Abstract
Matrigel, a basement-membrane matrix extracted from Engelbreth–Holm–Swarm mouse sarcomas, has been used for more than four decades for a myriad of cell culture applications. However, Matrigel is limited in its applicability to cellular biology, therapeutic cell manufacturing and drug discovery owing to its complex, ill-defined and variable composition. Variations in the mechanical and biochemical properties within a single batch of Matrigel — and between batches — have led to uncertainty in cell culture experiments and a lack of reproducibility. Moreover, Matrigel is not conducive to physical or biochemical manipulation, making it difficult to fine-tune the matrix to promote intended cell behaviours and achieve specific biological outcomes. Recent advances in synthetic scaffolds have led to the development of xenogenic-free, chemically defined, highly tunable and reproducible alternatives. In this Review, we assess the applications of Matrigel in cell culture, regenerative medicine and organoid assembly, detailing the limitations of Matrigel and highlighting synthetic scaffold alternatives that have shown equivalent or superior results. Additionally, we discuss the hurdles that are limiting a full transition from Matrigel to synthetic scaffolds and provide a brief perspective on the future directions of synthetic scaffolds for cell culture applications.
Toc Blurb
Matrigel is widely used for cell culture; however, its ill-defined composition, batch-to-batch variability, and animal-derived nature lead to experimental uncertainty and a lack of reproducibility. In this Review, we discuss the limitations of Matrigel and highlight synthetic alternatives for stem cell culture, regenerative medicine and organoid assembly.
Introduction
The origin of Matrigel dates back more than 40 years to the discovery of a murine tumour that produced large quantities of extracellular matrix (ECM) proteins reminiscent of a basement membrane1 — a specific ECM that serves as a structural support for cells in most epithelial and endothelial layers2. Later named the Engelbreth–Holm–Swarm (EHS) tumour, extracts from this basement-membrane-producing tumour were developed and marketed as Matrigel or EHS matrix3-5 (herein referred to as Matrigel). The primary components of Matrigel are four major basement membrane ECM proteins: laminin (~60%), collagen IV (~30%), entactin (~8%) and the heparin sulfate proteoglycan perlecan (~2–3%)6. Multiple isoforms of laminin have been identified in Matrigel, including β2, α5, α3 and α4, with the most predominate being α1, β1 and γ1, which make up the heterotrimer laminin 1 (also known as laminin 111)7,8. Laminin 1 contains multiple adhesion sites for the attachment of various cell types, including stem, epithelial, endothelial and tumour cells1,8-12. Moreover, the laminin-1-derived peptides Ile-Lys-Val-Ala-Val (IKVAV) and Try-Ile-Gly-Ser-Arg (YIGSR) promote differentiation13,14 and angiogenesis11,15, as well as tumour growth and metastasis16,17. Although collagen IV is most abundant, other collagens found in Matrigel include collagen I, XVIII, VI and III7. Matrigel also contains tumour-derived proteins, including growth factors, such as transforming growth factor (TGF) family peptides (for example, TGF-β) and fibroblast growth factors (FGFs)18,19, as well as enzymes, such as matrix metalloproteinases (MMPs)5,20. Collectively, these structural and biological proteins contribute to the biological function of Matrigel.
During preparation, the reconstituted form of Matrigel undergoes gelation at temperatures in the range 22–37 °C, during which entactin acts as a crosslinker between the laminin and collagen IV to create a hydrogel — a water-swollen, crosslinked network. Owing to its inherent bioactivity, Matrigel has been used for various applications for different cell types. As a thin gel coating, Matrigel has been used to culture and expand cells, such as human pluripotent stem cells (hPSCs)21, neurons22,23 and cardiomyocytes24. Thicker Matrigel coatings have been used to develop assays to investigate endothelial tubulogenesis25,26, and 3D Matrigel constructs allow for cell encapsulation in tissue engineering27,28 and organoid assembly29,30. In these contexts, Matrigel has been a useful, yet perhaps poorly understood, tool for cell culture.
The applicability of Matrigel is, however, severely limited by the variability in its composition and the presence of xenogenic contaminants. Indeed, multiple reports have indicated a need to use caution in interpreting results based on Matrigel-cultured cells18,31. However, researchers continue to use Matrigel for cell culture owing to its availability, ease of use and versatility for culturing different types of cells. The ubiquitous use of Matrigel may also in part be due to a historical lack of comparable synthetic alternatives. However, recently developed synthetic materials have shown results equivalent or superior to those of Matrigel. These synthetic alternatives can provide a chemically defined, xenogenic-free environment that can be modified for desired outcomes and provide reproducible results. In particular, synthetic materials used for cell culture, often termed scaffolds, have been designed and developed for stem cell culture, tissue engineering and organoid assembly for toxicant and therapeutic screening (Fig. 1).
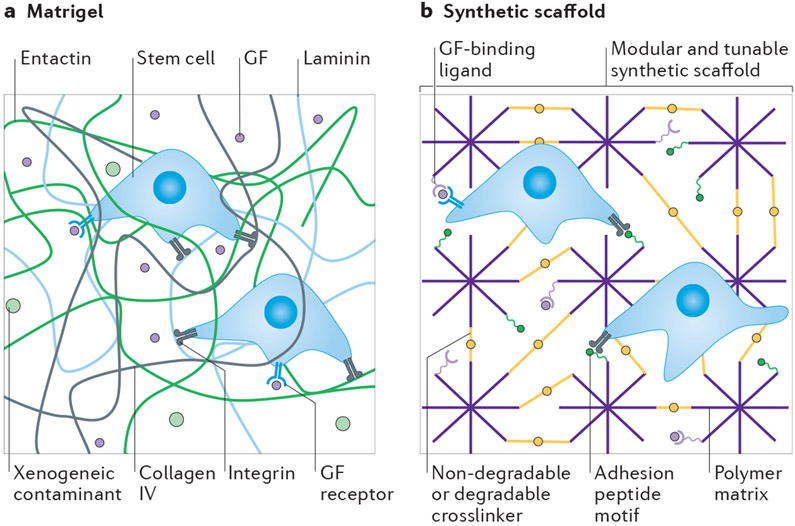
Fig. 1∣. Comparison of Matrigel and synthetic scaffolds.
a ∣ The composition of Matrigel is unamenable to modifications, ill-defined, complex and highly variable, resulting in heterogeneities in both biological and mechanical properties. As it is animal-derived, Matrigel may also contain xenogenic contaminants, and the presence of growth factors (GFs) and other biological proteins can lead to undesirable cellular effects. b ∣ Synthetic scaffolds are highly tunable and chemically defined. The mechanical, physical and biological properties of these scaffolds can be modified to direct cellular response while eliminating undesirable matrix-induced effects.
In this Review, we begin by briefly discussing the limitations of Matrigel, before assessing the use of Matrigel in three specific areas of research: stem cell culture, regenerative medicine and organoid assembly. For each application, we highlight key studies in which the performance of synthetic scaffolds has been directly compared with that of Matrigel and analyse the suitability of synthetic alternatives (Table 1). Lastly, we discuss the current impediments to replacing Matrigel with synthetic scaffolds and provide our perspective on the future of synthetic scaffolds for cell culture applications.
Table 1.
Synthetic scaffolds that have been directly compared with Matrigel
| Synthetic scaffold material | Cells and application | Refs. |
|---|---|---|
| Pluripotent stem cell culture and maintenance | ||
| PMEDSAH | Long-term 2D hESC and hiPSC culture and maintenance | 73-75 |
| PMVE-alt-MA | Long-term 2D hESC and hiPSC culture and maintenance | 76 |
| PAPA brushes tethered with cRGDfK | Long-term 2D hESC and hiPSC culture and maintenance | 80 |
| PEG thiol–ene hydrogels with cyclic RGD | Short-term 2D hESC culture and expansion | 81 |
| A peptide–acrylate surface generated from 2-hydroxyethyl methacrylate, 2-carboxyethyl acrylate and tetra(ethylene glycol) dimethacrylate and functionalized with a vitronectin-derived peptide | Long-term 2D hESC culture and maintenance | 83 |
| A poly(OEGMA-co-HEMA) film decorated with a vitronectin-derived peptide and developed through surface-initiated polymerization | Long-term 2D hiPSC culture and maintenance | 84 |
| PVA–IA hydrogels functionalized with a vitronectin-derived peptide | Long-term 2D hiPSC and hESC culture and maintenance | 85 |
| PSS and PAM copolymerized hydrogel PAM6-co-PSS2 | Long-term 2D hESC and hiPSC culture and maintenance | 92 |
| PAM hydrogels functionalized with a vitronectin-derived glycosaminoglycan-binding peptide | Long-term 2D hESC and hiPSC culture and maintenance | 94 |
| RGD-functionalized PEG hydrogel crosslinked using factor XIIIa | 3D Human fibroblast reprogramming to hiPSCs and 3D hiPSC culture | 100 |
| Stem cell differentiation | ||
| Self-assembled peptide nanofibre hydrogels functionalized with a peptide derived from brain ECM | Mouse neural stem cell differentiation into neurons, astrocytes and oligodendrocytes | 112 |
| RGD-functionalized and MMP-sensitive PEG thiol–ene hydrogel | hiPSC-derived endothelial cell and vascular morphogenesis | 113 |
| Electrospun synthetic polyamide nanofibres: (C28O4N4H47)n and (C28O4.4N4H47)n | Mouse ESC, hESC and iPSC differentiation into functional hepatocytes | 126 |
| MMP-sensitive PEG hydrogel crosslinked using factor XIIIa | Mouse ESC neuroepithelial differentiation | 128 |
| In vivo tissue regeneration | ||
| RGD-functionalized PEG–MAL protease-degradable hydrogels | Mouse muscle satellite cell engraftment in dystrophic aged skeletal muscle | 146 |
| RGD-functionalized maltodextrin-derived scaffolds | Myotubule formation from mouse myoblasts | 147 |
| Nanocomposite copolymer PLGA–PEG–PLGA hydrogel with Laponite | Mouse myoblast treatment of muscle injuries | 148 |
| Organoid assembly | ||
| Non-degradable PEG hydrogel functionalized with laminin-derived peptides and crosslinked using factor XIIIa | Mouse neuroepithelial tubule organoids | 132 |
| RGD-functionalized protease-degradable PEG–MAL hydrogel | Madin–Darby canine kidney cyst organoids | 109 |
| MMP-sensitive, heparin-functionalized biohybrid PEG hydrogel | Renal tubulogenesis, mammary epithelial morphogenesis and Alzheimer disease | 165-168 |
| Hydrolytically degradable PEG hydrogel functionalized with RGD and laminin and crosslinked using XIIIa | Mouse intestinal organoids | 162,163 |
| Protease-degradable, RGD-functionalized PEG–MAL hydrogel | Human intestinal organoids and lung organoids | 156,161 |
| Cell-based assays for preclinical tissue models, toxicant screening and drug discovery | ||
| MMP-degradable RGD-functionalized PEG thiol–ene hydrogel | Vascular toxicity screening | 81 |
| MMP-degradable, RGD-functionalized PEG thiol–ene hydrogel | Oestrogen-receptor-positive breast cancer assay | 169 |
cRGDfK, cyclo(Arg-Gly-Asp-d-Phe-Lys); ECM, extracellular matrix; ESC, embryonic stem cell; GLN, glutamine; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; iPSC, induced pluripotent stem cell; LYS, lysine; MAL, maleimide; MMP, metalloproteinase; PAM, polyacrylamide; PAPA, poly(acrylamide-co-propargyl acrylamide); PEG, polyethylene glycol; PLGA–PEG–PLGA, poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide); PMEDSAH, poly(2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide); PMVE-alt-MA, poly(methyl vinyl ether-alt-maleic anhydride); poly(OEGMA-co-HEMA), poly(oligo(ethylene glycol) methacrylate-co-2-hydroxy-ethyl methacrylate); PVA–IA, poly(vinyl alcohol-co-itaconic acid); PSS, poly(sodium 4-stryenesulfonate); RGD, Arg-Gly-Asp.
Limitations of Matrigel
Although Matrigel is commonly used as a cell culture tool7, it is inherently limited in its applicability for fundamental research, therapeutic cell manufacturing and cell-based assays, owing to its complex, ill-defined and variable composition5,32,33 (Fig. 1a). Inconsistencies in biochemical properties between batches of Matrigel — and within a single batch — has led to uncertainty and a lack of reproducibility in cell culture experiments18,19,34,35. More than 14,000 unique peptides and nearly 2,000 unique proteins have been identified in Matrigel4-6,8,13,14. The majority of those identified are structural proteins, but others include growth factors7,18,19, transcription factors7 and cytokines19. Numerous proteomic analyses on Matrigel have revealed considerable variability, with each new study discovering proteins that have not yet been recorded or not detecting proteins that had been previously reported7,18,19,36,37. For example, in one study, growth factors such as insulin-like growth factor 1 and epidermal growth factor, which are important and promiscuous signalling molecules, were expressed at quantifiable levels (on the order of nanograms per millilitre18) but were not detected in four independent Matrigel batches investigated in a later study19. The reported concentration of growth factors has also been inconsistent, including an order of magnitude difference in FGF2 and platelet-derived growth factor concentrations between batches19. Growth-factor-reduced (GFR) Matrigel is an alternative Matrigel product that is similar in structure to standard Matrigel but with lower growth factor concentrations18. When compared, 480 unique proteins were identified in standard Matrigel and 424 in GFR Matrigel, with only a ~53% batch-to-batch similarity in proteins between the two products7. This difference in protein content was not only attributed to the lower concentration of growth factors in GFR Matrigel, but also to variations in the structural protein content7.
The mechanical properties of Matrigel also show batch-to-batch variability. Although some variability in elastic modulus (or ‘stiffness’) can be attributed to different testing methods and temperatures38-40, inherent variability between batches and within a single batch have been identified41,42 (Fig. 1a). For example, using atomic force microscopy, the average elastic modulus of two batches of Matrigel was reported to be 400–420 Pa. However, a third batch had an average elastic modulus twice as high (840 Pa)35. Moreover, heterogeneities within the ECM resulted in local areas of the Matrigel with even higher elastic moduli (1–3 kPa)35. Using in situ mechanical interferometry to analyse local mechanical properties, the median elastic modulus of Matrigel was found to agree well with that of bulk measurements (~650 Pa)42. However, on the microscale, the Matrigel was non-uniform, with regions of higher elastic modulus (1–2 kPa)42. Optical thickness images revealed that these stiffer areas corresponded to areas of higher material density. Variations in the stiffness have also been attributed to the underlying substrate34 and the gradual changes in Matrigel thickness over time, perhaps caused by ECM remodelling42.
Another complexity inherent in Matrigel is the potential for antigenicity. The introduction of xenogenic contaminants from an animal-derived ECM such as Matrigel may limit the therapeutic potential of cells or tissues expanded in Matrigel-containing culture. Evidence of viral contamination, specifically lactate dehydrogenase-elevating virus (LDHV), has been found in multiple batches of animal-derived ECM products, including Matrigel43,44. LDHV is a natural mouse virus that infects macrophages and can affect the immune system and tumour behaviour44-46. Matrigel’s complexity and animal origin may also interfere with mechanistic studies of cell behaviour, making it difficult to distinguish biological effects caused by controlled experimental variables from those caused by Matrigel itself. The ambiguity in experimental results and the presence of xenogenic contaminants are often compounded when serum-containing media is used in conjunction with Matrigel (Box 1).
Box 1 ∣. Chemically defined, xeno-free cell culture.
Fully defined, xeno-free cell culture requires chemically defined, xenogenic-free media as well as a chemically defined scaffold184. For routine cell expansion, the media has traditionally included serum of human or animal origin, which is associated with now well-studied risks, including the potential for the transmission of prion, zoonotic or viral infections, and the potential for xenogeneic compounds to trigger undesirable immune responses185. Similar to Matrigel, serum is susceptible to batch-to-batch variability, raising concerns regarding the quality and concentration of proteins, and the potential effects on the reproducibility of experimental results186,187. Numerous serum-free, chemically defined media have been developed and shown to support the successful expansion of stem cells87,111,188-192. Synthetic scaffolds have been combined with chemically defined, xenogeneic media to develop a fully defined, xeno-free environment for cell culture for both fundamental research and cell manufacturing for therapeutic applications80,84-86,193. The proliferation and pluripotency maintenance of the cells cultured on synthetic scaffolds was similar to those cultured on Matrigel, while eliminating the possibility of xenogeneic contaminants32,80,84,156.
Synthetic alternatives to Matrigel
The limitations of Matrigel have driven the search for synthetic alternatives. Over the past two decades, numerous synthetic scaffolds, both 2D and 3D, have been developed using synthetic polymers. Unlike Matrigel, the physical, mechanical and biological properties of synthetic polymeric scaffolds can often be tuned independently by altering the composition, molecular weight, crosslinker, crosslink density and method of polymerization47,48 (Fig. 1b). The density and presentation of biofunctional moieties, often in the form of peptides, can also be controlled48. Owing to the diversity of scaffolds that have been designed and developed as alternatives to Matrigel, this Review is limited to describing only some of the key properties of the various scaffolds. An in-depth description of scaffold synthesis and characterization is beyond the scope of this Review. However, because many of the scaffolds presented here are derived from polyacrylamide (PAM) and polyethylene glycol (PEG), we provide an overview of these synthetic materials.
PAM is a synthetic polymer that forms a hydrogel upon reaction of an acrylamide monomer and bis-acrylamide crosslinker in the presence of ammonium persulfate and tetramethylethylenediamine. PAM is uncharged and bioinert, and therefore does not react with proteins or bind directly to cells49. However, these materials are commonly used for cell culture50,51 because the stiffness and biofunctionality of PAM hydrogels can be tuned, enabling user-defined control of cell–material interactions. For instance, cell-adhesion peptides and ECM proteins have been crosslinked to PAM hydrogels to engage cell interactions49. Owing to the toxicity of the hydrogel precursors and the polymerization reaction, however, PAM hydrogels are limited to 2D cell culture and cannot be used for 3D cell encapsulation47.
PEG is one of the most studied and widely used synthetic polymers for the construction of synthetic scaffolds52,53. This material is advantageous for cell culture as it is hydrophilic, bioinert and highly amenable to chemical modification54. PEG can be modified with diverse functional groups and formed into hydrogels using various polymerization techniques55,56. PEG hydrogels are often formed through photopolymerization, whereby multiarm PEG chains are functionalized with reactive groups (such as acrylate, norbornene or thiol), combined with a photoinitiator and then exposed to UV or visible light57-59. Other polymerization methods include Michael addition reactions, including the thiol-Michael addition reaction60, and enzymatic reactions using, for example, the activated transglutaminase enzyme factor XIIIa61,62. These polymerization techniques are typically non-toxic, which allows for cell encapsulation within the forming hydrogel47,63-65. Additionally, the thiol–ene chemistry permits cysteine-containing peptides, either as pendant peptides or crosslinkers, to be covalently tethered to the polymer, thus introducing biofunctionality into the otherwise inert system57,66.
Pluripotent stem cell culture
hPSCs, including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), proliferate indefinitely and maintain their ability to differentiate into cells from all three germ layers (the endoderm, mesoderm and ectoderm) when cultured in appropriate conditions67. The ability to expand and generate large numbers of hPSCs in vitro has great potential to serve as a feedstock for applications in disease modelling, drug screening and cellular therapies68-71. When they were first isolated, hESCs needed to be cultured on a feeder layer of mouse embryonic fibroblasts to maintain their pluripotency67. However, this method inevitably resulted in complications associated with co-culture, including the need to remove animal-derived contaminants. Matrigel was used in initial efforts to eliminate embryonic fibroblast feeder layers, and a pivotal study showed that it supported proliferation and maintenance of the stem cell phenotype of hESCs, as determined by a normal karyotype and high telomerase activity for up to 130 population doublings21. Although the use of Matrigel removed some complications associated with mouse fibroblast co-culture, it did not entirely rid the cultures of xenogeneic components that are undesirable for hPSC clinical applications72. Moreover, the ill-defined, animal-derived nature of Matrigel can influence cellular behaviour5,18, ultimately calling into question conclusions derived from stem cells grown on Matrigel.
Synthetic scaffolds that support hPSC proliferation and maintenance at similar or superior levels to those of Matrigel have been developed. For instance, the zwitterionic polymer, poly(2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide) (PMEDSAH) was the first fully synthetic polymer coating reported to sustain long-term culture of hESCs. The physical properties of the synthetic coating, including the hydrophilicity, thickness and surface charge, can be altered by varying the mode of polymerization and reaction time. Collectively, these physical properties influence the self-renewal of the hESCs73. Compared with Matrigel, the hESCs cultured on the PMEDSAH coating had a similar gene expression profile after 20 passages74,75. In another study, 90 polymers, varying in chemical composition and molecular weight, were evaluated for their ability to support the pluripotency of hPSCs. Of those screened, 16 polymers performed similarly to Matrigel and supported short-term proliferation and maintenance of hPSC pluripotency. However, poly(methyl vinyl ether-alt-maleic anhydride) was the only polymer to sustain long-term hPSC culture while reducing spontaneous differentiation of hESC and hiPSC lines to a similar extent as Matrigel76. Although the mechanism by which the polymer coating sustained long-term culture was not investigated, it was postulated that the anionic nature of the synthetic polymer mimics the structural and functional features of heparin, including its propensity for growth-factor binding, which may have a central role in regulating the self-renewal of hESCs.
Since these initial discoveries, various synthetic scaffolds have been developed to recapitulate the key cell–matrix interactions necessary for maintaining hPSC pluripotency. In addition to the physical properties, such as stiffness, topography and surface charge, the biochemical properties of the cellular microenvironment, including cell adhesivity, biochemical functionality and degradability, also have a key role in stem cell fate. Unlike Matrigel, the biochemical properties of synthetic scaffolds can also be tuned.
The cell–matrix interactions crucial for hPSC expansion and pluripotency can be reconstructed on synthetic scaffolds by incorporating cell-adhesion motifs. Integrin receptor subunits involved in hPSC adhesion to Matrigel include α5, α6, αv, β1 and β5 (refs7,34,77,78). Peptides that bind to these integrin receptors have been developed and presented on synthetic scaffolds in different combinations to promote cell adhesion and proliferation for long-term hPSC culture32,77. One of the most ubiquitously used peptides to encourage cell adhesion to synthetic scaffolds is the fibronectin-derived three-amino-acid peptide Arg-Gly-Asp (RGD), which binds to both αvβ3 and αvβ5 integrins79. In one study, RGD and a range of other peptides were covalently tethered to poly(acrylamide-co-propargyl acrylamide) (PAPA) brushes80. The PAPA coatings were prepared through photoinitiator-free photopolymerization using high-intensity UV light. Unlike Matrigel and other naturally derived scaffolds, the PAPA brushes offer a stable surface coating that has a longer shelf-life, are modifiable and can be sterilized using industry standard methodologies. A cyclic form of RGD , cyclo(Arg-Gly-Asp-d-Phe-Lys) (cRGDfK), was identified as the most effective peptide for hPSC culture; the cRGDfK peptide compared favourably with other peptides derived from laminin, fibronectin and vitronectin. The cRGDfk-coupled PAPA-coated scaffold maintained long-term undifferentiated cultures of three independent hPSC lines, similar to what is observed with Geltrex80 (the GFR Matrigel produced by Gibco), and eliminated karyotypic abnormalities observed in Geltrex-cultured cells. Moreover, cyclic RGD in a different form, cyclo(Arg-Gly-Asp-d-Phe-Cys), has also supported short-term hESC expansion81. Through high-throughput screening of an array of 64 PEG thiol–norbornene synthetic hydrogels of varying stiffness and cyclic RGD concentrations, several hydrogel formulations were identified that showed similar or enhanced maintenance of hESC pluripotency, as evaluated by NANOG expression, relative to that of hESCs cultured on Matrigel. One hydrogel formulation, containing 2 mM of cyclic RGD and with a modulus of 10 kPa, supported hESC expansion and pluripotency, even in the absence of a rho-associated protein kinase (ROCK) inhibitor, which is typically needed to maintain hPSC adhesion and expansion81.
In addition to RGD, other peptides, such as those derived from vitronectin (an ECM glycoprotein abundant in serum82 and present in Matrigel in only trace amounts6), have been tethered to synthetic scaffolds and the resulting materials investigated for their ability to maintain hPSC pluripotency. In one study, peptide sequences derived from natural ECM proteins, including laminin, bone sialoprotein and vitronectin, were conjugated to synthetic peptide–acrylate surfaces and screened for their ability to culture undifferentiated hESCs. Surfaces conjugated to the vitronectin-derived peptide supported hESC pluripotency to a level comparable to that of Matrigel for more than ten passages83. Moreover, a film composed of a copolymer of oligo-(ethylene glycol) methacrylate and 2-hydroxy-ethyl methacrylate (poly(OEGMA-co-HEMA)) functionalized with a vitronectin-derived peptide supported hiPSC self-renewal at a level similar to that of Matrigel for ten passages, but in a xeno-free and chemically defined media84. In a separate study, hPSC culture was investigated on poly(vinyl alcohol-co-itaconic acid) hydrogels of varying elasticities and grafted with a vitronectin-derived peptide. A hydrogel with an elasticity of 25 kPa and grafted with high concentrations (500–1,500 μg ml−1) of the vitronectin-derived peptide maintained hiPSC and hESC culture at levels similar to those of Matrigel for more than 20 passages under xeno-free conditions85. Synthemax, a commercially available, synthetic vitronectin scaffold functionalized with RGD, also supported hiPSC self-renewal to a similar extent as Matrigel86 but in chemically defined and growth-factor-free conditions87.
Synthetic scaffolds have also been used to mimic the role of heparin sulfate proteoglycans such as perlecan, a major component of Matrigel88, to support hPSC culture. Evidence suggests that heparin sulfate proteoglycans have a key role in maintaining the self-renewal of hPSCs, owing to their ability to bind to soluble basic fibroblast growth factor (bFGF), a crucial growth factor required for hPSC maintenance, and to protect bFGF from denaturation and proteolytic degradation89-91. In one study, a heparin-mimicking synthetic hydrogel was developed by copolymerizing poly(sodium 4-stryenesulfonate) (PSS) with PAM at different ratios. The resulting heparin-mimetic scaffold, PAM6-co-PSS2, supported long-term hPSC expansion and maintained pluripotency similar to Matrigel, as defined by NANOG and OCT4 expression, for more than 20 passages in a chemically defined media92. In addition, synthetic scaffolds that display proteoglycan-binding peptides, which can interact with glycosaminoglycans found on the surface of cells, are effective for sustained stem cell renewal93,94. For instance, PAM hydrogel scaffolds functionalized with a vitronectin-derived, glycosaminoglycan-binding peptide maintained hPSC pluripotency with similar gene expression profiles to those cultured on Matrigel. However, long-term hESC proliferation on these functionalized hydrogels was stiffness dependent: hESCs cultured on stiff hydrogels (10 kPa) proliferated into robust colonies whereas those on softer hydrogels (0.7 kPa and 3 kPa) eventually detached94.
hPSC culture and expansion using synthetic scaffolds has been extended from 2D surface coatings to 3D systems to further encourage pluripotency and self-renewal95-97. In contrast to 2D culture, the 3D environment allows for control over cell morphology and enhanced cell–cell interactions, which are both potent regulators of stem cell growth and phenotype98,99. For example, 3D PEG hydrogel scaffolds with customized stiffnesses, degradability and biochemical composition have promoted mouse ESC proliferation and hiPSC generation from somatic cells97,100. In one study, MMP-degradable, RGD-functionalized PEG hydrogel scaffolds, developed using factor-XIIa-mediated crosslinking of peptide-functionalized PEG monomers, increased the reprogramming efficiency of human fibroblasts into hiPSCs by 2.5-fold compared with a conventional 2D culture100. The 3D synthetic scaffold also supported homogenous hiPSC colony generation, which was not achievable in 3D Matrigel or 3D collagen scaffolds100. In a separate study, integrin-binding peptides — inspired by motifs involved in iPSC binding to Matrigel — were presented on a photopolymerized PEG thiol–ene hydrogel scaffold for 3D hiPSC culture97. The presentation of both a laminin-derived peptide, YIGSR, and an αvβ5-binding RGD-containing peptide on the scaffold were key to hiPSC pluripotency and enabled downstream differentiation into neural progenitor cells97. In both studies, the cell–matrix interactions that supported hiPSC culture in the 3D systems were different from those that supported culture in the 2D systems, indicating that stem cell–matrix interactions are system dependent101.
Regenerative medicine
Stem cell differentiation
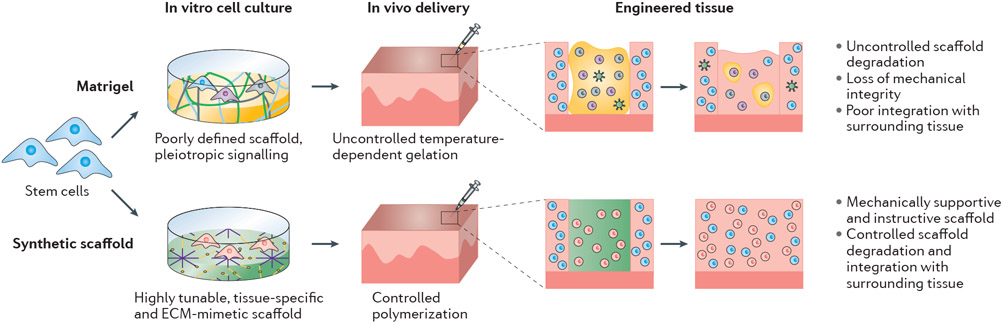
Interest in stem cells has increased owing to their tremendous potential for developing treatments in regenerative medicine102-104. However, before stem-cell-based therapies can be taken from ‘bench to bedside’, challenges associated with stem cell culture, such as directing lineage-specific stem cell differentiation, producing homogenous cell populations and ensuring localized in vivo delivery, must be addressed105. Various strategies have been developed to overcome these issues, including the development of cell culture environments that instruct stem cell behaviour. It is widely accepted that stem cell fate is directly affected by the interaction of the cells with their surrounding ECM98, whereby factors such as the composition, mechanics and architecture of the ECM act in concert to give rise to a series of spatially and temporally coordinated events that regulate cell differentiation and function. To unlock the full potential of stem cells in vitro, it has been posited that aspects of their in vivo native 3D environment must be reconstructed to provide the necessary cues106,107. Owing to the ill-defined composition of Matrigel, it is difficult to match the properties of Matrigel to the specific ECM requirements for different tissue types, and its spatially heterogeneous properties do not provide the tightly governed, spatio-temporal cues found during stem cell differentiation in vivo108,109 (Fig. 2). Together, these drawbacks limit the ability to control stem cell differentiation in Matrigel-based cultures. As an alternative to Matrigel, synthetic scaffolds have been used to identify appropriate environments to differentiate stem cells, maintain differentiated cell phenotypes and produce homogenous cell populations (Fig. 3).
Fig. 2∣. Advantages of synthetic scaffolds over Matrigel for cell culture, tissue engineering and organoid formation.
Synthetic alternatives to Matrigel provide a xenogenic-free, chemically defined and reproducible scaffold that can be tuned to guide cellular behaviour for a myriad of applications, including differentiation and organoid formation. The chemically defined nature of synthetic scaffolds also eliminates matrix-induced effects, providing a superior scaffold for toxicant and therapeutic screening assays.
Fig. 3∣. Comparison of Matrigel and synthetic scaffolds for stem cell differentiation and tissue engineering.
Unlike Matrigel, which is not tissue specific, synthetic scaffolds can be tuned (often through the addition of peptides) to provide specific biofunctionality to direct cell differentiation. The growth factors and other biologically active proteins in Matrigel lead to the generation of heterogeneous cell populations, whereas synthetic scaffolds generate pure populations of differentiated cells. In the context of in vivo delivery for tissue engineering applications, synthetic scaffolds can be delivered locally to the target site and be tuned to provide sustained mechanical support and biochemical instruction to transition from a cell-laden synthetic scaffold to neotissue. Conversely, the degradation of Matrigel is uncontrolled and its biofunctionality often leads to the formation of blood vessels. The potential for xenogenic contaminants in Matrigel or Matrigel-cultured cells prevents clinical application. Moreover, the handling of Matrigel in clinical settings is difficult owing to its gelation over a wide range of temperatures.
The advent of highly tunable synthetic scaffolds has made it possible for researchers to probe the role of mechanical and biochemical factors on stem cell fate. Notably, parameters such as scaffold stiffness and degradability, as well as the presence of tethered cell-adhesion peptides and growth factors, can be systematically varied to customize materials to encourage stem cell differentiation100,110,111. For example, self-assembled peptide nanofibre hydrogels, consisting of a peptide sequence derived from brain ECM that is known to inhibit neuronal apoptosis, supported stem cell differentiation into neurons, astrocytes and oligodendrocytes112. The synthetic hydrogel scaffolds also stimulated neuronal cell attachment, neurite outgrowth and the formation of active and functional synapses, overall showing superior cell survival and differentiation properties than those of Matrigel or collagen scaffolds112. Moreover, photopolymerizable PEG thiol–ene hydrogel scaffolds with cysteine-flanked MMP-sensitive crosslinks to encourage endothelial differentiation and vascular morphogenesis demonstrated similar gene expression profiles to those of cells cultured on Matrigel113. Several other synthetic hydrogel scaffolds have been found to support stem cell differentiation114-116; however, as they were not directly compared with Matrigel, they are not discussed in this Review.
In addition to biochemical cues, lineage-specific differentiation of stem cells is highly sensitive to mechanical and physical stimuli, such as scaffold stiffness117,118. Cell culture methods that recapitulate the stiffness of the natural tissue environment can direct stem cell differentiation. Soft scaffolds that mimic the elastic modulus of the brain (0.1–1 kPa) can be neurogenic, scaffolds of intermediate stiffness that mimic skeletal muscle (8–17 kPa) can be myogenic, and rigid scaffolds that mimic bone (25–40 kPa) can be osteogenic118. In comparison, Matrigel stiffness is relatively low (with an elastic modulus of ~400 Pa)35 and differs from that of most tissue-specific ECMs35. Although Matrigel stiffness can be increased slightly by increasing the overall protein concentration, this alters the biochemical composition and, thus, alters the biological functionality119. By contrast, the stiffness of synthetic hydrogel scaffolds can be varied over a wider range while maintaining their biochemical functionality110,120-122. For instance, in one study, the biochemical composition of a PEG hydrogel scaffold used to support adipogenic differentiation of human mesenchymal stem cells was identical to what was needed to support osteogenic differentiation, but the substrate stiffness requirements were drastically different120. The modularity of the PEG hydrogel scaffolds meant that the biochemical composition could be maintained while the stiffness of the scaffold was varied, enabling the physical and biological cues to be decoupled and the development of tissue-specific synthetic scaffolds.
Complex, yet defined, architectures that mimic cell morphologies and cell–matrix interactions in native tissues can also be achieved using synthetic scaffolds. Techniques such as electrospinning123,124, micropatterning125,126 and 3D printing127 have been developed to produce synthetic scaffolds that mimic the ECM down to the nanometre scale. These techniques have been used in several studies to control stem cell differentiation and/or maintain cell phenotype for a wide range of applications, although studies that report a direct comparison with Matrigel are limited. However, in one example, electrospun synthetic polyamide nanofibres, consisting of two polyamide polymers ((C28O4N4H47)n and (C28O4.4N4H47)n), promoted murine and human ESC and iPSC differentiation into functional hepatocytes. With these synthetic materials, the expression of hepatocyte-specific genes and albumin secretion was higher than on Matrigel or collagen, owing to manipulation of the cellular morphology128.
Emerging applications in regenerative medicine require pure populations of defined cells to be manufactured, but achieving this using Matrigel has been difficult100,129,130 (Fig. 4). Owing to heterogeneities between batches of Matrigel and within a single batch, the cells can experience different microenvironments, which can lead to different cell fates. In a 2017 protocol for the directed differentiation of iPSCs into functional cholangiocytes, variability in the differentiation efficiency between Matrigel batches was observed131. Heterogeneities have also been reported in neuroepithelial differentiation; colonies cultured on Matrigel were highly dissimilar in morphology and size, and exhibited both epithelial and mesenchymal phenotypes132. By contrast, colonies developed on PEG hydrogel scaffolds, generated using factor-XIIIa-mediated crosslinking, were homogenous and led to a pure population132. The mixed population present in Matrigel-cultured cells is postulated to be due to conflicting signals present in Matrigel that are not found in the chemically defined PEG. Furthermore, through manipulation of various properties, such as biofunctionalization with specific cell-binding peptides or enzymatically degradable crosslinks, synthetic scaffolds have been used to select for, or against, a certain cell type to achieve a more homogenous final population109,132.
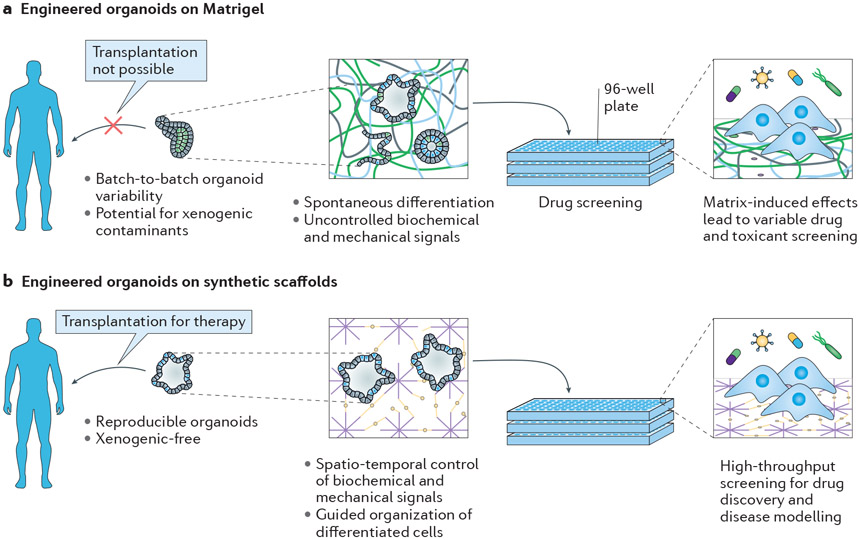
Fig. 4∣. Comparison of Matrigel and synthetic scaffolds for organoid assembly and preclinical tissue models.
a ∣ Matrigel scaffolds provide non-specific biochemical and mechanical signals for the spontaneous differentiation and self-assembly of cells into an organotypic model. The biological complexity of Matrigel leads to scaffold-induced effects, which affect the accuracy and reproducibility of preclinical models that rely on Matrigel-cultured cells. b ∣ Synthetic scaffolds have a chemically defined structure and the biological, mechanical and physical parameters can be tuned to guide organoid formation.
In vivo tissue regeneration
Scaffolds for tissue regeneration must provide a stable and supportive vehicle to deliver cells to the desired location in vivo. Materials that can be injected directly into the desired location (that is, the defect site), form a scaffold in situ and achieve a seamless transition from a cell-laden scaffold to neotissue are desirable, but require precise control of the formation and degradation of the material133,134 (Fig. 3). Matrigel gelation cannot be precisely controlled as it occurs over a wide temperature range (22–37 °C) and on timescales ranging from minutes to hours7,42. Protocols suggest gelling at physiological temperature, but gelation can occur at room temperature, making Matrigel difficult to prepare and handle in clinical settings40. Moreover, Matrigel degradation is not controllable. Matrigel degrades by exposure to MMPs, such as MMP2 and MMP9, but heterogeneities in Matrigel composition and crosslink density can result in unpredictable and non-uniform degradation135-137. This heterogeneous degradation jeopardizes the bulk material properties of Matrigel and limits its mechanical integrity138. Additionally, Matrigel contains growth factors and cytokines that can induce cell migration and angiogenesis, resulting in undesirable degradation and blood vessel formation when implanted in vivo139,140.
Synthetic scaffolds can substantially reduce complications associated with the in vivo administration of Matrigel. Some synthetic polymer precursors can be injected directly into a defect site, polymerized in situ and provide encapsulated cells with a space-filling scaffold that enables cells to produce their own ECM while simultaneously degrading the surrounding synthetic scaffold141-143. For instance, materials have been designed to photopolymerize on timescales on the order of seconds to ensure controlled cell delivery, and their ease of use has made them popular for tissue engineering applications59,64,144. Synthetic scaffolds can be designed to undergo multiple modes of degradation, including hydrolytic, enzymatic, physical (for example, thermal or pH) or a combination thereof145. Unlike Matrigel, the rate of degradation of these synthetic scaffolds can be tuned to match the rate of ECM deposition by manipulating the polymer concentration, crosslink density and peptide lability to ensure mechanical stability133,134.
In multiple in vivo studies, injectable synthetic scaffolds have shown similar, and in some instances better, tissue regeneration than Matrigel, demonstrating enhanced cell viability, engraftment and neotissue formation. For example, an enzymatically degradable, PEG–maleimide hydrogel functionalized with RGD was established as a cell-delivery system for treating muscle trauma in dystrophic mice. Specifically, mouse muscle satellite cells were encapsulated in the PEG hydrogel and delivered directly into the injured muscle. Compared with cells encapsulated in Matrigel or collagen, the hydrogel-delivered cells showed superior in vivo survival, proliferation and engraftment146. In another comparative study, six synthetic scaffolds derived from maltodextrin and of varying polymer molecular weight, crosslink density and RGD concentration were evaluated for their ability to serve as a vehicle and niche to transport mouse myoblasts in vivo147. After injection, a synthetic scaffold that supported skeletal myotubule formation similar to that in Matrigel-treated mice was identified. Injectable synthetic scaffolds can also be combined with other synthetic materials, such as microparticles or nanoparticles, to further direct cellular behaviour. For instance, mouse myoblasts encapsulated within a nanocomposite hydrogel scaffold comprising the biodegradable copolymer poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide) (PLGA–PEG–PLGA) and synthetic clay nanoparticles (Laponite) were used to treat skeletal muscle injuries, in vivo, in a mouse model148. In comparison to those treated with Matrigel or the PLGA–PEG–PLGA scaffold without the nanoparticles, the mice treated with the nanocomposite hydrogel scaffold exhibited considerably greater muscle tissue regeneration and functional recovery148. Although it was not investigated, it was postulated that the Laponite nanoparticles provide a large surface area and a highly anisotropic charged surface to facilitate strong adsorption of bioactive proteins and polysaccharides in situ, which can regenerate the native microenvironment and provide necessary cues to initiate tissue regeneration.
Organoid assembly
Organoids are stem cell or progenitor-derived tissues that exhibit key features found in organs in vivo, including characteristic tissue architecture, gene expression, cell function and multicellular complexity29,149-151. Within the past decade, notable progress has been made in developing various human organoids, including brain30,132, kidney152,153, retina154, lung155,156, prostate157, liver153,158,159 and gastrointestinal tissues156,160-163. These organoids have the potential to model embryonic development and disease, provide an in vitro platform for drug discovery and toxicity testing and serve as an implantable, cell-based therapy for tissue regeneration. Many of the organoid assembly protocols developed to date rely on the spontaneous differentiation and self-organization of cells, cell aggregates or embryoid bodies encapsulated in 3D Matrigel scaffolds160,164. However, owing to the inherent heterogeneity of Matrigel, this technique often results in batch-to-batch variability and organoids that are developmentally immature. The use of Matrigel in organoid culture also makes it difficult to decouple toxic or therapeutic effects from effects induced by the matrix itself81 (Fig. 4). Although the tremendous potential of organoids as a scientific and therapeutic tool remains, the lack of control over organoid formation, owing to the poorly defined Matrigel scaffold in which they are grown, impedes their advancement.
Scaffolds for organoid assembly
Synthetic scaffolds can be used to guide differentiation and influence organoid formation in a reproducible and controlled manner by recapitulating key cell–matrix interactions (Figs 2, 4). For example, a PEG hydrogel scaffold, crosslinked using factor XIIIa, was developed for the formation of neuroepithelial tubule organoids132, which required a scaffold of intermediate stiffness, non-degradable crosslinks and the presentation of laminin-derived peptides. A PEG–maleimide hydrogel scaffold, generated through Michael addition, was used to develop Madin–Darby canine kidney (MDCK) cyst organoids. Although the scaffold stiffness required for MDCK cyst organoid formation was the same as that for the formation of neuroepithelial tubule organoids (~4 kPa), the formation of MDCK cyst organoids required RGD in the place of laminin and degradable crosslinks to enable dynamic, cell-mediated remodelling of the microenvironment109. Similarly, a highly tunable biohybrid PEG hydrogel scaffold has been modified for a wide range of organotypic culture studies, including renal tubulogenesis, mammary epithelial morphogenesis, Alzheimer disease and acute myeloid leukemia165-168. Unlike the other scaffolds described in this Review, this biohybrid PEG scaffold contains the naturally derived glycosaminoglycan heparin and, thus, is not entirely synthetic. However, owing to its highly amenable nature, the scaffold was tuned for each application and in every case outperformed Matrigel166-168. These examples support the assertion that Matrigel’s ‘one-size-fits-all’ approach may not be appropriate for diverse organoid formation processes and that alternative synthetic scaffolds may provide superior tools.
One area of organoid research for which there are multiple studies that directly compare synthetic scaffolds with Matrigel is the formation of intestinal organoids from intestinal stem cells (ISCs). For example, the stiffness of a hydrolytically degradable, RGD-containing PEG hydrogel scaffold, generated through factor-XIIIa-mediated crosslinking, could be modulated to encourage ISC maturation. The mechanically dynamic hydrogel scaffold softened as it degraded, permitting the formation of organoids similar to those formed in Matrigel, with a similar gene expression profile, but only in the presence of the animal-derived protein laminin 1 (ref.163). A subsequent study reported a fully synthetic maleimide-terminated PEG hydrogel scaffold, polymerized through Michael-type addition, to eliminate the need for laminin 1 (refs156,161). In this case, the RGD-functionalized PEG hydrogel scaffold was tailored with protease-degradable crosslinkers to encourage cell-mediated degradation. Similar to intestinal organoids formed in Matrigel, the organoids formed in the PEG hydrogel scaffold remained viable and produced intestinal epithelium that resembled that of mature human intestine. The modular nature of the fully synthetic hydrogel allowed for further adaptation, and the same approach was used to generate other human organoids, such as lung156. These modifications of the scaffold are crucial for reproducible and controlled organoid assembly, and are not possible when using Matrigel to support organoids.
Organoid applications
Organoids offer an in vitro platform to evaluate drug efficacy and toxicity, and thereby aid drug discovery152. Through the use of patient-derived cells, organoids also offer the potential to accurately predict therapeutic response and guide personalized treatment strategies. However, although multiple types of organoids have been established as preclinical human tissue models, there is notable concern regarding the accuracy and reproducibility of Matrigel-cultured organoids in their response to chemical compounds (Fig. 4). In a study evaluating the effects of known toxicants on vascular tissue assembly by human umbilical vein endothelial cells (HUVECs), a customized PEG-based hydrogel scaffold combined with human endothelial cells was superior to the commonly used Matrigel-based assay in its ability to detect putative vascular disrupting compounds81. More than 500 hydrogel scaffolds were screened to identify the customized hydrogel that best supported human vascular tissue assembly by HUVECs, and the same screening approach also identified custom hydrogel scaffolds for hiPSC-derived endothelial cell assembly, hPSC expansion and human mesenchymal stromal cell expansion110. Additional drug-screening studies revealed that Matrigel can strongly influence cell-based assays owing to scaffold-induced effects, such as the introduction of xenogenic contaminants and growth factors into the culture environment169. In one study, prostate cancer cells cultured on synthetic polystyrene scaffolds were less responsive to drug treatment than those cultured on Matrigel170. By contrast, Matrigel and other naturally derived scaffolds have also been associated with enhanced tumorigenicity and chemotherapeutic drug resistance171. For organoids to be used in drug discovery and other cell-based assays, there is an imperative need for reproducible, standardized cell-based assays that are devoid of complicating components such as Matrigel.
Perspective
The importance of cautiously interpreting results from cell cultures that include Matrigel was first acknowledged in 1992 (ref.18). However, nearly 30 years later, Matrigel continues to be used for a myriad of applications. Other natural scaffolds that comprise purified proteins (for example, collagen type I, laminin and vitronectin) have been developed and found to be suitable for cell culture studies. However, these naturally derived products are also limited by batch-to-batch variability in composition and structure, as well as the inability to decouple biochemical and mechanical properties47,56. There are several potential reasons why Matrigel and other naturally derived products continue to be widely used. Historically, the primary reason has been the lack of synthetic alternatives that support the wide range of cell behaviours thought to be supported by Matrigel. However, the ongoing use of these naturally derived scaffolds can no longer be attributed to a lack of synthetic alternatives, as demonstrated by the range of studies described in this Review and the synthetic scaffolds emerging in the cell culture tools market. Synthetic scaffolds now have highly tunable biological, mechanical and degradation properties, and biofunctionalization can create a unique, fully defined microenvironment to guide stem cell expansion, differentiation or tissue formation. These synthetic scaffolds provide favourable alternatives to Matrigel, and the approaches recently used to customize synthetic scaffolds could result in materials that outperform naturally derived scaffolds.
The cost of a fully defined and synthetic cell culture environment, encompassing the synthetic scaffold and the chemically defined media, remains prohibitive. Although the cost of the raw materials to make PEG hydrogels is about half that of Matrigel161, the need for one or more synthetic peptides to provide the necessary biochemical cues to drive cellular behaviour can be prohibitively expensive for large-scale production171. However, recent advances in synthetic peptide synthesis and purification are generating more cost-effective options171,172. Another cost consideration when moving towards chemically defined cell culture environments is the requirement for recombinant growth factors, which are often found in Matrigel. Recent synthetic, xeno-free strategies to increase growth factor stability and availability can be applied to cell culture methods and may considerably reduce the costs associated with chemically defined conditions173. For example, an assortment of synthetic materials have been developed to sustain growth factor delivery over time to reduce the dosage needed compared with that for bulk administration174-177. For instance, long-term stabilization of bFGFs was achieved by electrostatically binding them to customized mineral-coated microparticles, reducing the required bFGF dosage for hPSC expansion by more than 80%178. Binding to the nanoparticles stabilizes bFGF and enables localized and sustained delivery 179. This approach could be generalized to other growth factors used in stem cell culture. Chemical compounds have also been used as analogues of recombinant growth factors to reduce costs and can prolong hPSC culture87. Ongoing developments in synthetic scaffolds that sequester growth factors and promote long-term growth factor stability180-183 could notably reduce the costs of chemically defined cell culture and make it economically viable for broad use.
Although synthetic scaffolds have proved to be promising alternatives to Matrigel, challenges remain in using them for cell culture, regenerative medicine and organoid growth. Similar to Matrigel, synthetic scaffolds do not provide a one-size-fits-all approach and can require considerable tuning to achieve a distinct set of physical and biochemical parameters to direct cellular behaviour. The process of screening multiple scaffolds of varying interdependent parameters can be time consuming, cost prohibitive and challenging, and those with little experience with synthetic materials may revert to the familiarity of Matrigel. Additionally, matching the fibre-like architecture to recapitulate the complexity of native tissues is difficult to achieve using synthetic scaffolds. As an alternative, optimized synthetic materials that provide a minimal initial set of conditions conducive to cell function, but then rely on cell-mediated processes to define the extracellular milieu, may produce scaffolds that are suitable for not just one purpose, but for several different cell types and applications.
Creating scaffolds in a form that are easy for an end user to employ is another major challenge. One approach involves providing precursor materials in the form of a kit, which requires the end user to form the scaffold themselves. This approach can be effective but also introduces the potential for user error and may require the end user to have specialized equipment for scaffold formation and quality control analysis. Another approach is to generate devices that are pre-coated with scaffold materials, such as pre-coated multi-well plates, which would require no additional modification or characterization by the end user. However, this approach requires coatings that are robust and reproducible, and the shelf life of the coated device would be an important additional parameter to consider. Although these challenges are not unique to synthetic scaffolds, and indeed are also among the limitations of naturally derived ECMs, they must be addressed in a manner that allows for widespread adoption.
There are several ways in which these challenges are being addressed in academia and industry. For example, in 2017 the US National Science Foundation established the Engineering Research Center for Cell Manufacturing Technologies (CMaT) to develop scalable and low-cost manufacturing of high-quality cells, with one focus being synthetic scaffolds. The demand for alternatives to Matrigel has also led to new product development at existing life science companies, including Corning’s Synthemax, as well as the formation of start-up companies such as Mosaic Biosciences, QGel and Stem Pharm, which provide synthetic scaffolds for the range of applications described in this Review. As research and development progress, it is important to maintain a collaborative dialogue between biologists, material scientists, engineers and clinicians across academia and industry, to not only improve synthetic scaffolds, but also to ensure their availability and ease of use to practitioners in stem cell therapy, regenerative medicine and drug discovery.
Acknowledgements
This research was funded by the US Environmental Protection Agency (STAR Grant no. 83573701), the US National Institutes of Health (award nos 1U01TR002383, R01HL093282 and 1R01NS109427) and the US National Science Foundation (award nos EEC1648035 and DMR 170179). E.A.A. acknowledges funding by the US National Institutes of Health (T32HL110853).
Footnotes
Competing interests
W.L.M is a co-founder and shareholder of Stem Pharm, Inc. E.A.A. declares no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
References
- 1.Orkin RW et al. A murine tumor producing a matrix of basement membrane. J. Exp. Med 145, 204–220 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBleu VS, Macdonald B & Kalluri R Structure and function of basement membranes. Exp. Biol. Med 232, 1121–1129 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Kubota Y, Kleinman HK, Martin GR & Lawley TJ Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol 107, 1589–1598 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinman HK et al. Basement membrane complexes with biological activity. Biochemistry 25, 312–318 (1986).This paper investigates the protein composition and biological activity of the basement membrane extract from EHS mouse chondrosarcomas; this extract was later developed and commercialized as Matrigel.
- 5.Kleinman HK & Martin GR Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol 15, 378–386 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Corning Incorporated Life Sciences. Corning Matrigel Matrix Frequently Asked Questions. https://www.corning.com/catalog/cls/documents/faqs/CLS-DL-CC-026-A4.pdf (2019).
- 7.Hughes CS, Postovit LM & Lajoie GA Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010).A full proteomic analysis of Matrigel and GFR Matrigel, reporting their complex, ill-defined and variable composition.
- 8.Timpl R et al. Laminin--a glycoprotein from basement membranes. J. Biol. Chem 254, 9933–9937 (1979). [PubMed] [Google Scholar]
- 9.Terranova VP, Aumailley M, Sultan LH, Martin GR & Kleinman HK Regulation of cell attachment and cell number by fibronectin and laminin. J. Cell. Physiol 127, 473–479 (1986). [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki T et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun 375, 27–32 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Ponce ML et al. Identification of Endothelial Cell Binding Sites on the Laminin γ1 Chain. Circ. Res 84, 688–694 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Ji L & Hua Z Functional Peptides from Laminin-1 Improve the Cell Adhesion Capacity of Recombinant Mussel Adhesive Protein. Protein Pept. Lett 24, 348–352 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Heaton MB & Swanson DJ The influence of laminin on the initial differentiation of cultured neural tube neurons. J. Neurosci. Res 19, 212–218 (1988). [DOI] [PubMed] [Google Scholar]
- 14.Farrukh A et al. Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis. Stem Cell Rep. 9, 1432–1440 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali S, Saik JE, Gould DJ, Dickinson ME & West JL Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis. BioResearch Open Access 2, 241–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engbring JA & Kleinman HK The basement membrane matrix in malignancy. J. Pathol 200, 465–470 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Kikkawa Y et al. Laminin-111-derived peptides and cancer. Cell Adh. Migr 7, 150–159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vukicevic S et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res 202, 1–8 (1992).This study identifies multiple active growth factors in Matrigel and suggests caution when interpreting cellular activity when cultured on Matrigel.
- 19.Talbot NC & Caperna TJ Proteome array identification of bioactive soluble proteins/peptides in Matrigel: relevance to stem cell responses. Cytotechnology 67, 873–883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillette KM, Forbes K & Sehgal I Detection of matrix metalloproteinases (MMP), tissue inhibitor of metalloproteinase-2, urokinase and plasminogen activator inhibitor-1 within matrigel and growth factor-reduced matrigel basement membrane. Tumori 89, 421–425 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Xu C et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol 19, 971–974 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Qian L & Saltzman WM Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials 25, 1331–1337 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Lee S-W et al. Optimization of Matrigel-based culture for expansion of neural stem cells. Anim. Cells Syst 19, 175–180 (2015). [Google Scholar]
- 24.Laflamme MA et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol 25, 1015–1024 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Ponce ML Tube formation: an in vitro matrigel angiogenesis assay. Methods Mol. Biol 467, 183–188 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Ponce ML In vitro Matrigel angiogenesis assays. Methods Mol. Med 46, 205–209 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Mondrinos MJ et al. Engineering Three-Dimensional Pulmonary Tissue Constructs. Tissue Eng. 12, 717–728 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Li Z & Guan J Hydrogels for Cardiac Tissue Engineering. Polymers 3, 740–761 (2011). [Google Scholar]
- 29.Lancaster MA & Knoblich JA Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benton G, Kleinman HK, George J & Arnaoutova I Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 128, 1751–1757 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Acuña R & García AJ Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 57–58, 324–333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polykandriotis E, Arkudas A, Horch RE, Kneser U & Mitchell G To Matrigel or Not to Matrigel. Am. J. Pathol 172, 1441–1442 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohen NT, Little LE & Healy KE Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases 4, 69–79 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Soofi SS, Last JA, Liliensiek SJ, Nealey PF & Murphy CJ The elastic modulus of MatrigelTM as determined by atomic force microscopy. J. Struct. Biol 167, 216–219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirami G et al. Identification of transferrin and inhibin-like proteins in Matrigel. In Vitro Cell. Dev. Biol. Anim 31, 404–411 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Hansen KC et al. An in-solution ultrasonication-assisted digestion method for improved extracellular matrix proteome coverage. Mol. Cell. Proteomics 8, 1648–1657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaman MH et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl Acad. Sci. USA 103, 10889–10894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semler EJ, Ranucci CS & Moghe PV Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol. Bioeng 69, 359–369 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Kane KIW et al. Determination of the rheological properties of Matrigel for optimum seeding conditions in microfluidic cell cultures. AIP Adv. 8, 125332 (2018). [Google Scholar]
- 41.Alcaraz J et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27, 2829–2838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed J, Walczak WJ, Petzold ON & Gimzewski JK In Situ Mechanical Interferometry of Matrigel Films. Langmuir 25, 36–39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson NC From bench to cageside: risk assessment for rodent pathogen contamination of cells and biologics. ILAR J. 49, 310–315 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H et al. Removal of lactate dehydrogenase-elevating virus from human-in-mouse breast tumor xenografts by cell-sorting. J. Virol. Methods 173, 266–270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammann CG, Messer RJ, Peterson KE & Hasenkrug KJ Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One 4, e6105 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riley V et al. The LDH virus: an interfering biological contaminant. Science 200, 124–126 (1978). [DOI] [PubMed] [Google Scholar]
- 47.Caliari SR & Burdick JA A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 13, 405–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Sun Q, Li Q, Kawazoe N & Chen G Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem 6, 499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer RS, Myers KA, Gardel ML & Waterman CM Stiffness-controlled three-dimensional extracellular matrices for high-resolution imaging of cell behavior. Nat. Protoc 7, 2056–2066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse JR & Engler AJ Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol 47, 10.16.1–10.16.16 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Pelham RJ Jnr & Wang, Y. l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. USA 94, 13661–13665 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zustiak SP & Leach JB Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 11, 1348–1357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krsko P & Libera M Biointeractive hydrogels. Mater. Today 8, 36–44 (2005). [Google Scholar]
- 54.Zhu J Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639–4656 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin C-C & Anseth KS PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 26, 631–643 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tibbitt MW & Anseth KS Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng 103, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fairbanks BD et al. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 21, 5005–5010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant S & Anseth K in Scaffolding In Tissue Engineering (eds Ma PX & Elisseeff J) 71–90 (CRC Press, 2005). [Google Scholar]
- 59.Nguyen KT & West JL Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 23, 4307–4314 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Nair DP et al. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater 26, 724–744 (2014). [Google Scholar]
- 61.Schense JC & Hubbell JA Cross-Linking Exogenous Bifunctional Peptides into Fibrin Gels with Factor XIIIa. Bioconjug. Chem 10, 75–81 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Ehrbar M et al. Biomolecular Hydrogels Formed and Degraded via Site-Specific Enzymatic Reactions. Biomacromolecules 8, 3000–3007 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Bryant SJ, Chowdhury TT, Lee DA, Bader DL & Anseth KS Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng 32, 407–417 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Roberts JJ & Bryant SJ Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials 34, 9969–9979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdick JA & Anseth KS Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23, 4315–4323 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Kharkar PM, Rehmann MS, Skeens KM, Maverakis E & Kloxin AM Thiol–ene click hydrogels for therapeutic delivery. ACS Biomater. Sci. Eng 2, 165–179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomson JA et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 68.Avior Y, Sagi I & Benvenisty N Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol 17, 170–182 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Singh VK, Kalsan M, Kumar N, Saini A & Chandra R Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol 3, 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi Y, Inoue H, Wu JC & Yamanaka S Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov 16, 115–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ortiz-Vitali JL & Darabi R iPSCs as a Platform for Disease Modeling, Drug Screening, and Personalized Therapy in Muscular Dystrophies. Cells 8, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hovatta O Derivation of human embryonic stem cell lines, towards clinical quality. Reprod. Fertil. Dev 18, 823–828 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Qian X, Villa-Diaz LG, Kumar R, Lahann J & Krebsbach PH Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials 35, 9581–9590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nandivada H et al. Fabrication of synthetic polymer coatings and their use in feeder-free culture of human embryonic stem cells. Nat. Protoc. 6, 1037–1043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villa-Diaz LG et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 28, 581–583 (2010).Along with Nandivada et al, this was one of the first studies to develop a fully synthetic, chemically defined scaffold for long-term hESC culture and to directly compare the performance with that of Matrigel.
- 76.Brafman DA et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 31, 9135–9144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng Y et al. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 24, 1056–1065 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowland TJ et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 19, 1231–1240 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Mondal G, Barui S & Chaudhuri A The relationship between the cyclic-RGDfK ligand and αvβ3 integrin receptor. Biomaterials 34, 6249–6260 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Lambshead JW et al. Long-Term Maintenance of Human Pluripotent Stem Cells on cRGDfK-Presenting Synthetic Surfaces. Sci. Rep 8, 701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen EH et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng 1, 0096 (2017).This study uses a high-throughput screening method of synthetic scaffolds to determine a synthetic alternative to Matrigel, finding that matrix-induced effects caused by the biological function of Matrigel can affect toxicity screenings.
- 82.Hayman EG, Pierschbacher MD, Suzuki S & Ruoslahti E Vitronectin—a major cell attachment-promoting protein in fetal bovine serum. Exp. Cell Res 160, 245–258 (1985). [DOI] [PubMed] [Google Scholar]
- 83.Melkoumian Z et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol 28, 606–610 (2010).An early report on tethering synthetic peptides to synthetic scaffolds that provides a direct comparison to Matrigel.
- 84.Deng Y et al. Long-term self-renewal of human pluripotent stem cells on peptide-decorated poly(OEGMA-co-HEMA) brushes under fully defined conditions. Acta Biomater. 9, 8840–8850 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Higuchi A et al. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep 5, 18136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin S, Yao H, Weber JL, Melkoumian ZK & Ye K A Synthetic, Xeno-Free Peptide Surface for Expansion and Directed Differentiation of Human Induced Pluripotent Stem Cells. PLOS ONE 7, e50880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuda S et al. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat. Biomed. Eng 2, 173–182 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Farach-Carson MC & Carson DD Perlecan—a multifunctional extracellular proteoglycan scaffold. Glycobiology 17, 897–905 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Furue MK et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl Acad. Sci. USA 105, 13409–13414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spivak-Kroizman T et al. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 79, 1015–1024 (1994). [DOI] [PubMed] [Google Scholar]
- 91.Vlodavsky I, Miao HQ, Medalion B, Danagher P & Ron D Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 15, 177–186 (1996). [DOI] [PubMed] [Google Scholar]
- 92.Chang C-W et al. Engineering cell–material interfaces for long-term expansion of human pluripotent stem cells. Biomaterials 34, 912–921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klim JR, Li L, Wrighton PJ, Piekarczyk MS & Kiessling LL A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods 7, 989–994 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Musah S et al. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS Nano 6, 10168–10177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerecht S et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 11298–11303 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lei Y & Schaffer DV A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl Acad. Sci. USA 110, E5039–5048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ovadia EM, Colby DW & Kloxin AM Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells. Biomater. Sci. 6, 1358–1370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy WL, McDevitt TC & Engler AJ Materials as stem cell regulators. Nat. Mater 13, 547–557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Folkman J & Moscona A Role of cell shape in growth control. Nature 273, 345–349 (1978). [DOI] [PubMed] [Google Scholar]
- 100.Caiazzo M et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater 15, 344–352 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Khalil AS, Xie AW & Murphy WL Context clues: the importance of stem cell–material interactions. ACS Chem. Biol 9, 45–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eve DJ The continued promise of stem cell therapy in regenerative medicine. Med. Sci. Monit 17, RA292-305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Helmy KY, Patel SA, Silverio K, Pliner L & Rameshwar P Stem cells and regenerative medicine: accomplishments to date and future promise. Ther. Deliv 1, 693–705 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffman T, Khademhosseini A & Langer R Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 25, 679–687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang NS, Varghese S & Elisseeff J Controlled differentiation of stem cells. Adv. Drug Deliv. Rev 60, 199–214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burdick JA & Vunjak-Novakovic G Engineered Microenvironments for Controlled Stem Cell Differentiation. Tissue Eng. Part A 15, 205–219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lutolf MP, Gilbert PM & Blau HM Designing materials to direct stem-cell fate. Nature 462, 433–441 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uriel S et al. Extraction and Assembly of Tissue-Derived Gels for Cell Culture and Tissue Engineering. Tissue Eng. Part C 15, 309–321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Enemchukwu NO et al. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J. Cell Biol 212, 113–124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le NNT, Zorn S, Schmitt SK, Gopalan P & Murphy WL Hydrogel arrays formed via differential wettability patterning enable combinatorial screening of stem cell behavior. Acta Biomater. 34, 93–103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koepsel JT, Brown PT, Loveland SG, Li W-J & Murphy WL Combinatorial screening of chemically defined human mesenchymal stem cell culture substrates. J. Mater. Chem 22, 19474–19481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koutsopoulos S & Zhang S Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, Matrigel and Collagen I. Acta Biomater. 9, 5162–5169 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Zhang J et al. A Genome-wide Analysis of Human Pluripotent Stem Cell-Derived Endothelial Cells in 2D or 3D Culture. Stem Cell Rep. 8, 907–918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farhat W et al. Hydrogels for Advanced Stem Cell Therapies: A Biomimetic Materials Approach for Enhancing Natural Tissue Function. IEEE Rev. Biomed. Eng 12, 333–351 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Tsou Y-H, Khoneisser J, Huang P-C & Xu X Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater 1, 39–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Donnelly H, Salmeron-Sanchez M & Dalby MJ Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 15, 20180388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vining KH & Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol 18, 728–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 119.Slater K, Partridge J & Nandivada H Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration. https://www.corning.com/catalog/cls/documents/application-notes/CLS-AC-AN-449.pdf (Corning, 2018).
- 120.Gobaa S et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Rape AD, Zibinsky M, Murthy N & Kumar S A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat. Commun 6, 8129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nemir S & West JL Synthetic Materials in the Study of Cell Response to Substrate Rigidity. Ann. Biomed. Eng 38, 2–20 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Sill TJ & von Recum HA Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Xu C, Inai R, Kotaki M & Ramakrishna S Electrospun Nanofiber Fabrication as Synthetic Extracellular Matrix and Its Potential for Vascular Tissue Engineering. Tissue Eng. 10, 1160–1168 (2004). [DOI] [PubMed] [Google Scholar]
- 125.Rashidi H, Yang J & M. Shakesheff K Surface engineering of synthetic polymer materials for tissue engineering and regenerative medicine applications. Biomater. Sci 2, 1318–1331 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Yim EKF, Pang SW & Leong KW Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res 313, 1820–1829 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu W et al. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol 40, 103–112 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Yamazoe T et al. A synthetic nanofibrillar matrix promotes in vitro hepatic differentiation of embryonic stem cells and induced pluripotent stem cells. J. Cell Sci 126, 5391–5399 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Franzin C et al. Single-cell PCR analysis of murine embryonic stem cells cultured on different substrates highlights heterogeneous expression of stem cell markers. Biol. Cell 105, 549–560 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Highet AR, Zhang VJ, Heinemann GK & Roberts CT Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta 33, 586–588 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Sampaziotis F et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat. Protoc 12, 814–827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ranga A et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl Acad. Sci USA 113, E6831–E6839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schneider MC et al. Local Heterogeneities Improve Matrix Connectivity in Degradable and Photoclickable Poly(ethylene glycol) Hydrogels for Applications in Tissue Engineering. ACS Biomater. Sci. Eng 3, 2480–2492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chu S et al. Understanding the Spatiotemporal Degradation Behavior of Aggrecanase-Sensitive Poly(ethylene glycol) Hydrogels for Use in Cartilage Tissue Engineering. Tissue Eng. Part A 23, 795–810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dolo V et al. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis 17, 131–140 (1999). [DOI] [PubMed] [Google Scholar]
- 136.Balduyck M et al. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clin. Exp. Metastasis 18, 171–178 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Wong AP, Cortez SL & Baricos WH Role of plasmin and gelatinase in extracellular matrix degradation by cultured rat mesangial cells. Am. J. Physiol 263, F1112–1118 (1992). [DOI] [PubMed] [Google Scholar]
- 138.Wolf M Influence of matrigel on biodistribution studies in cancer research. Pharm. 63, 43–48 (2008). [PubMed] [Google Scholar]
- 139.Shen D, Wen R, Tuo J, Bojanowski CM & Chan C-C Exacerbation of retinal degeneration and choroidal neovascularization induced by subretinal injection of Matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 38, 71–73 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kano MR et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J. Cell Sci 118, 3759–3768 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Lee JH Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res 22, 27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu L & Ding J Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev 37, 1473–1481 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Kretlow JD, Klouda L & Mikos AG Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev 59, 263–273 (2007). [DOI] [PubMed] [Google Scholar]
- 144.Pascual-Garrido C et al. Current and Novel Injectable Hydrogels to Treat Focal Chondral Lesions: Properties and Applicability. J. Orthop. Res 36, 64–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kharkar PM, Kiick KL & Kloxin AM Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev 42, 7335–7372 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han WM et al. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci. Adv 4, eaar4008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fernandes S, Kuklok S, McGonigle J, Reinecke H & Murry CE Synthetic matrices to serve as niches for muscle cell transplantation. Cells Tissues Organs 195, 48–59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagahama K et al. Nanocomposite injectable gels capable of self-replenishing regenerative extracellular microenvironments for in vivo tissue engineering. Biomater. Sci 6, 550–561 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Clevers H Modeling Development and Disease with Organoids. Cell 165, 1586–1597 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Camp JG et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci USA 112, 15672–15677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murrow LM, Weber RJ & Gartner ZJ Dissecting the stem cell niche with organoid models: an engineering-based approach. Development 144, 998–1007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Astashkina AI, Mann BK, Prestwich GD & Grainger DW A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 33, 4700–4711 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Takebe T et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Eiraku M et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Dye BR et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4, e05098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cruz-Acuña R et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol 19, 1326–1335 (2017).Cruz-Acuña et al. (2017, 2018) present a protocol to generate human intestinal and lung organoids using a fully synthetic, chemically defined PEG hydrogel scaffold.
- 157.Chua CW et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol 16, 951–961 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ardalani H et al. 3-D culture and endothelial cells improve maturity of human pluripotent stem cell-derived hepatocytes. Acta Biomater. 95, 371–381 (2019). [DOI] [PubMed] [Google Scholar]
- 159.Ramachandran SD et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLOS ONE 10, e0139345 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spence JR et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cruz-Acuña R et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc 13, 2102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gjorevski N et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Gjorevski N & Lutolf MP Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat. Protoc 12, 2263–2274 (2017). [DOI] [PubMed] [Google Scholar]
- 164.Fatehullah A, Tan SH & Barker N Organoids as an in vitro model of human development and disease. Nat. Cell Biol 18, 246–254 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Bray LJ et al. A three-dimensional ex vivo tri-culture model mimics cell-cell interactions between acute myeloid leukemia and the vascular niche. Haematologica 102, 1215–1226 (2017). (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Papadimitriou C et al. 3D Culture Method for Alzheimer’s Disease Modeling Reveals Interleukin-4 Rescues Aβ42-Induced Loss of Human Neural Stem Cell Plasticity. Dev. Cell 46, 85–101.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Nowak M, Freudenberg U, Tsurkan MV, Werner C & Levental KR Modular GAG-matrices to promote mammary epithelial morphogenesis in vitro. Biomaterials 112, 20–30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weber HM, Tsurkan MV, Magno V, Freudenberg U & Werner C Heparin-based hydrogels induce human renal tubulogenesis in vitro. Acta Biomater. 57, 59–69 (2017). [DOI] [PubMed] [Google Scholar]
- 169.Livingston MK et al. Evaluation of PEG-Based Hydrogel Influence on Estrogen-Receptor-Driven Responses in MCF7 Breast Cancer Cells. ACS Biomater. Sci. Eng 5, 6089–6098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Edmondson R, Adcock AF & Yang L Influence of Matrices on 3D-Cultured Prostate Cancer Cells’ Drug Response and Expression of Drug-Action Associated Proteins. PLOS ONE 11, e0158116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Collier JH & Segura T Evolving the use of peptides as biomaterials components. Biomaterials 32, 4198–4204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hosoyama K, Lazurko C, Muñoz M, McTiernan CD & Alarcon EI Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front. Bioeng. Biotechnol 7, 205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xie AW & Murphy WL Engineered biomaterials to mitigate growth factor cost in cell biomanufacturing. Curr. Opin. Biomed. Eng 10, 1–10 (2019). [Google Scholar]
- 174.Heidariyan Z et al. Efficient and cost-effective generation of hepatocyte-like cells through microparticle-mediated delivery of growth factors in a 3D culture of human pluripotent stem cells. Biomaterials 159, 174–188 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Bratt-Leal AM, Nguyen AH, Hammersmith KA, Singh A & McDevitt TC A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 34, 7227–7235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alberti K et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nat. Methods 5, 645–650 (2008). [DOI] [PubMed] [Google Scholar]
- 177.Platt MO et al. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi‐potent marrow stromal cells. J. Cell Physiol 221, 306–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu X et al. Nanostructured Mineral Coatings Stabilize Proteins for Therapeutic Delivery. Adv. Mater 29, 1701255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Khalil AS, Xie AW, Johnson HJ & Murphy WL Sustained release and protein stabilization reduce the growth factor dosage required for human pluripotent stem cell expansion. Biomaterials (2020). [DOI] [PMC free article] [PubMed]
- 180.Belair DG, Le NN & Murphy WL Design of Growth Factor Sequestering Biomaterials. Chem. Commun 50, 15651–15668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Belair DG & Murphy WL Specific VEGF sequestering to biomaterials: Influence of serum stability. Acta Biomater. 9, 8823–8831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan HJ et al. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 161, 190–202 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Jha AK et al. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials 47, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen KG, Mallon BS, McKay RDG & Robey PG Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 14, 13–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Julavijitphong S et al. A xeno-free culture method that enhances Wharton’s jelly mesenchymal stromal cell culture efficiency over traditional animal serum-supplemented cultures. Cytotherapy 16, 683–691 (2014). [DOI] [PubMed] [Google Scholar]
- 186.Thirumala S, Goebel WS & Woods EJ Manufacturing and banking of mesenchymal stem cells. Expert Opin. Biol. Ther 13, 673–691 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Halme DG & Kessler DA FDA regulation of stem-cell-based therapies. N. Engl. J. Med 355, 1730–1735 (2006). [DOI] [PubMed] [Google Scholar]
- 188.Xiao J, Yang D, Li Q, Tian W & Guo W The establishment of a chemically defined serum-free culture system for human dental pulp stem cells. Stem Cell Res. Ther 9, 191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hirata TM et al. Expression of multiple stem cell markers in dental pulp cells cultured in serum-free media. J. Endod 36, 1139–1144 (2010). [DOI] [PubMed] [Google Scholar]
- 190.Chen G et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Beers J et al. A cost-effective and efficient reprogramming platform for large-scale production of integration-free human induced pluripotent stem cells in chemically defined culture. Sci. Rep 5, 11319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie AW et al. Controlled Self-assembly of Stem Cell Aggregates Instructs Pluripotency and Lineage Bias. Sci. Rep 7, 14070 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leong MF et al. Electrospun polystyrene scaffolds as a synthetic substrate for xeno-free expansion and differentiation of human induced pluripotent stem cells. Acta Biomater. 46, 266–277 (2016). [DOI] [PubMed] [Google Scholar]