Abstract
The Atlantic Forest is considered the fourth most important biodiversity hotspot. Although almost 96% of its original area has been devastated, a large part of its remaining conserved area is inhabited by traditional communities. This research focused on two Quilombola communities who reside within the Núcleo Picinguaba of the Serra do Mar State Park, State of São Paulo, Brazil. The objective was to use a combination of ethnoecological and ecological approaches to select priority species for which to develop participatory conservation and sustainable management plans in protected areas in Brazil. We collaborated with community members to collect ethnobotanical and ethnoecological data and then measured the abundance of native species in local forests through phytosociological sampling. We used this information to assess the degree of threat to useful species using the Conservation Priority Index, adding an additional layer of analysis based on habitat successional categories. We then overlayed those useful species identified as highest risk locally with those federally listed as threatened or endangered. Based on this, we identified three species as priority for the development of sustainable management plans: Virola bicuhyba, Cedrella fissilis and Plinia edulis.
Introduction
Areas rich in biodiversity, with a large number of endemic species and which have a high degree of environmental degradation, were conceptualized by Myers [1] as a biodiversity hotspots. He thus mapped the priority areas of the planet for initiatives aimed at conservation.
Among these areas is the Atlantic Forest, the fourth most important hotspot among the 25 considered [2]. This biome had had almost 96% of its original area devastated [3], and its conservation is considered a challenge due to its high degree of disturbance, and that much of its remaining preserved area is inhabited by traditional communities [4].
According to Brazil’s constitutional decree No. 6,040 of February 7, 2007, traditional communities are culturally differentiated human groups that recognize themselves as such, and who occupy and use territories and natural resources as a condition for their cultural, religious and cultural reproduction. In Brazil, among the traditional communities, are the Quilombolas [4]. The Quilombolas are descendants of slaves of African origin who came to Brazil during the colonial (1530±1815), nited kingdom (1815±1822) and empire (1822±1889) periods. Some of these slaves fled the farms on which they were exploited, organizing communities of refugees called Quilombolas, in the local forests. Since that time, the Quilombolas have lived in villages where they have made a living from agriculture and use of forest resources [5]. According to Peralta [6], to date there is no certainty about how many Quilombola communities there are, however, data from the Brazilian Government estimates that there are about 3,000 Quilombola Communities in Brazil, with approximately 100 Quilombola communities in the Atlantic Forest [7]. Since these communities use the local flora as a means of meeting their basic demands for survival, it is essential that local use and conservation are compatible. Quilombola communities have lived and interacted with forests for a long time, developing detailed “traditional ecological knowledge” (TEK) [8]. TEK is developed through the process of observation and experimentation, transmitted among individuals and across generations [9] and is integral to the development of conservation and management plans in traditional communities today. The involvement and active participation of local residents is fundamental for the co-management, production, use and management of plant biodiversity resources [10]. Local and participatory management integrates local culture and knowledge and conservation [11].
There is a small but growing literature on traditional use of resources and biodiversity conservation in Quilombola communities. Hoffman [12] studied the impact of use on forest plants by a Quilombola community in Suriname. Austin-Ragosta [13] studied historical influences on the development of Jamaican Quilombola knowledge and biodiversity conservation, focusing on ethnomedicine. In Brazil, few studies have assessed traditional knowledge and biodiversity conservation in Quilombola communities. However, Crepaldi, Peixoto [14] and Conde and collaborators [5] evaluated the potential for sustainable harvest of plant resources based on traditional knowledge and species abundance in different Quilombola communities. Beyond Quilombola communities, many studies have used a combination of ecological and ethnographic approaches to assess sustainable resource use in local and indigenous communities [15, 16]. The Conservation Priority Index (IPC) is often used as a methodology for these assessments, especially in the context of traditional communities who use forest resources to meet many of their subsistence needs. This index assesses the conservation status of locally important plant resources by combing information on the local abundance of species in their natural environments, with the risk they face based on the method of harvest and the frequency and types of uses. Here, we adapt this method to include an additional consideration–the ecological successional habitat of the species.
The objective of this study was to use a combination of ethnobotanical and ecological approaches to select priority species for the development of participatory resource management plans in a protected area—Núcleo Picinguaba of the Serra do Mar State Park, State of São Paulo, Brazil. The broader goal is to foster the conservation and sustainable use of plant species in this region.
Methodology
Study area
Our research focused on two Quilombola communities (Fig 1), certified by Fundação Cultural Palmares since 2005 [17]. The first is Quilombo da Fazenda (QF), which dates back to the end of the 19th century and today consists of about 40 families (170 people). It overlaps with the protected area—the Núcleo Picinguaba of the Serra do Mar State Park, which represents the largest conservation park and portion of continuous conservation of the Atlantic Forest in Brazil. The second is Quilombo do Cambury (QC), which dates back more than 150 years and today has approximately 50 families (230 people). QC is locatedin the Serra da Bocaina Mosaic, in the north of São Paulo and Sul Fluminense, forming a significant ecological corridor for the protection of the Atlantic Forest [18]. Livelihoods in these communities center on subsistence agriculture and the use of forest resources.
Fig 1. Site of the Quilombo da Fazenda (QF) (in yellow) and Quilombo do Cambury (QC) (in red) in the Serra do Mar State Park–Nucleus Picinguaba (in green), in the State of São Paulo, Brazil.
Ethical aspects of research
Prior to data collection, all necessary legal licenses, as well as the participants’ consent to the use of the right to images, were obtained for the development of this study, as follows: 1) COTEC—Technical and Scientific Committee of Instituto Florestal, n°. 260108–009.510 / 2015for access to the Serra do Mar State Park area; 2) SISBIO—Biodiversity Information and Authorization System, n ° 51199–1 / 2015, for collecting and accessing plants in the Serra da Bocaina National Park; 3) SISGEN—National System for the Management of Genetic Heritage and Associated Traditional Knowledge, n. A648D14 to obtain prior informed consent and permission to inquire about traditional ecological knowledge; and 4) Research Ethics Committee No. 028525/2016 for the study to be carried out at the Federal University of São Paulo.
Project genesis (2015)
This project involves the collaboration of members of the two communities (QF and QC)—including 5 community partners, who actively participated in all phases of the project (from genesis and data analysis to publication), 19 interviewees who participated directly in the project, and 40 others who participated indirectly during the filming, workshops, assemblies and other activities developed with the communities, as well as a team of researchers with experience in agronomy, anthropology, botany, ecology, ethnobotany, pathophysiology, phytosociology and taxonomy of several universities (national and international) and the Botanical Garden of Brazil, including undergraduate and graduate students, in 4 phases [10]. This participatory ethnobotany approach was implemented with the support of the local communities, including those who resided in these area even before the creation of the integral protection area in the Park, to support actions and generate integrated knowledge to make sustainable management plans, for better use of local plant resources.
The first phase began in March 2015, with a workshop organized by the managers of the Picinguaba Center of the Serra do Mar State Park, Ubatuba, SP, Brazil, where Quilombolas communities participated. During this event, participants identified a clear need for managers to support projects related to local biodiversity and social and cultural aspects, including economic alternatives for residents. Therefore, throughout the year, five meetings were held involving members of the two communities (QF and QC) and the research team, to develop collaborative research with objectives that would be of common interest.
Collection of ethnobotanical and ethnoecological data (January 2016 to May 2018)
This study is part of the second phase of the project in which some members of the CQ and QF communities and university researchers co-developed project goals and methodologies, from the conception, sampling, collection and analysis of data [10, 19]. Meetings were held with the communities involved to co-define the objectives and activities of the study and community members were trained in data collection techniques including structured interview techniques [20], to document sociocultural data related to local knowledge (common name of the plant, part used, type of use, method of preparation, link between the collection of plants and the moon phase, possible restrictions to collection and collection instructions related to gender) and mainly herbal medicines (parts of prescribed plants, quantity and method of preparation, route of administration, time of use and possible contraindications). For the selection of the interviewees the 5 community collaborators invited all the 21 residents on the criteria of “being an expert in at least one of the following categories: medicinal, food / spices, civil construction, shipbuilding, handicrafts, combustion, others, hygiene / cosmetics, hunting, technology, dyeing and recreational [10, 21, 22]; 19 of them agreed to be part of the study. After obtaining the data on local knowledge, the community collaborators collected the specimens of species mentioned, which were identified and deposited in the Herbariums: Municipality of São Paulo (PMSP) and Instituto Florestal (SPSF).
During 178 days of fieldwork (see photos—bit.do/cee4, bit.do/cee5 and bit.do/cee6), 19 community members were interviewed by 5 community collaborators. In the QF 8 residents participated in the research, 5 women (62.5%) and 3 men (37.5%) with ages varying from 43 to 81 years old. All had incomplete elementary education, except one who has not studied. Occupations included artisans, farmers / farmers and one of the interviewees is a cook and works in the family restaurant. The 8 QF respondents generated a list of 92 plants. In the QC, 11 residents participated in the research, 2 women (18%) and 9 men (82%) aged between 35 and 65 years. All had incomplete elementary education, and worked as fishermen, cooks, farmers, bricklayers, and 6 of them live off the handicrafts they produce. The 11 QC respondents generated a list of 199 plants. This information was published in Yazbek [21] and Sauini [22]. Only 11.3% of the species were registered in both Quilombos. The categories dyes and foods / spices stand out for having the most common species in both communities, with 25% and 18.2%, respectively (Table 1).
Table 1. Number and percentage of plant species belonging to the 12 ethnobotanical categories reported by 11 interviewees of Quilombo do Cambury (QC) and eight of Quilombo da Fazenda (QF).
The species indicated in each quilombo total 199 (QC) and 92 (QF). As the same species may belong to more than one ethnobotanical category, they total 323 and 314 species, respectively.
| Ethnobotanical categories | N° species cited in QC | N° species cited in QF | Total species cited in quilombos | N° and (%) species coincident in both quilombos |
|---|---|---|---|---|
| 1.medicines | 90 | 157 | 247 | 29 (11,7%) |
| 2.food/spices | 71 | 72 | 143 | 26 (18,2%) |
| 3.construction | 44 | 33 | 77 | 8 (11,1%) |
| 4.shipbuilding | 41 | 5 | 46 | 2 (4,3%) |
| 5.handicraft | 30 | 15 | 45 | 4 (8,9%) |
| 6.tecnology | 5 | 11 | 16 | - |
| 7.combustion | 18 | 6 | 24 | 2 (8,3%) |
| 8.hunting | 5 | 4 | 9 | - |
| 9.tincture | 2 | 2 | 4 | 1 (25%) |
| 10.cosmetic | 6 | 4 | 10 | - |
| 11.recreative | 1 | - | 1 | - |
| 12.others | 10 | 5 | 15 | - |
| Total | 323 | 314 | 637 | 72 (11,3%) |
After the ethnobotanical and ethnoecological information was recorded, we carried out ecological studies (see below). The goal was to combine both sets of data to identify priority species for the development of sustainable use plans. Serra do Mar Park managers require these plans to allow residents to extract and market these plants in the form of crafts and others. This was one of the requests of the residents of these Quilombos and it can assist them in generating income, along with other activities they already perform with tourists.
Sampling of phytosociological data (January 2017 to May 2018)
Quantitative studies on vegetation structure were performed by phytosociological method to characterize the forest used by Quilombolas and to provide data on species density.
Maps derived from aerial images were contextualized and presented to community members, who were then asked to identify areas commonly used for the collection of plant resources. Six areas were identified, two of which were selected in QC (A1: 523.502E and 7.416.881S; A2: 523.764E and 7.416.768S) and two in QF (A3: 516.970E and 7.419.302S; A4: 516.397E and 7.419 .005S), as those areas most used their collection. Therefore, in a later phase, transections were carried out for sampling and data collection in the respective areas [23].
To identify the abundance of each species, ten 50x2m transects (adapted from Gentry [24]) were established, totaling 0.1 hectare in each Quilombola community (Fig 2). Trees, shrubs and tree ferns, with DBH (diameter at breast height or 1.30 m from the ground) equal to or above 4.8 cm., were sampled according Joly and collaborators [25]. For each individual, DBH, height and local name were noted. Fertile or vegetative samples were collected for later identification through pertinent bibliography and comparison with materials deposited in the PMSP and SP herbariums, adopting APG III [26]. The sampling effort was visualized using rarefaction curves for the sampling of each area from 100 randomizations, using EstimateS software [27], with the Jackknife-1 estimator. The number of individuals per species found in the transects was used to calculate relative density (see below).
Fig 2. 2m x 50m transection drawing (adapted from Gentry [24]) to identify the abundance of each species.
The current conservation status of all species sampled was then determined from official threatened species lists such as: National Center for Conservation of Flora [28], Ministry of the Environment [29], Secretariat of Environment of São Paulo [30] and International Union for Conservation of Nature [31].
Conservation priority analysis
To identify the degree of risk of collection of each species, we used the Conservation Priority Index (CPI) [5, 14, 32–35]. For all native species recorded from the transects, we carried out a bibliographic search to obtain the current state of conservation of these in Flora brasiliensis [36] and in the manual "Atlantic Forest Plants" [37].
The Conservation Priority Index was scored according to Table 2 and calculated using the following formula:
where:
B = biological value
RU = risk of use
and:
B = Rd x 10 Rd = (N / ni) x100
Rd = relative density
N = individuals of species x
ni = individuals of all sampled species
RU = 0.5 (C) + 0.5 (U) x 10
C = Collection risk based on the botanical part collected
U = Value over use. This is determined by the highest value between L and Div
Table 2. Scoring criteria used to determine priority species for conservation [5], where: Rd—relative density; C—collection risk based on the botanical part collected; L—use location based on the reference frequency; Div—diversity or plurality of use assigned to the species.
| Criteria | Score | |
|---|---|---|
| Rd | Occurrence between 0 and 1 or very low. | 10 |
| Occurrence between 1.1 and 3.5, or low. | 7 | |
| Occurrence between 3.6 and 7, or medium. | 4 | |
| Occurrence above 7 or moderate or high. | 1 | |
| C | Removal of specimen from offspring, excluding possibility of perpetuation of the species. | 10 |
| Removal of perennial structures without death, but actively influencing vegetative or flowering growth and perpetuation of species. | 7 | |
| Removal of permanent aerial parts without death only influences vegetative growth and energy production. | 4 | |
| The removal of transient aerial parts without direct influence on the life cycle of species. | 1 | |
| L | Above 20%, its use is considered high. | 10 |
| Between 10 and 20%, its use is considered moderately high. | 7 | |
| Up to 10%, its use is considered moderately low. | 4 | |
| Only mentioned in interviews. | 1 | |
| Div | For each use, add 1.42 to the Div value.—Considering (7) different use categories. | Maximum 10 |
The species were then classified into three groups [33, 38]:
Category 1 (species with a score ≥ 85): at risk of extinction at the site and therefore of conservation priority; in need of a sustainable use management plan;
Category 2 (species with a score between 85 and 60): can likely tolerate moderate levels of collection;
Category 3 (species with score ≤ 60): suitable for continued collection.
CPI based on successional categories
Finally, we then further divided species based on their successional categories. Although the CPI is recognized as the most efficient index to identify rare and impacted species in relation to the local vegetation [39], it doesn’t include species’ successional category, which may be relevant to conservation decisions. Therefore we classified species into three subdivisions based on local information as well as in other areas of the Atlantic Forest [40] as follows:
Subdivision A: includes old growth species (climax) and late secondary species found in more conserved forests;
Subdivision B: composed of early secondary species, uncommon in conserved areas, but more numerous in clearing areas and secondary forests;
Subdivision C: includes pioneer species—occurring in clearings, forest edges and degraded areas.
To classify species we used the works the works of Gandolfi and collaborators [41], Catharino and collaborators [42], and Barretto, Catharino [43]. We considered pioneer species as those with a short life cycle, fast growth and requiring high light for establishment and reproduction [43]. Early secondary species were considered to be fast-growing species with longer life cycles than the pioneer who show light-dependence but tolerate some shade. Late secondary species include long-lived species with shade-tolerant juveniles, these are generally slow-growing species typical of the mature canopy [43]. The ombrophilous category includes species that complete their entire life cycle in the shade of other trees, in the understory [42]. Plants considered as “conferatum”, undetermined or identified only at the genus level were grouped in the “unclassified” category.
Results
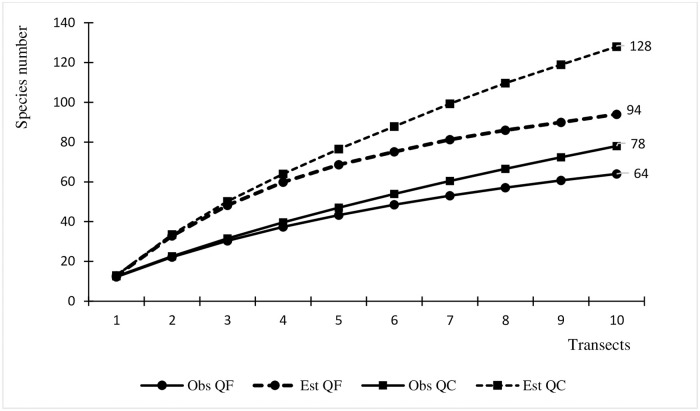
Fig 3 shows the rarefaction curve for the two study areas, with both observed values and those estimated with Jackknife 1 for QC (78–128) and QF (64–94), respectively.
Fig 3. Graph of rarefaction curve with the number of species in relation to transects performed for the two study areas (QC and QF), with both observed (Obs) values and those estimated (Est) by Jackknife 1 using EstimateS software.
Based on the combined ethnoecological data and the vegetation surveys, we assessed the conservation priority index for 113 species in 40 botanical families (Table 3).
Table 3.
List of native forest species from ethnobotanical collection in Quilombo do Cambury (QC) and Quilombo da Fazenda (QF) communities in alphabetical order of family, containing information on species, common name, relative density, biological value, risk of use, Conservation Priority Index (CPI), risk categories (category 1—species with a score ≥ 85; category 2—species with a score between 85 and 60; category 3—species with score ≤ 60), Subdivision as to the criteria of natural occurrence (A—includes late and climatic secondary species found in more conserved forests; B—composed of early secondary species, uncommon in conserved areas, but more numerous in clearing areas and secondary forests; C—includes pioneer species—occurring in clearings, forest edges and degraded areas; NC—no classification) and successional category (PI—pioneer; IS—initial secondary; LS—late secondary; UM—umbrophilous; NC—no classification).
| Family | Species | Common Name | Relative Density | Biological Value | Risk of Use | IPC | Risk Categories | Subdivision | Successional Category | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QC | QF | QC | QF | QC | QF | QC | QF | QC | QF | QC | QF | QC | QF | |||
| Anacardiaceae | Schinus terebinthifolius | Aroeira | 10 | 100 | 40 | 70 | 2 | C | PI | |||||||
| Annonaceae | Annona dolabripetala | Araticum | São-roque | 10 | 10 | 100 | 100 | 100 | 40 | 100 | 70 | 1 | 2 | A | A | LS |
| Annonaceae | Annona montana | Graviola | 10 | 100 | 25 | 62,5 | 2 | A | LS | |||||||
| Annonaceae | Guatteria australis | Astro-de-fisga | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Annonaceae | Xylopia brasiliensis | Canafista | 10 | 100 | 85 | 92,5 | 1 | B | IS | |||||||
| Apocynaceae | Malouetia cestroides | Guairana | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Apocynaceae | Tabernaemontana laeta | Guarana | 10 | 100 | 55 | 77,5 | 2 | C | PI | |||||||
| Araliaceae | Schfflera cf. Angustissima | Imbirotó | 7 | 70 | 70 | 70 | 3 | NC | NC | |||||||
| Arecaceae | Astrocaryum aculeatissimum | Brejaúba | 10 | 100 | 85 | 92,5 | 1 | A | UM | |||||||
| Arecaceae | Euterpe edulis | Juçara | Juçara | 1 | 1 | 10 | 10 | 25 | 100 | 17,5 | 55 | 3 | 3 | A | A | UM |
| Arecaceae | Geonoma sp. | Urecanga | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Arecaceae | Syagrus pseudococos | Patiuava | 10 | 100 | 100 | 100 | 1 | B | IS | |||||||
| Asteraceae | Vernonanthura beyrichii | Cambará | 10 | 100 | 55 | 77,5 | 2 | C | PI | |||||||
| Bignoniaceae | Cybistax antisyphilitica | Cinco-folhas | 10 | 100 | 55 | 77,5 | 2 | A | LS | |||||||
| Bignoniaceae | Handroanthus albus | Ipe-amarelo | Ipê-amarelo | 10 | 10 | 100 | 100 | 85 | 85 | 92,5 | 92,5 | 1 | 1 | A | A | LS |
| Bignoniaceae | Handroanthus impetiginosus | Ipê-roxo | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Bignoniaceae | Jacaranda puberula | Caroba-branca | Carobinha | 10 | 10 | 100 | 100 | 55 | 55 | 77,5 | 77,5 | 2 | 2 | B | B | IS |
| Bignoniaceae | Tabebuia cassinoides | Caxeta | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Boraginaceae | Cordia sellowiana | 7 | 70 | 100 | 85 | 1 | B | IS | ||||||||
| Boraginaceae | Cordia sp. 1 | Louro | Louro | 10 | 10 | 100 | 100 | 70 | 85 | 85 | 92,5 | 1 | 1 | NC | NC | NC |
| Boraginaceae | Cordia sp. 2 | Louro-pardo | 10 | 100 | 100 | 100 | 1 | NC | NC | |||||||
| Cannabaceae | Trema micrantha | Candiúva | 10 | 100 | 100 | 100 | 1 | C | PI | |||||||
| Caricaceae | Jacaratia spinosa | Mamão-do-mato | 10 | 100 | 25 | 62,5 | 2 | B | IS | |||||||
| Celastraceae | Monteverdia ardisiifolia | Guaracipó | 10 | 100 | 70 | 85 | 1 | A | UM | |||||||
| Chloranthaceae | Hedyosmum brasiliense | Congonha | 10 | 100 | 70 | 85 | 1 | B | IS | |||||||
| Chrysobalanaceae | Licania sp. | Milho-torrado | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Clusiaceae | Clusia criuva subsp. Parviflora | Figueira-braçadeira | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Clusiaceae | Garcinia gardneriana | Bacupari | Bacupari | 10 | 7 | 100 | 70 | 85 | 85 | 92,5 | 77,5 | 1 | 2 | A | A | LS |
| Combretaceae | Buchenavia kleinii | Angelim | 10 | 100 | 70 | 85 | 1 | A | LS | |||||||
| Erythroxylaceae | Erythroxylum pulchrum | Guará-cipó | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Euphorbiaceae | Actinostemon verticillatus | Sucanga | 10 | 100 | 100 | 100 | 1 | A | LS | |||||||
| Euphorbiaceae | Mabea piriri | Canudo-de-pito | Canudo-de-pito | 1 | 4 | 10 | 40 | 100 | 85 | 55 | 62,5 | 3 | 3 | C | C | PI |
| Euphorbiaceae | Maprounea sp. | Espera | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Euphorbiaceae | Tetrorchidium sp. | Bapeva | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Fabaceae | Albizia pedicellaris | Timbuíba | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Fabaceae | Albizia sp. | Timbuíva | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Fabaceae | Andira fraxinifolia | Sucupira | 10 | 100 | 70 | 85 | 1 | B | IS | |||||||
| Fabaceae | cf. Dalbergia frutescens | Braço-forte | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Fabaceae | cf. Hymenolobium janeirense | Guacuí | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Fabaceae | cf. Pterocarpus rohrii | Guaricica-amarela | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Fabaceae | cf. Swartzia oblata | Jatobá | 10 | 100 | 55 | 77,5 | 2 | NC | NC | |||||||
| Fabaceae | Hymenaea altíssima | Jatobá | Jatobá | 10 | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 1 | 1 | A | A | LS |
| Fabaceae | Inga cf. lenticellata | Ingá-ferro | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Fabaceae | Inga edulis | Ingá-de-metro | 10 | 100 | 55 | 77,5 | 2 | B | IS | |||||||
| Fabaceae | Inga marginata | Ingá-feijão | Ingá-feijão | 10 | 10 | 100 | 100 | 25 | 40 | 62,5 | 70 | 2 | 2 | B | B | IS |
| Fabaceae | Inga sp. | 10 | 100 | 70 | 85 | 1 | NC | NC | ||||||||
| Fabaceae | Myrocarpus frondosus | Cabreúva | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Fabaceae | Piptadenia gonoacantha | Caniveteiro | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Fabaceae | Pseudopiptadenia leptostachya | Cobi | 10 | 100 | 70 | 85 | 1 | B | IS | |||||||
| Fabaceae | Schizolobium parayba | Guapuruvu | 10 | 100 | 85 | 92,5 | 1 | C | PI | |||||||
| Fabaceae | Swartzia oblata | Barbatimão | Barbatimão | 10 | 10 | 100 | 100 | 70 | 100 | 85 | 100 | 1 | 1 | A | A | LS |
| Fabaceae | Swartzia simplex var. grandiflora | Laranjeira-do-mato | Canela-prego | 7 | 10 | 70 | 100 | 70 | 85 | 70 | 92,5 | 3 | 1 | A | A | LS |
| Fabaceae | Tachigali paratyensis | Ingá-flecha | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Fabaceae | Tachigali sp. 1 | Ingá-amarelo | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Fabaceae | Tachigali sp. 2 | Ingá-flecha | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Fabaceae | Tachigali sp. 3 | Ingá-fedido | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Lacistemaceae | Lacistema lucidum | Tatuzinho | Borrachudo | 7 | 10 | 70 | 100 | 85 | 85 | 77,5 | 92,5 | 2 | 1 | B | B | IS |
| Lamiaceae | Aegiphila integrifolia | Cajuja | Cajuja | 10 | 10 | 100 | 100 | 70 | 85 | 85 | 92,5 | 1 | 1 | C | C | PI |
| Lamiaceae | Vitex polygama | Tarumã | 10 | 100 | 70 | 85 | 1 | B | IS | |||||||
| Lauraceae | Aniba sp. | Canela-do-mato | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Lauraceae | Cryptocarya cf. mandioccana | Nóz-moscada | Nóz-moscada | 10 | 10 | 100 | 100 | 25 | 55 | 62,5 | 77,5 | 2 | 2 | NC | A | LS |
| Lauraceae | Cryptocarya saligna | Canela-sassafraize | 10 | 100 | 100 | 100 | 1 | A | LS | |||||||
| Lauraceae | Nectandra oppositifolia | Canela-do-mato | 10 | 100 | 85 | 92,5 | 1 | B | IS | |||||||
| Lecythidaceae | Cariniana estrellensis | Jequitibá | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Malvaceae | Eriotheca pentaphylla | Imbiruçu | 10 | 100 | 70 | 85 | 1 | A | LS | |||||||
| Melastomataceae | Huberia ovalifolia | Tinteiro | Tinteiro | 10 | 10 | 100 | 100 | 70 | 85 | 85 | 92,5 | 1 | 1 | A | A | UM |
| Melastomataceae | Miconia cinnamomifolia | Jacatirão | Jacatirão | 10 | 7 | 100 | 70 | 70 | 85 | 85 | 77,5 | 1 | 2 | B | B | IS |
| Melastomataceae | Miconia dodecandra | Pixirica | 10 | 100 | 25 | 62,5 | 2 | B | IS | |||||||
| Melastomataceae | Miconia prasina | Pixiricão | 10 | 100 | 25 | 62,5 | 2 | B | IS | |||||||
| Melastomataceae | Tibouchina pulchra | Quaresmeira | 7 | 70 | 100 | 85 | 1 | C | PI | |||||||
| Meliaceae | Cabralea canjerana | Ingá-cajarana | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Meliaceae | Cedrela cf. odorata | Cedro | 10 | 100 | 70 | 85 | 1 | NC | NC | |||||||
| Meliaceae | Cedrela fissilis | Cedro-rosa | Cedro-rosa | 10 | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 1 | 1 | A | A | LS |
| Meliaceae | Guarea macrophylla | Café-do-mato | Café-do-mato | 10 | 7 | 100 | 70 | 70 | 7 | 85 | 38,5 | 1 | 3 | A | A | UM |
| Moraceae | Brosimum guianense | Guaricica-da-vermelha | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Moraceae | Ficus adhatodifolia | Figueira-branca | Figueira | 10 | 10 | 100 | 100 | 70 | 100 | 85 | 100 | 1 | 1 | A | A | LS |
| Moraceae | Ficus gomelleira | Figueira-parda | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Moraceae | Sorocea cf. guilleminiana | Espineira-santa | Garapinha | 10 | 10 | 100 | 100 | 85 | 85 | 92,5 | 92,5 | 1 | 1 | NC | NC | NC |
| Myristicaceae | Virola bicuhyba | Bicuiba | 10 | 100 | 100 | 100 | 1 | A | LS | |||||||
| Myrtaceae | Campomanesia phaea | Cambuci | Cambuci | 10 | 100 | 55 | 77,5 | 2 | A | UM | ||||||
| Myrtaceae | Eugenia astringens | Araçarana | 10 | 100 | 85 | 92,5 | 1 | A | UM | |||||||
| Myrtaceae | Eugenia brasiliensis | Grumixama | 10 | 100 | 40 | 70 | 2 | A | UM | |||||||
| Myrtaceae | Eugenia cf. multicostata | Carambola-do-mato | 10 | 100 | 40 | 70 | 2 | NC | NC | |||||||
| Myrtaceae | Eugenia cf. stipitata | Araça-do-norte | 10 | 100 | 100 | 100 | 1 | NC | NC | |||||||
| Myrtaceae | Eugenia sulcata | Pitanga-do-mato | 10 | 100 | 25 | 62,5 | 2 | A | UM | |||||||
| Myrtaceae | Eugenia uniflora | Pitanga | Pitanga | 10 | 10 | 100 | 100 | 55 | 70 | 77,5 | 85 | 2 | 1 | A | A | UM |
| Myrtaceae | Myrcia neoriedeliana | Cambucá-do-mato | 10 | 100 | 40 | 70 | 2 | A | UM | |||||||
| Myrtaceae | Myrcia spectabilis | Arueira | Arco-de-peneira | 10 | 10 | 100 | 100 | 70 | 85 | 85 | 92,5 | 1 | 1 | B | B | IS |
| Myrtaceae | Plinia edulis | Cambucá | Cambucá | 10 | 10 | 100 | 100 | 100 | 40 | 100 | 70 | 1 | 2 | A | A | UM |
| Myrtaceae | Plinia sp. | Jaboticaba | 10 | 100 | 25 | 62,5 | 2 | NC | NC | |||||||
| Myrtaceae | Psidium cattleianum | Aracá | Araçá | 10 | 10 | 100 | 100 | 85 | 70 | 92,5 | 85 | 1 | 1 | B | B | IS |
| Myrtaceae | Psidium guajava | Goiaba | Goiaba | 10 | 10 | 100 | 100 | 70 | 100 | 85 | 100 | 1 | 1 | B | B | IS |
| Nyctaginaceae | Guapira nitida | 10 | 100 | 55 | 77,5 | 2 | A | UM | ||||||||
| Peraceae | Pera glabrata | Chile | Chile | 10 | 10 | 100 | 100 | 85 | 85 | 92,5 | 92,5 | 1 | 1 | B | B | IS |
| Phyllanthaceae | Hyeronima alchorneoides | Aricurana | Aricurana | 7 | 7 | 70 | 70 | 70 | 100 | 70 | 85 | 3 | 1 | B | B | IS |
| Phytolaccaceae | Gallesia integrifolia | Pau d’alho | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Primulaceae | Myrsine coriacea | Capororoca | Capororoca | 7 | 10 | 70 | 100 | 85 | 85 | 77,5 | 92,5 | 2 | 1 | C | C | PI |
| Primulaceae | Stylogyne lhotzkyana | Sapopema | 10 | 100 | 70 | 85 | 1 | A | UM | |||||||
| Rubiaceae | Bathysa mendoncaei | Sapopema | 10 | 100 | 85 | 92,5 | 1 | A | UM | |||||||
| Rubiaceae | cf. Bathysa | Aribarrosa | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Rubiaceae | Faramea hymenocalyx | Catinga-de-porca | 10 | 100 | 85 | 92,5 | 1 | A | UM | |||||||
| Rubiaceae | Rustia formosa | Manduberana | Guacá | 10 | 10 | 100 | 100 | 70 | 50 | 85 | 75 | 1 | 2 | A | A | UM |
| Rutaceae | Dictyoloma vandellianum | 10 | 100 | 25 | 62,5 | 2 | C | PI | ||||||||
| Rutaceae | Zanthoxylum rhoifolium | Mamica-de-moça | Mamica-de-porca | 10 | 10 | 100 | 100 | 70 | 100 | 85 | 100 | 1 | 1 | B | B | IS |
| Sapindaceae | Cupania oblongifolia | Cubatã | Cubatã | 10 | 7 | 100 | 70 | 100 | 85 | 100 | 77,5 | 1 | 2 | A | A | LS |
| Sapotaceae | Ecclinusa ramiflora | Guacá | 10 | 100 | 100 | 100 | 1 | A | LS | |||||||
| Sapotaceae | Pouteria caimito | Guapeva | 10 | 100 | 85 | 92,5 | 1 | A | LS | |||||||
| Sapotaceae | Pouteria sp. 2 | Guacuáuçu | 10 | 100 | 85 | 92,5 | 1 | NC | NC | |||||||
| Solanaceae | Solanum pseudoquina | Piloteira | Piloteira | 10 | 10 | 100 | 100 | 100 | 85 | 100 | 92,5 | 1 | 1 | C | C | PI |
| Urticaceae | Cecriopia glaziovii | Embaúba-vermelha | Embaúba | 10 | 10 | 100 | 100 | 100 | 100 | 100 | 100 | 1 | 1 | C | C | PI |
| Urticaceae | Cecropia pachystachya | Embaúba-branca | 10 | 100 | 40 | 70 | 2 | C | PI | |||||||
| Urticaceae | Pourouma guianensis | Baubu | 10 | 100 | 85 | 92,5 | 1 | B | IS | |||||||
| Verbenaceae | Citharexylum myrianthum | Tarumã | 10 | 100 | 70 | 85 | 1 | B | IS | |||||||
In the QC, 214 individuals were inventoried in the transects, distributed in 88 species from 37 families. The most abundant species were Palmito-jussara (Euterpe edulis) and Canudo-de-pito (Mabea piriri), representing 66 (30.8%) and 21 (9.8%) individuals, respectively.
In the QF, 158 individuals were sampled, distributed in 58 species from 28 families. The most abundant species were Palmito-jussara (Euterpe edulis) and Canudo-de-pito (Mabea piriri), representing 17 (10.7%) and 6 (3.7%) individuals, respectively.
In terms of the successional stage of the inventoried species, there are 18 pioneers (PI), 24 initial secondary (IS), 29 late secondary (LS), 17 umbrophilous (UM) and 25 without classification (NS).
Of the native species analyzed in relation to the Conservation Priority Index categories, in QC, 64 are in Category 1 (72.7% of the total sampled species), 12 of which are most relevant in that they have the maximum CPI value (100). In QF, 40 species are in Category 1 (68.9% of the total sampled species), 10 of which are the most relevant in terms of having the maximum CPI value (100).
In terms of conservation status in global conservation lists utilized, there were 11 species in the categories: "least concern" (LC), "almost threatened" (NT), "vulnerable" (VU) and "endangered" (EN) (Table 4).
Table 4. Local species listed of conservation concern and status (LC—least concern; NT—almost threatened; VU—vulnerable; EN—endangered).
| Specie | Conservation Status | |
|---|---|---|
| QC | Buchenavia kleinii | LC |
| Guatteria australis | LC | |
| Handroanthus albus | LC | |
| Cedrela fissilis | VU | |
| Plinia edulis | VU | |
| Virola bicuhyba | EN | |
| QF | Astrocaryum aculeatissimum | LC |
| Handroanthus albus | LC | |
| Erythroxylum pulchrum | LC | |
| Myrocarpus frondosus | LC | |
| Swartzia simplex var. grandiflora | LC | |
| Handroanthus impetiginosus | NT | |
| Cedrela fissilis | VU |
Discussion
The rarefaction curves both communities start to level off indicating sufficient sampling. The two stretches of forest sampled in the CQ are close to the Cambury beach access road and have had anthropogenic interventions in the past, such as shallow or selective botany exploration. In that region, there is a history of land use for agriculture [44], especially monocultures, initially sugarcane and then coffee [45]. The prevalence of umbrophilous species in the QF indicate that the access areas for collecting raw material are better preserved than in the QC (Fig 4).
Fig 4. Percentage of species occurring in the present sample as to their successional categories in QC and QF.
About 70% of species in both communities fell into the highest threat category (Category 1). These values are higher than those recorded in other studies. In Quilombola communities in the Atlantic Forest. Crepaldi, Peixoto [14] documented only 10.76% of the sampled species in the Cachoeira do Retiro Community (Espírito Santo) as Category 1. Conde and collaborators [5] documented 52% in the community of São Bento (Minas Gerais) and 56% in the community of São Sebastião da Boa Vista (Minas Gerais).
The high CPI values we recorded may be due in part to our sampling methodology, and demonstrate the importance of including successional category in this kind of analysis. For example, several common species from anthropogenic areas were classified in Category 1. This included Embauba-vermelha (Cecropia glaziovii), Cajuja (Aegiphila integrifolia) and Candiúva (Trema micrantha) in QC, and Capororoca (Myrsine coriacea), Caniveteiro (Piptadenia gonoacantha) and Guapuruvu (Schizolobium parahyba) in QF. All are pioneer species [43] and occur in clearings [46], forest edges and degraded areas. However, the areas identified by community members as the most important collection sites–and where the transects were therefore placed- were closed canopy areas (low light penetration). Therefore a low density of pioneer species is expected and the CPI values not fully representative.
Similarly, non-pioneer species included in Category 1 also included those found in low canopy cover forest environments and more associated with cleared environments and forest fragment borders. We also did not sample these habitats. In QC, this included Cedro-rosa (Cedrella fissilis), Cubatan (Cupania oblongifolia), Canafista (Xylopia brasiliensis) and Café-do-mato (Guarea macrophylla); and in QF Cedro-rosa (Cedrella fissilis) [44]. However, non-pioneer species included in Category 1 also included Guaracipó (Maytenus ardisiaefolia), Ingá-flecha (Tachigali paratyensis), Tinteiro (Huberia ovalifolia) and Figueira (Ficus adhatodifolia) in QC; and Guará-cipó (Erythroxylum pulchrum), Tinteiro (Huberia ovalifolia), Figueira (Ficus adhatodifolia) and Catinga-de-nut (Faramea hymenocalyx) in QF. These species are found in more conserved forests, and are recorded as naturally rare [43].
Selection of priority species for the development of sustainable use management plans
To select priority species, we focused on late and umbrophilous secondary plants. There were 8 late and umbrophilous secondary species with the highest CPI values (of 100) in QC (Annona dolabripetala, Actinostemon verticillatus, Hymenaea altíssima, Cedrela fissilis, Virola bicuhyba, Plinia edulis, Cupania oblongifolia, Ecclinusa ramiflora) and 5 species (Hymenaea altissima, Swartzia oblata, Cryptocarya saligna, Cedrela fissilis, Ficus adhatodifolia) in QF. When overlaid with the species officially listed as threatened or endangered at the level country, there candidate species emerged: Bicuíba (Virola bicyhyba), Cambucá (Plinia edulis) and Cedro-rosa (Cedrela fissilis). These represent priority species for which to develop sustainable use plans–they are both ethnobotanically highly important and ecologically at risk locally. Is important would highlight a chose to overlay the national priorities with the local priorities. Species that are of very high local priority may not be a national priority, but they might be the most important to address locally. Sustainable use plans can help conserve the species while contributing to the quality of life of local populations [47]:
- Priority 1—Endangered (EN)
- Virola bicuhyba (QC used as fuel)—According to CNCFlora [48], a loss of more than 65% of V. bicuhyba cover was reported within its known extent of occurrence; a population reduction of more than 60% was found in the last three generations of the taxon (estimated at about 30 years), caused mainly by selective extraction and habitat conversion, which will continue to cause future decline if nothing will be done according to its conservation. For these reasons, the species V. bicuhyba is considered threatened with extinction, requiring the creation of protected areas to ensure its survival and the development of specific legislation that regulates and controls its use in an appropriate manner. This species is of great importance to the regional economy in various locations and its total restriction can cause impacts.
- Priority 2—Vulnerable (VU)
- Plinia edulis (QF used as food)—According to CNCFlora [49] P. edulis is a species with edible and widely appreciated fruits and is therefore highly cultivated. However, it is quite rare in nature, with a population estimate of about 10,000 adult individuals. It is found outside protected areas and is therefore expected to face a population reduction of more than 10% over the next 30 years, considering a generation time of about ten years. In addition, the species occurs in places under strong anthropogenic pressure that have suffered habitat loss greater than 80%. It is therefore assumed that there has been a population reduction of more than 30% in the last 30 years. Thus, the species was therefore categorized as Vulnerable (VU).
- Cedrela fissilis (QC used for shipbuilding; QF used for construction, shipbuilding and medicine)—The species has historically been suffering from logging throughout its occurrence, which has led many of the subpopulations to extinction. In addition, most of its habitat has been completely degraded and converted into urban areas, pastures, plantations, among others. Due to these factors, it is suspected that C. fissilis has experienced a population decline of at least 30% over the last three generations, according to IUCN [50].
Conclusion
Our methodology allowed us to identify three species to prioritize for the co-development of sustainable management plans: these species are of high importance to the local communities, and both locally and globally threatened. The development of sustainable management plans requires consideration of harvest methods that will allow for their long-term resilience [51] as well as of potential alternatives, such as the promotion of species in agroforestry programs and/or the development of alternative uses for the species, that together can ensure the maintenance of cultural traditions and quality of life while preserving wildlife and the nature.
Acknowledgments
The authors would like to thank the interviewees and the other inhabitants of Quilombos Cambury and Fazenda for their contributions to this work.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
FAPESP - BIOTA PROGRAM (case number 2015 / 12046-0) and CNPq - Universal 2016 (case number 400802 / 2016-3).
References
- 1.Myers N. Threatened biotas: hotspots in tropical forests. Environmentalist 8: 1–20, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. Biodiversity hot spots for conservation priorities. Nature, 403: 853–858, 2000. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- 3.Ab’Saber NA. Os domínios de natureza no Brasil: potencialidades paisagísticas. Ateliê Editorial. 2003.
- 4.Cunha MC, Almeida MWB. Populações tradicionais e conservação ambiental In: Capobianco JPR, Veríssimo A, Moreira A, Santos I, Pinto LP. Biodiversidade na Amazônia brasileira: avaliação e ações prioritárias para a conservação, uso sustentável e repartição de benefícios. São Paulo, Estação Liberdade: Instituto Socioambiental, 2001. [Google Scholar]
- 5.Conde BE, Ticktin T, Fonseca AS, Macedo AL, Chedier LM, Rodrigues E, et al. Local ecological knowledge and its relationship with biodiversity conservation among two Quilombola groups living in the Atlantic Rainforest, Brazil. PLoS One, 12, 2017 10.1371/journal.pone.0187599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peralta RL. Desenvolvimento e sustentabilidade: novas interfaces para a luta quilombola. M.Sc. Thesis, Universidade Federal da Paraíba, João Pessoa, 2012. https://repositorio.ufpb.br/jspui/handle/tede/4516
- 7.Conde BE. Conhecimento ecológico local e sua interferência na conservação da biodiversidade botânica para três comunidades Quilombolas residentes em contexto de Floresta Atlântica. Tese de Doutorado. Universidade Federal de Juiz de Fora. 2016.
- 8.Diegues AC, Viana VM. (Orgs.) Comunidades tradicionais e manejo dos recursos naturais da Mata Atlântica. São Paulo: Nupeb, 2000. [Google Scholar]
- 9.Posey DA, Anderson AB. O reflorestamento indígena. In: Bologna G. 1990. Amazônia Adeus. Rio de Janeiro, Ed. Nova Fronteira, 1990.
- 10.Rodrigues E, Machado FCS, Conde BE, Cruz C, Barretto EHP, Santos G, et al. Participatory ethnobotany and conservation: a methodological case study conducted with quilombola communities in Brazil Atlantic Forest. Journal of Ethnobiology and Ethnomedicine, 16, 1–12, 2020. 10.1186/s13002-019-0351-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanazaki N, Zank S, Fonseca-Kruel VS, Schmidt IB. Indigenous and traditional knowledge, sustainable harvest, and the long road ahead to reach the 2020 Global Strategy for Plant Conservation objectives. Rodriguésia [online]. 2018, vol. 69, n. 4. 2018. [Google Scholar]
- 12.Hoffman B. Drums and arrows: ethnobotanical classification and use of tropical forest plants by a Maroon and Amerindian community in Suriname, with implications for biocultural conservation. PhD Thesis, University of Hawai’i at Manoa, Honolulu. 2009.
- 13.Austin-Ragosta S. Historical influences on the Development of Indigenous Jamaican Maroon Ethnomedicine: comparisons with West African and Arawak Ethnopharmacopoeia. Journal of Pan African Studies, 5(1): 278–280, 2012. [Google Scholar]
- 14.Crepaldi MOS, Peixoto AL. Use and knowledge of plants by “Quilombolas” as subsidies for conservation efforts in an area of the Atlantic Rainforest in Espırito Santo State, Brazil. Biodiversity and Conservation, 19: 37–60, 2010. [Google Scholar]
- 15.Ticktin T, Dacks R, Quazi S, Tora M, McGuigan A, Hastings Z, et al. Significant linkages between measures of biodiversity and community resilience in Pacific Island agroforests. Conservation Biology. v. 32, n.5, p. 1085–1095. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt IB, Figueiredo IB, Ticktin T. Sustainability of golden grass flower stalk harvesting in the Brazilian savanna. Ecological Sustainability for Non-Timber Forest Products: Dynamics and Case Studies of Harvesting, p. 199, 2015.
- 17.Fundação Cultural Palmares (FCP). 2005. Acessado em 20 de abril de 2016. http://www.palmares.gov.br/
- 18.Castro LMO. Ecoturismo e Inserção Comunitária em Unidades de Conservação: Uma análise comparativa entre as comunidades tradicionais do Núcleo Picinguaba do Parque Estadual Serra do Mar (PESM)–Ubatuba, SP. M.Sc. Thesis Universidade do Estado do Rio de Janeiro, 2015.
- 19.Yazbek P, Mata P, Passero F, Sauini T, Machado FCS, Garcia R, et al. Plants utilized as medicines by residents of Quilombo da Fazenda, Núcleo Picinguaba, Ubatuba, São Paulo, Brazil: A participatory survey. Journal of Ethnopharmacology, v. 244, p. 112123, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Bernard HR. Research Methods in Cultural Anthropology. 2 ed Newbury Park, USA: Sage Publications; 1988. [Google Scholar]
- 21.Yazbek P. Etnobotânica participativa: conservação e desenvolvimento local no Parque Estadual da Serra do Mar—Núcleo Picinguaba, Ubatuba, SP, Brasil. M.Sc. Thesis, Universidade Federal de São Paulo. 2018. https://bv.fapesp.br/pt/auxilios/93110/etnobotanica-participativa-conservacao-e-desenvolvimento-local-no-parque-estadual-da-serra-do-mar-/
- 22.Sauini T. Levantamento Etnobotânico Participativo entre os moradores do Quilombo Cambury, Ubatuba, SP, Brasil. M.Sc. Thesis, Universidade Federal de São Paulo. 2019.
- 23.Araújo EL, Ferraz EMN. Análise da vegetação nos estudos etnobotânicos. In: Albuquerque UP, Lucena RFP, Cruz da Cunha LVF. 2010. Métodos e Técnicas na Pesquisa Etnobiologica e Etnoecológica, Recife: NUPEA. 2010.
- 24.Gentry AH. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden, p. 1–34, 1988. [Google Scholar]
- 25.Joly CA, Assis MA, Bernacci LC, Tamashiro JY, Campos MCR, Gomes JAMA, et al. Florística e fitossociologia em parcelas permanentes da Mata Atlântica do sudeste do Brasil ao longo de um gradiente altitudinal. Biota Neotropica (Edição em Português. Online), v. 12, p. bn01812012012, 2012. [Google Scholar]
- 26.APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161:105–121. [Google Scholar]
- 27.Colwell RK. 2013. EstimateS: Statistical estimation of species richness and shared species from samples. Version 9.
- 28.CNCFlora. Centro de Conservação de Plantas. Lista Vermelha da flora brasileira. Accessed on 13 January 2019. http://www.cncflora.jbrj.gov.br/portal.
- 29.Brazil. Biodiversidade Brasileira Brasília: Ministério do Meio Ambiente, 2008. http://www.mma.gov.br/biodiversidade/biodiversidade-brasileira.
- 30.Secretaria de Meio Ambiente de São Paulo (SMA). Resolução SMA—57, de 05-VI-2016. Segunda revisão da lista oficial das espécies da flora ameaçadas de extinção no Estado de São Paulo. 2016.
- 31.International Union for Conservation of Nature (IUCN) ‘Red List of Threatened Plants’ 2019. Accessed in 17 May 2019. Avaliable from: http://www.iucn.org
- 32.Mander J, Quinn N, Mander M. Trade in wildlife medicinals in South Africa. Institute of Natural Resources Investigational Report No. 154, INR, Pietermaritzburg. 1997.
- 33.Dzerefos CM, Witkowski ETF. Density and potential utilization of medicinal grassland plants from Abe Bailey Nature Reserve, South Africa. Biodiversity and Conservation, 10: 1875–1896, 2001. [Google Scholar]
- 34.Oliveira RL, Neto EML, Araújo EL, Albuquerque UP. Conservation priorities and population structure of woody medicinal plants in an area of caatinga vegetation (Pernambuco State, NE Brazil). Environmental monitoring and assessment, v. 132, n. 1–3, p. 189–206, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Souza AS, Albuquerque UP, Nascimento ALB, Santoro FR, Torres-Avilez WM, Lucena RFP, et al. Temporal evaluation of the Conservation Priority Index for medicinal plants. Acta Botanica Brasilica, v. 31, p. 169–179, 2017. [Google Scholar]
- 36.Flora Brasiliensis: A obra. 2016. http://floradobrasil.jbrj.gov.br/.
- 37.Stehmann JR, Forzza RC, Salino A, Sobral M, Costa DP, Kamino LHY. Plantas da Floresta Atlântica. Rio de Janeiro, Jardim Botânico do Rio de Janeiro, 2009.
- 38.Albuquerque UP, Soldati GT, Sieber SS, Medeiros PM, Sá JC, Souza LC. Rapid ethnobotanical diagnosis of the Fulni-ô Indigenous lands (NE Brazil): floristic survey and local conservation priorities for medicinal plants. Meio Ambiente, Desenvolvimento e Sustentabilidade, 13 (2), 277–292. [Google Scholar]
- 39.Lucena RFP, Medeiros PM.; Araújo E. L.; A. G.C.; Albuquerque U.P. 2012. The Ecological Apparency Hypothesis and the Importance of Useful Plants: An Assessment Based on Use-Value. Journal of Environmental Management, 96: 106–115, 2012. 10.1016/j.jenvman.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 40.Ferreti ARA restauração da Mata Atlântica no litoral do Estado do Paraná: os trabalho da SPVS. In: Seminário Restauração Florestal, Curitiba, 2003. Fundamentos e estudo de casos. Colombo: Embrapa Florestas, 1–11, 2003
- 41.Gandolfi S, Leitão-Filho HF, Bezerra CL. Levantamento florístico e caráter sucessional das espécies arbustivo-arbóreas de uma floresta mesófila semidecídua no Município de Guarulhos, SP. Revista Brasileira de Biologia. 55: 753–767. 1995. [Google Scholar]
- 42.Catharino ELM, Bernacci LC, Franco GADC, Durigan G, Metzger JPW. Aspectos da composição e diversidade do componente arbóreo das florestas da Reserva Florestal do Morro Grande, Cotia, SP. Biota Neotropica 6: 1–28. 2006. [Google Scholar]
- 43.Barreto EHP, Catharino ELM. Florestas maduras da região metropolitana de São Paulo: diversidade, composição arbórea e variação florística ao longo de um gradiente litoral-interior, Estado de São Paulo, Brasil1. Hoehnea 42(3): 445–469. 2015. [Google Scholar]
- 44.FUNDART. Quilombos. Prefeitura municipal de Ubatuba. 2014. Accessed 18 june 2018. https://fundart.com.br/tradicao/comunidades/quilombos/.
- 45.São Paulo. Relatório pede reconhecimento de área quilombola em Ubatuba, 2007. http://www.saopaulo.sp.gov.br/spnoticias/ultimas-noticias/relatorio-pede-reconhecimento-de-area-quilombola-em- Diário Oficial do Estado de São Paulo, Poder Executivo, São Paulo, 30-VI-2016. Seção I, pp. 55–57. Accessed on 15 de August 2017.
- 46.Tabarelli M, Mantovani W. Clareiras naturais e a riqueza de espécies pioneiras em uma Floresta Atlântica Montana. Revista Brasileira de Biologia, v. 59, n. 2, p. 251–261, 1999. [Google Scholar]
- 47.Hanazaki N. Comunidades, conservação e manejo: o papel do conhecimento ecológico local. Biotemas, v. 16, n. 1, p. 23–47, 2003. [Google Scholar]
- 48.CNCFlora. Virola bicuhyba in Lista Vermelha da flora brasileira versão 2012.2 Centro Nacional de Conservação da Flora. Accessed on 13 January 2019. http://cncflora.jbrj.gov.br/portal/pt-br/profile/Virola bicuhyba
- 49.CNCFlora. Plinia edulis in Lista Vermelha da flora brasileira versão 2012.2 Centro Nacional de Conservação da Flora. Accessed on 13 January 2019. http://cncflora.jbrj.gov.br/portal/pt-br/profile/Plinia edulis
- 50.Barstow M. Cedrela fissilis. A Lista Vermelha da IUCN de Espécies Ameaçadas 2018. Accessed on 13 January 2019. 10.2305/IUCN.UK.2018-1.RLTS.T33928A68080477.en [DOI]
- 51.Ticktin T, Shackleton C. Harvesting non-timber forest products sustainably: opportunities and challenges In: Non-timber forest products in the global context. Springer, Berlin, Heidelberg, p. 149–169. 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.