Abstract
Background
CALM was a randomized phase 3 trial in patients with Crohn’s disease (CD) that demonstrated improved endoscopic outcomes when treatment was escalated based on cutoffs for inflammatory biomarkers, fecal calprotectin (FC), C-reactive protein (CRP), and CD Activity Index (CDAI) remission vs CDAI response alone. The purpose of this post hoc analysis of CALM was to identify drivers of treatment escalation and evaluate the association between biomarker cutoff concentrations and endoscopic end points.
Methods
The proportion of patients achieving CD Endoscopic Index of Severity (CDEIS) <4 and no deep ulcers 48 weeks after randomization was evaluated according to CRP <5 mg/L or ≥5 mg/L and FC <250 μg/g or ≥250 μg/g. Subgroup analyses were performed according to disease location, and sensitivity analyses were conducted in patients with elevated CRP and/or FC at baseline. The association between endoscopic end points and biomarker cutoffs was performed using χ 2 test.
Results
The proportion of patients who achieved the primary end point CDEIS <4 and no deep ulcers was significantly greater for those with FC <250 µg/g (74%; P < 0.001), with an additive effect for CRP <5 mg/L. The association of FC <250 µg/g with improved endoscopic outcomes was independent of disease location, although the greatest association was observed for ileocolonic disease. Fecal calprotectin <250 µg/g, CRP <5 mg/L, and CDAI <150 gave a sensitivity/specificity of 72%/63% and positive/negative predictive values of 86%/42% for CDEIS <4 and no deep ulcers 48 weeks after randomization.
Conclusion
This post hoc analysis of CALM demonstrated that a cutoff of FC <250 µg/g is a useful surrogate marker for mucosal healing in CD.
Keywords: biologics, Crohn’s disease, clinical pharmacology, inflammatory bowel disease
A post hoc analysis of a phase 3 trial in Crohn’s disease showed that a fecal calprotectin cutoff of <250 μg/g accurately classified mucosal healing at week 48 after randomization in a majority of patients.
INTRODUCTION
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by chronic inflammation of the gastrointestinal tract.1 Clinical symptoms such as abdominal pain, diarrhea, and fatigue contribute to low health-related quality of life, but destructive chronic inflammation can lead to irreversible intestinal damage such as fistulas or strictures that require surgical resection.1–4 Traditionally, the treatment goal for CD has been reduction of clinical symptoms, but increasing evidence has shown that clinical symptoms may not correlate with endoscopic activity and are therefore not reflective of underlying inflammation.5–7
Management of CD has moved toward the therapeutic goal of mucosal healing using objective biomarkers as surrogates of inflammatory activity.8 High levels of C-reactive protein (CRP) were shown to be associated with increased response to treatment with anti-tumor necrosis factor (TNF) antibodies in patients with CD.9 However, serologic markers are less sensitive and specific for intestinal inflammation than fecal markers such as lactoferrin or fecal calprotectin (FC).10, 11 Several studies have demonstrated a correlation of FC with endoscopic disease activity; however, these studies used different cutoff values of FC concentration, with a wide range of reported specificities for CD (approximately 50%–100%), although all reported relatively high sensitivity (>70%) for CD.5, 12–15
The CALM study was a randomized phase 3 trial in patients with CD that evaluated 2 treatment algorithms—one based on a “tight control” (TC) algorithm that monitored biomarkers (CRP and FC), symptoms (Crohn’s Disease Activity Index [CDAI]), and prednisone use, and the other based on clinical management (CM) that monitored only symptoms and prednisone use.16 The CALM study demonstrated that patients whose treatment was escalated based on biomarkers, symptoms, and prednisone use achieved improved clinical and endoscopic outcomes compared with those whose treatment was escalated based on symptoms and prednisone use alone.16 However, the relationship between biomarker cutoff levels and mucosal improvements has not been fully established. The purpose of this post hoc analysis was to demonstrate the association of the normalization CRP and FC with mucosal healing using data from CALM.
MATERIALS AND METHODS
Study Design and Patients
Details of the CALM study (NCT01235689) were reported previously.16 CALM was a multicenter, randomized, open-label, active-controlled, 48-week phase 3 trial to assess TC versus CM algorithms in adult patients with moderate to severe CD.16 After ≤8 weeks of prednisone induction therapy and mandated taper, patients were randomly assigned to the TC or CM groups in a 1:1 ratio, stratified by smoking status, weight, and disease duration. If failure criteria (Supplementary Table 1) were met at scheduled study visits, treatment was escalated stepwise from no treatment to adalimumab 160 mg at week 0, 80 mg at week 2, followed by 40 mg every other week, to adalimumab 40 mg every week, to adalimumab 40 mg every week plus 2.5 mg/kg azathioprine per day. Patients who did not meet a failure criterion remained on the same treatment option. Starting at weeks 23 and 35 after randomization, patients receiving weekly adalimumab could de-escalate to the previous treatment option if failure criteria were not met. Ileocolonoscopies were performed at study screening and at 48 weeks after randomization and were locally read for Crohn’s Disease Endoscopic Index of Severity (CDEIS) by site readers trained to assess endoscopies in a standardized manner. Fecal calprotectin concentrations were measured using a Calprotectin PhiCal enzyme-linked immunosorbent assay test (Genova Diagnostics, Asheville, NC, USA); CRP concentrations were measured using a particle-enhanced immunoturbidimetric assay and a turbidimetric/immunoturbidimetric assay (Genova Diagnostics).16 The primary end point in CALM was CDEIS <4 and no deep ulcers 48 weeks after randomization; endoscopic response was defined as CDEIS decrease >5 from baseline.
Data Analysis
Assessment of failure criteria
Patients randomized to the TC group (n = 122) were assessed for failure criteria 1 week before randomization (week −1), and at 11, 23, and 35 weeks after randomization. Failure criteria were assessed at each time point in patients who changed treatment option and were summarized as observed.
Association of biomarkers with endoscopic end points
The proportion of patients achieving the primary end point in CALM and endoscopic response were evaluated according to the biomarker cutoffs used in the study. Levels of FC were only quantitated if below 250 μg/g. Therefore, specific values of FC >250 μg/g were not available for analysis. Data were analyzed for all patients regardless of randomized group. Subgroup analyses by disease location at baseline and sensitivity analyses in patients with elevated CRP (≥5 mg/L) at baseline, elevated FC (≥250 μg/g) at baseline, and both CRP and FC elevated at baseline were performed.
Statistical analysis
The association between the endoscopic end points and biomarker cutoffs at 48 weeks after randomization was analyzed using the χ 2 test or Fisher exact test if ≥20% of the cells had expected cell count <5. Data were summarized as observed in patients who had both endoscopy and biomarker information at 48 weeks after randomization. Univariate logistic regression analyses were used to assess the associations between stool concentrations of FC and serum concentrations of CRP as predictive biomarkers measured at 11, 23, or 35 weeks after randomization in the treatment period, and to determine the odds ratio of achieving CDEIS <4 and no deep ulcers 48 weeks after randomization. Multivariate logistic regression analyses were used to assess CRP and CDAI in addition to FC as predictors of achieving CDEIS <4 and no deep ulcers at weeks 11, 23, and 35. Absolute values for CRP, CDAI, and FC were used for logistic regression analyses. R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for the regression analyses.
Ethical Considerations
The CALM study was conducted under a protocol approved by relevant ethics committees and institutional review boards, in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable local regulations.16
RESULTS
Study Population
A total of 244 patients were randomized in the CALM trial (CM, n = 122; TC, n = 122).16 The numbers of patients with available CRP, FC, and endoscopy data for the present analyses are shown in Supplementary Fig. 1. From week −1 to week 35, the proportion of patients who did not meet any failure criteria increased from approximately 10% to 80% (Supplementary Fig. 2A). At weeks 11 and 23 after randomization, treatment escalation decisions were primarily driven by the FC criterion, followed by CRP and CDAI criteria (Supplementary Fig. 2B). When multiple failure criteria were met, the most frequent reasons to escalate treatment included FC, CRP, or both (Supplementary Table 2).
Association of Biomarkers and Endoscopic Outcomes at Week 48 After Randomization
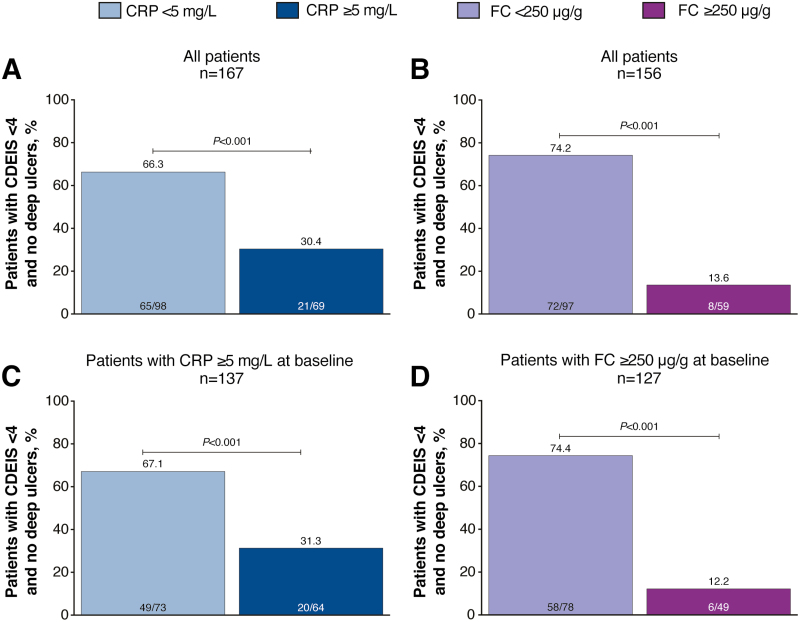
Because FC and CRP were the most common drivers of treatment escalation for patients in the TC group, we evaluated the association between endoscopic outcomes and FC and CRP cutoffs at week 48 after randomization for all patients in the TC and CM groups who completed the study and had available FC, CRP, and endoscopy data (n = 167). A significantly greater proportion of patients with CRP <5 mg/L at week 48 after randomization achieved the primary end point of mucosal healing (CDEIS <4) and no deep ulcers than those with CRP ≥5 mg/L at week 48 (66% vs 30%; P < 0.001; Fig. 1A). The odds ratio for achieving the primary end point was 4.5 (95% confidence interval [CI], 2.3–8.7) with CRP <5 mg/L. A significantly greater proportion of patients with FC <250 µg/g at week 48 after randomization achieved CDEIS <4 and no deep ulcers than those with FC ≥250 µg/g at week 48 (74% vs 14%; P < 0.001; Fig. 1B). The odds ratio for achieving the primary end point was 18.4 (95% CI, 7.7–44.0) with FC <250 µg/g. Results for patients with CRP ≥5 mg/L at baseline or FC ≥250 µg/g at baseline were similar to those reported for all patients (Fig. 1C, D).
FIGURE 1.
Proportion of patients achieving mucosal healing (CDEIS <4) and no deep ulcers in (A) all patients by CRP cutoff at week 48 after randomization, (B) all patients by FC cutoff at week 48 after randomization, (C) patients with CRP ≥5 mg/L at baseline, and (D) patients with FC ≥250 µg/g at baseline. P values were calculated using the χ 2 test or Fisher exact test if ≥20% of the cells had expected cell count <5.
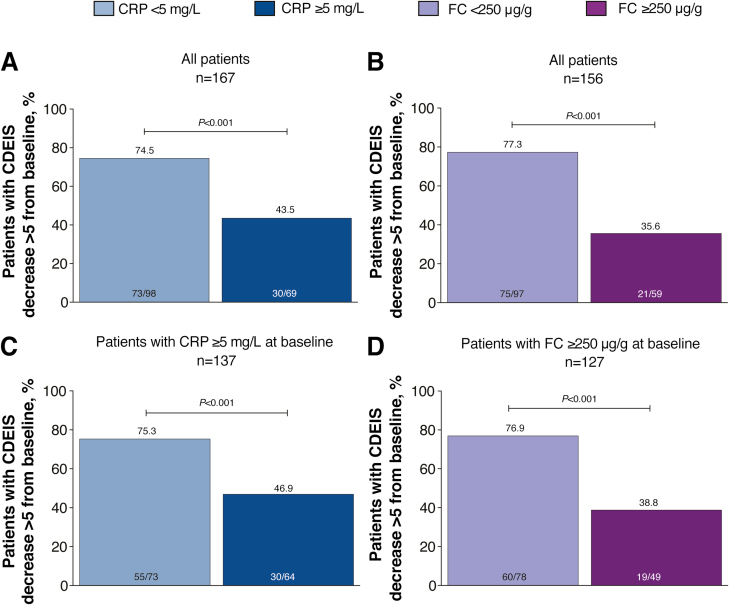
The association of endoscopic response (CDEIS decrease >5 points from baseline) with CRP or FC cutoffs was similar to that observed for the primary end point. More patients with CRP <5 mg/L at week 48 after randomization achieved endoscopic response compared with those with CRP ≥5 mg/L at week 48 (75% vs 44%; P < 0.001; Fig. 2A). The odds ratios for achieving endoscopic response were 3.8 (95% CI, 2.0–7.3) with CRP <5 mg/L and 6.2 (95% CI, 3.0–12.6) with FC <250 µg/g. A significantly greater proportion of patients with FC <250 µg/g at week 48 after randomization achieved endoscopic response than those with FC ≥250 µg/g at week 48 (77% vs 36%; P < 0.001; Fig. 2B). As observed for the primary end point, results for endoscopic response for patients with CRP ≥5 mg/L at baseline or FC ≥250 µg/g at baseline were similar to those reported for all patients (Fig. 2C, D).
FIGURE 2.
Proportion of patients achieving endoscopic response (CDEIS decrease >5 from baseline) in (A) all patients by CRP cutoff at week 48 after randomization, (B) all patients by FC cutoff at week 48 after randomization, (C) patients with CRP ≥5 mg/L at baseline, and (D) patients with FC ≥250 µg/g at baseline. P values were calculated using the χ 2 test or Fisher exact test if ≥20% of the cells had expected cell count <5.
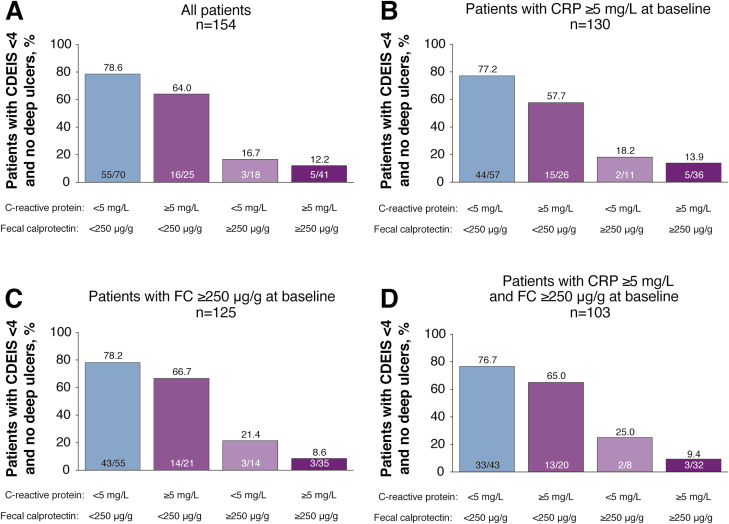
When analyzing the association of endoscopic outcomes with the combination of CRP and FC, a majority of patients (64%–79%) who achieved CDEIS <4 and no deep ulcers had either FC <250 µg/g or both FC <250 µg/g and CRP <5 mg/L at week 48 after randomization (Fig. 3A), indicating a small additive effect of CRP. Similar results were observed in sensitivity analyses of patients with CRP ≥5 mg/L at baseline (Fig. 3B), FC ≥250 µg/g at baseline (Fig. 3C), and both CRP ≥5 mg/L and FC ≥250 µg/g at baseline (Fig. 3D).
FIGURE 3.
Proportion of patients achieving mucosal healing (CDEIS <4) and no deep ulcers when evaluated by CRP and FC cutoffs at week 48 after randomization in (A) all patients, (B) patients with CRP ≥5 mg/L at baseline, (C) patients with FC ≥250 µg/g at baseline, and (D) patients with CRP ≥5 mg/L and FC ≥250 µg/g at baseline.
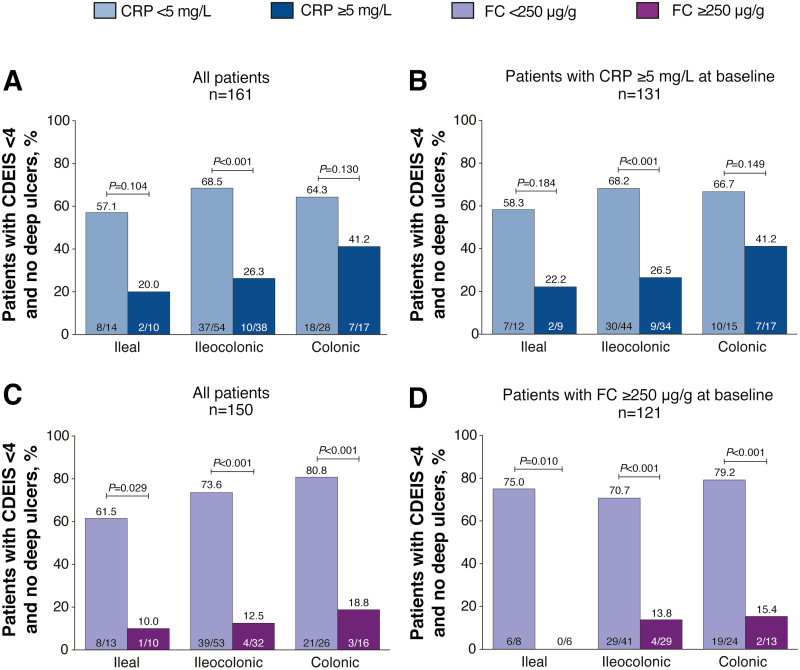
In subgroup analyses by disease location at baseline, a significant difference in the proportion of patients achieving CDEIS <4 and no deep ulcers or endoscopic response (CDEIS decrease >5 from baseline) at week 48 after randomization by CRP cutoffs was only observed for patients with ileocolonic disease (CDEIS <4 and no deep ulcers, 69% vs 26%, P < 0.001, Fig. 4A; CDEIS decrease >5 from baseline, 82% vs 45%, P < 0.001, Supplementary Fig. 3A). Results were similar when only patients with CRP ≥5 mg/L at baseline were included in the analysis (Fig. 4B; Supplementary Fig. 3B). A significantly greater proportion of patients with FC <250 µg/g at week 48 after randomization achieved CDEIS <4 and no deep ulcers compared with those with FC ≥250 µg/g, regardless of disease location at baseline (ileal, P = 0.029; ileocolonic and colonic, P < 0.001; Fig. 4C). Similar results were observed for patients with FC ≥250 µg/g at baseline (ileal, P = 0.010; ileocolonic and colonic, P < 0.001; Fig. 4D). A significantly greater proportion of those with FC <250 µg/g at week 48 achieved endoscopic response compared with those with FC ≥250 µg/g for patients with ileocolonic disease at baseline, and similar results were observed for patients with FC ≥250 µg/g at baseline (87% vs 34%, P < 0.001, Supplementary Fig. 3C; 83% vs 38%, P < 0.001, Supplementary Fig. 3D).
FIGURE 4.
Proportion of patients achieving mucosal healing (CDEIS <4) and no deep ulcers by disease location in (A) all patients by CRP cutoff at week 48 after randomization, (B) patients with CRP ≥5 mg/L at baseline, (C) all patients by FC cutoff at week 48 after randomization, and (D) patients with FC ≥250 µg/g at baseline. P values were calculated using the χ 2 test or Fisher exact test if ≥20% of the cells had expected cell count <5.
Predictive Performance of Biomarkers in Relation to Endoscopic Outcomes
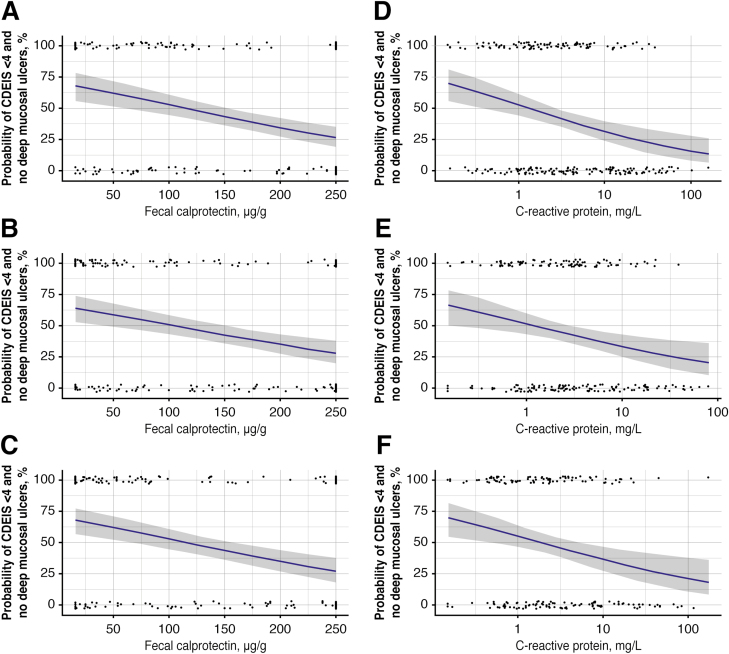
Univariate regression analyses revealed an approximately linear association between increasing levels of FC and CRP at weeks 11, 23, and 35 and decreasing likelihood of achieving CDEIS <4 and no deep ulcers at week 48 after randomization (Fig. 5). Multivariate logistic regression analyses showed a significant contribution of CRP to FC for weeks 11 (P = 0.02) and 23 (P = 0.04) as a predictor of CDEIS <4 and no deep ulcers—but not at week 35. No significant contribution of CDAI was observed. For all patients combined, FC <250 µg/g, CRP <5 mg/L, and CDAI <150 at week 11 gave a sensitivity of 70%, a specificity of 63%, a positive predictive value of 85%, and a negative predictive value of 42%, respectively, in predicting CDEIS <4 and no deep ulcers at week 48 after randomization. Similar sensitivity/specificity and predictive values were observed for FC <250 µg/g, CRP <5 mg/L, and CDAI <150 at weeks 23 and 35 (Table 1).
FIGURE 5.
Relationships of probabilities of achieving mucosal healing (CDEIS <4) and no deep ulcers versus stool concentrations of FC at weeks (A) 11, (B) 23, (C) 35, and serum concentrations of CRP at weeks (D) 11, (E) 23, and (F) 35. Dots represent the individual response status vs the corresponding biomarker concentration. Blue lines represent the relationship based on the fitted logistic regression model. The shaded regions represent the 95% CI of the relationship.
TABLE 1.
Predictive Values for Achievement of CDEIS <4 and No Deep Ulcers 48 Weeks After Randomization in Patients with FC <250 µg/g, CRP <5 mg/L, and CDAI <150
| Week | Sensitivity, % | PPV, % | Specificity, % | NPV, % | ROC AUC, 95% CI |
|---|---|---|---|---|---|
| 11 | 70 | 85 | 63 | 42 | 0.63, 0.58–0.69 |
| 23 | 72 | 82 | 62 | 50 | 0.66, 0.60–0.71 |
| 35 | 74 | 82 | 64 | 54 | 0.68, 0.62–0.74 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; ROC AUC, receiver operating characteristic area under the curve.
DISCUSSION
Primary results from the CALM study showed that patients with CD achieved better endoscopic outcomes when decisions to escalate treatment were based on biomarkers of inflammation, clinical symptoms, and prednisone use rather than on clinical symptoms and prednisone use alone.16 This post hoc analysis of CALM showed that FC and CRP were the main drivers of treatment escalation for patients in the TC group. Additionally, this analysis demonstrated that achieving FC <250 µg/g was strongly associated with the primary endoscopic outcome of mucosal healing, defined by CDEIS <4 and no deep ulcers in CALM. This association was independent of disease location. The proportion of patients who achieved the primary endoscopic outcome, when evaluated by both FC and CRP cutoffs at week 48 after randomization, was significantly greater for patients with FC <250 µg/g, with an additive effect of CRP <5 mg/L.
The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) consensus recommended use of biomarkers, including FC and CRP, only to facilitate patient monitoring, as the available evidence was insufficient to recommend treatment optimization based solely on biomarkers.17 Although increased levels of CRP have been associated with increased disease activity, not all patients with active disease have elevated CRP levels.18 In this study, approximately 30% of patients with elevated CRP achieved mucosal healing at week 48, indicating that CRP should not be used in isolation to guide treatment decisions. Fecal biomarkers such as FC may be more useful surrogate markers of endoscopic activity.19–21 Several studies have reported correlation of FC concentration with endoscopic disease activity in patients with CD receiving anti-inflammatory medications and in those receiving anti-TNF therapies.5, 6, 22 Measurement of FC can be an effective method of detecting endoscopic ulcerations, regardless of disease location.23 Elevated FC levels have been suggested as a predictor of relapse in patients with CD; the STORI trial demonstrated that FC ≥300 µg/g was an independent risk factor for relapse.24 Additionally, changes in FC levels before and after surgery were sensitive enough to monitor patients for recurrence of CD after intestinal resection.20
Recently, it was proposed that the STRIDE treat-to-target recommendations should be updated to include FC as a target for IBD.25 However, the appropriate cutoff point to define endoscopic activity has not been established. Several studies of FC in CD have evaluated different assays with cutoffs that ranged from 50 to 274 µg/g.5, 12, 13, 26 Different FC assays also have different sensitivity and specificity in detecting stool FC concentrations. In 77 patients with CD, an FC concentration of 200 µg/g had 70%/92% sensitivity/specificity for predicting endoscopically active disease (CDEIS ≥3). A post hoc analysis of FC levels in patients in the STORI trial suggested that FC ≤250 µg/g was the best cutoff to define mucosal inflammation; and a study of patients with CD also proposed a cutoff value of 250 µg/g with 94%/62% sensitivity/specificity for predicting endoscopic remission.5, 12 Although exploration of cutoff levels for FC and CRP to indicate mucosal healing was not the original objective of the CALM study, the results of this post hoc analysis of CALM support the cutoff proposed by earlier studies, as few patients with FC >250 µg/g at week 48 achieved the primary end point, and 79% of patients with baseline FC ≥250 µg/g achieved the primary end point if FC was <250 µg/g and CRP <5 mg/L 48 weeks after randomization. Additionally, the predefined combination of the biomarker cutoffs and CDAI <150 demonstrated some prognostic potential, with a positive predictive value of up to 86% for the primary end point. In this analysis, multivariate logistic regression showed no significant contribution of CDAI in prediction of endoscopic outcomes; this finding is supported by recent evidence that CDAI may not provide sufficient correlation with endoscopic scores and that the addition of biomarkers provides better predictive accuracy than CDAI alone.27, 28
The post hoc analysis was limited by the design of the CALM study, in which actual values of FC levels above 250 μg/g were not captured and were only classified as >250 μg/g. Therefore, FC levels were quantitated only when they were ≤250 μg/g, and no optimal FC cutoff using receiver operating characteristic analysis could be determined. Furthermore, because escalation decisions were made throughout the trial using the 250 μg/g cutoff, it could not be determined whether more patients would have met the primary end point if a lower cutoff was used. Other commercially available tests for FC may have different cutoff values; consequently, the results of the present study may not be generalizable. Another limitation of this post hoc analysis was that the predictive performance model did not take treatment escalation or de-escalation changes into account. However, as the goal of the predictive performance analysis was to show predictive values of specific thresholds (CDAI <150, FC <250 µg/g, and CRP <5 mg/L) at each time point, the results would not have been impacted by treatment changes. Finally, comparisons with other studies may be challenging, as there was a lack of an accepted definition of mucosal healing at the time of the CALM study, and the definition of mucosal healing is still evolving.29
The correlation of biomarker cutoffs with endoscopic response is important for future management of IBD, specifically CD. The goal of CD treatment is to induce and maintain deep remission (symptomatic and endoscopic remission) while avoiding long-term use of corticosteroids and immunomodulators, which are associated with increased risk of side effects.8 As treatment recommendations are updated to include biomarkers such as FC, identification of specific concentration cutoffs will be important for setting appropriate treatment goals.
CONCLUSION
The results of the CALM study demonstrated that use of a TC strategy including short-term normalization of inflammatory biomarkers was associated with improvements at 48 weeks after randomization in patients with CD. In the present analyses, cutoffs of CRP <5 mg/L at week 48 or FC <250 μg/g at week 48 after randomization accurately classified mucosal healing at week 48 in 66% and 74% of patients, respectively. Ultimately, these findings provide further support for the use of FC as a surrogate marker for mucosal inflammation when implementing a treat-to-target strategy for patients with CD. Nonetheless, a proportion of patients (FC <250 μg/g, 26%) were not classified correctly in terms of mucosal healing as defined in the CALM study. This implies that, although these biomarkers might be useful for guiding short- and medium-term treatment decisions, longer-term management should still involve endoscopic evaluation.
Supplementary Material
Author Contribution: WR, RP, AA, XH, SD, WJS, SS, GDH, QZ, EN, and J-FC contributed to the study concept and design. Acquisition of data was performed by WR, PB, FB, XH, ST, SD, GDH, QZ, and EN. Analysis and interpretation of data were conducted by WR, RP, PB, FB, AA, ST, SD, WJS, SS, SB, GDH, QZ, KK, EN, AAS, and J-FC. Statistical analysis was provided by QZ and AAS. Administrative, technical, or material support was provided by SS and QZ, and study supervision was provided by SS (local) and J-FC. All authors provided critical revision of the manuscript for important intellectual content and approved the final version of the article, including the authorship list.
Supported by: This work was funded by AbbVie Inc. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. The CALM study was funded by AbbVie Inc. The writing of this manuscript was funded by AbbVie Inc. Writing support was provided by Catherine DeBrosse, PhD, of Complete Publication Solutions, LLC (North Wales, PA, USA), an ICON plc company. AbbVie reviewed and approved the publication.
Conflicts of Interest: WR has received lecture fees from AbbVie, Actelion, Alfa Wasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor; was a consultant for AbbVie, Actelion, Alfa Wassermann, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor; and was an advisory board member for AbbVie, Actelion, Alfa Wassermann, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor. RP was a consultant and/or has received lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research, Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, MSD, Millennium Pharmaceuticals (now Takeda), Ocera Therapeutics, Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough, Synta Pharmaceuticals, Teva, UCB Pharma, and Warner Chilcott. PB has received financial support for research from AbbVie, Mundipharma, and Pfizer; lecture fee(s) from AbbVie, Takeda, and Janssen; and advisory board fees from Hospira, Janssen, MSD, Mundipharma, Roche, Pfizer, Sandoz, and Dr. Falk Benelux. FB has received personal fees from AbbVie, Celgene, Falk, Ferring, MSD, Janssen, Mundipharma, Takeda, and Pfizer/Hospira; grants from AbbVie, Chiesi Farmaceutici, MSD, Ipsen, and Roche; FB is the CEO of IBDIM as of October 2018. AA was a consultant or advisory member for AbbVie, Allergan, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Ferring, Hospira, Janssen, Lilly, MSD, Mundipharma, Mylan, Pfizer, Samsung Bioepis, Sandoz, Sofar, and Takeda; has received lecture fees from AbbVie, Amgen, AstraZeneca, Chiesi, Ferring, Hospira, Janssen, Medtronic, MSD, Mitsubishi Tanabe, Mundipharma, Nikkiso, Otsuka, Pfizer, Samsung Bioepis, Takeda, TiGenix, and Zambon; and research funding from MSD, Pfizer, and Takeda. XH received financial support for research from AbbVie, Abivax, Alfasigma, Celgene, Gilead, Eli Lilly, Enterome, Janssen, InDex Pharmaceuticals, Pfizer, Roche, Salix, Takeda, and Theravance; has served on advisory boards of AbbVie, Abivax, Arkopharma, Astellas, Janssen, Nutricia, Pfizer, and Takeda; and has received lecture fees from AbbVie, ARARD, Arkopharma, Baxter, Bristol-Myers Squibb, Ferring Pharmaceuticals, Janssen, MSD, Nutricia, Pfizer, Sanofi-Aventis, Takeda, and Tillotts.
ST received financial support for research from AbbVie, IOIBD, Lilly, UCB, Vifor, and Norman Collisson Foundation; lecture fees from AbbVie, Amgen, Asahi, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Cosmo, Ferring, Giuliani SpA, GlaxoSmithKline, Janssen, Lilly, MSD, Neovacs, NovoNordisk, Novartis, NPS Pharmaceuticals, Pfizer, Proximagen, Receptos, Shire, Sigmoid Pharma, Takeda, Topivert, UCB, VHsquared, and Vifor; and advisor fees from AbbVie, Amgen, Asahi, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Cosmo, Ferring, Giuliani SpA, GlaxoSmithKline, Janssen, Lilly, MSD, Neovacs, NovoNordisk, Novartis, NPS Pharmaceuticals, Pfizer, Proximagen, Receptos, Shire, Sigmoid Pharma, Takeda, Topivert, UCB, VHsquared, and Vifor; ST was also supported in part by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. SD has received lecture fees from AbbVie, Actelion, Alfa Wassermann, Astra Zeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor; has served as a consultant for AbbVie, Actelion, Alfa Wassermann, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor; and has served on the advisory boards for AbbVie, Actelion, Alfa Wassermann, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson & Johnson, Millennium, Merck & Co., NovoNordisk, Nycomed, Pfizer, Pharmacosmos, Schering-Plough, Salix, Takeda, UCB Pharma, and Vifor. WJS has received consulting fees from AbbVie, Akros Pharma, Allergan, Ambrx, Amgen, Ardelyx, Arena Pharmaceuticals, Atlantic Pharmaceuticals, Avaxia, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Conatus, Cosmo Technologies, Escalier Biosciences, Ferring, Ferring Research Institute, Forward Pharma, Galapagos, Genentech, Gilead Sciences, Immune Pharmaceuticals, Index Pharmaceuticals, Janssen, Kyowa Hakko Kirin Pharma, Lilly, MedImmune, Mesoblast, Miraca Life Sciences, Nivalis Therapeutics, Novartis, Nutrition Science Partners, Oppilan Pharma, Otsuka, Palatin, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Qu Biologics, Regeneron, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust [HART]; previously owned by the University of Western Ontario), Salix, Seattle Genetics, Seres Therapeutics, Shire, Sigmoid Biotechnologies, Takeda, Theradiag, Theravance, TiGenix, Tillotts Pharma, UCB Pharma, Vascular Biogenics, and Vivelix; has received research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, and Celgene/Receptos; and has received payments for lectures/speakers bureau from AbbVie, Janssen, and Takeda; and holds stock/stock options in Escalier Biosciences, Oppilan Pharma, Precision IBD, Progenity, and Ritter Pharmaceuticals.
SS has received consultancy and lecture fees from AbbVie, Falk Pharma, Ferring, Genentech, GlaxoSmithKline, MSD, Pfizer, Shire, and Takeda. GDH has received consulting and/or lecture fees from AbbVie, ActoGeniX, AIM, Boehringer Ingelheim GmbH, Centocor, ChemoCentryx, Cosmo Technologies, Elan Pharmaceuticals, enGene, Dr Falk Pharma, Ferring, Galapagos, Giuliani SpA, Given Imaging, GlaxoSmithKline, Janssen Biologics, MSD, Neovacs, NovoNordisk, Otsuka, PDL BioPharma, Pfizer, Receptos, Salix, SetPoint, Shire Pharmaceuticals, Schering-Plough, Takeda, Tillotts Pharma, UCB Pharma, Versant, and Vifor Pharma; has received research grants from AbbVie, Janssen, Given Imaging, MSD, Dr. Falk Pharma, and PhotoPill; and has received speaking honoraria from AbbVie, Tillotts, Tramedico, Ferring, MSD, UCB Pharma, Norgine, and Shire. J-FC has served as a consultant or an advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Johnson & Johnson, MedImmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, and Theradiag; has served as speaker for AbbVie, Ferring, Takeda, and Celgene Corporation; is a stock options holder of Intestinal Biotech Development and Genfit; and has received research grants from AbbVie and Takeda. SB, QZ, KK, EN, and AAS are employees of AbbVie and may own AbbVie stock and/or options.
DATA SHARING
AbbVie is committed to responsible data sharing regarding the clinical trials it sponsors. This includes access to anonymized, individual, and trial-level data (analysis data sets), in addition to other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided after review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
REFERENCES
- 1. Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet. 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 3. Ganz ML, Sugarman R, Wang R, et al. The economic and health-related impact of Crohn’s disease in the United States: evidence from a nationally representative survey. Inflamm Bowel Dis. 2016;22:1032–1041. [DOI] [PubMed] [Google Scholar]
- 4. Floyd DN, Langham S, Séverac HC, et al. The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60:299–312. [DOI] [PubMed] [Google Scholar]
- 5. Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. [DOI] [PubMed] [Google Scholar]
- 6. Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. [DOI] [PubMed] [Google Scholar]
- 7. Regueiro M, Kip KE, Schraut W, et al. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–126. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Hanauer S, Van Assche G, et al. Treating beyond symptoms with a view to improving patient outcomes in inflammatory bowel diseases. J Crohns Colitis. 2014;8:927–935. [DOI] [PubMed] [Google Scholar]
- 9. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 10. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langhorst J, Boone J, Lauche R, et al. Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: Post Hoc analysis of a prospective clinical trial. J Crohns Colitis. 2016;10:786–794. [DOI] [PubMed] [Google Scholar]
- 12. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 13. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 14. D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437. [DOI] [PubMed] [Google Scholar]
- 15. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 16. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 17. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 18. Florin TH, Paterson EW, Fowler EV, et al. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306–311. [DOI] [PubMed] [Google Scholar]
- 19. Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938–947.e1. [DOI] [PubMed] [Google Scholar]
- 21. Liverani E, Scaioli E, Digby RJ, et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. [DOI] [PubMed] [Google Scholar]
- 23. Buisson A, Mak WY, Andersen MJ, et al. Su1805 - Fecal calprotectin is highly effective to detect endoscopic ulcerations in Crohn’s disease regardless of disease location. Gastroenterology. 2018;154:S590–S591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70 e65; quiz e31. [DOI] [PubMed] [Google Scholar]
- 25. Pouillon L, Peyrin-Biroulet L. It is time to revise the STRIDE guidelines determining therapeutic goals for treat-to-target in inflammatory bowel disease. J Crohns Colitis. 2018;12:509. [DOI] [PubMed] [Google Scholar]
- 26. Lobatón T, López-García A, Rodríguez-Moranta F, et al. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641–e651. [DOI] [PubMed] [Google Scholar]
- 27. Morris MW, Stewart SA, Heisler C, et al. Biomarker-based models outperform patient-reported scores in predicting endoscopic inflammatory disease activity. Inflamm Bowel Dis. 2018;24:277–285. [DOI] [PubMed] [Google Scholar]
- 28. Tajra JB, Calegaro JU, de Paula AP, et al. Correlation and concordance measures between clinical, endoscopic and histological scores activity in Crohn’s disease under treatment. Scand J Gastroenterol. 2019;54:441–445. [DOI] [PubMed] [Google Scholar]
- 29. Bossuyt P, Louis E, Mary JY, et al. Defining endoscopic remission in ileocolonic Crohn’s disease: let’s start from scratch. J Crohns Colitis. 2018;12:1245–1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.