Abstract
Constipation is a common clinical manifestation of digestive system disorders and occurs worldwide. This study investigated the ability of Lactobacillus plantarum KSFY06 (LP‐KSFY06) to promote the action of geniposide in preventing montmorillonite‐induced constipation in Kunming mice, with the aim of providing a successful solution. The effects of LP‐KSFY06 and geniposide on constipation were measured, and the results showed that the protective effect of geniposide on constipation was enhanced by LP‐KSFY06 and that the combination resulted in increased weight, moisture content, and particle number of feces. The first black stool defecation time was decreased from 182 min to 87 min, which clearly indicates that defecating difficulty was alleviated in constipated mice. The synergic intervention of LP‐KSFY06 and geniposide (LP + G) assisted in maintaining the body weight of constipated mice. The LP + G intervention significantly increased serum levels of motilin (MTL, 167.8 pg/ml), acetylcholinesterase (AChE, 45.3 pg/ml), substance P (SP, 61.0 pg/ml), vasoactive intestinal peptide (VIP, 70.5 pg/ml), endothelin‐1 (ET‐1, 16.1 pg/ml), and gastrin (73.0 pg/ml) and remarkably decreased somatostatin (SS, 35.2 pg/ml) when compared to those indexes in the LP‐KSFY06 group and geniposide group. The LP + G treatment also significantly increased the mRNA expression of cluster of differentiation 117 (c‐Kit), stem cell factor (SCF), glial cell‐derived neurotrophic factor (GDNF), and remarkably downregulated the expression of inducible nitric oxide synthase (iNOS), transient receptor potential vanilloid‐1 (TRPV1), and cyclooxygenase‐2 (COX‐2). The experimental results showed that the combination treatment has the strongest prevention effect against constipation, and LP‐KSFY06 promotes the ability of geniposide to prevent constipation. Therefore, LP‐KSFY06 is a potential probiotic strain with the capacity to prevent montmorillonite‐induced constipation.
Keywords: constipation, geniposide, Lactobacillus plantarum, montmorillonite, mRNA
Combined effects of Lactobacillus plantarum KSFY06 and geniposide could inhibit the montmorillonite‐induced constipation in Kunming mice.
1. INTRODUCTION
Constipation is a clinical manifestation of digestive system disorders and is characterized by the formation of hard feces that are difficult to excrete. The prevalence of constipation ranges from 2% to 30%, especially in children and geriatric populations (Chen, Chao, & Pan, 2020; Zhao et al., 2019). Constipation can be divided into primary and secondary constipation, with primary constipation including three types (i.e., normal‐transit constipation, slow‐transit constipation, and defecatory disorders) (Lembo & Camilleri, 2003). Unhealthy eating and excretion habits, lack of exercise, neurological disorders, or irregular metabolic conditions can trigger constipation, although the exact pathogenesis of constipation is still unclear (Schuster, Kosar, & Kamrul, 2015). The duration of stools retained in the large intestine is prolonged when constipation occurs, and fecal moisture is subsequently absorbed, resulting in dry feces that inhibit the movement of the bowel and induce intestinal imbalance and bowel pain (Chen, Wu, Liao, & Yang, 2010; Khalif, Quigley, Konovitch, & Maximova, 2005). Constipation is a risk factor for irritable bowel syndrome and colorectal cancer, as well as other gastrointestinal disorders (Okawa, Fukudo, & Sanada, 2019; Shimotoyodome, Meguro, Hase, Tokimitsu, & Sakata, 2000). Administration of laxatives and prokinetic agents (motilin agonists, 5‐hydroxytryptamine modulators, opioid antagonists, and chloride‐channel activators) are common clinical treatments for constipation (Shin et al., 2014). Long‐term intake of anticonstipation agents, however, can cause drug dependence and is economically unfeasible due to direct and indirect health care expenses (Crowell, Harris, Lunsford, & Dibaise, 2009). Laxatives can also cause side effects such as diarrhea, upset stomach, stomach cramping, and vomiting.
Constipation is generally not regarded as a disease. It is a multifactorial disorder that can be managed by exercise and consumption of a healthy diet and additional water (Webster, Tummala, Diva, & Lappalainen, 2016). Nutritional therapy for constipation consists of consumption of fruits, vegetables, whole grains, and cereals, which are rich in dietary fiber, and can increase fecal mass and accelerate the small intestine passage, which subsequently reduces colonic transit time (Karabudak, Koksal, & Macit, 2019; Wang, Sun, Zhou, & Zhao, 2015). Moreover, increasing numbers of studies indicate that probiotics (i.e., Lactobacillus and Bifidobacterium) decrease whole‐gut transit time and increase defecation frequency by increasing intestinal motility, as well as softening fecal consistency (Eskesen et al., 2015; Wojtyniak & Szajewska, 2017).
Probiotics are defined as living microorganisms, and only when administered in adequate amounts can they confer beneficial health effects upon the host (FAO/WHO, 2006). Lactic acid bacteria (LAB) are an important group of probiotics and include Lactobacillus spp., Bifidobacterium spp., and Enterococcus spp., which are widely used as food supplements. LAB directly or indirectly act on the large intestine and other organs by influencing the intestinal flora, modulating intestinal permeability and immunological parameters, and producing regulatory or bioactive metabolites (Markowiak & Slizewska, 2017).
Geniposide is an iridoid glycoside extracted from the fruit of Gardenia jasminoides that is widely used in Asian countries. As a natural bioactive constituent, it has been proven that geniposide is a curative substance for diseases of the digestive system (Qian, Suo, Yi, Li, & Zhao, 2017). Lactobacillus strains also can effectively alleviate constipation (Chen et al., 2020; Zhao et al., 2019). Therefore, this study evaluated the potential synergic effect of the combination of Lactobacillus plantarum KSFY06 (LP‐KSFY06) and geniposide. We attempted to determine if the bacterial strain has the ability to increase the effectiveness of geniposide, which provides a theoretical basis for further in‐depth research, application, and industrial development.
2. MATERIALS AND METHODS
2.1. Experimental strain
Lactobacillus plantarum KSFY06 (LP‐KSFY06) was isolated from naturally fermented yogurt from Xinjiang, China. It was identified using Basic Local Alignment Search Tool (BLAST) at the National Center for Biotechnology Information (NCBI) website.
2.2. Design of a mouse constipation model
Fifty male, 6‐week‐old Kunming (KM) mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). They were acclimatized for 1 week at room temperature of 25 ± 2°C with relative humidity 55 ± 5% and a 12 hr light/12 hr dark cycle, with diet and water ad libitum. Then, the mice were randomly divided into 5 groups (n = 10/group): (a) normal, (b) model, (c) LP‐KSFY06, (d) geniposide, and (e) LP‐KSFY06 and geniposide (LP + G). All groups were fed a standard rodent AIN‐93G chow diet (Jiangsu Medicine Ltd.). The normal and model groups received a normal diet for 9 days, and the treatment groups received a normal diet with LP‐KSFY06 (0.5 × 107 colony‐forming units (CFU)/kg body weight), geniposide (50 mg/kg body weight), and LP + G (LP‐KSFY06 1 × 107 CFU/kg body weight and 50 mg/kg body weight). Montmorillonite powder suspension (30 mg/kg body weight) was orally administered to the model and intervention groups from d 7 to d 9 to induce constipation. Body weight (BW), stool weight, and moisture were recorded. This study was approved by the Ethics Committee of Chongqing Medical University (SCXK 2017‐0001).
2.3. Gastrointestinal transit ratio and defecation time
All groups were fasted for 24 hr with free access to water. The model and intervention groups received an oral administration of 0.2 ml of 10% activated carbon that was dissolved in 10% gum arabic, while the normal group received oral administration of 10% gum arabic. Half of the mice from each group were killed by CO2 following 30 min of treatment, and small intestines were collected. The gastrointestinal transit ratio for each mouse was calculated as:
The remaining 5 mice from each group were used to measure the defecation time of the first black stool.
2.4. Morphology of small intestinal villi from mice
All mice were killed using CO2, and the blood, colon tissue, and small intestine were collected. The small intestine of each mouse was cut into lengths of 2 cm and then fixed in 10% formalin. After dehydrating for 48 hr, transparency treatment, wax immersion, embedding, sectioning, and hematoxylin and eosin (H&E) staining were performed to observe the morphological changes of these tissues under optical microscopy (BX43, Olympus) (Chen et al., 2019).
2.5. Determination of serum gastrointestinal motility‐related biomarkers
The blood was centrifuged (1,500 g, 4°C, 10 min) to obtain serum, which was collected in Eppendorf tubes and stored at −80°C pending analysis. Then, serum levels of motilin (MTL), acetylcholinesterase (AChE), substance P (SP), vasoactive intestinal peptide (VIP), gastrin, somatostatin (SS), and endothelin‐1 (ET‐1) were measured by corresponding kits (Beijing Puer Weiye Biotechnology Co. Ltd.).
2.6. Total RNA extraction and quantitative PCR assay
The small intestine tissue from each mouse was washed with normal saline and homogenized. Total RNA was extracted with TRIzol reagent according to the manufacturer's protocol (Thermo Fisher Scientific). The purity and concentration of the extracted total RNA were determined by ultra‐micro spectrophotometry (Nano‐100, All for Life Science), and then, the RNA was diluted to 1 μg/μl. Total RNA samples were used as templates to generate cDNAs by reverse transcription (Thermo Fisher Scientific). Then, cDNAs (2 μl) were mixed with 2× SYBR Premix Ex Taq II (10 μl), 50× ROX reference dye (0.4 μl), total primer (2 μl, 10 μmol/L, Table 1), and distilled H2O (5.6 μl). The PCR was performed in an automatic thermocycler (QuantStudio 6 Flex PCR, Life Technologies) with the following parameters: 95°C for 60 s; 40 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 50 s; 75°C for 10 min. GAPDH was selected as an internal reference, and the relative expression of cluster of differentiation 117 (c‐Kit), stem cell factor (SCF) glial cell‐derived neurotrophic factor (GDNF), inducible nitric oxide synthase (iNOS), transient receptor potential vanilloid‐1 (TRPV1) and cyclooxygenase‐2 (COX‐2) mRNA was calculated using the 2−ΔΔCt formula (Zhao et al., 2019).
TABLE 1.
Stool status of mice during the experiment
| Item | Stool weight (g) | Stool water content (%) | ||
|---|---|---|---|---|
| Day 1–6 | Day 7–9 | Day 1–6 | Day 7–9 | |
| Normal | 1.01 ± 0.09a | 1.02 ± 0.05a | 48 ± 2.9a | 48 ± 2.9a |
| Model | 0.99 ± 0.05a | 0.49 ± 0.03c | 47 ± 2.5a | 20 ± 2.1e |
| LP‐KSFY06 | 1.02 ± 0.08a | 0.58 ± 0.04c | 47 ± 2.6a | 26 ± 1.0d |
| Geniposide | 1.01 ± 0.03a | 0.73 ± 0.05b | 48 ± 3.6a | 32 ± 4.2c |
| LP + G | 1.00 ± 0.04a | 0.83 ± 0.08b | 47 ± 2.8a | 38 ± 2.0b |
Values presented are the mean ± standard deviation (n = 10/group).
Day 1–6: treatment period without induction of constipation; Day 7–9: treatment period with induction of constipation.
LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide.
a‐eIn the same column means significant differences (p < .05) according to Duncan's multiple range test.
2.7. Statistical analysis
In this study, the experiments were carried out in triplicate, and the mean values were obtained. All the data, presented as the mean ± standard deviation, were analyzed by SPSS 22 software (SPSS Inc., Chicago, IL, USA). Significant differences among groups, presented by p < .05, were analyzed by one‐way analysis of variance (ANOVA). All figures were drawn using Origin 8.1 software.
3. RESULTS
3.1. Body weights of mice
The changes in BW shown in Figure 1 illustrated that gradual growth of mice in all groups occurred during the first six days. From day 7, the BWs of the model group were significantly decreased due to constipation induced by montmorillonite clay. The LP‐KSFY06 group exhibited the same tendency of BW. However, the treatment consisting of geniposide or LP + G inhibited weight loss compared with the model group (p < .05), and BW steadily increased.
FIGURE 1.
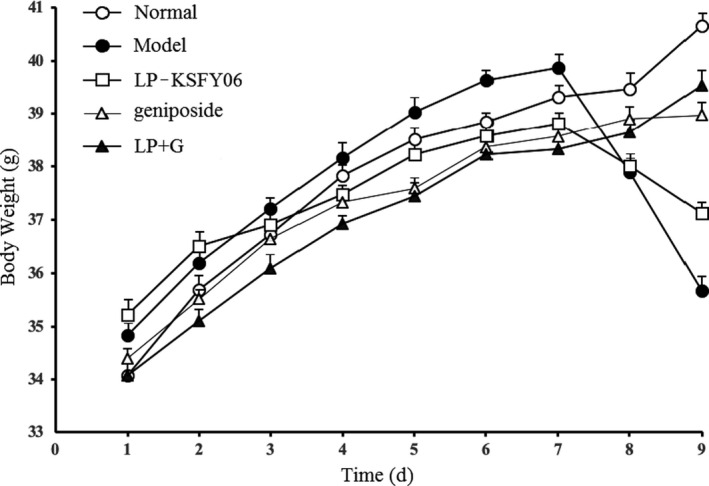
Body weight of mice during the experiment. LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide
3.2. Effect of LP‐KSFY06 and geniposide on fecal parameters
The fecal parameters shown in Table 1 indicate that montmorillonite significantly decreased the weight and water content of stool from 0.99 g to 0.49 g and 47% to 20%, respectively (p < .05). LP‐KSFY06 treatment enhanced the stool moisture of the constipated mice (p < .05), while there was no effect on stool weight (p > .05). The geniposide or LP + G intervention significantly increased the stool weight and water content (p < .05).
3.3. Gastrointestinal transit capability in constipation with different treatments
As shown in Table 2, montmorillonite did not influence the length of the small intestine, nor did the LP‐KSFY06 and geniposide. However, constipation without treatment significantly decreased the gastrointestinal transit capability, with the activated carbon propulsive rate reduced to 39.3% (p < .05), and increased the first black stool defecation time to 182 min (p < .05). Both LP‐KSFY06 and geniposide separately enhanced the gastrointestinal transit capability, and the combination of LP‐KSFY06 and geniposide (LP + G) exhibited more effective regulation.
TABLE 2.
Gastrointestinal transit capability and time to first black stool defection (n = 5)
| Group | Length of small intestine (cm) | Activated carbon propulsive rate (%) |
Time to first black stool defecation (min) |
|---|---|---|---|
| Normal | 50.4 ± 0.9a | 100.0 ± 0.0a | 67 ± 18e |
| Model | 49.8 ± 1.2a | 39.3 ± 1.8e | 182 ± 20a |
| LP‐KSFY06 | 50.2 ± 1.0a | 50.1 ± 1.6d | 133 ± 20b |
| Geniposide | 50.6 ± 1.0a | 69.1 ± 4.4c | 118 ± 19c |
| LP + G | 51.1 ± 1.2a | 80.3 ± 2.0b | 87 ± 21d |
Values presented are the mean ± standard deviation (n = 5/group).
Day 1–6: treatment period without induction of constipation; Day 7–9: treatment period with induction of constipation.
LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide.
a‐eIn the same column means significant differences (p < .05) according to Duncan's multiple range test.
3.4. Morphology of small intestinal villi from mice
Morphological section examination of small intestinal villi (Figure 2) in the model group revealed noticeable edema of the lamina propria, and irregular arrangement of the intestinal villi. All treatments significantly dissipated the edema of the lamina propria and repaired the intestinal villi. Moreover, the pathological changes of the intestinal villi in the LP + G group were recovered to a status similar to that in the normal group.
FIGURE 2.
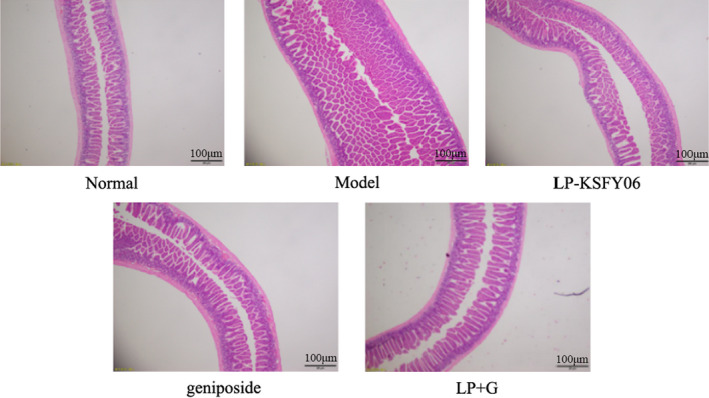
Hematoxylin–eosin (H&E) pathological observation of small intestinal villi from mice. Magnification 40×. LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide
3.5. Determination of serum gastrointestinal motility‐related biomarkers
The serum gastrointestinal motility‐related biomarkers were determined and are shown in Table 3. The results indicate that montmorillonite (model group) significantly decreased the levels of MTL, VIP, AchE, gastrin, and SP (p < .05), while it remarkably increased the level of ET‐1 and SS (p < .05). With the intervention of LP‐KSFY06, geniposide, or LP + G, these influenced biomarkers were attenuated. A more optimal regulatory effect was observed after LP + G treatment in constipated mice, and indexes of serum gastrointestinal motility were similar to those of the normal group.
TABLE 3.
Effect of Lactobacillus plantarum KSFY06 and geniposide on serum level (pg/mL) of gastrointestinal motility‐related biomarkers in mice with montmorillonite power‐induced constipation (n = 10)
| Group | MTL | ET‐1 | VIP | AchE | Gastrin | SP | SS |
|---|---|---|---|---|---|---|---|
| Normal | 192.5 ± 13.5a | 13.3 ± 1.6e | 80.4 ± 7.7a | 50.9 ± 15.7a | 90.6 ± 5.9a | 71.5 ± 4.3a | 28.3 ± 1.6d |
| Model | 92.7 ± 13.7e | 31.0 ± 6.4a | 38.4 ± 3.1e | 16.9 ± 5.8d | 41.5 ± 2.7e | 33.6 ± 2.2e | 61.5 ± 6.3a |
| LP‐KSFY06 | 127.7 ± 8.9d | 24.8 ± 2.7b | 49.1 ± 2.8d | 28.5 ± 3.0c | 45.6 ± 4.3d | 43.5 ± 1.8d | 48.1 ± 1.6b |
| Geniposide | 143.6 ± 6.9c | 18.8 ± 1.5c | 54.6 ± 4.8c | 37.6 ± 5.0b | 65.2 ± 3.8c | 54.3 ± 4.6c | 41.4 ± 1.9c |
| LP + G | 167.8 ± 14.8b | 16.1 ± 3.2d | 70.5 ± 4.9b | 45.3 ± 6.1a | 73.0 ± 4.6b | 61.0 ± 4.8b | 35.2 ± 2.9d |
Values presented are the mean ± standard deviation (n = 10/group).
Day 1–6: treatment period without induction of constipation; Day 7–9: treatment period with induction of constipation.
LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide.
Abbreviations: AChE, acetylcholinesterase; ET‐1, endothelin‐1; MTL, motilin; SP, substance P; SS, somatostatin; VIP, vasoactive intestinal peptide.
a‐eIn the same column means significant differences (p < .05) according to Duncan's multiple range test.
3.6. RNA expression in the mice small intestine
Compared with the normal group, the expression of c‐Kit, SCF, and GDNF mRNA in the model group was upregulated, and TRPV1, iNOS, and COX‐2 mRNA expression was downregulated (Figure 3). After intervention with LP‐KSFY06, geniposide, or LP + G, the changes induced by constipation were significantly attenuated in all the experimental groups (all p < .05), except for LP‐KSFY06 alone and its effects on TRPV1 and COX‐2. The efficacy of LP + G was superior to that of sole treatments with LP‐KSFY06 or geniposide. Moreover, LP‐KSFY06 improved the regulatory effect of geniposide on mRNA expression of some marker molecules in montmorillonite‐induced constipation.
FIGURE 3.
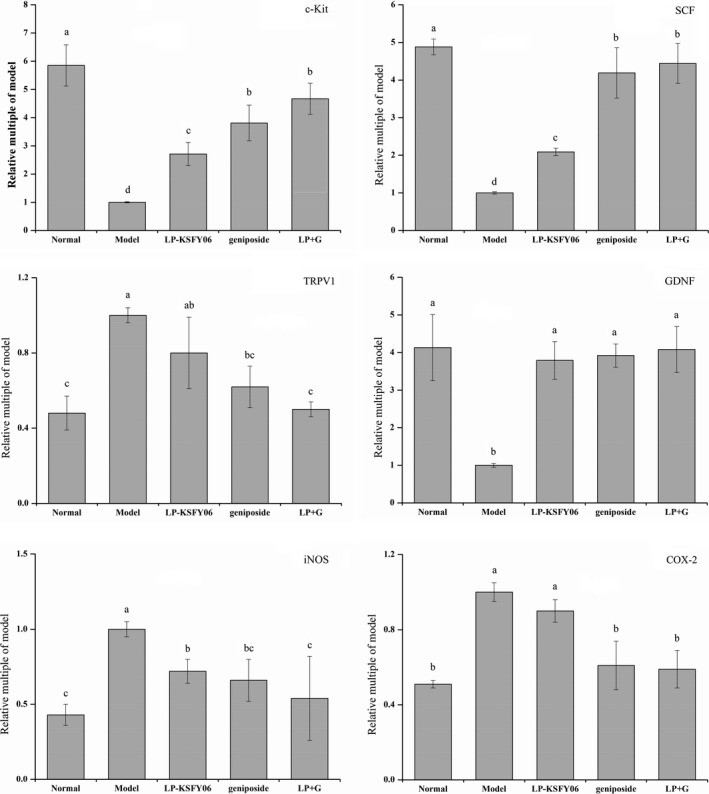
The cluster of differentiation 117 (c‐Kit), stem cell factor (SCF), glial cell‐derived neurotrophic factor (GDNF), inducible nitric oxide synthase (iNOS), transient receptor potential vanilloid‐1 (TRPV1), cyclooxygenase‐2 (COX‐2) mRNA expression in small intestine of mice. a–dThe different letters mean that there are significant differences (p < .05) between every two groups between every two groups according to Duncan's multiple range test. LP‐KSFY06: mice treated with 0.5 × 107 CFU/kg of Lactobacillus plantarum KSFY06; Geniposide: mice treated with 50 mg/kg body weight of geniposide; LP + G: mice treated with 1.0 × 107 CFU/kg of Lactobacillus plantarum KSFY06 and 50 mg/kg body weight of geniposide
4. DISCUSSION
Constipation is a common problem in humans, and current treatments do not provide relief of symptoms. Studies have shown that constipation is associated with gut microbiota dysbiosis, and decreased abundance of Lactobacillus, Bifidobacteria, Prevotella, and Bacteroides might be a potential cause or a consequence of altered gut motility (Attaluri, Jackson, Valestin, & Rao, 2010; Wojtyniak, Horvath, Dziechciarz, & Szajewska, 2017). With the recognition of the gut microbiota, probiotics can be used to modify health and disease, even though the exact mechanisms remain unclear. LAB probiotics are widely used in the food industry and have gradually developed into relatively indispensable functional foods that are eaten on a daily basis (Filanniono, Cagno, & Gobbetti, 2018; Venegas‐Ortega, Flores‐Gallegos, Martinez‐Hernandez, Aguilar, & Nevarez‐Moorillion, 2019). Studies have shown that LAB can alter the sensation and motility of the gut (Quigley, 2007; Rousseaux et al., 2007), soften the stool, and change secretion, as well as produce lactic acid and/or short‐chain fatty acids that can lower the intraluminal pH and promote defecation (Salminen & Salminen, 1997; Waller et al., 2011).
Patients with constipation usually exhibit difficulty with gastrointestinal peristalsis and voiding (Dimidi, Christodoulides, Fragkos, Scott, & Whelan, 2014; Suo et al., 2014), due to lower water content in the stool. In this study, montmorillonite‐induced constipation resulted in significantly decreased weight and moisture of stool, and decreased activated carbon propulsive rate that prolonged the time to first black stool defecation. Oral administration of LP‐KSFY06 (Table 1) increased gastrointestinal peristalsis (Table 2) and relieved intestinal damage. Moreover, LP‐KSFY06 promoted the interventional effects of geniposide.
Enteric nervous parameters secreted by the enteric nerve network in the gastrointestinal tract act as neuromodulators and neurotransmitters, promoting intestinal peristalsis and transportation of contents (Qian et al., 2015; Ramos et al., 2017). MLT, SP, and VIP are excitatory peptides, and AchE is an excitatory enzyme neurotransmitter, while ET‐1 and SS are inhibitory peptide neurotransmitters (Nassif et al., 1995; O'Dea, Brookes, & Wattchow, 2010; Wang, Hu, Xu, et al., 2017; Wang, Hu, Yan, et al., 2017). Gastrin, mainly secreted by the gastric sinus, promotes gastrointestinal motility, and the secretion is inhibited by SS (Jiang et al., 2020). In the current study, combination treatment with LP‐KSFY06 and geniposide (LP + G group) significantly enhanced levels of serum MTL, VIP, gastrin, AchE, and SP and inhibited serum ET‐1 and SS, of which the effects were remarkably superior to those of treatments with LP‐KSFY06 or geniposide alone. The serum levels of these biomarkers in the combination treatment group were similar to those in the normal group.
Apart from direct regulation of these neurotransmitters, LP‐KSFY06 also affects RNA expression in the small intestine. The interstitial cells of Cajal (ICC) are nerve‐like cells at the ends of motor neurons that maintained by c‐Kit, which promotes intestinal motility in the gastrointestinal system (Bansil & Turner, 2018; Lee, Park, Kamm, & Talbot, 2005; Yu, Crowell, Tihan, & Lacy, 2002). Low levels of ICC can be observed in patients with constipation (Sabri, Barksdale, & Lorenzo, 2003; Su et al., 2019). LAB could improve constipation by upregulating the expression of c‐Kit mRNA (Chen et al., 2020). SCF is a ligand for c‐Kit and necessary for the appropriate development and survival of ICC in the intestine (Wedel et al., 2002). In previous studies, the mRNA expression of c‐Kit and SCF in constipated mice or patients with constipation was decreased compared with that in normal mice and healthy individuals (Lee et al., 2005; Li et al., 2010). Our results also showed that there were lower mRNA levels of c‐Kit and SCF in model mice compared with those in the normal group. Additionally, combination LP‐KSFY06 and geniposide treatment enhanced the expression of c‐Kit and SCF mRNA (Figure 3).
Transient receptor potential cation channel subfamily V member 1 (TRPV1), a member of the TRPV group of transient receptor potential family of ion channels, is associated with the release of SP and closely related to defecation (Geppetti & Trevisani, 2004; Peng & Li, 2010), and its overexpression might indicate intestinal injury (Su et al., 2019). Treatment with LP‐KSFY06 reduced the increased expression level of TRPV1 to some degree, while the combination of LP‐KSFY06 and geniposide nearly eliminated the increased expression of TRPV1 in a manner similar to that in normal mice.
GDNF is a protein distributed in the gastrointestinal tract that modulates the growth and development of nerve cells (Allen, Watson, Shoemark, Barua, & Patel, 2013), repairs damaged nerve fibers, as well as promotes intestinal epithelial cell proliferation (Kalff, Schraut, Billiar, Simmons, & Bauer, 2000) and repairs the damaged intestinal tract (Su et al., 2019). As shown in Figure 3, the mRNA expression of GDNF in model mice was significantly inhibited by montmorillonite‐induced constipation. The inhibitory effects were totally negated, and there were no differences among mice treated with LP‐KSFY06, geniposide, or the combination.
Nitric oxide synthase (NOS) is the key to the produce endogenous NO from the esophagus to the anal sphincter. Activated inducible nitric oxide synthase (iNOS) hardly expresses in intestinal smooth muscle cells under normal circumstances which directly modulates intestinal dysmotility (Ma et al., 2019). However, a mass of NO derived from iNOS under inflammatory reaction will suppress the contractility of intestinal smooth muscle (Moojen et al., 1999; Toda & Okamura, 2016). Moreover, prostaglandin released by cyclooxygenase‐2 (COX‐2) also inhibits the intestinal smooth muscle contractility (Schwarz et al., 2001; Su et al., 2019). Inhibition of the expression of either iNOS or COX‐2 can significantly enhance the motility of the small intestine (Wen et al., 2006). In the current study, the mRNA expression of iNOS and COX‐2 significantly increased by 1.33‐fold and 0.95‐fold when compared to that in the normal group (Figure 3). After treatment with the combination of LP‐KSFY06 and geniposide, the mRNA expression of iNOS and COX‐2 was significantly decreased. However, LP‐KSFY06 showed no effect on regulating the expression of COX‐2 mRNA.
In this study, oral administration of LP‐KSFY06 and geniposide attenuated the montmorillonite‐induced constipation in mice. In addition, L. plantarum KSFY06 enhanced the effect of geniposide on constipation. The combination of KSFY06 and geniposide effectively increased stool weight and water content and increased the activated carbon propulsive rate, shortened the defecation time of the first black stool, and repaired intestinal villi. In addition, serum levels of MTL, VIP, AchE, gastrin, and SP were increased, and ET‐1 and SS were decreased to a greater degree after LP + G treatment compared with LP‐KSFY06‐ or geniposide‐treated mice. LP + G‐treated mice also demonstrated significantly increased mRNA expression levels of c‐Kit, SCF, and GDNF and decreased TRPV1, iNOS, and COX‐2. These results suggest that LP‐KSFY06 promoted geniposide attenuation of montmorillonite‐induced constipation in mice, and LP‐KSFY06 could be a potential agent that can be used for prevention or healing of constipation.
5. CONCLUSIONS
LP‐KSFY06 alleviated symptoms and reverted intestinal villi changes associated with constipation. Even though there were no differences in c‐Kit, SCF, GDNF, TRPV1, iNOS, and COX‐2 mRNA expression between the genisoside and LP + G groups, LP‐KSFY06 improved the regulatory effect of geniposide on gastrointestinal motility‐related biomarkers in constipated mice. This study indicated that L. plantarum KSFY06 may be an effective probiotic candidate with the ability to mitigate the adverse effects of constipation. The mechanism used by LP‐KSFY06 to promote geniposide's action will be more closely examined, and clinical trials will be carried out to verify their effectiveness in humans.
CONFLICT OF INTEREST
There are no conflicts of interest in this paper.
ACKNOWLEDGMENTS
This research was funded by Children's Research Institute of National Center for Schooling Development Programme and Chongqing University of Education (CSDP19FS01103), the Science, Technology Research Program of Chongqing Municipal Education Commission (KJZD‐K201901601), and Research Project of Chongqing University of Education (KY2015TBZC) and Scientific Research and Cultivation Project of the Affiliated Hospital of Stomatology of Chongqing Medical University (PYMS201603), China.
Gan Y, Liang J, Diao W, et al. Lactobacillus plantarum KSFY06 and geniposide counteract montmorillonite‐induced constipation in Kunming mice. Food Sci Nutr. 2020;8:5128–5137. 10.1002/fsn3.1814
Yi Gan and Jie Liang are equally contributed to this work.
Contributor Information
Fang Tan, Email: tanfang@foods.ac.cn.
Xin Zhao, Email: zhaoxin@cque.edu.cn.
DATA AVAILABILITY STATEMENT
No data were used to support this study.
REFERENCES
- Allen, S. J. , Watson, J. J. , Shoemark, D. K. , Barua, N. U. , & Patel, N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacology and Therapeutics, 138(2), 155–175. 10.1016/j.pharmthera.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Attaluri, A. , Jackson, M. , Valestin, J. , & Rao, S. S. C. (2010). Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. The American Journal of Gastroenterology, 105(6), 1407–1411. 10.1038/ajg.2009.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansil, R. , & Turner, B. S. (2018). The biology of mucus: Composition, synthesis and organization. Advanced Drug Delivery Reviews, 124, 3–15. 10.1016/j.addr.2017.09.023 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Chao, S. , & Pan, T. (2020). Lactobacillus paracasei subsp. paracasei NTU 101 lyophilized powder improves loperamide‐induced constipation in rats. Heliyon, 6, e03804 10.1016/j.heliyon.2020.e03804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Wu, J. , Liao, J. , & Yang, S. (2010). Polysaccharides of Tremella fuciformis as a diet for improvement of constipation in rats. Taiwan Nong Ye Yan Jiu, 59, 126–133. 10.1016/j.ijbiomac.2017.03.084 [DOI] [Google Scholar]
- Chen, L. , Zhang, J. , Suo, H. , Wang, W. , Wang, H. , Zhang, Y. , … Li, J. (2019). Preventive effects of different fermentation times of shuidouchi on diphenoxylate‐induced constipation in mice. Foods, 8(3), 86 10.3390/foods8030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, M. D. , Harris, L. A. , Lunsford, T. N. , & Dibaise, J. K. (2009). Emerging drugs for chronic constipation. Expert Opinion on Emerging Drugs, 14(3), 493–504. 10.1517/14728210903146890 [DOI] [PubMed] [Google Scholar]
- Dimidi, E. , Christodoulides, S. , Fragkos, K. S. , Scott, M. S. , & Whelan, K. (2014). The effect of probiotics on functional constipation in adults: A systematic review and meta‐analysis of randomized controlled trials. The American Journal of Clinical Nutrition, 100(4), 1075–1084. 10.3945/ajcn.114.089151 [DOI] [PubMed] [Google Scholar]
- Eskesen, D. , Jespersen, L. , Michelsen, B. , Whorwell, P. J. , Müller‐Lissner, S. , & Morberg, C. M. (2015). Effect of the probiotic strain Bifidobacterium animalis subsp. lactis BB‐12(R) on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: A randomised, double‐blind, placebo‐controlled, parallel‐group trial. The British Journal of Nutrition, 114(10), 1638–1646. 10.1017/S0007114515003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2006). Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutritional, 85.
- Filanniono, P. , Cagno, R. D. , & Gobbetti, M. (2018). Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Current Opinion Biotechnology, 49, 64–72. 10.1016/j.copbio.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Geppetti, P. , & Trevisani, M. (2004). Activation and sensitisation of the vanilloid receptor: Role in gastrointestinal inflammation and function. British Journal of Pharmacology, 141(8), 1313–1320. 10.1038/sj.bjp.0705768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Dong, J. , Jiang, S. , Liang, Q. , Zhang, Y. , Liu, Z. , … Kang, W. (2020). Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Research International, 136, 109316 10.1016/j.foodres.2020 [DOI] [PubMed] [Google Scholar]
- Kalff, J. C. , Schraut, W. H. , Billiar, T. R. , Simmons, R. L. , & Bauer, A. J. (2000). Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology, 118(2), 316–327. 10.1016/S0016-5085(00)70214-9 [DOI] [PubMed] [Google Scholar]
- Karabudak, E. , Koksal, E. , & Macit, M. S. (2019). The relationship between body weight, fiber and fluid intake status and functional constipation in young adults. Nutrition & Food Science, 49(1), 129–140. 10.1108/NFS-03-2018-0083 [DOI] [Google Scholar]
- Khalif, I. L. , Quigley, E. M. , Konovitch, E. A. , & Maximova, I. D. (2005). Alterations in thecolonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Digestive and Liver Disease, 37(11), 838–849. 10.1016/j.dld.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Lee, J. I. , Park, H. , Kamm, M. A. , & Talbot, I. C. (2005). Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow‐transit constipation and acquired megacolon. Journal of Gastroenterology and Hepatology, 20(8), 1292–1298. 10.1111/j.1440-1746.2005.03809.x [DOI] [PubMed] [Google Scholar]
- Lembo, A. , & Camilleri, M. (2003). Chronic constipation. The New England Journal of Medicine, 349(14), 1360–1368. 10.1016/j.rchipe.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Li, F. , Zhang, L. , Li, C. , Ni, B. , Wu, Y. , Huang, Y. , … Liu, B. (2010). Adenovirus‐mediated stem cell leukemia gene transfer induces rescue of interstitial cells of Cajal in ICC‐loss mice. International Journal of Colorectal Disease., 25(5), 557–566. 10.1007/s00384-010-0883-z [DOI] [PubMed] [Google Scholar]
- Ma, H. , Xiong, H. , Zhu, X. , Ji, C. , Xue, J. , Li, R. , … Cui, H. (2019). Polysaccharide from Spirulina platensis ameliorates diphenoxylate‐induced constipation symptoms in mice. International Journal of Biological Macromolecules, 133, 1090–1101. 10.1016/j.ijbiomac.2019.04.209 [DOI] [PubMed] [Google Scholar]
- Markowiak, P. , & Slizewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9(9), 1–30. 10.3390/nu9091021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moojen, T. M. , van Gulik, T. M. , Hoek, F. J. , Gouma, D. J. , Tytgat, G. N. , & Boeckxstaens, G. E. (1999). Possible role of nitric oxide in postoperative ileus: A comparative study. Neurogastroenterology and Motility, 11(5), 403–408. 10.1046/j.1365-2982.1999.00158.x [DOI] [PubMed] [Google Scholar]
- Nassif, A. , Longo, W. E. , Sexe, R. , Stratton, M. , Standeven, J. , Vernava, A. M. , & Kaminski, D. L. (1995). Clostridium difficile suppresses colonic vasoactive intestinal peptide associated with altered motility. Mediators of Inflammation, 4(6), 452–453. 10.1155/S096293519500072X [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea, C. J. , Brookes, J. H. , & Wattchow, D. A. (2010). The efficacy of treatment of patients with severe constipation or recurrent pseudo‐obstruction with pyridostigmine. Colorectal Disease: the Official Journal of the Association of Coloproctology of Great Britain and Ireland, 12(6), 540–548. 10.1111/j.1463-1318.2009.01838.x [DOI] [PubMed] [Google Scholar]
- Okawa, Y. , Fukudo, S. , & Sanada, H. (2019). Specific foods can reduce symptoms of irritable bowel syndrome and functional constipation: A review. BioPsychoSocial Medicine, 13(1), 10 10.1186/s13030-019-0152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , & Li, Y. J. (2010). The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. European Journal of Pharmacology, 627(1–3), 1–7. 10.1016/j.ejphar.2009.10.053 [DOI] [PubMed] [Google Scholar]
- Qian, Y. , Suo, H. , Du, M. , Zhao, X. , Li, J. , Li, G. , … Liu, Z. (2015). Preventive effect of Lactobacillus fermentum Lee on activated carbon‐induced constipation in mice. Food Science, 9(1), 272–278. 10.3892/etm.2014.2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. , Suo, H. , Yi, R. , Li, G. , & Zhao, X. (2017). Lactobacillus casei Qian enhances the preventive effect of geniposide on HCl/ethanol induced gastric injury in Mice. Food Science, 38(21), 230–240. 10.7506/spkx1002-6630-201721037 [DOI] [Google Scholar]
- Quigley, E. M. (2007). Bacteria: A new player in gastrointestinal motility disorders‐infections, bacterial overgrowth, and probiotics. Gastroenterology Clinics of North America, 36(3), 735–748. 10.1016/j.gtc.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Ramos, I. , Nuche‐Berenguer, B. , Moreno, P. , Mantey, S. A. , Iordanskaia, T. , & Jensen, R. T. (2017). Presence of the P21‐activated kinase, PAK4, in pancreatic acinar cells and its activation by numerous gastrointestinal hormones and growth factors. Gastroenterology, 152(5), S902 10.1016/S0016-5085(17)33082-2 [DOI] [Google Scholar]
- Rousseaux, C. , Thuru, X. , Gelot, A. , Barnich, N. , Neut, C. , Dubuquoy, L. , … Desreumaux, P. (2007). Lactobaccillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Natural Medicine, 13(1), 35–37. 10.1038/nm1521 [DOI] [PubMed] [Google Scholar]
- Sabri, M. , Barksdale, E. , & Lorenzo, C. D. (2003). Constipation and lack of colonic interstitial cells of Cajal. Digestive Diseases and Science, 48(5), 849–853. 10.1023/A:1023074823778 [DOI] [PubMed] [Google Scholar]
- Salminen, S. , & Salminen, E. (1997). Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scandinavian Journal of Gastroenterology, 32(222), 45–48. 10.1080/00365521.1997.11720717 [DOI] [PubMed] [Google Scholar]
- Schuster, B. G. , Kosar, L. , & Kamrul, R. (2015). Constipation in older adults: Stepwise approach to keep things moving. Canadian Family Yhysician Medecin, 61(2), 152–158. [PMC free article] [PubMed] [Google Scholar]
- Schwarz, N. T. , Kalff, J. C. , Turler, A. , Engel, B. M. , Watkins, S. C. , Billiar, T. R. , & Bauer, A. J. (2001). Prostanoid production via COX‐2 as a causative mechanism of rodent postoperative ileus. Gastroenterology, 121(6), 1354–1371. 10.1053/gast.2001.29605 [DOI] [PubMed] [Google Scholar]
- Shimotoyodome, A. , Meguro, S. , Hase, T. , Tokimitsu, I. , & Sakata, T. (2000). Decreased colonic mucus in rats with loperamide‐induced constipation. Comparative Biochemistry and Physiology, 126, 203–211. 10.1016/S1095-6433(00)00194-X [DOI] [PubMed] [Google Scholar]
- Shin, A. , Camilleri, M. , Kolar, G. , Erwin, P. , West, C. P. , & Murad, M. H. (2014). Systematic review with meta‐analysis: Highly selective 5‐HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Alimentary Pharmacology & Therapeutics, 39(3), 239–253. 10.1111/apt.12571 [DOI] [PubMed] [Google Scholar]
- Su, H. , Chen, J. , Miao, S. , Deng, K. , Liu, J. , Zeng, S. , … Lu, X. (2019). Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food and Chemical Toxicology, 134, 110838 10.1016/j.fct.2019.110838 [DOI] [PubMed] [Google Scholar]
- Suo, H. , Zhao, X. , Qian, Y. , Li, G. , Liu, Z. , Xie, J. , & Li, J. (2014). Therapeutic effect of activated carbon‐induced constipation mice with Lactobacillus fermentum Suo on treatment. International Journal of Molecular Sciences, 15(12), 21875–21895. 10.3390/ijms151221875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, N. , & Okamura, T. (2016). Cigarette smoking impairs nitric oxide‐mediated cerebral blood flow increase: Implications for Alzheimer's disease. Journal of Pharmacological Sciences, 131, 223–232. 10.1016/j.jphs.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Venegas‐Ortega, M. G. , Flores‐Gallegos, A. C. , Martinez‐Hernandez, J. L. , Aguilar, C. N. , & Nevarez‐Moorillion, G. V. (2019). Production of bioactive peptides from lactic acid bacteria: A sustainable approach for healthier foods. Comprehensive Reviews in Food Science and Food Safety, 18, 1039–1051. 10.1111/1541-4337.12455 [DOI] [PubMed] [Google Scholar]
- Waller, P. A. , Gopal, P. K. , Leyer, G. J. , Ouwehand, A. C. , Reifer, C. , Stewart, M. E. , & Miller, L. E. (2011). Dose‐response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scandinavian Journal of Gastroenterology, 46(9), 1057–1064. 10.3109/00365521.2011.584895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Hu, L. , Xu, Q. , Yin, B. , Fang, D. , Wang, G. , … Chen, W. (2017). Bifidobacterium adolescentis exerts strain‐specific effects on constipation induced by loperamide in BALB/c mice. International Journal of Molecular Sciences, 18(2), 318–340. 10.3390/ijms18020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Hu, L. , Yan, S. , Jiang, T. , Fang, S. , Wang, G. , … Chen, W. (2017). Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short‐chain fatty acids in mice with constipation. Food and Function, 8(5), 1966–1978. 10.1039/c7fo00031f [DOI] [PubMed] [Google Scholar]
- Wang, R. , Sun, P. , Zhou, Y. , & Zhao, X. (2015). Preventive effect of Dendrobium candidum Wall. ex Lindl. on activated carbon‐induced constipation in mice. Experimental and Therapeutic Medicine, 9(2), 563–568. 10.3892/etm.2014.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, L. , Tummala, R. , Diva, U. , & Lappalainen, J. (2016). A 12‐week extension study to assess the safety and tolerability of naloxegol in patients with noncancer pain and opioid‐induced constipation. Journal of Opioid Management, 12(6), 405–419. 10.5055/jom.2016.0360 [DOI] [PubMed] [Google Scholar]
- Wedel, T. , Spiegler, J. , Soellner, S. , Roblick, U. J. , Schiedeck, T. H. , Bruch, H. P. , & Krammer, H. J. (2002). Enteric nerves and interstitial cells of cajal are altered in patients with slow‐transit constipation and megacolon. Gastroenterology, 123(5), 1459–1467. 10.1053/gast.2002.36600 [DOI] [PubMed] [Google Scholar]
- Wen, S. , Huang, T. H. W. , Li, G. Q. , Yamahara, J. , Roufogalis, B. D. , & Li, Y. (2006). Bromelain improves decrease in defecation in postoperative rats: Modulation of colonic gene expression of inducible nitric oxide synthase. Life Sciences, 78(9), 995–1002. 10.1016/j.lfs.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Wojtyniak, K. , Horvath, A. , Dziechciarz, P. , & Szajewska, H. (2017). Lactovacillus casei rhamnosus Lcr35 in the management of functional constipation in children: A randomized trial. The Journal of Pediatrics, 184, 101–105. 10.1016/j.jpeds.2017.01.068 [DOI] [PubMed] [Google Scholar]
- Wojtyniak, K. , & Szajewska, H. (2017). Systematic review: Probiotics for functional constipation in children. European Journal of Pediatrics, 176(10), 1–8. 10.1007/s00431-017-2972-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. , Crowell, M. D. , Tihan, T. , & Lacy, B. E. (2002). Distribution of interstitial cells of cajal in the whole colon from patients with slow‐transit constipation. The American Journal of Gastroenterology, 97(9), S118 10.1016/S0002-9270(02)04831-1 [DOI] [Google Scholar]
- Zhao, X. , Qian, Y. , Li, G. , Yi, R. , Park, K. Y. , & Song, J. L. (2019). Lactobacillus plantarum YS2 (yak yogurt Lactobacillus) exhibited an activity to attenuate activated carbon‐induced constipation in male Kunming mice. Journal of Dairy Science, 102(1), 26–36. 10.3168/jds.2018-15206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


