Abstract
Flavonoids are now considered as an indispensable component in a variety of nutraceutical and pharmaceutical applications. Most recent researches have focused on the health aspects of flavonoids for humans. Especially, different flavonoids have been investigated for their potential antiviral activities, and several natural flavonoids exhibited significant antiviral properties both in vitro and in vivo. This review provides a survey of the literature regarding the evidence for antiviral bioactivities of natural flavonoids, highlights the cellular and molecular mechanisms of natural flavonoids on viruses, and presents the details of most reported flavonoids. Meanwhile, future perspectives on therapeutic applications of flavonoids against viral infections were discussed.
Keywords: Antiviral bioactivities, Natural flavonoids, Cellular and molecular mechanisms, Therapeutic applications
Introduction
Flavonoids comprise one of the largest groups of secondary metabolites found in biologically active plants, including vegetables, fruits, seeds, nuts, wine, and tea. Flavonoids are low molecular weight compounds with a simple 15 carbon backbone, and there are more than 9000 varieties of flavonoids that have been structurally identified. The natural flavonoids are an important source of medicines [1].
Typically, flavonoids are divided into flavones, flavonols, flavanones, flavanonols, flavanes, flavanols, chalcones, anthocyanidins, aurones, isoflavones, biflavones [2]. The carbon atoms in flavonoid molecules are assembled in two benzene rings, commonly denoted as A and B, which are connected by an oxygen-containing pyrene ring. A common part of the chemical structure of all flavonoids is the carbon skeleton based on the flavan system (C6–C3–C6) (Fig. 1). Aurone is a type of flavonoid with a heterocyclic ring containing a benzofuran element while biflavonoids are dimers of flavonoid moieties linked by a C–C or C–O–C bond. Condensation of A and B ring leads to the formation of chalcone, which undergoes cyclization involving isomerase and forms flavanone, the initial compound for the synthesis of other group flavonoids [3]. Although the various classes of flavonoids possess different structures, all flavonoids appear multi-bioactivities and complex roles in the system of biology.
Fig. 1.
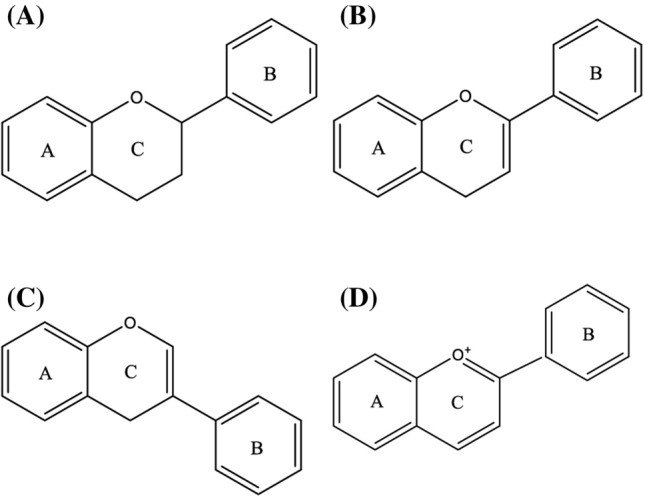
The basic scaffolds of flavonoids. a The scaffold of flavanones and catechins; b the scaffold of the flavones and flavonols; c the scaffold of isoflavone; d the scaffold of anthocyanins. The structures of aurones and biflavones were not involved in this figure since their structures containing some special elements which were described in the article
Most flavonoids, except for the subclass of catechins, are present in plants bound to sugars as β-glycosides. The common sources of natural flavones were the vegetables such as Chamomile tea (Matricaria chamomilla), leaves of parsley (Petroselinum crispum), celery (Apium graveolens) and spinach (Spinacia oleracea), roots of plants, propolis, and honey and so on [4]. Even the flavonoids could obtain from various of food and vegetables, the molecules with different structures are of different bioactivities. Since the first report in 1938, flavonoids were described as a broad spectrum of biological activities such as anti-inflammation, antioxidant, antibacterial, antiviral, anticancer, and neuroprotection [5]. In this review, we made a literature retrieval for the anti-virus activity of flavonoids. The antiviral activity of flavones was studied and reported from the 1990s, when apigenin showed synergistic effects to acyclovir on herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) in cell culture. Recently, flavones are reported the inhibitory activity on viruses, including A/FM1/1/47(H1N1), H3N2, H5N1 (strain A/Thailand/Kan-1/04), HBV, HCV, HIV, dengue virus (DENV-2), Sendai virus, Zika virus, Coxsackie virus (CVB3) and Japanese encephalitis virus (JEV) [6]. Especially, the latest study showed flavones efficiently inhibited SARS-CoV [7]. However, numerous positive findings have been reported on the in vitro efficacy of flavonoids, but less promising results have been obtained for most compounds in in vivo studies due to poor bioavailability of flavonoids. The low solubility of flavonoid aglycones in water, coupled with its short residence time in the intestine as well as its lower absorption, save humans from the acute toxic effects via the consumption of flavonoids, except for a rare occurrence of allergy [8]. Therefore, the efforts in enhancing the bioavailability of flavonoids upon intake by humans are vitally necessary in order to develop these natural compounds into potential antiviral drugs.
Generally speaking, the absorption of the dietary flavonoids liberated from the food will depend on their physicochemical properties such as molecular size, configuration, lipophilicity, solubility, and pKa [9]. In addition, flavonoid protein interactions are involved in flavonoid bioavailability. Depending upon structure, the flavonoid can be absorbed from the small intestine or has to go to the colon, where they are metabolized via microbial catabolism, conjugation in liver and enterocytes. Due to these conjugation reactions, no free flavonoid aglycones can be found in plasma or urine, except for catechins [10]. The sugar moiety of flavonoid glycosides is an important determinant of their bioavailability [11].
Overview of the Research on the Antiviral Effects of Flavonoids
Based on the literature published in the international journals, up to May 2020, more than 1000 researches on the anti-virus activities in vivo and in vitro and 100s of natural flavonoids have been tested in different viruses. But, only about decades were focused, such as coumarin, luteolin, and so on (Fig. 2).
Fig. 2.
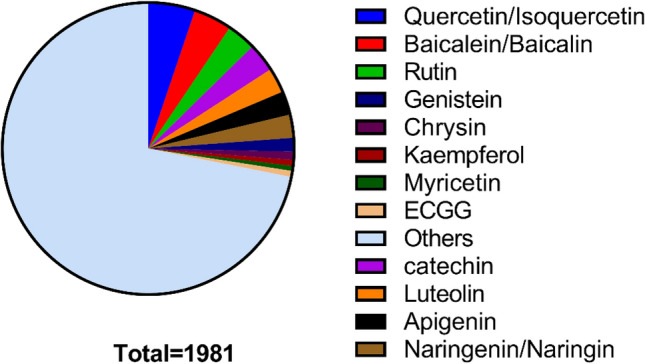
Literature review on the antiviral activities of natural flavonoids
Generally, all the bioactivities found in the flavonoids could be summarized to some main aspects. Flavonoids, including genistein, catechins, and so on, have been shown to reduce the infectivity of a variety of viruses affecting humans and animals, including adenovirus, HSV, HIV, porcine reproductive and respiratory syndrome virus, and rotavirus [12]. Current results about the mechanisms of action underlying their antiviral properties suggest a combination of effects on both the virus and the host cell. Flavonoids have been reported to affect virus adsorption, entry, replication, viral protein translation, the formation of certain virus envelope glycoprotein complexes, and virus release [13–16]. They also affect a variety of host cell signaling processes, including induction of gene transcription factors and secretion of cytokines [17, 18]. Although enormous promising results were from in vitro experiments, a few in vivo results can partly confirm their in vivo efficacy. Flavonoids possess antiviral properties against a wide range of viruses under both in vitro and in vivo conditions (Table 1).
Table 1.
The different viruses which inhibited by various flavonoids
| Viruses | Model | Flavonoids | References |
|---|---|---|---|
| Influenza virus | MDCK cells | Gallocatechin-7-gallate, catechins, apigenin, luteolin, 3-deoxysappanchalcone, scutellarin, galuteolin, vitexin, chrysin, kaempferol, quercetin, myricetin, rhamnocitrin, rutin, daidzein, genistein, sappanchalcone, baicalein, oroxylin A | Liu et al. [19], Yonekawa et al. [20] |
| HBV | Vero cells | Myricetin rhamnoside, myricetin-3-α-O-ramnosil (1 → 6)-α-galactoside, 5,3′-dihydroxy-3,6,7,8,4′- pentamethoxyflavone, 5-hydroxy-3,6,7,3′,4′- pentamethoxyflavone | Ortega et al. [21] |
| HCV | Huh-7.5 cells | Epigallocatechin gallate (EGCG), sorbifolin, pedalitin | Mekky et al. [22] |
| HIV-1 | CD4+ NKT cells, T cells | Hesperidin, linarin, catechins, genistein, herbacitrin, naringin, formononetin, biochanin A | Nzuza et al. [23] |
| HIV-2 | Vero cells | Genistein, formononetin, biochanin A | Patra [24] |
| HSV-1 | Vero and CV1 cells | Catechins, genistein,gorvanol A, kaempferol, 5,6,7-trimethoxyflavone, 5,3′-dihydroxy-3,6,7,8,4′- pentamethoxyflavone, 5-hydroxy-3,6,7,3′,4′- pentamethoxyflavone, coumestrol, houttuynoid A, chrysin | Li et al. [25] |
| HSV-2 | Vero cells | Genistein, coumestrol, houttuynoid A | Bús et al. [26] |
| HPV-1 | Human condyloma, Vero cells | Catechins, 5,3′-dihydroxy-3,6,7,8,4′- pentamethoxyflavone, 5-hydroxy-3,6,7,3′,4′- pentamethoxyflavone | Patra [24] |
| DENV-2 |
C6/36 Aedes albopictus mosquito cell, hepatocytes (Huh-7) |
Quercetin, quercitrin, kaempferitrin, chrysin | Patra [24] |
| Sendai virus (SeV) | Mice model | Baicalein | Dou et al. [27] |
| Zika virus (ZIKV) | Vero cells | Baicalein, baicalin, pinocembrin, chrysin, myricetin, luteolin, Epigallocatechin gallate, epicatechin gallate, gallocatechin gallate, quercetin-3-β-O-d-glucoside | Oo et al. [28] |
| CVB3 | Vero cells | Mosloflavone, oroxylin A, norwogonin, epigallocatechin-3-gallate | Patra [24] |
| JEV | A549 cells, BHK21 cells | Epigallocatechin-3-gallate (EGCG), luteolin, kaempferol | Patra [24] |
| EBV | Ramos cells | Genistein, quercetin, apigenin, luteolin, baicalein | Granato et al. [29] |
| Poliovirus | Vero cells | 5,6,7-Trimethoxyflavone, 3-methylkaempferol, 3(2H)-isoflavene | Ortega et al. [21] |
| RSV | Vero cells | Genistein, quercetin, baicalein, baicalin, epigallocatechin-3-gallate, proanthocyanidin | Zhang et al. [30] |
| Coronovirus | Vero cells | Quercetin, Luteolin, quercetin, quercetrin, kaempferol glycosides | Patra [24] |
| SARS-CoV | 3CL protease activity assay | Daidzein, rutin, genistein, icaritin, genistin, ipriflavone, (−) gallocatechin, (±)-epigallocatechin gallate, puerarin, (−)-epicatechin, glabridin, (±)-catechin, baicalein, diosmin, diosmetin, skullcapflavone II, orientin, acacetin, bacicalin, rhoifolin, hispidulin, sinensetin, oroxin B, pectolinarin, cirsiliol, homoplantaginin, amentoflavone, luteolin, herbaacetin, kaempferol, morin, myricetin, fisetin, quercitrin, queretin, helichrysetin, cardamonin, neodesperidin dishydrochalcone, mangiferin, auraptene | Jo et al. [7] |
| Human CMV | HEL 299 cells | Genistein, 5,6,7-Trimethoxyflavone | Patra [24] |
| Rotavirus | MA-104 cells, Caco2 cells | Genistein, epigallocatechin Gallate (EGCG), α-glucosyl hesperitin (GH) | Lipson et al. [31] |
| Adenovirus | Hep2 cells, SW480 cell, BCC-1/KMC cells | Catechins, genistein, quercetin | Patra [24] |
| SARS-CoV-2 | Vero cells | Baicalein, scutellarein, dihydromyricetin, quercetagetin, myricetin | Liu et al. [32] |
Mechanism of Antiviral Flavonoids
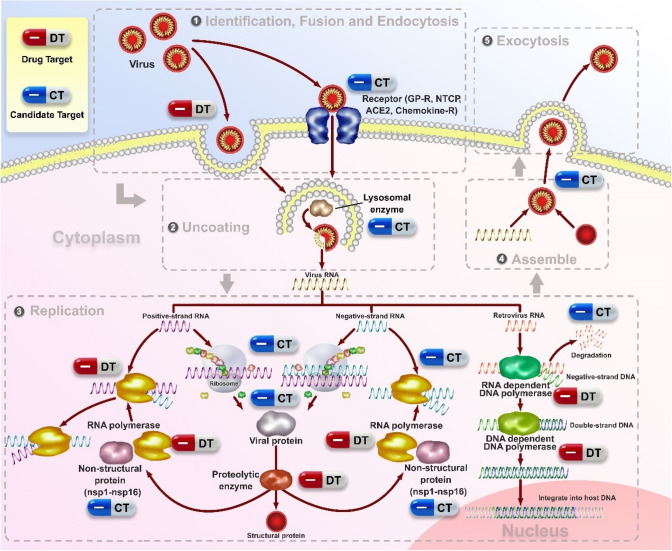
In many cases, DNA viruses utilize cellular enzymes for synthesis of their DNA genomes and mRNAs; all viruses utilize normal cellular ribosomes, tRNAs, and translation factors for the synthesis of their proteins. Most viruses commandeer the cellular machinery for macromolecular synthesis during the late phase of infection, directing it to synthesize large amounts of a small number of viral mRNAs and proteins instead of normal cellular macromolecules. The lytic cycle of viral replication includes adsorption, penetration, replication, and release [33] (Fig. 3). The outcome is the production of a new round of viral particles and the death of the cell. According to the lytic cycle of the virus, antiviral drugs can be categorized into the inhibitors of fusion, uncoating, nucleic acid synthesis, integration, protease, and release. Targeting chemokine receptors and glycoprotein (GP)-receptor interactions are also of the most attractive candidates to inhibit viral entry/fusion. Especially, viral enzymes, including RNA polymerase, DNA polymerase, and reverse transcriptase, were considered as alternative targets in many viral infections such as HBV [34, 35]. In addition to previously described viral targets, new classes of antiviral drugs targeting host factors involved in virus replication, virus-cell interactions, and the immune response have been introduced [36].
Fig. 3.
Potential drug targets (DT) and candidate targets (CT) of flavonoids in the viral life cycle. The viral life cycle can be divided into a sequence of stages (attachment and entry; uncoating, replication, assemble, and exocytosis), each of which is a potential site for pharmacologic intervention. Here we showed potential drug targets (DT) and candidate targets (CT) of antiviral flavonoids
Many reports on the antiviral activity of naturally occurring flavonoids are available. The structure–function relationship between flavonoids and their enzyme inhibitory activity has been observed. Baicalin was reported to interfere with the viral neuraminidase activity [37]. Flavan-3-o1 was more effective than flavones and flavonones in selective inhibition of HIV-1, HIV-2, and similar immunodeficiency virus infections. Baicalin inhibits HIV-1 infection and replication. Flavonoids such as demethylated gardenin A and robinetin are known to inhibit HIV-1 proteinase. It has also been reported that the flavonoids chrysin, acacetin, and apigenin prevent HIV-1 activation via a novel mechanism that probably involves inhibition of viral transcription [38, 39]. Various combinations of flavones and flavonols have been shown to exhibit antiviral synergism [21]. Kaempferol and luteolin show synergistic effects against herpes simplex virus (HSV). Synergism has also been reported between flavonoids and other antiviral agents. Quercetin is reported to potentiate the effects of 5-ethyl-2-dioxyuridine and acyclovir against HSV and pseudorabies infection [40]. Many flavonoids, namely, dihydroquercetin, dihydrofisetin, leucocyanidin, pelargonidin chloride, and catechin, show activity against several types of viruses, including HSV, respiratory syncytial virus, poliovirus, and Sindbis virus. Quercetin is reported to potentiate the effects of 5-ethyl-2-dioxyuridine and acyclovir against HSV and pseudorabies infection. Studies have displayed that flavonols are more active than flavones against herpes simplex virus type 1, and the activity order from strong to weak was found to be galangin, kaempferol, and quercetin. Zandi et al. studied the anti-dengue virus properties of quercetin, hesperetin, naringin, and daidzein at different stages of DENV-2 (dengue virus type-2) infection and replication cycle [41]. Quercetin was found to be most effective against DENV-2 in cells. In addition, flavonoids were reported anti-inflammation by diminishing inflammatory response and excessive immune response [42]. Therefore, flavonoids protect host cells from damage induced by a viral infection. For instance, selected flavonoids can reduce complement activation, thereby decreasing the adhesion of inflammatory cells to the endothelium and, in general, resulting in a diminished inflammatory response. Another feature of flavonoids is a reduction in the release of peroxidase. This reduction inhibits the production of reactive oxygen species by neutrophils by interfering with 1-antitrypsin activation and the metabolism of arachidonic acid [41, 43].
The flavonoids with anti-virus activities act on various targets, which are known targets with some anti-virus drugs. And some proteins and enzymes which some flavonoids interacted and include in the virus replications may be a candidate target for new anti-virus drugs. (Fig. 3).
Important Antiviral Natural Flavonoids
Quercetin and Isoquercitin
Quercetin is widely distributed in angiosperms such as Threevein Astere, Golden Saxifrage, berchemia lineata, gold, rhododendron dauricum, seguin loquat, purple rhododendron, Rhododendron micranthum, Japanese Ardisia Herb and Apocynum. Isoquercitin (IQ), a naturally occurring glycoside of quercetin also known as hirsutrin, isoquercetrin, quercetin3glucoside (Q3G), quercetin3Oβdglucoside. Naturally occurring quercetin compounds are mainly glycosides such as IQ and are commonly found in plants and the human diet.
The quercetin and IQ differ in their structures, bioavailability, absorption, and biological actions. Quercetin and IQ have various kinds of pharmacological functions and are mainly used for treating clinical bronchitis and phlegmatic inflammation. Currently, quercetin/IQ are reported antiviral activities by many researchers (Table 2).
Table 2.
Anti-virus activities of quercetin/IQ
| Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|
| A/Udorn/317/72 (H3N2) | Decreases superoxide and LPO associated viral infection | 1 mg/day | Kumar et al. [44] |
| Dengue virus | Inhibits virus replication | 20 mg/mL | Keivan Zandi et al. [43] |
| Japanese encephalitis virus | Inhibits virus adsorption; Interferes virus replication | IC50 (212.1 μg/mL) | Jefree Johari et al. [45] |
| Rhinovirus | Inhibits RV endocytosis and replication and the expression of chemokines and cytokines | 10 μM in vitro; 0.2 mg/kg in vivo | Shyamala Ganesan et al. [46] |
| Mayaro virus | Inhibits virus replication | 2 μg/mL | dos Santos et al. [47] |
| H1N1, H3N2, and H5N1 | Binds to Influenza hemagglutinin protein; inhibit viral-cell fusion | IC50 (7.756, 6.225, 2.738 μg/mL, respectively) | Wu et al. [48] |
| Epstein-Barr virus | Induces EBV gene transcription; reduces EBV latency; increases EBV progeny production; inhibits EBV infection | 62 μM | Lee et al. [49] |
| hepatitis C virus | Inhibits HCV replication, specific infectivity; affects virion integrity; hampers the localization of HCV core protein to LDs | 50 μM | Ángela Rojas et al. [50] |
| Influenza A H1N1 (A/PR/8/34) | Inhibits neuraminidase | 1.563 μg/mL; 240 mg/kg/days | Liu et al. [51] |
Baicalein
Scutellaria baicalensis Georgi is a medicinal plant with multiple pharmacological activities. Scutellaria baicalensis is the main component of Chinese patent medicine preparations for clinical use, such as Shuanghuanglian injection and Qingkailing injection. Baicalin and its active metabolite baicalein are the main pharmacologically active compounds in Scutellaria baicalensis. Modern research shows that baicalin has certain antiviral activity. Its antiviral pharmacological effect is a concrete manifestation of the heat-clearing and detoxifying effect in the classics of traditional Chinese medicine. With the development of research, details of the antiviral activities of baicalein were reported (Table 3).
Table 3.
Anti-virus activity of baicalin
| Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|
| A/FM1/1/47 (H1N1) | Interferes with neuraminidase activity | 1.2 μg/mL | Xu et al. [52] |
| Strain A/Thailand/K (H3N2) | Inhibits virus budding and neuraminidases | IC50:49.6 ± 1.07 μg/mL | Gao et al. [3] |
| an-1/04 (H5N1) | Interferes with H5N1 replication | IC50:18.79 ± 1.17 μM | Sithisarn et al. [15] |
| SARS-CoV | 3CLpro | 0.39 μM | Liu et al. (2020) |
| Zika virus | Inhibits virus replication | 0.004 µM | Oo et al. [28] |
| Dengue virus | Inhibits virus replication | IC50:13.5 ± 0.08 μg/mL | Moghaddam et al. [53] |
| Sendai virus | Interferes with neuraminidase | 0.70 μg/mL | Dou et al. [27] |
| Japanese encephalitis virus | Interactions with the E protein of DENV2 | 14.28 µg/mL | Johari et al. [45] |
| CVB3 | Inhibits virus replication | IC50:429.00 ± 22.06 μg/mL | Gao et al. [54] |
| Japanese encephalitis virus | Direct virucidal activity | 14.28 µg/mL | Johari et al. [45] |
| Human HIV-1 | Inhibits HIV-1 induced syncytium formation, HIV-1 p24 antigen, and HIV-1 RT production; inhibits Env-protein mediated fusion of HIV | 4.3 μM | Fesen et al. [55] |
| DENV-2 | Inhibits virus replication | 1.55 μg/mL | Zandi et al. [45] |
Apigenin
Apigenin, a member of the flavone family, is a nontoxic and nonmutagenic dietary flavonoid found in parsley, artichoke, basil, celery, and other plants. Apigenin (4′,5,7-trihydroxyflavone) contains a hydroxyl group in its B-ring, and hydroxyl groups in its C-ring. The apigenin contained plants are used for the treatment of different diseases and infections like diabetes, dysentery, hepatitis, blennorrhagia, cancer arthritis, inflammation, woods, hemorrhoids, and leishmanial ulcers. Especially, apigenin exhibits various antiviral activities against numerous viruses in vitro and in vivo: enterovirus 71 (EV71), hepatitis C virus (HCV), Human Immunodeficiency Virus (HIV), and adenoviruses. Apigenin exerted inhibitory effects on HCV replication by decreasing mature miR122 expression levels. Apigenin also inhibited FMDV (Foot and mouth disease virus) infection by suppressing IRES-driven translational activity inhibited FMDV infection at the post-entry stage. Apigenin inhibits EBV reactivation into the lytic cycle and virion production by EBV-positive NPC cells. The antiviral activity of apigenin is currently reported, as shown in Table 4.
Table 4.
Anti-virus activity of apigenin
| Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|
| EBV | Inhibits expression of EBV lytic proteins, Zta, Rta, EAD, and DNase | 200 to 295 μM (24 h); 69 to 158 μM (48 h) | An et al. [56] |
| African swine fever virus | Inhibits ASFV-specific protein synthesis and viral factory formation | IC50:212.1 ± 11.5 μM | Hakobyan et al. [57] |
| HCV | Inhibits HCV replication by decreasing mature miR122 expression | 5 μM | Shibata et al. [58] |
| SARS-CoV 3CLpro | Inhibits SARS-CoV 3CLpro | 280.8 μM | Ryu et al. [59] |
| PEDV | Interferes PEDV replication | Choi et al. [60] | |
| FMD virus | Inhibits cytopathogenic effect and FMDV replication | Qian et al. [61] | |
| HIV | Inhibits CYP3A4, slowdown elimination of PIs | Kehinde et al. [62] | |
| Influenza virus | 1.43 μg/mL | Liu et al. [51] | |
| Vaccinia virus | Inhibits VV replication | Chang et al. [63] | |
| PV-2 | 12.2–13.3 μM | Visintini Jaime et al. [64] |
Luteolin
Luteolin (3,4,5,7-tetrahydroxyflavone) is a pure yellow crystal representing the category of bioflavonoid. It is abundant in various medicinal herbs, fruits, and vegetables, e.g., broccoli, onion, parsley, green peppers, citrus, celery, and chamomile. Luteolin has many beneficial properties, including antioxidant, anti-inflammatory, anticancer, anti-diabetic, and cardio-protective effects and widely used in the development of different traditional medicines for the treatment of diseases. It is also well known to have good effects on anti-angiogenesis, anti-metastasis, anti-inflammation, and estrogenic regulation and can regulate many signal pathways. Besides, luteolin is considered to have potential clinical value for cancer prevention and therapies. Luteolin can obstruct the later stages of the DENV viral life cycle in infected cells by inhibiting the host proprotein convertase furin. Luteolin also exhibits inhibitory effects on Epstein-Barr Virus, Japanese encephalitis virus, HIV-1, Hepatitis B virus, Hepatitis C virus, enterovirus 71, coxsackievirus A16, and chikungunya virus (Table 5).
Table 5.
Anti-virus activity of luteolin
| Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|
| DENV | Inhibits proprotein convertase furin |
10 mM 100 mg/kg |
Peng et al. [65] |
| EBV | Inhibits viral lytic proteins expression and interferes with Sp1 binding to the IE gene promoters | NA cells (IC50 = 8.6–18.1 μM); HA cells (IC50 = 6–12.3 μM); B cells (IC50 = 6-8 μM) | Wu et al. [66] |
| Japanese Encephalitis Virus (JEV) | Inhibits JEV replication | IC50 = 4.56 μg/mL | Fan et al. [67] |
| Influenza virus A/Jinan/15/90 (H3N2) | Inhibits neuraminidase (NA) activities | IC50 = 7.15 μM | Liu et al. [51] |
|
Influenza virus A/Jiangxi/312/2006 (H3N2) A/Fort Monmouth/1/1947 (H1N1) |
Interferes with the virus at the early stages of its lifecycle and blocks influenza virus absorption and internalization | IC50 = 6.89 μM | Yan et al. [68] |
| HBV | Inhibits HBV transcription through ERK-mediated downregulation of HNF4α expression | 10–40 μM; 2 mg/kg | Bai et al. [69] |
| HIV-1 | Inhibits HIV-1 activity; infection by abrogating Tat-mediated LTR activity | 5–10 μM | Mehla et al. [70] |
| SARS-CoV | Binds to the surface spike protein of SARS-CoV and inhibits entry of the virus into host cells | EC50 = 10.6 μM | Yi et al. [71] |
| COVID-19 | Inhibits COVID-19 main protease Mpro | Khaerunnisa et al. [72] |
Isorhamnetin
Isorhamnetin (Iso) is a flavonoid compound extracted from the Chinese herb Hippophae rhamnoides L. Previous studies have revealed its anticancer, anti-inflammatory, and antioxidant activities. What's notable here is the antiviral activity of isorhamnetin. Oral administration of isorhamnetin in mice infected with the influenza A virus significantly decreased lung virus titer by 2-folds and decreased the virus titer in vivo using embryonated chicken eggs. Structure–activity relationship (SAR) showed the methyl group located on the B ring of isorhamnetin might contribute to its strong antiviral potency against the influenza virus in comparison with other flavonoids. In addition, isorhamnetin treatment reduced virus-induced ROS generation and blocked cytoplasmic lysosome acidification and the lipidation of microtubule associated protein1 light chain 3-B (LC3B). The evidence for the anti-virus activity of isorhamnetin was shown in Table 6.
Table 6.
Anti-virus activity of isorhamnetin
| Virus | Mechanism of action | Dose/concentration | In vitro/in vivo | References |
|---|---|---|---|---|
| EV71 virus | Inhibits EV71 RNA replication and protein synthesis | 10 mg/kg | In vivo | Dai et al. [73] |
| H1N1 virus | Reduces virus-induced active oxygen production, blocking cytoplasmic lysosomal acidification and lipid formation of microtubule-associated proteins | 1 mg/kg | In vivo | Enkhtaivan et al. [74] |
|
HHV1 virus HHV2 virus |
Adheres to the cell surface and reduces the interaction between cells and viruses | 100 g/mL | In vitro | Sochocka et al. [75] |
| Zika virus | Inhibits NS3–NS2B protease | 600 µM | Molecular docking study | Sonam et al. [76] |
Isoflavone
Isoflavones are polyphenolic secondary plant metabolites that are produced primarily from members of the Leguminosae. There are hundreds of naturally occurring isoflavones isolated and identified. Common isoflavones include daidzin, genistin, biochanin A, and formononetin. All isoflavones share the 3-phenylchromen-4-one backbone, which is always modified, mainly by O-substituents, glycosides, and/or prenylated derivatives. In plants consumed as part of the human diet (including dietary supplements), the highest concentrations of isoflavones have been observed in soy (Glycine max L.), red clover (Trifolium pratense), and kudzu. Isoflavones exhibit antioxidant, anticancer, antimicrobial, anti-inflammatory, antiosteoporotic, and estrogenic properties. Especially, isoflavones and their related flavonoid compounds exert antiviral properties both in vitro and in vivo against a wide range of viruses. Targets of Isoflavones reported affecting virus binding, entry, replication, viral protein translation, and formation of certain virus envelope glycoprotein complexes. Isoflavones also affect a variety of host cell signaling processes, including induction of gene transcription factors and secretion of cytokines (Table 7).
Table 7.
Anti-virus activity of isoflavones
| Isoflavone | Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|---|
| Genistein | Avian leucosis virus | Inhibits the late phase of ALV-J replicative cycle | 12.5–100 μM | Qian et al. [77] |
| Porcine reproductive and respiratory syndrome virus (PRRSV) | Activation of adaptive immune system pathways | Smith et al. [78] | ||
| Genistein | African swine fever virus | Disrupts viral DNA replication, blocking the transcription of late viral genes as well as the synthesis of late viral proteins, reducing viral progeny | IC50 = 13 μM, | Arabyan et al. [79] |
| Genistein | rotavirus | Inhibits rotavirus replication and upregulates AQP4 expression | 80 μM | Huang et al. [80] |
| Genistein | Herpes simplex virus | Inhibits virus replication | 40 μM | Argenta et al. [81] |
| KIN 101 | hepatitis C virus (HCV) and influenza virus | Activates the ISG54 promoter mediated nuclear translocation of IRF-3 | IC50 = 0.2 μM | Bedard et al. [82] |
| Deguelin | Human cytomegalovirus (HCMV) | Suppresses the production of the infectious virus; inhibits the lytic cycle | 250 nM | Nukui et al. [83] |
| HIV-1 | Inhibits HIV-1 entry into cell lines, primary human CD4+ T lymphocytes, and macrophages | IC50 = 81.6 ± 4.3 μM | Mediouni et al. [84] | |
| Daidzein | Dengue virus type-2 | Inhibits virus replication | IC50 = 142.6 μg/mL | Zandi et al. [43] |
Catechin/EGCG (Epigallocatechin-3-gallate)
Catechins are important ingredients from tea leaves and account for more than 75% of the polyphenol compounds in tea leaves. Catechins are members of the group of polyphenol compounds found in many medicinal plants, with a ring and the basic structure of flavan-3-ol and have intensive anti-oxidant and representative physiological activities.The major sources of catechins are Camellia sinensis (C. sinensis) and C. assumica. There are eight catechin: C ((−)-catechin), EC ((−)-epicatechin), ECG ((−)-epicatechingallate), EGC ((−)-epigallocatechin), EGCG ((−)-epigallocatechin gallate), GC ((−)-gallocatechin), CG ((−)-catechingallate), and GCG ((−)-gallocatechingallate). Because of the hydroxyl in the gallate group, Epigallocatechin-3-O-gallate (EGCG) and ECG are highly effective free-radical scavengers compared with many other standard anti-oxidants. According to the relationships between structure and antiviral activity of catechin derivatives, the 3-galloyl and 5′-OH group of catechin derivatives appear critical to antiviral activities. Most Catechin/EGCG were reported not to affect cell viability and proliferation but interfered with herpes simplex virus cell penetration and adhesion. Among these catechins, EGCG is the major catechin component of green tea (Cameria sinensis) and known to possess antiviral activities against a wide range of DNA viruses and RNA viruses. EGCG has been reported to possess a broad spectrum of antiviral activities against DNA viruses such as herpes simplex virus (HSV; Herpesviridae) adenovirus (Adenoviridae), human papilloma virus (HPV; Papovaviridae), and hepatitis B virus (HBV; Hepadnaviridae), and against (+)-RNA viruses such as hepatitis C virus (HCV; Flaviviridae), Zika virus (ZIKV; Flaviviridae), dengue virus (DENV; Flaviviridae), West Nile viruses (WNV; Flaviviridae), Chikungunya virus (CHIKV; Togaviridae), and Porcine Reproductive and Respiratory virus (PRRS; Atteriviridae), and (−)RNA viruses such as human immunodeficiency virus (HIV; Retroviridae), Ebola virus (EBOV; Filoviridae) and influenza virus (Table 8).
Table 8.
Anti-virus activity of catechin/EGCG
| Catechin | Virus | Mechanism of action | Dose/concentration | References |
|---|---|---|---|---|
| EGCG | HBV | Detrimental to HBV replication by altering lysosomal acidification | 25–50 μM | Zhong et al. [85] |
| EC, ECG, EGC and EGCG | HSV-1/HSV-2 | Destructive HSV-1 virions;competitively interacted with virion surface proteins |
HSV1:IC99 (IC50: 18.3–72.3 μM) HSV2:IC99 (IC50: 12.5–25 μM) |
Isaacs et al. [86] Colpitts et al. [87] |
| EGCG | EBV | Suppressed the synthesis of lytic protein; inhibited the lytic infection; reducing the DNA binding potency of nuclear antigen; inhibition of the MEK/ERK1/2 and PI3-K/Akt signaling pathways | IC50: 250 μM |
Weber et al. [88] Liu et al. [89] |
| EGCG | Adenovirus | Inhibited the attachment of adenovirus by interacting with virion surface proteins | IC50:20 µM | Liu et al. [90] |
|
EGCG, EC ECG |
HIV1/HIV2 | Inhibitory action against HIVRT; competitive inhibitors of the template-primer; noncompetitive inhibitors of Dttp; inhibits HIV entry |
EGCG:300 mg/kg/day; IC50:3.44 ± 1.07 µM GCG:2.45 ± 0.36 µM |
Yamaguchi et al. [91] Hartjen et al. [92] Rrapo et al. [93] |
| EGCG | HCV | Inhibitor of the HCV entry and viral RNA replication | IC50: 17.9 μM | Chen et al. [94] |
|
EGCG, EC ECG |
Influenza A/B | Inhibitory effects on the acidification of endosomes and lysosomes; |
EC: > 145.09 μg/mL EGC: 30.49 μg/mL EGCG: 56.49 μg/mL |
Imanishi [95] Yang et al [96] |
| EGCG | DENV, JEV and TBEV | Associated with the DENV2 E protein; destruction of the structure of ZIKV virions | > 100 μM | Carneiro et al. [97] |
| EGCG | Human T-cell Lymphotropic Virus-1 | Reduce the invasive potential of HTLV-1-positive leukemia cells; suppressing Tax expression; inhibiting the activation of NF-kB pathway and induction of MMP-9 transcription in HTLV-1 positive cells | 25 μM in HuT-102 | Harakeh et al. [98] |
|
EGCG GCC |
Rotaviruses enteroviruses | Interfering with virus adsorption; reduced reactive oxygen species (ROS) generation | GCG: 10 μM EGCG:10 μM | Ho et al. [99] |
| EGCG | Ebola virus (EBOV) | Reduced the production of new viruses via inhibiting HSPS5 | 10–100 μM | Reid et al. [100] |
Conclusion
The study of flavonoids is complex because of the heterogeneity of the different molecular structures and the scarcity of data on bioavailability. An important effect of flavonoids is the scavenging of oxygen-derived free radicals. Flavonoids possess anti-inflammatory, antiallergic, antiviral, and anticarcinogenic properties from the evidence obtained in vitro experimental systems. However, the clinical application of flavonoids is restricted by its low solubility and poor bioavailability. Although there are lots of advances in the antiviral pharmacology of natural flavonoids, further study is needed to elucidate the effects of flavonoids within the body and the degree and rate of absorption for the evaluation of druggability.
With the development of preparation techniques, newly developed flavonoids preparations exhibit better absorption and thus have higher bioavailability. Since flavonoids are frequently prescribed with other medications, understanding the compatibility of co-administrated drugs is of importance for clinical applications, and requires further research for better clarity. Furthermore, pharmacokinetic changes of flavonoids under different pathological conditions indicate clinical considerations of drug safety and the possible requirement of individualized antiviral therapy.
Acknowledgements
The work was supported by CAMS Innovation Fund for Medical Sciences (Grant No. 2017-I2M-1-010); National Key Research and Development Program (Grant No. 2018YFC0311005); National Science and Technology Major Projects (Grant No. 2018ZX09711001-012).
Compliance with Ethical Standards
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Congratulations to Academician Sun Handong for his outstanding scientific achievements and contributions. I sincerely thank him for his support and care for my work, and wish Professor Sun a happy eightieth birthday! Health and longevity!
References
- 1.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, Pizzolatti MG, Silva FR. Flavonoids: prospective drug candidates. Mini. Rev. Med. Chem. 2008;8(13):1429–1440. doi: 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]
- 2.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao L, Zu M, Wu S, Liu AL, Du GH. 3D QSAR and docking study of flavone derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Bioorg. Med. Chem. Lett. 2011;21(19):5964–5970. doi: 10.1016/j.bmcl.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales GB. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: questions, considerations and future perspectives. Proc. Nutr. Soc. 2017;76(3):175–181. doi: 10.1017/S0029665116002858. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Adv. Virol. 2017;162(9):2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Q, Zhang Y, Wang G, Hill L, Weng JK, Chen XY, Xue H, Martin C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellariabaicalensis. Sci. Adv. 2016;2(4):e1501780. doi: 10.1126/sciadv.1501780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules (Basel, Switzerland) 2019;24(2):370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murota K, Nakamura Y, Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018;82(4):600–610. doi: 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
- 11.Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radical Res. 1999;31(6):569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- 12.Huang HS, Ma CG, Chen ZW. Advances in the research on pharmacological actions of flavone compounds. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2000;25(10):589–592. [PubMed] [Google Scholar]
- 13.de Sousa LR, Wu H, Nebo L, Fernandes JB, da Silva MF, Kiefer W, Kanitz M, Bodem J, Diederich WE, Schirmeister T, Vieira PC. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015;23(3):466–470. doi: 10.1016/j.bmc.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Ozcelik B, Kartal M, Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49(4):396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 15.Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J., Jr Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97(1):41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu JF, Lima CS, Pereira CM, Bittar C, Batista MN, Nazare AC, Polaquini CR, Zothner C, Harris M, Rahal P, Regasini LO, Jardim ACG. Flavonoids from Pterogynenitens inhibit hepatitis C virus entry. Sci. Rep. 2017;7(1):16127. doi: 10.1038/s41598-017-16336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair MP, Kandaswami C, Mahajan S, Chadha KC, Chawda R, Nair H, Kumar N, Nair RE, Schwartz SA. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochem. Biophys. Acta. 2002;1593(1):29–36. doi: 10.1016/s0167-4889(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 18.Bae MJ, Shin HS, See HJ, Jung SY, Kwon DA, Shon DH. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016;6:32225. doi: 10.1038/srep32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AL, Liu B, Qin HL, Lee SM, Wang YT, Du GH. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtziarugulosa. Planta Med. 2008;74(8):847–851. doi: 10.1055/s-2008-1074558. [DOI] [PubMed] [Google Scholar]
- 20.Yonekawa M, Shimizu M, Kaneko A, Matsumura J, Takahashi H. Suppression of R5-type of HIV-1 in CD4(+) NKT cells by Vdelta1(+) T cells activated by flavonoid glycosides, hesperidin and linarin. Sci. Rep. 2019;9(1):7506. doi: 10.1038/s41598-019-40587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega JT, Serrano ML, Suarez AI, Baptista J, Pujol FH, Cavallaro LV, Campos HR, Rangel HR. Antiviral activity of flavonoids present in aerial parts of Marcetiataxifolia against hepatitis B virus, poliovirus, and herpes simplex virus in vitro. EXCLI J. 2019;18:1037–1048. doi: 10.17179/excli2019-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mekky RY, El-Ekiaby N, El Sobky SA, Elemam NM, Youness RA, El-Sayed M, Hamza MT, Esmat G, Abdelaziz AI. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Adv. Virol. 2019;164(6):1587–1595. doi: 10.1007/s00705-019-04232-x. [DOI] [PubMed] [Google Scholar]
- 23.Nzuza S, Zondi S, Owira PMO. Naringin prevents HIV-1 protease inhibitors-induced metabolic complications in vivo. PLoS ONE. 2017;12(11):e0183355. doi: 10.1371/journal.pone.0183355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patra AK, editor. Dietary phytochemicals and microbes. Dordrecht: Springer; 2012. pp. 93–126. [Google Scholar]
- 25.Li T, Liu L, Wu H, Chen S, Zhu Q, Gao H, Yu X, Wang Y, Su W, Yao X, Peng T. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuyniacordata Thunb. Antiviral Res. 2017;144:273–280. doi: 10.1016/j.antiviral.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Bus C, Kusz N, Jakab G, Senobar Tahaei SA, Zupko I, Endresz V, Bogdanov A, Burian K, Csupor-Loffler B, Hohmann J, Vasas A. Phenanthrenes from Juncuscompressus Jacq. with promising antiproliferative and anti-HSV-2 activities. Molecules. 2018;23(8):2085. doi: 10.3390/molecules23082085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou J, Chen L, Xu G, Zhang L, Zhou H, Wang H, Su Z, Ke M, Guo Q, Zhou C. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutinin-neuraminidase. Adv. Virol. 2011;156(5):793–801. doi: 10.1007/s00705-011-0917-z. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill S, Porter RK, McNamee N, Martinez VG, O'Driscoll L. 2-Deoxy-d-glucose inhibits aggressive triple-negative breast cancer cells by targeting glycolysis and the cancer stem cell phenotype. Sci. Rep. 2019;9(1):3788. doi: 10.1038/s41598-019-39789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granato M, Gilardini Montani MS, Zompetta C, Santarelli R, Gonnella R, Romeo MA, D'Orazi G, Faggioni A, Cirone M. Quercetin interrupts the positive feedback loop between STAT3 and IL-6, promotes autophagy, and reduces ROS, preventing EBV-driven B cell immortalization. Biomolecules. 2019;9(9):482. doi: 10.3390/biom9090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Li N, Niu F. Baicalein triazole prevents respiratory tract infection by RSV through suppression of oxidative damage. Microb. Pathog. 2019;131:227–233. doi: 10.1016/j.micpath.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Lipson SM, Karalis G, Karthikeyan L, Ozen FS, Gordon RE, Ponnala S, Bao J, Samarrai W, Wolfe E. Mechanism of anti-rotavirus synergistic activity by epigallocatechin gallate and a proanthocyanidin-containing nutraceutical. Food Environ. Virol. 2017;9(4):434–443. doi: 10.1007/s12560-017-9299-z. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Ye F, Sun Q, Liang H, Li C, Lu R, Huang B, Tan W, Lai L. Scutellariabaicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammadi Pour P, Fakhri S, Asgary S, Farzaei MH, Echeverria J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019;10:1207. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams J, Patel N, Mankaryous N, Tadros M, Miller CD. Nonnucleoside reverse transcriptase inhibitor resistance and the role of the second-generation agents. Ann. Pharmacother. 2010;44(1):157–165. doi: 10.1345/aph.1M359. [DOI] [PubMed] [Google Scholar]
- 35.Aarthy M, Singh SK. Discovery of potent inhibitors for the inhibition of dengue envelope protein: an in silico approach. Curr. Top. Med. Chem. 2018;18(18):1585–1602. doi: 10.2174/1568026618666181025100736. [DOI] [PubMed] [Google Scholar]
- 36.Prussia A, Thepchatri P, Snyder JP, Plemper RK. Systematic approaches towards the development of host-directed antiviral therapeutics. Int. J. Mol. Sci. 2011;12(6):4027–4052. doi: 10.3390/ijms12064027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hour MJ, Huang SH, Chang CY, Lin YK, Wang CY, Chang YS, Lin CW. Baicalein, ethyl acetate, and chloroform extracts of Scutellariabaicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza A viruses. Evid. Based Complemen. Altern. Med. 2013;2013:750803. doi: 10.1155/2013/750803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu AL, Wang HD, Lee SM, Wang YT, Du GH. Structure–activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008;16(15):7141–7147. doi: 10.1016/j.bmc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Fang JS, Lian WW, Pang XC, Liu AL, Du GH. In vitro antiviral effects and 3D QSAR study of resveratrol derivatives as potent inhibitors of influenza H1N1 neuraminidase. Chem. Biol. Drug Des. 2015;85(4):427–438. doi: 10.1111/cbdd.12425. [DOI] [PubMed] [Google Scholar]
- 40.Marunaka Y. Actions of quercetin, a flavonoid, on ion transporters: its physiological roles. Ann. N. Y. Acad. Sci. 2017;1398(1):142–151. doi: 10.1111/nyas.13361. [DOI] [PubMed] [Google Scholar]
- 41.Mello CDS, Valente LMM, Wolff T, Lima-Junior RS, Fialho LG, Marinho CF, Azeredo EL, Oliveira-Pinto LM, Pereira RCA, Siani AC, Kubelka CF. Decrease in dengue virus-2 infection and reduction of cytokine/chemokine production by Uncaria guianensis in human hepatocyte cell line Huh-7. Mem. Inst. Oswaldo Cruz. 2017;112(6):458–468. doi: 10.1590/0074-02760160323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barboza RS, Valente LMM, Wolff T, Assuncao-Miranda I, Neris RLS, Guimaraes-Andrade IP, Gomes M. Antiviral activity of Farameahyacinthina and Farameatruncata leaves on dengue virus type-2 and their major compounds. Chem. Biodivers. 2018;15(2):1700393. doi: 10.1002/cbdv.201700393. [DOI] [PubMed] [Google Scholar]
- 43.Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar P, Khanna M, Srivastava V, Tyagi YK, Raj HG, Ravi K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp. Lung Res. 2005;31(5):449–459. doi: 10.1080/019021490927088. [DOI] [PubMed] [Google Scholar]
- 45.Johari J, Kianmehr A, Mustafa MR, Abubakar S, Zandi K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012;13(12):16785–16795. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganesan S, Faris AN, Comstock AT, Wang Q, Nanua S, Hershenson MB, Sajjan US. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94(3):258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.dos Santos AE, Kuster RM, Yamamoto KA, Salles TS, Campos R, de Meneses MD, Soares MR, Ferreira D. Quercetin and quercetin 3-O-glycosides from Bauhinialongifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasites Vectors. 2014;7:130. doi: 10.1186/1756-3305-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu W, Li R, Li X, He J, Jiang S, Liu S, Yang J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8(1):6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M, Son M, Ryu E, Shin YS, Kim JG, Kang BW, Cho H, Kang H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget. 2015;6(14):12603–12624. doi: 10.18632/oncotarget.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rojas A, Del Campo JA, Clement S, Lemasson M, Garcia-Valdecasas M, Gil-Gomez A, Ranchal I, Bartosch B, Bautista JD, Rosenberg AR, Negro F, Romero-Gomez M. Effect of quercetin on hepatitis C virus life cycle: from viral to host targets. Sci. Rep. 2016;6:31777. doi: 10.1038/srep31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Zhao J, Li W, Shen L, Huang S, Tang J, Duan J, Fang F, Huang Y, Chang H, Chen Z, Zhang R. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016;6:19095. doi: 10.1038/srep19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu G, Dou J, Zhang L, Guo Q, Zhou C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol. Pharma. Bull. 2010;33(2):238–243. doi: 10.1248/bpb.33.238. [DOI] [PubMed] [Google Scholar]
- 53.Moghaddam E, Teoh BT, Sam SS, Lani R, Hassandarvish P, Chik Z, Yueh A, Abubakar S, Zandi K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang MJ, Yang CH, Jin Y, Wan CB, Qian WH, Xing F, Li X, Liu YY. Baicalin inhibits coxsackievirus B3 replication by reducing cellular lipid synthesis. Am. J. Chin. Med. 2020;48(1):143–160. doi: 10.1142/S0192415X20500081. [DOI] [PubMed] [Google Scholar]
- 55.Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994;48(3):595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 56.Wu CC, Fang CY, Cheng YJ, Hsu HY, Chou SP, Huang SY, Tsai CH, Chen JY. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017;24(1):2. doi: 10.1186/s12929-016-0313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakobyan A, Arabyan E, Avetisyan A, Abroyan L, Hakobyan L, Zakaryan H. Apigenin inhibits African swine fever virus infection in vitro. Adv. Virol. 2016;161(12):3445–3453. doi: 10.1007/s00705-016-3061-y. [DOI] [PubMed] [Google Scholar]
- 58.Shibata C, Ohno M, Otsuka M, Kishikawa T, Goto K, Muroyama R, Kato N, Yoshikawa T, Takata A, Koike K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology. 2014;462–463:42–48. doi: 10.1016/j.virol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 59.Ryu YB, Jeong HJ, Kim JH, Kim YM, Park JY, Kim D, Nguyen TT, Park SJ, Chang JS, Park KH, Rho MC, Lee WS. Biflavonoids from Torreyanucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, Kwon DH. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian S, Fan W, Qian P, Zhang D, Wei Y, Chen H, Li X. Apigenin restricts FMDV infection and inhibits viral IRES driven translational activity. Viruses. 2015;7(4):1613–1626. doi: 10.3390/v7041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kehinde I, Ramharack P, Nlooto M, Gordon M. The pharmacokinetic properties of HIV-1 protease inhibitors: a computational perspective on herbal phytochemicals. Heliyon. 2019;5(10):e02565. doi: 10.1016/j.heliyon.2019.e02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang C-W, Li H-C, Hsu C-F, Chang C-Y, Lo S-Y. Increased ATP generation in the host cell is required for efficient vaccinia virus production. J. Biomed. Sci. 2009;16(1):80–80. doi: 10.1186/1423-0127-16-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visintini Jaime MF, Redko F, Muschietti LV, Campos RH, Martino VS, Cavallaro LV. In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol. J. 2013;10(1):245. doi: 10.1186/1743-422X-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng M, Watanabe S, Chan KWK, He Q, Zhao Y, Zhang Z, Lai X, Luo D, Vasudevan SG, Li G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antiviral Res. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Wu CC, Fang CY, Hsu HY, Chen YJ, Chou SP, Huang SY, Cheng YJ, Lin SF, Chang Y, Tsai CH, Chen JY. Luteolin inhibits Epstein-Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antiviral Res. 2016;132:99–110. doi: 10.1016/j.antiviral.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Fan W, Qian S, Qian P, Li X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016;220:112–116. doi: 10.1016/j.virusres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 68.Yan H, Ma L, Wang H, Wu S, Huang H, Gu Z, Jiang J, Li Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019;73(3):487–496. doi: 10.1007/s11418-019-01287-7. [DOI] [PubMed] [Google Scholar]
- 69.Bai L, Nong Y, Shi Y, Liu M, Yan L, Shang J, Huang F, Lin Y, Tang H. Luteolin inhibits hepatitis B virus replication through extracellular signal-regulated kinase-mediated down-regulation of hepatocyte nuclear factor 4alpha expression. Mol. Pharm. 2016;13(2):568–577. doi: 10.1021/acs.molpharmaceut.5b00789. [DOI] [PubMed] [Google Scholar]
- 70.Mehla R, Bivalkar-Mehla S, Chauhan A. A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function. PLoS ONE. 2011;6(11):e27915. doi: 10.1371/journal.pone.0027915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, Zhang H, Luo H, Zhu L, Jiang P, Chen L, Shen Y, Luo M, Zuo G, Hu J, Duan D, Nie Y, Shi X, Wang W, Han Y, Li T, Liu Y, Ding M, Deng H, Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theoharides TC. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin, BioFactors. Oxford: England; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dai W, Bi J, Li F, Wang S, Huang X, Meng X, Sun B, Wang D, Kong W, Jiang C, Su W. Antiviral efficacy of flavonoids against enterovirus 71 infection in vitro and in newborn mice. Viruses. 2019;11(7):625. doi: 10.3390/v11070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Enkhtaivan G, Maria John KM, Pandurangan M, Hur JH, Leutou AS, Kim DH. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J. Biol. Sci. 2017;24(7):1646–1656. doi: 10.1016/j.sjbs.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sochocka M, Sobczynski M, Ochnik M, Zwolinska K, Leszek J. Hampering herpesviruses HHV-1 and HHV-2 infection by extract of Ginkgobiloba (EGb) and its phytochemical constituents. Front. Microbiol. 2019;10:2367. doi: 10.3389/fmicb.2019.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhargava S, Patel T, Gaikwad R, Patil UK, Gayen S. Identification of structural requirements and prediction of inhibitory activity of natural flavonoids against Zika virus through molecular docking and Monte Carlo based QSAR simulation. Nat. Prod. Res. 2019;33(6):851–857. doi: 10.1080/14786419.2017.1413574. [DOI] [PubMed] [Google Scholar]
- 77.Qian K, Gao AJ, Zhu MY, Shao HX, Jin WJ, Ye JQ, Qin AJ. Genistein inhibits the replication of avian leucosis virus subgroup J in DF-1 cells. Virus Res. 2014;192:114–120. doi: 10.1016/j.virusres.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Smith BN, Morris A, Oelschlager ML, Connor J, Dilger RN. Effects of dietary soy isoflavones and soy protein source on response of weanling pigs to porcine reproductive and respiratory syndrome viral infection. J. Anim. Sci. 2019;97(7):2989–3006. doi: 10.1093/jas/skz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arabyan E, Hakobyan A, Kotsinyan A, Karalyan Z, Arakelov V, Arakelov G, Nazaryan K, Simonyan A, Aroutiounian R, Ferreira F, Zakaryan H. Genistein inhibits African swine fever virus replication in vitro by disrupting viral DNA synthesis. Antiviral Res. 2018;156:128–137. doi: 10.1016/j.antiviral.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang H, Liao D, Liang L, Song L, Zhao W. Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected Caco-2 cells. Adv. Virol. 2015;160(6):1421–1433. doi: 10.1007/s00705-015-2404-4. [DOI] [PubMed] [Google Scholar]
- 81.Argenta DF, Silva IT, Bassani VL, Koester LS, Teixeira HF, Simoes CM. Antiherpes evaluation of soybean isoflavonoids. Adv. Virol. 2015;160(9):2335–2342. doi: 10.1007/s00705-015-2514-z. [DOI] [PubMed] [Google Scholar]
- 82.Bedard KM, Wang ML, Proll SC, Loo YM, Katze MG, Gale M, Jr, Iadonato SP. Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J. Virol. 2012;86(13):7334–7344. doi: 10.1128/JVI.06867-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nukui M, O'Connor CM, Murphy EA. The natural flavonoid compound deguelin inhibits HCMV lytic replication within fibroblasts. Viruses. 2018;10(11):614. doi: 10.3390/v10110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mediouni S, Jablonski JA, Tsuda S, Richard A, Kessing C, Andrade MV, Biswas A, Even Y, Tellinghuisen T, Choe H, Cameron M, Stevenson M, Valente ST. Potent suppression of HIV-1 cell attachment by Kudzu root extract. Retrovirology. 2018;15(1):64. doi: 10.1186/s12977-018-0446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong L, Hu J, Shu W, Gao B, Xiong S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015;6(5):e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC, Jr, Hillier S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008;52(3):962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J. Virol. 2014;88(14):7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003;58(2):167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 89.Liu S, Li H, Chen L, Yang L, Li L, Tao Y, Li W, Li Z, Liu H, Tang M, Bode AM, Dong Z, Cao Y. (−)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis. 2013;34(3):627–637. doi: 10.1093/carcin/bgs364. [DOI] [PubMed] [Google Scholar]
- 90.Liu S, Li H, Chen L, Yang L, Li L, Tao Y, Li W, Li Z, Liu H, Tang M, Bode AM, Dong Z, Cao Y. (−)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis. 2012;34(3):627–637. doi: 10.1093/carcin/bgs364. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002;53(1):19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 92.Hartjen P, Frerk S, Hauber I, Matzat V, Thomssen A, Holstermann B, Hohenberg H, Schulze W, Schulze Zur Wiesch J, van Lunzen J. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res. Ther. 2012;9(1):2. doi: 10.1186/1742-6405-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rrapo E, Zhu Y, Tian J, Hou H, Smith A, Fernandez F, Tan J, Giunta B. Green Tea-EGCG reduces GFAP associated neuronal loss in HIV-1 Tat transgenic mice. Am. J. Transl. Res. 2009;1(1):72–79. [PMC free article] [PubMed] [Google Scholar]
- 94.Chen C, Qiu H, Gong J, Liu Q, Xiao H, Chen XW, Sun BL, Yang RG. (−)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Adv. Virol. 2012;157(7):1301–1312. doi: 10.1007/s00705-012-1304-0. [DOI] [PubMed] [Google Scholar]
- 95.Roomi MW, Jariwalla RJ, Kalinovsky T, Roomi N, Niedzwiecki A, Rath M. Inhibition of cellular invasive parameters in influenza A virus-infected MDCK and Vero cells by a nutrient mixture. BioFactors (Oxford, England) 2008;33(1):61–75. doi: 10.1002/biof.5520330106. [DOI] [PubMed] [Google Scholar]
- 96.Yang ZF, Bai LP, Huang WB, Li XZ, Zhao SS, Zhong NS, Jiang ZH. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia. 2014;93:47–53. doi: 10.1016/j.fitote.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Harakeh S, Diab-Assaf M, Azar R, Hassan HM, Tayeb S, Abou-El-Ardat K, Damanhouri GA, Qadri I, Abuzenadah A, Chaudhary A, Kumosani T, Niedzwiecki A, Rath M, Yacoub H, Azhar E, Barbour E. Epigallocatechin-3-gallate inhibits tax-dependent activation of nuclear factor kappa B and of matrix metalloproteinase 9 in human T-cell lymphotropic virus-1 positive leukemia cells. Asian Pac. J. Cancer Prev. 2014;15(3):1219–1225. doi: 10.7314/apjcp.2014.15.3.1219. [DOI] [PubMed] [Google Scholar]
- 99.Ho HY, Cheng ML, Weng SF, Leu YL, Chiu DT. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009;57(14):6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- 100.Reid SP, Shurtleff AC, Costantino JA, Tritsch SR, Retterer C, Spurgers KB, Bavari S. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res. 2014;109:171–174. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]