Abstract
Introduction & Aim
Much is known about alcoholic hepatitis (AH) that is severe enough to require hospitalization. The characteristics of individuals with alcoholic hepatitis presenting with mild to moderate severity are not well understood. In this study we investigated the risk factors, characteristics, and outcomes of mild to moderate AH.
Methods
Two hundred and fifty five Individuals with AH enrolled into a multicenter, prospective, observational study between 12/2014 and 4/2018 were included. Participants were seen at enrollment, 6 months, and 12 months. Mild to moderate AH (M-AH) was defined as MELD 20 at presentation whereas severe AH as MELD ≥ 21.
Results
One hundred individuals had M-AH whereas 155 had severe AH. Individuals with M-AH were older (49 vs 44 years, p=0.01), had lower BMI (27 vs 31 kg/m2, p=0.0007) and more likely to be male (68% vs 55%, p=0.046) compared to severe AH group. A higher proportion in M-AH group consumed coffee in the last 5 years compared to the severe AH (29% vs 18%, p=0.03), and fewer had PNPLA3 risk allele G (p=0.019) compared to the severe AH group. Average drinks per drinking day (12.9 vs 10.7, p=0.13) and total number of drinks in last 30 day period (331 vs 280, p=0.14) were not different between two groups. Compared to severe AH, patients with M-AH had significantly lower mortality at 30 days (2% vs. 13.6%), 90 days (3% vs. 22.6%), and 12 months (10.4% vs. 31.4%) (p <0.001 for all).
Conclusions
Individuals with mild to moderate AH were older, less obese, drank coffee more often and carried more favorable PNPLA3 genotype compared to severe AH, despite similar alcohol consumption. Mild to moderate AH had substantial mortality with one in ten dying by 12 months. (Word Count 286)
Keywords: Alcoholic hepatitis, PNPLA3, Coffee drinking, MELD
Introduction
Alcoholic liver disease (ALD) represents a clinical and histopathological spectrum ranging from simple steatosis, alcoholic steatohepatitis, and cirrhosis (Sozio and Crabb, 2008) Alcoholic hepatitis (AH), the most severe clinical manifestation of ALD among excessive drinkers, is characterized by sudden onset of jaundice and liver-related complications. It is associated with a high short term mortality ranging 15% to 35% within the first month after the diagnosis (Liangpunsakul, 2011; Maddrey et al., 1978; Orntoft et al., 2014) However, majority of these estimates have come from hospitalized patients with severe AH who are participating in clinical trials. There are many patients with less severe AH who are not hospitalized and their clinical characteristics and outcomes are not well understood.
Model for End Stage Liver Disease (MELD) score, initially derived from a study to determine predictors of survival in a cohort of patients who underwent transjugular intrahepatic portosystemic shunt (TIPS), has been shown to be relevant in predicting 30- and 90-day mortality in patients with AH with similar sensitivity and specificity as Maddrey Discriminant function. (Dunn et al., 2005; Malinchoc et al., 2000) MELD score greater than 20 in AH patients is associated with a 90-day mortality rate of more than 20%. Therefore, patients with AH whose MELD > 21 are generally considered to have severe AH. (Dunn et al., 2005) (Dunn et al., 2005) Hence this serves as a good cut-off to categorize patients into those who present with severe and the rest who present with mild to moderate AH. Knowing the characteristics of individuals who present with mild-moderate AH would be instructive in understanding risk factors for severe presentation.
We have shown previously that PNPLA3 G allele among heavy drinkers was associated with alcoholic hepatitis. (Liangpunsakul et al., 2016) We also saw a lower prevalence of regular coffee consumption among heavy alcohol drinkers who develop AH. (Lianpunsakul, 2017)
We wanted to determine the clinical features, environmental risk factors such as coffee consumption and genetic risk factors such as PNPLA3 of individuals with AH who present with mild to moderate AH (M-AH) compared to severe AH. In this study, we report the risk factors, clinical characteristics and outcomes of patients presenting with mild to moderate AH (M-AH) who are enrolled into a prospective study and followed for twelve months following their enrollment.
Methods
The study consisted of individuals who were enrolled into TREAT 001 (NCT 02172898) study between 12/2014 and 04/2018. TREAT 001 was a multicenter, prospective, observational study of individuals with AH and heavy drinking controls. The design of this study has been previously described in detail (Liangpunsakul et al., 2016), but in brief, individuals with AH and heavy drinking controls without liver disease meeting predefined eligibility criteria were enrolled at three large liver centers in the United States and were followed for 12 months. This study was approved by the Institutional Review Boards at each of the participating centers and all participants signed an informed consent. Individuals who are included in this analysis have been part of several other publications by our group.
Demographic data, past medical history, clinical characteristics, questionnaires and blood samples were collected at the time of enrollment. Alcohol Use Disorders Identification Test (AUDIT) and the Time Line Follow-Back (TLFB) were used to characterize the alcohol consumption data. Coffee questionnaire captured duration and quantity per day of coffee and tea consumption. PNPLA3 genotype was determined according to methods previously described. The study protocol included an enrollment visit and visits at 6 and 12 months. Death was ascertained by direct family contact and in case of individuals who were lost to follow up it was through the National Death Registry. All subjects with a MELD score ≤ 20 at presentation were defined as having mild to moderate AH (M-AH) and those with MELD ≥ 21 was defined as severe AH.
Statistical analysis
Pearson’s chi-square test and two-sample t-tests were employed to determine the differences of the categorical and continuous variables, respectively, between patients with moderate and severe AH. When sample sizes were small, Fisher’s exact test was applied instead of Pearson’s chi-square test. Survival analysis between patients with moderate and severe AH was estimated using the Kaplan-Meier method, and the differences were compared using the log-rank test. A p-value <0.05 was considered statistically significant.
Results
During the study period, 255 individuals with AH were enrolled, of whom 100 had M-AH and 155 had severe AH. Baseline demographics and selected clinical characteristics of two groups are shown in Table 1. Among individuals with M-AH, the MELD score was between 11 and 20 in 93, 10 in 3, and 9 in 4 participants. Individuals with M-AH were older (49 vs 44 years, p=0.01) and were more likely to be male (68% vs 55%, p=0.046) when compared to those with severe AH. They also had a significantly lower BMI compared to those with severe AH (27 vs 31 kg/m2, p < 0.05). Although patients with M-AH had higher levels of ALT (82 vs 48 U/L, p<0.0001) and AST (163 vs 126 U/L, p=0.001), they demonstrated less severe liver synthetic dysfunction, as reflected in higher serum albumin (3.0 vs 2.7 gm/dL, p < 0.05), lower total bilirubin (6 vs 15 mg/dL, p < 0.0001) and lower international normalized ratio (1.4 vs 2.1, p < 0.0001).
Table 1:
Selected Demographic and Laboratory Characteristics of Individuals With Mild to Moderate and Severe AH¶
| Mild to Moderate AH (n=1100) | Severe AH (n=155) | P-value | |
|---|---|---|---|
| Age (years) | 49.0 ± 10.4 | 44.5 ± 10.9 | 0.001 |
| Male (%) | 68 | 55.5 | 0.046 |
| White (%) | 83 | 89 | 0.16 |
| PTSD (%) | 37 | 32 | 0.55 |
| BMI (Kg/m2) | 27.5 ± 6.0 | 30.8 ± 8.2 | 0.0007 |
| WBC (x 103/mm3) | 8.4 ± 5.4 | 13.5 ± 8.4 | <.0001 |
| Hemoglobin (gm/dl) | 10.3 ± 2.0 | 10.0 ± 2.0 | 0.17 |
| Platelet (x 103/mm3) | 131.9 ± 82.5 | 147.2 ± 83.1 | 0.15 |
| Total Bilirubin (mg/dl) | 5.7 ± 4.4 | 19.5 ± 10.9 | <.0001 |
| INR | 1.4 ± 0.3 | 2.1 ± 0.6 | <.0001 |
| AST (U/L) | 162.9 ± 110.5 | 126.5 ± 65.6 | 0.0011 |
| ALT (U/L) | 81.8 ± 82.5 | 48.3 ± 35.8 | <.0001 |
| Albumin (g/dl) | 3.0 ± 0.8 | 2.7 ± 0.6 | 0.009 |
| Creatinine (mg/dl) | 0.7 ± 0.2 | 1.3 ± 1.4 | <.0001 |
| MELD score | 16.1 ± 3.1 | 27.2 ± 5.8 | <.0001 |
Values are shown as means ± standard deviation unless otherwise specified
Abbreviations; BMI: Body Mass Iindex; WBC: White Blood Cells; INR; International Normalized Ratio; MELD score: Model for End Stage Liver Disease.
A higher proportion of individuals with M-AH were coffee drinkers in last five years compared to those with severe AH (29% vs 18%, p=0.03. (Table 2) There was no significant difference in average drinks per drinking day (12.9 vs 10.7, p=0.13) or total number of drinks in the last 30 days (331 vs 280, p=0.14) between two groups. (Table 2)
Table 2:
Coffee and Alcohol Consumption of Individuals with Mild to Moderate and Severe AH¶
| Mild to Moderate AH (n=100) | Severe AH (n=155) | P-value | |
|---|---|---|---|
| Years of alcohol drinking | 25.6 ± 14.5 | 25.1 ± 10.6 | 0.87 |
| Average alcohol Drinks per Drinking Day | 12.9 ± 11.8 | 10.7 ± 8.0 | 0.13 |
| Alcohol drinks per last 30 day period | 331.5 ± 273.3 | 280.1 ± 209.6 | 0.15 |
| Average alcohol consumed per drinking day (Grams) | 193.2 ± 177.0 | 160.5 ± 119.9 | 0.135 |
| Coffee drinking (%) | 29 | 18 | 0.035 |
| Years of drinking coffee? (if ≥ 5 years) | 25.6 ± 14.5 | 25.1 ± 10.6 | 0.8748 |
| Cups of caffeinated coffee per day (if ≥ 5 years) | 4.6 ± 8.1 | 2.3 ± 1.9 | 0.1439 |
Values are shown as means ± standard deviation unless otherwise specified
Fewer individuals with M-AH AH carried the PNPLA3 genotypes with high risk G allele (GG and GC) compared to those with severe AH. The frequency of PNPLA3 CC, CG, and GG genotypes in M-AH were 55%, 38%, and 7% respectively and it was significantly different from severe AH group (CC: 34%, CG: 52%, and GG: 13%, p=0.02).
There was a significant interaction between coffee drinking and the PNPLA3 genotype (p=0.0037). (Table 3) Among those carrying high risk PNPLA3 G allele, fewer proportion of coffee consumers presented with severe AH compared to non-consumers of coffee. (52.2% vs 74% respectively) Even among those with favorable PNPLA3 genotype profile (CC), fewer proportion of coffee consumers presented with severe AH compared to non-consumers of coffee. (33.3% vs 53.6%) (Table 3).
TABLE 3:
The relationship between PNPLA3 genotypes and coffee consumption and the severity of AH (N=174)
| Mild to moderate AH (n=69) | Severe AH (n=105) | P-value | |
|---|---|---|---|
| PNPLA3 CC genotype + Coffee drinking | 12 (66.7%) | 6 (33.3%) | 0.0037 |
| PNPLA3 CC genotype + No coffee drinking | 26 (46.4%) | 30 (53.6%) | |
| PNPLA3GG/GC genotype + Coffee drinking | 11 (47.8%) | 12 (52.2%) | |
| PNPLA3GG/GC genotype + No coffee drinking | 20 (26.0%) | 57 (74.0%) |
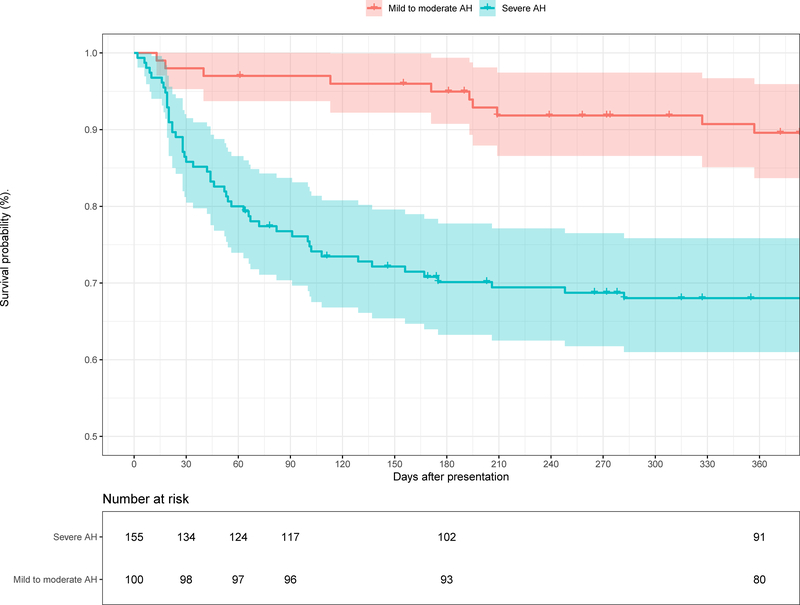
Compared to individuals with severe AH, patients with M-AH had significantly lower mortality at 30 days (2% vs. 13.6%, p<0.001), 90 days (3% vs. 22.6%, p<0.001), and 12 months (10.4% vs. 31.4%, p<0.001) (p <0.001 by Log-rank test). (Figure 1) The causes of death for individuals with M-AH were complications of end stage liver disease in 5, unknown causes in 2, complications from accidental falls in 2, clostridium difficile colitis in 1.
Figure 1.
Kaplan-Meier survival curves of Individuals with Mild to Moderate and Severe Alcoholic Hepatitis. The number of participants available at each time point is shown under the X-axis.
Discussion
Our study shows that a substantial proportion of AH patients (40%) seeking medical attention have mild to moderate AH, as defined by a MELD score 20. A majority of them with M-AH had MELD scores between 11 and 20 with a small minority presenting with MELD less than 11. Individuals with M-AH in our study were found to have lower BMI and fewer females compared to those with severe AH. While excess body weight and female sex were previously shown to be associated with an increased risk for AH (Liangpunsakul et al., 2016), our study is the first to observe that female sex and overweight are associated with severity of AH as well.
Lack of significant relationship between amount of alcohol consumed or duration of alcohol consumption by heavy drinkers and the disease severity is interesting. These results are consistent with our previous observation that the degree of alcohol consumption was not associated with the risk for AH among heavy drinkers.(Liangpunsakul et al., 2016) This suggests that once the level and duration of alcohol consumption reach a threshold it is the demographic, genetic, and environmental factors, which determine the risk for developing AH as well as its severity at presentation.
In addition to the demographic differences, we observed significant differences in environmental risk factors. Higher proportion of individuals with M-AH consumed coffee in the last five years compared to those with severe AH. This very interesting finding corroborates previous observations of coffee consumption as a protective risk modifier in development of hepatic fibrosis after adjusting for age, sex, BMI and liver disease diagnosis and alcohol intake. (Liu et al., 2015; Molloy et al., 2012)
We and others have previously shown that PNPLA3 G allele is associated with alcoholic cirrhosis and alcoholic hepatitis. (10, 11) Our data suggests that PNPLA3 G allele may also be associated with severity of AH. We have previously shown an interaction between PNPLA3 genotype and coffee drinking and the risk for AH among heavy drinkers. (Liangpunsakul, 2017) The current study suggests a similar interaction between coffee drinking and PNPLA3 genotype in modifying the AH severity. Coffee consumption in high risk PNPLA3 allele carriers, reduced the proportion of individuals presenting with severe AH to that seen in low risk PNPLA3 allele carriers who were not coffee consumers. Coffee consumption among the low risk PNPLA3 allele carriers, drove this proportion further down.
Although individuals with moderate AH fared better than those with severe AH, their mortality was still substantial with one year mortality of 10.4%. Another important observation is that a half of deceased individuals with mild to moderate AH died from reasons other than liver failure such as accidental falls (in 20%), infection (10%) and unknown (20%). Different strategies will likely be needed to prevent these non-hepatic causes of death in this patient population.
In summary, our data shows that in a population of AH patients, close to 40% present with M-AH. These patients are older, less likely to be female and have lower BMI compared to patients presenting with severe AH. Although their level of alcohol consumption was similar, patients with M-AH more commonly report coffee consumption and have lower frequency of carrying high risk PNPLA3 G allele compared to severe AH, suggesting the role of genetic and environmental factors in modifying disease severity. Their mortality is substantial with 1-year mortality of 10.4%, with half of deaths due to non-liver related causes.
Acknowledgments
Source of Funding: The Translational Research and Evolving Alcoholic Hepatitis Treatment (TREAT) Consortium was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (grants 5U01AA021883–04, 5U01AA021891–04, 5U01AA021788–04, 5U01AA021840–04).
Roles of authors
All authors have read and approved the manuscript for submission. All have made a substantial contribution to the conception, design, gathering, analysis and/or interpretation of data and a contribution to the writing and intellectual content of the article; and acknowledge that they have exercised due care in ensuring the integrity of the work.
Disclosures: Drs. Chalasani and Sanyal have number of consulting agreements and research grants from pharmaceutical companies but none are deemed relevant for this paper. Dr. Shah has research grant support from Generon, but is not relevant for this paper. Other authors declare no conflicts of interest.
Footnotes
Members of the TREAT Consortium
TREAT Consortium: The consortium was established with the funding from the NIH/NIAAA to study the pathogenesis and new treatments for AH. It consists of three academic centers in the US: Indiana University (Indianapolis, IN), Mayo Clinic (Rochester, MN), and Virginia Commonwealth University (Richmond, VA).
Indiana University, Indianapolis, IN: David Crabb, MD, Naga Chalasani, MD, Suthat Liangpunsakul, MD, Barry Katz, PhD, Spencer Lourens, PhD, Andy Borst, BS, Ryan Cook, MPH, Andy Qigui Yu, PhD, David Nelson, PhD, Romil Saxena, MD, Jennifer Lehman, RN, Kayla Gelow, BS.
Mayo Clinic, Rochester, MN: Vijay Shah, MD, Gregory Gores, MD, Patrick Kamath, MD, Vikas Verma, PhD, Sarah Wilder, RN, BSN, Amy Olofson, RN, Amanda Schimek
Virginia Commonwealth University, Richmond, VA: Arun Sanyal, MD, Puneet Puri, MD, Susan Walker, RN, MSN
The National Institute of Alcohol Abuse and Alcoholism (NIAAA): Scientific/Program Collaborator: Svetlana Radaeva, PhD.
References
- Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KVN, Malinchoc M, Kamath PS, Shah V, 2005. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 41, 353–358. 10.1002/hep.20503 [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, 2017. The interaction between PNPLA3 genotype and coffee drinking and the risk for acute alcoholic hepatitis. [DOI] [PMC free article] [PubMed]
- Liangpunsakul S, 2011. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J. Clin. Gastroenterol 45, 714–719. 10.1097/MCG.0b013e3181fdef1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S, Puri P, Shah VH, Kamath P, Sanyal A, Urban T, Ren X, Katz B, Radaeva S, Chalasani N, Crabb DW, Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium, 2016. Effects of Age, Sex, Body Weight, and Quantity of Alcohol Consumption on Occurrence and Severity of Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol 14, 1831–1838.e3. 10.1016/j.cgh.2016.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang X, Wu G, Chen L, Hu P, Ren H, Hu H, 2015. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLOS ONE 10, e0142457 10.1371/journal.pone.0142457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI, 1978. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 75, 193–199. [PubMed] [Google Scholar]
- Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC, 2000. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31, 864–871. 10.1053/he.2000.5852 [DOI] [PubMed] [Google Scholar]
- Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA, 2012. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 55, 429–436. 10.1002/hep.24731 [DOI] [PubMed] [Google Scholar]
- Orntoft NW, Sandahl TD, Jepsen P, Vilstrup H, 2014. Short-term and long-term causes of death in patients with alcoholic hepatitis in Denmark. Clin. Gastroenterol. Hepatol 12, 1739–1744.e1. 10.1016/j.cgh.2014.04.020 [DOI] [PubMed] [Google Scholar]
- Sozio M, Crabb DW, 2008. Alcohol and lipid metabolism. Am. J. Physiol. Endocrinol. Metab 295, E10–16. 10.1152/ajpendo.00011.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]