Abstract
Aim
Reduced calcium sensing receptor (CaSR) expression has been implicated in parathyroid tumorigenesis, but the underlying mechanism remains elusive. Accordingly, we aimed to explore the epigenetic changes (DNA methylation and histone modifications) involved in CaSR regulation in sporadic parathyroid adenomas and correlate epigenetic state with disease indices.
Experimental Design
Forty sporadic parathyroid adenomas and 10 control parathyroid tissues were studied. Real-time quantitative PCR (qPCR) for mRNA and immunohistochemistry for protein expression of CaSR were performed. The methylation status of the CaSR promoter 2 was determined by bisulphite sequencing analysis of sodium bisulphite-converted DNA. To determine the role of histone modifications in the CaSR regulation, chromatin immunoprecipitation-qPCR assay was performed.
Results
Real-time qPCR revealed reduced CaSR mRNA expression with a fold reduction of 0.12 (P < 0.0001) in parathyroid adenomas. Immunohistochemistry revealed reduced protein expression of CaSR in 90% (36/40) of adenomas. The promoter 2 region of CaSR displayed significant hypermethylation in 45% (18/40) of the adenomas compared with the controls (6.7%; 1 of 10) (P < 0.002). Bisulphite sequencing analysis revealed maximum methylated CpG at glial cell missing 2 binding site on the CaSR promoter 2 compared to other CpG sites. The methylation status of CaSR correlated directly with plasma intact parathyroid hormone levels in patients with parathyroid adenoma. With chromatin immunoprecipitation-qPCR analysis, H3K9me3 levels showed increased enrichment by 10-fold in adenomas and correlated with CaSR-mRNA expression (r = 0.61; P < 0.003). Treatment with 5-aza-2′deoxycytidine restored the expression of CaSR in a parathyroid cell line.
Conclusion
Our data suggest that hypermethylation and increased H3K9me3 of the CaSR promoter 2 are involved in silencing CaSR expression in sporadic parathyroid adenoma.
Keywords: primary hyperparathyroidism, calcium sensing receptor, promoter methylation, histone methylation, 5-aza 2 ʹ deoxycytidine, glial cell missing 2
Primary hyperparathyroidism (PHPT) is characterized by hypercalcemia and non-suppressed or elevated intact parathyroid hormone (iPTH) levels. The molecular mechanisms involved in parathyroid tumorigenesis are not fully elucidated except for the well-established roles of cyclin D1 and multiple endocrine neoplasia 1 (MEN1) in the pathogenesis of PHPT (1-4). However, cyclin D1 is also overexpressed in some sporadic parathyroid adenomas as a result of tissue-specific DNA rearrangements (2). Reduced calcium sensing receptor (CaSR) expression has been reported in parathyroid tumors (5-7), which results in failure to sense serum calcium levels correctly and increases the set point for PTH secretion resulting in parathyroid cell proliferation and adenoma formation (8). The mechanism of underexpression of CaSR in sporadic parathyroid tumors is only partially understood, but no mutations in the coding region of CaSR have been reported in patients with sporadic PHPT (9). In addition, reduced CaSR expression has been reported in various cancers such as prostate, renal, colon, ovarian, and breast cancers (10-14).
The CaSR gene has 2 promoters, an upstream promoter P1 and a downstream promoter P2: promoter P1 contains a TATA box and a CCAAT box near the transcription start site (TSS); and promoter P2 is rich in guanine-cytosine (GC) nucleosides. Epigenetics are heritable changes in gene transcription without changes in the DNA sequence and are mediated by 2 major mechanisms: DNA methylation and histone modifications. Trimethylation of histone H3 at lysine 9 (H3K9me3) and 27 (H3K27me3) induces transcriptional silencing. Accordingly, in the present study, we explored the role of CaSR in pathogenesis of parathyroid tumorigenesis by investigating promoter DNA methylation, histone modifications (H3K9me3 and H3K27me3), and acetylation of histone H3 at lysine 9 (H3K9ac). We have also checked the effect of an epigenetic drug in the PTHC-1 cell line and its possible role in reversal of CaSR expression.
Materials and Methods
Patients and parathyroid tissues
Forty parathyroid adenomatous and 10 control parathyroid tissues were collected during required parathyroid or thyroid surgeries. The study was approved by the institutional ethics committee, Post Graduate Institute of Medical Education and Research, Chandigarh, India. Written informed consent was obtained from all study participants. The tissue samples were examined and confirmed either as adenoma or normal parathyroid tissues by an experienced endocrine pathologist (U.N.S.). All samples were stored in at –80°C until the final molecular analyses.
Relevant clinical manifestations were recorded and biochemical measurements like serum calcium (reference range [RR], 8.2-10.2 mg/dL), serum phosphorus (RR, 2.7-4.5 mg/dL), serum creatinine (RR, 0.50-1.20 mg/dL), serum albumin (RR, 3.5-5.5 g/dL), and alkaline phosphatase (ALP) (RR, 40-129 U/L) were measured by Olympus auto-analyzer, and the serum calcium level was adjusted with the serum albumin level. The plasma iPTH (RR, 15-65 pg/mL) and 25-hydroxyvitamin D (RR, 11.1-42.9 ng/mL) were measured using immunochemiluminescence (ELECSYS- 2010: Roche Diagnostics, Germany).
Gene expression analysis
Total RNA was extracted from both adenoma and normal parathyroid tissue and reverse transcribed as described previously (5). CaSR mRNA levels were quantified by quantitative reverse transcriptase (qRT)-PCR on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, USA) using 18s rRNA as housekeeping gene and a specific set of primers (For CaSR, forward primer 5′-GACCCCTTACATAGATTACACGC-3′ and reverse primer 5′-CTCCACAGGATTTTCTCCTC-3′). qRT-PCR reaction conditions were reported previously (4). Experiments were performed in duplicate with 2 non-template controls as negative control. For all experimental samples, the relative value was normalized to the 18s rRNA of the same sample. The 2-(ΔΔCt) method was used to quantify relative expression of CaSR as a fold change.
Immunohistochemistry
Paraffin-embedded tissue blocks were retrieved and 4 to 5-micron sections were cut for immunohistochemistry staining as described previously (15). The immunohistochemistry slides were graded semiquantitatively based on percentage of positive cells and staining intensity by an endocrine pathologist for protein expression of CaSR. Membranous staining for CaSR was regarded as positive. Approximately 200 cells were counted at 6 different regions for each section under 40× magnification. The staining intensity for the tumors and normal parathyroid tissue sections was calculated as weak positive (+; <40%), moderate positive (++; 40%-60%) and strong positive (+++; >60%) cells (5).
Bisulfite-specific PCR and sequencing
Genomic DNA was extracted from parathyroid adenoma and control parathyroid tissue samples by DNeasy blood and tissue kit (Qiagen, USA). DNA was bisulphite modified using EZ DNA Methylation-GoldTM Kit (Zymo Research Corporation, USA) involving chemical reaction that changed all cytosine to uracil. To identify the CpG island in the CaSR promoter 2 region and primers for bisulphite-specific PCR, online tool meth primer was used. Primer sequences for CaSR forward 5′-ATTTATTTTACTGTGAAT-3′ and CaSR reverse 5′-GAAGGAGGGAGCTGTTTGCCAGC-3′ were used to amplify 306-bp sequence (nucleotide –436 to –140 relative to TSS) containing 21 CpG sites. PCR amplification was carried out with EpiTaq polymerase (Takara, Japan). Amplified PCR products were run on 2% agarose gels, isolated, and sent for sequencing on ABI 3730 XL DNA Analyzer (Thermo Fisher Scientific, USA). The sequencing data were analyzed by online software BISMA (bisulfite sequencing and DNA methylation analysis) (4). A methylation density of >10% was regarded as methylated for the specific gene examined (16).
Chromatin immunoprecipitation of H3K9me3, H3K27me3, and H3K9ac
Enrichment of trimethylated H3K9, trimethylated H3K27, and acetylated H3K9 within the promoter 2 region of CaSR were analyzed by chromatin immunoprecipitation (ChIP)-qPCR assay. The experiment was performed as described previously (17). In brief, a total of 25 to 30 mg homogenized frozen tissues were fixed with 1% paraformaldehyde at room temperature for 10 minutes, stopped with 0.125 M glycine, and lysed for 10 minutes on ice. The lysate was digested using the homogenizer in 1 mL lysis buffer (NaCl 150 mM, Tris HCl [pH 7.5], 25 mM, EDTA 5 mM, Trition X 100 1%, SDS 0.1%, sodium deoxycholate 0.5%, PMSF 1 mM, sodium butyrate 10 mM) 1XPIC followed by resuspension in lysis buffer for sonication 15 cycles (30 seconds on and 30 seconds off) using sonicator (Diagenode, Belgium). Cross-linked DNA was immunoprecipitated with nonspecific IgG Ab (45% of total supernatant) for isotype control and immunoprecipitation with antihistone H3 trimethyl K9, H3 trimethyl K27, and H3 acetyl K9 antibodies (45% of total supernatant; H3K9me3, H3K27me3, and H3K9ac; Abcam, Cambridge) at 4°C overnight, pulled down by protein A/G beads, washed, reverse cross-linked, and purified ChIP DNA by phenol, chloroform isoamyl alcohol method for qPCR. Primers for ChIP-qPCR assay were designed by using primer blast provided by NCBI and sequences were CaSR forward primer 5′-TTGGCCATAATGAGGATGTG-3′ and CaSR reverse primer 5′-GGGCGATGTACGTTCTTCCT-3′. For ChIP-qPCR analysis, both input and immunoprecipitated DNA were quantified by real-time PCR with ChIP primers. The assays were performed in duplicate and the levels of histone modifications were calculated by the fold enrichment method relative to the nonspecific IgG antibody and normalized to the input DNA.
Parathyroid cell culture and effect of DNA methyltransferase inhibitor
PTHC-1 cell line was established from rat parathyroid tissue and used for the in vitro treatments because there is lack of human parathyroid cell line (18). PTHC-1 cells show epithelial morphology and parathyroid functional features. The cells were cultured in 25-cm2 or 75-cm2 tissue culture flasks supplemented with nutrient mixture F-12 Ham Coon’s modification (Sigma-Aldrich, USA) containing 10% fetal bovine serum (Gibco, Thermo Fisher Scientific), and 100 U/mL penicillin/streptomycin (Gibco, Thermo Fisher Scientific) and then incubated at 37°C in 5% CO2. Cell culture medium was changed every third day and their doubling time was 15 hours. After reaching 70% to 80% confluency, PTHC-1 cells were treated with a DNA methyltransferase inhibitor (ie, 5-Aza-2′-deoxycytidine [DAC]; Sigma Aldrich) in desired concentrations at indicated time points with suitable controls. The concentration of DAC was standardized by studying 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide cell viability assay.
PTHC-1 cells were seeded, allowed to grow overnight, and treatment was performed using 10 µM DAC for 72 hours (fresh drug was changed every 24 hours), to study the effect of change in expression of CaSR and thereby reactivate CaSR expression in PTHC-1 cells by qRT-PCR and immunofluorescence.
Effect of DAC treatment on CaSR expression in PTHC-1
Total RNA was isolated from treated and untreated parathyroid cultured cells, reverse transcribed and qRT-PCR analysis was performed as described previously for tissue samples. Rat-specific CaSR primers (forward primer 5′-GCGGGCTGGTACGACATCCT-3′ and reverse primer 5′-GCCCGAGATGTTTTCTGGAGTCCT-3′) were used during the experiments. Experiments were performed in triplicate with 2 non-template controls as negative control. Data were normalized to the expression of β-actin as a housekeeping gene for cell line and relative expression was calculated using 2-(ΔΔCt) value.
Immunofluorescence staining of CaSR in rat parathyroid continuous cell line (PTHC-1)
After treatments, PTHC-1 cells were grown on 8-well glass-chambered slides and were fixed with 4% paraformaldehyde in 1× PBS for 20 minutes and washed extensively with 1× PBS. Cells were permeabilized with 0.2% Triton-X in 1× PBS for 20 minutes, and blocked with 2% BSA in PBS for 1 hour. Primary antibody for CaSR (Thermo Fisher Scientific) was diluted in 2% BSA and applied to the cells. Slides were incubated for 4 hours at room temperature. After washing with 1× PBS, secondary antibody-labeled fluorochrome (1:500) was applied on the cells for 1 hour at room temperature in the dark. Cells were washed once for 5 minutes with 1X PBS. Nuclear staining and mounting were performed with AntiFade Gold reagent (Thermo Fisher Scientific). Whole slide images were acquired using EVOS microscope with appropriate fluorescence wavelengths per the fluorescent labels used.
Statistical analysis
The statistical analyses were performed using GraphPad Prism 6. The nonparametric test was used to evaluate the differences in all the study parameters between the control and the parathyroid adenoma tissue samples. All values are presented as mean ± SD except the ChIP-qPCR data, which was expressed as mean ± SEM. A P value < 0.05 was considered statistically significant. Mann-Whitney, χ 2, and t tests were used as appropriate to compare the differences in study data between the control and adenoma samples. Pearson correlation test was used to examine the relationship among various continuous variables.
Results
Demographics and clinical and biochemical features of patients with sporadic PHPT
The mean age of the patients was 43 ± 13.5y (range, 18-65 years) with a female to male ratio of 3:1 (31 women and 9 men); all PHPT patients were symptomatic. The most common presenting symptoms were bone pain in 29 (72%), followed by weakness and fatigue in 22 (55%), kidney stones in 13 (32%), gall stone disease in 8 (20%), fractures in 8 (20%), and pancreatitis in 4 (10%). The mean preoperative serum calcium level was 12.0 ± 1.0 mg/dL (range, 10.05-14.5 mg/dL) and median iPTH level was 558 pg/mL (interquartile range: 213, 1060 pg/mL), mean serum ALP level was 285.9 ± 235.6 U/L (range, 72-883) and mean serum creatinine was 0.98 ± 0.54 mg/dL (range, 0.34-2.6). Mean serum 25-hydroxyvitamin D level was 19.5 ± 13.3 ng/mL (range, 3-62 ng/mL) and 16 (40%) patients had vitamin D deficiency (<20 ng/mL) at the time of presentation (Table 1).
Table 1.
The Baseline Biochemical Characteristics and Tumor Weight of Sporadic PHPT Patients
| Biochemical Parameters (Reference Range) | Parathyroid Adenoma (N = 40) Mean ± SD (Minimum-Maximum) |
|---|---|
| Serum calcium (8.6-10.2 mg/dL) | 12.0 ± 1.0 (10.5-14.5) |
| Serum phosphorous (2.4-4.5 mg/dL) | 2.1 ± 0.5 (1.25-3.1) |
| Plasma intact parathyroid hormone (10-65 pg/mL) | 558 (213, 1060)a |
| Plasma 25 (OH) D (11-42 ng/mL) | 19.5 ± 13.3 (3-61.6) |
| Serum alkaline phosphatase (40-129 U/L) | 285.9 ± 235.6 (72-883) |
| Serum Creatinine (0.6-1.2 mg/dL) | 0.98 ± 0.54 (0.34-2.6) |
| Tumor weight (g) | 2 (1, 4.5)a |
a Data represented as median (1st, 3rd interquartile range).
Reduced CaSR expression in sporadic parathyroid adenomas
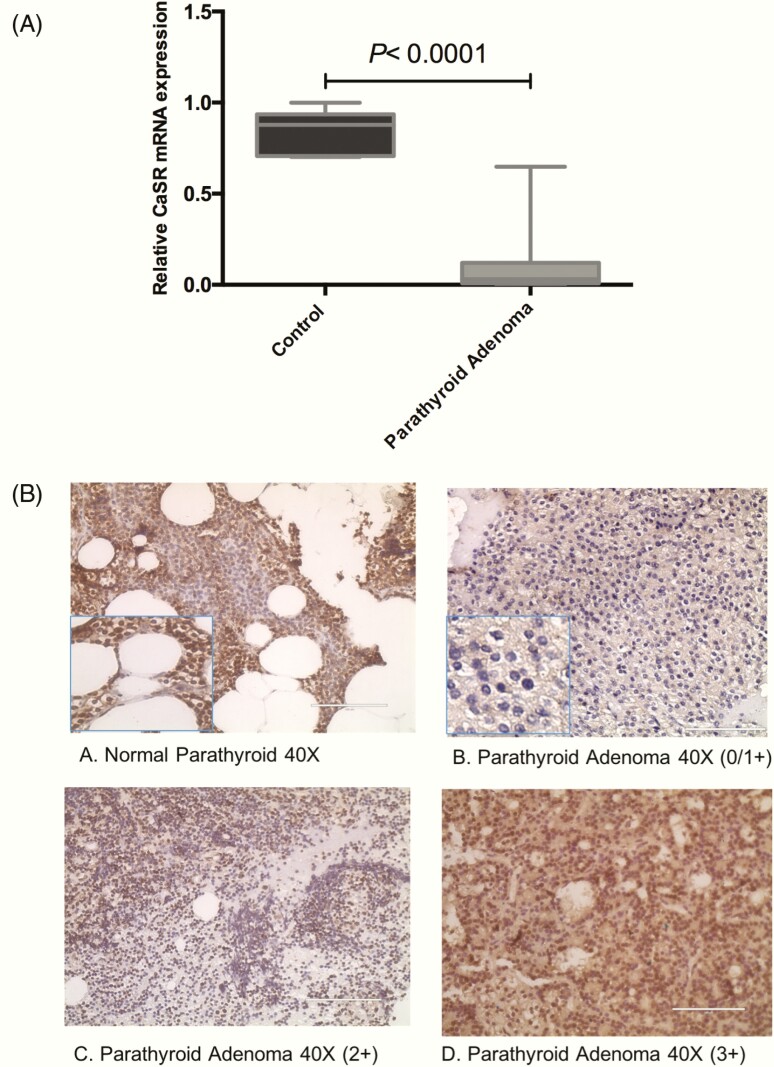
Relative quantification of CaSR mRNA by qRT-PCR revealed a significant reduced expression in 90% (n = 36) of sporadic parathyroid adenomatous tissues with a mean fold value of 0.12 ± 0.20 (median = 0.03; interquartile range, 0.0008, 0.64) relative to the control parathyroid tissue samples (P < 0.0001; Fig. 1A).
Figure 1.
Calcium sensing receptor (CaSR) gene and protein expression analysis in sporadic parathyroid adenoma (N = 40). (A) Box-and-whisker plot representing relative mRNA expression of CaSR in parathyroid adenoma compared with control parathyroid (***P < 0.0001). (B) Representative photomicrographs showing CaSR expression in parathyroid tissue sections (A) normal and (B-D) show intensity in adenoma as 1+, 2+, and 3+, respectively. Images captured at magnification at 40×, scale bar = 400 µm.
All normal parathyroid tissue sections showed 3+ intensity (range, >60%-100% positivity) membranous staining of CaSR protein. We observed 1+ intensity (range, 0%-40% positivity) in 18 (45%), 2+ intensity (range, >40%-60% intensity) in 16 (40%), and 3+ intensity only in 6 (15%) parathyroid adenomatous tissues (Fig. 1B). In parathyroid adenoma cases, the mean proportion of positive cells was 47 ± 21%, whereas 79 ± 2% positive cells were seen in normal parathyroid tissue samples. The present findings showed a significant loss of CaSR expression at the protein level in parathyroid adenoma compared to normal parathyroid tissue sections (P < 0.0002). There was no significant correlation between the tumor weight and patient’s biochemical parameters such as serum calcium, phosphorous, ALP, creatinine, iPTH, or 25-hydroxy D.
Status of CaSR promoter 2 and methylation pattern and correlation with mRNA expression
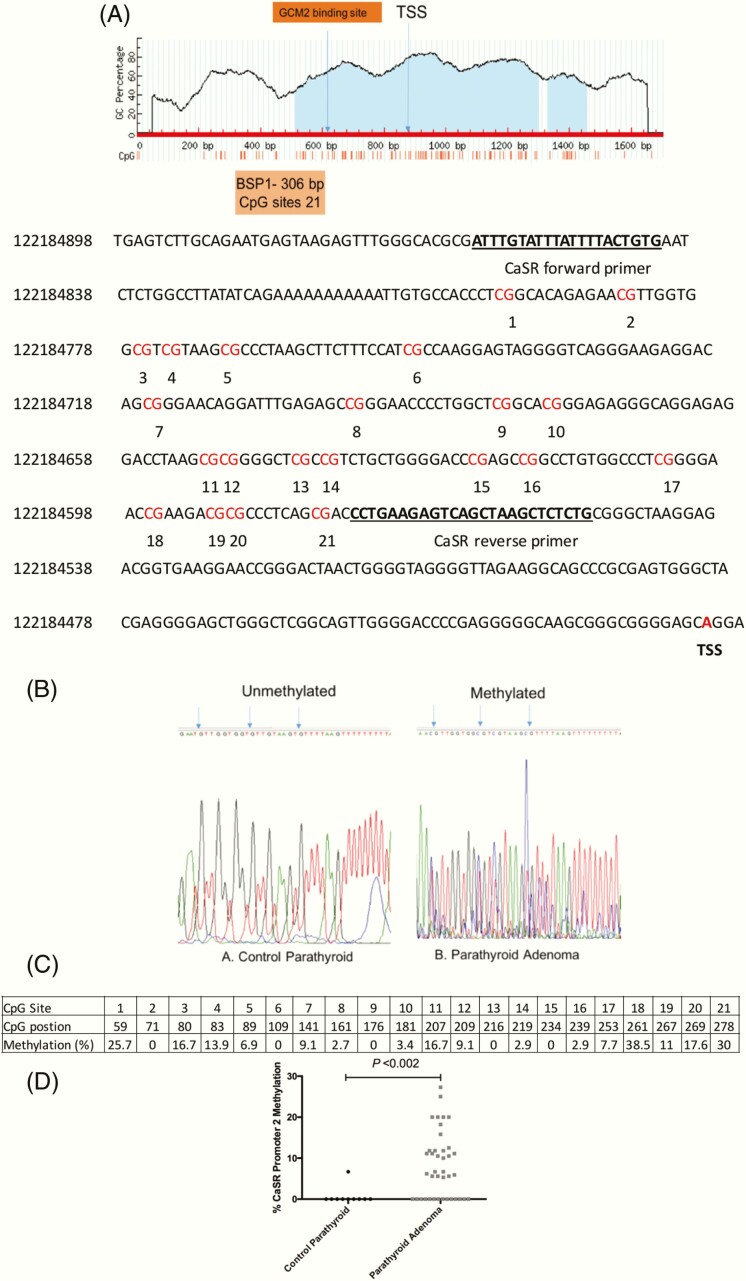
To further understand the reason for reduced CaSR expression, we analyzed the methylation status of the CaSR promoter 2. The CaSR promoter 2 region (nucleotides, –436 to –140 relative to TSS) containing 21 CpG sites with a product size of 306 bp was analyzed (Fig. 2A). The bisulphite sequencing analysis revealed that 45% (n = 18) of parathyroid adenoma cases were methylated with a mean methylation density of 15.5 ± 5.5 (10-27.3). Five cases had mean methylation density of <10, whereas 17 cases did not show methylation at any of the CpG sites in CaSR promoter 2. In contrast, except for a single control sample with a methylation density of 6.7%, all control parathyroid tissues did not show hypermethylation of CpG sites. Representative chromatograms of bisulphite sequencing representing unmethylated and methylated CpG sites in promoter 2 region of CaSR are in Fig. 2B. CpG site 18 was methylated in 38.5% parathyroid adenoma samples compared with the other sites on the CaSR promoter 2 region (Fig. 2C). Further, in silico analysis revealed that binding elements for a parathyroid specific transcription factor, Glial cell missing 2 (GCM2), (nucleotides, –166 to –156 relative to TSS) are present on the 18th and 20th CpG sites of CaSR promoter 2. So, hypermethylation at the 18th CpG site might alter the binding site for GCM2 and lead to reduced CaSR expression. Methylation densities of control and parathyroid adenoma samples for CaSR promoter 2 are shown as individual values in the scatter plot (Fig. 2D). Pearson correlation analysis revealed that plasma iPTH level was directly associated with methylation of CaSR promoter 2 region (r = 0.57; P < 0.002).
Figure 2.
Calcium sensing receptor (CaSR) promoter 2 methylation in sporadic parathyroid adenoma (N = 40). (A) CaSR promoter 2 region showing 21 CpG sites (red) including the transcription start site (TSS; red bold) selected for bisulfite sequencing PCR. (B) Representative chromatograms showing methylated site (blue arrow) in adenoma compared with control parathyroid tissue samples. (C) Tabular representation showing average percentage methylation of individual CpG sites by bisulfite sequencing analysis. (D) Individual value scatter plot for control parathyroid and parathyroid adenomas representing percentage methylation of CaSR promoter 2 gene.
Finally, the CaSR gene expression levels were compared between hypermethylated and unmethylated parathyroid adenoma groups. The CaSR expression was significantly lower in the hypermethylated group compared with the unmethylated group (0.02 ± 0.02 vs 0.19 ± 0.26; P < 0.01).
ChIP-qPCR analysis for H3K9me3, H3K27me3, and H3K9ac at CaSR promoter 2
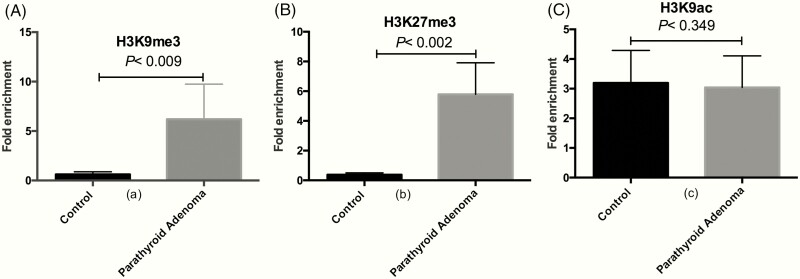
ChIP-qPCR analysis showed that H3K9me3 and H3K27me3 levels in CaSR promoter 2 were increased by 10-fold (P < 0.009; Fig. 3A) and 15-fold (P < 0.002; Fig. 3B) in sporadic parathyroid adenomatous tissues compared with control parathyroid tissue samples. In contrast to the increased levels of H3K9me3 and H3K27me3 in CaSR promoter 2, H3K9ac levels were decreased by 1.2-fold (P = 0.349; Fig. 3C). The H3K9me3 modification, known to be associated with transcriptionally inactive loci, correlated with CaSR mRNA levels (r = 0.61; P < 0.003). We did not find any correlation between H3K9me3 levels and biochemical indices or with the weight of the parathyroid adenoma in patients with PHPT.
Figure 3.
Chromatin immunoprecipitation (ChIP)-quantitative PCR for CaSR 2 promoter region in sporadic parathyroid adenoma. Quantification of fold enrichment in (A) H3K9me3 (**P < 0.009), (B) H3K27me3 (**P < 0.002), and (C) H3K9ac (P = 0.349) levels relative to nonspecific IgG as negative control and normalized with input DNA. Bars are represented as mean fold enrichment ± SEM (n = 23).
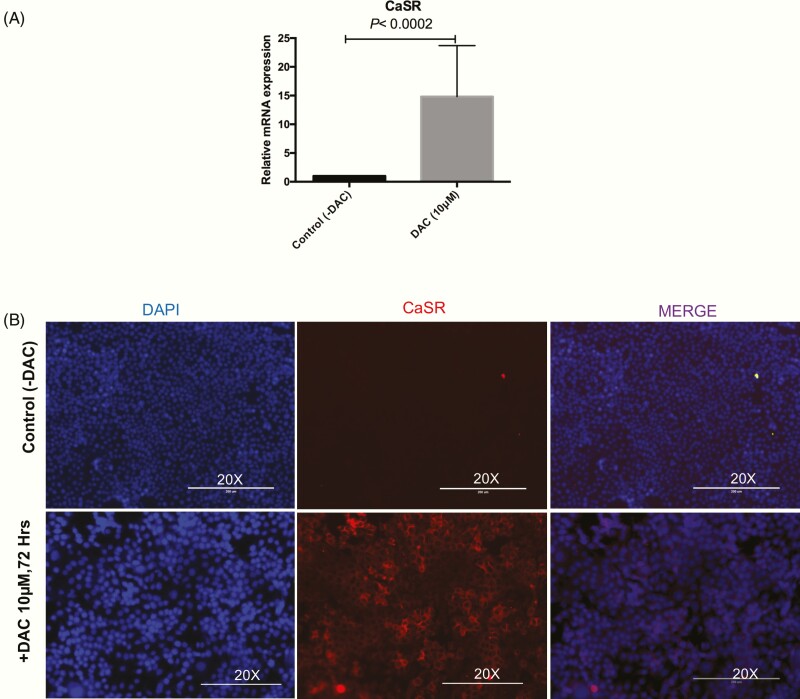
Impact of DAC on CaSR expression in PTHC-1 cells
qRT-PCR was performed to assess the effect of DAC treatment on restoration of CaSR expression in the PTHC-1 cell line. The qRT-PCR analysis indicated a significant increase in CaSR gene expression in PTHC-1 cells treated (mean ± SEM: 14.8 ± 8.8) with 10 µM DAC for 72 hours compared with control (P < 0.0002; Fig. 4A). Immunofluorescence experiments were performed to evaluate the efficiency of the treatment with 10 µM DAC on the protein expression of CaSR in PTHC-1 cells. Compared with control cells, we observed increased CaSR protein expression in DAC treated PTHC-1 cells (Fig. 4B). Taken together, these results suggest that the treatment with DAC in PTHC-1 cells for 72 hours may restore the expression of CaSR.
Figure 4.
Calcium sensing receptor (CaSR) expression is restored in parathyroid cancer cell line upon treatments with epigenetic drug. (A) Relative gene expression of CaSR in the experimental groups treated with DAC (10 µM) vs control at 72 hours as determined by quantitative reverse transcriptase-PCR and normalized with b actin. The results are shown as mean ± SEM values, and P values were evaluated by analyzing t tests from 3 independent experiments. (B) Representative images indicated CaSR protein expression before (–) and after (+) DAC treatment in PTHC-1 cells by immunofluorescence. PTHC-1 cells were fixed and stained with CaSR antibody (red) primarily located in the cytoplasm. Nuclei were stained with DAPI (blue). Images captured at magnification, 20×. Scale bar = 200µm.
Discussion
In the present study, we comprehensively explored the epigenetic mechanisms of under-expression of CaSR in sporadic parathyroid adenomas (5, 7). We found a higher proportion of methylated CpG sites and increased enrichment of histone modifications (H3K9me3 and H3K27me3) in the CaSR promoter 2. In addition, we also demonstrated that the DNA methylation inhibitor (DAC) restores CaSR transcription and protein levels in PTHC-1 cells.
In the present study, the mean age of symptomatic PHPT patients (n = 40) was 43 years. The most common presenting complaint was bone pain followed by weakness and fatigue, renal stones, gallstones, and fractures. These clinical findings, biochemistry, and hormonal profiles were comparable to the symptomatic PHPT reported from the Indian subcontinent (8, 19, 20).
We observed a reduction of CaSR both at the gene and protein levels in sporadic parathyroid adenomatous tissues compared with control parathyroid tissue, similar to previous studies (7, 21-23). We did not find any significant correlation between gene and protein expression of CaSR with disease parameters. It seems likely that hypercalcemia with elevated serum iPTH, or consequences of adenoma formation resulting from increased cell proliferation, are actually responsible for decreased expression of CaSR in parathyroid tumors (7, 22, 24). Previously, our group observed a similar reduction in CaSR expression while studying the methylation patterns in CaSR promoter 1 in parathyroid adenomas. In that study, we did not observe significant methylation in CaSR upstream of promoter 1, which has 11 CpG sites (5, 25).
CaSR is a G protein-coupled receptor that senses the extracellular calcium and thus is involved in calcium homeostasis (26, 27). The CaSR gene has 2 promoters (P1 and P2), and the promoter 2 region has a large CpG island. Thus, it is likely that epigenetic modifications such as promoter methylation and histone modifications on CaSR promoter 2 are responsible for “gene silencing” (4). In the present study, we observed hypermethylation of CaSR promoter 2 in 45% of sporadic parathyroid adenoma cases. We found a strong association between the CaSR promoter 2 methylation and plasma iPTH levels in patients with PHPT, implicating the role of hypermethylation at CaSR promoter 2 in parathyroid tumorigenesis and possibly in the severity of disease expression. Previous studies have emphasized the role of promoter DNA methylation in the reduction of CaSR expression in colon cancer and neuroblastoma (13, 28). However, in correlation analysis, no evident relationship was found between the CaSR hypermethylation and gene expression, perhaps because the methylation of a specific promoter region could not affect the expression of the whole gene. GCM2, a parathyroid specific transcription factor binding site (nucleotide –166 to –156 relative to TSS) was present in CaSR promoter 2. We found maximum methylation at the specific CpG sites at the GCM2 binding site compared with other CpG sites on CaSR promoter 2. More intriguing is that hypermethylation at CpG sites might lead to less or no binding of GCM2 in CaSR promoter 2, which may subsequently influence the expression of CaSR in sporadic parathyroid adenoma.
Also, previous studies have suggested that decreased CaSR might be the initial event in the parathyroid tumorigenesis (29, 30). In sporadic parathyroid adenomas, underexpression of CaSR is consistent with a low response to the inhibitory effects of elevated iPTH and to receptor silencing, which may affect optimal calcium sensing (31, 32).
Thus, our observations emphasize that other epigenetic regulators such as modifications in histone markers might be involved in decreased CaSR expression in the pathogenesis of parathyroid tumor. The gain or loss of particular histone modifications has been shown to correlate with the expression level of genes (33). Our findings of increased histone methylation (H3K9me3 and H3K27me3) and histone deacetylation (H3K9ac) are in line with previous studies linking DNA methylation and transcriptional silencing (34). The present study, for the first time, suggests that higher H3K9me3 levels are significantly related to decreased CaSR mRNA expression in sporadic parathyroid adenomas. In addition, we suggest that H3K9me3 levels catalyzed by histone methyl transferase may serve as a potential regulatory histone modification causing reduction in CaSR expression in parathyroid tumors.
Significantly increased H3K27me3 levels on CaSR promoter 2 in sporadic parathyroid adenoma correlated with tumor weight of the excised parathyroid gland. This observation is in agreement with a previous study on malignant parathyroid tumors that showed H3K27me3 levels were mediated by enhanced zeste homolog 2 (EZH2), resulting in gene silencing by hypermethylated cancer 1 gene (35). Thus, higher H3K27me3 levels having EZH2 activity may be oncogenic, and may play a crucial role in parathyroid tumorigenesis. However, this observation could illustrate that all modifications are not equally important, probably because of a high extent of redundancy. Besides, the levels of a single modification (H3K9me3) can faithfully influence CaSR gene expression. Collectively, our current experiments demonstrate that among the many proposed mechanisms for decreased CaSR expression, promoter hypermethylation, and histone methylation (H3K9me3) at CaSR promoter 2 are the most likely proximate causes in the sporadic pathogenesis of sporadic parathyroid adenomas.
Finally, we have shown that in the PTHC-1-cell line, treatment with DAC, a DNA methyltransferase 1 (DNMT1) inhibitor, restored the expression of CaSR. Moreover, DAC is highly recommended for reversal of epigenetic modifications and has been approved for the treatment of acute myeloid leukemia and colorectal cancer (13, 36, 37). We have studied the effect of DAC on epigenetically inactivated CaSR gene and showed that after DAC treatment in PTHC-1 cells, there was a statistically significant increase in reactivation of CaSR. Further, we have also confirmed increased protein expression of CaSR in response to DAC treatment by immunofluorescence microscopy.
Our results from DAC treatment of PTHC-1 cells are similar to those in a previous study in colorectal cancer where CaSR was silenced because of epigenetic modifications, and treatment with DAC in Coga and HT29 colorectal cancer cell lines caused restoration of CaSR at both transcriptional and protein levels (13). Other previous studies on cancer cell lines such as HCT116, SW48, and SW480 showed that DAC treatment induces demethylation by inhibiting DNMT1 activity and promotes re-expression of genes such as clusterin, IL-23 receptor, transketolase-like 1, and tubulin alpha 3C (36). These results imply that DAC, a known DNMT1 inhibitor, induces reactivation of transcriptionally silenced CaSR by reversal of epigenetic modification.
Conclusion
This is the first and the most comprehensive study assessing epigenetic mechanisms involved in parathyroid tumorigenesis. Our results suggest that CaSR underexpression in sporadic parathyroid adenomas is mediated by promoter methylation and histone modification. Finally, for the first time, we provide new insight using DAC, a potential therapeutic target for patients with symptomatic PHPT.
Acknowledgments
P.S. was supported by a research fellowship from the Indian Council of Medical Research (3/1/3-JRF2014/HRD-57), New Delhi; and this work was supported by a grant from the Department of Science and Technology, DST-SERB (EMR/2016/005956), New Delhi; and Intramural research grant (no. 71/8-Edu-15/2544) from the Postgraduate Institute of Medical Education and Research, Chandigarh. This work was also supported in part by The National Institutes of Health grant DK43858 to S.D.R.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- CaSR
reduced calcium sensing receptor
- ChIP
chromatin immunoprecipitation
- DAC
5-Aza-2′-deoxycytidine
- DNMT1
DNA methyltransferase 1
- GC
guanine-cytosine
- GCM2
glial cell missing 2
- EZH2
enhanced zeste homolog 2
- iPTH
intact parathyroid hormone
- MEN1
multiple endocrine neoplasia 1
- PHPT
primary hyperparathyroidism
- qRT
quantitative reverse transcriptase
- RR
reference range
- TSS
transcription start site
Additional Information
Disclosure Summary: No potential conflicts of interest were disclosed.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Grolmusz VK, Borka K, Kövesdi A, et al. MEN1 mutations and potentially MEN1-targeting miRNAs are responsible for menin deficiency in sporadic and MEN1 syndrome-associated primary hyperparathyroidism. Virchows Arch. 2017;471(3): 401-411. [DOI] [PubMed] [Google Scholar]
- 2. Imanishi Y, Hosokawa Y, Yoshimoto K, et al. Primary hyperparathyroidism caused by parathyroid-targeted overexpression of cyclin D1 in transgenic mice. J Clin Invest. 2001;107(9): 1093-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cetani F, Saponaro F, Borsari S, Marcocci C. Familial and hereditary forms of primary hyperparathyroidism. Front Horm Res. 2019;51:40-51. [DOI] [PubMed] [Google Scholar]
- 4. Arya AK, Bhadada SK, Singh P, et al. Promoter hypermethylation inactivates CDKN2A, CDKN2B and RASSF1A genes in sporadic parathyroid adenomas. Sci Rep. 2017;7(1):3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varshney S, Bhadada SK, Saikia UN, et al. Simultaneous expression analysis of vitamin D receptor, calcium-sensing receptor, cyclin D1, and PTH in symptomatic primary hyperparathyroidism in Asian Indians. Eur J Endocrinol. 2013;169(1):109-116. [DOI] [PubMed] [Google Scholar]
- 6. Kantham L, Quinn SJ, Egbuna OI, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297(4):E915-E923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sudhaker Rao D, Han ZH, Phillips ER, Palnitkar S, Parfitt AM. Reduced vitamin D receptor expression in parathyroid adenomas: implications for pathogenesis. Clin Endocrinol (Oxf). 2000;53(3):373-381. [DOI] [PubMed] [Google Scholar]
- 8. Bhadada SK, Arya AK, Mukhopadhyay S, et al. Primary hyperparathyroidism: insights from the Indian PHPT registry. J Bone Miner Metab. 2018;36(2):238-245. [DOI] [PubMed] [Google Scholar]
- 9. Cetani F, Pinchera A, Pardi E, et al. No evidence for mutations in the calcium-sensing receptor gene in sporadic parathyroid adenomas. J Bone Miner Res. 1999;14(6):878-882. [DOI] [PubMed] [Google Scholar]
- 10. Brennan SC, Thiem U, Roth S, et al. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta. 2013;1833(7):1732-1744. [DOI] [PubMed] [Google Scholar]
- 11. Bernichtein S, Pigat N, Barry Delongchamps N, et al. Vitamin D3 prevents calcium-induced progression of early-stage prostate tumors by counteracting TRPC6 and calcium sensing receptor upregulation. Cancer Res. 2017;77(2):355-365. [DOI] [PubMed] [Google Scholar]
- 12. Campos-Verdes LM, Costa-Silva DR, da Silva-Sampaio JP, et al. Review of polymorphism of the calcium-sensing receptor gene and breast cancer risk. Cancer Invest. 2018;36(2):1-7. [DOI] [PubMed] [Google Scholar]
- 13. Fetahu IS, Höbaus J, Aggarwal A, et al. Calcium-sensing receptor silencing in colorectal cancer is associated with promoter hypermethylation and loss of acetylation on histone 3. Int J Cancer. 2014;135(9):2014-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan S, Yuan C, Yang Q, et al. A genetic polymorphism (rs17251221) in the calcium-sensing receptor is associated with ovarian cancer susceptibility. Oncol Rep. 2015;34(4):2151-2155. [DOI] [PubMed] [Google Scholar]
- 15. Singh P, Vadi SK, Saikia UN, et al. Minimally invasive parathyroid carcinoma—a missing entity between parathyroid adenoma and carcinoma: scintigraphic and histological features. Clin Endocrinol (Oxf). 2019;91(6):842-850. [DOI] [PubMed] [Google Scholar]
- 16. Juhlin CC, Kiss NB, Villablanca A, et al. Frequent promoter hypermethylation of the APC and RASSF1A tumour suppressors in parathyroid tumours. PLoS One. 2010;5(3):e9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahat B, Mahajan A, Bagga R, Hamid A, Kaur J. Epigenetic modifications at DMRs of placental genes are subjected to variations in normal gestation, pathological conditions and folate supplementation. Sci Rep. 2017;7:40774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabbri S, Ciuffi S, Nardone V, et al. PTH-C1: a rat continuous cell line expressing the parathyroid phenotype. Endocrine. 2014;47(1):90-99. [DOI] [PubMed] [Google Scholar]
- 19. Shah VN, Bhadada SK, Bhansali A, et al. Effect of gender, biochemical parameters & parathyroid surgery on gastrointestinal manifestations of symptomatic primary hyperparathyroidism. Indian J Med Res. 2014;139(2):279-284. [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao L, Liu JM, He XY, et al. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: data from 2000 to 2010 in a single clinical center. J Clin Endocrinol Metab. 2013;98(2):721-728. [DOI] [PubMed] [Google Scholar]
- 21. Farnebo F, Kytölä S, Teh BT, et al. Alternative genetic pathways in parathyroid tumorigenesis. J Clin Endocrinol Metab. 1999;84(10):3775-3780. [DOI] [PubMed] [Google Scholar]
- 22. Garner SC, Hinson TK, McCarty KS, Leight M, Leight GS Jr, Quarles LD. Quantitative analysis of the calcium-sensing receptor messenger RNA in parathyroid adenomas. Surgery. 1997;122(6):1166-1175. [DOI] [PubMed] [Google Scholar]
- 23. Kifor O, Moore FD Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81(4):1598-1606. [DOI] [PubMed] [Google Scholar]
- 24. Carling T, et al. Reduced parathyroid vitamin D receptor messenger ribonucleic acid levels in primary and secondary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85(5):2000-2003. [DOI] [PubMed] [Google Scholar]
- 25. Varshney S, Bhadada SK, Sachdeva N, et al. Methylation status of the CpG islands in vitamin D and calcium-sensing receptor gene promoters does not explain the reduced gene expressions in parathyroid adenomas. J Clin Endocrinol Metab. 2013;98(10):E1631-E1635. [DOI] [PubMed] [Google Scholar]
- 26. Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42(1):35-70. [DOI] [PubMed] [Google Scholar]
- 27. Brown EM, Vassilev PM, Quinn S, Hebert SC. G-protein-coupled, extracellular Ca(2+)-sensing receptor: a versatile regulator of diverse cellular functions. Vitam Horm. 1999;55:1-71. [DOI] [PubMed] [Google Scholar]
- 28. Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16(Spec No 1):R50-R59. [DOI] [PubMed] [Google Scholar]
- 29. Chakravarti B, Dwivedi SK, Mithal A, Chattopadhyay N. Calcium-sensing receptor in cancer: good cop or bad cop? Endocrine. 2009;35(3):271-284. [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez-Rodero S, Delgado-Álvarez E, Fernández AF, Fernández-Morera JL, Menéndez-Torre E, Fraga MF. Epigenetic alterations in endocrine-related cancer. Endocr Relat Cancer. 2014;21(4):R319-R330. [DOI] [PubMed] [Google Scholar]
- 31. Corbetta S, Mantovani G, Lania A, et al. Calcium-sensing receptor expression and signalling in human parathyroid adenomas and primary hyperplasia. Clin Endocrinol (Oxf). 2000;52(3):339-348. [DOI] [PubMed] [Google Scholar]
- 32. Brown EM, Broadus AE, Brennan MF, et al. Direct comparison in vivo and in vitro of suppressibility of parathyroid function by calcium in primary hyperparathyroidism. J Clin Endocrinol Metab. 1979;48(4):604-610. [DOI] [PubMed] [Google Scholar]
- 33. Mellor J, Dudek P, Clynes D. A glimpse into the epigenetic landscape of gene regulation. Curr Opin Genet Dev. 2008;18(2):116-122. [DOI] [PubMed] [Google Scholar]
- 34. Geiman TM, Robertson KD. Chromatin remodeling, histone modifications, and DNA methylation-how does it all fit together? J Cell Biochem. 2002;87(2):117-125. [DOI] [PubMed] [Google Scholar]
- 35. Svedlund J, Barazeghi E, Stålberg P, et al. The histone methyltransferase EZH2, an oncogene common to benign and malignant parathyroid tumors. Endocr Relat Cancer. 2014;21(2):231-239. [DOI] [PubMed] [Google Scholar]
- 36. Mossman D, Kim KT, Scott RJ. Demethylation by 5-aza-2’-deoxycytidine in colorectal cancer cells targets genomic DNA whilst promoter CpG island methylation persists. BMC Cancer. 2010;10:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]