Abstract
Neutrophils are essential soldiers of the immune response and their role have long been restricted to their activities in defence against microbial infections and during the acute phase of the inflammatory response. However, increasing number of investigations showed that neutrophils are endowed with plasticity and can participate in the orchestration of both innate and adaptive immune responses. Neutrophils have an impact on a broad range of disorders, including infections, chronic inflammations, and cancer. Neutrophils are present in the tumour microenvironment and have been reported to mediate both pro-tumour and anti-tumour responses. Neutrophils can contribute to genetic instability, tumour cell proliferation, angiogenesis and suppression of the anti-tumour immune response. In contrast, neutrophils are reported to mediate anti-tumour resistance by direct killing of tumour cells or by engaging cooperative interactions with other immune cells. Here we discuss the current understandings of neutrophils biology and functions in health and diseases, with a specific focus on their role in cancer biology and their prognostic significance in human cancer.
Abbreviations: 3-MCA, 3-methylcholathrene; ACKR2, atypical chemokine receptor 2; APC, antigen presenting cell; G-CSF, granulocytes colony stimulating factor; G-MDSCs, granulocytes-myeloid-derived suppressor cells; GM-CSF, granulocytes-macrophages colony stimulating factor; GMPs, granulocyte monocyte progenitors; iNOS, inducible nitric oxide synthase; M-MDSCs, monocytes-myeloid-derived suppressor cells; MMP-9, matrix metallopeptidase 9; MPO, myeloperoxidase; NETs, neutrophil extracellular traps; NK, natural killer; PRRs, pattern recognition receptors; ROS, eactive oxygen species; TANs, tumour-associated neutrophils; TCR, T cell receptor; TILs, tumour infiltrating leukocytes; TME, tumour microenvironment; TRAIL, TNF-related apoptosis inducing ligand; TRPM2, transient receptor potential cation channel subfamily M, member 2; UTC, unconventional T cell
Keywords: Neutrophils, Tumour-associated neutrophil, Inflammation, Cancer-related inflammation, Tumour microenvironment
1. Introduction
Neutrophils are the essential players in the early response against pathogens and during acute inflammation [1,2]. In response to injury and inflammatory stimuli, neutrophils are rapidly recruited in the tissues [3] where they engage multiple cross-talks with immune and non-immune cells [4]. Accordingly, neutrophils play an important role in the regulation of innate and adaptive immune responses and are part of the pathogenesis of numerous diseases [2]. In addition, a growing number of studies showed that neutrophils are endowed with plasticity and heterogeneity which have long been underestimated [[5], [6], [7], [8]].
Cancer-related inflammation has emerged as a hallmark of tumour biology [9,10] and the tumour microenvironment (TME) consists of both stromal and inflammatory cells, including neutrophils [11]. The role of neutrophils in tumour biology has long been underestimated while particular emphasis has been placed on the role of other myeloid cells, such as macrophages [12,13]. However, recent findings showed that neutrophils are an important component of the TME and have highlighted their importance in tumour progression as well as in the orchestration of pathways leading to tumour resistance [[14], [15], [16], [17], [18]]. These contrasting findings are likely the results of the previously underestimated plasticity and heterogeneity of neutrophils in cancer [8,16,19]. In humans, the density of tumour-associated neutrophils (TANs) can have prognostic significance for patient’s outcome [[20], [21], [22], [23]].
Here we review the current understandings on neutrophils biology from their development to the mechanisms of their trafficking and functions in health and disease, with a focus on their role in tumour biology and their prognostic significance in human cancer.
2. Neutrophil development and mobilization in steady state
2.1. Neutrophil development
Neutrophils have a short lifespan and their presence in the circulation requires a continuous production in the bone marrow (BM), which is mainly regulated by the production of granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) [1,[24], [25], [26]]. In humans, up to 2 × 1011 neutrophils are produced daily during the process of granulopoiesis in the BM and released in the bloodstream [1]. The process of granulopoiesis has been extensively studied under both steady state and emergency conditions [25,27,28].
In the BM, hematopoietic stem cells (HSC) differentiate into lymphoid-primed multipotent progenitors (LMPPs) or common myeloid progenitors (CMPs) which can both give rise to granulocyte monocyte progenitors (GMPs). GMPs will commit to the generation of neutrophils upon receiving G-CSF and GM-CSF signals produced by bone marrow stromal cells [15,26,29]. Accordingly, G-CSF−/− and GM-CSF-/- mice present a severe and chronic neutropenia [30]. The process of granulopoiesis is regulated by the successive expression of specific transcription factors (TF) and deficiency in these TFs in mice has been associated with neutropenia [[31], [32], [33], [34], [35]]. In particular, CCAAT/enhancer-binding proteins (C/EBPs) are a family of six TFs among which the members C/EBPα, C/EBPβ and C/EBPε are involved in neutrophil development [[36], [37], [38], [39]]. Specifically, C/EBPα acts at the GMP stage of the process and its disruption in mice lead to a complete loss of mature neutrophils by blocking the transition from CMPs to GMPs [40]. C/EBPβ is not necessary for the development of neutrophils in steady state but plays an important role in emergency granulopoiesis (see below) [36,37]. On the other hand, C/EBPε drives GMP to myelocyte transition and may be important in the terminal step of the granulopoiesis [31,41]. Other TFs, such as the growth factor independent-1 (Gfi-1) have also been implicated in the process of granulopoiesis [33,42].
Transcription factors control gene expression profiles, that define the molecular signatures of immature cell subsets revealing their heterogeneity [36,43]. State of the art technologies, such as total and single-cell RNA sequencing (scRNAseq) and mass cytometry by time-of-flight (CyTOF), have been employed to characterize neutrophil differentiation and distinct cellular phenotypes have been identified from GMP to mature neutrophils [[43], [44], [45], [46]]. In particular, a population of proliferative Gr1+ CD11b+ CXCR4hi CD117int CXCR2− neutrophil precursors (defined as preNeu) with a potential to differentiate into non-proliferative immature and then mature neutrophils has been identified in mouse BM [47]. Recently, progenitors of the preNeu population have been described as early committed neutrophil progenitors (proNeu1), which gives rise to an intermediate progeny (proNeu2) and then to the subsequent populations [48]. These neutrophils progenitors are present in mice and humans and their phenotypic characteristics are outlined in Table 1 . A study previously described an early unipotent neutrophil progenitor (defined as NeP), comprising different clusters of cells [46]. It is conceivable that this heterogenous population represents a mixture of neutrophil progenitors described as proNeu1, proNeu2 and preNeu [[46], [47], [48]].
Table 1.
Subsets of immature and mature neutrophils in the bone marrow.
| Nuclear morphology of immature and mature mouse neutrophils | Surface markers in Human | Surface markers in Mouse | |
|---|---|---|---|
| Granulocyte monocyte progenitor (GMP) |
 |
Lin−, CD34+, CD38+, CD45RA+, CXCR4+, CXCR2− | Lin−, Sca1−, CD117hi, CD11b−, CD34+, CD16/32+, CXCR4+, CXCR2−, Gr1−, Ly6C− |
| Early committed neutrophil progenitor1 (proNeu1) |  |
CD49dhi, CD34+, CD81hi, CD11b−, CD38hi, CD24lo | Lin−, CD115−, Flt3−, Ly6C+, CD117hi, CD34+, CD16/32+, CD106−, CD11blo, |
| Early committed neutrophil progenitor2 (proNeu2) |
 |
CD49dint, CD34−, CD81lo, CD11b−, CD38lo, CD24hi | Lin−, CD115−, Flt3−, Ly6C+, CD117hi, CD34+, CD16/32+, CD106+, CD11bhi |
| Committed proliferative neutrophil precursor (preNeu) |
 |
Lin−, Siglec8−, CD15+, CD34−, CD66b+, CD101−, CD49dlo, CD81lo, CD11blo, CXCR4+, CXCR2− | Lin−, CD115−, Siglec-F−, CD117int, Gr1+, CD11b+, Ly6Glo, CXCR4hi, CXCR2− |
| Band Neutrophil |  |
Siglec8−, CD66b+, CD15+, CD33mid, CD49d−, CD10−, CD16low, CD101+, CD34−, CD81lo, CD11blo, CXCR4+, CXCR2+ | Lin−, CD115−, Siglec-F−, CD117−, Gr1+, CD11b+, CD101−, Ly6Glo/int, CXCR4lo, CXCR2− |
| Mature Neutrophil |  |
Siglec8−, CD66b+, CD15+, CD49d−, CD10+, CD101+, CD16hi, CD34−, CD81lo, CD11bhi, CXCR4−, CXCR2+ | Lin−, CD115−, Siglec-F−, CD117−, Gr1+, CD11b+, CD101+, Ly6Ghi, CXCR4−, CXCR2+ |
2.2. Neutrophil mobilization
The release of neutrophils in the bloodstream is a process controlled by the regulation of the expression of genes coding for CXCR4 and CXCR2 in neutrophil precursors [49]. In steady state, CXCL12 is constitutively expressed by BM stromal cells which supports the retention of CXCR4+ neutrophils inside the BM. During their maturation, expression of CXCR4 on the surface of neutrophils is downregulated, while the expression of CXCR2 is increased, leading to their release in the blood via the interaction with CXC chemokines (e.g. CXCL1, CXCL2) present in the circulation [15,24,49,50]. Circulating aged neutrophils upregulate the expression of CXCR4 in order to migrate into the BM where they are eliminated by macrophages [51].
3. Neutrophil development and mobilization during inflammation
In steady state, BM is the unique site of haematopoiesis, but during inflammatory conditions, such as cancer and systemic infection, the spleen can be involved in the generation of neutrophils [52,53]. In inflammatory conditions, expression levels of granulopoietic cytokines, such as G-CSF, GM-CSF and IL-6 are increased, leading to the process of emergency granulopoiesis [27,54]. Interestingly, despite that G-CSF−/− and GM-CSF-/- mice present a severe neutropenia in steady state, the expression of IL-6 in these mice can be sufficient for the production of neutrophils during emergency granulopoiesis [15,30]. However, the phenotype and effector functions of neutrophils produced in G-CSF−/− and GM-CSF-/- mice remain to be fully elucidated.
Neutrophil development during inflammation is also controlled by the expression of specific transcription factors [37,55,56]. Increased expression of G-CSF and G-CSF receptor signalling triggers the phosphorylation of signal transducer and activator of transcription 3 (STAT3) which directly enhances C/EBPβ expression in progenitor cells [27]. In a negative feedback loop, the phosphorylation of STAT3 activates the suppressor of cytokine signalling3 (SOCS3), which in turn, by inhibiting the activation of STAT3, prevents an excessive production of neutrophils [27].
Elevated number of circulating neutrophils have been reported in inflammatory conditions, including cancer [7,57,58]. Accordingly, the production of cytokines and chemokines (such as Il-17, IL-1β, G-CSF, CXCL1, CXCL2) involved in the development and mobilization of neutrophils is upregulated in tumour-bearing host [15]. Tumour cells themselves or tumour-associated immune cells can produce these cytokines. For instance, tumour-associated macrophages (TAMs) produce IL-1β, which stimulates T cells to produce IL-17. In turn, IL-17 increases the expression of G-CSF and the release of neutrophils in the circulation (see also the paragraph 6.2) [15]. The pressure created by the tumours on neutrophil mobilization can lead to the release of immature neutrophils in the circulation [8,14,59]. In mouse models of cancer, the frequency of Ly6Ghigh CD101− immature neutrophils in the blood has been positively correlated with the tumour burden [47]. In both human and mice, NePs (see above), are reported to infiltrate the tumour tissue [14,46,47,60]. In particular, NePs have been identified in the blood and tumour of melanoma patients and reported to promote tumour growth in mice through their immunosuppressive activities (see below) [46]. Studies described pro-tumoral capacity for immature neutrophils through their T-cell suppression ability [14,61]. However, not all immature neutrophils show immunosuppressive proprieties [62,63] and further investigations are needed to fully understand the exact mechanism in which these cells affect tumour growth.
4. Neutrophil recruitment in tissues
Once in the circulation, neutrophils are ready to migrate into the tissues [3]. Different conditions provide chemotactic gradients for neutrophil recruitment. In particular, Neutrophils express the chemokine receptors CXCR1 and CXCR2 and the presence of their ligands provides important chemotactic pathways for neutrophil recruitment to the tissues [64,65].
Extravasation of neutrophils across the vascular endothelium is a multistep process orchestrated by adhesion molecules present on vascular endothelial cells and neutrophils. This process has been extensively studied and involves the following steps: capture, rolling, arrest, firm adhesion and transmigration [1]. Neutrophil capture and rolling on endothelium occur through the initial interaction of P-selectin glycoprotein ligand 1 (PSGL1) expressed on neutrophils with P-selectin and E-selectin expressed on endothelial cells. In addition, engagement of l-selectin expressed by neutrophils with sialylated ligands on endothelial cells is also involved in the rolling of neutrophils and activation of integrins [66,67]. Lymphocyte function-associated antigen 1 (LFA-1) is the principal integrin which mediates arrest and firm adhesion of rolling neutrophils. LFA-1 binds to its ligands intercellular adhesion molecule 1 (ICAM1) and ICAM2 expressed on the endothelium [68]. Once arrested on the endothelium, neutrophils are ready to transmigrate from the vasculature to the tissue where they can play different roles depending on the tissue context or inflammatory situation (see below).
In addition to their migration in tissues, evidences showed that neutrophils can migrate from the inflamed tissues to the circulation through the process of reverse migration, which has been associated with the disruption of endothelial cells tight junctions and an altered production of chemoattractant molecules [[69], [70], [71]]. The process of reverse migration has been proposed to prevent excessive inflammation and tissue damage through the removal of neutrophils but could also act as a trigger of inflammation in a secondary site through the migration of activated neutrophils [70,72]. Therefore, while neutrophil trafficking to inflamed tissues has been extensively studied, the mechanism and role of reverse migration remain poorly understood and future investigations are needed to decipher the different aspects of this process.
4.1. Neutrophil recruitment in cancer
As mentioned above, neutrophils express CXCR1 and CXCR2, which can bind to their ligands CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8 released by tumour cells, stromal cells and leukocytes in the TME [50,73]. In addition to chemokines, tumour cells and tumour-infiltrating leukocytes are reported to produce cytokines involved in the development, mobilization and recruitment of neutrophils. For instance, T cells are shown to produce GM-CSF, TNFα, IL-17, CXCL1 and CXCL2, which can directly or indirectly recruit neutrophils to the TME [14,15,[74], [75], [76]]. Tumour-associated neutrophils (TANs) themselves produce CXCL1 and CXCL2, which in turn enhance their recruitment in the TME [77]. In different models of primary carcinogenesis, deficiency in CXCR2 has been associated with a dramatic reduction of neutrophil recruitment in the TME associated with reduction in tumour growth [78,79]. In addition to chemokines, tumour-derived oxysterols are also shown to trigger the recruitment of neutrophils to the TME through a CXCR2 dependent mechanism [80].
Recent investigations showed that neutrophils were present in the TME at the very early stages of tumour growth. Indeed, intravital multiphoton imaging in transplantable tumour models of mice with neutrophil-specific fluorescence revealed that neutrophils were present in the TME as early as 3 h after tumour cell injection and were present in the tissue up to 3 days after tumour cell injection [81]. Here, intra-tumoral neutrophils showed lower motility compared to peri-tumoral neutrophils, suggesting that the TME can affect neutrophil motility and functions [81].
5. Neutrophil functions
5.1. The role of neutrophils in steady state
The classical view of neutrophil roles has long been limited to their effector functions in the elimination of pathogens. However, a growing body of evidence showed the recruitment of neutrophils into naive tissues and their role in maintaining tissue homeostasis, such as in the lung where pulmonary endothelium retains CXCR4+ neutrophils through the expression of CXCL12 [3,82,83]. Under homeostatic condition, neutrophils are also found in liver, spleen and lymph nodes where they play different roles [3,84,85]. Indeed, liver infiltrating neutrophils can participate in lipid metabolism [84], whereas neutrophils present in the spleen provide helper signals to B cells through the production of B-cell activating factor (BAFF), a proliferation-inducing ligand (APRIL) and IL-21 [85]. In lymph nodes, neutrophils are mostly located in proximity of T cells and natural killer (NK) cells. These neutrophils express high amount of major histocompatibility complex II (MHCII), suggesting a role in CD4+ T cell activation [86].
Neutrophils have been also detected in other tissues, such as intestine, white adipose tissue, skin, and skeletal muscle but their role remains to be elucidated [3].
5.2. The role of neutrophils in infections
Neutrophils can detect a wide range of ligands expressed by microbes through their broad repertoire of cell associated and soluble pattern recognition receptors (PRRs) and represent the first line of defence against invading pathogens [2,29,87]. Neutrophils can eliminate pathogens through phagocytosis and production of ROS into the phagocytic vacuole, discharge of cytoplasmic granules containing microbicidal components, release of neutrophil extracellular traps (NETs), or indirectly by secreting pro-inflammatory cytokines and chemokines (e.g. TNFα, IL-6, IL-1β and IL-8) [2,88,89], which promote the recruitment and activation of other immune cells [[90], [91], [92], [93]].
In addition to their role in defence against pathogens, NETs are reported to drive thrombosis [93,94]. In Coronavirus disease 2019 (COVID-19) patients, increased plasma levels of NETs were correlated with the disease severity and in autopsy lung samples from patients, neutrophils undergoing NETosis were shown to colocalize with platelets in structures consistent with blood vessels, suggesting the role of NETs in COVID-19 related thrombosis [95]. These results suggest NETs formation as a potential target for COVID-19 treatment. In a more general view, these results highlight that an excessive presence of activated neutrophils in tissues can be deleterious for the host [96,97]. Thus, an efficient resolution of inflammation is crucial for the restoration of tissue integrity and function after an infection. Neutrophils are actively involved in this process through the production of pro-resolving lipid mediators (including resolvins and protectin D1). These molecules can block the infiltration of neutrophils in tissues and contribute to chemokines and cytokines scavenging [2,98,99]. In addition, phagocytosis of apoptotic neutrophils changes the phenotype of macrophages to produce anti-inflammatory cytokines such as TGFβ and IL-10 [100].
5.3. The role of neutrophils in inflammatory and autoimmune diseases
A growing number of studies showed that neutrophils can be involved in different inflammatory and autoimmune diseases, such as in cardiovascular disorders, multiple sclerosis (MS), systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [2,[101], [102], [103], [104]].
Proteins stored in neutrophils granules, such as MPO and LL-37 have been associated with the process of atherosclerosis through the activation of endothelial cells and the recruitment of inflammatory monocytes, respectively [105,106]. NETs have also been associated with cardiovascular diseases through different mechanisms, including activation of endothelial cells, plasmacytoid dendritic cells (pDCs) and macrophages [107,108]. Accordingly, neutrophil depletion in mice decreased the number of monocytes in aortic lysates and reduced the plaque sizes [109].
Neutrophils have also been involved in autoimmune diseases [104]. For instance, a population of low density neutrophils producing pro-inflammatory cytokines (e.g. IFNα, TNFα) and NETs has been identified in SLE patients [110]. Impaired clearance of apoptotic neutrophils by macrophages is also observed in these patients [111]. Interestingly, NETs can activate pDCs, which in turn produce IFNα and IL-6 to promote B cell differentiation into plasma cells [112,113]. Neutrophils can also support the B cell response through the production of BAFF [114,115].
Collectively, these data suggest that neutrophils, neutrophil-derived molecules and NETs represent important targets for therapy in patients with inflammatory and autoimmune disorders.
6. Neutrophil heterogeneity
6.1. Neutrophils heterogeneity in steady state
In steady state, both circulating and tissue neutrophils are endowed with functional and phenotypic characteristics [16,116]. Accordingly, neutrophil heterogeneity has been associated with different parameters, such as maturation, ageing or activation states in response to signals from the tissue microenvironment, such as cytokines [19].
The BM is the principal site of granulopoiesis and elimination of aged neutrophils. Interestingly, in vivo studies revealed specialized subsets of neutrophils in the BM displaying niche-supportive and HSC-supportive functions. In particular, a subset of granulocytes expressing the histidine-decarboxylase and histamine was shown to support the quiescence of myeloid-biased HSCs [117]. Moreover, BM neutrophils can secrete prostaglandin E2 which promotes the retention of HSC in the BM through the production of osteopontin by the preosteoblasts [118].
Neutrophils are released in the blood following a circadian rhythm and circulating neutrophils present different phenotypes over the course of the day in both humans and mice [19,51,119,120]. In particular, mature neutrophils freshly released from the BM are described as CD62L+ and CXCR2+ neutrophils. Within ∼6 h in the circulation, neutrophils downregulate the expression of CD62 L and upregulate the expression of CXCR4 and CD11b and acquire a hypersegmented nuclei. These CXCR4+ CD11b+ CD62Llow neutrophils exhibit increased integrin activation and a significant capacity to form NETs in inflamed mouse venules [119,121]. These phenotypic changes were linked to a circadian transcriptional oscillation of the transcription factor BMAL1 which controls the expression of CXCL2 by neutrophils. In turn, CXCL2 acts on CXCR2 to drive neutrophil ageing [119].
6.2. Neutrophils heterogeneity in cancer
Heterogeneity and plasticity of tumour infiltrating leukocytes (TILs) have been well characterized. Macrophages served as a paradigm for this phenomenon with classically activated M1 macrophages and alternatively activated M2 macrophages exerting anti-tumour and pro-tumour activities, respectively [13,122]. TANs can also undergo two different polarization states and by analogy with the nomenclature of M1 and M2 macrophages, TANs are classified into anti-tumour N1 neutrophils and pro-tumour N2 neutrophils. Here, TGFβ presents in the TME drives the polarization towards the N2 phenotype [44]. N1 TANs have been characterized by their cytotoxic activity towards tumour cells, their capacity to produce T cell chemokines CCL3, CXCL9 and CXCL10 and to sustain CD8+ T cells activation [77]. In contrast, N2 TANs showed a pro-tumour phenotype characterized by the release of proangiogenic factors (e.g. matrix metallopeptidase 9 (MMP-9)) and the inhibition of CD8+ T cells activation through secretion of arginase 1 (Arg1) [44,77].
Neutrophil differentiation and maturation trajectories are profoundly altered in tumour-bearing mice [57,123]. In advanced neoplasia, immature myeloid cells endowed with immunosuppressive properties appear in the circulation, primary tumours and metastases, often referred to as monocytes or granulocytes-myeloid-derived suppressor cells (M-MDSCs or G-MDSCs) [46,124,125]. G-MDSCs are functionally characterized by their ability to suppress T cells proliferation and activation ex vivo [126]. However, there is no consensus regarding their phenotypic characterization based on surface molecule expression. In human, G-MDSCs are described as CD15+ CD66b+ CD33dim HLA-DR− rendering them indistinguishable from the other neutrophil subsets [127,128].
Besides their heterogeneity in gene expression profile and surface molecule expression, neutrophils were classified by their sedimentation proprieties into low density neutrophils (LDNs), normal density neutrophils (NDNs) and high density neutrophil (HDNs) [16,17,129]. LDNs were reported to include both immature and mature neutrophils and to accumulate in cancer [8]. LDNs were described to be generally endowed with immunosuppressive proprieties [8,22,130]. However, immunosuppressive neutrophils (CD66b+, CD10+) have been also observed in the HDN fraction [131], highlighting the urge for an accurate system to define the neutrophil subsets in cancer.
7. The role of neutrophils in cancer
7.1. Interactions between neutrophils and tumour cells in the primary tumour
Cancer-related inflammation has emerged as a hallmark of cancer [9,10] and neutrophils were found to infiltrate different types of neoplasia, including non-small cell lung cancer (NSCLC), colorectal cancer (CRC), gastric cancer, hepatocellular carcinoma, melanoma, breast cancer and renal carcinoma [15,16,[20], [21], [22],132].
In the TME, neutrophils represent a source of cytokines, chemokines and growth factors that sustain tumour growth and progression including epidermal growth factor (EGF), hepatocytes growth factor (HGF) and platelet-derived growth factor (PDGF) [16,133]. Other neutrophil-derived mediators that influence tumour progression are found in neutrophil granules. For instance, NE has been shown to favour the proliferation of different cancer cell types in vitro, such as human oesophageal cell lines and mammary cell lines through the transactivation of the EGF receptor (EGFR) and TLR4, and human prostate cancer cell lines through the activation of the mitogen activation protein kinase (MAPK) pathway [[134], [135], [136]]. In a genetically engineered mouse model of lung adenocarcinoma induced by oncogenic Kras, NE-deficient mice displayed reduced tumour cells proliferation and tumour growth. Mechanistically, in vitro experiments showed that NE acted by degrading the insulin receptor substrate 1 (IRS-1), which usually inhibits the phosphoinositide 3-kinase (PI3K) in tumour cells. In turn, PI3K can interact with the cytoplasmic domain of the PDGFR, which has a potent mitogenic action on human and mouse lung cancer cells [137] (Fig. 1 ).
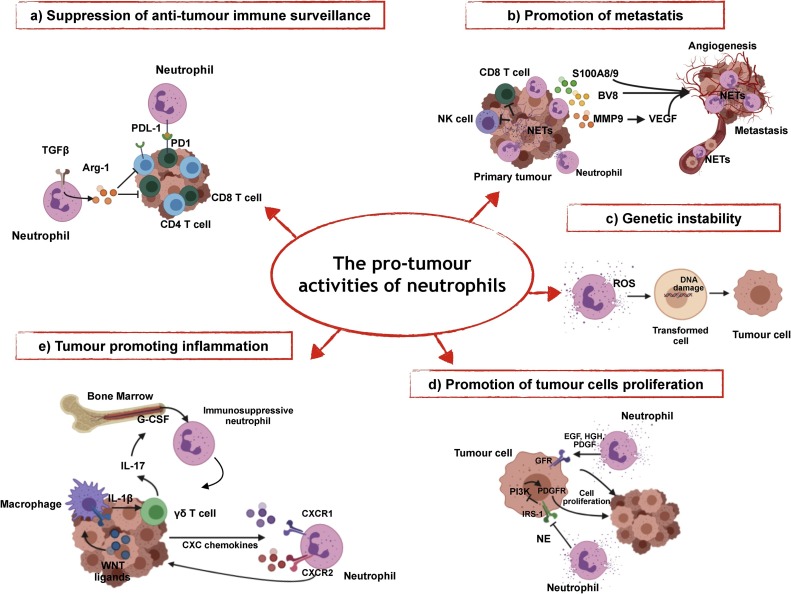
Fig. 1.
The pro-tumour activities of neutrophils.
a) Neutrophils can express ligands of immune checkpoints expressed on T cells, such as PD-L1. In response to TGFβ, neutrophils produce Arg1, leading to T cell dysfunction and suppression of the T cell-mediated anti-tumour response. b) Neutrophil-derived mediators, such as Bv8, MMP9, S100A8, S100A9, and NETs play an important role in the formation of metastasis through different mechanisms, such as tumour angiogenesis or entrapment and migration of circulating cancer cells. c) Neutrophil-derived ROS sustain DNA damage and genetic instability in epithelial cells; d) Neutrophils elastase (NE) and neutrophil-derived growth factors (i.e. EGF, HGF, PDGF) sustain the proliferation of tumour cells. e) Tumour cells drive the production of IL-1β in macrophages, which in turn activates the expression of IL-17 in γδ T cell, leading to the activation of the IL-17/G-CSF pathway and the recruitment of immunosuppressive neutrophils in the pre-metastatic lungs.
In addition to their role against microbial infections, NETs were observed in different tumours (e.g. liver, breast and intestinal) and were associated with the presence of metastasis (see below) [[138], [139], [140], [141], [142], [143]]. A recent study showed that NETs were able to interfere with lymphocytes cytotoxicity in the primary tumour. in vivo and in vitro experiments demonstrated that NETs can form a protective layer on tumour cells that acts as a shield against the cytotoxic activity of CD8+ T cells and NK cells (Fig. 1). Interestingly, pharmacological inhibition of NETs synergized with combination immunotherapy of anti-PD1 plus anti-CTLA4 to delay tumour progression in mice injected with 4T1 breast cancer cell line [144].
In tumour, neutrophil-derived ROS have been associated with increased DNA damage and genetic instability in epithelial cells [145] suggesting that neutrophils were involved in the initiation stage of carcinogenesis (Fig. 1). Accordingly, neutropenic mice (Csf3−/− mice) showed reduced urethane-induced lung carcinogenesis and forcing the recruitment of neutrophils in Csf3−/− mice by G-CSF was sufficient to restore tumour formation in the lung. The detrimental activity of neutrophils was linked to the production of ROS, which acts as an amplifier of DNA damage induced by urethane carcinogenesis [146]. In apparent contrast, in 3-methylcholathrene(3-MCA)-induced sarcomagenesis, neutrophils were found to be crucial for the initiation of an early anti-tumour response. Indeed, neutrophils amplified the production of IL-12 in macrophages, which induced type 1 polarization and IFNγ production in a subset of unconventional T cell (CD4- CD8- TCRβ+) [17]. These contrasting reports are likely related to the plasticity and heterogeneity of neutrophils in different tissue contexts and conditions.
ROS were shown to be a potent arm for neutrophil anti-tumour activity, through which they can directly kill tumour cells [44,147]. in vivo evidences showed that neutrophils can kill tumour cells through a cell-contact dependent mechanism and the generation of ROS (i.e. H2O2). Cancer cells undergoing epithelial-mesenchymal transition (EMT) upregulated the transient receptor potential cation channel, subfamily M, member 2 (TRPM2), an H2O2-dependent Ca2+ channel, which induces a lethal influx of Ca2+ in target cells [148] (Fig. 2 ). Accordingly, reduced TRPM2 expression in cancer cells protected them from the cytotoxic activity of neutrophils. Moreover, the expression of TRPM2 is associated with increased release of CXCL2, a potent neutrophil chemoattractant. These data suggest that TRPM2 has a dual role in tumour, by promoting the recruitment of neutrophils in the TME and the elimination of cancer cells [149].
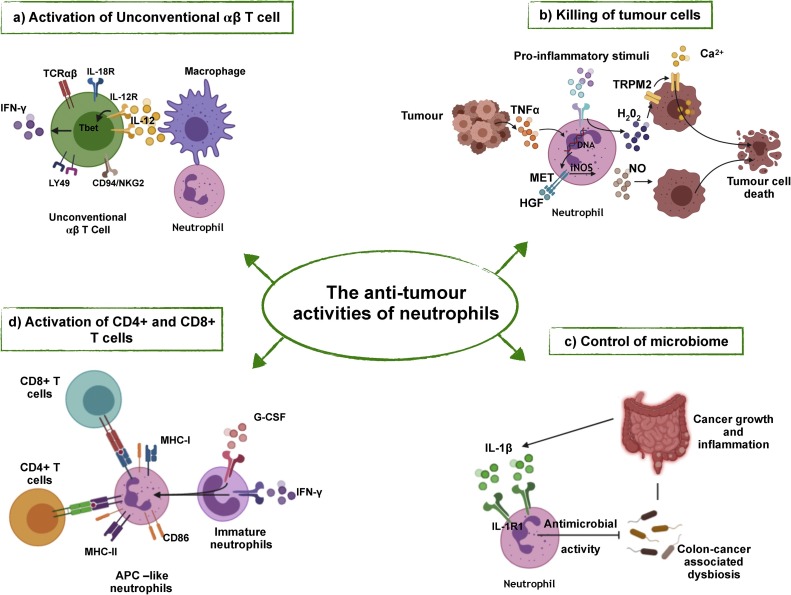
Fig. 2.
The anti-tumour activities of neutrophils.
a) Neutrophils enhance the expression of IL-12 by macrophages, which leads to the polarization of UTCαβ towards a type 1 activation state with increased expression of Tbet and IFNγ. b) Neutrophils can kill tumour cells through the release of ROS (i.e. H2O2) or RNI (NO) induced by various stimuli. c) In response to IL-1β, neutrophils restrict the invasion of bacteria and the subsequent tumour-promoting inflammation. d) In response to GM-CSF and IFNγ, immature neutrophils acquire an APC-like phenotype and can activate CD4+ and CD8+ T cells.
Neutrophil-mediated killing of tumour cells can also be induced by the expression of TNF-related apoptosis inducing ligand (TRAIL) by neutrophils [150] and TNFα. Tumour cell-derived TNFα induced the expression of the hepatocyte growth factor receptor (HGFR, also called MET) in neutrophils [151]. This induction is crucial for neutrophil transmigration through inflamed endothelium, the production of inducible nitric oxide synthase (iNOS) and NO by neutrophils in response to HGF. In turn, NO kills cancer cells [151] (Fig. 2). In contrast, the HGF-MET pathway was also shown to drive an immunosuppressive phenotype in neutrophils associated with limited expansion of anti-tumour T cells [152]. Therefore, the impact of MET expression on neutrophils and its possible therapeutic applications is still to be fully clarified.
7.2. The role of neutrophils in metastasis
The accumulation of neutrophils in tissues distant from the primary tumour has been shown to contribute to the formation of the pre-metastatic niche. In the pre-metastatic lung, neutrophils were found to promote the formation of metastasis via the secretion of several mediators, such as the proangiogenic factors Bv8 (also called prokineticin 2) and MMP-9 [76], the chemoattractant molecules S100A8 and S100A9 [14], NE and cathepsin G that mediate the degradation of thrombospondin-1 (Tsp-1) [153], the pro-inflammatory cytokine IL-1β and the leukotriene B4 (LTB4) [18]. In particular, neutrophil-derived MMP-9 was shown to induce the liberation and the activation of VEGF and the subsequent production of new vessels. in vivo studies have shown that Bv8, produced by neutrophils, was responsible for the poor efficacy of anti-VEGF therapy. Thus, the inhibition of IL-17\G-CSF axis was shown to increase the therapeutic efficacy of anti-VEGF treatment [[154], [155], [156], [157]].
The use of oncogene-driven cancer model has highlighted how the interaction between tumour cells and neutrophils was involved in metastasis formation [14,18,158,159]. In K14cre; Cdh1fl/fl; Trp53fl/fl (KEP) mouse model of breast cancer, the accumulation of neutrophils in the pre-metastatic lung with immunosuppressive features was associated with the formation of lung metastasis [14]. Further studies have demonstrated that tumour cells, through the secretion of WNT ligands induced a systemic pro-inflammatory cascade starting by the secretion of IL-1β by macrophages [159]. In turn, IL-1β induced the production of IL-17 by γδ T cells, leading to a G-CSF-dependent expansion of neutrophils and their polarization in an immunosuppressive phenotype [14,160] (Fig. 1). Accordingly, IL-17 derived from CD4 + T cells or γδ T cells drives most of the neutrophils-derived pro-tumour activities described above.
Studies showed that NETs can support the formation of metastasis through their ability to trap disseminating cancer cells and to facilitate their seeding and proliferation in a distant anatomical site [[138], [139], [140], [141], [142], [143]]. In an in vivo model of lung metastasis, sustained lung inflammation and cancer cell-derived G-CSF induced NETs formation, which in turn promoted the proliferation of dormant cancer cells. Accordingly, administration of DNase to eliminate NETs reduced the formation of lung metastasis [142]. In ovarian cancer, the formation of new metastasis in the omentum was also dependent on the formation of NETs in the pre-metastatic niche [141]. Mechanistically, recent investigations have provided new insights regarding the molecular interaction between cancer cells and NETs. In particular, cancer cells express the coiled-coil domain containing protein-25 (CCDC25), which acts as an extracellular DNA sensor and can activate cell motility [161]. Therefore, the formation of NETs in the liver or lung induced the migration of cancer cells and CCDC25-deficient mice present a reduced formation of metastasis. Moreover, in silico analyses showed that CCDC25 was expressed by several cancer cells in human and in cohorts of patients with breast cancer or colon cancer, the expression of CCDC25 was correlated with poor clinical outcome [161].
In apparent contrast with the previous findings, neutrophils recruitment and activation in the metastatic niche was found to be important for the reduction of metastasis formation through the killing of cancer cells [162,163]. Genetic ablation of the atypical chemokine receptor 2 (ACKR2) resulted in increased levels of chemokine receptors (i.e. CCR1, CCR2 and CCR5) in hematopoietic progenitors and the release from the BM of activated neutrophils endowed with cytotoxic activity towards tumour cells and anti-metastatic potential [163]. ACKR2 deficient mice transplanted with 4T1 breast cancer cell lines or intravenously injected with B16F10 melanoma cell lines showed protection against metastasis formation [163]. Thus, targeting ACKR2 unleashed anti-metastatic potential of neutrophils and represents an interesting novel myeloid checkpoint for innovative therapeutic strategies.
A recent study has demonstrated a mechanism that may explain, at least in part, why breast cancer patients develop metastasis [162]. The injection of human breast cancer cells with low spontaneous metastatic potential in non-obese diabetic (NOD)/severe combined immunodeficient (SCID) mice, resulted in reprogramming of neutrophils in the pre-metastatic lung. Breast cancer cells with low spontaneous metastatic potential secreted high amount of CCL2 compared to cancer cells with high metastatic potential. CCL2 induced the recruitment of IFNγ-producing CCR2+ monocytes in the pre-metastatic lung, leading to upregulation of the transmembrane protein 173 (TMEM173, also called STING) on neutrophils and activation of their cytotoxic activity [162].
Collectively, these studies underlined the relevance of neutrophils in the TME, both in the primary site and in the metastatic niche. Neutrophils with pro-tumour activities are associated to all steps of tumorigenesis, from the promotion of genetic instability, through the proliferation, dissemination and implantation of cancer cells to distal anatomical sites. However, when properly activated, neutrophils can kill tumour cells and participate to the anti-tumour response. Thus, studies aimed at clarifying the pathways that trigger neutrophils cytotoxicity against cancer cells, may be useful to pave the way for new therapeutic strategies targeting neutrophils.
7.3. The interaction between neutrophils and immune cells in cancer
Once recruited into inflamed tissues, neutrophils can engage bi-directional interactions with non-immune (e.g. platelets, mesenchymal cells) or immune cells (e.g. DCs, macrophages, NK cells, B cells, T cells) [2]. These interactions can occur in a contact-depend manner or via the secretion of soluble mediators. In these cross-talks, neutrophils receive signals that modulate their survival or activation status, while on the other hand neutrophils amplify, suppress or initiate the innate and adaptive immune responses [4,16]. These interactions have been shown to be relevant in the TME. For instance, tumour-entrained neutrophils can produce chemokines, including CXCL10, CCL2, CCL3, CXCL1 and CXCL2, responsible for the recruitment of T cells and other leukocytes [2,16].
In non-small cell lung cancer (NSCLC), phenotypic analysis of neutrophils in early stage patients revealed that neutrophils can acquire antigen presenting cell (APC)-like features and activate CD4+ T cells and CD8+ T cells [60]. These APC-like neutrophils derived from a subset of immature progenitors (CD11b+ CD15hi CD10– CD16int/low) that in response to GM-CSF and IFNγ present in the TME increased the expression of MHCII and CD86 (Fig. 2). On the same line, neutrophils isolated from colorectal cancer (CRC) patients were able to enhance CD8+ T cell responsiveness to T cell receptor (TCR) triggering [164].
Neutrophils have been described to be important in orchestrating the establishment of an effective anti-tumour immunity in sarcoma [17]. In a model of 3-MCA-induced sarcoma, neutrophils were found to engage a tripartite interaction with macrophages and a subset of UTCαβ. Neutrophils, by amplifying the production of IL-12 by macrophages, were essential to promote type I polarization and IFNγ production in UTCαβ, which support tumour resistance in vivo (Fig. 2). Interestingly, in silico analyses indicated that this neutrophil dependent anti-tumour axis was relevant in selected human tumours, including undifferentiated pleomorphic sarcoma (UPS), CRC and ovarian cancer [17].
As mentioned above, neutrophils in the TME can produce different mediators including ROS, reactive nitrogen intermediates (RNI) and Arg1 involved in the suppression of both innate and adaptive immune cells effector functions. In a mammary transplantable tumour model, c-Kit+ immature neutrophils were shown to be sensitive to glucose limitation. In this condition, neutrophils use mitochondrial fatty acid oxidation to support NAPDH oxidase-dependent ROS production which inhibits the T cells response [165]. This mitochondrial fitness is driven by c-Kit and was especially observed in splenic neutrophils [165].
In response to TGFβ found in TME, neutrophils produced high amount of Arg1, which reduced the availability of l-arginine in the TME and lead to T cell dysfunction and suppression of the T cell-mediated anti-tumour response [44] (Fig. 1). Notably, Arg1 produced by neutrophils from renal carcinoma and NSCLC cancer patients inhibited T cell response [166,167].
Recent investigations showed an interaction between neutrophils and the microbiota in cancer [160,[168], [169], [170], [171]]. Microbiota can regulate the granulopoiesis through the activation of the IL-17/G-CSF pathway in the intestine [172]. In CRC, neutrophils were reported to have a tumour suppressive effect through the expression of antimicrobial peptides, which limit the invasion of bacteria and the subsequent tumour-promoting inflammation [[168], [169], [170]] (Fig. 2). In addition, the process of lung carcinogenesis induced by Kras mutation coupled with p53 loss has been described to be associated with dysbiosis of the airway microbiota, which stimulates IL-17 production by resident γδ T cells resulting in neutrophilia and tumour growth [160].
Neutrophils engage important bi-directional interactions also with innate lymphoid cells (ILCs), particularly with NK cells. For instance, neutrophils have been shown to promote the metastatic spread by preventing NK cells-mediated clearance of disseminating cancer cells [144,173]. in vivo experiments showed that in the absence of NK cells, neutrophils acquired a pro-tumour phenotype characterized by increased expression of VEGF-A [174]. These results suggest that NK cells can control neutrophils pro-tumour activities.
Neutrophils can express ligands of checkpoint receptors, such as PD-L1 and V-domain immunoglobulin suppressor of T-cell activation (VISTA) involved in the suppression of the T cell response by driving checkpoint engagement and T cell exhaustion (Fig. 1). PD-L1 expressing neutrophils were found in hepatocellular carcinoma and gastric cancer and were associated with a bad prognosis [175,176]. In a murine transplantable model of melanoma, blockade of VISTA induced the production of IL-12 in tumour associated monocytes and dendritic cells, via a MyD88-dependent pro-inflammatory response [177]. However, the inhibition of VISTA on neutrophils did not alter their immunosuppressive phenotype, suggesting that combination therapy, with VISTA inhibition and reprogramming or depletion of neutrophils, should be considered in this model.
Collectively, these findings suggest that neutrophils can amplify or suppress the immune anti-tumour immune response, through different mechanisms. Deciphering these mechanisms may provide new therapeutic approaches to reprogram neutrophils in an anti-tumour activation state.
8. Clinical significance of neutrophils in cancer patients
8.1. Prognostic significance of neutrophils in cancer patients
High density of TANs or a high level of absolute neutrophil count (ANC) in the blood are generally associated with a poor prognosis in cancer patients [16,[20], [21], [22]]. Accordingly, blood neutrophil to lymphocytes ratio (NLR) has been proposed as a clinical biomarker in patients with cancer. This parameter is easy to obtain in clinical practice and can be monitored over time. A systematic review of electronic databases has been conducted to explore the significance of blood NLR in a variety of solid tumours. In 40,559 patients, with 22 different types of cancer, blood NLR was associated with faster tumour progression and reduced overall survival (OS) of patients in the majority of solid tumours [23]. However, it is important to note that the validity of the NLR parameter has been questioned. Indeed, NLR is a dynamic parameter that can undergo variations over time and can be affected by other clinical parameters, such as infections. Therefore, it remains to establish an optimal and uniform threshold to define a low, normal and high NLR in cancer patients [178].
Beside circulating neutrophils, the density of TANs has been also associated with outcome of patients with cancer. Neutrophils represent a significant proportion of tumour-infiltrating leukocytes in different subtype of solid tumours, including NSCLC, hepatocellular carcinoma, renal carcinoma, CRC and gastric cancer [20,21,132,164,175,176,[179], [180], [181]]. In most cases, density of TANs has been associated with a bad prognosis, likely due to their immunosuppressive features and their tumour promoting functions (see above). For example, TANs represent the dominant leukocytes population in NSCLC and were found inversely associated with T cell infiltration [180].
In other tumour contexts, such as in CRC, endometrial cancer, invasive ductal breast carcinoma, low grade glioma and undifferentiated pleomorphic sarcoma (UPS), density of TANs appear to correlate with a good prognosis [17,60,164,179,182,183]. In CRC patients, TANs were frequently colocalized with CD8+ T cells and can enhance their responsiveness to TCR triggering [164]. In patients with UPS neutrophil infiltration and a neutrophil signature were associated with a type I immune response and a better prognosis [17].
Thus, currently available data on the occurrence and significance of neutrophils in human cancer suggest that circulating and tumour infiltrating neutrophils may have opposite prognostic significance in different tumour contexts.
8.2. Neutrophils in response to therapy
High levels of circulating neutrophils, NLR or density of TANs have been associated with a poor response to different treatments, including chemotherapy, radiotherapy and immunotherapy, in the majority of solid tumours [[184], [185], [186], [187], [188], [189], [190]]. Some exception are CRC, gastric cancer and ovarian cancer where higher levels of TANs have been associated with a good response to therapy [179,183,[191], [192], [193], [194], [195], [196]].
For instance, high levels of CD66b+ neutrophils infiltration in stage (I-IV) CRC patients were found to correlate with a better clinical outcome [179,183] and in a cohort of stage III CRC patients and gastric cancer patients treated or not with 5-FU-based chemotherapy, high levels of TANs infiltration have been associated with a better response to therapy [179,195]. Interestingly, similar findings have been observed in patients with ovarian cancer treated with a platinum-based chemotherapy [193]. Therefore, further investigations are needed to define the molecular and cellular mechanisms by which neutrophils can improve the efficacy of chemotherapy in specific cancer subtypes.
9. Conclusions
The role of neutrophils has long been considered restricted to the elimination of invading pathogens and to the acute phase of inflammation. However, a large number of studies have challenged this view and neutrophils are now recognized for their role in the activation and orientation of both the innate and adaptive immune responses. Moreover, neutrophils are endowed with a previously underestimated plasticity and can engage complex cross-talks with immune and non-immune cells, depending on the tissue and inflammatory contexts. Accordingly, neutrophils can participate in the pathogenesis of different disorders such as infections, chronic inflammation, autoimmunity and cancer.
The role of neutrophils in cancer has long been considered insignificant and was neglected. However, neutrophilia is observed in cancer and neutrophils represent an important component of the TME. Therefore, the clinical significance of circulating neutrophils, NLR and TANs has been proposed in human cancer and mostly associated with a poor prognosis.
Neutrophils play a dual role in cancer and can support both pro-tumour and anti-tumour mechanisms. Neutrophils can sustain genetic instability, tumour cell proliferation and metastasis and can suppress the immune response. In contrast, neutrophils can kill tumour cells and can be part of the anti-tumour immune response. This dual role is probably related to the diversity and plasticity of neutrophils in different tumour contexts, leading to the formation of different subtypes of neutrophils with opposite functions. Therefore, a better dissection of TANs diversity in different cancer subtypes is required to define a comprehensive nomenclature of neutrophils in cancer and to identify the pathways that shape neutrophils in an anti-tumour activation state. We assume that further therapeutic approaches targeting neutrophils in cancer represent a new frontier in cancer immunotherapy.
Aknowledgements
Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) (PRIN 2017K7FSYBto SJ, Ministero della Salute (GR-2016-02361263to SJ) and Fondazione AIRC per la ricerca sul cancro (AIRC IG-22815 to SJ) are gratefully acknowledged.
References
- 1.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 3.Casanova-Acebes M., Nicolas-Avila J.A., Li J.L., Garcia-Silva S., Balachander A., Rubio-Ponce A., Weiss L.A., Adrover J.M., Burrows K., A.G. N, Ballesteros I., Devi S., Quintana J.A., Crainiciuc G., Leiva M., Gunzer M., Weber C., Nagasawa T., Soehnlein O., Merad M., Mortha A., Ng L.G., Peinado H., Hidalgo A. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018;215(11):2778–2795. doi: 10.1084/jem.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scapini P., Cassatella M.A. Social networking of human neutrophils within the immune system. Blood. 2014;124(5):710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 5.Garley M., Jablonska E. Heterogeneity among neutrophils. Arch Immunol Ther Exp (Warsz) 2018;66(1):21–30. doi: 10.1007/s00005-017-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong C.W. Current understanding in neutrophil differentiation and heterogeneity. Immune Netw. 2017;17(5):298–306. doi: 10.4110/in.2017.17.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecot P., Sarabi M., Pereira Abrantes M., Mussard J., Koenderman L., Caux C., Bendriss-Vermare N., Michallet M.C. Neutrophil heterogeneity in Cancer: from biology to therapies. Front. Immunol. 2019;10:2155. doi: 10.3389/fimmu.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagiv J.Y., Michaeli J., Assi S., Mishalian I., Kisos H., Levy L., Damti P., Lumbroso D., Polyansky L., Sionov R.V., Ariel A., Hovav A.H., Henke E., Fridlender Z.G., Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Fridman W.H., Zitvogel L., Sautes-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 12.Cortese N., Donadon M., Rigamonti A., Marchesi F. Macrophages at the crossroads of anticancer strategies. Front. Biosci. Landmark Ed. (Landmark Ed) 2019;24:1271–1283. doi: 10.2741/4779. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., Verstegen N.J.M., Ciampricotti M., Hawinkels L., Jonkers J., de Visser K.E. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16(7):431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 16.Jaillon S., Ponzetta A., Di Mitri D., Santoni A., Bonecchi R., Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer. 2020;20(9):485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 17.Ponzetta A., Carriero R., Carnevale S., Barbagallo M., Molgora M., Perucchini C., Magrini E., Gianni F., Kunderfranco P., Polentarutti N., Pasqualini F., Di Marco S., Supino D., Peano C., Cananzi F., Colombo P., Pilotti S., Alomar S.Y., Bonavita E., Galdiero M.R., Garlanda C., Mantovani A., Jaillon S. Neutrophils driving unconventional t cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178(2):346–360. doi: 10.1016/j.cell.2019.05.047. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wculek S.K., Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng L.G., Ostuni R., Hidalgo A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19(4):255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 20.Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A., Bruneval P., Fridman W.H., Becker C., Pages F., Speicher M.R., Trajanoski Z., Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D., Diehn M., West R.B., Plevritis S.K., Alizadeh A.A. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaul M.E., Fridlender Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019;16(10):601–620. doi: 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- 23.Templeton A.J., McNamara M.G., Seruga B., Vera-Badillo F.E., Aneja P., Ocana A., Leibowitz-Amit R., Sonpavde G., Knox J.J., Tran B., Tannock I.F., Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 24.Aroca-Crevillen A., Adrover J.M., Hidalgo A. Circadian features of neutrophil biology. Front. Immunol. 2020;11:576. doi: 10.3389/fimmu.2020.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo A., Chilvers E.R., Summers C., Koenderman L. The neutrophil life cycle. Trends Immunol. 2019;40(7):584–597. doi: 10.1016/j.it.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence S.M., Corriden R., Nizet V. The ontogeny of a neutrophil: mechanisms of granulopoiesis and homeostasis. Microbiol. Mol. Biol. Rev. 2018;82(1) doi: 10.1128/MMBR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manz M.G., Boettcher S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014;14(5):302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 28.Yvan-Charvet L., Ng L.G. Granulopoiesis and Neutrophil Homeostasis: A Metabolic, Daily Balancing Act. Trends Immunol. 2019;40(7):598–612. doi: 10.1016/j.it.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Walker F., Zhang H.H., Matthews V., Weinstock J., Nice E.C., Ernst M., Rose-John S., Burgess A.W. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111(8):3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- 31.Ai Z., Udalova I.A. Transcriptional regulation of neutrophil differentiation and function during inflammation. J. Leukoc. Biol. 2020;107(3):419–430. doi: 10.1002/JLB.1RU1219-504RR. [DOI] [PubMed] [Google Scholar]
- 32.Cowland J.B., Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016;273(1):11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 33.Hock H., Hamblen M.J., Rooke H.M., Traver D., Bronson R.T., Cameron S., Orkin S.H. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18(1):109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q., Dong F. Gfi-1 inhibits the expression of eosinophil major basic protein (MBP) during G-CSF-induced neutrophilic differentiation. Int. J. Hematol. 2012;95(6):640–647. doi: 10.1007/s12185-012-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul F., Arkin Y., Giladi A., Jaitin D.A., Kenigsberg E., Keren-Shaul H., Winter D., Lara-Astiaso D., Gury M., Weiner A., David E., Cohen N., Lauridsen F.K., Haas S., Schlitzer A., Mildner A., Ginhoux F., Jung S., Trumpp A., Porse B.T., Tanay A., Amit I. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell. 2015;163(7):1663–1677. doi: 10.1016/j.cell.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Bjerregaard M.D., Jurlander J., Klausen P., Borregaard N., Cowland J.B. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood. 2003;101(11):4322–4332. doi: 10.1182/blood-2002-03-0835. [DOI] [PubMed] [Google Scholar]
- 37.Hirai H., Zhang P., Dayaram T., Hetherington C.J., Mizuno S., Imanishi J., Akashi K., Tenen D.G. C/EBPbeta is required for’ emergency’ granulopoiesis. Nat. Immunol. 2006;7(7):732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 38.Morosetti R., Park D.J., Chumakov A.M., Grillier I., Shiohara M., Gombart A.F., Nakamaki T., Weinberg K., Koeffler H.P. A novel, myeloid transcription factor, C/EBP epsilon, is upregulated during granulocytic, but not monocytic, differentiation. Blood. 1997;90(7):2591–2600. [PubMed] [Google Scholar]
- 39.Ohlsson E., Schuster M.B., Hasemann M., Porse B.T. The multifaceted functions of C/EBPalpha in normal and malignant haematopoiesis. Leukemia. 2016;30(4):767–775. doi: 10.1038/leu.2015.324. [DOI] [PubMed] [Google Scholar]
- 40.Zhang P., Iwasaki-Arai J., Iwasaki H., Fenyus M.L., Dayaram T., Owens B.M., Shigematsu H., Levantini E., Huettner C.S., Lekstrom-Himes J.A., Akashi K., Tenen D.G. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21(6):853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Gery S., Gombart A.F., Fung Y.K., Koeffler H.P. C/EBPepsilon interacts with retinoblastoma and E2F1 during granulopoiesis. Blood. 2004;103(3):828–835. doi: 10.1182/blood-2003-01-0159. [DOI] [PubMed] [Google Scholar]
- 42.Hock H., Hamblen M.J., Rooke H.M., Schindler J.W., Saleque S., Fujiwara Y., Orkin S.H. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431(7011):1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 43.Grassi L., Pourfarzad F., Ullrich S., Merkel A., Were F., Carrillo-de-Santa-Pau E., Yi G., Hiemstra I.H., Tool A.T.J., Mul E., Perner J., Janssen-Megens E., Berentsen K., Kerstens H., Habibi E., Gut M., Yaspo M.L., Linser M., Lowy E., Datta A., Clarke L., Flicek P., Vingron M., Roos D., van den Berg T.K., Heath S., Rico D., Frontini M., Kostadima M., Gut I., Valencia A., Ouwehand W.H., Stunnenberg H.G., Martens J.H.A., Kuijpers T.W. Dynamics of transcription regulation in human bone marrow myeloid differentiation to mature blood neutrophils. Cell Rep. 2018;24(10):2784–2794. doi: 10.1016/j.celrep.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giladi A., Paul F., Herzog Y., Lubling Y., Weiner A., Yofe I., Jaitin D., Cabezas-Wallscheid N., Dress R., Ginhoux F., Trumpp A., Tanay A., Amit I. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat. Cell Biol. 2018;20(7):836–846. doi: 10.1038/s41556-018-0121-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y.P., Padgett L., Dinh H.Q., Marcovecchio P., Blatchley A., Wu R., Ehinger E., Kim C., Mikulski Z., Seumois G., Madrigal A., Vijayanand P., Hedrick C.C. Identification of an early unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone marrow. Cell Rep. 2018;24(9):2329–2341. doi: 10.1016/j.celrep.2018.07.097. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evrard M., Kwok I.W.H., Chong S.Z., Teng K.W.W., Becht E., Chen J., Sieow J.L., Penny H.L., Ching G.C., Devi S., Adrover J.M., Li J.L.Y., Liong K.H., Tan L., Poon Z., Foo S., Chua J.W., Su I.H., Balabanian K., Bachelerie F., Biswas S.K., Larbi A., Hwang W.Y.K., Madan V., Koeffler H.P., Wong S.C., Newell E.W., Hidalgo A., Ginhoux F., Ng L.G. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48(2):364–379. doi: 10.1016/j.immuni.2018.02.002. e8. [DOI] [PubMed] [Google Scholar]
- 48.Kwok I., Becht E., Xia Y., Ng M., Teh Y.C., Tan L., Evrard M., Li J.L.Y., Tran H.T.N., Tan Y., Liu D., Mishra A., Liong K.H., Leong K., Zhang Y., Olsson A., Mantri C.K., Shyamsunder P., Liu Z., Piot C., Dutertre C.A., Cheng H., Bari S., Ang N., Biswas S.K., Koeffler H.P., Tey H.L., Larbi A., Su I.H., Lee B., St John A., Chan J.K.Y., Hwang W.Y.K., Chen J., Salomonis N., Chong S.Z., Grimes H.L., Liu B., Hidalgo A., Newell E.W., Cheng T., Ginhoux F., Ng L.G. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity. 2020 doi: 10.1016/j.immuni.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Eash K.J., Greenbaum A.M., Gopalan P.K., Link D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 2010;120(7):2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capucetti A., Albano F., Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casanova-Acebes M., Pitaval C., Weiss L.A., Nombela-Arrieta C., Chevre R., A.G. N, Kunisaki Y., Zhang D., van Rooijen N., Silberstein L.E., Weber C., Nagasawa T., Frenette P.S., Castrillo A. A. Hidalgo, Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortez-Retamozo V., Etzrodt M., Newton A., Rauch P.J., Chudnovskiy A., Berger C., Ryan R.J., Iwamoto Y., Marinelli B., Gorbatov R., Forghani R., Novobrantseva T.I., Koteliansky V., Figueiredo J.L., Chen J.W., Anderson D.G., Nahrendorf M., Swirski F.K., Weissleder R., Pittet M.J. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109(7):2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boettcher S., Manz M.G. Regulation of inflammation- and infection-driven hematopoiesis. Trends Immunol. 2017;38(5):345–357. doi: 10.1016/j.it.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Hirai H., Yokota A., Tamura A., Sato A., Maekawa T. Non-steady-state hematopoiesis regulated by the C/EBPbeta transcription factor. Cancer Sci. 2015;106(7):797–802. doi: 10.1111/cas.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satake S., Hirai H., Hayashi Y., Shime N., Tamura A., Yao H., Yoshioka S., Miura Y., Inaba T., Fujita N., Ashihara E., Imanishi J., Sawa T., Maekawa T. C/EBPbeta is involved in the amplification of early granulocyte precursors during candidemia-induced “emergency” granulopoiesis. J. Immunol. 2012;189(9):4546–4555. doi: 10.4049/jimmunol.1103007. [DOI] [PubMed] [Google Scholar]
- 57.Casbon A.J., Reynaud D., Park C., Khuc E., Gan D.D., Schepers K., Passegue E., Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112(6):E566–75. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt H., Bastholt L., Geertsen P., Christensen I.J., Larsen S., Gehl J., von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br. J. Cancer. 2005;93(3):273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackey J.B.G., Coffelt S.B., Carlin L.M. Neutrophil maturity in Cancer. Front. Immunol. 2019;10:1912. doi: 10.3389/fimmu.2019.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singhal S., Bhojnagarwala P.S., O’Brien S., Moon E.K., Garfall A.L., Rao A.S., Quatromoni J.G., Stephen T.L., Litzky L., Deshpande C., Feldman M.D., Hancock W.W., Conejo-Garcia J.R., Albelda S.M., Eruslanov E.B. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung Cancer. Cancer Cell. 2016;30(1):120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J., Nefedova Y., Lei A., Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin. Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andreu P., Johansson M., Affara N.I., Pucci F., Tan T., Junankar S., Korets L., Lam J., Tawfik D., DeNardo D.G., Naldini L., de Visser K.E., De Palma M., Coussens L.M. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomihara K., Guo M., Shin T., Sun X., Ludwig S.M., Brumlik M.J., Zhang B., Curiel T.J., Shin T. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J. Immunol. 2010;184(11):6151–6160. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 64.Ha H., Debnath B., Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–1588. doi: 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stadtmann A., Zarbock A. CXCR2: From Bench to Bedside. Front. Immunol. 2012;3:263. doi: 10.3389/fimmu.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillipson M., Kubes P. The neutrophil in vascular inflammation. Nat. Med. 2011;17(11):1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zarbock A., Ley K., McEver R.P., Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liew P.X., Kubes P. The neutrophil’s role during health and disease. Physiol. Rev. 2019;99(2):1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 69.Colom B., Bodkin J.V., Beyrau M., Woodfin A., Ody C., Rourke C., Chavakis T., Brohi K., Imhof B.A., Nourshargh S. Leukotriene B4-Neutrophil elastase Axis Drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42(6):1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nourshargh S., Renshaw S.A., Imhof B.A. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol. 2016;37(5):273–286. doi: 10.1016/j.it.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Woodfin A., Voisin M.B., Beyrau M., Colom B., Caille D., Diapouli F.M., Nash G.B., Chavakis T., Albelda S.M., Rainger G.E., Meda P., Imhof B.A., Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Oliveira S., Rosowski E.E., Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat. Rev. Immunol. 2016;16(6):378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.SenGupta S., Subramanian B.C., Parent C.A., TANned Getting. How the tumor microenvironment drives neutrophil recruitment. J. Leukoc. Biol. 2019;105(3):449–462. doi: 10.1002/JLB.3RI0718-282R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aruga A., Aruga E., Cameron M.J., Chang A.E. Different cytokine profiles released by CD4+ and CD8+ tumor-draining lymph node cells involved in mediating tumor regression. J. Leukoc. Biol. 1997;61(4):507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- 75.Charles K.A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R.G., Kollias G., Smyth J.F., Balkwill F.R., Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Invest. 2009;119(10):3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kowanetz M., Wu X., Lee J., Tan M., Hagenbeek T., Qu X., Yu L., Ross J., Korsisaari N., Cao T., Bou-Reslan H., Kallop D., Weimer R., Ludlam M.J., Kaminker J.S., Modrusan Z., van Bruggen N., Peale F.V., Carano R., Meng Y.G., Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fridlender Z.G., Albelda S.M. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 78.Jamieson T., Clarke M., Steele C.W., Samuel M.S., Neumann J., Jung A., Huels D., Olson M.F., Das S., Nibbs R.J., Sansom O.J. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 2012;122(9):3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh H., Wang D., Daikoku T., Sun H., Dey S.K., Dubois R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(5):631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raccosta L., Fontana R., Maggioni D., Lanterna C., Villablanca E.J., Paniccia A., Musumeci A., Chiricozzi E., Trincavelli M.L., Daniele S., Martini C., Gustafsson J.A., Doglioni C., Feo S.G., Leiva A., Ciampa M.G., Mauri L., Sensi C., Prinetti A., Eberini I., Mora J.R., Bordignon C., Steffensen K.R., Sonnino S., Sozzani S., Traversari C., Russo V. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 2013;210(9):1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sody S., Uddin M., Gruneboom A., Gorgens A., Giebel B., Gunzer M., Brandau S. Distinct spatio-temporal dynamics of tumor-associated neutrophils in small tumor lesions. Front. Immunol. 2019;10:1419. doi: 10.3389/fimmu.2019.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolas-Avila J.A., Adrover J.M., Hidalgo A. Neutrophils in homeostasis, immunity, and Cancer. Immunity. 2017;46(1):15–28. doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Devi S., Wang Y., Chew W.K., Lima R., A.G. N, Mattar C.N., Chong S.Z., Schlitzer A., Bakocevic N., Chew S., Keeble J.L., Goh C.C., Li J.L., Evrard M., Malleret B., Larbi A., Renia L., Haniffa M., Tan S.M., Chan J.K., Balabanian K., Nagasawa T., Bachelerie F., Hidalgo A., Ginhoux F., Kubes P. L.G. Ng, Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 2013;210(11):2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansuy-Aubert V., Zhou Q.L., Xie X., Gong Z., Huang J.Y., Khan A.R., Aubert G., Candelaria K., Thomas S., Shin D.J., Booth S., Baig S.M., Bilal A., Hwang D., Zhang H., Lovell-Badge R., Smith S.R., Awan F.R., Jiang Z.Y. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17(4):534–548. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puga I., Cols M., Barra C.M., He B., Cassis L., Gentile M., Comerma L., Chorny A., Shan M., Xu W., Magri G., Knowles D.M., Tam W., Chiu A., Bussel J.B., Serrano S., Lorente J.A., Bellosillo B., Lloreta J., Juanpere N., Alameda F., Baro T., de Heredia C.D., Toran N., Catala A., Torrebadell M., Fortuny C., Cusi V., Carreras C., Diaz G.A., Blander J.M., Farber C.M., Silvestri G., Cunningham-Rundles C., Calvillo M., Dufour C., Notarangelo L.D., Lougaris V., Plebani A., Casanova J.L., Ganal S.C., Diefenbach A., Arostegui J.I., Juan M., Yague J., Mahlaoui N., Donadieu J., Chen K., Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011;13(2):170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lok L.S.C., Dennison T.W., Mahbubani K.M., Saeb-Parsy K., Chilvers E.R., Clatworthy M.R. Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc Natl Acad Sci U S A. 2019;116(38):19083–19089. doi: 10.1073/pnas.1905054116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jaillon S., Ponzetta A., Magrini E., Barajon I., Barbagallo M., Garlanda C., Mantovani A. Fluid phase recognition molecules in neutrophil-dependent immune responses. Semin. Immunol. 2016;28(2):109–118. doi: 10.1016/j.smim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Griffin G.K., Newton G., Tarrio M.L., Bu D.X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F.W., Croce K.J., Lichtman A.H. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012;188(12):6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tecchio C., Micheletti A., Cassatella M.A. Neutrophil-derived cytokines: facts beyond expression. Front. Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dohrmann S., Cole J.N., Nizet V. Conquering neutrophils. PLoS Pathog. 2016;12(7) doi: 10.1371/journal.ppat.1005682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 93.Sollberger G., Tilley D.O., Zychlinsky A. Neutrophil extracellular traps: the biology of chromatin externalization. Dev. Cell. 2018;44(5):542–553. doi: 10.1016/j.devcel.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 94.Perdomo J., Leung H.H.L., Ahmadi Z., Yan F., Chong J.J.H., Passam F.H., Chong B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019;10(1):1322. doi: 10.1038/s41467-019-09160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020 doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bordon J., Aliberti S., Fernandez-Botran R., Uriarte S.M., Rane M.J., Duvvuri P., Peyrani P., Morlacchi L.C., Blasi F., Ramirez J.A. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int. J. Infect. Dis. 2013;17(2):e76–83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 97.Mortaz E., Alipoor S.D., Adcock I.M., Mumby S., Koenderman L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018;9:2171. doi: 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sansbury B.E., Spite M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 2016;119(1):113–130. doi: 10.1161/CIRCRESAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ariel A., Fredman G., Sun Y.P., Kantarci A., Van Dyke T.E., Luster A.D., Serhan C.N. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7(11):1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weidenbusch M., Anders H.J. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J. Innate Immun. 2012;4(5–6):463–477. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013;210(7):1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thieblemont N., Wright H.L., Edwards S.W., Witko-Sarsat V. Human neutrophils in auto-immunity. Semin. Immunol. 2016;28(2):159–173. doi: 10.1016/j.smim.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Martini E., Kunderfranco P., Peano C., Carullo P., Cremonesi M., Schorn T., Carriero R., Termanini A., Colombo F.S., Jachetti E., Panico C., Faggian G., Fumero A., Torracca L., Molgora M., Cibella J., Pagiatakis C., Brummelman J., Alvisi G., Mazza E.M.C., Colombo M.P., Lugli E., Condorelli G., Kallikourdis M. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation. 2019;140(25):2089–2107. doi: 10.1161/CIRCULATIONAHA.119.041694. [DOI] [PubMed] [Google Scholar]
- 104.Wang X., Qiu L., Li Z., Wang X.Y., Yi H. Understanding the multifaceted role of neutrophils in Cancer and autoimmune diseases. Front. Immunol. 2018;9:2456. doi: 10.3389/fimmu.2018.02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doring Y., Drechsler M., Soehnlein O., Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler. Thromb. Vasc. Biol. 2015;35(2):288–295. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 106.Jerke U., Rolle S., Purfurst B., Luft F.C., Nauseef W.M., Kettritz R. beta2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. J. Biol. Chem. 2013;288(18):12910–12919. doi: 10.1074/jbc.M112.434613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doring Y., Manthey H.D., Drechsler M., Lievens D., Megens R.T., Soehnlein O., Busch M., Manca M., Koenen R.R., Pelisek J., Daemen M.J., Lutgens E., Zenke M., Binder C.J., Weber C., Zernecke A. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125(13):1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 108.Qi H., Yang S., Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front. Immunol. 2017;8:928. doi: 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drechsler M., Megens R.T., van Zandvoort M., Weber C., Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 110.Villanueva E., Yalavarthi S., Berthier C.C., Hodgin J.B., Khandpur R., Lin A.M., Rubin C.J., Zhao W., Olsen S.H., Klinker M., Shealy D., Denny M.F., Plumas J., Chaperot L., Kretzler M., Bruce A.T., Kaplan M.J. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren Y., Tang J., Mok M.Y., Chan A.W., Wu A., Lau C.S. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48(10):2888–2897. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 112.Jego G., Palucka A.K., Blanck J.P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 113.Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., Bassett R., Amuro H., Fukuhara S., Ito T., Liu Y.J., Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3(73) doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pers J.O., Daridon C., Devauchelle V., Jousse S., Saraux A., Jamin C., Youinou P. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann. N. Y. Acad. Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 115.Coquery C.M., Wade N.S., Loo W.M., Kinchen J.M., Cox K.M., Jiang C., Tung K.S., Erickson L.D. Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silvestre-Roig C., Fridlender Z.G., Glogauer M., Scapini P. Neutrophil diversity in health and disease. Trends Immunol. 2019;40(7):565–583. doi: 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen X., Deng H., Churchill M.J., Luchsinger L.L., Du X., Chu T.H., Friedman R.A., Middelhoff M., Ding H., Tailor Y.H., Wang A.L.E., Liu H., Niu Z., Wang H., Jiang Z., Renders S., Ho S.H., Shah S.V., Tishchenko P., Chang W., Swayne T.C., Munteanu L., Califano A., Takahashi R., Nagar K.K., Renz B.W., Worthley D.L., Westphalen C.B., Hayakawa Y., Asfaha S., Borot F., Lin C.S., Snoeck H.W., Mukherjee S., Wang T.C. Bone marrow myeloid cells regulate myeloid-biased hematopoietic stem cells via a histamine-dependent feedback loop. Cell Stem Cell. 2017;21(6):747–760. doi: 10.1016/j.stem.2017.11.003. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawano Y., Fukui C., Shinohara M., Wakahashi K., Ishii S., Suzuki T., Sato M., Asada N., Kawano H., Minagawa K., Sada A., Furuyashiki T., Uematsu S., Akira S., Uede T., Narumiya S., Matsui T., Katayama Y. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood. 2017;129(5):587–597. doi: 10.1182/blood-2016-07-725754. [DOI] [PubMed] [Google Scholar]
- 119.Adrover J.M., Del Fresno C., Crainiciuc G., Cuartero M.I., Casanova-Acebes M., Weiss L.A., Huerga-Encabo H., Silvestre-Roig C., Rossaint J., Cossio I., Lechuga-Vieco A.V., Garcia-Prieto J., Gomez-Parrizas M., Quintana J.A., Ballesteros I., Martin-Salamanca S., Aroca-Crevillen A., Chong S.Z., Evrard M., Balabanian K., Lopez J., Bidzhekov K., Bachelerie F., Abad-Santos F., Munoz-Calleja C., Zarbock A., Soehnlein O., Weber C., Ng L.G., Lopez-Rodriguez C., Sancho D., Moro M.A., Ibanez B., Hidalgo A. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390–402. doi: 10.1016/j.immuni.2019.01.002. e10. [DOI] [PubMed] [Google Scholar]
- 120.Ella K., Csepanyi-Komi R., Kaldi K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016;57:209–221. doi: 10.1016/j.bbi.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 121.Zhang D., Chen G., Manwani D., Mortha A., Xu C., Faith J.J., Burk R.D., Kunisaki Y., Jang J.E., Scheiermann C., Merad M., Frenette P.S. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525(7570):528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galdiero M.R., Bonavita E., Barajon I., Garlanda C., Mantovani A., Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Alshetaiwi H., Pervolarakis N., McIntyre L.L., Ma D., Nguyen Q., Rath J.A., Nee K., Hernandez G., Evans K., Torosian L., Silva A., Walsh C., Kessenbrock K. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 2020;5(44) doi: 10.1126/sciimmunol.aay6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19(2):108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moses K., Klein J.C., Mann L., Klingberg A., Gunzer M., Brandau S. Survival of residual neutrophils and accelerated myelopoiesis limit the efficacy of antibody-mediated depletion of Ly-6G+ cells in tumor-bearing mice. J. Leukoc. Biol. 2016;99(6):811–823. doi: 10.1189/jlb.1HI0715-289R. [DOI] [PubMed] [Google Scholar]
- 128.Scapini P., Marini O., Tecchio C., Cassatella M.A. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol. Rev. 2016;273(1):48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 129.Hassani M., Hellebrekers P., Chen N., van Aalst C., Bongers S., Hietbrink F., Koenderman L., Vrisekoop N. On the origin of low-density neutrophils. J. Leukoc. Biol. 2020;107(5):809–818. doi: 10.1002/JLB.5HR0120-459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Condamine T., Kumar V., Ramachandran I.R., Youn J.I., Celis E., Finnberg N., El-Deiry W.S., Winograd R., Vonderheide R.H., English N.R., Knight S.C., Yagita H., McCaffrey J.C., Antonia S., Hockstein N., Witt R., Masters G., Bauer T., Gabrilovich D.I. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest. 2014;124(6):2626–2639. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marini O., Costa S., Bevilacqua D., Calzetti F., Tamassia N., Spina C., De Sabata D., Tinazzi E., Lunardi C., Scupoli M.T., Cavallini C., Zoratti E., Tinazzi I., Marchetta A., Vassanelli A., Cantini M., Gandini G., Ruzzenente A., Guglielmi A., Missale F., Vermi W., Tecchio C., Cassatella M.A., Scapini P. Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129(10):1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- 132.Eruslanov E.B., Singhal S., Albelda S.M. Mouse versus human neutrophils in Cancer: a major knowledge gap. Trends Cancer. 2017;3(2):149–160. doi: 10.1016/j.trecan.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Granot Z., Jablonska J. Distinct functions of neutrophil in Cancer and its regulation. Mediators Inflamm 2015. 2015 doi: 10.1155/2015/701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caruso J.A., Akli S., Pageon L., Hunt K.K., Keyomarsi K. The serine protease inhibitor elafin maintains normal growth control by opposing the mitogenic effects of neutrophil elastase. Oncogene. 2015;34(27):3556–3567. doi: 10.1038/onc.2014.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lerman I., Garcia-Hernandez M.L., Rangel-Moreno J., Chiriboga L., Pan C., Nastiuk K.L., Krolewski J.J., Sen A., Hammes S.R. Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Mol. Cancer Res. 2017;15(9):1138–1152. doi: 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wada Y., Yoshida K., Tsutani Y., Shigematsu H., Oeda M., Sanada Y., Suzuki T., Mizuiri H., Hamai Y., Tanabe K., Ukon K., Hihara J. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol. Rep. 2007;17(1):161–167. [PubMed] [Google Scholar]
- 137.Houghton A.M., Rzymkiewicz D.M., Ji H., Gregory A.D., Egea E.E., Metz H.E., Stolz D.B., Land S.R., Marconcini L.A., Kliment C.R., Jenkins K.M., Beaulieu K.A., Mouded M., Frank S.J., Wong K.K., Shapiro S.D. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010;16(2):219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Kuttner V., Bruzas E., Maiorino L., Bautista C., Carmona E.M., Gimotty P.A., Fearon D.T., Chang K., Lyons S.K., Pinkerton K.E., Trotman L.C., Goldberg M.S., Yeh J.T., Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409) doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guglietta S., Chiavelli A., Zagato E., Krieg C., Gandini S., Ravenda P.S., Bazolli B., Lu B., Penna G., Rescigno M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016;7:11037. doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee W., Ko S.Y., Mohamed M.S., Kenny H.A., Lengyel E., Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019;216(1):176–194. doi: 10.1084/jem.20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H., Nakasone E.S., Hearn S.A., Kuttner V., Qiu J., Almeida A.S., Perurena N., Kessenbrock K., Goldberg M.S., Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016;8(361) doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Windt D.J., Sud V., Zhang H., Varley P.R., Goswami J., Yazdani H.O., Tohme S., Loughran P., O’Doherty R.M., Minervini M.I., Huang H., Simmons R.L., Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Teijeira A., Garasa S., Gato M., Alfaro C., Migueliz I., Cirella A., de Andrea C., Ochoa M.C., Otano I., Etxeberria I., Andueza M.P., Nieto C.P., Resano L., Azpilikueta A., Allegretti M., de Pizzol M., Ponz-Sarvise M., Rouzaut A., Sanmamed M.F., Schalper K., Carleton M., Mellado M., Rodriguez-Ruiz M.E., Berraondo P., Perez-Gracia J.L., Melero I. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856–871. doi: 10.1016/j.immuni.2020.03.001. e8. [DOI] [PubMed] [Google Scholar]
- 145.Gungor N., Knaapen A.M., Munnia A., Peluso M., Haenen G.R., Chiu R.K., Godschalk R.W. F.J. Van Schooten, Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25(2):149–154. doi: 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- 146.Wculek S.K., Bridgeman V.L., Peakman F., Malanchi I. Early neutrophil responses to chemical carcinogenesis shape long-term lung Cancer susceptibility. iScience. 2020;23(7) doi: 10.1016/j.isci.2020.101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Granot Z., Henke E., Comen E.A., King T.A., Norton L., Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gershkovitz M., Caspi Y., Fainsod-Levi T., Katz B., Michaeli J., Khawaled S., Lev S., Polyansky L., Shaul M.E., Sionov R.V., Cohen-Daniel L., Aqeilan R.I., Shaul Y.D., Mori Y., Karni R., Fridlender Z.G., Binshtok A.M., Granot Z. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78(10):2680–2690. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- 149.Gershkovitz M., Fainsod-Levi T., Zelter T., Sionov R.V., Granot Z. TRPM2 modulates neutrophil attraction to murine tumor cells by regulating CXCL2 expression. Cancer Immunol. Immunother. 2019;68(1):33–43. doi: 10.1007/s00262-018-2249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koga Y., Matsuzaki A., Suminoe A., Hattori H., Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64(3):1037–1043. doi: 10.1158/0008-5472.can-03-1808. [DOI] [PubMed] [Google Scholar]
- 151.Finisguerra V., Di Conza G., Di Matteo M., Serneels J., Costa S., Thompson A.A., Wauters E., Walmsley S., Prenen H., Granot Z., Casazza A., Mazzone M. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–353. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Glodde N., Bald T., van den Boorn-Konijnenberg D., Nakamura K., O’Donnell J.S., Szczepanski S., Brandes M., Eickhoff S., Das I., Shridhar N., Hinze D., Rogava M., van der Sluis T.C., Ruotsalainen J.J., Gaffal E., Landsberg J., Ludwig K.U., Wilhelm C., Riek-Burchardt M., Muller A.J., Gebhardt C., Scolyer R.A., Long G.V., Janzen V., Teng M.W.L., Kastenmuller W., Mazzone M., Smyth M.J., Tuting T., Holzel M. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit Cancer immunotherapy. Immunity. 2017;47(4):789–802. doi: 10.1016/j.immuni.2017.09.012. e9. [DOI] [PubMed] [Google Scholar]
- 153.El Rayes T., Catena R., Lee S., Stawowczyk M., Joshi N., Fischbach C., Powell C.A., Dannenberg A.J., Altorki N.K., Gao D., Mittal V. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112(52):16000–16005. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Albini A., Bruno A., Noonan D.M., Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front. Immunol. 2018;9:527. doi: 10.3389/fimmu.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chung A.S., Wu X., Zhuang G., Ngu H., Kasman I., Zhang J., Vernes J.M., Jiang Z., Meng Y.G., Peale F.V., Ouyang W., Ferrara N. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013;19(9):1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 156.Phan V.T., Wu X., Cheng J.H., Sheng R.X., Chung A.S., Zhuang G., Tran C., Song Q., Kowanetz M., Sambrone A., Tan M., Meng Y.G., Jackson E.L., Peale F.V., Junttila M.R., Ferrara N. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci U S A. 2013;110(15):6079–6084. doi: 10.1073/pnas.1303302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shojaei F., Wu X., Qu X., Kowanetz M., Yu L., Tan M., Meng Y.G., Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106(16):6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jackstadt R., van Hooff S.R., Leach J.D., Cortes-Lavaud X., Lohuis J.O., Ridgway R.A., Wouters V.M., Roper J., Kendall T.J., Roxburgh C.S., Horgan P.G., Nixon C., Nourse C., Gunzer M., Clark W., Hedley A., Yilmaz O.H., Rashid M., Bailey P., Biankin A.V., Campbell A.D., Adams D.J., Barry S.T., Steele C.W., Medema J.P., Sansom O.J. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal Cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36(3):319–336. doi: 10.1016/j.ccell.2019.08.003. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wellenstein M.D., Coffelt S.B., Duits D.E.M., van Miltenburg M.H., Slagter M., de Rink I., Henneman L., Kas S.M., Prekovic S., Hau C.S., Vrijland K., Drenth A.P., de Korte-Grimmerink R., Schut E., van der Heijden I., Zwart W., Wessels L.F.A., Schumacher T.N., Jonkers J., de Visser K.E. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538–542. doi: 10.1038/s41586-019-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jin C., Lagoudas G.K., Zhao C., Bullman S., Bhutkar A., Hu B., Ameh S., Sandel D., Liang X.S., Mazzilli S., Whary M.T., Meyerson M., Germain R., Blainey P.C., Fox J.G., Jacks T. Commensal microbiota promote lung Cancer development via gammadelta t cells. Cell. 2019;176(5):998–1013. doi: 10.1016/j.cell.2018.12.040. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang L., Liu Q., Zhang X., Liu X., Zhou B., Chen J., Huang D., Li J., Li H., Chen F., Liu J., Xing Y., Chen X., Su S., Song E. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 162.Hagerling C., Gonzalez H., Salari K., Wang C.Y., Lin C., Robles I. M. Van Gogh, A. Dejmek, K. Jirstrom, Z. Werb, Immune effector monocyte-neutrophil cooperation induced by the primary tumor prevents metastatic progression of breast cancer. Proc Natl Acad Sci U S A. 2019;116(43):21704–21714. doi: 10.1073/pnas.1907660116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Massara M., Bonavita O., Savino B., Caronni N., Mollica Poeta V., Sironi M., Setten E., Recordati C., Crisafulli L., Ficara F., Mantovani A., Locati M., Bonecchi R. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat. Commun. 2018;9(1):676. doi: 10.1038/s41467-018-03080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Governa V., Trella E., Mele V., Tornillo L., Amicarella F., Cremonesi E., Muraro M.G., Xu H., Droeser R., Daster S.R., Bolli M., Rosso R., Oertli D., Eppenberger-Castori S., Terracciano L.M., Iezzi G., Spagnoli G.C. The interplay between neutrophils and CD8(+) t cells improves survival in human colorectal Cancer. Clin. Cancer Res. 2017;23(14):3847–3858. doi: 10.1158/1078-0432.CCR-16-2047. [DOI] [PubMed] [Google Scholar]
- 165.Rice C.M., Davies L.C., Subleski J.J., Maio N., Gonzalez-Cotto M., Andrews C., Patel N.L., Palmieri E.M., Weiss J.M., Lee J.M., Annunziata C.M., Rouault T.A., Durum S.K., McVicar D.W. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat. Commun. 2018;9(1):5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu C.Y., Wang Y.M., Wang C.L., Feng P.H., Ko H.W., Liu Y.H., Wu Y.C., Chu Y., Chung F.T., Kuo C.H., Lee K.Y., Lin S.M., Lin H.C., Wang C.H., Yu C.T., Kuo H.P. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(-)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rodriguez P.C., Ernstoff M.S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A.C. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brennan C.A., Garrett W.S., Microbiota Gut. Inflammation, and colorectal Cancer. Annu. Rev. Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dmitrieva-Posocco O., Dzutsev A., Posocco D.F., Hou V., Yuan W., Thovarai V., Mufazalov I.A., Gunzer M., Shilovskiy I.P., Khaitov M.R., Trinchieri G., Waisman A., Grivennikov S.I. Cell-type-Specific responses to Interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal Cancer. Immunity. 2019;50(1):166–180. doi: 10.1016/j.immuni.2018.11.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., Taniguchi K., Yu G.Y., Osterreicher C.H., Hung K.E., Datz C., Feng Y., Fearon E.R., Oukka M., Tessarollo L., Coppola V., Yarovinsky F., Cheroutre H., Eckmann L., Trinchieri G., Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang D., Frenette P.S. Cross talk between neutrophils and the microbiota. Blood. 2019;133(20):2168–2177. doi: 10.1182/blood-2018-11-844555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Deshmukh H.S., Liu Y., Menkiti O.R., Mei J., Dai N., O’Leary C.E., Oliver P.M., Kolls J.K., Weiser J.N., Worthen G.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014;20(5):524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spiegel A., Brooks M.W., Houshyar S., Reinhardt F., Ardolino M., Fessler E., Chen M.B., Krall J.A., DeCock J., Zervantonakis I.K., Iannello A., Iwamoto Y., Cortez-Retamozo V., Kamm R.D., Pittet M.J., Raulet D.H., Weinberg R.A. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ogura K., Sato-Matsushita M., Yamamoto S., Hori T., Sasahara M., Iwakura Y., Saiki I., Tahara H., Hayakawa Y. NK cells control tumor-promoting function of neutrophils in mice. Cancer Immunol. Res. 2018;6(3):348–357. doi: 10.1158/2326-6066.CIR-17-0204. [DOI] [PubMed] [Google Scholar]
- 175.He G., Zhang H., Zhou J., Wang B., Chen Y., Kong Y., Xie X., Wang X., Fei R., Wei L., Chen H., Zeng H. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015;34:141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang T.T., Zhao Y.L., Peng L.S., Chen N., Chen W., Lv Y.P., Mao F.Y., Zhang J.Y., Cheng P., Teng Y.S., Fu X.L., Yu P.W., Guo G., Luo P., Zhuang Y., Zou Q.M. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66(11):1900–1911. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu W., Dong J., Zheng Y., Zhou J., Yuan Y., Ta H.M., Miller H.E., Olson M., Rajasekaran K., Ernstoff M.S., Wang D., Malarkannan S., Wang L. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol. Res. 2019;7(9):1497–1510. doi: 10.1158/2326-6066.CIR-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ivars Rubio A., Yufera J.C., de la Morena P., Fernandez Sanchez A., Navarro Manzano E., Garcia Garre E., Garcia Martinez E., Marin Zafra G., Sanchez Canovas M., Garcia Torralba E., Ayala de la Pena F. Neutrophil-lymphocyte ratio in metastatic breast cancer is not an independent predictor of survival, but depends on other variables. Sci. Rep. 2019;9(1):16979. doi: 10.1038/s41598-019-53606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galdiero M.R., Bianchi P., Grizzi F., Di Caro G., Basso G., Ponzetta A., Bonavita E., Barbagallo M., Tartari S., Polentarutti N., Malesci A., Marone G., Roncalli M., Laghi L., Garlanda C., Mantovani A., Jaillon S. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer. 2016;139(2):446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 180.Kargl J., Busch S.E., Yang G.H., Kim K.H., Hanke M.L., Metz H.E., Hubbard J.J., Lee S.M., Madtes D.K., McIntosh M.W., Houghton A.M. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kuang D.M., Zhao Q., Wu Y., Peng C., Wang J., Xu Z., Yin X.Y., Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011;54(5):948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 182.Blaisdell A., Crequer A., Columbus D., Daikoku T., Mittal K., Dey S.K., Erlebacher A. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28(6):785–799. doi: 10.1016/j.ccell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wikberg M.L., Ling A., Li X., Oberg A., Edin S., Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum. Pathol. 2017;68:193–202. doi: 10.1016/j.humpath.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 184.Bilen M.A., Dutcher G.M.A., Liu Y., Ravindranathan D., Kissick H.T., Carthon B.C., Kucuk O., Harris W.B., Master V.A. Association between pretreatment neutrophil-to-Lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin. Genitourin. Cancer. 2018;16(3):e563–e575. doi: 10.1016/j.clgc.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Capone M., Giannarelli D., Mallardo D., Madonna G., Festino L., Grimaldi A.M., Vanella V., Simeone E., Paone M., Palmieri G., Cavalcanti E., Caraco C., Ascierto P.A. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cassidy M.R., Wolchok R.E., Zheng J., Panageas K.S., Wolchok J.D., Coit D., Postow M.A., Ariyan C. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. 2017;18:56–61. doi: 10.1016/j.ebiom.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cha Y.J., Park E.J., Baik S.H., Lee K.Y., Kang J. Clinical significance of tumor-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in patients with stage III colon cancer who underwent surgery followed by FOLFOX chemotherapy. Sci. Rep. 2019;9(1):11617. doi: 10.1038/s41598-019-48140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chua W., Charles K.A., Baracos V.E., Clarke S.J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br. J. Cancer. 2011;104(8):1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Russo A., Franchina T., Ricciardi G.R.R., Battaglia A., Scimone A., Berenato R., Giordano A., Adamo V. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J. Cell. Physiol. 2018;233(10):6337–6343. doi: 10.1002/jcp.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu J., Ni C., Ma C., Zhang L., Jing X., Li C., Liu Y., Qu X. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin. Transl. Oncol. 2017;19(8):989–996. doi: 10.1007/s12094-017-1630-5. [DOI] [PubMed] [Google Scholar]
- 191.Manfroi B., Moreaux J., Righini C., Ghiringhelli F., Sturm N., Huard B. Tumor-associated neutrophils correlate with poor prognosis in diffuse large B-cell lymphoma patients. Blood Cancer J. 2018;8(7):66. doi: 10.1038/s41408-018-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Matsumoto Y., Mabuchi S., Kozasa K., Kuroda H., Sasano T., Yokoi E., Komura N., Sawada K., Kimura T. The significance of tumor-associated neutrophil density in uterine cervical cancer treated with definitive radiotherapy. Gynecol. Oncol. 2017;145(3):469–475. doi: 10.1016/j.ygyno.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 193.Posabella A., Kohn P., Lalos A., Wilhelm A., Mechera R., Soysal S., Muenst S., Guth U., Stadlmann S., Terracciano L., Droeser R.A., Zeindler J., Singer G. High density of CD66b in primary high-grade ovarian cancer independently predicts response to chemotherapy. J. Cancer Res. Clin. Oncol. 2020;146(1):127–136. doi: 10.1007/s00432-019-03108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schiffmann L.M., Fritsch M., Gebauer F., Gunther S.D., Stair N.R., Seeger J.M., Thangarajah F., Dieplinger G., Bludau M., Alakus H., Gobel H., Quaas A., Zander T., Hilberg F., Bruns C.J., Kashkar H., Coutelle O. Tumour-infiltrating neutrophils counteract anti-VEGF therapy in metastatic colorectal cancer. Br. J. Cancer. 2019;120(1):69–78. doi: 10.1038/s41416-018-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang H., Liu H., Shen Z., Lin C., Wang X., Qin J., Qin X., Xu J., Sun Y. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric Cancer. Ann. Surg. 2018;267(2):311–318. doi: 10.1097/SLA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 196.Zhou S.L., Zhou Z.J., Hu Z.Q., Huang X.W., Wang Z., Chen E.B., Fan J., Cao Y., Dai Z., Zhou J. Tumor-associated neutrophils recruit macrophages and T-Regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646–1658. doi: 10.1053/j.gastro.2016.02.040. e17. [DOI] [PubMed] [Google Scholar]