Abstract
Introduction:
Regulatory T cells (Treg) and their role in health and disease is being intensively investigated. Today, human Treg emerge as a highly heterogeneous subset of CD4+ T cells which mediate immune suppression but also regulate responses of non-immune cells. In cancer, Treg occupy a critical although not yet entirely understood role.
Areas covered:
Newly acquired insights into Treg indicate a much greater plasticity and functional heterogeneity of this T cell subset than was previously known. Functional redundancy of Treg and their interactions with a variety of immune and non-immune cellular targets emphasize the central role Treg play in cancer. Treg not only regulate the host responses to cancer; they may also regulate responses to immune therapies. The impact of immune checkpoint blockade on Treg survival, stability and suppressive activity remains to be elucidated. T cell reprogramming by tumor-derived factors, including tumor-derived exosomes (TEX), plays a key role in shaping the Treg repertoire in the tumor microenvironment (TME). The reprogrammed or induced iTreg acquire capabilities to strongly down-regulate anti-tumor immune responses by mechanisms that are specific for each TME. Therapeutic silencing of such Treg calls for the discrimination of “bad” from “good” Treg subsets, an approach that remains elusive in the absence of a definitive “Treg signature.”
Expert opinion:
Context-related plasticity and heterogeneity of Treg in the TME are significant barriers to selective therapeutic depletion of those Treg subsets that are reprogramed by the tumor to suppress anti-tumor immunity.
Keywords: regulatory T cells, Treg, Cancer, Tumor Microenvironment, Immune suppression
1.0. Introduction
T regulatory cells (Treg) were discovered more than 20 years ago and have been a subject of intense interest and investigation ever since [1]. Yet, many question about Treg origin, development, functions and their clinical significance in cancer remain unanswered. Much of the data available for Treg comes from experiments performed in mice. Although we have made substantial progress in enlarging the knowledge of Treg-mediated suppression in man, and Treg have even become a target of anti-cancer therapies in the clinic, large gaps exist in our understanding of the mechanisms regulating Treg activities. How precisely Treg responds to the immune check point blockade remains an open issue. Much conflicting information has recently accumulated in this regard, adding confusion to already existing contradictory data about clinical significance of Treg accumulations in various tumor tissues and the impact of the accumulating Treg on cancer progression and metastasis [2, 3, 4, 5, 6]. Although in autoimmune diseases Treg functional deficiencies clearly underlie the disease pathology, and although it is accepted that Treg functions are essential for health, their role in cancer remains a topic of controversy. It is not clear whether Treg are good or bad in cancer or whether they should be therapeutically targeted rather than left undisturbed. To answer these and other questions concerning the role human Treg play in cancer I have undertaken the task of reviewing the recently generated and published information. As much has been recently accomplished, and as the available data are extensive, it will be necessary to select the topics for discussion. The objective is to highlight the available information that best illustrates the heterogeneity of Treg and the regulatory mechanisms that drive Treg functions in the tumor microenvironment (TME), especially when immunotherapies are used. The overreaching goal is to demonstrate how these mechanisms relate to cancer therapy, progression and outcome.
1.1. Treg in the tumor microenvironment
Treg accumulate at tumor sites and in the peripheral circulation of patients with cancer [7, 8, 9, 10]. They are variably increased in the frequency among tumor-infiltrating lymphocytes (TILs), and in the patients’ peripheral circulation, they represent a significantly increased proportion of lymphocytes (e.g., up to 10% or more) relative to that seen in healthy blood donors (HD). Treg found at tumor sites differ phenotypically and functionally from conventional T cells (Tconv). They are similar to in vitro induced Treg defined as iTreg. [11] There are no specific markers that identify human iTreg. All we can say is that upon isolation from the tumor, they mediate vigorous immune suppression of various immune cells and co-express numerous immune checkpoint inhibitory receptors (ICIRs), e.g., cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed cell death 1 (PD-1), T-cell Ig and mucin-domain containing-3 (TIM3), lymphocyte activation gene 3 (LAG3) and T-cell Ig and ITIM domain (TIGIT) (Figure 1). They are CD4+, variably CD25hi or CD25low and may or may not express FOXP3, a linage-specific transcription factor of Treg and a master regulator of their function [12, 13, 14, 15]. Treg that are FOXP3+ are also specifically hypomethylated at the DNA loci that code for genes regulating Treg immune suppressor proteins/functions. This region is referred to as Treg specific demethylation region (TSDR), and it appears to be a stable epigenetic modification that is highly specific for functional Treg. Tconv do not have this specific hypomethylation, and thus it could be considered as a bona fide biomarker of Treg, and it has been widely used to confirm the Treg status of CD4+ T cells [16]. However, it has been reported that human iTreg isolated from solid tumors may be FOXP3(−), which detracts from the TSDR status as a marker of all Treg.
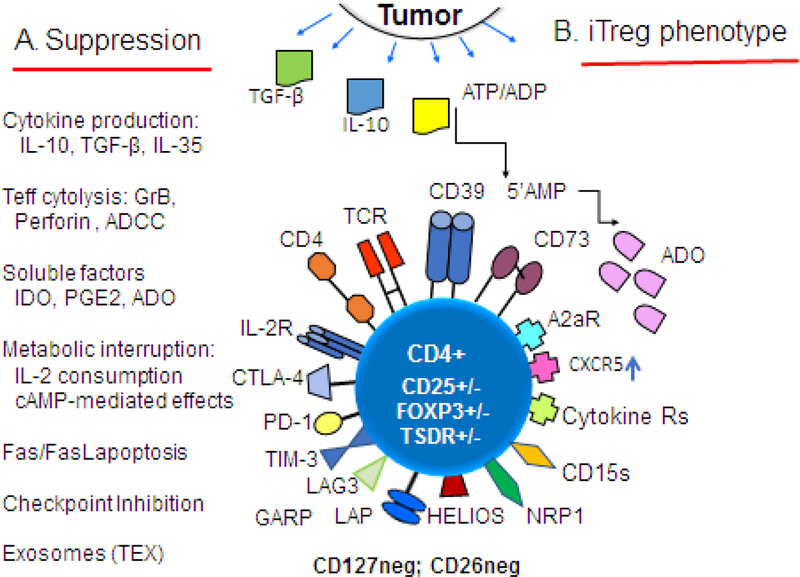
Figure 1.
Functional and phenotypic characteristics of iTreg in the tumor microenvironment (TME). Tumor-derived TGF-β, IL10 and exosomes promote differentiation of T cells infiltrating the tumor into Treg. In A, various molecular pathways and mechanisms responsible for suppression mediated by iTreg are listed. Tumors produce cytokines, soluble factors or exosomes which reprogram functions of Treg that accumulate in the TME, converting them into highly effective suppressor cells that serve to promote tumor escape and its progression. In B, iTreg that accumulate in the TME are activated and up-regulate expression levels of a wide variety of the surface receptors, including checkpoint inhibitory receptors and proteins mediating immunosuppressive functions such as TGF-β-associated LAP and GARP, CD39.CD73 ectonucleotidases. iTreg may or may not be FOXP3+, TSDR+ and CD25hi. They are negative for CD127 (IL7R-α) and CD26 (adenosine deaminase). Abbreviations: ATP, adenosine triphosphate; ADP, adenosine diphosphate; 5’AMP, adenosine-5’monophosphate; ADO adenosine; TCR, T cell receptor; CTLA-4, cytotoxic lymphocyte antigen-4; PD-1, programmed death-1; TIM-3, T-cell immunoglobulin mucin-3; LAG-3, latency-associated protein; GARP, glycoprotein A repetition predominant; NRP-1, neuropilin-1; CD15s, sialyl Lewis X.
Much has been written about clinical significance of Treg accumulating in human tumors. The availability of “immune signatures” of tumors, currently almost routinely obtained in pathology, provide data that discriminate tumors richly-infiltrated with immune cells from “non-inflamed” tumors containing scarce immune cell infiltrates [17]. These data indicate that Treg are prominently present among TILs in tumors that contain large infiltrations of immune cells but are rarely seen in the TME of tumors that are poorly infiltrated [18]. By determining the ratios of Treg to T effector cells (Teff) in immune infiltrates of various human tumors, it has been possible to use the Treg/Teff ratios to estimate prognosis of patients with cancer [19, 20]. The approach is based on the presumption that an excess of Treg, which mediate immune suppression in the TME and thus promote tumor progression, would associate with unfavorable prognosis and poor patient survival. However, the Treg/Teff ratio does not inform whether the Treg are pro-cancer or anti-cancer; Treg with either attribute may influence prognosis. Thus, the rationale for the prognostic use of the ratio is weak. When the assembled data from numerous studies were examined, the excess of Treg (i.e., the high Treg/Teff ratio) correlated with poor outcome only in some tumors (e.g., ovarian and pancreatic carcinomas, lung cancer, melanoma and glioblastoma), while in other tumor types (e.g., colorectal and breast carcinomas, bladder cancer or head and neck cancer) the high Treg/Teff ratios correlated with favorable prognosis and improved overall survival (OS) [21, 22, 23, 24, 25, 26, 27, 28]. This association of the Treg excess with improved OS is seen mainly in chronically inflamed tumors, such as colorectal carcinomas, and has been explained by the ability of Treg to suppress and control “tumor promoting inflammation” (TPI). The discrepancy in prognostic significance of the Treg/Teff ratio in various tumors might reflect functional hetrogenity of Treg that is not yet identified by phenotype. Concerns also arise about how rigorously Treg were being identified in these studies performed independently at various centers. These concerns are legitimate given the fact that no specific marker or markers are available for Treg and that the commonly used phenotype of CD4+CD25+ FOXP3+ cells to identify Treg is likely to include CD4+ Teff that are transiently FOXP3+ and omit iTreg cells that may be FOXP3low [29, 30]. Clearly, without a marker that can specifically identify Treg, the results of correlative prognostic studies that have appeared in the current literature remain open to criticism.
1.2. Origin and nomenclature of human Treg
In considering Treg identity, their origin from the thymus is clear: Treg are a subset of CD4+ T cells and are generated in the thymus. Extensive studies performed in mice indicate that the Treg development begins with a T cell receptor (TCR) signal delivered by an antigen presenting cell (APC) to CD4+CD8(−) thymocytes (Figure 2). A fraction of these thymocytes activated by the high-affinity binding of self-antigen/MHC complexes to the TCR is selected to acquire CD25 and signaling via STAT5, and is responsive to IL-2 [31]. Subsequently, these thymocytes express FOXP3 and after moving to the periphery, differentiate into natural thymus-derived Treg (tTreg) capable of mediating immune suppression. Thymocytes with moderate-affinity TCR for self peptides differentiate into CD4+Teff, while those with low affinity TCR for self- peptides are destined to die in the thymus [32]. In addition to tTreg, another category of Treg emerges in the circulation and is referred to as peripheral Treg (pTreg). These may be derived by conversion of conventional CD4+ T cells (Teff) potentially mediated by a broad variety of stimulatory signals [33, 34]. It is a subset of these Teff that presumably gives rise to induced Treg (iTreg) accumulating in the circulation and in the tumor of patients with cancer. The process of conversion or tumor-driven programming is not well defined, and it may be driven by cytokines, growth factors, Ag/Ab complexes, exosomes or microbiota [35]. It does not lead to expansion of antigen-specific iTreg, as illustrated in vitro by the observation that iTreg stimulated to proliferate by an antigen develop into a population of suppressor cells with unrestricted specificity [36, 37]. The fate of CD4+ T cells converted to iTreg is also uncertain, as their migration to the tumor and their stability in the TME are poorly defined and remain under intense investigation [38].
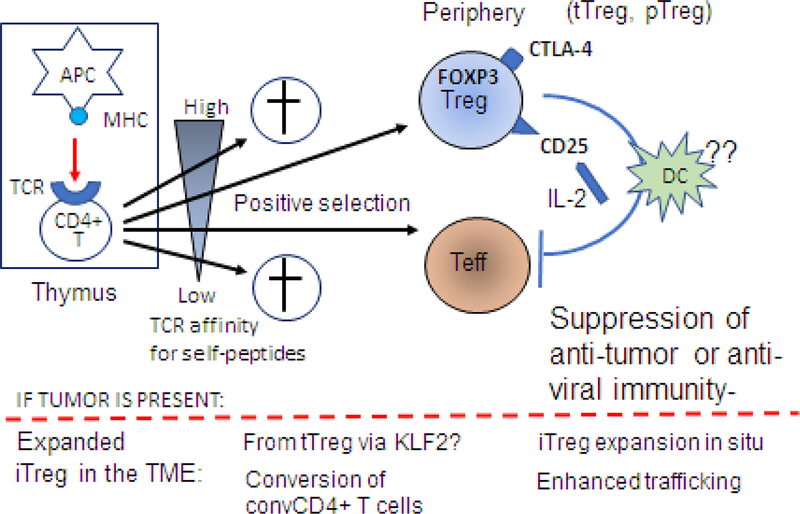
Figure 2.
Treg origin and development: Natural Treg are generated in the thymus from naïve CD4+CD8- thymocytes which interact with self-reactive thymic antigen-presenting cells (APCs) expressing self-peptide/MHC complexes. Stimulation of CD4+CD8- thymocytes by a T cell receptor (TCR) signal and continuous activation by IL-2 binding to CD25 (IL-2 receptor) leads to FOXP3 expression and differentiation into Treg. Note that thymocytes with the low affinity for self-peptides as well as those with very high affinity for self-peptides are eliminated. Only thymocytes with the high/intermediate affinity for self-peptides are positively selected for development and give rise to CD4+ effector T cells (Teff) or to CD4+FOXP3+ CD25+ tTreg and pTreg. In the periphery, Treg upregulate expression levels of CD25 and CTLA4. Upon interaction with an APC in the presence of IL-2, peripheral Treg become fully suppressive Treg that inhibit anti-tumor functions of Teff. It remains unclear how exactly this conversion happens, but it appears that iIn the presence of tumor-derived factors, pTreg and/or tTreg become inducible (i)Treg. The conversion of tTreg might require the KLF2 transcription factor. It is also possible that convTCD4+ T cells can undergo reprogramming to iTreg. This conversion is accompanied by an expansion of iTreg and their migration to the tumor site.
In the TME, iTreg express a unique phenotype (Figure 2) that differs from those of CD4+Teff and natural tTreg [8, 14, 39]. They may or may not be FOXP3+ and their TSDR status is not definitive [40, 41]. Type I regulatory cells (Tr1) identified in mice regulate inflammation and are present in the TME [42, 43]. Tr1 cells are CD4+ CD49b+ LAG3+ secrete IL-10 and are FOXP3(-) [42, 43, 44]. They are rich in highly up-regulated check point inhibitory receptors (ICIRs), adenosine receptors (ADORs), transforming growth factor receptors (TGFRs) and other biologically-active receptors and ligands (Figure 1). Among the various markers, a combination of CTLA-4, CD39, GARP, and TIM3 worked best in discriminating iTreg from Teff according to one study [14]. Others suggest that expression levels of HELIOS, neuropilin 1 (NRP1) or sialyl Lewis X (CD15s) are better discriminators of Treg [45, 46, 47, 48]. In the absence of biomarker specific for Treg, the origin, differentiation and nomenclature of this T cell subset remains speculative. It is clear, however, that iTreg present in the TME are highly immunosuppressive and use various suppressive mechanisms to prevent Teff from exercising anti-tumor functions. The mechanisms used by iTreg to mediate immune suppression have been extensively examined [45, 46, 47, 49, 50, 51, 52, 53, 54, 55, 56], are listed in Figure 1 and are described in detail in ref [5]. These are numerous and varied, begging the question of why such a large armamentarium of suppression mechanisms is necessary and how it is used in different tumor types. The rational explanation may be that this “mechanistic redundancy” is necessary to enable tumor escape from the host immune system, and it provides the tumor with the capability to select the most efficient mechanism of escape.
Recently, a classification of CD4+FOXP3+ Treg based on co-expression levels of FOXP3, CD25 and CD45RA, the latter a marker of naïve T cells [57], was introduced by Sakaguchi and colleagues [58]. This classification divides CD4+FOXP3+CD25+ T cells into three fractions with different functional attributes: (a) Fraction I consisting of CD4+CD45RA+ FOXP3lowCD25low naive T cells with weak suppressor activity; (b) Fraction II consisting of CD4+CD45RA-FOXP3hi CD25hi effector Treg (eTreg) with strong suppressor functions; and (c) Fraction III containing CD4+CD45RA-FOXP3lowCD25low T cells with no suppressor activity considered to be non-Treg [58]. Naive Treg in Fraction I are T cells that have recently emerged from the thymus, have not yet been activated in the periphery and have weak suppressive activity. Upon TCR stimulation these cells proliferate, developing into highly suppressive eTreg producing IL10 and TGF-β (Fraction II). Non-Treg in Fraction III mediate immune stimulation rather than immune suppression and produce inflammatory cytokines, including IL-2, IFN-γ and IL-17 [58, 59]. This functional and phenotypic classification of human Treg emphasizes their enormous heterogeneity as well as plasticity, both features likely determined by contextual interactions in the TME. This classification of Treg is not universally accepted, and at least one report states that the Fraction III cells (presumably non-Treg) are ~50% FOXP3+ Helios+ with demethylated TCRs [60].
1.3. Intra-tumoral eTreg (II) may be derived from peripheral blood Treg?
Sakaguchi’s schema for the classification of human Treg indicates that the highly suppressive eTreg (Fraction II) represent from 2–5% of peripheral blood Treg, while in tumor tissues they account for a large proportion (20–50%) of intra-tumoral Treg [59]. The origin of these intra-tumoral Treg and their relationship with the Treg in peripheral blood are unclear. A recent study, compared the eTreg (Fr II) in the peripheral blood of patients with breast cancer with their counterparts isolated from paired tumor specimens [61]. Using an extensive panel of phenotypic and functional ex vivo assays, the authors showed that intratumoral Treg originate primarily from peripheral blood Treg II. This conclusion was strongly supported by the following data: (a) although there were more Treg II in the tumor than in the blood, suppressor functions were comparable on per cell basis; (b) phenotypically, the Treg II in tumor and blood were similar, with comparable expression levels of activation markers as well inhibitory and co-stimulatory receptors; (c) the TCR repertoire of Treg II in blood and tumor was similar (i.e. ,there was a clonal overlap); (d) the high cytokine signaling index (CSI) for Treg II, comprising ex vivo responses to immunosuppressive TGFβ and IL-10 vs responses to IL-4 or IFN-γ, and measured at breast cancer diagnosis correlated with worse clinical outcome in two independent patient cohorts; (e) the chemokine receptor profile of Treg II in blood and tumor was comparable, with elevated expression levels of CCR2, CCR4, CXCR6, suggesting that recruitment of Treg II into human breast tumors is mediated via chemokine signaling, as also previously reported by others [62, 63, 64]. In aggregate, these new data strongly support the recruitment of Treg II to tumors from the Treg II fraction present in the peripheral blood, although they do not rule out the possibility that intra-tumoral Treg II could migrate to the periphery. Since the CSI reflected the intra-tumoral immune suppression and predicted clinical outcome, it could potentially be developed as a blood-based measurement to evaluate the immune status of cancer patients. Further, if Treg II in the blood are precursors of highly suppressive intra-tumoral Treg II, they might be promising and readily accessible therapeutic targets in the future.
1.4. Treg signaling in the TME
iTreg accumulating in the TME are under a persistent pressure exerted by the tumor which is attempting to escape from the host immune cells [5, 65]. Thus, signals received by these Treg are dominated by tumor-derived immunosuppressive proteins or extracellular vesicles (EVs) carrying inhibitory cargos [66]. The result of this type of signaling should be immune suppression. For example, PD-L1 presented by the tumor, APC or EVs and binding to PD-1 on the surface of iTreg initiates a signal that should be immunosuppressive [67]. However, in iTreg, PD-L1 signaling leads to activation and expansion of Treg [68]. Although iTreg in the TME are induced to up-regulate multiple ICIRs, including PD-1, CTLA-4, TIM-3, LAG3 and TIGIT [12, 49], signaling via these receptors does not result in suppression of iTreg but rather in their invigoration (Figure 3A). In contrast to all other immune cells, signaling via the immune checkpoint inhibitory receptors (ICIRs) expressed by iTreg promotes their activities and survival [69]. The molecular pathways underlying this response are not entirely clear, but it has been suggested that since iTreg have increased levels of intracellular PTEN [70], signals received by ICIRs may be transmitted via PTEN. Exogenously-activated PTEN silences the Akt and mTOR pathways, directing the signal to STAT5, inducing STAT5 phosphorylation and thus promoting Treg expansion, their inhibitory functions and their stability [71]. PD-1 inhibits excessive T cell activation by suppressing TCR- and CD28-mediated signals [72, 73]. Blockade of PD-1 by ICIs removes “the breaks” allowing for T cell activation. In Treg, which are dependent on TCR and CD28 signals for survival and functions, blocking of PD-1 seems to activate immunosuppressive functions [74] (Figure 3B). In fact, PD-1 blockade was reported to promote Treg differentiation and apoptosis [69]. Further, it has been also reported that dying Treg exert enhanced immunosuppressive activity [75, 76]. Since anti-PD-1 antibodies induce death of at least some Treg through antibody-mediated cellular cytotoxicity (ADCC) [77], the overall effects of the PD-1 blockade may be the enhancement of Treg suppressive functions. These data suggest that the blockade of ICIRs on T cells has unexpected effects on Teff and Treg subsets. While immune competence of Teff is restored, Treg suppressor functions are not reduced, as might be expected, but are enhanced by the blockade.
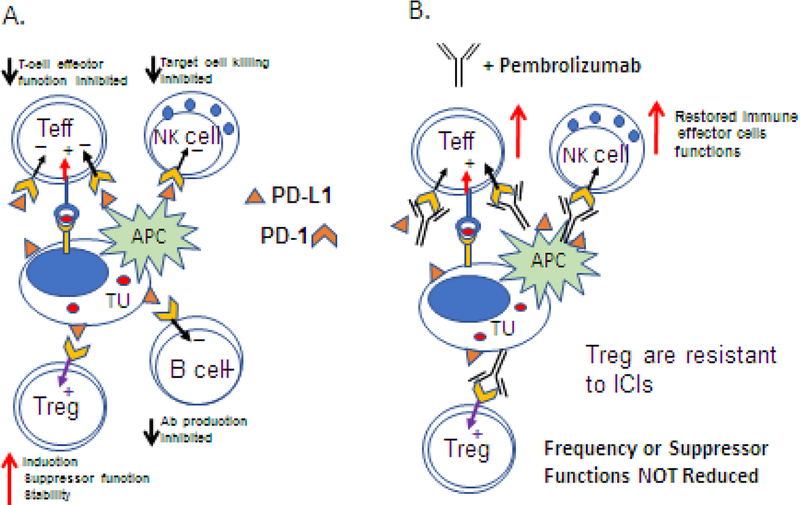
Figure 3.
PD-1-/PD-L1 signaling in the TME in the absence (A) or in the presence (B) of pembrolizumab (an antibody blocking PD-1/PD-L1 interactions). In A, tumor-derived or APC-derived PD-L1 binds to PD-1 receptors on immune cells and inhibits anti-tumor functions of T, B and NK cells, but not of Treg. PD-L1 signals induce up-regulation of suppressor functions and increase Treg stability. In B, pembrolizumab restores functions of T, B and NK cells. However, neither the frequency not suppressor functions of Treg are not reduced. In the presence of pembrolizumab, Treg at the tumor site are resistant to its rejuvenating functions and retain their suppressor functions.
We and others have reported that human iTreg express ectonucleotidases, CD39 and CD73, hydrolyze ATP to 5’AMP then to ADO and suppress functions of Teff via the binding of ADO to A2AR on the cell surface [78, 79, 80, 81]. We also reported that human Treg express low levels of adenosine deaminase (CD26), an enzyme responsible for the degradation of ADO to inosine [82]. In contrast to Teff, which overexpress CD26 and rapidly convert ADO to inosine, Treg produce and secrete ADO, which accumulates in the pericellular space and is re-utilized via its binding to A2ARs expressed on the cell surface of Treg [82]. Interestingly, this autocrine utilization of ADO by Treg appears to promote their suppressor functions and stability. In Teff, which also express A2ARs, ADO signaling leads to cAMP-mediated down-regulation or a loss of effector functions [78]. This is another example of differences in processing of extracellular signals between Treg and Teff in the TME. This differential signaling via the A2aRs, ICIRs or other surface receptors in Treg relative to Teff emerges as an important characteristic of Treg that might allow them to survive in the hostile highly immunosuppressive TME and retain functionality.
1.5. Treg and exosome-mediated reprogramming
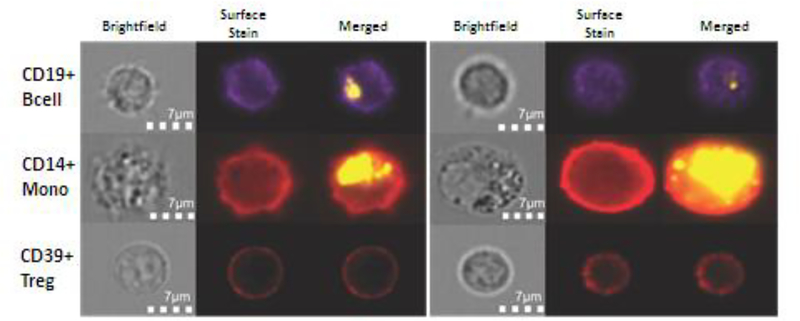
Tumor-derived exosomes, TEX, represent an intercellular communication system in the TME [83]. Tumors secrete masses of TEX which carry immunosuppressive receptors and ligands, as illustrated in Figure 4, and deliver them to recipient cells in the TME [84]. Infiltrating immune cells are targets for TEX and are reprogrammed upon co-incubation with TEX [85]. In addition to a profile of immunoregulatory proteins on the surface membrane, exosomes carry nucleic acids: mRNA, miRNA, DNA as well as enzymes, cytokines and other soluble factors in the vesicle lumen and can alter the Treg transcriptome [85, 86]. Ex vivo co-incubation studies of primary human T cells and PKH26-labeled TEX (Figure 5) showed that tumor-derived exosomes are readily taken up by monocytes, B cells, natural killer (NK) cells but not by T cells. Only Treg were internalizing labeled exosomes, albeit not as efficiently as other immune cells [87]. Despite the apparent lack of exosome up-take, functions of T cells were profoundly altered upon co-incubation. In early experiments with TEX derived from tumor cell lines, we showed that TEX inhibited STAT5 signaling in primary CD8+ T cells but up-regulated STAT5 phosphorylation in CD4+ T cells [88]. These signals translated into enhanced CD4+ T cell proliferation and suppressor functions, largely mediated via apoptosis of CD8+ T lymphocytes [89]. We and others have also demonstrated that co-incubation of CD4+ T cells with TEX induced differentiation and expansion of Treg and promoted suppression activity mediated by these Treg [90, 91]. Additional ex vivo studies with CD4+CD39+ Treg subsets isolated from PBMC of healthy donors and co-incubated with TEX indicated that TEX delivered a series of suppressive signals to Treg and rapidly up- or down-regulated expression levels of multiple immunoregulatory genes [54, 85]. Functions of Treg were re-programmed as a result of this interaction [85]. In parallel experiments, the same TEX co-incubated with CD4+ T cells or APCs also induced changes in the transcript levels of recipient cells that were qualitatively and quantitatively different from those seen in CD8+ T cells [85]. Our results suggested that TEX reprogram CD4+ T cells differently than CD8+ recipient cells. Multiple signals carried and delivered by TEX to recipient cells simultaneously alter signaling on the cell surface as well as inside the recipient cells [92]. While the cell surface signals might involve a receptor/ligand-type interactions [93], the up-take and transfer of miRNAs may be largely responsible for transcriptional alterations in recipient cells [94]. Furthermore, not only the molecular profile of the TEX cargo but also the nature and activation state of recipient cells may determine the extent and degree of reprogramming induced by TEX [66]. Since Treg do not easily or readily internalize TEX, which in the TME carry a predominantly immunosuppressive cargo, it is likely that receptor-ligand signaling on the surface of Treg drives reprogramming by simultaneous activation of not one but many suppressive molecular pathways, such as the ADO, TGFβ, TNFR2 or Fas/FasL pathways [86]. Immunosuppressive activities of TEX are evident not only in the tumor but extend to the periphery, as virus-size exosomes are ubiquitous in all body fluids [84]. While TEX have been extensively studied in vitro and ex vivo, studies in mice injected with TEX confirm potent immunoregulatory roles of TEX in the tumor development or metastasis [95, 96, 97]
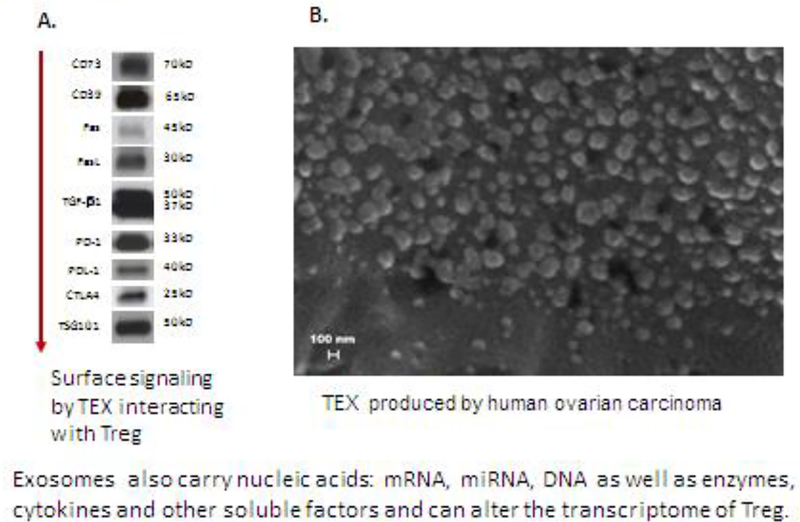
Figure 4.
Tumor-derived exosomes in the TME or in the plasma of cancer patients carry an excess of immunoinhibitory proteins and immune checkpoint inhibitors. In A, Western blots of exosomes isolated by size exclusion chromatography from plasma of a cancer patient. TSG101 is an endocytic marker confirming the exosome origin from the endocytic compartment of the parent tumor cell. In B, a scanning electron microscope image of exosomes isolated from plasma of a patient with ovarian carcinoma (courtesy of Dr. Marta Szajnik; reproduced under the terms of the Creative Commons Attribution License from ref. 143).
Figure 5.
Uptake and internalization of PKH26-labeled tumor-derived exosomes (TEX) by human monocytes or lymphocytes. Representative data from 2/5 independent experiments (two different fields shown) with isolated immune cell subsets obtained from normal human PBMC and co-incubated with labeled TEX for 24–48h prior to analysis by an Amnis Image Stream analyzer. (Reproduced are under the terms of the Creative Commons Attribution License with permission from ref. 85.)
1.6. Immune therapies and Treg
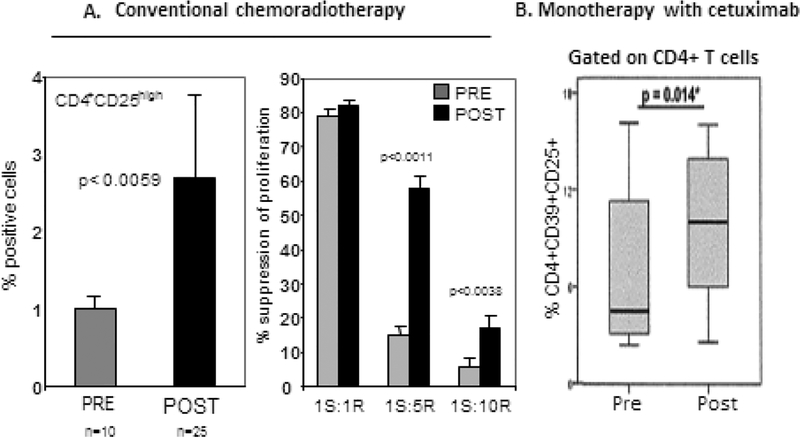
Based on the data suggesting that Treg inhibit anti-tumor functions of effector immune cells, the notion has emerged and persists that Treg are “bad” and have to be depleted if anti-cancer therapies are to be successful. This notion was further encouraged by observations that conventional anti-cancer therapies, such as, e.g., cisplatin in HNSCC [98] or immune monotherapies such as cetuximab, an FDA approved Ab for therapy of advanced/relapsed HNSC, increase the frequency and suppressor functions of Treg, especially in patients who did not respond to immunotherapy [99] (Figure 6). These data reinforced the perception that Treg represent a barrier that should be removed for anti-tumor therapies to be effective. Various therapeutic approaches targeting Treg have been introduced and progressed to clinical trials in the last decade (see Table 1). Interestingly, despite mechanistically diverse approaches, immunologic, pharmacologic or chemo-radiation based, Treg-depleting regiments have not been successful [5, 56]. While they significantly reduced Treg numbers, they never succeeded in sustained depletion of Treg, which invariably rebound, often to exceed their former frequency and suppression levels (reviewed in [7, 100]. In retrospect, this result is not unexpected: Treg are necessary for health, and their elimination or imbalance threatens to disturb the basic survival mechanism.
Figure 6.
Increased frequency and suppressor functions in PBMC of patients with head and neck squamous cell carcinoma (HNSCC) treated with oncological therapies. In A, the left panel illustrates the frequency of CD4+CD25+ T cells prior to and post conventional chemoradiotherapy, and in the right panel, suppressor functions of these cells measured in proliferation assays (reproduced with minor alterations with permission from ref. 144). In B, the percentage of CD4+CD39+CD25+ Treg in the peripheral blood of HNSCC patients prior to and post therapy with cetuximab (reproduced from permission from ref. 99.)
Table 1.
Therapeutic depletion of human Treg a
| Therapies Used | References |
|---|---|
| Blocking of IL-2R | [133, 134] |
| Anti-CD25 Ab (daclizumab) | |
| Anti-CD25Ab + radiation | |
| Denileukin diftitox (ONTAK): Killing CD25 + Treg | [135] |
| Agonistic Abs | |
| Anti-OX40 | [136] |
| Anti-GITR | [137] |
| Immune checkpoint blockade | [59, 130] |
| Anti-CCR4 Abs targeting eTreg | [138] |
| Low-dose metronomic cyclophosphamide | |
| Alone | [139] |
| With other immune therapies | [140] |
| Metabolic regulation of Treg | [141] |
| Targeting “fragility” of Treg | [142] |
| Interrupting Treg stabilizing pathways | [143] |
Selected therapies are presented
1.7. Modulation of Treg by immune checkpoint inhibition
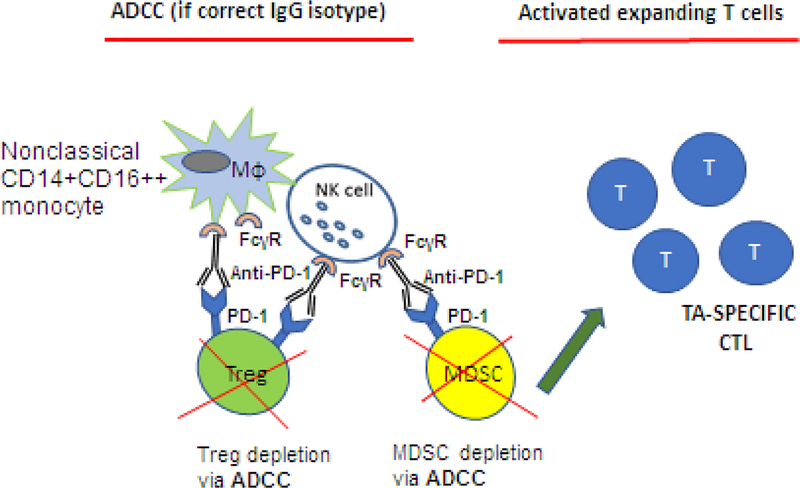
With the advent of checkpoint inhibitory Ab therapies, e.g., ipilimumab, the expectation was that suppressor activity of Treg, constitutively expressing CTLA-4 and overexpressing other ICIRs, would be silenced upon Ab-mediated disruption of negative signaling. While CTLA blockade by ipilimumab released Teff cells from suppression and rejuvenated their anti-tumor activities, Treg-mediated suppression, phenotype, frequency and stability were not altered [101, 102]. Again, responses of Treg to the immune checkpoint blockade differed from those of Teff (Figure 3B). Since ipilimumab is fully human IgG1, it can engage FcγRIIIA (CD16) expressed on monocytes or NK cells and mediate antibody dependent cellular cytotoxicity (ADCC) targeting CTLA-4+ Treg [77]. Indeed, in the FcR-deficient mice, anti-tumor activity of anti-CTLA4-specific mAb was shown to be dependent on ADCC of Treg [103]. Romano et al reported that ipilimumab-dependent cell mediated cytotoxicity of melanoma Treg was mediated ex vivo by non-classical monocytes and led to lysis of Treg [104]. They also reported that melanoma patients who responded to ipilimumab therapy had a high frequency of non-classical monocytes in PBMC at baseline [104]. These data suggested that anti-CTLA-4 therapy may target and deplete Treg in the circulation of melanoma patients (Figure 7). On the other hand, a study from my laboratory indicated that only a proportion (~30%) of CTLA-4+Treg isolated from PBMC of patients with melanoma co-incubated with ipilimumab were sensitive to lysis by NK cells, as measured in flow cytometry-based ADCC assays. The remaining Treg were resistant, survived and proliferated in culture (TW, unreported data). Several clinical studies have reported strong correlations between clinical efficacy of ipilimumab and reduction of Treg numbers in tumor tissues [105, 106]. It is possible that ipilimumab targets the Treg in tumor tissues that express high levels of CTLA4, leaving Treg with low CTLA4 expression undisturbed, As discussed above, dying Treg were reported to exert vigorous suppressor functions [75, 76], perhaps due to the release of various suppressive factors [7], and this suppression may interfere with ipilimumab-induced activation of Teff anti-tumor functions. Another anti-CTLA4 mAb, tremelimumab, was reported to effectively activate Teff without affecting Treg [107]. Studies of the effects of ipilimumab therapy on Treg have not provided definitive answers and are continuing.
Figure 7.
ADCC as a potential mechanism responsible for elimination of Treg in the presence of anti-CTLA-4 mAbs. Ipilimumab (anti-CTLA-4 Ab) which has an IgG1 isotype, could mediate ADCC and eliminate Treg and myeloid-derived suppressor cells (MDSC) in addition to blocking the immune checkpoint signaling. As a consequence of ADCC, tumor-associated antigens (TAAs) are released, processed by APCs and presented to T cells. TAA-activated cytolytic T cells expand and mediate anti-tumor activities.
Effects of anti-PD-1 mAbs, e.g., pembrolizumab, on Treg, which express comparable levels of PD-1 as Teff remains controversial. As illustrated in Figure 3B, there is evidence suggesting that PD-1 signaling in Treg promotes suppressor function and increases Treg stability and survival [108]. Thus, Treg and Teff respond differentially to PD-1/PD-L1 signaling. When PD-L1 on the tumor or APC interacts with PD-1 on the surface of immune cells, negative signaling disrupts functions of all immune cells expressing PD-1 except for Treg functions. When a checkpoint inhibitory Ab such as, e.g., pembrolizumab, is introduced, negative signaling driven by PD-1/PD-L1 is blocked, and Teff regain their anti-tumor effector activity: they are re-juvenated. In contrast, suppressive functions of Treg are not blocked or decreased; Treg remain functional and their frequency (based on the phenotype) is not altered in vitro [101] or in vivo [109]. These data suggest that Treg may be resistant to the ICI blockade with anti-PD1 mAbs. It has been suggested that this resistance of Treg to ICIs could be in part responsible for the unresponsiveness of some cancer patients to Abs blocking immune checkpoints.
It has been suggested that Treg are especially effective inhibitors of self-antigen driven responses, such as exist in autoimmune diseases [110]. In contrast, Treg in the TME are driven by antigen-MHC complexes presented by the tumor or APC. Inefficient responses of Treg to ICIs could be explained by preferential self-antigen reactivity of Treg [111]. Thus, Treg may efficiently suppress responses of Teff to shared tumor-associated antigens (TAAs), which are considered to be mostly self, but may be less involved or less interested in suppression of strong neo-antigen-driven responses that characterizes the checkpoint blockade restores and promotes [112]. This hypothetical explanation introduces a possibility that Treg selectively regulate responses to certain antigenic stimuli and again emphasizes functional heterogeneity of this regulatory T cell subset.
1.8. The role of multiple ICIRs on Treg
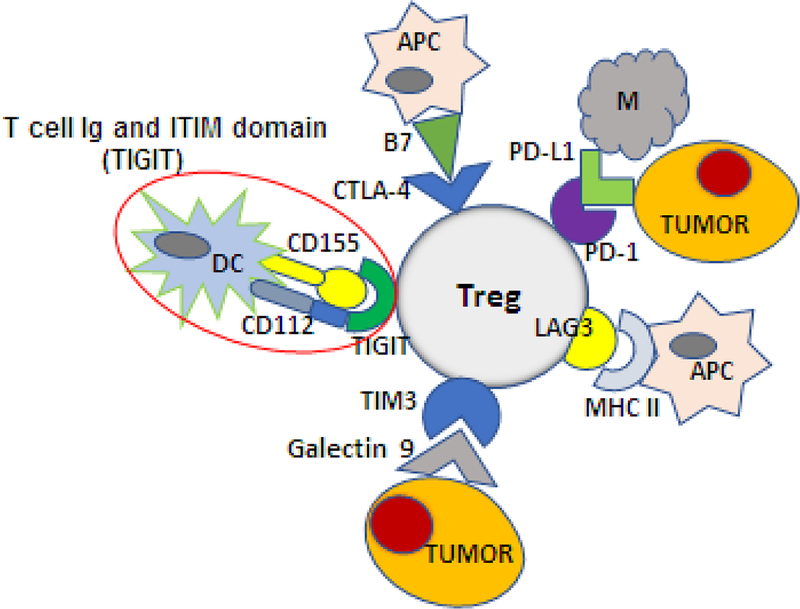
The question of why Treg express and up-regulate multiple ICIRs is being slowly unraveled. Treg found in the TME emerge as highly interactive cells: the multiple ICIRs enable these Treg to interact with various immune and non-immune cell types present in the TME, as previously suggested (Figure 8). Treg in the TME are modulated by a variety of signals, and expression pf PD-1, CTLA4,Tim-3, LAG3 and TIGIT, to name a few, could regulate Treg responses to environmental activating signals, maintaining the balance between excessive stimulation and inhibition [113]. An illustration of how such interactions occur is provided by the co-expression of CTLA-4 and CD28 on Treg. The two receptors compete for the ligands, CD80 and CD86, expressed on APC with a result that high-affinity binding of these ligands to CTLA-4 delivers a strong negative signal that blocks co-stimulation, while lower-affinity binding of the ligands to CD28 leads to co-stimulation [114, 115]. More recently, Fourcade and colleagues explained how TIGIT, an ICIR expressed on Treg in human melanoma regulates functions of Treg [116]. Signaling via TIGIT is strongly inhibitory (e.g., promotes IL-10 secretion by Treg). In Treg, TIGIT is opposed by the co-receptor CD226 (DNAM1), which is stimulatory and promotes IFN-γ secretion [107]. The ligands are CD155 (PVR) and CD112 (nectin-2) expressed on APCs as well as tumor and other cells. Binding of CD155 to TIGIT on Treg is inhibitory, while its binding to CD226 is stimulatory. In melanoma, TIGIT is up-regulated in PBMCs and TILs, and the high ratio of TIGIT+/CD226+ Treg favors negative signaling. In fact, TIGIT+ Treg mediate higher suppression than TIGIT(−) Treg and are more stable in the TME. Importantly, a high TIGIT/CD226 ratio in melanoma Treg correlated with poor clinical outcome to therapy with ipilimumab or pembrolizumab [116]. These data illustrate the complexity of signals processed by Treg in the TME. Better understanding of these signaling events is clinically important, because it may identify new therapeutic targets, such as e.g., TIGIT, whose suppression on Treg is expected to provide an opportunity for the stimulatory CD226 to become engaged in restoration of immune anti-tumor responses. Moreover, the above examples of co-receptor-mediated signaling in Treg indicates that these cells are critically involved in regulation of anti-tumor immunity and of responses to Immune therapies in patients with cancer.
Figure 8.
Activated Treg in the TME engage a variety of immune or non-immune cells via highly up-regulated immune checkpoint inhibitory receptors (ICIRs), such as CTLA-4, PD-1, LAG-3, TIM-3 and TIGIT. The ligands for these ICIRs are present on various tissue and immune cells. Using the ICIRs that bind cognate ligands expressed by surrounding cells, Treg can readily interact with these cells and regulate their responses. For example, TIGIT, an ICIR expressed on the Treg surface, can bind two different ligands present on APCs, CD155 (a parvovirus receptor, PVR) and CD112 (nectin-2), resulting in distinct functional outcomes.
1.9. Unanswered questions and conclusions
The most urgent and so far unanswered question about human Treg concerns the absence of a definitive marker for these T cells. While a variety of markers, such as HELIOS, NRP1 or CD15s, are enriched in expression on some Treg, neither is a unique Treg marker and neither is expressed on all subsets of Treg [14, 45, 46, 117]. Consequently, a question about the “transcriptional signature” of Treg is frequently posed. The FOXP3 signature does not seem to meet the requirement for specificity, as it often is absent or only weakly positive in Treg present in the TME [38, 41]. Evidence for “Treg-locking” transcription factors, such as EOS, IRF4, SATIB1 and GATA1, which are up-regulated in the TME, positively regulate FOXP3 mRNA at post-transcriptional level and whose expression levels are dependent on tumor-derived signals and factors, has been provided by Hancock et al [14]. Whether this “Treg-locking” signature is operating in all or only some tumors remains to be determined.
Increasingly often, the notion that FOXP3+ Treg represent a stable T cell linage has been challenged. It has become apparent that Treg can lose FOXP3 expression, as discussed above, and that this loss may be associated with conversion of Treg to potent Teff cells capable of inducing autoimmune disease [40]. Because the maintenance of Treg stability is critical for survival of the host, the studies addressing the fate of Treg in physiological and pathological conditions are critically important.
The primary target(s) of Treg-mediated suppression are not well defined. In many studies of Treg, especially in vivo, APCs rather than responder T cells emerge as primary targets of suppression [111, 118]. Treg (as well as tumor-derived exosomes [119]) down-regulate expression of co-stimulatory molecules on APCs and interfere with the antigen processing machinery (APM) components in APCs [120].
The related concern of whether Treg-mediated suppression is antigen specific or antigen-non-specific has not been adequately addressed so far [37]. The perception that Treg activation is antigen-specific but becomes broadly unrestricted once activated Treg initiate suppression appears to be commonly accepted; however, in some studies, Treg are featured as antigen-specific suppressor T cells [110].
The potential of Treg to mediate numerous mechanisms of immune suppression has been a subject of considerable interest. Clearly, the functional redundancy evident in the presence of multiple mechanisms of suppression in Treg is a biologically important characteristic [37, 121, 122]. It endows Treg with the ability to mediate suppression under various conditions and points to the critical role of these cells in control of inflammatory and immune responses.
Finally, a recent application of immune check point inhibitors in cancer immunotherapy brings forward a number of questions regarding the fate of Treg during such immunotherapy and of the exact role of numerous ICIRs present on activated Treg in functional repertoire of these cells.
Perhaps one the most intriguing and previously unsuspected insights into Treg functions is their functional diversity. This is true not only when examining the numerous molecular pathways Treg engage to execute suppression, as previously reviewed [5] but also in the Treg ability to engage in a broad variety of non-immunosuppressive functions [123]. For example, recent data point to Treg involvement in limiting TPI in response to gut microbes in colorectal cancer CRC [124, 125], in regulation of MMPs which serve as invasion-promoting factors [126] or in modulating angiogenesis via production of NRP1 [127]. Further, Treg have been implicated in regulating tissue repair, e.g. by production of amphiregulin, in protecting tissues from damage during infections [128] and in regulating differentiation of stem cells [129]. These emerging data suggest that Treg serve as diverse regulatory elements in many different tissues and organs in health and many different diseases, including cancer.
2.0. Expert opinion
Our understanding of the role of Treg in human cancer has been largely based on in vitro-acquired data and experimental mouse models. More recently, the view of Treg in cancer progression has recently undergone a substantial change. Initially perceived as highly effective suppressors of anti-tumor immune responses that had to be eliminated because they interfered with immunotherapy of cancer, Treg currently emerge as a universal mechanism of immune homeostasis that tumors have hijacked and shaped to promote tumor escape from the host immune system. The concept of therapeutic removal of such hijacked Treg to facilitate responses to immune therapies has not met with success in the clinic so far, for reasons that are now becoming clear. Nevertheless, murine experiments show that partial elimination of Treg from the TME may be therapeutically beneficial, especially with other immune therapies [130, 131]. Treg regulatory functions are essential for health and life; their complete removal is not an option, and their enormous contextual heterogeneity prevents the selection for therapeutic elimination of those Treg subsets that are reprogramed and engaged in suppression of anti-tumor responses.
Interestingly, certain subsets of Treg appear to be resistant to immune checkpoint inhibitors, such as ipilimumab or pembrolizumab, which rejuvenate functions of effector immune cells but have no effects on the stability and suppression mediated by Treg. This differential response of Treg and Teff cells to the ICIR blockade, emphasizes the existence of substantial differences in molecular signaling between various T cell subsets. Because these differences may be regulated at the level of signals generated in the TME of each tumor, targeting of Treg and their removal could present a significant therapeutic barrier. Until a marker or markers for Treg are found that reliably measure their presence in the TME and allow for Treg isolation and subtyping to define their functions, Treg-targeted therapies are not likely to be successful. The ubiquitous presence of tumor-derived exosomes (TEX) in the TME and reprogramming of immune cells, including Treg, by TEX making them resistant to immune therapies, represents a new, previously unforeseen, barrier that will have to be addressed. The future of Treg-directed therapies depends on our ability to effectively target defined subsets of Treg that have been hijacked by the tumor without interfering with Treg subsets that protect us from autoimmune disease or serve as regulatory cells in various physiological as well as pathological events. Rapid progress in genetic, molecular and technical aspects of cellular immunology and intense focus on the use of Treg for cellular biotherapy, e.g., for the prevention of GVHD after hematopoietic stem cell transplantation [132], provide hope that solutions for many of the above listed barriers are around the corner.
Article highlights.
Elevated Treg/Teff cell ratios in tumors are prognostically useful
iTreg accumulating in the tumor microenvironment (TME) are reprogrammed
Origins of tTreg, pTreg and iTreg
Treg nomenclature and control of cancer prognosis by eTreg
Mechanisms involved in Treg-mediated suppression
Up-regulated ICIRs on Treg and Treg signaling in the TME
Emerging role of tumor-derived exosomes in Treg reprogramming
Attempts at silencing “bad” Treg in cancer
Treg modulation by immune checkpoint inhibition
Contextual heterogeneity and broad functional profile of Treg
Funding
This work has been supported in part by NIH grants R01 CA168628 and R21 CA204644 to TLW. This project used UPMC Hillman Cancer Center shared resources that are supported in part by NIH award P30 CA047904.
Footnotes
Declaration of Interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Articles of special interest have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. August 1;155(3):1151–64. [PubMed] [Google Scholar]; * This is a classic paper in which the authors “reintroduce” Treg and provide evidence for their regulatory role in vivo.
- 2.Syn NL, Teng MWL, Mok TSK, et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017. December;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 3.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019. January 31. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa H, Sido JM, Reyes EE, et al. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci U S A. 2016. May 31;113(22):6248–53. doi: 10.1073/pnas.1604765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteside TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018. April;22(4):353–363. doi: 10.1080/14728222.2018.1451514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014. May;259(1):173–91. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther. 2015;4:159–71. doi: 10.2147/ITT.S55415. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A thorough discussion of the role of Treg in cancer immunology.
- 8.Ondondo B, Jones E, Godkin A, et al. Home sweet home: the tumor microenvironment as a haven for regulatory T cells. Front Immunol. 2013;4:197. doi: 10.3389/fimmu.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011. July;60(7):909–18. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001. August 1;167(3):1245–53. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014. May;259(1):88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016. May 17;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Shayan G, Avery L, et al. Tumor-infiltrating Tim-3(+) T cells proliferate avidly except when PD-1 is co-expressed: Evidence for intracellular cross talk. Oncoimmunology. 2016;5(10):e1200778. doi: 10.1080/2162402X.2016.1200778. PubMed PMID: 27853635; PubMed Central PMCID: PMCPMC5087305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akimova T, Zhang T, Negorev D, et al. Human lung tumor FOXP3+ Tregs upregulate four “Treg-locking” transcription factors. JCI Insight. 2017. August 17;2(16). doi: 10.1172/jci.insight.94075. [DOI] [PMC free article] [PubMed] [Google Scholar]; * An excellent overview of transcriptional activation of human Treg and their phenotypic profiles and suppressor functions.
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003. April;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Arvey A, Chinen T, et al. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014. August 14;158(4):749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012. March 15;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 18.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006. January 15;12(2):465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 19.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009. January 10;27(2):186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 20.Mandal R, Senbabaoglu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016. October 20;1(17):e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baras AS, Drake C, Liu JJ, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016. May;5(5):e1134412. doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004. September;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]; * One of the first papers on the predictive role Treg might play in cancer progression and disease outcome.
- 23.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005. December 20;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006. December 1;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 25.Shang B, Liu Y, Jiang SJ, et al. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015. October 14;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010. June 1;126(11):2635–43. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 27.Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009. October;137(4):1270–9. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke CM, Wu K, Zhao E, et al. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010. August 15;127(4):748–58. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]
- 29.deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012. June 1;18(11):3022–9. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 30.Devaud C, Darcy PK, Kershaw MH. Foxp3 expression in T regulatory cells and other cell lineages. Cancer Immunol Immunother. 2014. September;63(9):869–76. doi: 10.1007/s00262-014-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3(+) regulatory T cells. Immunol Rev. 2014. May;259(1):11–22. doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001. April;2(4):301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008. June;28(6):870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009. June 19;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000. January 1;164(1):183–90. [DOI] [PubMed] [Google Scholar]
- 37.Shevach EM. Foxp3(+) T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front Immunol. 2018;9:1048. doi: 10.3389/fimmu.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A thoughtful discussion of what we currently know about Treg, what we do not know and what needs to be accomplished to establish the biological significance of Treg.
- 38.Miyao T, Floess S, Setoguchi R, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012. February 24;36(2):262–75. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012. August;22(4):327–34. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009. September;10(9):1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Zhang W, Guo J, et al. Activation and Functional Specialization of Regulatory T Cells Lead to the Generation of Foxp3 Instability. J Immunol. 2017. April 1;198(7):2612–2625. doi: 10.4049/jimmunol.1601409. [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, Vent-Schmidt J, McGeough MD, et al. Tr1 Cells, but Not Foxp3+ Regulatory T Cells, Suppress NLRP3 Inflammasome Activation via an IL-10- Dependent Mechanism. J Immunol. 2015. July 15;195(2):488–97. doi: 10.4049/jimmunol.1403225. [DOI] [PubMed] [Google Scholar]
- 43.Chihara N, Madi A, Karwacz K, et al. Differentiation and Characterization of Tr1 Cells. Curr Protoc Immunol. 2016. April 1;113:3 27 1–3 27 10. doi: 10.1002/0471142735.im0327s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan H, Zhang P, Kong X, et al. Primary Tr1 cells from metastatic melanoma eliminate tumor-promoting macrophages through granzyme B- and perforin-dependent mechanisms. Tumour Biol. 2017. April;39(4):1010428317697554. doi: 10.1177/1010428317697554. [DOI] [PubMed] [Google Scholar]
- 45.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010. April 1;184(7):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyara M, Chader D, Sage E, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A. 2015. June 9;112(23):7225–30. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Evidence is provided that CD15s might qualify as a specific marker for “true” Treg in humans.
- 47.Delgoffe GM, Woo SR, Turnis ME, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013. September 12;501(7466):252–6. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012. February 1;188(3):976–80. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines (Basel). 2016. August 6;4(3). doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007. August 1;13(15 Pt 1):4345–54. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 51.Mellor AL, Lemos H, Huang L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Front Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010. September 3;285(36):27571–80. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper provides support for the role of adenosine (ADO) and prostaglandin E2 as major participants in Treg-mediated immune suppression in cancer.
- 53.Grossman WJ, Verbsky JW, Barchet W, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004. October;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Schuler PJ, Saze Z, Hong CS, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin Exp Immunol. 2014. August;177(2):531–43. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton AM, Donovan EE, Piccirillo CA, et al. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004. June 1;172(11):6519–23. [DOI] [PubMed] [Google Scholar]
- 56.Ayala M, Li Z, DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology. 2019 2019. doi: 10.1111/imm.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This review discusses expanding new modality specifically targeting tumor-infiltrating Treg by reprogramming of their functions from suppressor to stimulatory in situ.
- 57.Pike KA, Tremblay ML. Regulating naive and memory CD8 T cell homeostasis--a role for protein tyrosine phosphatases. FEBS J. 2013. January;280(2):432–44. doi: 10.1111/j.1742-4658.2012.08587.x. [DOI] [PubMed] [Google Scholar]
- 58.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016. June;22(6):679–84. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017. January;27(1):109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golding A, Hasni S, Illei G, et al. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013. November;65(11):2898–906. doi: 10.1002/art.38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Simons DL, Lu X, et al. Connecting blood and intratumoral Treg activity in predicting future relapse in breast cancer. Nat Immunol. 2019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loyher PL, Rochefort J, Baudesson de Chanville C, et al. CCR2 Influences T Regulatory Cell Migration to Tumors and Serves as a Biomarker of Cyclophosphamide Sensitivity. Cancer Res. 2016. November 15;76(22):6483–6494. doi: 10.1158/0008-5472.CAN-16-0984. [DOI] [PubMed] [Google Scholar]
- 63.Redjimi N, Raffin C, Raimbaud I, et al. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012. September 1;72(17):4351–60. doi: 10.1158/0008-5472.CAN-12-0579. [DOI] [PubMed] [Google Scholar]
- 64.Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009. February 1;182(3):1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou W Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006. April;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 66.Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017. December;13(28):2583–2592. doi: 10.2217/fon-2017-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016. Feb;39(1):98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009. December 21;206(13):3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** These studies define a mechanism for iTreg cell development and function and a new strategy for controlling Treg cell plasticity.
- 69.Asano T, Meguri Y, Yoshioka T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017. April 13;129(15):2186–2197. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng W, Chen JQ, Liu C, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016. February;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma MD, Shinde R, McGaha TL, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015. November;1(10):e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017. March 31;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017. March 31;355(6332):1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levine AG, Arvey A, Jin W, et al. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014. November;15(11):1070–8. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Togashi Y, Nishikawa H. Suppression from beyond the grave. Nat Immunol. 2017. November 16;18(12):1285–1286. doi: 10.1038/ni.3870. [DOI] [PubMed] [Google Scholar]; * An interesting paper reporting that dying Treg exert enhanced immunosupressive activity suggesting that the overall effects of the PD-1 blockade may be the enhancement of Treg-mediated suppression.
- 76.Maj T, Wang W, Crespo J, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017. December;18(12):1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013. August 26;210(9):1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010. March 5;285(10):7176–86. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007. June 11;204(6):1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mandapathil M, Szczepanski M, Harasymczuk M, et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology. 2012. August 1;1(5):659–669. doi: 10.4161/onci.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017. September;189(3):259–267. doi: 10.1111/cei.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A review of immunoinhibitory activities and mechanisms mediated by tumor-derived exosomes in cancer.
- 84.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018. February;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016. February 4;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017. June;31(6):1259–1268. doi: 10.1038/leu.2017.91. [DOI] [PubMed] [Google Scholar]
- 87.Muller L, Simms P, Hong CS, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6(8):e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009. September 15;183(6):3720–30. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009. February 1;182(3):1469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szajnik M, Czystowska M, Szczepanski MJ, et al. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010. July 22;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The first report providing evidence that tumor-derived extracellular vesicles (EVs) promote in vitro expansion and sustain suppressive activity in human Treg.
- 91.Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2015. January;107(1):363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 92.Hong CS, Sharma P, Yerneni SS, et al. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep. 2017. October 31;7(1):14684. doi: 10.1038/s41598-017-14661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Semin Cell Dev Biol. 2017. July;67:23–28. doi: 10.1016/j.semcdb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ludwig N, Yerneni SS, Razzo BM, et al. Exosomes from HNSCC Promote Angiogenesis through Reprogramming of Endothelial Cells. Mol Cancer Res. 2018. November;16(11):1798–1808. doi: 10.1158/1541-7786.MCR-18-0358. [DOI] [PubMed] [Google Scholar]
- 96.Wortzel I, Dror S, Kenific CM, et al. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019. May 6;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Greening DW, Gopal SK, Xu R, et al. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015. April;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 98.Schuler PJ, Harasymczuk M, Schilling B, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin Cancer Res. 2013. December 1;19(23):6585–96. doi: 10.1158/1078-0432.CCR-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015. June 1;75(11):2200–10. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baur AS, Lutz MB, Schierer S, et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013. September 26;122(13):2185–94. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 101.Toor SM, Syed Khaja AS, Alkurd I, et al. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol. 2018. February;191(2):189–197. doi: 10.1111/cei.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007. July;12(7):873–83. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 103.Bulliard Y, Jolicoeur R, Windman M, et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013. August 26;210(9):1685–93. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015. May 12;112(19):6140–5. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This report calls attention to non-classical monocytes in patients with melanoma, which mediate ipilimumab-dependent cytotoxicity (ADCC) resulting in lysis of Treg.
- 105.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008. February 26;105(8):3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008. September 30;105(39):14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan S, Burt DJ, Ralph C, et al. Tremelimumab (anti-CTLA4) mediates immune responses mainly by direct activation of T effector cells rather than by affecting T regulatory cells. Clin Immunol. 2011. January;138(1):85–96. doi: 10.1016/j.clim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 108.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012. June 4;209(6):1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Togashi Y, Kamada T, Sasaki A, et al. Clinicopathological, genomic and immunological features of hyperprogressive disease during PD-1 blockade in gastric cancer patients. J Clin Oncol. 2018 2018;36(15_supple):4106–4106. [Google Scholar]
- 110.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004. June 7;199(11):1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeda Y, Nishikawa H, Sugiyama D, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. 2014. December 19;346(6216):1536–40. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 112.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015. April 3;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. 2018. January;96(1):21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000. July 17;192(2):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994. August;1(5):405–13. [DOI] [PubMed] [Google Scholar]
- 116.Fourcade J, Sun Z, Chauvin JM, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018. July 26;3(14). doi: 10.1172/jci.insight.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sarris M, Andersen KG, Randow F, et al. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008. March;28(3):402–13. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holt MP, Punkosdy GA, Glass DD, et al. TCR Signaling and CD28/CTLA-4 Signaling Cooperatively Modulate T Regulatory Cell Homeostasis. J Immunol. 2017. February 15;198(4):1503–1511. doi: 10.4049/jimmunol.1601670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ludwig S, Sharma P, Theodoraki MN, et al. Molecular and Functional Profiles of Exosomes From HPV(+) and HPV(−) Head and Neck Cancer Cell Lines. Front Oncol. 2018;8:445. doi: 10.3389/fonc.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caridade M, Graca L, Ribeiro RM. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Front Immunol. 2013. November 18;4:378. doi: 10.3389/fimmu.2013.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shevach EM, McHugh RS, Piccirillo CA, et al. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001. August;182:58–67. [DOI] [PubMed] [Google Scholar]
- 122.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008. July;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vasanthakumar A, Kallies A. The Regulatory T Cell: Jack-Of-All-Trades. Trends Immunol. 2015. December;36(12):756–758. doi: 10.1016/j.it.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Russler-Germain EV, Rengarajan S, Hsieh CS. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017. November;10(6):1375–1386. doi: 10.1038/mi.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol. 2012. August;24(4):392–7. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 126.Wang BQ, Zhang CM, Gao W, et al. Cancer-derived matrix metalloproteinase-9 contributes to tumor tolerance. J Cancer Res Clin Oncol. 2011. October;137(10):1525–33. doi: 10.1007/s00432-011-1010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matkar PN, Jong ED, Ariyagunarajah R, et al. Jack of many trades: Multifaceted role of neuropilins in pancreatic cancer. Cancer Med. 2018. October;7(10):5036–5046. doi: 10.1002/cam4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arpaia N, Green JA, Moltedo B, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015. August 27;162(5):1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ali N, Zirak B, Rodriguez RS, et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell. 2017. June 1;169(6):1119–1129 e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor NA, Vick SC, Iglesia MD, et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J Clin Invest. 2017. September 1;127(9):3472–3483. doi: 10.1172/JCI90499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colbeck EJ, Jones E, Hindley JP, et al. Treg Depletion Licenses T Cell-Driven HEV Neogenesis and Promotes Tumor Destruction. Cancer immunology research. 2017. November;5(11):1005–1015. doi: 10.1158/2326-6066.CIR-17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016. February 25;127(8):1044–51. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huss DJ, Mehta DS, Sharma A, et al. In vivo maintenance of human regulatory T cells during CD25 blockade. J Immunol. 2015. January 1;194(1):84–92. doi: 10.4049/jimmunol.1402140. [DOI] [PubMed] [Google Scholar]
- 134.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009. September;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]; ** This report provides evidence that a peptide-based vaccine in patients with metastatic breast cancer is made more effective by the use of daclizumab, which induced prolonged elimination of CD25+ FoxP3+ Tregs.
- 135.Lutz MB, Baur AS, Schuler-Thurner B, et al. Immunogenic and tolerogenic effects of the chimeric IL-2-diphtheria toxin cytocidal agent Ontak((R)) on CD25(+) cells. Oncoimmunology. 2014;3:e28223. doi: 10.4161/onci.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014. April;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 137.Schaer DA, Budhu S, Liu C, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer immunology research. 2013. November;1(5):320–31. doi: 10.1158/2326-6066.CIR-13-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013. October 29;110(44):17945–50. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012. July 15;72(14):3439–44. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009. May 11;206(5):1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang H, Franco F, Ho PC. Metabolic Regulation of Tregs in Cancer: Opportunities for Immunotherapy. Trends Cancer. 2017. August;3(8):583–592. doi: 10.1016/j.trecan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 142.Overacre-Delgoffe AE, Vignali DAA. Treg Fragility: A Prerequisite for Effective Antitumor Immunity? Cancer immunology research. 2018. August;6(8):882–887. doi: 10.1158/2326-6066.CIR-18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szajnik M, Derbis M, Lach M, et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol Obstet (Sunnyvale). 2013. April 29;Suppl 4:3. doi: 10.4172/2161-0932.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Strauss L, Bergmann C, Gooding W, et al. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007. November 1;13(21):6301–11. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]