Abstract
This study investigated the effects of dietary selenium-enriched yeast (Se yeast) supplementation on the laying performance, egg quality, plasma antioxidant balance, and egg selenium (Se) content in laying Longyan ducks. A total of 480 32-week-old ducks were randomly divided into four dietary treatments, each consisting of six replicates of 20 ducks. The dietary treatments were a control basal diet and basal diets with supplementation of 0.05, 0.15, and 0.25 mg Se/kg via Se yeast. The analyzed Se contents of the four diets were 0.15, 0.21, 0.36, and 0.43 mg Se/kg, respectively. Dietary Se yeast supplementation had no apparent effects on laying performance and egg quality (p > 0.05), but it improved the antioxidant balance of ducks, as inferred by greater glutathione peroxidase and catalase activities, and decreased the malondialdehyde content in plasma of ducks (p < 0.05). It was suggested that the Se content in the basal diet containing 0.15 mg/kg of Se requirement is adequate for productive performance, but not for the antioxidant balance of laying ducks. Besides that, the Se contents in the yolk, albumen, and whole egg increased linearly as the Se supplementation levels increased. With more feeding days, the Se contents in the yolk and whole egg from non-Se-yeast-supplemented ducks increased linearly (p < 0.05), while those from Se-yeast-supplemented ducks showed a quadratic relationship (p < 0.05). In conclusion, the Se content of the basal diet at 0.15 mg/kg was adequate for laying performance and egg quality traits in laying ducks. Dietary Se yeast supplementation is beneficial to improve the antioxidant balance of laying ducks and increase the Se deposition in eggs for producing Se-enriched eggs. Based on the quadratic model or the quadratic broken-line model analyses, supplemental 0.19 mg Se/kg via Se yeast, with a total equivalent of 0.34 mg Se/kg in the diet, could provide the optimum antioxidant balance in laying ducks. Dietary supplementation of 0.25 mg Se/kg via Se yeast, with a total equivalent of 0.40 mg Se/kg in the diet, could lead to achieving the desired Se content in the whole egg.
Keywords: antioxidant balance, egg selenium content, laying ducks, selenium-enriched yeast, selenium supplementation
Introduction
As a key component of glutathione peroxidase and selenoproteins, selenium (Se) is essential for the execution of oxidoreductase functions and plays a crucial role in various biological processes of animals, such as the fertilization capacity of spermatozoa, embryonic and post-natal development, and immune responses (1–5). Therefore, Se-enriched animal products are gaining popularity for improving the Se health status of their human consumers. A fair number of studies tried to produce Se-enriched animal products via feed-based nutritional interventions to increase Se deposition in meat, egg, and milk (6–10). For example, Se deposition in egg yolk and albumen rose as dietary Se supplement levels increased, regardless of the Se source used (3, 11, 12). Other reports, however, did not find any elevated Se deposition in eggs from hens that were fed an insufficient Se-supplemented diet below the requirement of laying hens (13–15). Previous studies on laying hens and quails showed that those given organic Se sources, because of its higher bioavailability, received greater egg Se deposition compared with those given inorganic Se sources (16–18). Organic selenium-enriched yeast (Se yeast), containing 50–75% selenomethionine (SeMet), has been widely used in poultry diets (19–21). SeMet could be incorporated into proteins in place of Met and then enter into a long-term body pool, with a slower turnover rate than sodium selenite (SS), resulting in greater Se deposition in the eggs than that obtained by SS (22–24). For example, 34.26–37.12 μg Se per egg was provided by hens fed a Se-yeast-supplemented diet for 84 days, values 67.94–67.96% greater than those available from a SS-supplemented diet (25). Furthermore, it is speculated that egg deposition efficiency mainly depends on dietary Se level and Se sources, as well as the experimental feeding time. Additionally, there can be a wide variation in Se deposition efficiency in eggs among different domestic avian species. The Se contents of egg albumen and yolk in ducks were higher than those found in chicken, turkeys, and geese when these birds were offered the same feed type and schedule (26). Most studies of egg Se deposition have focused on laying hens that received diets with Se supplementation, leaving ducks rarely investigated in this respect (27–29). Accordingly, here we tested to investigate the effects of Se supplementation above its basal requirement, using Se yeast as an organic Se source, on laying performance, egg quality, egg Se deposition, and plasma antioxidant indices in ducks.
Materials and Methods
The protocol was reviewed and approved by the Animal Care and Use Committee of South China Agricultural University.
Longyan duck (Anas platyrhynchos) is a local breed of ducks for the dual production of eggs and meat in South China. A total of 480 32-week-old Longyan duck layers, during the middle–late laying period, with a similar initial laying rate (56.28 ± 1.97 %), were randomly divided into four treatments, each consisting of six replicates of 20 ducks. Two duck layers were housed in a single wire cage (40 cm length × 40 cm width × 50 cm height) equipped with three ladders and allowed ad libitum access to tap water. The 80-g feed was provided twice daily at 08:00 and 16:00 h. The corn–soybean meal basal diet served as the control diet; it was formulated to satisfy the basic nutrient requirements for Longyan laying ducks, containing 0.15 mg/kg of Se (Table 1). The three dietary Se treatments were formulated by supplementing the basal diet with 0.05, 0.15, or 0.25 mg/kg of Se via Se yeast (2,000 mg Se/kg Se yeast, Boshan Biological Feed Co., Ltd., Guangzhou, China). The total analyzed Se contents of the four diets were thus 0.15, 0.21, 0.36, and 0.43 mg Se/kg, respectively. The experimental time lasted for 28 days. During this period, eggs were collected from egg trays and the total egg weight of each replicate was recorded daily. The feed-to-egg ratio was calculated as the egg mass divided by feed intake. Two eggs per replicate were randomly selected, and the albumen and yolk were separated for Se assay. Another two eggs were broken and their egg albumen and yolk were mixed to prepare the whole egg samples. The Se contents in egg albumen, egg yolk, and the whole egg samples were measured at days 1, 7, 14, 21, and 28 of the experimental period. The experiment was conducted in a tropical area (Shantou City, China; 116° E longitude, 231° N latitude). The birds were kept in a semi-open laying house at 30 ± 2°C with 60–65% relative humidity and maintained on a 16-h light and 8-h dark photoperiod cycle.
Table 1.
Ingredients and nutrient levels of the basal diet (as-fed basis).
| Ingredients | Composition, % | Nutrient | Nutrient levels |
|---|---|---|---|
| Corn | 52.8 | ME, MJ/kg | 10.5 |
| Soybean meal | 23.0 | Crude protein, % | 17.0 |
| Wheat bran | 11.8 | Calcium, % | 3.50 |
| Fish meal | 2.00 | Available phosphorus, % | 0.350 |
| Limestone | 8.30 | Methionine, % | 0.402 |
| Calcium hydrophosphate | 0.70 | Methionine + cystine, % | 0.673 |
| Sodium chloride | 0.20 | Lysine, % | 0.956 |
| DL-Methionine | 0.14 | Seleniuma, mg/kg | 0.15 |
| L-Lysine hydrochloride | 0.060 | ||
| Premixb | 1.00 | ||
| Total | 100 |
Measured value of Se level; levels of other nutrients were calculated values. The total analyzed Se contents of the four treatment diets were 0.15, 0.21, 0.36, and 0.43 mg Se/kg, respectively.
Supplied per kilogram of diet: 16,000 IU of vitamin A, 4,000 IU of vitamin D3, 40 mg of vitamin E, 8 mg of vitamin K3, 4 mg of vitamin B1, 12 mg of vitamin B2, 8 mg of vitamin B6, 0.04 mg of vitamin B12, 0.2 mg of D-biotin, 24 mg of D-pantothenic acid, 2 mg of folic acid, 80 mg of nicotinamide, 50 mg of choline chloride, 6.4 mg of Cu, 70 mg of Fe, 80 mg of Zn, 96 mg of Mn, 0.56 mg of I, 0.32 mg of Co, 0.15 mg of Se.
Egg Quality
At the end of the experiment, 24 eggs per treatment (four eggs per replicate) were randomly selected for egg quality measurement. The eggs were weighed individually, and then their respective length and width were recorded with an egg form coefficient measuring instrument (FHK Fujihira Industry Co., Ltd., Tokyo, Japan) before breaking. Eggshell strength was measured with an egg force reader (Tenovo International Co., Ltd., Beijing, China), and eggshell thickness (average of three locations around the eggshell) was determined with an eggshell thickness gauge (FHK Fujihira Industry Co., Ltd., Tokyo, Japan). Yolk color, albumen height, and Haugh unit were all assayed by the egg analyzer (ORKA Food Technology Co. Ltd., Ramat HaSharon, Israel). The yolks were detached with an egg separator, weighed, and expressed as a percentage of egg weight. The weight of dried eggshells with their shell membranes was also weighed and likewise expressed as a percentage of egg weight. The albumen weight was calculated by subtracting the yolk and shell weight from the total egg weight, expressed also as a percentage of egg weight.
Selenium Assay
The Se content assay was performed as described by Meng et al. (28). In brief, a given homogenized egg sample or feed sample was mixed with HNO3-HClO4 (4:1) and heated until white fumes appeared; at this point, hydrochloric acid solution was added to digest it. Next, the digested sample was cooled down and diluted with ultrapure water for its measurement with an atomic fluorescence spectrophotometer (SK-2003A, Beijing Jinsuokun Technology Development Co., Ltd., Beijing, China).
Plasma Biochemical Assay
On day 28, one duck per replicate was randomly selected for drawing of blood sample from its wing vein. These blood samples were centrifuged at 3,000 rpm for 10 min, and the ensuing plasma samples were kept in Eppendorf tubes at −20°C until their analysis. Total protein (TP), albumin (ALB), alanine transaminase (ALT) enzyme, and aspartate transaminase (AST) enzyme levels were determined by an automatic biochemical analyzer (BS-5800M, Mindray Bio-Medical Electronics Co. Ltd., Shenzhen, China). Globulin (GLB) was calculated by subtracting ALB from TP from which the ALB/GLB ratio was then obtained. Total antioxidant capability (TAC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) in plasma were determined by following the manufacturer's instructions of the respective assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and then the MDA/TAC ratio was calculated as described by Attia et al. (30).
Statistical Analysis
Data were analyzed by one-way ANOVA using the PROC GLM procedure of the Statistical Analysis system v9.2 (SAS Inst. Inc., Cary, NC, USA). Orthogonal polynomial contrasts were used to determine the form of the effect (linear, quadratic, or cubic) as a function of higher Se levels in the diet or more experimental days. Data on laying rate, yolk proportion, albumen proportion, and eggshell proportion were transformed into arc sine values before statistical analysis. Each replicate served as the experimental unit for all statistical analyses. Data were deemed significant at p < 0.05. The regression of antioxidant indices was estimated by quadratic model or quadratic broken-line model analyses to discern the optimum antioxidant balance. The quadratic regression model is Y = A + BX + CX2, where Y means the antioxidant indices in plasma, and Se supplementation from Se yeast was estimated when the concentration of antioxidant indices reached the maximum response. The quadratic broken-line model for regression is Y = L + U × (R – X)2, where L is the plateau at values of x > R, R is the abscissa of the breakpoint, and the value of (R – X) is zero at values of X > R (31). Y means the antioxidant indices in plasma, L means the concentration of antioxidant indices at the plateau, and R means the Se supplementation from Se yeast. The plateau breakpoint for each Se response curve was determined by straight or quadratic broken-line model analyses to discern the optimum Se content in the whole egg (31). The straight broken-line model is Y = L + U × (R – X), and the quadratic broken-line model is Y = L + U × (R – X)2, where Y means the Se content in eggs, L means the Se content at the plateau, and R means the experimental day.
Results
Laying Performance
Dietary Se yeast supplementation had negligible effects (p > 0.05) on the laying rate, egg weight, egg mass, and feed/egg ratio in laying ducks (Table 2).
Table 2.
Effect of dietary Se yeast supplementation on the laying performance of Longyan laying ducks (n = 6)a.
| Items | Supplemented Se level from Se yeast, mg/kg | SEM | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.15 | 0.25 | Treatment | Linear | Quadratic | Cubic | ||
| Laying rate, % | 59.2 | 55.8 | 54.6 | 58.8 | 2.40 | 0.466 | 0.973 | 0.120 | 0.893 |
| Egg weight, g | 56.6 | 56.7 | 57.1 | 57.1 | 0.49 | 0.841 | 0.409 | 0.787 | 0.832 |
| Egg mass, g/day/bird | 33.5 | 31.6 | 31.2 | 33.6 | 1.40 | 0.501 | 0.877 | 0.138 | 0.872 |
| Feed: egg ratio | 5.02 | 5.29 | 5.36 | 4.97 | 0.222 | 0.528 | 0.794 | 0.153 | 0.909 |
Se yeast, selenium-enriched yeast.
SEM, standard error of the mean.
Means were calculated using six replicates per treatment (20 ducks per replicate).
Egg Quality
Dietary Se yeast supplementation had no apparent effect (p > 0.05) on the egg quality of laying ducks, in terms of the yolk proportion, albumen proportion, eggshell proportion, egg shape index, eggshell strength, eggshell thickness, albumen height, Haugh unit, and egg yolk color (Table 3).
Table 3.
Effect of dietary Se yeast supplementation on the egg quality of Longyan laying ducks (n = 6)a.
| Items | Supplemented Se level from Se yeast, mg/kg | SEM | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.15 | 0.25 | Treatment | Linear | Quadratic | Cubic | ||
| Yolk proportion, % | 30.1 | 30.9 | 31.1 | 30.7 | 0.89 | 0.856 | 0.677 | 0.470 | 0.882 |
| Albumen proportion, % | 54.5 | 50.2 | 54.2 | 55.3 | 1.21 | 0.578 | 0.378 | 0.406 | 0.440 |
| Eggshell proportion, % | 15.1 | 16.0 | 14.7 | 14.1 | 0.70 | 0.260 | 0.103 | 0.512 | 0.287 |
| Egg shape index | 73.9 | 74.1 | 72.4 | 72.1 | 1.09 | 0.454 | 0.128 | 0.923 | 0.575 |
| Eggshell strength, kg/cm2 | 4.15 | 4.32 | 3.94 | 4.01 | 0.178 | 0.407 | 0.257 | 0.859 | 0.204 |
| Eggshell thickness, mm | 0.341 | 0.342 | 0.342 | 0.343 | 0.006 | 0.538 | 0.362 | 0.414 | 0.380 |
| Albumen height, mm | 6.02 | 6.26 | 6.38 | 5.85 | 0.285 | 0.550 | 0.640 | 0.163 | 0.936 |
| Haugh unit | 77.9 | 78.9 | 80.5 | 76.2 | 2.04 | 0.510 | 0.608 | 0.157 | 0.702 |
| Egg yolk color | 4.46 | 4.65 | 4.20 | 4.55 | 0.204 | 0.448 | 0.812 | 0.414 | 0.157 |
Se yeast, selenium-enriched yeast; SEM, standard error of the mean.
Means were calculated using six replicates per treatment (four eggs per replicate).
Plasma Biochemical and Antioxidant Indices
Dietary Se yeast supplementation had no discernable impact (p > 0.05) on the TP, ALB, and GLB contents and ALB/GLB ratio, as well as the activities of ALT, AST, and SOD in the duck's plasma (Table 4). However, in response to increasing Se supplementation from Se yeast, the CAT activity increased in a linear way (r2 = 0.472, p < 0.001), while GSH-Px activity was elevated in both linear (r2 = 0.296, p < 0.001) and quadratic manner (r2 = 0.955, p < 0.001). For TAC, it was enhanced in a linear (r2 = 0.168, p < 0.001), quadratic (r2 = 0.861, p < 0.001), and cubic manner (r2 = 0.955, p < 0.001) with increasing Se supplementation from Se yeast. By contrast, negative linear (r2 = 0.453, p < 0.001), quadratic (r2 = 0.950, p < 0.001), and cubic relationship (r2 = 0.974, p < 0.001) was detected between the MDA content in plasma and dietary-supplemented Se level from Se yeast. The MDA/TAC ratio was decreased in a linear (r2 = 0.390, p < 0.001), quadratic (r2 = 0.894, p < 0.001), and cubic manner (r2 = 0.922, p < 0.05) with increasing Se supplementation from Se yeast. The highest GSH-Px and TAC activities and the lowest MDA content in plasma characterized those ducks fed a diet with 0.15 mg/kg Se supplementation from Se yeast. According to the regression (Table 5), the Se supplementation from Se yeast ranged from 0.11 to 0.32 mg/kg, and the average value was 0.19 mg/kg when the concentration of antioxidant indices reached the maximum response.
Table 4.
Effect of dietary Se yeast supplementation on the biochemical and the antioxidant indices in plasma of Longyan laying ducks (n = 6)a.
| Items | Supplemented Se level from Se yeast, mg/kg | SEM | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.15 | 0.25 | Treatment | Linear | Quadratic | Cubic | ||
| TP, mg/ml | 5.54 | 5.59 | 5.20 | 5.22 | 0.176 | 0.424 | 0.177 | 0.755 | 0.458 |
| ALB, mg/ml | 1.54 | 1.50 | 1.37 | 1.34 | 0.163 | 0.846 | 0.433 | 0.857 | 0.917 |
| GLB, mg/ml | 4.00 | 4.10 | 3.83 | 3.88 | 0.278 | 0.941 | 0.675 | 0.926 | 0.679 |
| ALB/GLB ratio | 0.407 | 0.378 | 0.427 | 0.350 | 0.074 | 0.991 | 0.845 | 0.855 | 0.964 |
| ALT, U/L | 3.07 | 3.13 | 3.02 | 3.17 | 0.303 | 0.993 | 0.911 | 0.863 | 0.819 |
| AST, U/L | 2.73 | 2.75 | 2.36 | 2.05 | 0.347 | 0.576 | 0.175 | 0.863 | 0.813 |
| GSH-Px, mmol/ml | 173c | 274b | 365a | 272b | 6.5 | <0.001 | <0.001 | <0.001 | 0.393 |
| SOD, U/ml | 22.2 | 22.8 | 25.1 | 26.3 | 4.61 | 0.910 | 0.478 | 0.956 | 0.939 |
| CAT, U/ml | 1.71c | 2.51bc | 3.28ab | 3.90a | 0.376 | 0.003 | <0.001 | 0.470 | 0.712 |
| TAC, U/ml | 3.53c | 5.65b | 10.6a | 5.61b | 0.251 | <0.001 | <0.001 | <0.001 | <0.001 |
| MDA, nmol/ml | 32.8a | 23.4b | 18.8c | 22.5b | 0.38 | <0.001 | <0.001 | <0.001 | <0.001 |
| MDA/TAC ratio | 9.55a | 4.16b | 1.78b | 4.03c | 0.373 | <0.001 | <0.001 | <0.001 | 0.0152 |
Se yeast, selenium-enriched yeast; SEM, standard error of the mean; TP, total protein; Alb, albumin; Glb, globulin; ALT, alanine transaminase enzyme; AST, aspartate transaminase enzyme; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; TAC, total antioxidant capability; MDA, malondialdehyde.
Means were calculated using six replicates per treatment (one bird per replicate).
Mean values within a row with different letters were significantly different (p < 0.05).
Table 5.
Estimated change of antioxidant indices in plasma with the Se supplementation from Se yeast (n = 6)a.
| Variation | Equation of regression | Estimated Se supplementation from Se yeast (mg/kg) | Estimated maximum response | P-values | R2 |
|---|---|---|---|---|---|
| Quadratic regression equationb | |||||
| GSH-Px, mmol/ml | Y = 170.6 + 2581.7X – 8693.9X2 | 0.15 | 362 | <0.001 | 0.955 |
| CAT, U/ml | Y = 1.77 + 13.78X – 21.32X2 | 0.32 | 3.99 | <0.001 | 0.486 |
| TAC, U/ml | Y = 2.88 + 96.95X – 340.19X2 | 0.14 | 9.79 | <0.001 | 0.861 |
| MDA/TAC ratio | Y = 9.16 – 101.36X + 325.78X2 | 0.16 | 1.27 | <0.001 | 0.894 |
| Quadratic broken-line regression equationc | |||||
| GSH-Px, mmol/ml | Y = 318.4 – 11565.1 × (0.11 – X)2 | 0.11 | 318 | <0.001 | 0.732 |
| CAT, U/ml | Y = 3.99 – 21.32 × (0.32 – X)2 | 0.32 | 3.99 | <0.001 | 0.486 |
| TAC, U/ml | Y = 8.03 – 195.3 × (0.15 – X)2 | 0.15 | 8.03 | <0.001 | 0.512 |
Se yeast, selenium-enriched yeast; GSH-Px, glutathione peroxidase; CAT, catalase; TAC, total antioxidant capability; MDA, malondialdehyde.
Means were calculated using six replicates per treatment (one bird per replicate).
The quadratic model for regression is Y = A + BX + CX2. Y means the antioxidant indices in plasma, and the Se supplementation from Se yeast was estimated when the concentration of the antioxidant indices reached the maximum response.
The quadratic broken-line model for regression is Y = L + U × (R – X)2, where L is the plateau at values of X > R, R is the abscissa of the breakpoint, and the value of (R – X) is zero at values of X > R. Y means the antioxidant indices in plasma, L means the concentration of antioxidant indices at the plateau, and R means the Se supplementation from Se yeast.
Se Contents
With a greater Se supplementation from Se yeast, the Se contents of the yolk, albumen, and whole egg all increased linearly (p < 0.05) on days 7, 14, 21, and 28 (Figures 1A–C). With more time (i. e., days of the experiment), the Se contents of the yolk and the whole egg increased linearly (p < 0.05) in the control group (Figures 1A,C), whereas those of the yolk, albumen, and whole egg all increased quadratically (p < 0.05) for the Se-yeast-supplementation treatments (i. e., the basal diet plus 0.05, 0.15, and 0.25 mg Se/kg from Se yeast, Figures 1A–C). Based on the broken-line analysis, the estimated experimental days for reaching the plateaued response of Se content in the whole egg declined as the supplemented Se level from Se yeast increased (Table 6). The maximum Se contents of whole eggs from ducks receiving diets with 0.05, 0.15, and 0.25 mg/kg Se supplementation via Se yeast were estimated to be 0.37, 0.44, and 0.53 mg/kg on days 18, 13, and 12, respectively (Table 6).
Figure 1.
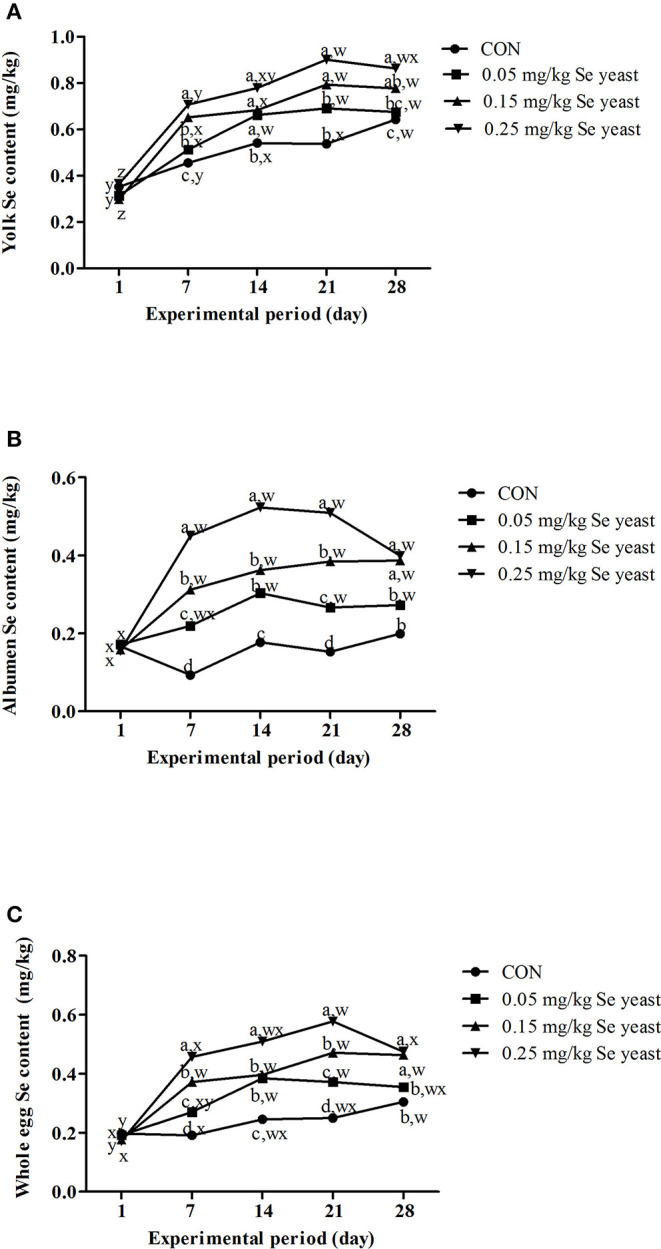
(A) Effect of dietary Se yeast supplementation on the dynamic change of yolk Se contents (mg/kg, fresh weight basis) during the 28-d feeding period. (B) Effect of dietary Se yeast supplementation on the dynamic change of albumen Se contents (mg/kg, fresh weight basis) during the 28-d feeding period. (C) Effect of dietary Se yeast supplementation on the dynamic change of the whole egg Se contents (mg/kg, fresh weight basis) during the 28-d feeding period. CON = the basal diet with 0.15 mg Se/kg from premix, 0.05 mg/kg Se yeast = the basal diet plus 0.05 mg/kg of Se from Se yeast, 0.15 mg/kg Se yeast = the basal diet plus 0.15 mg/kg of Se from Se yeast, 0.25 mg/kg Se yeast = the basal diet plus 0.25 mg/kg of Se from Se yeast. Values on the same day with different superscripts (a, b, c, d) are significantly different at p < 0.05. Values on the same line with different superscripts (w, x, y, z) are significantly different at p < 0.05. Egg Se contents for each treatment data on the same experimental day are means of 6 replicates (2 eggs per replicate).
Table 6.
Estimated change of Se content in eggs with the increase in experimental days.
| Variation | Supplemented Se level from Se yeast, mg/kg | Regression equationsa | Estimated days | Estimated Se content in eggs, mg/kg | P-values | R2 |
|---|---|---|---|---|---|---|
| Yolk Se content (mg/kg) | 0 | Y = 0.71 – 0.0095 × (36.0 – X) | 36 | 0.71 | <0.001 | 0.510 |
| 0.05 | Y = 0.68 – 0.0012 × (18.9 – X)2 | 19 | 0.68 | <0.001 | 0.622 | |
| 0.15 | Y = 0.75 – 0.0036 × (12.3 – X)2 | 13 | 0.75 | <0.001 | 0.650 | |
| 0.25 | Y = 0.87 – 0.0013 × (20.0 – X)2 | 20 | 0.87 | <0.001 | 0.647 | |
| Albumen Se content (mg/kg) | 0.05 | Y = 0.28 – 0.00050 × (16.3 – X)2 | 17 | 0.28 | 0.018 | 0.492 |
| 0.15 | Y = 0.38 – 0.0012 × (14.4 – X)2 | 15 | 0.38 | <0.001 | 0.805 | |
| 0.25 | Y = 0.48 – 0.0043 × (9.7 – X)2 | 10 | 0.48 | <0.001 | 0.704 | |
| Whole-egg Se contents (mg/kg) | 0 | Y = 0.33 – 0.0042 × (36.7 – X) | 37 | 0.33 | 0.001 | 0.567 |
| 0.05 | Y = 0.37 – 0.00068 × (17.7 – X)2 | 18 | 0.37 | 0.001 | 0.650 | |
| 0.15 | Y = 0.44 – 0.0018 × (12.9 – X)2 | 13 | 0.44 | <0.001 | 0.724 | |
| 0.25 | Y = 0.53 – 0.0029 × (11.8 – X)2 | 12 | 0.53 | <0.001 | 0.877 |
The regression equation for the non-Se-yeast-supplementation group was the linear broken-line model Y = L + U × (R – X), while for the Se-yeast-supplementation treatment groups, it was the quadratic broken-line model Y = L + U × (R – X)2, where L is the plateau at values of X > R, R is the abscissa of the breakpoint, and the value of (R – X) is zero at values of X > R. Y means the Se content in eggs, L means the Se content at the plateau, and R means the experimental day.
Discussion
It is generally recognized that Se is an essential element underpinning reproductive performance in poultry. Dietary Se supplementation with different levels (0.10–1.00 mg/kg) and sources (SS, Se yeast, and nano-Se or Se-enriched probiotics) improves both the laying performance and the egg quality of hens and quails (8, 11, 12, 14, 28, 32). In our study, supplementation with 0.05–0.25 mg Se/kg in the form of Se yeast included in the basal diet of Longyan laying ducks had no significant effect on their laying performance. These results agree with studies of Shanma laying ducks and Hy-Line, Leghorn, and Brown Warren laying hens that were fed diets supplemented with Se sources varying from 0.08 to 0.50 mg Se/kg (25, 33–35). Besides that, it was reported that egg production was not significantly affected by the level (0.10–0.40 mg/kg) and the source of Se, and no significant effect on the interaction between these variables was observed either (36). The divergent results for the laying performance may be due to the differences in Se sources and levels, avian species, age, and rearing environmental conditions. For example, Longyan laying ducks, a local breed of ducks for the dual production of eggs and meat in South China, which were used in our experiment, had a relatively low laying performance compared to the commercial-strain hens during the mid–late laying period. Besides that, this experiment was conducted in a tropical area with high temperature and relative humidity, which resulted in a slight decrease in the overall egg production performance of birds during the experimental period. Concerning egg quality, Baylan et al. (8) showed that, for quail eggs, their eggshell weight and eggshell thickness increased with inorganic or organic Se supplementation (0.10–0.30 mg Se/kg from SS or Se yeast), but excessive dietary Se can impair eggshell quality, as revealed by the reduced eggshell thickness in ducks and the lower eggshell proportion and strength in hens (29, 35). Those significant differences on laying performance and egg quality by dietary Se treatments were not observed in our study, which could be mainly explained by poultry breed, Se content of the basal diet, Se sources, and the duration of the experiment. We thus speculated that the Se content of the basal diet at 0.15 mg/kg was adequate for laying performance and egg quality traits in ducks. Moreover, the 28-day experimental period might be too short to induce a significant effect on the laying performance and egg quality of the duck breed that we studied.
As a component of the GSH-Px enzyme and selenoproteins, Se can improve the antioxidant capacity and reduce the lipid peroxides (37). The beneficial effect of enhanced antioxidant activity was observed in chicken broilers, laying hens, and pigeons when fed diets containing 0.10–0.90 mg Se/kg supplementation, regardless of the Se sources used (SS, Se yeast, nano-Se, or DL-SeMet) (7, 15, 38–40). Jing et al. (15) demonstrated that, compared to inorganic sources, organic Se sources fostered a better antioxidant balance by increasing the activities of GSH–Px and SOD and decreasing the MDA content in plasma. We found that plasma GSH-Px and CAT activities in laying ducks fed Se-yeast-supplemented diets rose remarkably relative to layers fed with the basal diet only. Furthermore, plasma TAC activity was increased when the laying ducks were given Se-yeast-supplemented diets, a result that is consistent with other findings reported for laying hens (28). Additionally, dietary Se yeast supplementation reduced the plasma MDA content, pointing to less lipid peroxidation damage, which could reflect the greater antioxidant capacity in these ducks. By comparison with the results of laying performance and egg quality, it was implied that the basal diet with 0.15 mg Se/kg was adequate for productive performance but not for antioxidant balance. In addition, it was acknowledged that Se is key to the maintenance of various physiological and biochemical functions within a small amount, while an excessive dose may lead to harmful effects (41). The Se supplementation at 0.15 and 0.25 mg/kg diet via Se yeast improves the antioxidant balance of the laying ducks in our study, and the average value of Se supplementation level from Se yeast was 0.19 mg/kg for optimum antioxidant balance. Heat stress affects the growth performance in broilers and the laying performance in laying hens, leading to a compromising efficiency in poultry production (42–44). So far, the potential enhancement of antioxidant ability from organic Se sources has been applied to alleviate the negative effect on poultry production when this is subjected to environmental stress challenge (45, 46). For example, many studies in broilers showed that the dosage of Se, added at a range of 0.3–1.0 mg/kg to the diet, improved the growth performance of chicken and quails when exposed to heat stress, contributing to the improvement of immune function and the increased activities of glutathione, GSH-Px, and SOD (47–53). It is also implied that a greater amount of Se is required for enhancing selenium-dependent antioxidant enzyme activities and gene expression relative to the glutathione system to alleviate oxidative damage induced by heat stress in poultry (54–59). According to the positive result of antioxidant ability, we will further investigate the Se requirement as well as the effect of Se yeast supplementation in diets in duck layers under heat-stressed condition in our next study.
Se deficiency could impair human health, given its association with a weaker immune system and a greater risk of susceptibility to various diseases (37, 60). The mineral contents of eggs also reflected the nutrition and the health status of laying hens (61). So far, one of the effective ways to improve Se intake is to consume some functional food, such as Se-enriched egg, meat, and milk products (10, 27, 62). However, the Se content in ordinary eggs collected from the retail market is under detectable levels (41, 63). Many studies have demonstrated that organic Se supplementation of the diets of laying hens translates into greater Se content and Se deposition efficiency in their eggs compared to using inorganic Se (16, 17, 64, 65). Since ducks may have a greater Se transfer rate among poultry, they could be an easier source to obtain Se-enriched eggs from than hens, turkeys, and geese (26). For this reason, how the supplemental Se level from the Se yeast source above the Se requirement affects egg Se deposition in laying ducks was investigated in the present study. Our results showed that egg Se deposition in ducks increased as dietary Se yeast supplementation increased, corroborating previous studies of laying hens that received diets with Se supplementation levels of 0.20 to 1.00 mg Se/kg (11, 12, 66, 67). Moreover, the Se content of whole eggs could be achieved at 0.53 mg/kg in laying ducks fed a diet containing 0.25 mg Se/kg from Se yeast for 13 days, which was less than the 0.50 mg/kg in laying hens fed Se-yeast-supplemented diets containing 0.30 to 0.50 mg Se/kg for 28 days, though the basal diets in the above-mentioned condition already contained 0.15 mg/kg Se (25, 28, 68). Therefore, we suggest that Se deposition efficiency might be greater for laying ducks than for laying hens. Interestingly, the Se contents of eggs from laying ducks fed the Se-yeast-supplemented diets were increased quadratically as the feeding days increased. The days required to attain the maximum Se deposition in whole eggs reduced as dietary Se yeast supplementation increased. From the broken-line analyses, the days were shorter for duck layers fed diets with 0.25 or 0.15 mg Se/kg supplementation via Se yeast (12 or 13 days) to peak the Se content in a whole egg than that fed diet with 0.05 mg Se/kg supplementation via Se yeast or the control basal diet (18 or 37 days). Therefore, to produce duck Se-enriched eggs, we recommend feeding ducks a diet (containing 0.15 mg Se/kg) supplemented with additional 0.15–0.25 mg Se/kg via Se yeast for no more than 12–13 days.
Conclusions
Our results showed that dietary Se yeast supplementation had no apparent effects on laying performance and egg quality, but it improved the glutathione peroxidase and catalase activities and decreased the malondialdehyde content in the plasma of ducks. Also, the Se contents in the egg yolk, albumen, and whole egg increased linearly as dietary Se levels increase. From the findings, the Se content of the basal diet at 0.15 mg/kg was adequate for laying performance and egg quality traits. Dietary Se yeast supplementation is beneficial to improve the antioxidant balance of laying ducks and increase Se deposition in eggs to produce Se-enriched eggs. Based on the quadratic model or the quadratic broken-line model analyses, supplemental 0.19 mg Se/kg via Se yeast, with a total equivalent of 0.34 mg Se/kg in the diet, could provide the optimum antioxidant balance in laying ducks. Dietary supplementation of 0.25 mg Se/kg via Se yeast, with a total equivalent of 0.40 mg Se/kg in the diet, could lead to achieving the desired Se content in the whole egg.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Animal Care and Use Committee of South China Agricultural University.
Author Contributions
XZ designed this study, carried out the experiments and measurements, and drafted the manuscript. LT and SZ helped with the analysis of the samples. ZL, HYa, and JC assisted with the duck trial. HYe and WW helped with the data analysis. LY and YZ participated in the study's design, coordination, and manuscript writing. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- Se
selenium
- Se yeast
selenium-enriched yeast
- SeMet
selenomethionine
- SS
sodium selenite
- TP
total protein
- ALB
albumin
- GLB
globulin
- ALT
alanine transaminase
- AST
aspartate transaminase
- TAC
total antioxidant capability
- SOD
superoxide dismutase
- GSH-Px
glutathione peroxidase
- CAT
catalase
- MDA
malondialdehyde.
Footnotes
Funding. This study was sponsored by National Natural Science Foundation of China (31972584), Guangdong Provincial Natural Science Foundation for Starting Ph.D. (2017A030310398 and 2018A030310202), National Key Research Program (2016YFD0500509-07), and National Waterfowl Industry Program in China (CARS-42-15).
References
- 1.Dalgaard TS, Briens M, Engberg RM, Lauridsen C. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim Feed Sci Technol. (2018) 238:73–83. 10.1016/j.anifeedsci.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortier ME, Audet I, Giguere A, Laforest JP, Bilodeau JF, Quesnel H, et al. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J Anim Sci. (2012) 90:231–40. 10.2527/jas.2010-3340 [DOI] [PubMed] [Google Scholar]
- 3.Yuan D, Zhan X, Wang Y. Effects of selenium sources and levels on reproductive performance and selenium retention in broiler breeder, egg, developing embryo, and 1-day-old chick. Biol Trace Elem Res. (2011) 144:705–14. 10.1007/s12011-011-9111-0 [DOI] [PubMed] [Google Scholar]
- 4.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. (1999) 285:1393–6. 10.1126/science.285.5432.1393 [DOI] [PubMed] [Google Scholar]
- 5.Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. (2014) 94:739–77. 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esmaeili S, Khosravi-Darani K. Selenium-enriched yeast: as selenium source for nutritional purpose. Cur Nutri Food Sci. (2014) 10:49–56. 10.2174/157340131001140328115753 [DOI] [Google Scholar]
- 7.Cai S, Wu C, Gong L, Song T, Wu H, Zhang L. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci. (2012) 91:2532–9. 10.3382/ps.2012-02160 [DOI] [PubMed] [Google Scholar]
- 8.Baylan M, Canogullari S, Ayasan T, Copur G. Effects of dietary selenium source, storage time, and temperature on the quality of quail eggs. Biol Trace Elem Res. (2011) 143:957–64. 10.1007/s12011-010-8912-x [DOI] [PubMed] [Google Scholar]
- 9.Juniper DT, Phipps RH, Jones AK, Bertin G. Selenium supplementation of lactating dairy cows: effect on selenium concentration in blood, milk, urine, and feces. J Dairy Sci. (2006) 89:3544–51. 10.3168/jds.S0022-0302(06)72394-3 [DOI] [PubMed] [Google Scholar]
- 10.Krstic B, Jokic Z, Pavlovic Z, Zivkovic D. Options for the production of selenized chicken meat. Biol Trace Elem Res. (2012) 146:68–72. 10.1007/s12011-011-9229-0 [DOI] [PubMed] [Google Scholar]
- 11.Pan CL, Zhao YX, Liao SF, Chen F, Qin SY, Wu XS, et al. Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J Agric Food Chem. (2011) 59:11424–31. 10.1021/jf202014k [DOI] [PubMed] [Google Scholar]
- 12.Scheideler SE, Weber P, Monsalve D. Supplemental vitamin E and selenium effects on egg production, egg quality, and egg deposition of alpha-tocopherol and selenium. J Appl Poult Res. (2010) 19:354–60. 10.3382/japr.2010-00198 [DOI] [Google Scholar]
- 13.Slupczynska M, Jamroz D, Orda J, Wiliczkiewicz A, Krol B. Long-term supplementation of laying hen diets with various selenium sources as a method for the fortification of eggs with selenium. J Chem. (2018) 2018:1–7. 10.1155/2018/7986591 [DOI] [Google Scholar]
- 14.Han XJ, Qin P, Li WX, Ma QG, Ji C, Zhang JY, et al. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult Sci. (2017) 96:3973–80. 10.3382/ps/pex216 [DOI] [PubMed] [Google Scholar]
- 15.Jing CL, Dong XF, Wang ZM, Liu S, Tong JM. Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci. (2015) 94:965–75. 10.3382/ps/pev045 [DOI] [PubMed] [Google Scholar]
- 16.Chantiratikul A, Chinrasri O, Chantiratikul P. Effect of selenium from selenium-enriched kale sprout versus other selenium sources on productivity and selenium concentrations in egg and tissue of laying hens. Biol Trace Elem Res. (2018) 182:105–10. 10.1007/s12011-017-1069-0 [DOI] [PubMed] [Google Scholar]
- 17.Tufarelli V, Ceci E, Laudadio V. 2-Hydroxy-4-Methylselenobutanoic acid as new organic selenium dietary supplement to produce selenium-enriched eggs. Biol Trace Elem Res. (2016) 171:453–8. 10.1007/s12011-015-0548-4 [DOI] [PubMed] [Google Scholar]
- 18.Chinrasri O, Chantiratikul P, Maneetong S, Chookhampaeng S, Chantiratikul A. Productivity and selenium concentrations in egg and tissue of laying quails fed selenium from hydroponically produced selenium-enriched kale sprout (Brassica oleracea var. Alboglabra L.). Biol Trace Elem Res. (2013) 155:381–6. 10.1007/s12011-013-9824-3 [DOI] [PubMed] [Google Scholar]
- 19.Surai PF. Selenium in poultry nutrition 1. antioxidant properties, deficiency and toxicity. Worlds Poult Sci J. (2002) 58:333–47. 10.1079/WPS20020026 [DOI] [Google Scholar]
- 20.Surai PF. Selenium in poultry nutrition 2. reproduction, egg and meat quality and practical applications. Worlds Poult Sci J. (2002) 58:431–50. 10.1079/WPS20020032 [DOI] [Google Scholar]
- 21.Surai PF, Kochish II, Fisinin VI, Velichko OA. Selenium in poultry nutrition: from sodium selenite to organic selenium sources. J Poult Sci. (2018) 55:79–93. 10.2141/jpsa.0170132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrauzer GN. The nutritional significance, metabolism and toxicology of selenomethionine. Adv Food Nutri Res. (2003) 47:73–112. 10.1016/S1043-4526(03)47002-2 [DOI] [PubMed] [Google Scholar]
- 23.Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr. (2015) 35:109–34. 10.1146/annurev-nutr-071714-034250 [DOI] [PubMed] [Google Scholar]
- 24.Payne RL, Lavergne TK, Southern LL. Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult Sci. (2005) 84:232–7. 10.1093/ps/84.2.232 [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Qu L, Shen MM, Hu YP, Guo J, Dou TC, et al. Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult Sci. (2018) 97:3102–8. 10.3382/ps/pey161 [DOI] [PubMed] [Google Scholar]
- 26.Nisianakis P, Giannenas I, Gavriil A, Kontopidis G, Kyriazakis I. Variation in trace element contents among chicken, turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Biol Trace Elem Res. (2009) 128:62–71. 10.1007/s12011-008-8249-x [DOI] [PubMed] [Google Scholar]
- 27.Bennett DC, Cheng KM. Selenium enrichment of table eggs. Poult Sci. (2010) 89:2166–72. 10.3382/ps.2009-00571 [DOI] [PubMed] [Google Scholar]
- 28.Meng T, Liu YL, Xie CY, Zhang B, Huang YQ, Zhang YW, et al. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res. (2018) 189:548–55. 10.1007/s12011-018-1490-z [DOI] [PubMed] [Google Scholar]
- 29.Arpasova H, Petrovic V, Mellen M, Kacaniova M, Cobanova K, Leng L. The effects of supplementing sodium selenite and selenized yeast to the diet for laying hens on the quality and mineral content of eggs. J Anim Feed Sci. (2009) 18:90–100. 10.22358/jafs/66371/2009 [DOI] [Google Scholar]
- 30.Attia YA, Al-Harthi MA, Abo El-Maaty HM. The effects of different oil sources on performance, digestive enzymes, carcass traits, biochemical, immunological, antioxidant, and morphometric responses of broiler chicks. Front Vet Sci. (2020) 7:181. 10.3389/fvets.2020.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins K, Saxton A, Southern L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. (2006) 84:155–65. 10.2527/2006.8413_supplE155x [DOI] [PubMed] [Google Scholar]
- 32.Abd El-Hack ME, Mahrose K, Askar AA, Alagawany M, Arif M, Saeed M, et al. Single and combined impacts of vitamin A and selenium in diet on productive performance, egg quality, and some blood parameters of laying hens during hot season. Biol Trace Elem Res. (2017) 177:169–79. 10.1007/s12011-016-0862-5 [DOI] [PubMed] [Google Scholar]
- 33.Li JK, Wang XL. Effect of dietary organic versus inorganic selenium in laying hens on the productivity, selenium distribution in egg and selenium content in blood, liver and kidney. J Trace Elem Med Biol. (2004) 18:65–8. 10.1016/j.jtemb.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 34.Invernizzi G, Agazzi A, Ferroni M, Rebucci R, Fanelli A, Baldi A, et al. Effects of inclusion of selenium-enriched yeast in the diet of laying hens on performance, eggshell quality, and selenium tissue deposition. Ital J Anim Sci. (2013) 12:1–8. 10.4081/ijas.2013.e1 [DOI] [Google Scholar]
- 35.Chen W, Zhang HX, Wang S, Ruan D, Xie XZ, Yu DQ, et al. Estimation of dietary selenium requirement for Chinese egg-laying ducks. Anim Prod Sci. (2015) 55:1056–63. 10.1071/AN13447 [DOI] [Google Scholar]
- 36.Attia YA, Abdalah AA, Zeweil HS, Bovera F, El-Din AAT, Araft MA. Effect of inorganic or organic selenium supplementation on productive performance, egg quality and some physiological traits of dual-purpose breeding hens. Czech J Anim Sci. (2010) 55:505–19. 10.17221/1702-CJAS [DOI] [Google Scholar]
- 37.Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Asp Med. (2005) 26:256–67. 10.1016/j.mam.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Yang HM, Cao W, Li YB. Effect of selenium supplementation on pigeon reproductive performance, selenium concentration and antioxidant status. Poult Sci. (2017) 96:3407–13. 10.3382/ps/pex121 [DOI] [PubMed] [Google Scholar]
- 39.Petrovic V, Boldizarova K, Faix S, Mellen M, Arpasova H, Leng L. Antioxidant and selenium status of laying hens fed with diets supplemented with selenite or Se-yeast. J Anim Feed Sci. (2006) 15:435–44. 10.22358/jafs/66914/2006 [DOI] [Google Scholar]
- 40.Perić L, Milošević N, Žikić D, Kanački Z, DŽinić N, Nollet L, et al. Effect of selenium sources on performance and meat characteristics of broiler chickens. J Appl Poult Res. (2009) 18:403–9. 10.3382/japr.2008-00017 [DOI] [Google Scholar]
- 41.Korish MA, Attia YA. Evaluation of heavy metal content in feed, litter, meat, meat products, liver, and table eggs of chickens. Animals. (2020) 10:727. 10.3390/ani10040727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attia YA, Al-Harthi MA, Sh. Elnaggar A. Productive, physiological and immunological responses of two broiler strains fed different dietary regimens and exposed to heat stress. Ital J Anim Sci. (2018) 17:686–97. 10.1080/1828051X.2017.1416961 [DOI] [Google Scholar]
- 43.Attia YA, Abd El AE-HE, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, et al. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. Springerplus. (2016) 5:1619 10.1186/s40064-016-3304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Harthi MA. The effect of natural and synthetic antioxidants on performance, egg quality and blood constituents of laying hens grown under high ambient temperature. Ital J Anim Sci. (2014) 13:3239 10.4081/ijas.2014.3239 [DOI] [Google Scholar]
- 45.Habibian M, Sadeghi G, Ghazi S, Moeini MM. Selenium as a feed supplement for heat-stressed poultry: a review. Biol Trace Elem Res. (2015) 165:183–93. 10.1007/s12011-015-0275-x [DOI] [PubMed] [Google Scholar]
- 46.Shakeri M, Oskoueian E, Le HH, Shakeri M. Strategies to combat heat stress in broiler chickens: unveiling the roles of selenium, vitamin E and vitamin C. Vet Sci. (2020) 7:71. 10.3390/vetsci7020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarica S, Ozdemir D. The effects of dietary oleuropein and organic selenium supplementation in heat-stressed quails on tonic immobility duration and fluctuating asymmetry. Ital J Anim Sci. (2018) 17:145–52. 10.1080/1828051X.2017.1351325 [DOI] [Google Scholar]
- 48.Habibian M, Ghazi S, Moeini MM. Effects of dietary selenium and vitamin E on growth performance, meat Yield, and selenium content and lipid oxidation of breast meat of broilers reared under heat stress. Biol Trace Elem Res. (2016) 169:142–52. 10.1007/s12011-015-0404-6 [DOI] [PubMed] [Google Scholar]
- 49.Habibian M, Ghazi S, Moeini MM, Abdolmohammadi A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int J Biometeorol. (2014) 58:741–52. 10.1007/s00484-013-0654-y [DOI] [PubMed] [Google Scholar]
- 50.Harsini SG, Habibiyan M, Moeini MM, Abdolmohammadi AR. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res. (2012) 148:322–30. 10.1007/s12011-012-9374-0 [DOI] [PubMed] [Google Scholar]
- 51.Liao X, Lu L, Li S, Liu S, Zhang L, Wang G, et al. Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biol Trace Elem Res. (2012) 150:158–65. 10.1007/s12011-012-9517-3 [DOI] [PubMed] [Google Scholar]
- 52.Khajali F, Raei A, Aghaei A, Qujeq D. Evaluation of a dietary organic selenium supplement at different dietary protein concentrations on growth performance, body composition and antioxidative status of broilers reared under heat stress. Asian Australas J Anim Sci. (2010) 23:501–7. 10.5713/ajas.2010.90448 [DOI] [Google Scholar]
- 53.Mahmoud KZ, Edens FW. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus). Comp Biochem Physiol B Biochem Mol Biol. (2003) 136:921–34. 10.1016/S1096-4959(03)00288-4 [DOI] [PubMed] [Google Scholar]
- 54.Xu D, Li W, Li B, Tian Y, Huang Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on endoplasmic reticulum stress and apoptosis in chicken spleen induced by heat stress. Rsc Adv. (2017) 7:7519–25. 10.1039/C6RA27730F [DOI] [Google Scholar]
- 55.Xu D, Li W, Huang Y, He J, Tian Y. The effect of selenium and polysaccharide of atractylodes macrocephala koidz. (PAMK) on immune response in chicken spleen under heat stress. Biol Trace Elem Res. (2014) 160:232–7. 10.1007/s12011-014-0056-y [DOI] [PubMed] [Google Scholar]
- 56.Sarica S, Ozdemir D, Ozturk H. The effects of dietary oleuropein and organic selenium supplementation on performance and heat shock protein 70 response of brain in heat-stressed quail. Ital J Anim Sci. (2015) 14:226–32. 10.4081/ijas.2015.3737 [DOI] [Google Scholar]
- 57.Khan AZ, Kumbhar S, Hamid M, Afzal S, Parveen F, Liu Y, et al. Effects of selenium-enriched probiotics on heart lesions by influencing the mRNA expressions of selenoproteins and heat shock proteins in heat stressed broiler chickens. Pak Vet J. (2016) 36:460–4. Available online at: http://www.pvj.com.pk/archive/Volume_36_Issue_4_2016.htm [Google Scholar]
- 58.Xu D, Tian Y. Selenium and polysaccharides of atractylodes macrocephala koidz play different roles in improving the immune response induced by heat stress in chickens. Biol Trace Elem Res. (2015) 168:235–41. 10.1007/s12011-015-0351-2 [DOI] [PubMed] [Google Scholar]
- 59.Khan AZ, Khan IU, Khan S, Afzal S, Hamid M, Tariq M, et al. Selenium-enriched probiotics improve hepatic protection by regulating pro-inflammatory cytokines and antioxidant capacity in broilers under heat stress conditions. J Adv Vet Anim Res. (2019) 6:355–61. 10.5455/javar.2019.f354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surai PF. Selenium in Nutrition and Health. Nottingham: Nottingham University Press; (2006). [Google Scholar]
- 61.Attia YA, Al-Harthi MA, Shiboob MM. Evaluation of quality and nutrient contents of table eggs from different sources in the retail market. Ital J Anim Sci. (2014) 13:3294 10.4081/ijas.2014.3294 [DOI] [Google Scholar]
- 62.Fisinin VI, Papazyan TT, Surai PF. Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit Rev Biotechnol. (2009) 29:18–28. 10.1080/07388550802658030 [DOI] [PubMed] [Google Scholar]
- 63.Khan SA, Khan A, Khan SA, Beg MA, Ali A, Damanhouri G. Comparative study of fatty-acid composition of table eggs from the Jeddah food market and effect of value addition in omega-3 bio-fortified eggs. Saudi J Biol Sci. (2017) 24:929–35. 10.1016/j.sjbs.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jlali M, Briens M, Rouffineau F, Mercerand F, Geraert PA, Mercier Y. Effect of 2-hydroxy-4-methylselenobutanoic acid as a dietary selenium supplement to improve the selenium concentration of table eggs. J Anim Sci. (2013) 91:1745–52. 10.2527/jas.2012-5825 [DOI] [PubMed] [Google Scholar]
- 65.Utterback PL, Parsons CM, Yoon I, Butler J. Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult Sci. (2005) 84:1900–1. 10.1093/ps/84.12.1900 [DOI] [PubMed] [Google Scholar]
- 66.Wang ZG, Pan XJ, Zhang WQ, Peng ZQ, Zhao RQ, Zhou GH. Methionine and selenium yeast supplementation of the maternal diets affects antioxidant activity of breeding eggs. Poult Sci. (2010) 89:931–7. 10.3382/ps.2009-00268 [DOI] [PubMed] [Google Scholar]
- 67.Pavlovic Z, Miletic I, Jokic Z, Sobajic S. The effect of dietary selenium source and level on hen production and egg selenium concentration. Biol Trace Elem Res. (2009) 131:263–70. 10.1007/s12011-009-8369-y [DOI] [PubMed] [Google Scholar]
- 68.Pan CL, Huang KH, Zhao YX, Qin SY, Chen F, Hu QH. Effect of selenium source and level in hen's diet on tissue selenium deposition and egg selenium concentrations. J Agric Food Chem. (2007) 55:1027–32. 10.1021/jf062010a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.


