders. In higher plants, the most common UFAs are three 18-carbon species, namely, oleic (18:1), linoleic (18:2), and α-linolenic (18:3) acids. These simple compounds act as ingredients and modulators of cellular membranes in glycerolipids, reserve of carbon and energy in triacylglycerol, stocks of extracellular barrier constituents (e.g., cutin and suberin), precursors of various bioactive molecules (e.g., jasmonates and nitroalkenes), and regulators of stress signaling. Nevertheless, they are also potential inducers of oxidative stress. In this review, we will present an overview of these roles and then shed light on genetic engineering of FA synthetic genes for improving plant/crop stress tolerance.
Keywords: land plants, unsaturated fatty acids, stress-related roles, genetic engineering, stress tolerance
Introduction
Land plants are living in a harsh environment that imposes strikingly manifold stresses on their health and productivity. Biotic stressors include viruses, bacteria, fungi, nematodes, and arthropods, among others. The first four pathogens cause various diseases such as leaf spot, stem rust, and root rot, while arthropods are herbivorous or parasitic and may serve as pathogen vector. Abiotic stressors include temperature (low or high), water (deficit or excess), ultraviolet, salt, heavy metals, and so on. High salinity, for example, can induce ionic toxicity, osmotic pressure, oxidative damage, and nutrient deficiency (Zhao et al., 2010; Feng et al., 2014a; Feng et al., 2014b), thereby strongly suppressing the whole lifecycle of most plants (Guo et al., 2012; Guo et al., 2015; Guo et al., 2018). More seriously, in the context of climate change and soil salinization, cold/heat, drought and salt usually arise together, posing an increasingly severe threat to plant subsistence and cultivation worldwide (Yuan et al., 2015; He et al., 2018). Even worse is that biotic stresses may occur simultaneously with them.
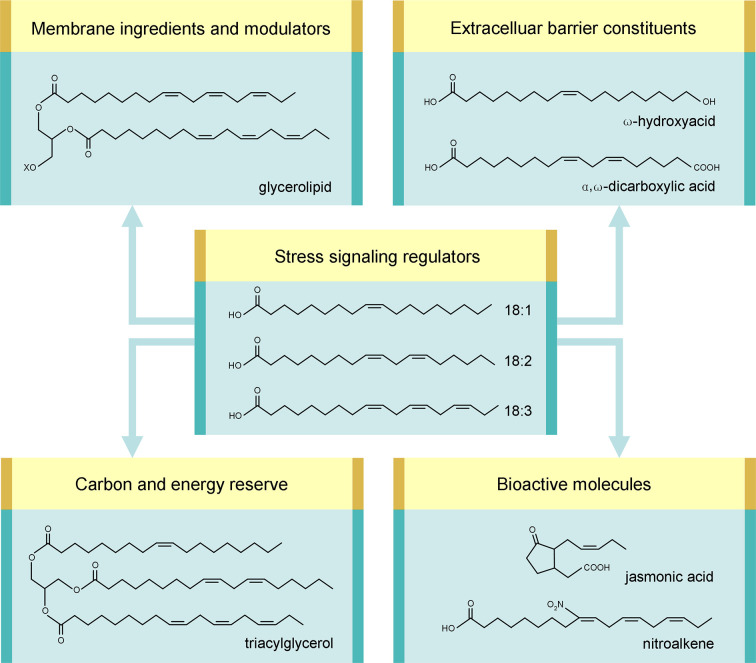
Being sessile organisms, land plants cannot but face the stresses and hence have evolved elaborate strategies to avoid or abide the adverse effects. Unsaturated fatty acids (UFAs) are coming into the limelight as one of the general defense systems against various biotic and abiotic stresses (He et al., 2018). In higher plants, the most common UFAs are three 18-carbon (C18) species: 18:1 (oleate), 18:2 (linoleate), and 18:3 (α-linolenate), where m:n denotes an FA with m carbon atoms and n cis-double bonds (He et al., 2020). Here, we will present an overview of their roles in stress defense as follows: (1) ingredients and modulators of cellular membranes in glycerolipids; (2) reserve of carbon and energy in triacylglycerol (TAG); (3) stocks of extracellular barrier constituents; (4) precursors of various bioactive molecules; (5) regulators of stress signaling (Figure 1). In addition, light will be shed on genetic engineering of FA synthetic genes for improving stress tolerance. For better understanding, the metabolism of C18 UFAs (Figure 2), as well as the intertwinement between C18 UFAs and reactive species (RS) (Figure 3), will be briefly introduced first.
Figure 1.
Multiple roles of C18 unsaturated fatty acids in stress defense. Examples of corresponding derivatives are shown. “X” stands for the head group of glycerolipid.
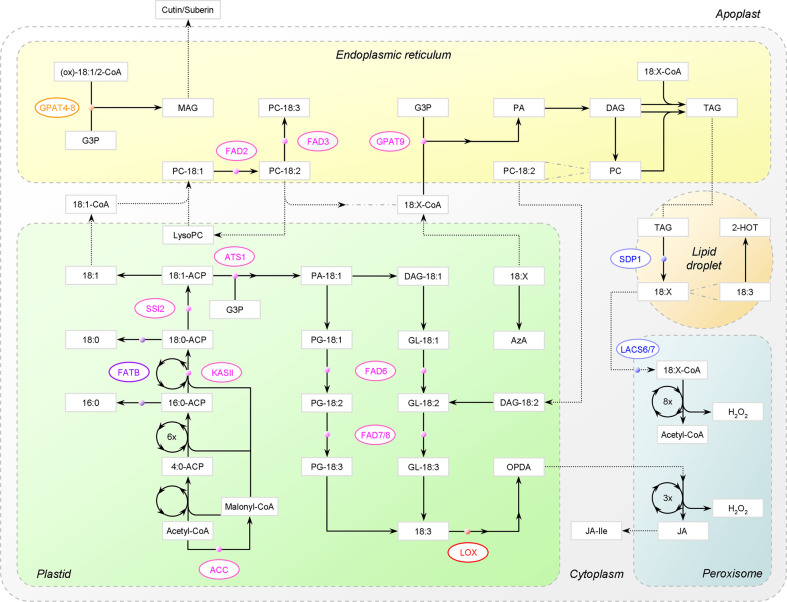
Figure 2.
Major metabolic pathways of C18 unsaturated fatty acids (UFAs) in Arabidopsis. FA synthesis in plastids and β-oxidation in peroxisomes are depicted as circle. PC-18:2 is used as an example substrate to show the acyl editing way of 18:1 esterification and the eukaryotic way of glycolipid synthesis. Dotted arrow denotes that trafficking is involved. For simplicity, the bypath in JA synthesis, connections between acyl-CoA pools, and connections between 18:X and membrane lipids are not shown. ACC, acetyl-CoA carboxylase; ACP, acyl-carrier protein; KASII, 3-ketoacyl-ACP synthase II; FATB, acyl-ACP thioesterase B; SSI2, stearoyl-ACP desaturase; G3P, glycerol-3-phosphate; GPAT, G3P acyltransferase; ATS1, plastidial GPAT; PA, phosphatidic acid; DAG, diacylglycerol; PG, phosphatidylglycerol; GL, glycolipids, PC, phosphatidylcholine; FAD, fatty acid desaturase; (ox)-18:1/2-CoA, unmodified or oxygenated 18:1/18:2-CoA; MAG, monoacylglycerol; 18:X, C18 UFAs; TAG, triacylglycerol; SDP1, TAG lipase; LACS, long-chain acyl-CoA synthetase; 2-HOT, 2-hydroxy-octadecatrienoic acid; AzA, azelaic acid; LOX, 13-lipoxygenase; OPDA, 12-oxo-phytodienoic acid; JA, jasmonic acid; JA-Ile, jasmonoyl-isoleucine.
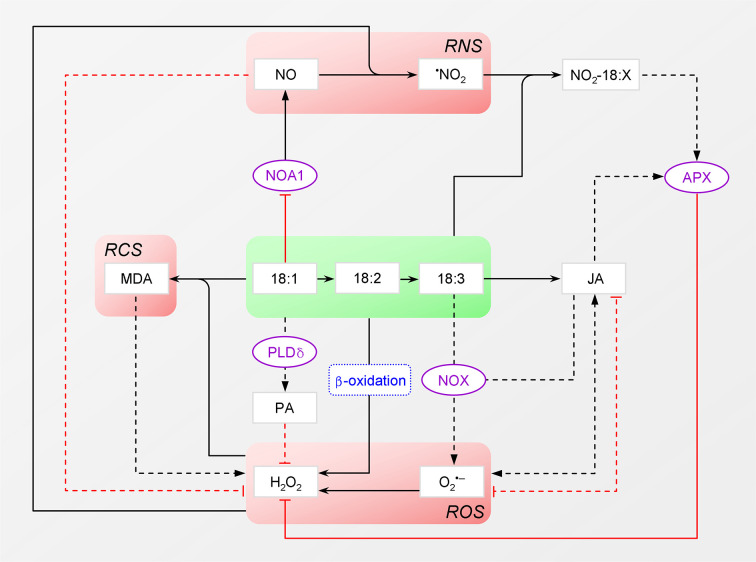
Figure 3.
Intertwinement between C18 unsaturated fatty acids and reactive species. Reaction is distinguished from activation and catalysis with different arrowhead. Dashed line denotes indirect effect (e.g., via signaling). Please note that the crosstalk between JA and NO is not shown. ROS, reactive oxygen species; RCS, reactive carbonyl species; RNS, reactive nitrogen species; MDA, malondialdehyde; PLDδ, plasma membrane phospholipase D; PA, phosphatidic acid; NOX, NADPH oxidase; JA, jasmonic acid; APX, ascorbate peroxidase; 18:X, C18 unsaturated fatty acids; NOA1, NITRIC OXIDE ASSOCIATED 1.
Metabolism of C18 Unsaturated Fatty Acids
As depicted in Figure 2, C18 UFAs stem from acetyl-coenzyme A (CoA) via de novo FA synthesis in plastids. 18:0-ACP, the C18 end product attached to acyl-carrier protein, is mostly destined for the unsaturation program conducted by a cascade of FA desaturases (FADs). Once produced, it is rapidly converted to 18:1-ACP by stearoyl-ACP desaturase (SAD). However, not until being esterified into membrane glycerolipids will 18:1(Δ9) be processed to 18:2(Δ9, 12) by ω-6 FADs and further to 18:3(Δ9, 12, 15) by ω-3 FADs, where Δ and ω stand for the carboxylic and methyl ends, respectively [for more, see reviews (Ohlrogge and Browse, 1995; He et al., 2020)].
There are two pathways for membrane glycerolipid biogenesis: the “prokaryotic” one in plastids and the “eukaryotic” one in the endoplasmic reticulum (ER). Both are based on sequential acylation of glycerol-3-phosphate (G3P), with phosphatidic acid (PA) and diacylglycerol (DAG) being the intermediates. The first reaction is catalyzed by G3P acyltransferase (GPAT), rendering it a key enzyme. With the emergence of substrate glycerolipids, C18 polyunsaturated FAs (PUFAs) are then generated by the ω-6 and ω-3 pair of FADs, i.e., plastidial FAD6-FAD7/8 or ER FAD2-FAD3 (Ohlrogge and Browse, 1995; He et al., 2020).
In the eukaryotic pathway, phosphatidylcholine (PC) is the vector for FA desaturation. However, nascent 18:1 exported out of plastids appears to prefer the acyl editing way to be directly incorporated into PC rather than the multistep de novo way (He et al., 2020). The exchanged acyl chains (e.g., 18:2) can then enter other metabolic fates, for example, the production of TAG prevailing in seed cells or cutin unlocked in epidermal cells. Of note, unlike PC and TAG that are directly converted from DAG, cutin or suberin is assembled via monoacylglycerol (MAG) (Beisson et al., 2012).
Further, C18 UFAs, esterified or released, can be modified into a great variety of bioactive molecules, such as jasmonic acid (JA), azelaic acid (AzA), and 2-hydroxy-octadecatrienoic acid (2-HOT) from oxidation. Nevertheless, when incorporated into TAG, their major fate is to retrograde into acetyl-CoA via β-oxidation in peroxisomes. Notably, FAs removed from membrane lipids for turnover will be deposited in TAG first (Fan et al., 2017).
Intertwinement Between C18 Unsaturated Fatty Acids and Reactive Species
A common stress consequence is the burst of reactive oxygen species (ROS), e.g., superoxide anion (O2•–) and hydrogen peroxide (H2O2). ROS can readily attack various biomolecules, with lipids, proteins, carbohydrates, and nucleic acids all being endangered (Saxena et al., 2016). Their excess can thereby bring cells to oxidative catastrophe, including membrane injury, photoinhibition augment, and gene mutation (Sharma et al., 2012; Sui, 2015). Nevertheless, these inevitable toxic byproducts of aerobic metabolism are tactically exploited as signaling molecules to coordinate diverse biological processes, particularly stress defense. They are also actively produced as poisons to retort biotic intruders. Plants have therefore developed sophisticated producing and scavenging systems to adjust ROS levels according to the context. For instance, NADPH oxidase (NOX, or RBOH for respiratory burst oxidase homolog) generates O2•–, whereas ascorbate peroxidase (APX) eliminates H2O2 (Gechev et al., 2006; Choudhury et al., 2017).
Due to the presence of double bond, C18 UFAs are vulnerable to ROS. In fact, malondialdehyde (MDA), a product of their peroxidation, is widely used as an indicator of oxidative stress (Guo et al., 2012). Notably, MDA is a latent reactive carbonyl species (RCS) capable of launching a new round of attack under acidic conditions. By forming covalent adducts termed advanced lipoxidation end-products (ALEs), this RCS can cause protein malfunction and consequent ROS proliferation (Farmer and Davoine, 2007; Deng et al., 2010). C18 UFAs are hence turned from victims into accomplices. Moreover, FA β-oxidation is a source of H2O2, while 18:3 is a likely stimulator of NOX, which will be discussed later on.
On the contrary, the chemical nature also renders C18 UFAs intrinsic antioxidants; that is, they can directly react with and thus consume ROS. And their oxidation gives rise to various oxylipins, as represented by the stress hormone JA, which, in turn, modulates ROS levels and signaling (see below). Interestingly, 18:3, though less effective than 18:1, can also stimulate the plasma membrane (PM) phospholipase D (PLDδ) that generates PA to attenuate H2O2-induced cell death in Arabidopsis (Wang and Wang, 2001; Zhang et al., 2003). Further, the participation of nitric oxide (NO) (see below), the primary reactive nitrogen species (RNS), adds one more layer of complexity to the intertwinement between C18 UFAs and RS (Figure 3).
Multiple Roles of C18 Unsaturated Fatty Acids in Stress Defense
In planta, C18 UFAs are utilized as raw material to produce numerous aliphatic compounds, including membrane glycerolipids, TAG, cutin/suberin, jasmonates, and nitroalkenes (NO2-FAs). It is remarkable that all these products, as well as C18 UFAs themselves, take part in plant defense against various biotic and abiotic stresses. C18 UFAs are therefore implicated, directly and indirectly, in stress defense via multiple mechanisms (examples listed in Table 1), which will be discussed hereinafter.
Table 1.
Mechanisms for C18 unsaturated fatty acids in stress defense.
| Related molecule | Mechanism |
|---|---|
| Membrane glycerolipids | Maintaining proper membrane fluidity required for multiple membrane-dependent processes, e.g., Ca2+ signaling |
| Modulating directly activities of membrane-bound proteins, e.g., plasma membrane (PM) H+-ATPase | |
| Mitigating stress-enhanced photoinhibition on photosystem II | |
| Signaling to regulate stress defense, e.g., phosphatidic acid (PA) can antagonize H2O2 in inducing cell death | |
| Triacylglycerol | Supplying carbon and energy for stress survival or recovery |
| Cutin/Suberin | Constructing physically and chemically resistant barrier |
| Jasmonates | Signaling to organize local and/or systemic defense |
| Nitroalkenes | Signaling to motivate heat shock proteins and antioxidant enzymes |
| 118:1/2/3 | Stimulating PM phospholipase D (PLDδ) and thus PA production |
| 18:1 | Suppressing NITRIC OXIDE ASSOCIATED 1 (NOA1) and thus NO production |
| 18:3 | Stimulating PM NADPH oxidase (NOX) and thus O2•– production |
Ingredients and Modulators of Cellular Membranes
A main building block of biomembranes is glycerolipid composed of a glycerol core attached with a polar “head” group and two nonpolar “tails” derived primarily from C16/C18 FAs (Figure 1). The acyl chains inside the bilayer have a profound impact on membrane properties. Particularly, their unsaturation degree is a major factor shaping membrane fluidity. A higher unsaturation degree can lead to a more fluid state because a cis-double bond creates a kink as steric hindrance in intermolecular package (He et al., 2018). Of the membrane-constituting UFAs, C18 species are prominent modulators of unsaturation degree, since trans-16:1 has a high melting point and 16:3 is only present in several plants (Ohlrogge and Browse, 1995).
Biomembranes serve as not only structural barrier for cells and intracellular organelles, but functional platform for multiple cellular processes, including substance exchange, signal transduction, and many metabolic reactions. A notable example is that the signaling of Ca2+, a versatile second messenger active in plant response to virtually every stress, is based on membrane isolation and transportation. Ca2+ signaling is ignited by sharp influx of the cation into the cytosol through its channels, including the PM cyclic nucleotide-gated channels (CNGCs) and the tonoplast TWO PORE CHANNEL 1 (TPC1) (Lu et al., 2016; Zheng et al., 2017), and is quickly quenched by efflux through Ca2+-ATPases and Ca2+/H+ exchangers (Han et al., 2011; Han et al., 2012).
Some other ion transporters, including the K+ rectifier ARABIDOPSIS K+ TRANSPORTER 1 (AKT1) (Ren et al., 2013) and the Na+/H+ antiporter SALT OVERLY SENSITIVE 1 (SOS1) (Deng et al., 2016; Kong et al., 2016), maintain a properly high K+/Na+ ratio in the cytoplasm, which is critical for salt tolerance (Chen et al., 2005; Feng et al., 2015). These secondary transporters are energized by virtue of the electrochemical gradient created by transmembrane proton pumps, e.g., PM H+-ATPase, vacuolar H+-ATPase (V-ATPase), and vacuolar H+-translocating inorganic pyrophosphatase (V-PPase) (Chen et al., 2010; Yang et al., 2010; Yuan et al., 2016). Notably, the PM H+-ATPase is a key site responsive to not only salt, but other stresses like cold and heavy metals that also involve active transport across the PM (Martz et al., 2006; Shi et al., 2008; Janicka-Russak et al., 2012).
Besides bulk lipids that can alter membrane fluidity, the conformation and function of a membrane-bound protein may be affected by annular (selectively enriched) or non-annular (tightly bound) lipids (Contreras et al., 2011). As in the case of the PM H+-ATPase, its catalytic activity has an absolute requirement for phospholipid, albeit the mechanisms underlying such regulation are still uncertain [see reviews (Kasamo, 2003; Morales-Cedillo et al., 2015)]. When the stimulatory effect of PC was tested in vitro, the ATPase activity decreased with increased FA chain length or unsaturation degree, and maximum activation was achieved with the combination of 14:0 and 18:1 (Kasamo, 1990). Free FAs and lysoPC, the hydrolytic products of PC, could also activate the enzyme, with 18:3 being the most effective C18 species (Palmgren et al., 1988). In vivo, however, the ATPase activity appears to have a correlation with double bond index (DBI) rather than individual C18 UFAs (Martz et al., 2006; Shi et al., 2008).
Membrane fluidity is susceptible to multiple stresses, especially extreme temperatures. However, both cold-induced rigidification and heat-induced fluidization are detrimental to membrane function, resulting in protein deactivation, electrolyte leakage, and even cytoskeleton destabilization (Sangwan et al., 2002; Liu et al., 2013; He et al., 2018). In fact, being a thermodynamic property, membrane fluidity may serve as a sensor in temperature signaling (Ruelland and Zachowski, 2011). Interestingly, the effects of cold and heat can be mimicked at 25°C by dimethylsulfoxide (DMSO) and benzyl alcohol (BA), respectively. In alfalfa (Medicago sativa), the two chemicals induced opposite changes in membrane fluidity coupled with cytoskeleton disassembly, which triggered Ca2+ signaling to activate two distinct mitogen-activated protein kinase (MAPK) cascades, leading to corresponding stress response (Sangwan et al., 2002).
Plants are poikilothermic organisms, highlighting the especial importance of membrane remodeling, not to mention the pressure from climate change. Adjusting the unsaturation degree of FA tails is an efficient strategy favored by plants in offsetting thermal perturbations to keep membrane fluidity in the optimal range, as manifested by the temperature sensitivity of FAD mutants [see review (Wallis and Browse, 2002)] or transformants (Table 2). Particularly, the unsaturation degree of chloroplast phosphatidylglycerol (PG) is a major factor affecting cold tolerance [see reviews (Nishida and Murata, 1996; Iba, 2002)].
Table 2.
Altered stress tolerance upon genetic manipulation of fatty acid synthetic genes.
| Enzyme | Genea | Genetic manipulationb | Stress tolerancec | Reference |
|---|---|---|---|---|
| Stearoyl-ACP desaturase | SSI2/FAB2 | OE in Brassica napus from Sapium sebiferum | ↑freezing | (Peng et al., 2018) |
| ω-6 Fatty acid desaturase | FAD2 | OE in Oryza sativa | ↑cold | (Shi et al., 2012) |
| ω-3 Fatty acid desaturase | FAD3 | OE in Nicotiana tabacum from B. napus | ↑drought | (Zhang et al., 2005) |
| OE in Lycopersicon esculentum | ↑cold | (Yu et al., 2009) | ||
| OE in L. esculentum | ↑salt | (Wang et al., 2014) | ||
| OE in N. tabacum from Chorispora bungeana | ↑cold, drought and salt | (Shi et al., 2018) | ||
| FAD7 | OE in N. tabacum from Arabidopsis thaliana | ↑cold | (Kodama et al., 1994) | |
| CS in N. tabacum from A. thaliana | ↑heat | (Murakami et al., 2000) | ||
| AS in N. tabacum from A. thaliana | ↓drought and salt | (Im et al., 2002) | ||
| FAD8 | OE in N. tabacum from A. thaliana | ↑drought | (Zhang et al., 2005) | |
| Glycerol-3-phosphate acyltransferase | ATS1/ACT1 | OE in N. tabacum from A. thaliana | ↑cold | (Murata et al., 1992) |
| AS in L. esculentum | ↑heat | (Sui et al., 2007) | ||
| OE in A. thaliana from Suaeda salsa | ↑salt | (Sui et al., 2017) | ||
| OE in A. thaliana from Ammopiptanthus mongolicus | ↑cold, freezing and oxidative | (Xue et al., 2019) |
Arabidopsis gene name.
OE, overexpression; CS, co-suppression; AS, antisense suppression.
”↑”, enhanced; “↓”, reduced.
Being the sole phospholipid species present in thylakoid membranes, PG is an indispensable constituent of the membrane-bound photosynthetic apparatus, e.g., photosystem II (PSII) (Wada and Murata, 2007). PSII is subject to photoinhibition, wherein the D1 protein of the reaction center is caught in an endless loop of photodamage and repair (Takahashi and Murata, 2008; Liu et al., 2016). PG desaturation is capable of protecting PSII from cold-enhanced photoinhibition (Moon et al., 1995). This may apply to other stresses that can potentiate the process as well (Takahashi and Murata, 2008). Under saline conditions, alleviated PSII photoinhibition has been observed to be accompanied with elevated UFA content in membrane lipids (Sui et al., 2010; Sui and Han, 2014; Liu et al., 2017). Indeed, specifically raising the unsaturation level of PG could accelerate the repair of D1 (Sun et al., 2010).
However, it should be pointed out that the relationship between membrane (particularly PM) lipid unsaturation and salt tolerance are elusive (examples listed in Table 3). For salt-sensitive species, it is plausible that increase and decrease in the unsaturation degree reflect defense and damage, respectively, depending on their sensitivity to certain salinity. This is supported by the behavior of peanut (Arachis hypogaea) that is moderately tolerant to salt — the DBI of total leaf lipids went up at 150 mM NaCl, but fell down at higher concentrations (Liu et al., 2017; Sui et al., 2018). Notably, choline (Salama and Mansour, 2015) and silicon (Liang et al., 2006) were able to reverse the reductions in PM unsaturation of wheat (Triticum sativum) and PM fluidity of barley (Hordeum vulgare), respectively, which contributed to improved salt tolerance.
Table 3.
Changes in membrane lipid unsaturation under salt stress.
| Species | Tissue | Tolerance | Membrane | Lipid unsaturationa | Reference | |||
|---|---|---|---|---|---|---|---|---|
| no/lower NaCl | highest NaCl | change | ||||||
| single | Brassica oleracea | root | tolerant | plasma | 137* | 171* | ↑ | (López-Pérez et al., 2009) |
| Carthamus tinctorius | root | tolerant | total | 85.21* | 126.37* | ↑ | (Harrathi et al., 2012) | |
| Suaeda salsa | leaf | halophyte | total | 126.7* | 144.7* | ↑ | (Sui et al., 2010) | |
| Triticum aestivum | root | sensitive | plasma | 1.9 | 1.5 | ↓ | (Mansour et al., 1994) | |
| Glycine max | root | sensitive | plasma | 1.04 | 0.79 | ↓ | (Surjus and Durand, 1996) | |
| pair | Hordeum maritimum | root | tolerant | total | 1.30 | 1.26 | → | (Chalbi et al., 2013) |
| Hordeum vulgare | sensitive | 0.46 | 1.02 | ↑ | ||||
| Thellungiella halophila | leaf | halophyte | total | 73.59* | 124.79* | ↑ | (Sui and Han, 2014) | |
| Arabidopsis thaliana | sensitive | 104.03* | 51.06* | ↓ | ||||
| Buchloe dactyloides | root | tolerant | plasma | 23* | 123* | ↑ | (Lin and Wu, 1996) | |
| sensitive | – | 66* | ↑ | |||||
| Lycopersicon esculentum | callus | tolerant | plasmab | 45.5* | 32.4* | ↓ | (Kerkeb et al., 2001) | |
| sensitive | 46.1* | n/a | n/a | |||||
| Zea mays | root | tolerant | plasma | 0.62 | 0.24 | ↓ | (Salama et al., 2007) | |
| sensitive | 0.54 | 0.46 | ↓ | |||||
| Brassica napus | root | tolerant | plasma | 0.60, 0.65 | 0.28, 0.22 | ↓ | (Zamani et al., 2010) | |
| sensitive | 0.54-0.55 | 0.44-0.46 | ↓ | |||||
Unsaturation to saturation ratio if not labeled; bphosphatidylcholine unsaturation; “*”, double bond index; “–”, Not detected; “↑”, increase; “→”, little change; “↓”, decrease.
Phosphatidylcholine unsaturation.
Surprisingly, increasing, maintaining and decreasing the unsaturation degree are instrumental for different salt-tolerant species. Of species like maize (Zea mays), the tolerant varieties exhibited a greater decline than did the sensitive ones upon NaCl treatment (Table 3). Such change was assumed to reduce PM fluidity and thus the permeability and import of Na+ and Cl−, thereby alleviating salt stress. Another possibility is that, in these species, while other factors (e.g., sterol composition) are more involved in modulating PM properties, UFA content is lowered to mitigate PM susceptibility to salt-induced oxidative damage [see review (Guo et al., 2019)]. Nevertheless, gain- or loss-of-function of FADs has demonstrated the positive effect of FA unsaturation on salt tolerance [Table 2 and (Guo et al., 2019)]. Of note, even though tomato (Lycopersicon esculentum or Solanum lycopersicum) fell into the decreasing group (Table 3), FAD3 overexpression did enhance the ability of early seedlings to resist high salinity (Table 2).
Reserve of Carbon and Energy
FAs are incorporated into TAG (Figure 1) and packaged in lipid droplets (or oil bodies) as carbon and energy reserve. In seed cells, TAG is accumulated for fueling seedling establishment, which is fundamental to plant revival after escaping biotic and abiotic stresses via seed dormancy. When conditions become suitable for germination, FAs are channeled to metabolic breakdown via the β-oxidation spiral operating in glyoxysomes, the specialized peroxisomes (Figure 2). Acetyl-CoA is thereby regenerated, which is a core metabolite for energy production via mitochondrial respiration and for carbohydrate anabolism via the glyoxylate cycle and gluconeogenesis [see reviews (Graham, 2008; Xu and Shanklin, 2016)]. Noteworthily, acetyl-CoA is also the donor for histone acetylation, conferring peroxisomal FA β-oxidation a regulatory role in nuclear epigenetic modification, which may affect diverse cellular processes (Wang et al., 2019).
In Arabidopsis, the mobilization process has been characterized, though not fully understood. Firstly, FAs are liberated by a series of lipases, including the TAG lipase SUGAR-DEPENDENT1 (SDP1) (Eastmond, 2006) and the MAG lipase MAGL8 (Kim et al., 2016). A likely route for peroxisome import is that FAs are transferred as CoA esters across the envelope by the peroxisomal ATP-binding cassette (ABC) transporter PXA1 (also ABCD1, CTS or PED3); however, CoA will be lost due to the intrinsic acyl-CoA thioesterase activity of PXA1 (De Marcos Lousa et al., 2013). LACS6 and -7, two long-chain acyl-CoA synthetases, are then required to re-activate FAs. Subsequently, stepwise chain truncation by β-oxidation gives rise to acetyl-CoA per round [for detail, see review (Graham, 2008)].
Notably, TAG is more than an energy-dense storage form. It appears to be a key intermediate in peroxisomal degradation of membrane-constituting FAs. In other words, to enter β-oxidation, FAs removed from membrane lipids will be deposited in TAG first (Fan et al., 2017). In vegetative tissues, despite the high synthetic capacity, TAG yield is constrained due to biased partitioning toward membrane glycerolipid production and rapid SDP1-driven TAG turnover (Xu and Shanklin, 2016). Upon carbon depletion that can be induced by extended darkness, chloroplast lipids will be mobilized as substitutes for respiration. However, when Arabidopsis sdp1 mutants were under this situation, 16:3 unique to the plastid-intrinsic monogalactosyldiacylglycerol (MGDG) accumulated in TAG. The even distribution of 16:3 across the three positions of TAG suggested that 16:3 was first hydrolyzed and then exported to the ER for de novo TAG synthesis (Fan et al., 2017).
Indeed, PLASTID LIPASE 1 (PLIP1), a thylakoid-associated phospholipase A1, has been identified to drive the turnover of PG with sn-2 trans-16:1 by cleaving sn-1 PUFAs, primarily 18:3. In seed cells, the liberated 18:3 is eventually incorporated into TAG, with PC serving as either DAG or acyl donor (Wang et al., 2017). Of note, free FAs, as well as PA and DAG, are cytotoxic. TAG accumulation hence plays a pivotal role in sequestering them into lipid droplets, thereby buffering lipid homeostasis and protecting cells against lipotoxic death due to FA overload (Fan et al., 2017), which can be a consequence of robust membrane remodeling in stress response. Under heat stress, for instance, HEAT INDUCIBLE LIPASE 1 (HIL1) removes 18:3 from MGDG to decrease membrane fluidity, resulting in increased level of 18:3-containing TAG (Higashi et al., 2018).
Nevertheless, FA β-oxidation is a double-edged sword for plants in that a byproduct is H2O2 (Figure 2). For H2O2 scavenging, peroxisomes deploy double insurance — catalase (CAT) present in the matrix to protect themselves plus the APX system associated with the membrane to prevent leakage into the cytoplasm (Graham, 2008). If H2O2 generation exceeds detoxification, it is natural that FA β-oxidation will be turned into an inducer of oxidative stress and ultimate cell death, as happened under extended darkness, rendering TAG accumulation another protecting role (Fan et al., 2017).
TAG mobilization may therefore be subjected to opposite regulation, according to whether the supply of carbon skeletons and energy or the avoidance of excess ROS is given priority. To counteract salt stress, melatonin, a phytohormone to be licensed, promotes TAG breakdown in parallel with ROS scavenging, so that enough ATP is provided to sustain the enhanced PM H+-ATPase activity, thereby improving K+/Na+ homeostasis in sweet potato (Ipomoea batatas) (Yu et al., 2018). In contrast, Arabidopsis pxa1 mutants, in which FA import into peroxisomes is blocked, exhibit reduced ROS accumulation and enhanced salt tolerance (Yu et al., 2019).
Stocks of Extracellular Barrier Constituents
To cope with and adapt to the harsh environment during terrestrial colonization, land plants have developed the frontier tissue with physically and chemically resistant barriers. Basically, the whole plant body is enveloped by a monolayer epidermis. In organs undergoing secondary growth, e.g., roots and stems of woody species, a multilayer periderm evolved instead. Epidermis and periderm are both equipped with FA-derived biopolyesters (cutin and suberin, respectively) to specialize the cell walls. Their principal roles are to participate in restricting non-stomatal fluxes of water, solutes and gases, as well as defending abiotic and biotic stresses, particularly desiccation and wounding. Unlike cutin that is specific to epidermis, suberin is also present in some internal tissues like root endodermis and appears at abscission zones for sealing [for review, see (Pollard et al., 2008; Beisson et al., 2012; Graça, 2015; Fich et al., 2016)].
Cutin is made mostly of C16 (16:0) and C18 (18:0-18:2) FA derivatives generated from ω-terminal and/or mid-chain oxidation, including ω-hydroxyacids, α, ω-dicarboxylic acids (DCAs) (Figure 1), epoxyacids, and polyhydroxyacids (Beisson et al., 2012). Based on the ester bond formed between carboxyl (–COOH) and hydroxyl (–OH), these monomers are linked directly to each other or, to a much lesser extent, via a bridging molecule such as glycerol or ferulic acid (Pollard et al., 2008; Deng et al., 2015). The resultant macromolecule serves as the framework to be embedded with and coated by cuticular waxes, a complex mixture consisting chiefly of saturated very long-chain FA (VLCFA) derivatives. A translucent hydrophobic film termed cuticle is thereby constructed on the polysaccharide matrices of primary cell walls to seal the aerial surface of epidermis (Fich et al., 2016; He et al., 2018).
Different from cutin, suberin utilizes saturated VLCFAs in addition to C16/18 FAs as monomer precursors to be oxygenated, and usually contains a higher amount of DCAs, primary alcohols, glycerol, and phenolics (Beisson et al., 2012). Particularly, it is covalently combined with lignin-like polyaromatics, in which phenolics are interlinked by C–C or ether (C–O–C) bonds that are more hydrolysis-resistant. A typical polylamellate secondary wall known as suberin lamellae is thereby built beneath the primary wall of a suberized cell (Pollard et al., 2008; Graça, 2015). Of note, herein, suberin denotes the aliphatic polyester only rather than a macromolecule containing polyaliphatic and polyaromatic domains, as was proposed by Graça, (2015).
The monomer composition of cutin or suberin can vary greatly with plant species, organ, and developmental stage. In Arabidopsis, for example, the cutin of leaves and stems has an unusual high content of 18:2-derived DCA (Bonaventure et al., 2004), whereas that of flowers is mainly assembled from 16:0-derived dihydroxyacid (Li-Beisson et al., 2009). Moreover, it is worth noting that unmodified FAs may also appear in cutin or suberin, acting like primary alcohols as dead ends in the polyester (Pollard et al., 2008; Graça, 2015).
The major themes of monomer biosynthesis common to cutin and suberin have been delineated in Arabidopsis. For polymerization, the aliphatic monomers are in the form of sn-2 MAG. The intermediates are generated from free FAs via acyl activation, oxidation, and esterification to glycerol, albeit the order of this pathway has not been fully determined (Pollard et al., 2008; Fich et al., 2016). LACS members are required in that the ER-localized GPAT members utilize acyl-CoAs as substrates. Of acyl oxidation reactions, hydroxylation is better characterized, with members of the CYP86 and CYP77 subfamilies of cytochrome P450 monooxygenases being ω-terminal and mid-chain hydroxylases, respectively (Beisson et al., 2012).
It appears that different isozymes are used for different context, as exemplified by GPATs — GPAT4/8 in leaves and stems vs. GPAT6 in flowers for cutin, while GPAT5 in roots and seeds vs. GPAT7 likely in wounded tissues for suberin (Yang et al., 2012). Notably, GPAT substrate specificity is a major factor accounting for the difference in monomer composition between cutin and suberin. GPAT4, -6, and -8 are strongly biased toward C16/18 ω-oxidized acyl-CoAs, whereas GPAT5 can accept a broader chain length range of ω-oxidized and unmodified acyl-CoAs (Yang et al., 2012). Interestingly, ectopic expression of GPAT5 together with CYP86A1 or -B1 resulted in the appearance of C20-24 suberin monomers in stem cutin (Li et al., 2007; Molina et al., 2009).
To assemble the apoplastic polyesters, the materials need to be exported from the ER to and then across the PM. For cutin, additional transit through the cell wall onto the outer surface follows. ABC transporter members are engaged in channeling the PM for cutin/suberin (Beisson et al., 2012). Particularly, the half-transporter ABCG11 is shared by them (Panikashvili et al., 2010). Recently, LTPG15, a PM glycosylphosphatidylinositol (GPI)-anchored lipid transfer protein, was identified to participate in suberin monomer export in seed coat (Lee and Suh, 2018). Like transport means, both polymerization mechanism and polymer architecture remain largely elusive. The formation of cutin is beginning to be understood with the discovery of CUTIN SYNTHASE 1 (CUS1), a cuticle-localized member of the GDSL lipase/hydrolase superfamily (Girard et al., 2012; Yeats et al., 2012). Nevertheless, given that the CUS1 null mutant of tomato is not fully deprived of fruit cutin, non-catalytic ways cannot be ruled out yet (Yeats et al., 2012; Fich et al., 2016).
In addition, there are other protective biopolymers composed of aliphatics and aromatics, including cutan and sporopollenin. Cutan is present in a few plants as an additional cuticle fraction (Gupta et al., 2006), with 18:2 appearing to be preferred over 16:0 among the FA precursors (Villena et al., 1999). Sporopollenin is the basic component of the exine that covers a spore/pollen grain, with 18:1 being one of the aliphatic sources [see review (Ariizumi and Toriyama, 2011)]. Being refractory to degradation, however, the two macromolecules are so far less characterized.
Precursors of Various Bioactive Molecules
As aforementioned, C18 UFAs, esterified or released, can be modified into a great variety of bioactive molecules. Besides JA, AzA and 2-HOT from oxidation, examples include NO2-FAs and N-acylethanolamines (NAEs) from nitridation. Like JA and NO2-FAs (Figure 1) addressed below, NAEs, though less understood, are signaling molecules implicated in diverse biological processes [see review (Hou et al., 2016)]. By contrast, AzA and 2-HOT are more specific for defending biotic stresses. While 2-HOT (from 18:3 of TAG) is an antifungal compound [see review (Shimada et al., 2018)], AzA (from C18 UFAs of galactolipids) is an inducer of systemic acquired resistance (SAR) against secondary pathogen infection by promoting G3P biosynthesis [see review (Lim et al., 2017)].
Jasmonates
JA and its derivatives, as well as its precursor 12-oxo-phytodienoic acid (OPDA), compose the jasmonate family of phytohormones. JA is derived from 18:3 or 16:3 of plastidial glycerolipids via clear enzymatic pathways, with 13-lipoxygenase (LOX) catalyzing the first oxygenation step. Recently, it was observed that OPDA can directly enter β-oxidation to create 4,5-didehydro-JA first, thus bypassing the canonical octadecanoid pathway sketched in Figure 2 [for more, see reviews (Wasternack and Strnad, 2018; Ruan et al., 2019)]. Another notable update is that PLIP2 and PLIP3, two putative paralogs of the aforecited PLIP1, are capable of triggering JA synthesis in response to abscisic acid (ABA) by releasing 18:3 from PG and MGDG, respectively (Wang et al., 2018). A new node is then added to the crosstalk between the two prominent stress hormones, accounting for the induction effect of ABA on JA.
Well-known in organizing wounding response, JA is active in combating various other stresses, including drought (Liu et al., 2018; Luo et al., 2019) and salt (Yang et al., 2017; Yuan et al., 2019). Particularly, it is of economic interest that methyl JA (MeJA) is capable of alleviating chilling injury to fruits and vegetables, and thus can be applied to maintain their post-harvest quality (González-Aguilar et al., 2000; Ding et al., 2002). Moreover, it is noteworthy that MeJA can induce epigenetic changes of defense-related genes, thereby priming plants against future biotic and abiotic stresses (Laura et al., 2018). In stress response, JA extensively interplays with other signaling molecules involved, as a synergist or antagonist. Here, the focus is on the intricate crosstalk between JA and H2O2.
Briefly, in canonical JA signaling, the primary ligand is the conjugate jasmonoyl-isoleucine (JA-Ile). The receptor, however, requires two components. One is CORONATINE INSENSITIVE 1 (COI1), a subunit of the Skp1/Cullin/F-box (SCF) E3 ubiquitin ligase; the other is the jasmonate ZIM domain (JAZ) family of transcription factors (TFs) that, through direct interaction, can repress various other TFs, typically the key activator MYC2. Upon elicitation, JA-Ile mediates the binding of COI1 to JAZs, leading to their ubiquitination and consequent proteasomal degradation. MYC2 is then released to turn on its target genes, thereby launching robust JA response [for more, see reviews (Wasternack and Hause, 2013; Ali and Baek, 2020)]. More specifically, in cold tolerance, TFs belonging to the C-REPEAT BINDING FACTOR/DEHYDRATION RESPONSIVE ELEMENT BINDING FACTOR 1 (CBF/DREB1) family play a central role via activating downstream targets such as the cold-regulated (COR) genes (He et al., 2016; Wang et al., 2016). These TFs themselves are subject to transactivation by the inducer of CBF expression (ICE) TFs. However, ICEs are repressed by JAZs, rendering JA a positive regulator of this anti-cold cascade (Hu et al., 2013).
As already mentioned, H2O2 possesses signaling property. In fact, plant cells employ diverse enzymes to actively produce H2O2 to launch local and/or systemic defense against biotic and abiotic stresses. The major one is the PM-localized NOX that generates O2•–. This radical is soon converted to H2O2 spontaneously or by superoxide dismutase (SOD). A core component of H2O2 signaling is the MAPK pathway composed of three kinases: MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK/MKK), and MAPK. Of note, MAPK cascades can differentially decipher H2O2 signals. MPK3 and MPK6, two MAPKs, are more characterized as stress-related transducers. Moreover, reversible thiol oxidation of target proteins may offer a direct and important way for H2O2 signal transduction [for more, see reviews (Jalmi and Sinha, 2015; Liu and He, 2017; He et al., 2018)].
JA can affect H2O2 producing and/or scavenging processes, depending on the context. When tomato leaves were mechanically wounded, JA was rapidly synthesized and then increased NOX activity to accumulate H2O2 as its second messenger to switch on an array of defensive genes, e.g., those encoding for proteinase inhibitors (Orozco-Cárdenas et al., 2001). Actually, JA biosynthesis per se is a source of H2O2 (Figure 2), in which three or two rounds of β-oxidation are involved (Ruan et al., 2019). In contrast, when Arabidopsis was infected by the bacterium Pseudomonas syringae DC3000, MeJA induced NATA1 expression to reduce H2O2 generation from polyamine oxidation. The encoded N-ACETYLTRANSFERASE ACTIVITY 1 acetylated putrescine, the precursor of spermidine and spermine, thereby lessening their amount for oxidation (Lou et al., 2016). Moreover, as demonstrated by mounting evidence, JA can modulate the expression and/or activities of various antioxidant enzymes, including CAT, APX and SOD (Hanaka et al., 2016; Ali and Baek, 2020).
Inversely, H2O2 can up-regulate JA biosynthetic genes (e.g., LOX), as has been observed in tobacco (Nicotiana tabacum) (Vandenabeele et al., 2003). This effect is likely mediated by MPK3, given that wound-induced protein kinase (WIPK), a tobacco MPK3, is required for JA production (Seo et al., 1999), and that introducing a peanut ortholog into tobacco resulted in higher transcription of LOX (Kumar et al., 2009). Notably, in tomato (Kandoth et al., 2007) and rice (Oryza sativa) (Wang et al., 2013), MPK3 targets LOX as well. Interestingly, in Arabidopsis, JA can activate the MKK3-MPK6 cascade to repress MYC2 expression, thereby fine-tuning its own signaling (Takahashi et al., 2007). Since MAPK cascades can shape H2O2 signaling via modulating, for example, NOX activity (Jalmi and Sinha, 2015), it is possible that they also participate in mediating JA regulation on H2O2. Further, if JA-induced MPK6 activation is independent of H2O2 (e.g., via Ca2+), then MPK6 renders a converging node for the two signaling pathways. Similarly, Ca2+ may also participate in the crosstalk between JA and H2O2 as a mediator and integrator.
Nitroalkenes
Nitro-FAs, primarily NO2-FAs, are newly recognized signaling molecules common to plants and animals. These species are created by the spontaneous reaction between UFA and nitrogen dioxide (·NO2) (Figure 3), an RNS derived from NO, nitrite (NO2ˉ), or peroxynitrite (ONOOˉ). Endogenous NO2-FAs can be free, lipid-esterified or protein-adducted [see reviews (Schopfer et al., 2011; Mata-Pérez et al., 2018)]. In the plant kingdom, the first to be discovered were NO2-18:1-Cys adducts in fresh olives and conjugated NO2-18:2 in extra virgin olive oil (Fazzari et al., 2014). Subsequently, NO2-18:3 was identified in several model species, including Arabidopsis, rice and pea (Pisum sativum) (Mata-Pérez et al., 2016; Mata-Pérez et al., 2017).
Based on their chemical nature, there are two ways known for NO2-FAs to exert signaling character in animals. One is to be NO donors that can release the gasotransmitter into aqueous microenvironments, thereby triggering NO signaling to regulate diverse biological processes (Mata-Pérez et al., 2018). Notably, NO is capable of protecting plants against a wide spectrum of stresses, not least oxidative stress (Kong et al., 2016; He et al., 2018). The other is to be protein modulators that can induce post-translational nitroalkylation. Like aldehyde, the electron-withdrawing NO2 group turns the UFA moiety into an electrophile, enabling it to attack nucleophiles, including Cys, His and Lys residues, via a reversible reaction termed “Michael addition”. The modification activates or represses target proteins, including signal transducers, TFs and metabolic enzymes, leading to substantial reprogramming (Schopfer et al., 2011).
In Arabidopsis, NO2-18:3 has been found to be significantly elevated under various abiotic stresses, including cold, salt, cadmium, and mechanical wounding. Transcriptomic analysis revealed that it can induce the expression of heat shock proteins (HSPs) and an APX isoform (Mata-Pérez et al., 2016). HSPs are representative molecular chaperones serving as a universal salvation system against not only heat but all other adverse conditions that can cause protein damage. They can prevent denatured proteins from aggregation, assist them in refolding or promote their proteolysis, thereby restoring cellular homeostasis (Wang et al., 2004; He et al., 2018). By reducing H2O2 to water, APXs are key components of the ROS scavenging system that copes with oxidative stress (Pang et al., 2011; Li et al., 2012; Cao et al., 2017). Therefore, NO2-18:3 is capable of motivating two general defense systems, which might be a conserved mechanism adopted by both plants and animals.
Regulators of Stress Signaling
Apart from being components of signaling lipids such as PA [see reviews (Zhao, 2015; Hou et al., 2016)], C18 UFAs per se are regulators of stress response. In fact, as aforecited, they can activate PLDδ to produce PA (Wang and Wang, 2001). The involvement of 18:1 in plant immunity against pathogen infection has long been disclosed by the suppressor of SA insensitive 2 (ssi2) mutant of Arabidopsis, which is deficient in SSI2/FAB2, the major SAD isoform (Kachroo et al., 2001). The mutant has higher resistance to the biotrophic fungus Peronospora parasitica, owing to the accumulation of salicylic acid (SA), another stress hormone, and the constitutive activation of its downstream pathogenesis-related (PR) genes. However, a concurrent phenotype is higher susceptibility to the necrotrophic fungus Botrytis cinerea, resulting from the impairment in some branches of JA response, e.g., defensin (PDF1.2) induction. Of note, this cannot be rescued by exogenous JA and it is the reduced level of chloroplast 18:1 that accounts for the alterations in the two hormonal signaling pathways [see review (Lim et al., 2017)].
Later, it was elucidated that 18:1 regulates defense signaling via suppressing NO production (Mandal et al., 2012). In plants, besides nitrate reductase (NR), NITRIC OXIDE ASSOCIATED 1 (NOA1), a protein bearing GTPase activity, is involved in NO biosynthesis, though the mechanism remains unknown. In chloroplast nucleoids, 18:1 physically interacts with NOA1 and promotes its degradation in a protease-dependent manner. Decreased 18:1 content thus leads to increased NOA1 level. Meanwhile, the expression of NIA1 and NIA2, two NRs, is also up-regulated. As a result, NO accumulates to launch its downstream signaling, thereby reprogramming the immune response, with SA and JA pathways being affected. Notably, ssi2 phenotypes can be fully restored by disrupting NOA1 together with either NR.
However, 18:3 may regulate defense signaling via enhancing ROS production (Yaeno et al., 2004). The hypersensitive response (HR) of plants to prevent pathogen spread is characterized by oxidative burst and programmed cell death (PCD), with NOX being the key enzyme. In vitro assay demonstrated that 18:3 could effectively enhance NOX activity, suggesting its regulatory role in HR. Indeed, the fad7fad8 double mutant of Arabidopsis, which is defective in chloroplast 18:3 synthesis, showed reductions in ROS level, PCD progress, and disease resistance following inoculation with P. syringae DC3000. Of note, the regulatory roles of 18:1 and 18:3 are not confined to biotic stresses, since NO and ROS are also potent organizers in abiotic stress response.
Genetic Engineering of Fatty Acid Synthetic Genes for Improving Stress Tolerance
There are a growing number of cases succeeding in improving the ability of plants to deal with various abiotic stresses via genetically manipulating FA synthetic enzymes, in particular ω-3 FADs and plastidial GPAT (examples listed in Table 2). It should be highlighted that genes from extremophytes are good candidates for transgenesis, as exemplified by FAD3 from Chorispora bungeana, a perennial crucifer inhabiting periglacial regions (Shi et al., 2018), and GPAT from Ammopiptanthus mongolicus, an evergreen broadleaf shrub inhabiting desert (Xue et al., 2019). Both of them conferred multistress tolerance on transgenic plants. Nevertheless, gene silencing of FAD7 in tobacco (Murakami et al., 2000) or GPAT in tomato (Sui et al., 2007) enabled plants to endure high temperatures. Therefore, in order not to compromise heat tolerance, using synthetic stress-inducible promoters (Hou et al., 2012) to drive gene expression could be a method of choice.
Plastidial GPAT has attracted much interest in genetic engineering in that its substrate selectivity determines the unsaturation degree of chloroplast PG. GPAT from chilling-resistant spinach (Spinacia oleracea), but not chilling-sensitive squash (Cucurbita moschata), has a pronounced preference for 18:1-ACP, which matters because the second acylation almost exclusively utilizes 16:0-ACP [see review (He et al., 2020)]. An interesting phenomenon is that the chilling behavior of tobacco (intermediate) was shaped by GPATs from squash (sensitive) and Arabidopsis (resistant), respectively (Murata et al., 1992), whereas the salt susceptibility of Arabidopsis (glycophyte) was relieved by GPAT from Suaeda salsa (euhalophyte) (Sui et al., 2017). In fact, S. salsa also exhibited chilling tolerance under saline situations (Cheng et al., 2014).
To favor the anabolic flow toward C18 UFAs, other enzymes can be manipulated together with FADs. Candidates include acetyl-CoA carboxylase (ACC), 3-ketoacyl-ACP synthase II (KASII), and acyl-ACP thioesterase B (FATB) (Figure 2). ACC catalyzes the first step of FA synthesis and the plastid-targeting expression of Arabidopsis ACC1 in Brassica napus increased 18:1 content (Roesler et al., 1997). KASII elongates 16:0-ACP to 18:0-ACP and its transactivation in B. napus increased total C18 FA content (Gupta et al., 2012). FATB releases 16:0 and 18:0 from ACP and its knockdown by an artificial microRNA in Camelina sativa increased 18:1 content (Ozseyhan et al., 2018). In addition, as essential cofactor for FA synthesis in plastids, ACP can also affect FA composition and stress tolerance. For example, introducing ACP1 from peanut into tobacco resulted in higher levels of 18:2 and 18:3 coupled with lower susceptibility to cold (Tang et al., 2012).
Last but not least, it was newly reported that strong co-suppression may pose an obstacle to direct FAD2 overexpression (Du et al., 2019). This can be overcome by the mutation of RDR6 in Arabidopsis; however, the expense of impaired post-transcriptional gene silencing will be increased viral sensitivity and disturbed gene regulation. But for the transgenerational instability due to co-suppression (Du et al., 2019), the undesired effect can instead be taken advantage of to achieve high 18:1 content that is good for oil stability and human health. In fact, to this end, FAD2 has become a “hotspot” for targeted disruption by using the powerful genome editing tool CRISPR/Cas9 (He et al., 2020). Nevertheless, it should be noted that, in Arabidopsis, FAD2 is required for establishing a proper ratio of 18:2 to 18:1 that allows the ER membrane to tolerate ER stress (Nguyen et al., 2019).
Conclusion and Perspectives
In stress response, C18 UFAs are deeply involved and strikingly versatile. A notable feature is that the chemical nature of double bond appears to render them redox sensors in planta. Remarkably, oxylipins, the inevitable products from ROS attack, are also exploited in a smart way like ROS; that is, they are actively produced by specific enzymes as signaling molecules or biotoxins to retort the elicitors. It should be pinpointed that C18 UFAs are also “Janus” molecules that each has a positive face as general defender against biotic and abiotic stresses in concomitance with a negative face as potential inducer of oxidative stress. Moreover, there are still some aspects of FA/acyl-lipid metabolism remain to be elucidated, particularly the trafficking of the intermediates between different cellular compartments and the functions of a large number of lipases that are tagged hitherto as predicted.
Land plants are routinely confronted with an unpredictable combination of stresses in the field, especially under the context of climate change, soil salinization, and environmental pollution. It is thus getting imperative to equip them with multistress tolerance (He et al., 2018). The ability of C18 UFAs to combat a broad spectrum of stresses renders their synthetic genes good candidates for genetic engineering, as has been proven by the foregoing successful cases. Of note, C18 UFAs draw much interest not only for anti-stress roles, but for wholesome properties and industrial applications (He et al., 2020). Therefore, manipulating FA composition and increasing oil yield of crops are also prompted by the ever-increasing demand for high-quality edible oils and staple oleochemicals.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by grants from Key R&D project of Shandong Province (2019GSF107020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ali M. S., Baek K. H. (2020). Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 21, 621. 10.3390/ijms21020621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T., Toriyama K. (2011). Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 62, 437–460. 10.1146/annurev-arplant-042809-112312 [DOI] [PubMed] [Google Scholar]
- Beisson F., Li-Beisson Y., Pollard M. (2012). Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 15, 329–337. 10.1016/j.pbi.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Bonaventure G., Beisson F., Ohlrogge J., Pollard M. (2004). Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: Occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 40, 920–930. 10.1111/j.1365-313X.2004.02258.x [DOI] [PubMed] [Google Scholar]
- Cao S., Du X. H., Li L. H., Liu Y. D., Zhang L., Pan X., et al. (2017). Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ. J. Plant Physiol. 64, 224–234. 10.1134/s1021443717020029 [DOI] [Google Scholar]
- Chalbi N., Hessini K., Gandour M., Mohamed S. N., Smaoui A., Abdelly C., et al. (2013). Are changes in membrane lipids and fatty acid composition related to saltstress resistance in wild and cultivated barley? J. Plant Nutr. Soil Sci. 176, 138–147. 10.1002/jpln.201100413 [DOI] [Google Scholar]
- Chen Z., Newman I., Zhou M., Mendham N., Zhang G., Shabala S. (2005). Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 28, 1230–1246. 10.1111/j.1365-3040.2005.01364.x [DOI] [Google Scholar]
- Chen M., Song J., Wang B. S. (2010). NaCl increases the activity of the plasma membrane H+-ATPase in C-3 halophyte Suaeda salsa callus. Acta Physiol. Plant. 32, 27–36. 10.1007/s11738-009-0371-7 [DOI] [Google Scholar]
- Cheng S., Yang Z., Wang M. J., Song J., Sui N., Fan H. (2014). Salinity improves chilling resistance in Suaeda salsa. Acta Physiol. Plant. 36, 1823–1830. 10.1007/s11738-014-1555-3 [DOI] [Google Scholar]
- Choudhury F. K., Rivero R. M., Blumwald E., Mittler R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J. 90, 856–867. 10.1111/tpj.13299 [DOI] [PubMed] [Google Scholar]
- Contreras F. X., Ernst A. M., Wieland F., Brügger B. (2011). Specificity of intramembrane protein-lipid interactions. Cold Spring Harb. Perspect. Biol. 3, a004705. 10.1101/cshperspect.a004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C., van Roermund C. W., Postis V. L., Dietrich D., Kerr I. D., Wanders R. J., et al. (2013). Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. U. S. A. 110, 1279–1284. 10.1073/pnas.1218034110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Xu L., Zeng X., Li Z., Qin B., He N. (2010). New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: It’s interaction with malondiadehyde. J. Biomed. Nanotechnol. 6, 318–324. 10.1166/jbn.2010.1130 [DOI] [PubMed] [Google Scholar]
- Deng Y. Q., Feng Z. T., Yuan F., Guo J. R., Suo S. S., Wang B. S. (2015). Identification and functional analysis of the autofluorescent substance in Limonium bicolor salt glands. Plant Physiol. Biochem. 97, 20–27. 10.1016/j.plaphy.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Deng Y. Q., Bao J., Yuan F., Liang X., Feng Z. T., Wang B. S. (2016). Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 79, 391–399. 10.1007/s10725-015-0143-x [DOI] [Google Scholar]
- Ding C. K., Wang C. Y., Gross K. C., Smith D. L. (2002). Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 214, 895–901. 10.1007/s00425-001-0698-9 [DOI] [PubMed] [Google Scholar]
- Du C., Chen Y., Wang K., Yang Z., Zhao C., Jia Q., et al. (2019). Strong co-suppression impedes an increase in polyunsaturated fatty acids in seeds overexpressing FAD2. J. Exp. Bot. 70, 985–994. 10.1093/jxb/ery378 [DOI] [PubMed] [Google Scholar]
- Eastmond P. J. (2006). SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18, 665–675. 10.1105/tpc.105.040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Yu L., Xu C. (2017). A central role for triacylglycerol in membrane lipid breakdown, fatty acid β-oxidation, and plant survival under extended darkness. Plant Physiol. 174, 1517–1530. 10.1104/pp.17.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Davoine C. (2007). Reactive electrophile species. Curr. Opin. Plant Biol. 10, 380–386. 10.1016/j.pbi.2007.04.019 [DOI] [PubMed] [Google Scholar]
- Fazzari M., Trostchansky A., Schopfer F. J., Salvatore S. R., Sánchez-Calvo B., Vitturi D., et al. (2014). Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS One 9, e84884. 10.1371/journal.pone.0084884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. T., Deng Y. Q., Fan H., Sun Q. J., Sui N., Wang B. S. (2014. a). Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica 52, 313–320. 10.1007/s11099-014-0032-y [DOI] [Google Scholar]
- Feng Z. T., Sun Q. J., Deng Y. Q., Sun S. F., Zhang J. G., Wang B. S. (2014. b). Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol. Plant. 36, 2729–2741. 10.1007/s11738-014-1644-3 [DOI] [Google Scholar]
- Feng Z. T., Deng Y. Q., Zhang S. C., Liang X., Yuan F., Hao J. L., et al. (2015). K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 238, 286–296. 10.1016/j.plantsci.2015.06.021 [DOI] [PubMed] [Google Scholar]
- Fich E. A., Segerson N. A., Rose J. K. (2016). The plant polyester cutin: Biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 67, 207–233. 10.1146/annurev-arplant-043015-111929 [DOI] [PubMed] [Google Scholar]
- Gechev T. S., Van Breusegem F., Stone J. M., Denev I., Laloi C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28, 1091–1101. 10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- Girard A. L., Mounet F., Lemaire-Chamley M., Gaillard C., Elmorjani K., Vivancos J., et al. (2012). Tomato GDSL1 is required for cutin deposition in the fruit cuticle. Plant Cell 24, 3119–3134. 10.1105/tpc.112.101055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Aguilar G. A., Fortiz J., Cruz R., Baez R., Wang C. Y. (2000). Methyl jasmonate reduces chilling injury and maintains postharvest quality of mango fruit. J. Agric. Food Chem. 48, 515–519. 10.1021/jf9902806 [DOI] [PubMed] [Google Scholar]
- Graça J. (2015). Suberin: the biopolyester at the frontier of plants. Front. Chem. 3:62. 10.3389/fchem.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I. A. (2008). Seed storage oil mobilization. Annu. Rev. Plant Biol. 59, 115–142. 10.1146/annurev.arplant.59.032607.092938 [DOI] [PubMed] [Google Scholar]
- Guo Y. H., Jia W. J., Song J., Wang D. A., Chen M., Wang B. S. (2012). Thellungilla halophila is more adaptive to salinity than Arabidopsis thaliana at stages of seed germination and seedling establishment. Acta Physiol. Plant. 34, 1287–1294. 10.1007/s11738-012-0925-y [DOI] [Google Scholar]
- Guo J. R., Suo S. S., Wang B. S. (2015). Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci. Res. 25, 335–344. 10.1017/s0960258515000239 [DOI] [Google Scholar]
- Guo J. R., Li Y. D., Han G. L., Song J., Wang B. S. (2018). NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 45, 350–361. 10.1071/fp17181 [DOI] [PubMed] [Google Scholar]
- Guo Q., Liu L., Barkla B. J. (2019). Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 20, 4264. 10.3390/ijms20174264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. S., Collinson M. E., Briggs D. E. G., Evershed R. P., Pancost R. D. (2006). Reinvestigation of the occurrence of cutan in plants: Implications for the leaf fossil record. Paleobiology 32, 432–449. 10.1666/05038.1 [DOI] [Google Scholar]
- Gupta M., DeKelver R. C., Palta A., Clifford C., Gopalan S., Miller J. C., et al. (2012). Transcriptional activation of Brassica napus β-ketoacyl-ACP synthase II with an engineered zinc finger protein transcription factor. Plant Biotechnol. J. 10, 783–791. 10.1111/j.1467-7652.2012.00695.x [DOI] [PubMed] [Google Scholar]
- Han N., Shao Q., Bao H. Y., Wang B. S. (2011). Cloning and characterization of a Ca2+/H+ antiporter from halophyte Suaeda salsa L. Plant Mol. Biol. Rep. 29, 449–457. 10.1007/s11105-010-0244-7 [DOI] [Google Scholar]
- Han N., Lan W. J., He X., Shao Q., Wang B. S., Zhao X. J. (2012). Expression of a Suaeda salsa vacuolar H+/Ca2+ transporter gene in Arabidopsis contributes to physiological changes in salinity. Plant Mol. Biol. Rep. 30, 470–477. 10.1007/s11105-011-0353-y [DOI] [Google Scholar]
- Hanaka A., Wojcik M., Dresler S., Mroczekzdyrska M., Maksymiec W. (2016). Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with Cu? Ecotoxicol. Environ. Saf. 124, 480–488. 10.1016/j.ecoenv.2015.11.024 [DOI] [PubMed] [Google Scholar]
- Harrathi J., Hosni K., Karray-Bouraoui N., Attia H., Marzouk B., Magné C., et al. (2012). Effect of salt stress on growth, fatty acids and essential oils in safflower (Carthamus tinctorius L.). Acta Physiol. Plant. 34, 129–137. 10.1007/s11738-011-0811-z [DOI] [Google Scholar]
- He Y. A., Li Y. P., Cui L. X., Xie L. X., Zheng C. K., Zhou G. H., et al. (2016). Phytochrome B negatively affects cold tolerance by regulating OsDREB1 gene expression through phytochrome interacting factor-like protein OsPIL16 in rice. Front. Plant Sci. 7, 1963. 10.3389/fpls.2016.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., He C. Q., Ding N. Z. (2018). Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9, 1771. 10.3389/fpls.2018.01771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Qin C. X., Wang X., Ding N. Z. (2020). Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 11, 390. 10.3389/fpls.2020.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Okazaki Y., Takano K., Myouga F., Shinozaki K., Knoch E., et al. (2018). Remodels chloroplastic monogalactosyldiacylglycerol by liberating α-linolenic acid in Arabidopsis leaves under heat stress. Plant Cell 30, 1887–1905. 10.1105/tpc.18.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Chen L. J., Wang J. Y., Xu D. F., Dai L. X., Zhang H., et al. (2012). Construction of stress responsive synthetic promoters and analysis of their activity in transgenic Arabidopsis thaliana. Plant Mol. Biol. Rep. 30, 1496–1506. 10.1007/s11105-012-0464-0 [DOI] [Google Scholar]
- Hou Q., Ufer G., Bartels D. (2016). Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 39, 1029–1048. 10.1111/pce.12666 [DOI] [PubMed] [Google Scholar]
- Hu Y., Jiang L., Wang F., Yu D. (2013). Jasmonate regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25, 2907–2924. 10.1105/tpc.113.112631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. (2002). Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 53, 225–245. 10.1146/annurev.arplant.53.100201.160729 [DOI] [PubMed] [Google Scholar]
- Im Y. J., Han O., Chung G. C., Cho B. H. (2002). Antisense expression of an Arabidopsis omega-3 fatty acid desaturase gene reduces salt/drought tolerance in transgenic tobacco plants. Mol. Cells 13, 264–271. [PubMed] [Google Scholar]
- Jalmi S. K., Sinha A. K. (2015). ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 6, 769. 10.3389/fpls.2015.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabała K., Burzyński M. (2012). Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Biol. 63, 4133–4142. 10.1093/jxb/ers097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P., Shanklin J., Shah J., Whittle E. J., Klessig D. F. (2001). A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. U. S. A. 98, 9448–9453. 10.1073/pnas.151258398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth P. K., Ranf S., Pancholi S. S., Jayanty S., Walla M. D., Miller W., et al. (2007). Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. U. S. A. 104, 12205–12210. 10.1073/pnas.0700344104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K. (1990). Mechanism for activation of plasma membrane H+-ATPase from rice (Oryza sativa L.) culture cells by molecular species of a phospholipid. Plant Physiol. 93, 1049–1052. 10.1104/pp.93.3.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K. (2003). Regulation of plasma membrane H+-ATPase activity by the membrane environment. J. Plant Res. 116, 517–523. 10.1007/s10265-003-0112-8 [DOI] [PubMed] [Google Scholar]
- Kerkeb L., Donaire J. P., Venema K., Rodríguez-Rosales M. P. (2001). Tolerance to NaCl induces changes in plasma membrane lipid composition, fluidity and H+-ATPase activity of tomato calli. Physiol. Plant. 113, 217–224. 10.1034/j.1399-3054.2001.1130209.x [DOI] [PubMed] [Google Scholar]
- Kim R. J., Kim H. J., Shim D., Suh M. C. (2016). Molecular and biochemical characterizations of the monoacylglycerol lipase gene family of Arabidopsis thaliana. Plant J. 85, 758–771. 10.1111/tpj.13146 [DOI] [PubMed] [Google Scholar]
- Kodama H., Hamada T., Horiguchi G., Nishimura M., Iba K. (1994). Genetic enhancement of cold tolerance by expression of a gene for chloroplast [omega]-3 fatty acid desaturase in transgenic tobacco. Plant Physiol. 105, 601–605. 10.1104/pp.105.2.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. Q., Wang T., Li W. J., Tang W., Zhang D. M., Dong H. Z. (2016). Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 38, 61. 10.1007/s11738-016-2079-9 [DOI] [Google Scholar]
- Kumar K. R. R., Srinivasan T., Kirti P. B. (2009). A mitogen-activated protein kinase gene, AhMPK3 of peanut: Molecular cloning, genomic organization, and heterologous expression conferring resistance against Spodoptera litura in tobacco. Mol. Genet. Genomics 282, 65–81. 10.1007/s00438-009-0446-6 [DOI] [PubMed] [Google Scholar]
- Laura B., Silvia P., Francesca F., Benedetta S., Carla C. (2018). Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 228, 166–177. 10.1016/j.jplph.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Lee S. B., Suh M. C. (2018). Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein 15 affects seed coat permeability in Arabidopsis. Plant J. 96, 1206–1217. 10.1111/tpj.14101 [DOI] [PubMed] [Google Scholar]
- Li Y., Beisson F., Koo A. J., Molina I., Pollard M., Ohlrogge J. B. (2007). Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. U. S. A. 104, 18339–18344. 10.1073/pnas.0706984104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Pang C. H., Ding F., Sui N., Feng Z. T., Wang B. S. (2012). Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S. Afr. J. Bot. 78, 235–245. 10.1016/j.sajb.2011.09.006 [DOI] [Google Scholar]
- Liang Y., Zhang W., Chen Q., Liu Y., Ding R. (2006). Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 57, 212–219. 10.1016/j.envexpbot.2005.05.012 [DOI] [Google Scholar]
- Li-Beisson Y., Pollard M., Sauveplane V., Pinot F., Ohlrogge J., Beisson F. (2009). Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. U. S. A. 106, 22008–22013. 10.1073/pnas.0909090106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G. H., Singhal R., Kachroo A., Kachroo P. (2017). Fatty acid- and lipid-mediated signaling in plant defense. Annu. Rev. Phytopathol. 55, 505–536. 10.1146/annurev-phyto-080516-035406 [DOI] [PubMed] [Google Scholar]
- Lin H., Wu L. (1996). Effects of salt stress on root plasma membrane characteristics of salt-tolerant and salt-sensitive buffalograss clones. Environ. Exp. Bot. 36, 239–254. 10.1016/0098-8472(96)01025-8 [DOI] [Google Scholar]
- Liu Y., He C. (2017). A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 11, 192–204. 10.1016/j.redox.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Ji S. X., Fang X. L., Wang Q. G., Li Z., Yao F. Y., et al. (2013). Protein kinase LTRPK1 influences cold adaptation and microtubule stability in rice. J. Plant Growth Regul. 32, 483–490. 10.1007/s00344-012-9314-4 [DOI] [Google Scholar]
- Liu X. X., Fu C., Yang W. W., Zhang Q., Fan H., Liu J. (2016). The involvement of TsFtsH8 in Thellungiella salsuginea tolerance to cold and high light stresses. Acta Physiol. Plant. 38, 62. 10.1007/s11738-016-2080-3 [DOI] [Google Scholar]
- Liu S. S., Wang W. Q., Li M., Wan S. B., Sui N. (2017). Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol. Plant. 39, 207. 10.1007/s11738-017-2501-y [DOI] [Google Scholar]
- Liu S., Lv Z., Liu Y., Li L., Zhang L. (2018). Network analysis of ABA-dependent and ABA-independent drought responsive genes in Arabidopsis thaliana. Genet. Mol. Biol. 41, 624–637. 10.1590/1678-4685-GMB-2017-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pérez L., del Carmen Martínez-Ballesta M., Maurel C., Carvajal M. (2009). Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry 70, 492–500. 10.1016/j.phytochem.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Lou Y., Bor M., Yan J., Preuss A. S., Jander G. (2016). Arabidopsis NATA1 acetylates putrescine and decreases defense-related hydrogen peroxide accumulation. Plant Physiol. 171, 1443–1455. 10.1104/pp.16.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Zhang Y. Y., Tang S. K., Pan J. B., Yu Y. K., Han J., et al. (2016). AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J. Exp. Bot. 67, 809–819. 10.1093/jxb/erv500 [DOI] [PubMed] [Google Scholar]
- Luo Z., Kong X., Zhang Y., Li W., Zhang D., Dai J., et al. (2019). Leaf-derived jasmonate mediates water uptake from hydrated cotton roots under partial root-zone irrigation. Plant Physiol. 180, 1660–1676. 10.1104/pp.19.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M. K., Chandra-Shekara A. C., Jeong R. D., Yu K., Zhu S., Chanda B., et al. (2012). Oleic acid-dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell 24, 1654–1674. 10.1105/tpc.112.096768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M. M. F., van Hasselt P. R., Kuiper P. J. C. (1994). Plasma membrane lipid alterations induced by NaCl in winter wheat roots. Physiol. Plant. 92, 473–478. 10.1111/j.1399-3054.1994.tb08838.x [DOI] [Google Scholar]
- Martz F., Sutinen M., Kiviniemi S., Palta J. P. (2006). Changes in freezing tolerance, plasma membrane H+-ATPase activity and fatty acid composition in Pinus resinosa needles during cold acclimation and de-acclimation. Tree Physiol. 26, 783–790. 10.1093/treephys/26.6.783 [DOI] [PubMed] [Google Scholar]
- Mata-Pérez C., Sánchez-Calvo B., Padilla M. N., Begara-Morales J. C., Luque F., Melguizo M., et al. (2016). Nitro-fatty acids in plant signaling: Nitro-linolenic acid induces the molecular chaperone network in Arabidopsis. Plant Physiol. 170, 686–701. 10.1104/pp.15.01671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Pérez C., Sánchez-Calvo B., Padilla M. N., Begara-Morales J. C., Valderrama R., Corpas F. J., et al. (2017). Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 11, 554–561. 10.1016/j.redox.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Pérez C., Padilla M. N., Sánchez-Calvo B., Begara-Morales J. C., Valderrama R., Chaki M., et al. (2018). Biological properties of nitro-fatty acids in plants. Nitric Oxide 78, 176–179. 10.1016/j.niox.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Molina I., Li-Beisson Y., Beisson F., Ohlrogge J. B., Pollard M. (2009). Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol. 151, 1317–1328. 10.1104/pp.109.144907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon B. Y., Higashi S., Gombos Z., Murata N. (1995). Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. U. S. A. 92, 6219–6223. 10.1073/pnas.92.14.6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Cedillo F., González-Solís A., Gutiérrez-Angoa L., Cano-Ramírez D. L., Gavilanes-Ruiz M. (2015). Plant lipid environment and membrane enzymes: the case of the plasma membrane H+-ATPase. Plant Cell Rep. 34, 617–629. 10.1007/s00299-014-1735-z [DOI] [PubMed] [Google Scholar]
- Murakami Y., Tsuyama M., Kobayashi Y., Kodama H., Iba K. (2000). Trienoic fatty acids and plant tolerance of high temperature. Science 287, 476–479. 10.1126/science.287.5452.476 [DOI] [PubMed] [Google Scholar]
- Murata N., Shizaki-Nishizawa O., Higashi S., Hayash H., Tasaka Y., Nishida I. (1992). Genetically engineered alteration in the chilling sensitivity of plants. Nature 356, 710–713. 10.1038/356710a0 [DOI] [Google Scholar]
- Nguyen V. C., Nakamura Y., Kanehara K. (2019). Membrane lipid polyunsaturation mediated by FATTY ACID DESATURASE 2 (FAD2) is involved in endoplasmic reticulum stress tolerance in Arabidopsis thaliana. Plant J. 99, 478–493. 10.1111/tpj.14338 [DOI] [PubMed] [Google Scholar]
- Nishida I., Murata N. (1996). Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 541–568. 10.1146/annurev.arplant.47.1.541 [DOI] [PubMed] [Google Scholar]
- Ohlrogge J., Browse J. (1995). Lipid biosynthesis. Plant Cell 7, 957–970. 10.1105/tpc.7.7.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas M. L., Narváez-Vásquez J., Ryan C. A. (2001). Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13, 179–191. 10.1105/tpc.13.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozseyhan M. E., Li P., Na G. N., Li Z., Wang C., Lu C. (2018). Improved fatty acid profiles in seeds of Camelina sativa by artificial microRNA mediated FATB gene suppression. Biochem. Biophys. Res. Commun. 503, 621–624. 10.1016/j.bbrc.2018.06.051 [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Ulvskov P., Jorgensen P. L. (1988). Modulation of plasma membrane H+-ATPase from oat roots by lysophosphatidylcholine, free fatty acids and phospholipase A2. Physiol. Plant. 74, 11–19. 10.1111/j.1399-3054.1988.tb04934.x [DOI] [Google Scholar]
- Pang C. H., Li K., Wang B. S. (2011). Overexpression of SsCHLAPXs confers protection against oxidative stress induced by high light in transgenic Arabidopsis thaliana. Physiol. Plant. 143, 355–366. 10.1111/j.1399-3054.2011.01515.x [DOI] [PubMed] [Google Scholar]
- Panikashvili D., Shi J. X., Bocobza S., Franke R. B., Schreiber L., Aharoni A. (2010). The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol. Plant. 3, 563–575. 10.1093/mp/ssp103 [DOI] [PubMed] [Google Scholar]
- Peng D., Zhou B., Jiang Y., Tan X. F., Yuan D. Y., Zhang L. (2018). Enhancing freezing tolerance of Brassica napus L. by overexpression of a stearoyl-acyl carrier protein desaturase gene (SAD) from Sapium sebiferum (L.) Roxb. Plant Sci. 272, 32–41. 10.1016/j.plantsci.2018.03.028 [DOI] [PubMed] [Google Scholar]
- Pollard M., Beisson F., Li Y., Ohlrogge J. B. (2008). Building lipid barriers:Biosynthesis of cutin and suberin. Trends Plant Sci. 13, 236–246. 10.1016/j.tplants.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Ren X. L., Qi G. N., Feng H. Q., Zhao S., Zhao S. S., Wang Y., et al. (2013). Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 74, 258–266. 10.1111/tpj.12123 [DOI] [PubMed] [Google Scholar]
- Roesler K., Shintani D., Savage L., Boddupalli S., Ohlrogge J. (1997). Targeting of the Arabidopsis homomeric acetyl-Coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol. 113, 75–81. 10.1104/pp.113.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J., Zhou Y., Zhou M., Yan J., Khurshid M., Weng W., et al. (2019). Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 20, 2479. 10.3390/ijms20102479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelland E., Zachowski A. (2011). How plants sense temperature. Environ. Exp. Bot. 69, 225–232. 10.1016/j.envexpbot.2010.05.011 [DOI] [Google Scholar]
- Salama K. H. A., Mansour M. M. F. (2015). Choline priming-induced plasma membrane lipid alterations contributed to improved wheat salt tolerance. Acta Physiol. Plant. 37, 170. 10.1007/s11738-015-1934-4 [DOI] [Google Scholar]
- Salama K. H. A., Mansour M. M. F., Ali F. Z. M., Abou-hadid A. F. (2007). NaCl-induced changes in plasma membrane lipids and proteins of Zea mays L. cultivars differing in their response to salinity. Acta Physiol. Plant. 29, 351–359. 10.1007/s11738-007-0044-3 [DOI] [Google Scholar]
- Sangwan V., Örvar B. L., Beyerly J., Hirt H., Dhindsa R. S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31, 629–638. 10.1046/j.1365-313X.2002.01384.x [DOI] [PubMed] [Google Scholar]
- Saxena I., Srikanth S., Chen Z. (2016). Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 7:570. 10.3389/fpls.2016.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer F. J., Cipollina C., Freeman B. A. (2011). Formation and signaling actions of electrophilic lipids. Chem. Rev. 111, 5997–6021. 10.1021/cr200131e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Sano H., Ohashi Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. 10.1105/tpc.11.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26. 10.1155/2012/217037 [DOI] [Google Scholar]
- Shi Y., An L., Zhang M., Huang C., Zhang H., Xu S. (2008). Regulation of the plasma membrane during exposure to low temperatures in suspension-cultured cells from a cryophyte (Chorispora bungeana). Protoplasma 232, 173–181. 10.1007/s00709-008-0291-1 [DOI] [PubMed] [Google Scholar]
- Shi J., Cao Y., Fan X., Li M., Wang Y., Ming F. (2012). A rice microsomal delta-12 fatty acid desaturase can enhance resistance to cold stress in yeast and Oryza sativa. Mol. Breed. 29, 743–757. 10.1007/s11032-011-9587-5 [DOI] [Google Scholar]
- Shi Y., Yue X., An L. (2018). Integrated regulation triggered by a cryophyte ω-3 desaturase gene confers multiple-stress tolerance in tobacco. J. Exp. Bot. 69, 2131–2148. 10.1093/jxb/ery050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T. L., Hayashi M., Hara-Nishimura I. (2018). Membrane dynamics and multiple functions of oil bodies in seeds and leaves. Plant Physiol. 176, 199–207. 10.1104/pp.17.01522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui N., Han G. L. (2014). Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol. Plant. 36, 983–992. 10.1007/s11738-013-1477-5 [DOI] [Google Scholar]
- Sui N., Li M., Shu D. F., Zhao S. J., Meng Q. W. (2007). Antisense-mediated depletion of tomato chloroplast glycerol-3-phosphate acyltransferase affects male fertility and increases thermal tolerance. Physiol. Plant. 130, 301–314. 10.1111/j.1399-3054.2007.00907.x [DOI] [Google Scholar]
- Sui N., Li M., Li K., Song J., Wang B. S. (2010). Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 48, 623–629. 10.1007/s11099-010-0080-x [DOI] [Google Scholar]
- Sui N., Tian S. S., Wang W. Q., Wang M. J., Fan H. (2017). Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front. Plant Sci. 8, 1337. 10.3389/fpls.2017.01337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui N., Wang Y., Liu S. S., Yang Z., Wang F., Wan S. B. (2018). Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front. Plant Sci. 9, 7. 10.3389/fpls.2018.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui N. (2015). Photoinhibition of Suaeda salsa to chilling stress is related to energy dissipation and water-water cycle. Photosynthetica 53, 207–212. 10.1007/s11099-015-0080-y [DOI] [Google Scholar]
- Sun Y. L., Li F., Su N., Sun X. L., Zhao S. J., Meng Q. W. (2010). The increase in unsaturation of fatty acids of phosphatidylglycerol in thylakoid membrane enhanced salt tolerance in tomato. Photosynthetica 48, 400–408. 10.1007/s11099-010-0052-1 [DOI] [Google Scholar]
- Surjus A., Durand M. (1996). Lipid changes in soybean root membranes in response to salt treatment. J. Exp. Bot. 47, 17–23. 10.1093/jxb/47.1.17 [DOI] [Google Scholar]
- Takahashi S., Murata N. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13, 178–182. 10.1016/j.tplants.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Takahashi F., Yoshida R., Ichimura K., Mizoguchi T., Seo S., Yonezawa M., et al. (2007). The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19, 805–818. 10.1105/tpc.106.046581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G. Y., Wei L. Q., Liu Z. J., Bi Y. P., Shan L. (2012). Ectopic expression of peanut acyl carrier protein in tobacco alters fatty acid composition in the leaf and resistance to cold stress. Biol. Plant. 56, 493–501. 10.1007/s10535-012-0057-7 [DOI] [Google Scholar]
- Vandenabeele S., Van Der Kelen K., Dat J., Gadjev I., Boonefaes T., Morsa S., et al. (2003). A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl. Acad. Sci. U. S. A. 100, 16113–16118. 10.1073/pnas.2136610100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena J. F., Domínguez E., Stewart D., Heredia A. (1999). Characterization and biosynthesis of non-degradable polymers in plant cuticles. Planta 208, 181–187. 10.1007/s004250050548 [DOI] [PubMed] [Google Scholar]
- Wada H., Murata N. (2007). The essential role of phosphatidylglycerol in photosynthesis. Photosynth. Res. 92, 205–215. 10.1007/s11120-007-9203-z [DOI] [PubMed] [Google Scholar]
- Wallis J. G., Browse J. (2002). Mutants of Arabidopsis reveal many roles for membrane lipids. Prog. Lipid Res. 41, 254–278. 10.1016/S0163-7827(01)00027-3 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang X. (2001). A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 127, 1102–1112. 10.1104/pp.010444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Shoseyov O., Altman A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252. 10.1016/j.tplants.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Wang Q., Li J., Hu L., Zhang T., Zhang G., Lou Y. (2013). OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant Cell Rep. 32, 1075–1084. 10.1007/s00299-013-1389-2 [DOI] [PubMed] [Google Scholar]
- Wang H., Yu C., Tang X., Zhu Z. J., Ma N. N., Meng Q. W. (2014). A tomato endoplasmic reticulum (ER)-type omega-3 fatty acid desaturase (LeFAD3) functions in early seedling tolerance to salinity stress. Plant Cell Rep. 33, 131–142. 10.1007/s00299-013-1517-z [DOI] [PubMed] [Google Scholar]
- Wang F., Guo Z., Li H., Wang M., Onac E., Zhou J., et al. (2016). Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol. 170, 459–471. 10.1104/pp.15.01171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Froehlich J. E., Zienkiewicz A., Hersh H. L., Benning C. (2017). A plastid phosphatidylglycerol lipase contributes to the export of acyl groups from plastids for seed oil biosynthesis. Plant Cell 29, 1678–1696. 10.1105/tpc.17.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Guo Q., Froehlich J. E., Hersh H. L., Zienkiewicz A., Howe G. A., et al. (2018). Two abscisic acid-responsive plastid lipase genes involved in jasmonic acid biosynthesis in Arabidopsis thaliana. Plant Cell 30, 1006–1022. 10.1105/tpc.18.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang C., Liu X., Cheng J., Li S. (2019). Peroxisomal β-oxidation regulates histone acetylation and DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 116, 10576–10585. 10.1073/pnas.1904143116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058. 10.1093/aob/mct067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Strnad M. (2018). Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 19, 2539. 10.3390/ijms19092539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Shanklin J. (2016). Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 67, 179–206. 10.1146/annurev-arplant-043015-111641 [DOI] [PubMed] [Google Scholar]
- Xue M., Guo T., Ren M., Wang Z., Tang K., Zhang W. (2019). Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. 143, 375–387. 10.1016/j.plaphy.2019.07.019 [DOI] [PubMed] [Google Scholar]
- Yaeno T., Matsuda O., Iba K. (2004). Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J. 40, 931–941. 10.1111/j.1365-313X.2004.02260.x [DOI] [PubMed] [Google Scholar]
- Yang M. F., Song J., Wang B. S. (2010). Organ-specific responses of vacuolar H+-ATPase in the shoots and roots of C-3 halophyte Suaeda salsa to NaCl. J. Integr. Plant Biol. 52, 308–314. 10.1111/j.1744-7909.2010.00895.x [DOI] [PubMed] [Google Scholar]
- Yang W., Simpson J. P., Li-Beisson Y., Beisson F., Pollard M., Ohlrogge J. B. (2012). A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: Substrate specificity, sn-2 preference, and evolution. Plant Physiol. 160, 638–652. 10.1104/pp.112.201996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang Y., Wei X. C., Zhao X., Wang B. S., Sui N. (2017). Transcription profiles of genes related to hormonal regulations under salt stress in sweet sorghum. Plant Mol. Biol. Rep. 35, 586–599. 10.1007/s11105-017-1047-x [DOI] [Google Scholar]
- Yeats T. H., Martin L. B., Viart H. M., Isaacson T., He Y., Zhao L., et al. (2012). The identification of cutin synthase: Formation of the plant polyester cutin. Nat. Chem. Biol. 8, 609–611. 10.1038/nchembio.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Wang H. S., Yang S., Tang X. F., Duan M., Meng Q. W. (2009). Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol. Biochem. 47, 1102–1112. 10.1016/j.plaphy.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Yu Y., Wang A., Li X., Kou M., Wang W., Chen X., et al. (2018). Melatonin-stimulated triacylglycerol breakdown and energy turnover under salinity stress contributes to the maintenance of plasma membrane H+-ATPase activity and K+/Na+ homeostasis in sweet potato. Front. Plant Sci. 9, 256. 10.3389/fpls.2018.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Fan J., Xu C. (2019). Peroxisomal fatty acid β-oxidation negatively impacts plant survival under salt stress. Plant Signal. Behav. 14, 1561121. 10.1080/15592324.2018.1561121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F., Lyu M. J. A., Leng B. Y., Zheng G. Y., Feng Z. T., Li P. H., et al. (2015). Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell Environ. 38, 1637–1657. 10.1111/pce.12514 [DOI] [PubMed] [Google Scholar]
- Yuan F., Lyu M. J. A., Leng B. Y., Zhu X. G., Wang B. S. (2016). The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol. Biol. 91, 241–256. 10.1007/s11103-016-0460-0 [DOI] [PubMed] [Google Scholar]
- Yuan F., Liang X., Li Y., Yin S., Wang B. (2019). Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor. Funct. Plant Biol. 46, 82–92. 10.1071/FP18120 [DOI] [PubMed] [Google Scholar]
- Zamani S., Bybordi A., Khorshidi S., Nezami T. (2010). Effects of NaCl salinity levels on lipids and proteins of canola (Brassica napus L.) cultivars. Adv. Environ. Biol. 4, 397–403. [Google Scholar]
- Zhang W., Wang C., Qin C., Wood T., Olafsdottir G., Welti R., et al. (2003). The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15, 2285–2295. 10.1105/tpc.013961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Barg R., Yin M., Gueta-Dahan Y., Leikin-Frenkel A., Salts Y., et al. (2005). Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 44, 361–371. 10.1111/j.1365-313X.2005.02536.x [DOI] [PubMed] [Google Scholar]
- Zhao K. F., Song J., Fan H., Zhou S., Zhao M. (2010). Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J. Integr. Plant Biol. 52, 468–475. 10.1111/j.1744-7909.2010.00947.x [DOI] [PubMed] [Google Scholar]
- Zhao J. (2015). Phospholipase D and phosphatidic acid in plant defence response: from protein-protein and lipid-protein interactions to hormone signalling. J. Exp. Biol. 66, 1721–1736. 10.1093/jxb/eru540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Liao C. C., Zhao S. S., Wang C. W., Guo Y. (2017). The glycosyltransferase QUA1 regulates chloroplast-associated calcium signaling during salt and drought stress in Arabidopsis. Plant Cell Physiol. 58, 329–341. 10.1093/pcp/pcw192 [DOI] [PubMed] [Google Scholar]