Abstract
Background
Diagnosis and monitoring of inflammatory bowel diseases (IBDs) utilize invasive methods including endoscopy and tissue biopsy, with blood tests being less specific for IBDs. Substantial evidence has implicated involvement of the neurohormone serotonin (5-hydroxytryptamine, 5-HT) in the pathophysiology of IBDs. The current study investigated whether serum 5-HT is elevated in patients with active ulcerative colitis (UC) or Crohn’s disease (CD).
Methods
Serum samples were obtained from a German cohort of 96 CD and UC patients with active disease, refractory disease, or remission of disease based upon their disease activity index (DAI) and disease history. High pressure liquid chromatography with tandemmass spectrometry was used to measure 5-HT, tryptophan (TRP), and kynurenine (KYN) levels in the serum samples, and Luminex Multiplex ELISA was used to measure cytokine levels. Intestinal mucosal biopsies were obtained from a separate cohort of healthy and CD patients, and the immunoreactivity of the serotonin transporter (SERT) was determined.
Results
There was no statistically significant difference in TRP or KYN levels between disease categories in either UC or CD. Interestingly, 5-HT levels were significantly elevated in patients with active CD but not active UC when compared with the levels in remission or refractory disease. Serum 5-HT was superior to C-reactive protein and circulating cytokines in differentiating between disease categories in CD. Additionally, SERT immunoreactivity was decreased in the ileum and colon of patients with CD compared to healthy controls.
Conclusion
We have shown that the serum 5-HT can differentiate between active disease and refractory disease or remission among CD patients, emphasizing the potential suitability of serum 5-HT as an auxiliary measure in diagnosing active CD.
Keywords: serotonin, 5-hydroxytryptamine, serotonin transporter, SERT, tryptophan, kynurenine
Serum serotonin levels discriminate active disease from refractory disease or disease in remission in Crohn’s disease but not in ulcerative colitis. C-reactive protein, tryptophan, kynurenine, and cytokines poorly discriminate between disease states. Serotonin transporter immunofluorescence was decreased in Crohn’s disease.
INTRODUCTION
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders of the gastrointestinal (GI) tract that can be categorized into 2 major subtypes, ulcerative colitis (UC) and Crohn’s disease (CD). Though the pathophysiology of IBDs are not entirely clear, it is believed that both genetic and environmental factors are involved in the initiation and progression of intestinal inflammation.1 Diagnosing IBDs and prognostic evaluation primarily relies on expensive and time-consuming endoscopic, histological, and radiographic tests, with noninvasive tests being less commonly used.2 Although endoscopy with biopsy is the gold standard of diagnosis, it can be uncomfortable for the patient and carries several risks.3 Accurate diagnosis, evaluation of severity, definition of remission status, and categorization into UC and CD subtypes also continue to pose a challenge for clinicians.4 Such concerns warrant new methods for the diagnosis and monitoring of IBDs that are less invasive, more accurate, and less expensive.
In this regard, the tryptophan (TRP)-derived enteric neurotransmitter and hormone serotonin (5-hydroxytryptamine, 5-HT) has been shown to be involved in the pathogenesis of IBDs. In the GI tract, 5-HT increases motility,5 participates in neuronal reflexes,6 and regulates transport of fluid and electrolytes.7–9 Several clinical studies have shown that the prevalence of the 5-HT producing epithelial cells of the GI tract, enterochromaffin cells, is altered in IBD patients.10–13 In accordance with these observations, patients with IBDs have also been found to have altered mucosal 5-HT content12, 14 and an abundance of mRNA encoding the rate-limiting enzyme of 5-HT synthesis, tryptophan hydroxylase I (TPH-1).12, 14, 15 The most consistent observation, however, is the decrease in expression of the serotonin transporter (SERT; SLC6A4) in the mucosa of IBD patients.15–17 SERT is expressed on several cell types in the GI tract including epithelial cells, enteric neurons, and circulating platelets, where it functions to regulate extracellular availability of 5-HT via internalization of 5-HT into the intracellular compartment.18
Several studies have examined the levels of circulating 5-HT in the context of different diseases. Conditions with elevated circulating 5-HT include primary pulmonary dysfunction,19, 20 cardiac dysfunction,21–23 certain malignancies,24, 25 and epilepsy.25, 26 Conversely, decreased circulating 5-HT has been observed in several neurological and psychiatric conditions27–29 and in rheumatologic conditions.30, 31 Although the primary source of circulating 5-HT is the GI tract (as platelets do not synthesize 5-HT but can only reuptake 5-HT from the GI tract via SERT), few studies have measured circulating 5-HT in GI conditions such as IBDs. A study by Sikander et al showed a significant increase in serum 5-HT levels in patients with UC compared with healthy controls regardless of their remission status.32 Recently, Shajib et al demonstrated an increase in both plasma 5-HT and platelet-depleted plasma 5-HT in patients with CD compared with healthy controls.16 An intriguing study by Wang et al found a correlation between circulating 5-HT and ileal pouch inflammatory scores.33 However, additional studies are warranted to examine how the levels of circulating 5-HT and other tryptophan metabolites vary with different disease activity indices in UC and CD.
Besides its conversion to 5-HT via TPH-1, an alternative metabolic fate of TRP is catabolism into kynurenine (KYN) via the rate limiting enzyme indoleamine 2,3-dioxygenase (IDO) 1.34 Expression and activity of IDO1 is increased in response to inflammatory mediators such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β.34 Kynurenine is converted further into nicotinamide derivatives and other metabolites that play roles in immune modulation, cell proliferation, and cellular energy generation.34 Additionally, condensation products of KYN are high-affinity ligands for the aryl hydrocarbon receptor (AhR), which plays roles in mucosal homeostasis at barrier organs, xenobiotic detoxification, and immunity.35 When KYN and TRP levels were measured in CD patients, several studies found an increase in the serum KYN/TRP ratio and a decrease in serum TRP level, which were more pronounced with increased disease severity.36–38 Studies examining KYN and TRP levels during UC show a similar decrease in serum TRP but changes in serum KYN are inconsistent.37–39 When the studies examining the serotonergic system and the kynurenine pathway in IBD are taken collectively, it is clear that there are alterations in these TRP metabolites that should be further investigated.
Thus, the current studies were undertaken in an attempt to identify consistent noninvasive indicators of IBD severity. We first measured serum levels of TRP, KYN, and 5-HT in patients with CD or UC. We next compared the ability of these metabolites to discriminate between active disease, refractory disease, or remission to the well-known inflammatory marker C-reactive protein (CRP). Our next step was to see if circulating cytokines were able to discriminate between CD disease states and determine whether levels of these cytokines correlated with levels of 5-HT. In a separate cohort of CD patients, we examined the expression of SERT in ileal and colonic biopsies to postulate a mechanism contributing to alterations in circulating 5-HT levels.
MATERIALS AND METHODS
Study Population and Sample Collection
Serum samples were collected from patients with confirmed diagnosis of UC or CD at the Department of Internal Medicine at the University Medical Center in Regensburg, Germany, between 2005 and 2010. Informed consent was provided before donation of serum. Patients were diagnosed based upon the European Crohn’s and Colitis organization (ECCO) clinical consensus guidelines by 4 experienced IBD physicians at the University Medical Center in Regensburg based upon clinical, radiographic, endoscopic, and histopathological criteria.40, 41 Patients were categorized as having active disease, refractory disease, or being in remission based upon clinical evaluation. Active disease includes patients who experience acute relapses followed by symptom-free periods after a short course of steroids or other immunosuppressive therapy. Samples were collected during an acute relapse in their disease. For CD, a Crohn’s Disease Activity Index (CDAI) of <150 indicated remission, and a CDAI of >150 indicated active disease. For both UC and CD, refractory disease was defined by a persistence of clinical symptoms despite adequate drug therapy, which brings about an improvement, but no complete and lasting (ie, less than 2 relapses per year) remission. Patient characteristics and laboratory data were obtained by abstracting from the medical record. Laboratory values including C-reactive protein levels were obtained at the time of sample collection and were utilized in analysis when available. Serum was isolated by centrifugation of blood after collection and was stored at −80°C. All serum samples are de-identified and are labeled with a bar code for identification by an honest broker. Clinical data were obtained or updated, respectively, for each time point of sample procurement separately by the treating physician at the University Medical Center in Regensburg. Once collected, data were transferred and stored in a secure coded pseudonymized database for analysis.
Biopsy samples were collected from patients diagnosed with CD and from healthy individuals undergoing biopsy sampling for other reasons according to the approved protocol (No: IRB 19–1944) at the University of Chicago.
Liquid Chromatography With Tandem Mass Spectrometry Measurements
High pressure liquid chromatography with tandem mass spectrometry (LC-MS/MS) was used to measure serum TRP, KYN, and 5-HT levels using a protocol adopted from a previous report.42 All solvents used for LC-MS/MS were of LC-MS grade and purchased from Thermo Fisher (Waltham, MA). All TRP, KYN, and 5-HT calibration standards were purchased from Sigma-Aldrich (St. Louis, MO). Internal standards were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Briefly, 10 μL of serum sample or calibration standard was combined with 10 μL of internal standard mixture containing 10 μM TRP-D5, 1 μM 5HT-D4, and 10 μL of 0.1% formic acid and 1% acetonitrile in water before the addition of 150 μL of ice-cold methanol. After precipitation for 30 minutes at −20°C, the samples were centrifuged at 10,000 G for 10 minutes at 4°C. The supernatant was obtained, evaporated under a gentle stream of nitrogen, and reconstituted in an equal volume of 0.1% formic acid: 1% acetonitrile in water before injection of 5 μL onto a Kinetix C18 (100 × 2.1 mm, 2.6 μm particle size) in an Agilent 1260 UHPLC system coupled to a triple quadrupole mass spectrometer (SCIEX QTRAP 5500). Mobile phase A was composed of 0.1% formic acid in water, and mobile phase B was composed of 0.1% acetonitrile in water. The LC method is given in Supplementary Table 1. For detection, the mass spectrometer was operated in electrospray ionization positive mode (ESI+) with multiple reaction monitoring (MRM). Parameters for MRM are given in Supplementary Table 2. The average retention times were 2.15 minutes for TRP, 1.2 minutes for KYN, and 0.89 minutes for 5-HT. For quantification, levels of TRP and KYN were normalized to TRP-D5, and levels of 5-HT were normalized to 5-HT-D4. Calibration standards used to generate the standard curves for quantification of TRP ranged from 1 μM to 100 μM, and calibration standards for KYN and 5-HT ranged from 10 nM to 10 μM. When performing the LC-MS/MS measurements, we ran the patient samples alongside 3 samples of fresh serum collected the week prior from healthy volunteers and found that levels of 5-HT were comparable, posing no concern of long-term storage on stability. Additionally, the levels of 5-HT we measured are comparable with what has been measured previously in human serum.32, 36, 37
Multiplex ELISA
A custom high-sensitivity multiplex ELISA (R&D Systems, Minneapolis, MN) was used to quantify cytokines in CD patient serum. The cytokines measured were interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-7 (IL-7), interleukin-17A (IL-17A), interleukin-22 (IL-22), and tumor necrosis factor-α (TNF-α). The assay was performed according to the manufacturer’s instructions, and the plate was read using a Luminex Magpix instrument. The assay was performed with selected samples (13 remission, 12 active, 15 refractory) due to technical limitations. The 5 samples omitted included 3 gross outliers based upon LC-MS/MS measurements and 2 samples from patients receiving selective serotonin reuptake inhibitors (SSRIs).
Hematoxylin and Eosin Staining
Formalin-fixed paraffin embedded 5-μM thick sections from healthy patient ileum (n = 9) and colon (n = 3) and from patients with Crohn’s colitis (n = 3) and ileitis (n = 10) were used for staining. The slides were stained per the manufacturer’s protocol (Syteck laboratories Inc., Logan, UT,) and hematoxylin and eosin (H&E) staining kit.
Histopathological Scoring
Histopathological scoring was conducted in a blinded manner in H&E stained sections.
A 2-digit histopathological score was assigned to each sample. The first digit denoted the presence or absence of IBD; absence of IBD is denoted as 1 and presence of IBD as 2. The second digit represents the activity of inflammation in which scores from 1 to 4 were assigned based on the following criteria: (1) quiescent/inactive (absence of architectural distortion, increased lamina propria lymphocytes), (2) mild active (presence of architectural distortion, lamina propria lymphocytes, and intra-epithelial granulocytes), (3) moderate active (presence of features in 2 and crypt abscesses), and (4) severe active (presence of all features in 3 and erosions).43
Immunostaining
Immunofluorescence staining was performed in formalin-fixed paraffin embedded in 5-μM thick sections of ileum (n = 5) and colon (n = 3) from healthy individuals and patients with Crohn’s colitis and ileitis. The staining was conducted as described previously.44 Briefly, the slides were first deparaffinized in coupling jars by immersing in xylene. This was followed by hydration of slides in solutions of ethanol for 5 minutes each in the following order: 100%, 95%, 70% v/v, and finally in distilled water. Afterward, antigen retrieval was performed in a steamer using citrate buffer (pH = 6) for 30 minutes. The slides were then washed in tris buffered saline (TBS) containing 0.05% v/v Tween (polysorbate) 20 (0.05% TBST). This was followed by permeabilization in 0.1% triton X in TBS. The tissue sections were encircled with a water repellant pap pen (Vector laboratories, Burlingame, CA) and were blocked with 10% normal goat serum (NGS) for 1 hour in a humidified container. Afterward, the blocking solution was removed and was replaced with 1% NGS containing primary antibody solution for SERT (ImmunoStar Inc. Hudson, WI) at a ratio of 1:100 and incubated at 4°C overnight. The antibodies have been previously validated by us utilizing peptide competition assays.45 The next day, slides were washed with TBS and incubated for 1 hour at room temperature, with a secondary antirabbit Alexa Fluor 488 (Invitrogen, Carlsbad, CA) antibody at a ratio of 1:100 in 1% NGS. The slides were washed and mounted using Prolong diamond antifade mounting reagent with DAPI (Invitrogen). The slides were stored at −20°C until imaged. Imaging was conducted at integrated light microscopy core facility at the University of Chicago with the aid of Olympus IX-81 at 40X magnification. SERT intensity on the apical membrane was quantified by ImageJ software analyzing 10 separate areas per patient in 3 to 5 patients per group.
Statistics
Data are presented as box plots by the method of Tukey. Patient characteristics were analyzed by the nonparametric Kruskal-Wallis test and χ 2 for means and proportions, respectively. Both metabolite and cytokine data were not normally distributed, so the nonparametric Mann-Whitney U test was used for comparison of 2 groups, and the nonparametric Kruskal-Wallis test was used followed by the Dunn multiple comparisons test for analysis of more than 2 groups. For correlation analysis, Pearson R was computed. Two patients with active CD taking selective serotonin reuptake inhibitors had very low serum 5-HT levels and were omitted from all analysis besides CRP comparison. SERT immunofluorescence intensity was analyzed between groups by nested t test. A probability cutoff of P < 0.05 was considered as statistically significant.
RESULTS
Patient Characteristics
A total of 96 patients with a confirmed diagnosis of IBD were included in the study, with 45 patients meeting diagnostic criteria for CD and 46 patients meeting criteria for UC. Among the 45 patients with CD, 15 patients were in remission, 15 patients had active disease, and 15 patients had disease refractory to treatment. Among the 45 patients with UC, 15 patients were in remission, 15 patients had active disease, and 16 patients had disease refractory to treatment. There was no significant difference between mean ages (P = 0.633) or gender frequency (P = 0.223) among the 6 groups. There was also no difference in hemoglobin (P = 0.357), hematocrit (P = 0.461), leukocyte count (P = 0.292), or platelet count (P = 0.250). Two CD patients taking SSRIs were omitted from metabolite analysis due to the confounding effects on 5-HT levels,46 and an additional 3 patients were omitted from cytokine analysis due to technical limitations. Additional patient parameters including medications, localization of disease, and disease course are displayed in Supplementary Table 3.
Serum Serotonin is Elevated During Active Crohn’s Disease but not Ulcerative Colitis
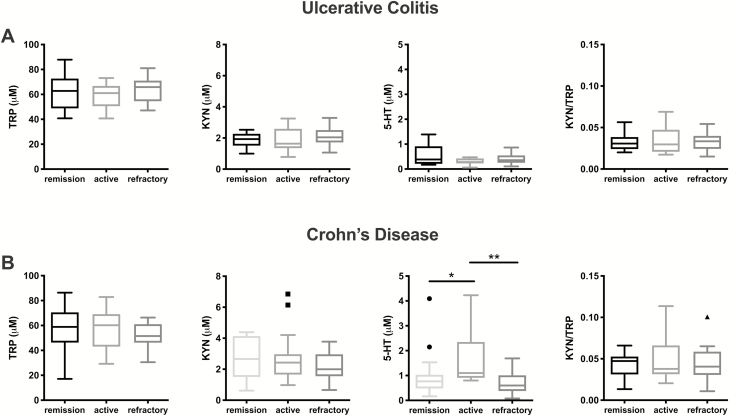
We first measured levels of TRP, 5-HT, and KYN in the serum of patients with UC and CD and compared these among disease categories. In UC, none of the metabolites measured discriminated between patients in remission, those with active disease, or those with refractory disease (Fig. 1A). In CD, there was no statistically significant difference in TRP or KYN levels between disease categories, but there was a significant increase in 5-HT in patients with active disease (Fig. 1B). The KYN/TRP ratio was also computed in all patient groups as an index which represents IDO activity. However, there was no statistically significant difference in the KYN/TRP ratio among disease categories in UC or CD.
FIGURE 1.
Measurement of TRP, KYN, and 5-HT in UC and CD patient serum by LC-MS/MS. Levels of each metabolite and the KYN/TRP ratio as an indication of IDO activity were compared among disease activity categories in (A) UC (remission N = 15, active N = 13, refractory N = 15) and (B) CD (remission N = 15, active N = 15, refractory N = 16) by the Kruskal-Wallis test followed by the Dunn multiple comparisons test. Data are presented as box plots by the method of Tukey. *P < 0.05, **P < 0.01.
C-reactive Protein Levels Poorly Discriminate Between Disease Categories in Ulcerative Colitis and Crohn’s Disease
Circulating CRP is one of the most widely used biomarkers of increased disease activity in IBD.47 We next determined the difference between CRP levels among UC and CD patients in remission, active disease, or disease refractory to treatment (Fig. 2A–B). In UC, there was a trend toward decreased CRP levels in patients in remission when compared to patients with active disease (P = 0.13) or refractory disease (P = 0.060). However, CRP levels failed to differentiate between disease states in CD. These data suggest that there is a need for new noninvasive biomarkers that have a stronger association with disease activity in UC and CD.
FIGURE 2.
Comparison of CRP and 5-HT in serum of UC and CD patients. Levels of CRP were compared among disease activity categories in (A) UC (remission N = 14, active N = 13, refractory N = 13) and (B) CD (remission N = 15, active N = 14, refractory N = 16) by the Kruskal-Wallis test followed by the Dunn multiple comparisons test. C, Levels of CRP were compared between patients with active UC (N = 13) and active CD (N = 14). D, Levels of serum 5-HT were compared between patients with active UC (N = 15) and active CD (N = 13). Data were analyzed for (C) and (D) using the Mann-Whitney U test. Data are presented as box plots by the method of Tukey. ****P < 0.0001.
The abilities of CRP and 5-HT to differentiate between active UC and CD were then compared. There was no statistically significant difference between CRP levels in patients with active UC or CD (Fig. 2C). In contrast, serum 5-HT levels were markedly increased in active CD when compared with 5-HT levels in active UC (Fig. 2D). These results suggest that 5-HT is more selectively elevated in active CD when compared with CRP.
Serum Cytokine Levels in Crohn’s Disease Patients
Previous studies have shown that the levels of some serum cytokines are altered in CD.48 To see if serum cytokines discriminate among patients in remission, with active disease, or with refractory disease, we measured and compared serum levels of IL-1β, IL-6, IL-7, IL-17A, IL-22, and TNF-α among the patient groups (Fig. 3). There were no statistically significant differences in cytokine levels other than decreased TNF-α in patients with refractory disease.
FIGURE 3.
Measurement of cytokines in CD patient serum by multiplex ELISA. Levels of IL-1β, IL-6, IL-7, IL-17A, IL-22, and TNF-α were compared among disease activity categories in CD (remission N = 13, active N = 12, refractory N = 15) by the Kruskal-Wallis test followed by the Dunn multiple comparisons test. Data are presented as box plots by the method of Tukey. *P < 0.05.
Next, we determined whether serum levels of TRP, KYN, 5-HT, or the KYN/TRP ratio correlated with serum cytokine and CRP levels (Table 1). Interestingly, TRP levels negatively correlated with CRP (R = −0.443; P = 0.002), IL-1β (R = −0.318; P = 0.046), IL-6 (R = −0.403; P = 0.010), IL-7 (R = −0.435; P = 0.005), and IL-17A (R = −0.344; P = 0.030). Both KYN and 5-HT levels were independent of CRP and cytokine levels, whereas the KYN/TRP ratio was positively correlated with TNF-α levels (R = −0.400; P = 0.011). Collectively, these results support the concept that serum 5-HT is superior to serum cytokines in differentiating active CD from remission or refractory disease and that 5-HT levels are independent from levels of serum cytokines.
TABLE 1.
Correlation of Serum TRP Metabolite Levels with CRP and Cytokine Levels
| CRP | IL-1β | IL-6 | IL-7 | IL-17A | IL-22 | TNF-α | |
|---|---|---|---|---|---|---|---|
| TRP | −0.443** | −0.318* | −0.403** | −0.435** | −0.344* | 0.122 | −0.237 |
| KYN | 0.012 | −0.090 | −0.018 | −0.078 | 0.024 | 0.015 | 0.202 |
| 5-HT | 0.022 | 0.131 | −0.023 | −0.204 | 0.192 | 0.053 | 0.144 |
| KYN/TRP | 0.211 | 0.142 | 0.246 | 0.159 | 0.207 | 0.019 | 0.400* |
Data expressed as Spearman correlation coefficient. *P < 0.05, **P < 0.01.
SERT Immunoreactivity is Significantly Reduced in Ileal and Colonic Mucosa of Crohn’s Disease Patients
Previous studies have demonstrated decreased expression of intestinal SERT in mucosal biopsies from patients with various inflammatory conditions of the intestine such as UC, diverticulitis, and celiac disease.15, 17, 49, 50 A recent study has shown a decrease in SERT mRNA in CD patients.16 However to our knowledge, SERT immunoreactivity in the intestinal mucosa of CD patients has not yet been investigated. Therefore, we examined the expression of SERT in both ileum and colon of healthy subjects and patients with CD at different stages of disease activity. We have previously shown that SERT is highly expressed in the ileum and is localized predominantly on the apical membrane of enterocytes.45 Our observations corroborated with these findings in which SERT expression was higher in the ileum (Fig. 4A–C) compared with the colon (Fig. 5A–C) in individuals with no apparent inflammation. SERT stained in green was predominant on the apical membrane of the ileal and colonic mucosa (Figs. 4 and 5, lower panels). Images from the corresponding H&E stained sections are also shown (Figs. 4 and 5, upper panels). Crohn’s disease patients with mild, moderate, and severe inflammation as determined by blinded histopathological scoring had lower SERT immunoreactivity compared with individuals with no inflammation, showing almost no SERT expression in severely inflamed mucosa (Fig. 4A–F). Quantification of apical SERT immunofluorescence intensity showed a significant reduction in SERT immunoreactivity in CD (Fig. 4G). Similarly in the colon, colitis that was mild and severe in nature showed significantly lower SERT staining compared with noninflamed tissues (Fig. 5). These data agree with previous findings in which SERT expression was found to be lower during inflammation and may, in part, explain the higher circulating 5-HT during active CD.
FIGURE 4.
Histology and corresponding SERT staining in ileum of healthy individuals and patients with Crohn’s ileitis. A, B, and C, Representative images of H&E and SERT immunostaining (green) of 3 healthy individuals with a 2-digit histopathological score of 1 1. SERT staining is highly expressed on the apical membrane in all 3 individual,s indicating normal ileal morphology. D, Representative images of a patient with a histopathological score of 2 2 indicating mild enteritis. E, Representative images of a patient with a histopathological score of 2 3 indicating moderate enteritis. F, Representative images of a patient with a histopathological score of 2 4 indicating severe enteritis. G, Graphical representation of quantified SERT intensity in the ileum (n = 5) of normal and Crohn’s (mild-2, moderate-2, and severe-1) patients. Data presented as mean ± SEM, ****P < 0.0001 vs normal ileum.
FIGURE 5.
Histology and corresponding SERT staining in colon of healthy individuals and patients with Crohn’s colitis. A, B, and C, Representative images of H&E and SERT immunostaining (green) of 3 individuals with healthy colon. (Colonic mucosa without diagnostic abnormality with a 2-digit histopathological score of 1 1). Green SERT staining is confined to the apical membrane of all 3 individuals. D, Representative images of a patient with a histopathological score of 2 4 indicating severe colitis. E and F, Representative images of 2 patients with a histopathological score of 2 2, indicating mild colitis. G, Graphical representation of quantified SERT intensity in the colon (n = 3) of normal and Crohn’s (mild-2 and severe-1) patients. Data presented as mean ± SEM, *P < 0.05 vs normal colon.
DISCUSSION
Currently, monitoring disease states and response to medications in IBDs consists mainly of invasive methods such as endoscopy, colonoscopy, and tissue biopsy. Although noninvasive markers of inflammation such as C-reactive protein, erythrocyte sedimentation rate (ESR), and fecal calprotectin may be useful, they are nonspecific to inflammation in the gut and may be elevated in other disease processes.51 Serum markers such as CRP and ESR are sensitive markers for inflammation, but conditions such as bacterial infections, viral infections, and even anemia can make their interpretation vexing. This is consistent with our inability to show a difference in CRP levels among both UC and CD patients in different disease states. Furthermore, CRP has been noted to have different responses in IBDs with higher concentration being noted in CD patients when compared with UC.52 Fecal calprotectin is more sensitive than serum markers for detection of gut inflammation,53 but increased levels have also been noted in gastrointestinal infections, neoplasia, and polyps,54 further reaffirming the need to explore novel markers of disease activity in IBDs. The data presented in the current study demonstrate elevated serum levels of 5-HT in active CD and provide evidence that 5-HT can discriminate between disease activity states in CD better than CRP. Interestingly, UC patients did not show this augmentation in 5-HT levels compared with remission or refractory disease. Our studies further provide a potential mechanism linking elevated 5-HT levels with a decrease in epithelial SERT protein in the ileum and colon of patients with active CD.
As mentioned previously, only few studies have measured circulating 5-HT in IBD patients. Our finding of increased plasma 5-HT in active CD is consistent with the results of Shajib et al who showed an overall elevated plasma 5-HT in CD patients when compared with levels in healthy controls, and an elevation in 5-HT in CD patients experiencing symptoms when compared with CD patients not experiencing symptoms.16 Interestingly, they also found that circulating 5-HT levels were similar in CD patients without symptoms and healthy subjects, which supports our utilization of patients in remission as a control group for comparison. Our findings were also similar to those of Sikander et al, showing no difference in serum 5-HT between active UC and remission.32 However, it is important to note that they showed slightly elevated 5-HT in active UC when compared with a healthy control.
We did not find a difference in TRP, KYN, or KYN/TRP levels among our patients with UC or CD. In contrast to our results, some studies have shown decreases in circulating TRP and increases in KYN/TRP in active CD compared with inactive or less severe disease.36, 37 However, more recent studies have been in agreement with our results. Sofia et al compared the ratio of several KYN metabolites to TRP in the serum of UC patients with increasing endoscopic inflammatory score and found no difference in KYN/TRP between groups.39 Similarly, Dudzińska et al did not find a difference in either TRP levels or KYN/TRP when compared among groups of CD or UC patients with active disease or in remission.38 It is interesting to note that both of these studies found increases in a downstream KYN metabolite, called kynurenic acid, in IBD patients with active disease, which will be subjected to investigation in our future studies.
In the search for further molecular biometric markers and comparing these to 5-HT, we measured and compared the levels of IL-1β, IL-6, IL-7, IL-17A, IL-22, and TNF-α in different disease activity categories within patients with CD. We found no significant changes in cytokine levels among disease states, except for a decreased level of TNF-α in refractory disease. Intriguingly, levels of IL-22 were elevated in patients in remission when compared with other disease states. Even though this difference was not statistically significant, this trend is consistent with the main function of IL-22, which is to support and maintain the epithelial gut barrier by promoting intestinal-stem-cell-mediated epithelial regeneration.55 Interleukin-7 levels were also noted to be slightly more elevated in patients with refractory CD and patients in remission when compared with active CD. However, this elevation in IL-7 was not significant. Interleukin-7 has been noted to be valuable for T-cell proliferation and is also produced in the gut, regulating the proliferation of lymphocytes in the intestinal mucosa.56 We agree with the conclusion from Korolkova et al who found some differences in the serum cytokine levels when comparing UC and CD and concluded that no cytokine profile was able to distinguish UC from CD, and therefore, there is a need to find unique molecular biomarkers.48
There have been several roles proposed for 5-HT in the intestinal inflammatory response that characterizes IBDs. Once released by EC cells into the extracellular space, 5-HT can bind to one of many subtypes of membrane-bound 5-HT receptors (5-HTRs).18 However, attempts to identify the pro-inflammatory or anti-inflammatory nature of 5-HTR subtypes have produced contradictory results.57–60 The overall role of 5-HT in IBDs seems to be pro-inflammatory because deletion61 or pharmacological inhibition62 of TPH-1 in animal models of IBDs reduced the severity of the inflammation. Recently, we showed that 5-HT, which is taken up intracellularly via SERT, can activate AhR.63, 64 As activation of the AhR pathway has been shown to be protective against intestinal inflammation,65–67 it is plausible that disruption of the SERT-5-HT-AhR axis has an additional role in IBD pathogenesis.
The increase in serum 5-HT in patients with CD might be explained by our histopathological finding showing for the first time decreased intestinal epithelial SERT expression by immunofluorescence. In consonance with our findings, Shajib et al previously reported reduction in SERT mRNA expression in patients with active CD.16 Interestingly, Minderhoud et al measured SERT mRNA in the ileum and colon of healthy controls and patients with CD in remission and found no difference between these groups.68 However, studies in the past have also shown a decrease in mucosal SERT expression in UC as well.12, 15 The reason why we did not find increased 5-HT serum levels in UC patients could be due to several factors. Microbial imbalances may attribute to this finding given that dysregulation of gut microbiota has been reported to influence 5-HT levels in patients with IBDs.69 An alternative explanation that may better explain our findings is the localization of SERT and the distribution of the disease. Previous studies from our laboratory show predominant apical localization of SERT in native human and rodent small intestine, with maximum expression in the ileum and very low expression in the colon.45 Similarly, Chen et al showed SERT immunoreactivity in murine small intestine in the apical and subapical mucosal epithelium and in the neurons.70 Given that UC involves the rectum and colon, and only in rare instances involves a small part of the terminal ileum (ie, “backwash” ileitis), it is reasonable to expect that decreased uptake via SERT expressed by the mucosal epithelium is not a mechanism that would lead to increased 5-HT levels in UC.
Our results that show decreased SERT immunoreactivity in CD provide a postulated mechanism to explain the increase in circulating 5-HT in CD (Fig. 6). Namely, there is a decrease in SERT protein expression on the intestinal epithelial cells, which increases extracellular availability of 5-HT, which in turn increases the amount of 5-HT that is able to be taken up by circulating platelets. Additionally, it is likely that the disruption of tight junction proteins, which has long been established as a key characteristic of IBDs,71 introduces a paracellular pathway to allow luminal 5-HT to access the platelet-containing blood vessels in the basolateral compartment. Unlike inflammatory markers such as CRP, ESR, and circulating cytokines, greater than 95% of total body 5-HT is produced by the GI tract, which likely contributes to its superiority in distinguishing between disease states in CD.18, 51 In future studies, it will be interesting to compare the ability of 5-HT to discriminate between disease activity states to that of newer inflammatory markers such as fecal calprotectin.
FIGURE 6.
Proposed mechanism for the increase in circulating 5-HT in CD. 5-HT secreted from EC cells is released into the extracellular compartments. Under healthy conditions, SERT is expressed at high levels on the enterocytes and is able to reuptake 5-HT back into the intracellular compartment. During CD, SERT expression is significantly reduced, and inflammation induced tight junction disruption allows increased passage of 5-HT to the basolateral compartment. High extracellular 5-HT gains access to blood via the leaky vasculature and is taken up by circulating platelets also via SERT. 5-HT is represented by the orange pentagons.
The utilization of selective serotonin reuptake inhibitors (SSRIs) in IBDs is a topic currently under investigation. Several case-control studies and case reports have shown an association between SSRI use and the development of microscopic colitis.72–75 However, 2 studies have shown a benefit of SSRIs in IBDs when using rate of escalation of therapy as a surrogate for disease severity.76, 77 The authors, however, stated that such a proxy measure of disease activity limits the validity of their studies. Additionally, the authors mention that the psychological benefits of SSRIs could also confound their results, as many IBD patients experience comorbid depression and anxiety.78 Nonetheless, additional, properly controlled, and high-powered studies are needed to determine the true effects of SSRIs on IBDs. Of note, 2 patients in our study with active CD were taking SSRIs. Due to the known effect of SSRIs on decreasing circulating 5-HT due to inhibition of SERT on circulating platelets, which limits their ability to uptake 5-HT from the GI tract,79 these patients were excluded from our analysis. This limits the applicability of our results to patients taking SSRIs or other medications known to inhibit SERT activity (including tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors).
In conclusion, we show that circulating 5-HT levels are elevated in patients with active CD when compared with the levels in CD patients in remission or with refractory disease and provide a plausible mechanism to explain these findings. We also demonstrate that 5-HT is superior to CRP and circulating cytokines in stratifying between disease categories in CD. We hope to expand these studies to larger cohorts in the future for confirmation and design more elaborate studies to enhance our understanding of 5-HT and TRP metabolism in IBDs. These additional studies will also inform the potential utilization of serum 5-HT to aid in differentiating between disease states in CD.
Supplementary Material
ACKNOWLEDGEMENTS
Authors would like to thank Andrea Dirmeier and Claudia Kunst, PhD, from the University Medical Center in Regensburg for their generosity in sharing the serum samples and corresponding clinical data utilized in the study.
Author Contribution: RKG contributed to the initial conceptualization of the study. CRM contributed to the evolution of conceptualization. CRM, DJ, WP, CRW, and LY contributed to the investigations. RKG, CRW, WAA, BJ, and JB contributed resources to the study. RKG, CRW, and WAA supervised the study. CM, DJ, and WP wrote the original draft. And all authors reviewed and edited the manuscript.
Supported by: These studies were supported by the National Institute of Diabetes and Digestive and Kidney Disease grants R01 DK098170 (RKG), R01 DK109709 (WAA), F30 DK113703 (CRM), the Crohn’s and Colitis Foundation CCFA 581138 (RG), and the Department of Veterans Affairs BX 000152 (WAA), VA Research Career Scientist Award 1IK6BX005243 (WAA).
Conflicts of Interest: The authors declare no conflicts of interest.
REFERENCES
- 1. Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor S, Lobo AJ. Diagnosis and treatment of inflammatory bowel disease. Practitioner. 2016;260:19–23. [PubMed] [Google Scholar]
- 3. Froehlich F, Gonvers JJ, Vader JP, et al. Appropriateness of gastrointestinal endoscopy: risk of complications. Endoscopy. 1999;31:684–686. [DOI] [PubMed] [Google Scholar]
- 4. Feakins RM. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology. 2014;64:317–335. [DOI] [PubMed] [Google Scholar]
- 5. Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- 6. Kim M, Cooke HJ, Javed NH, et al. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121:1400–1406. [DOI] [PubMed] [Google Scholar]
- 7. Amin MR, Ghannad L, Othman A, et al. Transcriptional regulation of the human Na+/H+ exchanger NHE3 by serotonin in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2009;382:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saksena S, Gill RK, Tyagi S, et al. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(-)/OH- exchange activity in Caco-2 cells by serotonin. J Biol Chem. 2005;280:11859–11868. [DOI] [PubMed] [Google Scholar]
- 9. Gill RK, Saksena S, Tyagi S, et al. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology. 2005;128:962–974. [DOI] [PubMed] [Google Scholar]
- 10. Bishop AE, Pietroletti R, Taat CW, et al. Increased populations of endocrine cells in Crohn’s ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–396. [DOI] [PubMed] [Google Scholar]
- 11. El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. [DOI] [PubMed] [Google Scholar]
- 12. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. [DOI] [PubMed] [Google Scholar]
- 13. Ahonen A, Kyösola K, Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1–7. [PubMed] [Google Scholar]
- 14. Kidd M, Gustafsson BI, Drozdov I, et al. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil. 2009;21:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tada Y, Ishihara S, Kawashima K, et al. Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. J Gastroenterol Hepatol. 2016;31:1443–1452. [DOI] [PubMed] [Google Scholar]
- 16. Shajib MS, Chauhan U, Adeeb S, et al. Characterization of serotonin signaling components in patients with inflammatory bowel disease. J Can Assoc Gastroenterolo. 2018;1:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giuffrida P, Vanoli A, Biletta E, et al. Increase in chromogranin A- and serotonin-positive cells in pouch mucosa of patients with ulcerative colitis undergoing proctocolectomy. Dig Liver Dis. 2018;50:1205–1213. [DOI] [PubMed] [Google Scholar]
- 18. Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kéreveur A, Callebert J, Humbert M, et al. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler Thromb Vasc Biol. 2000;20:2233–2239. [DOI] [PubMed] [Google Scholar]
- 20. Lau WK, Chan-Yeung MM, Yip BH, et al. ; COPD Study Group of the Hong Kong Thoracic Society The role of circulating serotonin in the development of chronic obstructive pulmonary disease. Plos One. 2012;7:e31617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Odaka Y, Takahashi J, Tsuburaya R, et al. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur Heart J. 2017;38:489–496. [DOI] [PubMed] [Google Scholar]
- 22. Rouzaud Laborde C, Delmas C, Mialet-Perez J, et al. First evidence of increased plasma serotonin levels in Tako-Tsubo cardiomyopathy. Biomed Res Int. 2013;2013:847069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selim AM, Sarswat N, Kelesidis I, et al. Plasma serotonin in heart failure: possible marker and potential treatment target. Heart Lung Circ. 2017;26:442–449. [DOI] [PubMed] [Google Scholar]
- 24. Fröbe A, Čičin-Šain L, Jones G, et al. Plasma free serotonin as a marker for early detection of breast cancer recurrence. Anticancer Res. 2014;34:1167–1169. [PubMed] [Google Scholar]
- 25. Mamdouh F, Abdel Alem S, Abdo M, et al. Serum serotonin as a potential diagnostic marker for hepatocellular carcinoma. J Interferon Cytokine Res. 2019;39:780–785. [DOI] [PubMed] [Google Scholar]
- 26. Murugesan A, Rani MRS, Hampson J, et al. Serum serotonin levels in patients with epileptic seizures. Epilepsia. 2018;59:e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nedvídková J, Papezová H, Haluzík M, et al. Interaction between serum leptin levels and hypothalamo-hypophyseal-thyroid axis in patients with anorexia nervosa. Endocr Res. 2000;26:219–230. [DOI] [PubMed] [Google Scholar]
- 28. Marcinko D, Pivac N, Martinac M, et al. Platelet serotonin and serum cholesterol concentrations in suicidal and nonsuicidal male patients with a first episode of psychosis. Psychiatry Res. 2007;150:105–108. [DOI] [PubMed] [Google Scholar]
- 29. Ren C, Liu J, Zhou J, et al. Low levels of serum serotonin and amino acids identified in migraine patients. Biochem Biophys Res Commun. 2018;496:267–273. [DOI] [PubMed] [Google Scholar]
- 30. Russell IJ, Michalek JE, Vipraio GA, et al. Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome. J Rheumatol. 1992;19:104–109. [PubMed] [Google Scholar]
- 31. Klavdianou K, Liossis SN, Papachristou DJ, et al. Decreased serotonin levels and serotonin-mediated osteoblastic inhibitory signaling in patients with ankylosing spondylitis. J Bone Miner Res. 2016;31:630–639. [DOI] [PubMed] [Google Scholar]
- 32. Sikander A, Sinha SK, Prasad KK, et al. Association of serotonin transporter promoter polymorphism (5-HTTLPR) with microscopic colitis and ulcerative colitis. Dig Dis Sci. 2015;60:887–894. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Gong H, Lopez R, et al. Correlation between serum serotonin and endoscopy inflammation scores in patients with ileal pouches. J Crohns Colitis. 2013;7:e133–e142. [DOI] [PubMed] [Google Scholar]
- 34. Routy JP, Routy B, Graziani GM, et al. The Kynurenine pathway is a double-edged sword in immune-privileged sites and in cancer: implications for immunotherapy. Int J Tryptophan Res. 2016;9:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stockinger B, Di Meglio P, Gialitakis M, et al. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. [DOI] [PubMed] [Google Scholar]
- 36. Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153:1504–1516.e2. [DOI] [PubMed] [Google Scholar]
- 38. Dudzińska E, Szymona K, Kloc R, et al. Increased expression of kynurenine aminotransferases mRNA in lymphocytes of patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2019;12:1756284819881304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sofia MA, Ciorba MA, Meckel K, et al. Tryptophan metabolism through the Kynurenine pathway is associated with endoscopic inflammation in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Travis SPL, Stange EF, Lémann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut 2006;55:i16–i35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stange EF, Travis SP, Vermeire S, et al. ; European Crohn’s and Colitis Organisation (ECCO) European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis. 2008;2:1–23. [DOI] [PubMed] [Google Scholar]
- 42. Fuertig R, Ceci A, Camus SM, et al. LC-MS/MS-based quantification of kynurenine metabolites, tryptophan, monoamines and neopterin in plasma, cerebrospinal fluid and brain. Bioanalysis. 2016;8:1903–1917. [DOI] [PubMed] [Google Scholar]
- 43. Shen L, Weber CR. Pathological diagnosis of inflammatory bowel disease. In: Cohen RD, ed. Inflammatory Bowel Disease. Totowa, NJ: Humana Press; 2017:121–136. [Google Scholar]
- 44. Jayawardena D, Guzman G, Gill RK, et al. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am J Physiol Gastrointest Liver Physiol. 2017;313:G16–G25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gill RK, Pant N, Saksena S, et al. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol. 2008;294:G254–G262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Epperson N, Czarkowski KA, Ward-O’Brien D, et al. Maternal sertraline treatment and serotonin transport in breast-feeding mother-infant pairs. Am J Psychiatry. 2001;158:1631–1637. [DOI] [PubMed] [Google Scholar]
- 47. Norouzinia M, Chaleshi V, Alizadeh AHM, Zali MR. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench. 2017;10:155–167. [PMC free article] [PubMed] [Google Scholar]
- 48. Korolkova OY, Myers JN, Pellom ST, et al. Characterization of serum cytokine profile in predominantly colonic inflammatory bowel disease to delineate ulcerative and Crohn’s colitides. Clin Med Insights Gastroenterol. 2015;8:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–43.e1. [DOI] [PubMed] [Google Scholar]
- 50. Costedio MM, Coates MD, Danielson AB, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vermeire S. Review article: genetic susceptibility and application of genetic testing in clinical management of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24 Suppl 3:2–10. [DOI] [PubMed] [Google Scholar]
- 52. Saverymuttu SH, Hodgson HJ, Chadwick VS, et al. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut. 1986;27:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ashorov O, Hamouda D, Dickman R, et al. Clinical accuracy of a new rapid assay for fecal calprotectin measurement. Clin Lab. 2020;66:4. [DOI] [PubMed] [Google Scholar]
- 54. Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watanabe M, Watanabe N, Iwao Y, et al. The serum factor from patients with ulcerative colitis that induces T cell proliferation in the mouse thymus is interleukin-7. J Clin Immunol. 1997;17:282–292. [DOI] [PubMed] [Google Scholar]
- 57. Spohn SN, Bianco F, Scott RB, et al. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology. 2016;151:933–944.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim JJ, Bridle BW, Ghia JE, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795–4804. [DOI] [PubMed] [Google Scholar]
- 59. Guseva D, Holst K, Kaune B, et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516–1529. [DOI] [PubMed] [Google Scholar]
- 60. Rapalli A, Bertoni S, Arcaro V, et al. Dual role of endogenous serotonin in 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Front Pharmacol. 2016;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Margolis KG, Stevanovic K, Li Z, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. [DOI] [PubMed] [Google Scholar]
- 63. Manzella C, Singhal M, Alrefai WA, et al. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci Rep. 2018;8:6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Manzella CR, Ackerman M, Singhal M, et al. Serotonin modulates AhR activation by interfering with CYP1A1-mediated clearance of AhR ligands. Cell Physiol Biochem. 2020;54:126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takamura T, Harama D, Matsuoka S, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–689. [DOI] [PubMed] [Google Scholar]
- 66. Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 2011;120:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furumatsu K, Nishiumi S, Kawano Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56:2532–2544. [DOI] [PubMed] [Google Scholar]
- 68. Minderhoud IM, Oldenburg B, Schipper ME, et al. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–720. [DOI] [PubMed] [Google Scholar]
- 69. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keszthelyi D, Penders J, Masclee AA, et al. Is microscopic colitis a drug-induced disease? J Clin Gastroenterol. 2012;46:811–822. [DOI] [PubMed] [Google Scholar]
- 73. Verhaegh BP, de Vries F, Masclee AA, et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:1004–1013. [DOI] [PubMed] [Google Scholar]
- 74. Khan WI. The Role of 5-HT dysregulation in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:259–261. [PMC free article] [PubMed] [Google Scholar]
- 75. Mikocka-Walus AA, Turnbull DA, Moulding NT, et al. Antidepressants and inflammatory bowel disease: a systematic review. Clin Pract Epidemiol Ment Health. 2006;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hall BJ, Hamlin PJ, Gracie DJ, et al. The effect of antidepressants on the course of inflammatory bowel disease. Can J Gastroenterol Hepatol. 2018;2018:2047242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kristensen MS, Kjærulff TM, Ersbøll AK, et al. The influence of antidepressants on the disease course among patients with Crohn’s disease and ulcerative colitis—a Danish nationwide register–based Cohort Study. Inflamm. Bowel Dis. 2018;14:829–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mikocka-Walus A, Knowles SR, Keefer L, et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752–762. [DOI] [PubMed] [Google Scholar]
- 79. Epperson CN, Jatlow PI, Czarkowski K, et al. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003;112:e425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.