Abstract
Context
Glucocorticoids (GCs) are commonly prescribed, but their use is associated with adverse metabolic effects. 5α-reductase inhibitors (5α-RI) are also frequently prescribed, mainly to inhibit testosterone conversion to dihydrotestosterone. However, they also prevent the inactivation of GCs.
Objective
We hypothesized that 5α-RI may worsen the adverse effects of GCs.
Design
Prospective, randomized study.
Patients
A total of 19 healthy male volunteers (age 45 ± 2 years; body mass index 27.1 ± 0.7kg/m2).
Interventions
Participants underwent metabolic assessments; 2-step hyperinsulinemic, euglycemic clamp incorporating stable isotopes, adipose tissue microdialysis, and biopsy. Participants were then randomized to either prednisolone (10 mg daily) or prednisolone (10 mg daily) plus a 5α-RI (finasteride 5 mg daily or dutasteride 0.5 mg daily) for 7 days; metabolic assessments were then repeated.
Main Outcome Measures
Ra glucose, glucose utilization (M-value), glucose oxidation, and nonesterified fatty acids (NEFA) levels.
Results
Co-administration of prednisolone with a 5α-RI increased circulating prednisolone levels (482 ± 96 vs 761 ± 57 nmol/L, P = 0.029). Prednisolone alone did not alter Ra glucose (2.55 ± 0.34 vs 2.62 ± 0.19 mg/kg/minute, P = 0.86), M-value (3.2 ± 0.5 vs 2.7 ± 0.7 mg/kg/minute, P = 0.37), or glucose oxidation (0.042 ± 0.007 vs 0.040 ± 0.004 mmol/hr/kg/minute, P = 0.79). However, co-administration with a 5α-RI increased Ra glucose (2.67 ± 0.16 vs 3.05 ± 0.18 mg/kg/minute, P < 0.05) and decreased M-value (4.0 ± 0.5 vs 2.6 ± 0.4 mg/kg/minute, P < 0.05), and oxidation (0.043 ± 0.003 vs 0.036 ± 0.002 mmol/hr/kg, P < 0.01). Similarly, prednisolone did not impair insulin-mediated suppression of circulating NEFA (43.1 ± 28.9 vs 36.8 ± 14.3 μmol/L, P = 0.81), unless co-administered with a 5α-RI (49.8 ± 8.6 vs 88.5 ± 13.5 μmol/L, P < 0.01).
Conclusions
We have demonstrated that 5α-RIs exacerbate the adverse effects of prednisolone. This study has significant translational implications, including the need to consider GC dose adjustments, but also the necessity for increased vigilance for the development of adverse effects.
Keywords: prednisolone, finasteride, dutasteride, insulin, hyperinsulinemic euglycemic clamp
In the United Kingdom and United States, 2% to 3% percent of the population is currently prescribed glucocorticoid (GC) therapy (1). Glucocorticoid use, both acute and chronic, is known to be associated with a number of significant adverse effects. Recurrent short-course administration is also associated with increased morbidity and mortality (2). Adverse metabolic features include obesity, skeletal muscle myopathy, hypertension, insulin resistance and diabetes mellitus and are collectively termed “Iatrogenic Cushing’s syndrome.”
5 α -reductases (5 α R) have a crucial role in the metabolism of testosterone and GCs (3). They metabolize testosterone to the more potent androgen, 5 α -dihydrotestosterone, and inactivate cortisol to 5 α -dihydrocortisol, which is then in turn metabolized to tetrahydrocortisol by 3 α -hydroxysteroid dehydrogenase. There are 2 isoforms of 5 α R: 5 α R type 1 (5 α R1) is found in the liver, nongenital skin, muscle, adipose tissue, and brain whilst 5 α R type 2 (5 α R2) is mainly expressed in the male reproductive tissues such as prostate, epididymis, and seminal vesicles but also in the liver (3). 5 α R, therefore, simultaneously enhances androgens and limits active GC availability and represents a potent prereceptor regulatory step in steroid hormone action.
The 5 α R have an established role in the regulation of metabolic phenotypes. 5 α R1 knock-out (KO) male mice are glucose intolerant and have a higher incidence of hepatosteatosis and liver fibrosis (4, 5). Cross-sectional studies in humans have shown that 5 α R activity correlates positively with body mass index (BMI) (6–8) and tracks longitudinally over time, with both weight and insulin resistance (6). Conversely, weight loss is associated with reduced 5 α R activity (6–8). 5 α R inhibitors (5α-RI) such as dutasteride and finasteride are prescribed widely for their antiandrogenic effects in conditions such as benign prostate hyperplasia (BPH), prostate cancer, alopecia, as well as in some patients with polycystic ovary syndrome (PCOS). Dutasteride is a nonselective 5α-RI inhibiting both 5 α R1 and 5 α R2, whilst finasteride is a selective 5 α R2 inhibitor. The ability of these drugs to regulate metabolic phenotypes has only been examined in a very small number of studies. Dutasteride (and not finasteride) treatment worsened skeletal muscle and hepatic insulin sensitivity and increased hepatic triglyceride accumulation (9, 10). Most recently, analysis of data from primary care prescriptions has suggested a significant association between 5α-RI prescriptions and the incidence of type 2 diabetes (T2D) (11).
Patients who are prescribed GCs often have other comorbidities necessitating treatment with other medications and there are numerous examples of drug interactions altering GC exposure, leading to clinical signs and symptoms of GC excess. Such medications include protease inhibitors, antifungals, antibiotics, immunosuppressive medications, and combined oral contraceptives (12–14). Despite the well-recognized role of 5 α R in GC metabolism (including synthetic GCs (3)), their ability to negatively impact upon the adverse effect profile associated with prescribed GC has not been explored.
We have therefore undertaken a detailed, proof-of-concept experimental medicine study in healthy volunteers, to test the hypothesis that 5α-RIs can worsen the metabolic impact of prescribed prednisolone, putatively, through decreased metabolism and generation of inactive metabolites, and/or increased prednisolone clearance (15, 16).
Materials and Methods
Clinical protocol
The clinical protocol received full ethical approval from the Wales 7 Research Ethics Committee (reference 15/WA/0071) (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/find-it-2/). Nineteen healthy male volunteers were recruited from local advertisement, the Oxford Biobank, (reference 08/H0606/107 + 5), National Health Service hospitals and General Practice surgeries. All were aged 18–65 years, had a BMI 20–35kg/m2, did not have T1D or T2D, were normotensive, had not used GC therapy within the last 6 months, and were not on any medications known to impact upon GC metabolism. The participants attended the Clinical Research Unit, Churchill Hospital (Oxford, United Kingdom) at 8:00 am and underwent subcutaneous abdominal adipose biopsy after an overnight fast (from 12:00 am). Aspiration of adipocytes (approximately 1 g of tissue) was achieved using a needle and syringe and liposuction following administration of local anesthetic. At 9:00 am an adipose microdialysis catheter (CMA Microdialysis, Solna, Sweden) was inserted under local anesthetic into the subcutaneous abdominal adipose tissue 5 cm to 1 side of the umbilicus. Using the microdialysis pump, a microdialysate solution (0.9% sterile saline solution) was introduced into the catheter (perfusion rate = 0.3 μl/minute). Samples were collected every 30 minutes until the completion of the hyperinsulinemic clamp (see below).
Two-step hyperinsulinemic euglycemic clamp.
On commencement of the 2-step hyperinsulinemic euglycemic clamp, a bolus of [U-13C]-glucose (Cambridge Isotope Laboratories, Andover, MA) was administered (2 mg/kg over 1 minute followed by a continuous infusion in 0.9% saline [20 μg/kg/minute]). At the same time a [2,2-2H2]-palmitate (Cambridge Isotope Laboratories, Andover, MA) in human serum albumin infusion was started (0.03 μmol/kg/minute). Blood glucose was monitored at 15-minute intervals during the initial 120-minute (t = 0–120 minutes) basal phase. At t = 120 minutes, an insulin infusion (Actrapid; Novo Nordisk, Denmark) was infused at 20 mU/m2/minute (low-dose) alongside an infusion of 20% dextrose supplemented with [U-13C]-glucose enriched to 4%; blood glucose levels were monitored at 5-minute intervals (t = 120–360 minutes). At t = 240 minutes, the insulin rate was increased to 100 mU/m2/minute (high-dose) and continued for another 120 minutes (t = 240–360 minutes). In addition to blood glucose sampling, blood samples were taken at 3 time points in the last 30 minutes of each phase (basal, low-, and high-insulin) for steady-state measurements of insulin, whole body glucose turnover (glucose production, glucose disposal [Gd] glucose), endogenous glucose production (EGP) rate, and lipolysis (Ra palmitate). Glucose and palmitate disposal rates were calculated using a modified version of the Steele equations (17, 18). Exhaled breath samples were collected from the participants at 60-minute intervals throughout the study to allow for analysis of glucose oxidation (13CO2 production).
Participants were then randomized to 1 of 3 drug regimen arms: prednisolone 10 mg once daily (OD), prednisolone 10 mg and finasteride 5 mg OD, or prednisolone 10 mg and dutasteride 0.5 mg OD. The medications were taken for 7 days and the participants returned to the research facility on the last day of administration, and all investigations were repeated.
Biochemical and stable isotope analysis
Cholesterol, liver biochemistry, and plasma glucose were measured using standard laboratory methods (Roche Modular system, Roche Ltd, Lewes, United Kingdom). Insulin was measured using a commercially available colorimetric ELISA (Mercodia, Uppsala, Sweden) with an in-house coefficient of variation of less than 15%. Nonesterified fatty acids (NEFA), beta-hydroxybutyrate (OHB), and glycerol serum concentrations were measured using the 600/650 iLAB clinical chemistry analyzer (Instrumentation Laboratory, Milano, Italy). Microdialysis samples were analyzed using a mobile photometric, enzyme-kinetic analyzer (CMA ISCUS Flex, Solna, Sweden) for glycerol, pyruvate, glucose, and lactate.
Prednisolone, prednisone, cortisol, and cortisone were extracted from participants’ serum, calibration standards, and quality control by liquid:liquid extraction using diethyl ether. The upper solvent layer containing the steroids was separated from the bottom aqueous layer using an ice bath (Thermo Fisher Scientific, Neslab CB-60 Thermo Electron Corporation) filled with ethanol (VWR). A rotary evaporator was used to dry down extracts and they were then reconstituted in 50:50 methanol water before analysis on the mass spectrometer.
Analysis was performed by LC-MS/MS, specifically a Shimadzu HPLC system coupled to an API 5000 tandem mass spectrometer (Sciex, Warrington, United Kingdom). The ion source used was atmospheric pressure ionisation and the software was Analyst version 1.7.
Plasma enrichment of [U-13C]-glucose was measured using gas chromatography-mass spectrometry (model 5973; Agilent Technologies, Cheshire, United Kingdom). The plasma lipids were extracted according to the method of Folch (19) and the NEFA fraction isolated by solid phase extraction (Bond Elut NH2- Aminopropyl columns). Following methylation of the NEFA fraction, the samples were run on GC (model 5890; Agilent Technologies, Cheshire, United Kingdom) to determine the relative amount of individual fatty acids. Whole body lipolysis (Ra palmitate) was calculated using extracted total circulating lipid and by measuring deuterium ([2,2-2H2]-palmitate) enrichment in the NEFA fraction. [2,2-2H2]-palmitate enrichments were determined by gas chromatography-mass spectrometry using a 5890 GC coupled to a 5973N MSD (Agilent Technologies, San Diego, CA). Ions with mass-to-charge ratios (m/z) of 270 (M + 0) and 272 (M + 2) were determined by selected ion monitoring (20).
Ribonucleic acid extraction and Ribonucleic acid sequencing
Total ribonucleic acid (RNA) from adipose tissue biopsies was extracted using the Tri-Reagent system (Sigma-Aldrich, Dorset, United Kingdom). Assessment of RNA quality was performed using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Hemel Hempstead, United Kingdom). Total RNA was enriched for polyA-tailed mRNA using oligo (dT) beads. The Illumina TruSeq Stranded mRNA HT Sample Prep Kit was used to prepare cDNA libraries for sequencing. In-house 8 bp indexes (21) were used to multiplex samples (10-plex), which were then sequenced over 1 lane of an Illumina HiSeq4000 machine using a HiSeq 3000/4000 PE Cluster Kit and an SBS Kit. Paired-end sequencing (75 bp) was performed at a depth of ~25 million read pairs per sample.
Reads were mapped with STAR 2.5.1b (22) on default settings with GENCODE version 19 (23) as transcriptome reference and GRCh37 asa genome reference. Gene level read counts for all protein-coding and long intergenic noncoding RNA (lincRNA) transcripts present in GENCODE version 19 were quantified in a strand-specific manner with featureCounts (24) from the Subread package v1.5.0-p2. For plotting purposes, we also normalized the gene counts to transcripts per million.
Statistical approach
Data are presented as mean ± SE unless otherwise stated. In the first instance, data was analyzed as 3 groups: prednisolone only, prednisolone + finasteride, and prednisolone + dutasteride. Delta changes for the variables were calculated as the difference: follow-up (7 days post-treatment) minus baseline. A Shapiro-Wilk test was run to check for the normality of distribution of the data. For normally distributed variables, 1-way ANOVA was conducted to determine if there were differences between the 3 groups both for baseline measures and delta changes. For non-normally distributed variables, Kruskal-Wallis test was done instead. There were no outliers, as assessed by boxplot, and there was homogeneity of variances, as assessed by Levene’s test of homogeneity of variances.
In cases where the differences between the 3 groups was not significant, further stratification was done by merging the 2 groups that received both prednisolone and a 5α-RI, thus dividing the participants into 2 groups: prednisolone only and prednisolone + 5α-RI. Paired t-tests were then used to compare individual variables before and after intervention within each participants’ group where the data was normally distributed and, in cases the data was not normally distributed, the Wilcoxon test was used instead. Absolute change between the 2 groups, as well as post-treatment comparisons, were calculated using unpaired t-test where the data was normally distributed or using the Mann-Whitney test where the data was not normally distributed.
Statistical analyses were performed using SPSS, version (IBM, Chicago, IL) and GraphPad Prism 8 software package (GraphPad Software, La Jolla, CA) for MacOS. Area under the curve (AUC) analysis was performed using the trapezoidal method.
For RNA-sequencing data, differential expression analysis was performed using edgeR (25) in R 3.2.2 on normalized gene counts for all autosomal protein-coding and lincRNA genes that were expressed at > 2 count per million in all samples. A paired model was fitted to the data, and significance was determined by empirical Bayes moderated t-statistics implemented in edgeR. Differentially regulated genes were defined by a false discovery rate (Benjamini-Hochberg method) adjusted P-value < 5%.
Results
Clinical characteristics
Nineteen healthy male volunteers were recruited (mean age 45 ± 2years, BMI 27.1 ± 0.7 kg/m2). There were no significant differences when comparing participants randomized to prednisolone treatment alone (n = 6), prednisolone + finasteride (n = 7), or prednisolone + dutasteride (n = 6). Subsequently, the 2 arms that received prednisolone + 5α-RI were merged and post hoc analysis was undertaken as 2 groups; prednisolone alone (n = 6) and prednisolone + 5α-RI (n = 13). Detailed demographic and biochemical data are presented in Table 1.
Table 1.
Clinical characteristics and changes in fasting biochemistry before and after randomization to 7 days of treatment with either prednisolone (10 mg once daily) or prednisolone (10 mg once daily) and a 5α-RI (finasteride [5 mg once daily] or dutasteride [0.5 mg once daily])
| Prednisolone | Prednisolone + 5α-RI | |||
|---|---|---|---|---|
| Clinical Variable | Before | After | Before | After |
| Age, y | 46.7 ± 3.9 | 44.5 ± 2.5 | ||
| Weight, kg | 89.4 ± 2.8 | 86.2 ± 3.9 | ||
| BMI, kg/m2 | 27.8 ± 1.2 | 26.7 ± 0.9 | ||
| SBP, mm Hg | 140.8 ± 3.1 | 141.8 ± 3.0 | ||
| DBP, mm Hg | 86.7 ± 2.5 | 82.0 ± 2.6 | ||
| HbA1c, mmol/mol (20-42) | 34.3 ± 1.2 | 32.1 ± 1.0 | ||
| Fasting glucose, mmol/L | 4.8 ± 0.2 | 5.1 ± 0.3 | 4.5 ± 0.1 | 4.7 ± 0.1 |
| Fasting insulin, pmol/L | 20.4 ± 6.2 | 33.2 ± 9.9 | 33.4 ± 6.9 | 29.4 ± 4.2 |
| HDL cholesterol, mmol/L | 1.4 ± 0.1a | 1.5 ± 0.1a | 1.2 ± 0.1 | 1.3 ± 0.1 |
| Total cholesterol, mmol/L | 5.1 ± 0.5 | 5.0 ± 0.4 | 4.8 ± 0.3 | 4.9 ± 0.4 |
| AST, IU/L (15-42) | 21.7 ± 1.9 | 18.2 ± 1.7 | 19.0 ± 1.3 | 19.5 ± 2.6 |
| Bilirubin, mmol/L (<21) | 19.2 ± 4.0 | 14.0 ± 2.5 | 12.9 ± 1.8 | 11.2 ± 1.4 |
| ALT, IU/L (10-45) | 22.7 ± 3.4 | 24.8 ± 4.1 | 22.1 ± 3.4 | 20.9 ± 3.5 |
| ALP, IU/L (30–130) | 58.7 ± 6.3 | 47.0 ± 11.2 | 62.0 ± 6.6a | 55.8 ± 5.3a |
| Albumin, g/L (32-50) | 37.5 ± 1.1 | 37.5 ± 0.7 | 37.6 ± 0.6 | 36.2 ± 0.6 |
| Palmitic acid 16:00, % | 32.0 ± 3.3c | 28.0 ± 1.2 | 27.5 ± 1.3c | 26.5 ± 1.0 |
| Stearic acid 18:00, % | 22.9 ± 1.9a | 19.2 ± 1.9a | 21.6 ± 1.4a | 18.5 ± 0.9a |
| Oleic acid 18: 1n-9, % | 21.6 ± 4.1a | 28.3 ± 1.7a | 26.9 ± 2.5b | 31.6 ± 2.0b |
| Linoleic acid 18: 2n-6, % | 13.1 ± 1.2 | 16.0 ± 2.9 | 15.0 ± 1.8 | 13.6 ± 1.2 |
| Fasting triglycerides, μmol/L | 659 ± 140 | 568 ± 138 | 823 ± 116 | 786 ± 91 |
| Serum prednisolone, nmol/L | 482 ± 96* | 761 ± 57* | ||
| Serum prednisone, nmol/L | 95 ± 16 | 105 ± 9 | ||
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; DHT, dihydrotestosterone; HDL, high-density lipoprotein; SBP, systolic blood pressure. Local reference ranges are included in parentheses.
a P < 0.05, before vs after treatment.
b P < 0.01, before vs after treatment.
c P < 0.05, before treatment between the 2 groups.
*P < 0.05, after vs after treatment.
Circulating prednisolone levels were significantly high in participants taking 5α-RIs compared to those taking prednisolone alone (482 ± 96 vs 761 ± 57 nmol/L, P = 0.029). There was no difference in circulating prednisone levels (95 ± 16 vs 105 ± 9 nmol/L, P = 0.54) (Fig. 1A, Table1).
Figure 1.
Effect of prednisolone and co-administration with a 5α-RI on circulating prednisolone and prednisone levels (A), glucose production (Ra Glucose) (B, C), endogenous glucose production (EGP) rate (D, E) and glucose utilization (M-value) (F, G) during a two-step hyperinsulinemic euglycemic clamp. Absolute change refers to the difference between pre- and post-treatment values (C, E, G). Data shown are from low-dose insulin infusion (B–G). Data are medians and error bars are IQR. Datapoints represent individual patients. White circles represent pre-treatment levels and black squares post-treatment levels (7 days), light grey circles are prednisolone alone and dark grey squares, prednisolone + 5α-RI.
There was no impact of either prednisolone alone or the co-administration of prednisolone + 5α-RI on fasting glucose or insulin levels, or on circulating lipids and liver chemistry. In addition, the changes observed in the composition of the fatty acid pool were similar in both groups (Table1).
5α-RI co-administration with prednisolone impairs hepatic insulin sensitivity.
Basal Ra glucose was not altered by treatment with prednisolone alone. However, when combined with 5α-RIs, Ra glucose increased significantly; the absolute change between the 2 groups was not significant (P = 0.30) (Table 2, Fig. 1B and 1C). The EGP rate was unchanged by prednisolone treatment, but following 5α-RI co-administration the EGP significantly increased, which was consistent with worsening hepatic insulin sensitivity (Table 2, Fig. 1D and 1E). The absolute change between the 2 groups was not statistically significant (P = 0.15).
Table 2.
The effect of prednisolone and co-administration of prednisolone and a 5α-RI on glucose and lipid metabolism during a 2-step hyperinsulinemic euglycemic clamp
| Prednisolone | Prednisolone + 5α-RI | |||||
|---|---|---|---|---|---|---|
| Metabolic Variable | Before | After | Absolute Change | Before | After | Absolute Change |
| NEFAs, μmol/L | ||||||
| Basal | 490.3 ± 53.0 | 393.5 ± 67.3 | -96.8 ± 63.9 | 405.8 ± 30.3 | 465.9 ± 39.2 | 60.1 ± 45.2 |
| Low insulin | 43.1 ± 28.9 | 36.8 ± 14.3* | -6.3 ± 16.5a | 49.8 ± 8.6b | 88.5 ± 13.5b,* | 38.7 ± 10.9a |
| High insulin | 13.8 ± 4.8 | 16.3 ± 4.0 | 2.5 ± 3.3 | 22.6 ± 4.3 | 20.4 ± 3.0 | -2.2 ± 3.6 |
| Glycerol, μmol/L | ||||||
| Basal | 33.0 ± 3.1 | 29.9 ± 4.2 | -3.2 ± 4.6 | 27.2 ± 1.9 | 29.2 ± 2.6 | 2.0 ± 3.7 |
| Low insulin | 8.2 ± 1.5b | 9.9 ± 1.5b | 1.8 ± 0.3 | 7.1 ± 0.9b | 10.8 ± 1.1b | 3.7 ± 1.2 |
| High insulin | 6.9 ± 1.4 | 7.1 ± 0.9 | 0.3 ± 0.8 | 6.0 ± 0.6 | 7.4 ± 1.0 | 1.4 ± 1.0 |
| β-hydroxybutyrate, μmol/L | ||||||
| Basal | 135.2 ± 35.5 | 93.5 ± 39.0 | -41.7 ± 43.8 | 69.0 ± 14.1 | 87.8 ± 19.3 | 18.7 ± 21.1 |
| Low insulin | 15.9 ± 2.7 | 16.7 ± 1.8* | 0.8 ± 2.0 | 19.9 ± 2.1a | 25.6 ± 2.2a,* | 5.7 ± 2.1 |
| High insulin | 9.0 ± 1.2 | 10.0 ± 1.8 | 1.0 ± 2.3 | 13.6 ± 1.0 | 13.6 ± 0.9 | 0.0 ± 0.8 |
| M-value, mg/kg・minute | ||||||
| Low insulin | 3.2 ± 0.5 | 2.7 ± 0.7 | -0.5 ± 0.5 | 4.0 ± 0.5a | 2.6 ± 0.4a | -1.4 ± 0.5 |
| High insulin | 11.5 ± 1.2 | 12.0 ± 1.9 | -1.2 ± 2.0 | 10.9 ± 1.1 | 9.8 ± 0.9 | -1.1 ± 0.9 |
| M/I-value, mg/kg ・minute・pmol・L | ||||||
| Low insulin | 0.026 ± 0.005a | 0.015 ± 0.003a | -0.010 ± 0.003 | 0.026 ± 0.004a | 0.017 ± 0.002a | -0.010 ± 0.003 |
| High insulin | 0.027 ± 0.006 | 0.045 ± 0.015 | 0.015 ± 0.009 | 0.027 ± 0.004 | 0.026 ± 0.003 | -0.001 ± 0.002 |
| Ra glucose, mg/kg ・minute | ||||||
| Basal | 2.55 ± 0.34 | 2.62 ± 0.19 | 0.07 ± 0.38 | 2.67 ± 0.16a | 3.05 ± 0.18a | 0.38 ± 0.13 |
| EGP, mg/kg ・minute | ||||||
| Low insulin | 1.40 ± 0.26 | 1.29 ± 0.29 | -0.11 ± 0.36 | 1.11 ± 0.16a | 2.05 ± 0.30a | 0.93 ± 0.40 |
| Gd, mg/kg ・minute | ||||||
| Low insulin | 2.5 ± 0.6a | 1.8 ± 0.6a | -0.8 ± 0.2 | 2.9 ± 0.5 | 2.0 ± 0.3 | -0.8 ± 0.4 |
| High insulin | 11.0 ± 2.2 | 10.4 ± 2.4 | -0.6 ± 1.5 | 8.8 ± 1.1 | 8.4 ± 1.1 | -0.4 ± 1.2 |
| Ra palmitate, mg/kg ・minute | ||||||
| Basal | 1.98 ± 0.26 | 1.73 ± 0.17 | -0.26 ± 0.32 | 1.60 ± 0.09 | 1.85 ± 0.21 | 0.25 ± 0.20 |
| Low insulin | 0.66 ± 0.11 | 0.63 ± 0.09 | -0.03 ± 0.08 | 0.60 ± 0.05 | 0.96 ± 0.30 | 0.35 ± 0.26 |
| High insulin | 0.51 ± 0.06 | 0.46 ± 0.05 | -0.05 ± 0.04 | 0.49 ± 0.04 | 0.53 ± 0.08 | 0.04 ± 0.06 |
| Breath 13-CO2 AUC, mmol/h/kg | ||||||
| Low insulin | 0.042 ± 0.007 | 0.040 ± 0.004 | -0.002 ± 0.005 | 0.043 ± 0.003b | 0.036 ± 0.002b | -0.007 ± 0.002 |
| High insulin | 0.073 ± 0.003 | 0.056 ± 0.007 | -0.018 ± 0.009 | 0.070 ± 0.003c | 0.060 ± 0.002c | -0.010 ± 0.002 |
| Adipose microdialysis | ||||||
| Glycerol AUC, μmol/L・hour | ||||||
| Basal | 227.7 ± 37.1 | 242.1 ± 50.8 | 12.0 ± 23.5 | 336.4 ± 80.8 | 235.7 ± 23.2 | -77.4 ± 53.4 |
| Low insulin | 124.0 ± 38.0 | 151.7 ± 34.9 | 23.1 ± 17.5 | 199.9 ± 32.9 | 199.4 ± 39.1 | -0.4 ± 26.2 |
| High insulin | 68.0 ± 6.7 | 98.8 ± 24.9 | 25.7 ± 21.8 | 140.4 ± 34.0 | 136.8 ± 39.1 | -3.0 ± 22.6 |
| Pyruvate AUC, μmol/L・hour | ||||||
| Basal | 127.3 ± 11.4 | 132.2 ± 47.7 | 4.1 ± 35.9 | 141.8 ± 18.9 | 133.0 ± 17.2 | -6.8 ± 15.5 |
| Low insulin | 116.2 ± 17.3 | 136.0 ± 19.9 | 16.5 ± 9.6 | 144.7 ± 16.9 | 117.3 ± 21.4 | -23.2 ± 22.8 |
| High insulin | 110.6 ± 15.5 | 106.1 ± 22.6 | -3.8 ± 24.2 | 127.9 ± 22.3 | 123.2 ± 29.4 | -4.0 ± 18.0 |
| Lactate AUC, mmol/l ・hour | ||||||
| Basal | 1.7 ± 0.5 | 1.9 ± 0.7 | 0.2 ± 0.7 | 1.8 ± 0.4 | 1.5 ± 0.2 | -0.2 ± 0.3 |
| Low insulin | 3.0 ± 1.5 | 2.4 ± 0.6 | -0.5 ± 1.2 | 2.4 ± 0.5 | 1.7 ± 0.3 | -0.5 ± 0.6 |
| High insulin | 3.3 ± 1.4 | 1.9 ± 0.4 | -1.2 ± 0.9 | 2.4 ± 0.5 | 1.7 ± 0.4 | -0.5 ± 0.5 |
| Glucose AUC, mmol/l ・hour | ||||||
| Basal | 3.9 ± 0.5 | 3.7 ± 0.5 | -0.2 ± 0.6 | 4.2 ± 0.3 | 4.3 ± 0.3 | 0.1 ± 0.3 |
| Low insulin | 3.5 ± 0.7 | 3.9 ± 0.5 | 0.4 ± 0.9 | 3.3 ± 0.3 | 3.1 ± 0.4 | -0.2 ± 0.4 |
| High insulin | 3.5 ± 0.7 | 3.5 ± 0.7 | 0.0 ± 0.9 | 3.2 ± 0.4 | 2.6 ± 0.3 | -0.5 ± 0.4 |
Data shown are before, after, and absolute change following randomization to 7 days of treatment with either prednisolone (10 mg once daily) or prednisolone (10 mg once daily) and a 5aRI (finasteride [5 mg once daily] or dutasteride [0.5 mg once daily]).
Abbreviations: EGP, endogenous glucose production; Gd, glucose disposal; NEFA, nonesterified fatty acids.
a P < 0.05, before vs after treatment.
b P < 0.01, before vs after treatment.
c P < 0.001, before vs after treatment.
*P < 0.05, after vs after treatment.
5α-RIs increase GC-induced peripheral insulin resistance and glucose oxidation.
Prednisolone alone had no impact on the M-value during the low- or high-dose insulin infusion. However, when combined with a 5α-RI, under low-dose insulin infusion it decreased significantly (Table 2, Fig. 1F and 1G). Glucose disposal was decreased by prednisolone alone. Although the magnitude in reduction of Gd with prednisolone + 5α-RI was similar to prednisolone alone, this did not reach statistical significance (P = 0.080) (Table 2). There were no differences in the absolute changes between the groups (Table 2).
13CO2 production from the infused [U-13C]-glucose was used as a marker of glucose uptake and subsequent oxidation. Co-administration of prednisolone + 5α-RI (but not prednisolone alone) decreased glucose oxidation across the 2-step clamp (both low- and high-dose insulin phases) (Table 2). The absolute change between the 2 groups, under both low- and high-dose insulin phases, was not different (Table 2).
5α-RI co-administration with prednisolone impairs adipose tissue insulin sensitivity.
Prednisolone alone had no impact on insulin-mediated suppression of circulating NEFA levels during the low- or high-dose insulin infusion. However, when combined with a 5α-RI, there was a significant reduction in the ability of low-dose insulin (but not high-dose insulin) to suppress circulating NEFA levels (Table 2, Fig. 2A–2C).
Figure 2.
Effect of prednisolone and co-administration with a 5α-RI on circulating non-esterified fatty acids (NEFA) (A–C), glycerol (D–F), and β-hydroxybutyrate (OHB) (G–I) levels across a 2-step hyperinsulinemic euglycemic clamp. Absolute change refers to the difference between pre and post-treatment values (C, F, I). Data are medians and error bars are IQR. Datapoints represent individual patients. White circles represent pre-treatment levels and black squares post-treatment levels (7 days), light grey circles are prednisolone alone and dark grey squares, prednisolone + 5α-RI.
Prednisolone alone, as well as prednisolone + 5α-RI were equally effective in impairing the ability of insulin to suppress circulating glycerol under low- but not high-dose insulin infusions. There was no significant difference in the absolute change between the 2 groups across the clamp (Table 2, Fig. 2D–2F).
Adipose tissue lipolysis (as measured using [2,2-2H2]-palmitate) was decreased by insulin (both low- and high-dose), but there was no impact of prednisolone or prednisolone + 5α-RI treatment (Table 2). Insulin decreased fatty acid oxidation, as measured by circulating levels of beta-hydroxybutyrate. Prednisolone had no impact on the ability of insulin to suppress beta-hydroxybutyrate levels, but when co-administered with a 5α-RI, insulin-mediated suppression of beta-hydroxybutyrate was impaired (Table 2, Fig. 2G and 2H). The absolute change between the 2 groups was different but did not reach statistical significance (P = 0.17) (Table 2, Fig. 2I). There were no differences following high-dose insulin infusion (Table 2).
Adipose tissue microdialysis was used to specifically examine the impact of treatment upon subcutaneous adipose tissue. Insulin decreased adipose tissue interstitial glycerol levels in a dose-dependent manner (Table 2). However, neither prednisolone alone nor prednisolone + 5α-RI had any impact on subcutaneous adipose interstitial fluid levels of glycerol, glucose, pyruvate, and lactate or their response to low- and high-dose insulin infusion (Table 2).
Subcutaneous adipose tissue gene expression.
RNA sequencing analysis identifed only 11 genes (PLA2G2A, ETNK2, MALL, EDN1, SOX7, FAM166B, LINC00844, ARNTL, KRT1, NFKBIA, GADD45B) that were regulated by prednisolone treatment, several of which are recognized GC-targets (including endothelin 1, aryl hydrocarbon receptor nuclear translocator-like, NFKB inhibitor alpha and growth arrest, and DNA damage inducible beta) (26–29). The expression of only a single gene changed following prednisolone + 5α-RI treatment (ribosomal protein L41 pseudogene 1).
Finasteride and dutasteride have a similar action to augment the metabolic impact of prednisolone.
A post hoc subgroup analysis was used to compare the administration of either finasteride or dutasteride. There were no differences in the fasting basal metabolic parameters between the subgroups (data not shown). Prednisolone levels were similar in those individuals treated with either prednisolone + finasteride or prednisolone + dutasteride (806 ± 66 vs 709 ± 99 nmol/L, P = 0.89). In both cases, levels were higher than in participants treated with prednisolone alone (482 ± 96 vs 806 ± 66 vs 709 ± 99 nmol/L, P = 0.046).
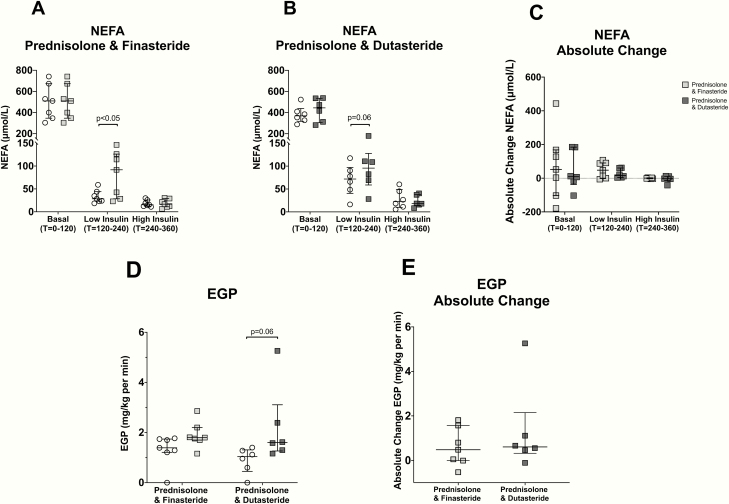
The low-dose insulin-mediated suppression of NEFA was similar in individuals treated with finasteride + prednisolone (P < 0.05) and dutasteride + prednisolone (P = 0.058); there was no significant difference between the groups (Fig. 3A–3C). Changes in EGP were similar in individuals treated with either finasteride or dutasteride and there was no significant difference between the groups (Fig. 3D and 3E).
Figure 3.
Effect of co-administration of prednisolone with finasteride or dutasteride on circulating nonesterified fatty acids (NEFA) (A–C) levels across a 2-step hyperinsulinemic euglycemic clamp and endogenous glucose production (EGP) (D, E) during the low-dose insulin phase of a 2-step hyperinsulinemic euglycemic clamp. Absolute change refers to the difference between pre- and post-treatment values. Data are medians and error bars are IQR. Datapoints represent individual patients. White circles represent pre-treatment levels, light grey squares are post-treatment (7 days) finasteride + prednisolone and dark grey squares, post-treatment (7 days) with dustasteride + prednisolone.
Interestingly, the changes in glycerol, OHB, M-value, and Gd under high-dose insulin were more marked in individuals treated with finasteride (Table 3). Changes in other metabolic variables were not different between the two groups and are summarized in Table 3.
Table 3.
The impact of specific 5α-RI (finasteride 5 mg once daily or dutasteride 0.5 mg once daily) co-administered with prednisolone (10 mg once daily) on glucose and lipid metabolism during a 2-step hyperinsulinemic euglycemic clamp
| Prednisolone + Finasteride | Prednisolone + Dutasteride | |||||
|---|---|---|---|---|---|---|
| Metabolic Variable | Before | After | Absolute Change | Before | After | Absolute Change |
| NEFA, μmol/L | ||||||
| Basal | 427.0 ± 49.6 | 500.2 ± 61.9 | 73.2 ± 76.9 | 381.1 ± 33.3 | 425.9 ± 44.9 | 44.8 ± 47.3 |
| Low insulin | 33.1 ± 5.4a | 81.8 ± 19.0a | 48.7 ± 17.8 | 69.3 ± 14.2 | 96.3 ± 20.6 | 27.0 ± 11 |
| High insulin | 18.2 ± 2.7 | 18.2 ± 3.4 | 0.0 ± 1.1 | 27.8 ± 8.6 | 23.0 ± 5.2 | -4.8 ± 8.0 |
| Glycerol, μmol/L | ||||||
| Basal | 29.0 ± 3.1 | 31.5 ± 4.2 | 2.6 ± 6.3 | 25.2 ± 2.0 | 26.6 ± 2.6 | 1.3 ± 3.8 |
| Low insulin | 6.7 ± 1.0 | 11.3 ± 2.0 | 4.7 ± 1.9 | 7.5 ± 1.5 | 10.0 ± 1.0 | 2.5 ± 1.3 |
| High insulin | 5.7 ± 0.7 | 8.1 ± 1.8 | 2.4 ± 1.7 | 6.3 ± 1.1 | 6.6 ± 0.6 | 0.2 ± 0.7 |
| β-hydroxybutyrate, μmol/L | ||||||
| Basal | 59.9 ± 8.5 | 88.3 ± 29.4 | 28.5 ± 28.4 | 78.2 ± 27.7 | 87.2 ± 27.9 | 9.0 ± 33.4 |
| Low insulin | 18.5 ± 1.9a | 26.9 ± 3.6a | 8.4 ± 3.0 | 21.3 ± 3.8 | 24.3 ± 2.6 | 3.0 ± 2.8 |
| High insulin | 14.9 ± 1.1 | 14.1 ± 1.5 | -0.8 ± 1.5 | 12.2 ± 1.5 | 13.1 ± 1.2 | 0.9 ± 0.8 |
| M-value, mg/kg・minute | ||||||
| Low insulin | 3.7 ± 0.4 | 2.5 ± 0.6 | -1.1 ± 0.6 | 4.3 ± 1.1 | 2.7 ± 0.5 | -1.6 ± 0.9 |
| High insulin | 12.9 ± 0.8a | 9.9 ± 1.3a | -2.9 ± 1.2a | 8.7 ± 1.8 | 9.6 ± 1.3 | 1.0 ± 0.9a |
| M/I-value, mg/kg ・minute・pmol・L | ||||||
| Low insulin | 0.024 ± 0.003 | 0.018 ± 0.004 | -0.006 ± 0.004 | 0.029 ± 0.008 | 0.015 ± 0.003 | -0.014 ± 0.006 |
| High insulin | 0.034 ± 0.004 | 0.028 ± 0.005 | -0.005 ± 0.003 | 0.019 ± 0.005 | 0.023 ± 0.003 | 0.004 ± 0.003 |
| Ra glucose, mg/kg ・minute | ||||||
| Basal | 2.81 ± 0.20 | 3.07 ± 0.18 | 0.26 ± 0.12 | 2.52 ± 0.27 | 3.04 ± 0.36 | 0.52 ± 0.24 |
| EGP, mg/kg ・min | ||||||
| Low insulin | 1.30 ± 0.23 | 1.90 ± 0.20 | 0.60 ± 0.32 | 0.89 ± 0.21 | 2.22 ± 0.63 | 1.32 ± 0.80 |
| Gd, mg/kg ・minute | ||||||
| Low insulin | 2.7 ± 0.4 | 1.9 ± 0.5 | -0.8 ± 0.6 | 3.1 ± 1.0 | 2.2 ± 0.5 | -0.9 ± 0.7 |
| High insulin | 10.8 ± 1.3 | 8.3 ± 1.2 | -2.5 ± 1.4a | 6.5 ± 1.4 | 8.7 ± 1.9 | 2.2 ± 1.7a |
| Ra palmitate, mg/kg ・minute | ||||||
| Basal | 1.70 ± 0.11 | 2.11 ± 0.36 | 0.41 ± 0.34 | 1.48 ± 0.14 | 1.54 ± 0.10 | -0.05 ± 0.15 |
| Low insulin | 0.62 ± 0.08 | 1.24 ± 0.54 | 0.63 ± 0.47 | 0.58 ± 0.07 | 0.62 ± 0.05 | 0.04 ± 0.06 |
| High insulin | 0.58 ± 0.06 | 0.63 ± 0.14 | 0.05 ± 0.12 | 0.39 ± 0.04 | 0.41 ± 0.03 | 0.02 ± 0.03 |
| Breath 13-CO2 AUC, mmol/hr/kg | ||||||
| Low insulin | 0.039 ± 0.003 | 0.033 ± 0.002* | -0.005 ± 0.002 | 0.048 ± 0.006 | 0.040 ± 0.002* | -0.008 ± 0.005 |
| High insulin | 0.065 ± 0.004a | 0.056 ± 0.003a | -0.009 ± 0.003 | 0.076 ± 0.005b | 0.064 ± 0.004b | -0.010 ± 0.003 |
Data shown are before, after, and absolute change following randomization to 7 days of treatment with either prednisolone (10 mg once daily), prednisolone (10 mg once daily) and finasteride (5 mg once daily) or prednisolone (10 mg once daily) and dutasteride (0.5 mg once daily).
Abbreviations: EGP, endogenous glucose production; Gd, glucose disposal; NEFA, nonesterified fatty acids.
a P < 0.05, before vs after treatment.
b P < 0.01, before vs after treatment.
*P < 0.05, after vs after treatment.
Discussion
In this proof-of-concept, experimental medicine study, we have shown that the metabolic impact of prednisolone (10 mg daily) for 7 days is relatively modest. However, when co-administered with a 5α-RI, circulating prednisolone levels are increased and the adverse metabolic effects of prednisolone on peripheral, hepatic, and adipose tissue insulin sensitivity are augmented. Whilst in the acute phases of immune and inflammatory conditions, doses of prednisolone higher than 10 mg are often used; longer-term maintenance doses are often lower and, therefore, the co-administration of drugs that can impact on prednisolone metabolism may well have a clinical impact. Given that combined administration is not infrequent either in men with BPH or in women with PCOS, these data have broad clinical implications.
Previous studies have examined the metabolic effects of short-term isolated GC treatment, including both prednisolone and hydrocortisone. High doses (30–75 mg) of prednisolone for a 1- to 15-day duration causes pancreatic beta-cell dysfunction and reduced glucose tolerance (30, 31). In healthy male volunteers, treatment with low (7.5 mg) and high (30 mg) dose prednisolone for 2 weeks decreased the ability of insulin to suppress EGP and lipolysis, and high-dose (but not low-dose) treatment decreased glucose disposal and increased fasting insulin levels (32, 33). In patients with inflammatory rheumatologic disease, a 7- to 10-day course of prednisolone (6 mg) increased basal EGP, reduced glucose disposal, and increased peripheral insulin resistance (34, 35). These data are consistent with the current study, although the magnitude of effect that we observed with prednisolone treatment alone was less than in the published studies, and this is likely to reflect the fact that this was a shorter treatment duration (1 week). Both dose and duration are important, and we have shown that acute administration of a high dose of intravenous hydrocortisone (0.2 mg/kg/hour) increases EGP, limits glucose disposal, and induces systemic insulin resistance (36).
The role of 5 α R in the regulation of metabolic phenotypes is still not entirely understood. Cross-sectional observation studies provided the first evidence of dysregulation of 5 α R activity, demonstrating increased activity with weight gain and insulin resistance and reduced activity with weight loss (6–8). In rodent models, 5 α R1 KO male mice are more prone to the development of glucose intolerance as well as hepatosteatosis and liver fibrosis (4, 5). It is important to note, however, that mice (contrasting with humans) do not express 5 α R2 in the liver and therefore direct extrapolation to clinical studies cannot be made.
Very few translational, interventional clinical studies have been performed. A retrospective clinical analysis has suggested that long-term dutasteride treatment is associated with hyperglycemia and adverse circuiting lipid profiles (37). In smaller mechanistic studies, isolated treatment with dutasteride alone has demonstrated increased skeletal muscle and hepatic insulin resistance and increased hepatic triglyceride content (9, 10). Finasteride was without effect, suggestive of a specific role for 5 α R1. More recently, data have been published suggesting an increased risk of incident T2D associated with both dutasteride and finasteride (11), although the analysis did not examine the impact of the co-prescription of these medications with GCs.
Building on the established role of the 5 α Rs in glucocorticoid metabolism (38), the aim of the current study was to test the impact of co-administration of prednisolone and 5α-RIs. We have previously shown that both finasteride (5 mg daily) and dutasteride (0.5 mg daily) (in the absence of exogenous glucocorticoid, for a 3-week duration) have no impact on fasting glucose, fasting insulin, M-value across a 2-step hyperinsulinemic euglycemic clamp, Ra glucose, M-value, glucose disposal (Gd), circulating NEFA, or Ra glycerol (10). We have therefore concluded that the changes that we observed in the prednisolone + 5α-RI arm of this study are due to the combination of treatment rather than the 5α-RI treatment alone. This study therefore provides the first evidence to suggest that co-administration of GC and 5α-RI can precipitate the development of adverse metabolic consequences.
There are very few differences in GC metabolites when comparing individuals taking finasteride or dutasteride (10). The limited additional impact of combined 5 α R1 and 5 α R2 inhibition suggests that 5 α R2 may be most critical for glucocorticoid metabolism. Our data would endorse this observation; our subgroup analysis showed the impact of finasteride was similar (or indeed slightly more marked) than that of dutasteride.
There are many examples of co-prescriptions of medications altering GC half-life and availability with resultant adverse metabolic effects. In particular, ritonavir, itraconazole, erythromycin, cyclosporin, and oral contraceptives have been shown to increase circulating GC levels (12, 13, 39). In contrast, drugs such as carbamazepine, phenytoin, phenobarbital, and rifampicin decrease GC levels due to increased P-450 activity (12, 13, 39). In cases were these medications are co-administered with GCs dose adjustments and vigilance as to the development of adverse effects need to be considered.
There are a number of limitations to this study; the sample size is modest, although it does reflect the complex and sensitive nature of the investigations that were performed. The study was powered to detect a 15% change in EGP, and sample size estimates suggested that 8 participants taking both prednisolone and 5α-RIs would be needed (additional volunteers were recruited to account for potential drop-outs or failed sample analysis). We did not include a dedicated arm of participants treated with 5α-RI alone, as we have already reported findings from participants treated in this way (10). Whilst it is established that 5 α Rs are able to metabolize prednisolone and prednisone (40), there are very limited data with respect to other synthetic GCs and, therefore, it may not be possible to extrapolate our findings to all prescribed steroids across all routes of administration.
In conclusion, we have demonstrated for the first time that co-administration of prednisolone with a 5α-RI worsens metabolic phenotypes. These data not only demonstrate the potent ability of the pre-receptor 5 α R system to regulate exogenous GC action, but raise important clinical questions with respect to vigilance and surveillance for adverse effects as well as the potential need to consider dose adjustments.
Acknowledgments
This work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, the Medical Research Council UK (program grant MR/P011462/1 to JWT); by the British Heart Foundation (senior fellowship to LH FS/15/56/31645) and a University of Oxford / Novo Nordisk clinical research training fellowship (awarded to AM); by the Exchange in Endocrinology Expertise program of the European Union of Medical Specialists and the European Society of Endocrinology (3E fellowship and Short-Term Fellowship, to RP). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care UK.
Financial support: Medical Research Council (program grant to J.W.T. ref. MR/P011462/1); NIHR Oxford Biomedical Research Centre (principal investigator award to J.W.T.); University of Oxford/Novo Nordisk clinical fellowship (A.M.); British Heart Foundation (senior fellowship to L.H. ref. FS/15/56/31645).
Additional Information
Disclosure Summary: J.W.T. has been an advisory board member for Novo Nordisk, Pfizer, and Poxel.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. Qjm. 2000;93(2):105–111. [DOI] [PubMed] [Google Scholar]
- 2. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294–298. [DOI] [PubMed] [Google Scholar]
- 3. Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. [DOI] [PubMed] [Google Scholar]
- 4. Dowman JK, Hopkins LJ, Reynolds GM, et al. Loss of 5α-reductase type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice. Endocrinology. 2013;154(12):4536–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Livingstone DE, Barat P, Di Rollo EM, et al. 5α-Reductase type 1 deficiency or inhibition predisposes to insulin resistance, hepatic steatosis, and liver fibrosis in rodents. Diabetes. 2015;64(2):447–458. [DOI] [PubMed] [Google Scholar]
- 6. Crowley RK, Hughes B, Gray J, et al. Longitudinal changes in glucocorticoid metabolism are associated with later development of adverse metabolic phenotype. Eur J Endocrinol. 2014;171(4):433–442. [DOI] [PubMed] [Google Scholar]
- 7. Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008;57(10):2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM. Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes. 2008;57(6):1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Upreti R, Hughes KA, Livingstone DE, et al. 5α-reductase type 1 modulates insulin sensitivity in men. J Clin Endocrinol Metab. 2014;99(8):E1397–E1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hazlehurst JM, Oprescu AI, Nikolaou N, et al. Dual-5α-Reductase Inhibition Promotes Hepatic Lipid Accumulation in Man. J Clin Endocrinol Metab. 2016;101(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei L, Lai EC, Kao-Yang YH, Walker BR, MacDonald TM, Andrew R. Incidence of type 2 diabetes mellitus in men receiving steroid 5α-reductase inhibitors: population based cohort study. Bmj. 2019;365:l1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liapi C CG. Glucocorticoids. In: AJe Jaffe SJ, ed. Pediatric Pharmacology. 2nd ed. Philadelphia: WB Saunders Co; 1992:466–475. [Google Scholar]
- 13. Foisy MM, Yakiwchuk EM, Chiu I, Singh AE. Adrenal suppression and Cushing’s syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9(6):389–396. [DOI] [PubMed] [Google Scholar]
- 14. Alberts P, Nilsson C, Selen G, et al. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology 2003; 144:4755–4762 [DOI] [PubMed] [Google Scholar]
- 15. Kozower M, Veatch L, Kaplan MM. Decreased clearance of prednisolone, a factor in the development of corticosteroid side effects. J Clin Endocrinol Metab. 1974;38(3):407–412. [DOI] [PubMed] [Google Scholar]
- 16. Ahi S, Beotra A, Dubey S, Upadhyay A, Jain S. Simultaneous identification of prednisolone and its ten metabolites in human urine by high performance liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2012;4(6):460–467. [DOI] [PubMed] [Google Scholar]
- 17. Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36(8):914–924. [DOI] [PubMed] [Google Scholar]
- 18. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. [DOI] [PubMed] [Google Scholar]
- 19. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 20. Umpleby AM. Hormone measurement guidelines: tracing lipid metabolism: the value of stable isotopes. J Endocrinol. 2015;226(3):G1–10. [DOI] [PubMed] [Google Scholar]
- 21. Lamble S, Batty E, Attar M, et al. Improved workflows for high throughput library preparation using the transposome-based Nextera system. BMC Biotechnol. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrow J, Frankish A, Gonzalez JM, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. [DOI] [PubMed] [Google Scholar]
- 25. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venneri MA, Hasenmajer V, Fiore D, et al. circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J Clin Endocrinol Metab. 2018;103(8):2998–3009. [DOI] [PubMed] [Google Scholar]
- 27. Lee H, Kim M, Park YH, Park JB. Dexamethasone downregulates SIRT1 and IL6 and upregulates EDN1 genes in stem cells derived from gingivae via the AGE/RAGE pathway. Biotechnol Lett. 2018;40(3):509–519. [DOI] [PubMed] [Google Scholar]
- 28. Sasse SK, Altonsy MO, Kadiyala V, Cao G, Panettieri RA Jr, Gerber AN. Glucocorticoid and TNF signaling converge at A20 (TNFAIP3) to repress airway smooth muscle cytokine expression. Am J Physiol Lung Cell Mol Physiol. 2016;311(2):L421–L432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mostafa MM, Rider CF, Shah S, et al. Glucocorticoid-driven transcriptomes in human airway epithelial cells: commonalities, differences and functional insight from cell lines and primary cells. BMC Med Genomics. 2019;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. den Uyl D, van Raalte DH, Nurmohamed MT, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: balance between diabetogenic effects and inflammation reduction. Arthritis Rheum. 2012;64(3):639–646. [DOI] [PubMed] [Google Scholar]
- 31. van Raalte DH, Nofrate V, Bunck MC, et al. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162(4): 729–735. [DOI] [PubMed] [Google Scholar]
- 32. van Raalte DH, Brands M, van der Zijl NJ, et al. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia. 2011;54(8):2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Raalte DH, Diamant M, Ouwens DM, et al. Glucocorticoid treatment impairs microvascular function in healthy men in association with its adverse effects on glucose metabolism and blood pressure: a randomised controlled trial. Diabetologia. 2013;56(11):2383–2391. [DOI] [PubMed] [Google Scholar]
- 34. Petersons CJ, Mangelsdorf BL, Jenkins AB, et al. Effects of low-dose prednisolone on hepatic and peripheral insulin sensitivity, insulin secretion, and abdominal adiposity in patients with inflammatory rheumatologic disease. Diabetes Care. 2013;36(9):2822–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersons CJ, Mangelsdorf BL, Poljak A, et al. Low dose prednisolone and insulin sensitivity differentially affect arterial stiffness and endothelial function: an open interventional and cross-sectional study. Atherosclerosis. 2017;258:34–39. [DOI] [PubMed] [Google Scholar]
- 36. Hazlehurst JM, Gathercole LL, Nasiri M, et al. Glucocorticoids fail to cause insulin resistance in human subcutaneous adipose tissue in vivo. J Clin Endocrinol Metab. 2013;98(4): 1631–1640. [DOI] [PubMed] [Google Scholar]
- 37. Traish A, Haider KS, Doros G, Haider A. Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction. Horm Mol Biol Clin Investig 2017;30 [DOI] [PubMed] [Google Scholar]
- 38. Nixon M, Upreti R, Andrew R. 5α-Reduced glucocorticoids: a story of natural selection. J Endocrinol. 2012;212(2): 111–127. [DOI] [PubMed] [Google Scholar]
- 39. Saberi P, Phengrasamy T, Nguyen DP. Inhaled corticosteroid use in HIV-positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Med. 2013;14(9):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Renner E, Horber FF, Jost G, Frey BM, Frey FJ. Effect of liver function on the metabolism of prednisone and prednisolone in humans. Gastroenterology. 1986;90(4):819–828. [DOI] [PubMed] [Google Scholar]