Abstract
Noncommunicable diseases have increasingly grown the cause of morbidities and mortalities worldwide. Among them, cardiovascular diseases (CVDs) continue to be the major contributor to deaths. CVDs are common in the urban community population due to the substandard living conditions, which have a significant impact on the healthcare system, and over 23 million human beings are anticipated to suffer from the CVDs before 2030. At the moment, CVD physicians are immediately advancing both primary and secondary prevention modalities in high‐risk populations. The cornerstone of CVD prevention is a healthy lifestyle that is more cost‐effective than the treatments after disease onset. In fact, in the present scenario, comprehensive research conducted on food plant components is potentially efficacious in reducing some highly prevalent CVD risk factors, such as hypercholesterolemia, hypertension, and atherosclerosis. Polyphenols of olive oil (OO), virgin olive oil (VOO), and extra virgin olive oil contribute an essential role for the management of CVDs. Olive oil induces cardioprotective effects due to the presence of a plethora of polyphenolic compounds, for example, oleuropein (OL), tyrosol, and hydroxytyrosol. The present study examines the bioavailability and absorption of major olive bioactive compounds, for instance, oleacein, oleocanthal, OL, and tyrosol. This review also elucidates the snobbish connection of olive polyphenols (OP) and the potential mechanism involved in combating various CVD results taken up from the in vitro and in vivo studies, such as animal and human model studies.
Keywords: atherosclerosis, bioactive compounds, cardiovascular diseases, hypercholesterolemia, hypertension, olive polyphenols, potential mechanism
This review also elucidates the snobbish connection of olive polyphenols (OP) and the potential mechanism involved in combating various CVD results taken up from the in vitro and in vivo studies, such as animal and human model studies.
1. INTRODUCTION
The noncommunicable diseases account for over 75% of mortalities worldwide particularly in underdeveloped and developing countries and take place almost equally in men and women (Townsend et al., 2016). Cardiovascular diseases (CVDs) are often called “silent killers,” and it is estimated that approximately 17.7 million people died from CVDs representing 31% of all global deaths (Roth, et al., 2017). In addition, CVDs are a group of disorders such as chronic heart disease, stroke, rheumatic heart disease, peripheral arterial disease, congenital heart disease, pulmonary embolism, and deep vein thrombosis and generally proliferate by accumulation of fatty deposits on the inner walls of the blood vessels causing a blockage and stoppage of blood circulation to the arms, legs, brain, and heart (Lanier, Bury, & Richardson, 2016; Roth et al., 2017). Strokes can also be produced by blood loss from a blood vessel. According to the WHO, it is estimated that 7.4 million deaths were due to coronary heart disease and 6.7 million people died due to stroke in 2015. Hypertension is also the most frequent and important intermediate risk factor in addition to diabetes, overweight, obesity, and hyperlipidemia (Roth et al., 2017) Vascular dysfunction has been studied over the past decades, and numerous advances have been made regarding its specific risk factors on the health (Buckland & Gonzalez, 2015). There are two main reasons for vascular diseases, that is, coronary heart diseases and cerebrovascular diseases, such as thromboembolism and atherosclerosis. Atherosclerosis is mainly caused by the accumulation of cholesterol, fat, and other compounds in or on the artery walls, which results in restriction of blood flow to the organs (Mendis et al., 2015). If atherogenesis is not reverted, it will result in smooth muscle cell procreation, production of the extracellular matrix and fibrous tissue, and formation of the necrotic core. Physical disruption of atherosclerotic plaque can cause arterial occlusion, clot formation, and arterial thrombosis (Longo & Mattson, 2014). A healthy lifestyle and diet are fundamental factors for promoting and maintaining good health to decrease the risk of CVDs. The key to good cardiovascular health is to manage the behavioral risk factors such as smoking, less physical activity, harmful use of alcohol, and not as much healthy diet. According to various clinical trials, strategies that are planned to detect and modify the behavioral risk factors can lead to a decrease in hypertension and atherosclerosis development, and also erode the incidence of cardiovascular events. Many medications reduce the risks of CVDs, but most of them exert a wide range of side effects in patients, such as flushing, fatigue, headache, shortness of breath, and dizziness (Blumenthal, Merz, Bittner, & Gluckman, 2008). Among all the behavioral CVD risk factors, a diet is most important and has a huge impact on human health and CVD deaths. The Mediterranean diet (MD) is the heritage of millennia and one of the healthiest diet patterns globally, especially in Mediterranean countries, and eliminates the risk factors for CVDs that represent the principal cause of deaths worldwide. Indeed, MD had been popular in everyday life and declared as an Intangible Cultural Heritage of Humanity by UNESCO in 2010. Traditional MD is considered by the less intake of meat and meat products, more intake of vegetables, fruits, legumes, cereals, and nuts, a low consumption of alcohol, a moderate intake of seafood and fish, and regular use of olive oil (OO), mainly VOO and extra virgin olive oil (EVOO), which intake typically ranges between 25 and 50 ml (approximately two tablespoons) per day. Worldwide cultivation of olive trees (Olea europaea L) is more than 8 million ha, and almost 98% of them are cultivated in the Mediterranean basin (Peralbo‐Molina & Luque de Castro, 2013). Spain has the highest number of olive trees and olive orchard (IOC, 2012). In 2014/2015, the total world olive oil (OO) production was 2.39 million tons, and among this, European Union produced 1.53 million tons and it was mainly used for human consumption (IOC, 2014). Besides OO production, olive trees are also used for table olive (TO) production. OO and TO are the two main foods of the MD (Obied et al., 2012). The contemporary world needs natural polyphenols for medications of different types of diseases and increasing interest in the studies of OO and its polyphenols. Therefore, this review discusses the advance research conducted on OO and its polyphenol bioavailability and health benefits with special emphasis regarding the CVDs.
2. COMMERCIAL CLASSIFICATION AND CHEMICAL CONTENTS OF OLIVE OIL
Nowadays, OO consumption is abruptly increased on account of the health‐promoting components such as polyphenols, tocopherols and carotenoids (Dias et al., 2018. Further, OO is very important for reducing the growth of foodborne pathogens and stimulation of growth of probiotic microorganisms such as L. Acidophilus and B. Bifidum, in addition to antioxidant activity, which make it very popular edible oil in the world (Borges, Alberto, et al., 2017; Borges, Carlos, Alberto, Vique‐Cabrera, & Seiquer, 2017). The classification of OO is dependent on sensory characteristics (aroma, flavor, and off‐flavor) and physicochemical properties (particularly free acidity, peroxide value, and absorption at specific ultraviolet wavelengths). Furthermore, EVOO has good quality due to the maximum presence of physicochemical quality attributes and sensory properties as compared to VOO and LOO. However, VOO has shown off moderate physicochemical quality parameters. The key components of OO are saponifiable lipids (about 98%), which contain mostly triglycerols. The fatty acid present in triglycerols is a monounsaturated fatty acid, particularly oleic acid (55%–83%), which is demonstrated to have numerous health benefits (Ambra, Natella, Lucchetti, Forte, & Pastore, 2017), while saturated fatty acid such as palmitic acid (7.5%–20%) and a polyunsaturated fatty acid such as linoleic acid (2.5%–21%) are also present in the OO (Ramírez‐Tortosa, Granados, & Quiles, 2006). The minor fraction (only 2%) has more than 230 complicated chemical compounds, and it is said to contribute to the organoleptic properties of OO. It also possesses some phenolic compounds (hydrophilic phenols) such as hydroxytyrosol, oleuropein (OL), and tyrosol (Robles‐Almazan et al., 2018). Currently, almost 36 phenolic compounds have been isolated from EVOO and identified, which are present at a wide range of concentrations (0.02–600 mg/kg). In addition, phenolic acids (vanillic acid, syringic acid, gallic acid, etc), flavonoids (eriodictyol, apigenin, luteolin, etc.), secoiridoids (oleacein, oleocanthal, etc.), and lignans ((+) ‐pinoresinol, (+)‐1‐hydroxypinoresinol, (+) 1‐acetoxypinoresinol) are also a part of phenolic compounds (Table 1) (Quirantes‐Pine et al., 2012; Ramírez‐Tortosa et al., 2006). Besides, sterol, 4 methyl sterols, phenolic alcohols, such as triterpenic alcohols, triterpenic dialcohols, fatty alcohols (Srigley, Oles, Reza, Kia, & Mossoba, 2016), hydrocarbons such as carotene, b‐carotene, squalene (Dias et al., 2018; Eggersdorfer & Wyss, 2018), lipophilic phenols, in particular, tocopherols (Borges, Alberto, et al., 2017; Borges, Carlos, et al., 2017), xanthophylls, color pigments (pheophytins, chlorophylls), ketones, waxes, and esters are also a part of the fractions of olive oil (Lombardo, Grasso, Lanciano, Loria, & Monetti, 2018). However, it should be well‐known fact that the composition of more or less 230 complex chemical compounds of OO affected by farming methods, irrigation techniques, climate conditions, geographical regions, variety, and process, that is, extraction, can also affect the chemical contents of OO extract from olive fruit (OF) (Squeo et al., 2016). In case of VOO and EVOO, about 95% of them come from the mesocarp (fleshy mesocarp and epicarp) and only 5% come from the seed of the OF such as embryo and endosperm, after twice pressing, that is, by cold pressing without any chemicals, with small amount of heat applied (Borges, Alberto, et al., 2017; Borges, Carlos, et al., 2017). In another study, the crop year and the density of olive tree affected the chemical composition of the OO obtained from OF (Rodrigues et al., 2018). In this sense, it can be concluded that the strength of the biological effects of OO differs as a result of chemical composition.
TABLE 1.
Distribution of bioactive compounds present in various parts of olive
| Classification | Compounds | OF | VOO | EVOO | OMW | OPW | OL |
|---|---|---|---|---|---|---|---|
| Phenolic acids | p‐coumaric acid | – | 0.81 ± 0.40 mg/kg | 3.28 ± 0.02 mg/kg | – | 0.549 ± 0.038 g of tyrosol | – |
| Vanillic acid | 0.058 ± 0.01 mg/g | 2.00 ± 0.33 mg/kg | – | 10.4 ± 0.66 mg/kg | 45.0 ± 16.9 lg/g DM | 0.002 ± 0.0002 mg/g | |
| Caffeic acid | 2.17 ± 0.02 mg/g | 23.8 ± 0.62 mg/kg | 0.35 ± 0.00 mg/kg | 20.7 ± 0.56 mg/kg | 33.3 ± 24.7 lg/g of DM | 0.081 ± 0.001 mg/g | |
| Ferulic acid | – | 4.6 ± 0.8 mg/kg | 0.01 ± 0.00 mg/kg | 7.2 ± 0.08 | – | – | |
| Syringic acid | 0.5 6 ± 0.1 mg/kg | 15.1 ± 0.74 mg/kg | – | 22.4 ± 0.38 | 0.2 ± 0.1 lg/g of DM | – | |
| Flavonoids | Apigenin | 6.1 6 ± 0.6 mg/kg | 0.91 ± 0.08 mg/kg | 1.72 ± 0.01 mg/kg | – | 3.3 ± 1.4 lg/g of DM | – |
| Luteolin | 0.38 ± 0.06 mg/g | 2.68 ± 0.25 mg/kg | 15.54 ± 0.13 mg/kg | – | 105.1 ± 32.2 lg/g of DM | 0.081 ± 0.002 mg/g | |
| Luteolin−7‐O‐glucoside | 0.43 ± 0.03 mg/g | 0.43 ± 0.03 mg/g | – | – | 32.0 ± 22.1 lg/g of DM | 0.008 ± 0.0002 mg/g | |
| Rutin | 4.3 6 ± 0.3 mg/kg | 0.002 ± 0.0002 mg/g | – | 11.7 ± 0.39% | 0.44%TPC | 0.13 ± 0.09 mg/g | |
| Secoiridoids | Oleuropein | 6.53 ± 0.01 mg/g | 140 ± 2.99 mg/kg | – | 83.0 ± 3.60 mg/kg | 12.4 ± 740.1 lg/g of DM | 0.042 ± 0.001 mg/g |
| p‐HPEA‐EDA | 41.1 ± 19.8 mg/kg | 59.91 ± 6.95 mg/kg | 55.31 ± 0.33 mg/kg | – |
3.41 %TPC |
– | |
| 3,4‐DHPEA‐EDA | 384.0 ± 10.8 mg/kg | 75.47 ± 23.91 mg/kg | 42.02 ± 0.38 mg/kg | – | – | – | |
| 3,4‐DHPEA‐EA | 186 ± 1.2 mg/kg | 33.71 ± 1.88 mg/kg | 60.22 ± 0.51 mg/kg | – |
1.04 %TPC |
– | |
| Lignans | (+)‐pinoresinol | – | 8.8 ± 0.01 mg/kg | 0.42 mg/100 g | – | 0.96%TPC | – |
| 1‐acetoxypinoresinol | – | 27.1 ± 1.15 mg/kg 1 | – | – | 2.3%TPC | – | |
| Hydroxytyrosol | 0.076 ± 0.001 mg/g | 41.3 ± 1.04 mg/kg | 1.78 ± 0.01 mg/kg | 20.7 ± 0.56 mg/kg | 1.0 ± 2.5 | 0.54 ± 0.02 mg/g | |
| Tyrosol | 84.5 ± 6 3.5 mg/kg | 23.8 ± 0.62 mg/kg | 0.25 ± 0.00 mg/kg | 13.5 ± 1.04 mg/kg | 282.4 ± 107.5 | – | |
| 3,4‐DHPEA‐AC | 58.7 ± 0.7 mg/kg | 42.29 ± 3.55 mg/kg | 0.24 ± 0.00 mg/kg | – | 28.4 ± 14.5 | – |
Adopted from (Ambra et al., 2017; Aludatt et al., 2010; Aggoun et al., 2016; Arslan 2012; Brahim, Kelebek, Ammar, Abichou, & Bouaziz, 2017; Cioffi et al., 2010; El‐Abbassi, Kiai, & Hafidi, 2012; Franco et al., 2014; Leouifoudi et al., 2014; Xie, Huang, Zhang, & Zhang, 2015; Zamora‐Ros, Knaze, & González, 2012).
Abbreviations: DM, Dry matter; EVOO, Extra virgin olive oil; OF, Olive fruit; OL, Olive leaves; OMW, Olive mill water; OPW, Olive pomace waste; TPC, Total phenolic content; VOO, Virgin olive oil.
3. OLIVE OIL POLYPHENOL (OOP) BIOAVAILABILITY
At the time of justification of the health benefits of food components, it is essential to consider the difference between bioavailability and absorbed contents of OOP (Difonzo et al., 2017). Altogether during in vitro (cell culture, gastrointestinal digestion) and in vivo (animal and human) investigation, OOP identified was sooner or later got absorbed and was available for storage and physiological events; furthermore, it was also participated in the biological functions quantitatively (Rodriguez‐Concepcion et al., 2018; Mosele et al., 2014). Nowadays, it is considered that in the absorption of lipophilic compounds, in particular, carotenoids (Rodrigues et al., 2018), they are unable to dissolve in the aqueous environment until their release from the matrix, and they need to be merged into colloidal solution. In this sense, the lipids and bile components get developed and possibly picked up by the apical surface of the enterocytes from which lipophilic compounds can be absorbed in a long run, and are further incorporated into circulation (Meléndez‐Martínez et al., 2017). It is noted that the OOPs, for example, hydroxytyrosol and tyrosol, are well reviewed as a bioavailability aspect (Robles‐Almazan et al., 2018; Rosignoli, Fuccelli, Sepporta, & Fabiani, 2016). Moreover, some OOPs such as hydroxytyrosols were properly absorbed in the gastrointestinal tract, but their improper bioavailability resulted in the formation of glucuronide, sulfate, and rapid metabolism (Torre et al., 2008; Mateos et al., 2016). Generally, the low bioavailability of OOP results in an incomplete intestinal absorption and abrupt biotransformation favoring urinary excretion. It is said that the OOP and their secoiridoid derivatives such as oleuropein aglycones, OL, elenolic acid, dialdehydes such as lignans (acetoxypinoresinol), and verbascoside are rapidly hydrolyzed through the gastrointestinal tract. Mode of absorption for hydroxytyrosol and tyrosol is mainly dose‐dependent (Robles‐Almazan et al., 2018). Almost after 1 hr of consumption, their peak plasma level was detected (Miro‐Casas et al., 2003), whereas peak urine concentrations were found after 2 hr of consumption (Miro Casas et al., 2001). The results of in vitro methods (GI and cell culture) also claimed the bioavailability of OOP (Soler et al., 2010; Mosele et al., 2014; Mateos et al., 2016; Pinto et al., 2011; Rosignoli et al., 2016). Provided evidences regarding bioavailability and metabolism of OOP in the human body, animal, GI tract, and cell culture demand further studies that are required to understand the metabolism and bioavailability of OOP.
4. OP COMBATING CVDs
This section of the present review describes the studied published olive and its polyphenolic compounds combating CVDs. The summary of key studied results also presented in Table 2 and Figure 1.
TABLE 2.
Summary of effect of olive polyphenols on CVDs
| Disease | Parts used | Study type | Observations | References |
|---|---|---|---|---|
| Atherosclerosis | OO | In vivo | OO protected from atherosclerosis as compared to saturated fatty acids rich diet in niacin‐treated mice | Montserrat‐de la Paz et al. (2016) |
| EVOO | In vivo | EVOO polyphenols improved endothelial function and lowered lipid accumulation within the atherosclerotic lesion of Apo E‐deficient mice. | Claro et al. (2015) | |
| VOO and thyme | Randomized, double‐blind, crossover, controlled trial | Incorporation of thyme into VOO improved the lipoprotein particle atherogenic ratios in 33 hypercholesterolemic individuals after 3 weeks | Fernández‐Castillejo et al. (2016) | |
| VOO and thyme | Randomized, double‐blind, crossover, controlled trial | VOO and thyme 25 ml/day for 3 weeks improved endothelial function in 12 healthy subjects | Valls et al. (2017) | |
| Squalene | In vivo | Administration of squalene to atherosclerotic rabbits reversed endothelial activation and lowered cellularity in gingival mucosa | Bullon et al. (2009) | |
| In vivo | Administration of hydroxytyrosol at 4 mg/kg bw with presence of saturated fat and cholesterol, reduced the size of atherosclerotic lesions when compared with animals receiving this diet without hydroxytyrosol | Gonzalez‐Santiago et al. (2006) | ||
| Platelet aggregation | OO | Hydroxytyrosol and oleuropein inhibited collagen‐induced platelet activation or ADP | Petroni et al. (1995) | |
| Oleocanthal and oleacein possessed antiplatelet activity by inhibiting COX and 5‐ LOX inhibitor | Beauchamp et al. (2005) and Vougogiannopoulou et al. (2014) | |||
| OOP | In vitro | OLP inhibited platelet function in blood taken from 11 healthy males. | Singh et al. (2008) | |
| (olive oil polyphenols | OOP inhibited platelet aggregation by cAMP‐PDE inhibition | Dell’Agli et al. (2008) | ||
| EVOO | Randomized crossover | Weekly consumption of EVOO (40 ml) rich in oleocanthal prevented from platelet aggregation. | Agrawal et al. (2017) | |
| Hyperlipidemia | OO | Daily intake of OO (25 ml) didn't promote postprandial lipemia | Weinbrenner, Fitó, et al. (2004) | |
| (olive polyphenols) | A double‐blind, randomized, placebo‐controlled study | OP (250−1,000 mg for 12 months) to the 64 osteopenic patients women (age: 49 to 68 years) significantly reduced total and LDL cholesterol level | Filip et al. (2015). | |
| OP | Randomized, crossover, control study | OP significantly increased HDL level in 47 healthy European male volunteers | Hernáez et al. (2014) | |
| VOO | Double‐blind, randomized, crossover, control trial | VOO (25 ml/day) reduced LDL/HDL particles, HDL cholesterol/HDL‐P ratios, small HDL/large HDL and LP‐IR to the 33 hypercholesterolemic individuals | Ferna´ndez‐Castillejo et al. (2016) | |
| OLP | human | OLP enrich extract (136 mg oleuropein; 6 mg hydroxytyrosol) for 6 weeks significantly reduced the blood lipid profile in prehypertensive male (60n, aged: 45 years). | Lockyer, Rowland, Spencer, Yaqoob, and Stonehouse (2017) | |
| OL | A double‐blind, randomized, controlled, longitudinal | OL extract (1.200 mg/day for 28 days) decreased total cholesterol, LDL cholesterol, total cholesterol/HDL cholesterol ratio, oxidized LDL, and GGT in Granada, Spain 39 hypercholesterolemic subjects (aged 45.0 ± 8.8 years) | Fonolla, Diaz‐Ropero, de la Fuente, & Quintela, (2010) | |
| Inflammation | OOP (olive oil polyphenols) | OOP reduced an eicosanoid inflammatory mediators derived from arachidonic acid (thromboxane B2 and 6‐keto‐prostaglandin F1a) and other inflammatory markers, high‐sensibility C‐reactive protein or IL−6 | Bogani et al. (2007) and Fito et al. (2008) | |
| OL | In vitro | Andalusian OL extract inhibited pro‐inflammatory mediator NO in LPS stimulated RAW264.7 cells | Talhaoui et al. (2016) | |
| EVOO polyphenols | Hydroxytyrosol and tyrosol inhibited MAPK phosphorylation, ROS production and reduced cytokine secretion induced by the oxysterols in PBMCs | Serra et al. (2017) | ||
| OLP (olive leaves polyphenols | double‐blind, randomized, crossover study | OLP (10 mg HT, 51 mg oleuropein) for 4 weeks modulated IL−8 production in 18 healthy volunteers (9 female, 9 male). | Lockyer et al. (2017) | |
| EVOO | EVOO exhibited inflammatory activity by decreasing NO, ROS production, modulate COX−2, iNOS, and mPGES−1 protein expressions, reduced MAPK phosphorylation and prevented nuclear NFkB translocation in LPS‐stimulated murine macrophages. | C´ardeno et al. (2014) | ||
| OO | Meta‐analysis and systematic review | OO (1–50 mg) decreased C‐reactive protein and interleukin−6 | Schwingshackl et al. (2015) | |
| OLE polyphenols | Double‐blind randomized crossover | OLE polyphenols (6 mg hydroxytyrosol, 136 mg oleuropein) for 6 weeks reduced the interleukin−8 in 60 experimental participants. | Lockyer et al. (2017) | |
| Antihypertensive | OO and VOO | Oleic acid made structure alternations in membrane lipid (HII phase propensity) in that way to handle G protein‐mediated signaling which regulated phospholipase C and adenylyl cyclase, results decreased in BP | Tere´s et al. (2008) | |
| OO | Oleanolic acid decreased smooth muscle cell Ca by NO release from dependent endothelium and results relaxation. | Rodriguez‐Rodriguez et al. (2008) | ||
| OO | In vivo | OO improved endothelial function in SMRA in SHR rats by modulating an agonist‐mediated EDHF/NO response, which resulted repair dysfunctional endothelium with hypertension | Rodriguez‐Rodriguez et al. (2009) | |
| OLE polyphenols | Double‐blind randomized crossover | OLE polyphenols (6 mg hydroxytyrosol, 136 mg oleuropein) for 6 weeks reduced BP without the alternation in inflammation, glucose metabolism and vascular function biomarkers in 60 experimental participants. | Lockyer et al. (2017) | |
| EVOO | Randomized, single‐blind placebo control | Replacing EVOO in American diet reduced BP after 3‐month consumption of EVOO in old overweight/obese (age > 65) participant (41n). | Rozati et al. (2015) | |
| OO | Polyphenols in OO stimulated NO level which results reduction of BP. | Ferrara et al. (2000) | ||
| OL | In vivo | Oleuropein reduced SBP in male SHR Sprague Dawley rats | Ghibu et al. (2015) | |
| OL | OL extract EFLA®943 (500−1,000 mg) tablets to hypertensive monozygotic twins (40n, age: 16–60), significant reduction in BP was observed after 8 weeks | Perrinjaquet‐Moccetti et al. (2008) | ||
| OL | Human | OL extract EFLA®943 (500−1,000 mg twice daily) tablets and Captopril (12.5 mg twice daily) to stage−1 decreased hypertension in patients (aged 25–60 year) after 8 weeks. | Susalit et al. (2011) | |
| OO | Human | A decrease in BP was observed in stage 1 essential hypertension in young women (24n) when they consumed polyphenol enriched in OO (34 mg/day) | Moreno‐Luna et al. (2012) | |
| pomace olive oil | In vivo | OPO (10 mg/kg/day bw) to the spontaneously hypertensive SHR rats for 8 weeks modulated endothelial NO | Valero‐Mun˜ oz et al. (2014) | |
| OO | Meta‐analysis and systematic review | OO consumption (1–50 mg) increased flow‐mediated dilatation and effective for the endothelial function | (Schwingshackl et al., 2015) | |
| OL | In vivo | OL extract (30 mg/kg/day bw 5 weeks) to SHR rat reduced SBP by modulating the pro‐oxidative and pro‐inflammatory status and improved vascular function. | Romero et al. (2016) | |
| Antioxidant | OO | Human | OO (2.7 to 216 mg/kg) for 14 to 21 days protected from oxidative stress in healthy male participants. | Covas, Nyyssönen, et al. (2006) |
| EVOO | Human | EVOO (147 to 592 mg/kg) for 8 weeks prevented from DNA damage in 10 healthy postmenopausal women Florence, Italy | Salvini et al. (2006) | |
| EVOO | Human | EVOO significantly increased antioxidant enzyme activity CAT, GPX, SOD, decrease in CAT and increase in SOD gene expression was observed | Oliveras‐López et al. (2014) | |
| EVOO | In vivo | EVOO (330 µl/BW), its lipophilic (3 ml/BW) and hydrophilic (3 ml/BW) fraction for 21 days protected oxidative stress by increasing CAT, GPX, SOD, GSH, NPSH, vitamin C level and decreased plasma LDH, CK, MDA and AOPP level in cardiotoxic rats | Ghorbel et al. (2015) | |
| Olive leaves (OL). | Cohort, pigs | OL extract 50–100 g/kg bw for 8 weeks to the pigs significantly protected RBCs hemolysis from AAPH or H2O2 initiators in dose‐dependent manner | Paiva‐Martins et al. (2014) | |
| olive cake, added with thyme | In vivo | Olive cake, added with thyme extract significantly influenced the plasma and erythrocyte antioxidant status in rat dose‐dependent and time‐dependent manner by inhibiting (DPPH and FRAP), decreased (SOD and GPx) and increased (CAT) level in rats. | Rubió et al. (2014) | |
| VOO | In vitro | VOO protected from RBCs hemolysis from AAPH or H2O2 initiators in time (2−4 hr) and dose‐dependent manner (10–80 μM). | Paiva‐Martins et al. (2015) | |
| EVOO | In vitro | Bioaccessible fractions of EVOO protected from oxidative stress induced by t‐BOOH. | Borges et al. (2015) | |
| EVOO | In vitro | EVOO increased GSH levels and nonsignificant effects on ROS level. | Kouka et al. (2017) | |
| VOO | In vitro | VOO showed strong antioxidant activity in ABTS, DPPH, ORAC assays and decreased ROS level in Caco−2 cells. | Quintero‐Florez et al. (2017) |
Abbreviations: 5‐ LOX, 5‐lipoxygenase; AAPH, 2,2'‐Azobis (2‐amidinopropane) dihydrochloride, ABTS, 2,2'‐azino‐bis (3‐ethylbenzothiazoline‐6‐sulfonic acid) DPPH, 2,2‐diphenyl‐1‐picrylhydrazyl, ADP, Adenine diphosphate; AOPP Advanced oxidation protein products, BP, Blood pressure; cAMP‐PDE, Cyclic adenosine monophosphate‐phosphodiesterase; CAT, Catalase; CK, Creatine kinase, COX, Cyclooxygenase; EDHF, Endothelium‐derived hyperpolarizing factor; EVOO, Extra virgin olive oil; FRAP, Fluorescence recovery after photobleaching; GGT, Gamma‐glutamyltransferase; GPx, Glutathione peroxidase, GSH, Glutathione, H2O2, Hydrogen peroxide, HDL, High‐density lipoprotein; IL‐6, Interleukin‐6; iNOS, Inducible nitric oxide synthase; LDH, Lactate dehydrogenase, LDL, Low‐density lipoprotein; LP‐IR, Lipoprotein insulin resistance index; LPS, Lipopolysaccharide; MAPK, Mitogen‐activated protein kinase; MDA, Malondialdehyde, mPGES‐ 1, Microsomal prostaglandin E synthase‐1; NFkB, Nuclear factor kappa‐light‐chain‐enhancer of activated B cells; NO, Nitric oxide; NPSH, Nonprotein thiols, OL, Olive leaves; OLP, Olive leave polyphenols; OO, Olive oil; OOP, Olive oil polyphenols; OP, Olive polyphenols; OPO, Olive pomace oil; ORAC, Oxygen radical absorbance capacity, PBMCs, Peripheral blood mononuclear cells; RBCs, Red blood cells, ROS, Reactive oxygen species; SBP, Systolic blood pressure; SHR, Spontaneously hypertensive; SMRA, Small mesenteric resistance arteries; SOD, Superoxide dismutase, t‐BOOH, t‐butyl hydroperoxide, VOO, Virgin olive oil.
FIGURE 1.
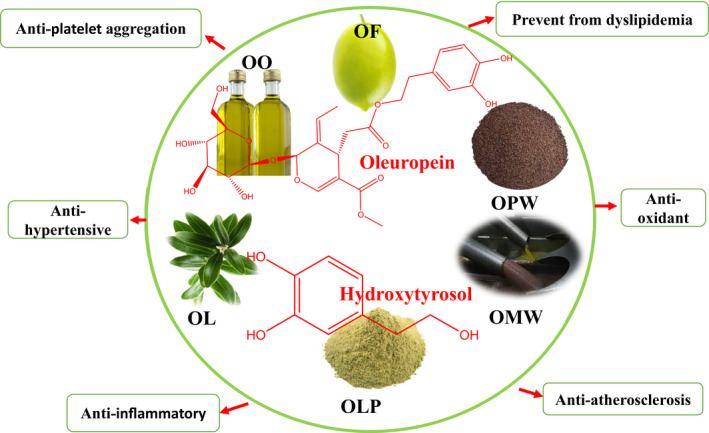
Effects of olive polyphenols on CVDs
5. OP and OXIDATIVE STRESS
The cells in the cardiovascular system contentiously generate reactive oxygen species (ROS) that serve as signaling molecules and also alter or damage the lipids, protein, and DNA, even impair the vascular function and structure (Leopold & Loscalzo, 2009; Lubos, Loscalzo, & Handy, 2011). These ROS are a fundamental part of maintaining cellular homeostatic and work in the Fenton environment, which is balanced by antioxidants (extra‐ or intracellular). It is established that when the ROS level is more than the cellular antioxidant capacity, it produces oxidative stress. At the moment when this ROS level increases, the various kinds of antioxidant system control the ROS level into the cell. These enzymatic and nonenzymatic antioxidants, such as glutathione peroxidases, catalase, superoxide dismutases (reduce the lipid hydroperoxides to lipid hydroxides), ascorbic acid, β‐carotene, α‐tocopherol, and reduced glutathione, play an important role to minimize the oxidative stress. Furthermore, this oxidative stress facilitates atherosclerosis, thrombus formation (oxidizes lipids), and endothelial dysfunction (immune responses and activates inflammation) and eventually results in atherosclerotic plaque formation under several complex mechanisms (Leopold & Loscalzo, 2009; Lubos et al., 2011). The bioactive compounds in virgin olive oil (VOO) alter the postprandial hemostatic profile and protect from oxidative stress (Ruano et al., 2007). A number of studies confirmed that various parts of olive such as OO (Covas, Nyyssönen, et al., 2006), EVOO (Borges, Cabrera‐Viqueb, & Seiquer, 2015; Kouka et al., 2017), VOO (Covas, Nyyssönen, et al., 2006; Quintero‐Florez et al., 2017), leaves (Paiva‐Martins et al., 2014), cake (Rubió et al., 2014), wastewater, and pomace oil have strong antioxidant activity due to the presence of tangible amount of biologically active compounds. Around 50% of biologically active compounds present in olive are hydroxytyrosol and their derivatives as described in Table 2 (Gonzalez‐Santiago, Fonolla, & Lopez‐Huertas, 2010; Raederstorff, 2009). It has been observed that these bioactive compounds are well absorbed even at low concentration (25 ml, 22 g olive oil) in the gut and transferred to bloodstream and protect from ROS formation (Covas, Nyyssönen, et al., 2006; Covas, Ruiz‐Gutiérrez, et al., 2006; Gonzalez‐Santiago et al., 2010; Weinbrenner, Fitó, et al., 2004). However, an earlier study conducted by Vissers, Zock, and Katan (2004) reported that OO did not take a large part in the oxidation process. But, the results of Covas, Nyyssönen, et al. (2006) did not support the previous finding of Vissers et al. (2004). They reported that daily consumption of OO about 2.7–216 mg/kg for 14–21 days prevented healthy male participant from oxidative stress. In another in vivo study, Weinbrenner, Fitó, et al. (2004) documented that OO prevents DNA oxidation. Daily consumption of EVOO (about 147–592 mg/kg) for 8 weeks also prevented DNA damage in 10 healthy postmenopausal women from Florence, Italy (Salvini et al., 2006). One more study by Oliveras‐López, Berná, Jurado‐Ruiz, de la Serrana, and Martín (2014) observed that daily consumption of EVOO polyphenolic‐rich extract significantly improves the plasma antioxidant status in healthy adults. EVOO was daily given (15 ml/day) to healthy adults (45n) for 30 days, and their plasma antioxidant activity (CAT, GPX, SOD) was evaluated. The results revealed that EVOO significantly escalated antioxidant enzyme activity of CAT (42 ± 6–83 ± 13), GPX (90 ± 5–112 ± 6), and SOD (809 ± 191 to 588 ± 146), whereas a decrease in CAT and increase in SOD gene expression were observed without altering other metabolic parameters. Ghorbel et al. (2015) figured out that administration of EVOO (330 µl/BW), and its lipophilic (3 ml/BW) and hydrophilic (3 ml/BW) fractions for 21 days protected oxidative stress by increasing CAT, GPX, SOD, GSH, NPSH, and vitamin C level and decreasing plasma LDH, CK, MDA, and AOPP level in cardiotoxic rats (aluminum trichloride 50 mg//kg bw and acrylamide 20 mg/kg bw). The results of cohort animal studies on pigs also confirmed the strong antioxidant activity of olive leaves (OL). OL extract 50–100 g/kg bw was given to the pigs for 8 weeks. The results demonstrated that OL extracts significantly protected RBCs hemolysis from AAPH or H2O2 initiators in a dose‐dependent manner (Paiva‐Martins et al., 2014). Additionally, in vivo research conducted by Rubió et al. (2014) reported that olive cake (OC), added with thyme extract, significantly influenced the plasma and erythrocyte antioxidant status in rat. OC, thyme, and their combination (1.5 g/kg bw) were intragastrically gavaged to the rats and elucidated the antioxidant activity of plasma (DPPH and FRAP) and erythrocyte (SOD, CAT, and GPx) assays. The outcomes from that report illustrated that individual and combined extract had a significant effect on plasma and erythrocyte, and antioxidant activity dose depended on time and the manner by inhibiting DPPH and FRAP, decreasing SOD and GPx, and increasing CAT level in rats. Paiva‐Martins et al. (2015) also confirmed that active compounds, that is, 3, 4‐dihydroxyphenylethanolelenolic acid dialdehyde (3, 4‐DHPEA‐EDA) and 3, 4‐dihydroxy phenyl ethanol‐elenolic acid (3, 4‐DHPEA‐EA), in VOO protect from oxidative stress. These compounds also protect from RBC hemolysis and from AAPH or H2O2 initiators in time‐dependent (2–4 hr) and dose‐dependent (10–80 μM) manner.
The bioaccessible fractions of six Spanish monovarietal EVOO (Arbequina, Cornicabra, Hojiblanca, Manzanilla, Picual, and Picudo) possessed strong antioxidant activity in vitro (ABTS, DPPH, and FRAP) and Caco‐2 cells (Borges et al., 2015). Furthermore, bioaccessible fractions of all varieties except Picudo also protect from oxidative stress induced by t‐BOOH. Recently, two studies also supported the previous findings. Kouka et al. (2017) isolated major active compounds in EVOO and investigated antioxidant activity by in vitro assay. The results revealed that EVOO polyphenolic compounds show strong antioxidant activity by increasing GSH levels and have significant effects on the ROS level. However, antioxidant activity was more in hydroxytyrosol as compared to a polyphenolic fraction of EVOO. In another study, Quintero‐Florez et al. (2017) investigated that olive cultivars (Chetoui, Blanqueta, Habichuelero, and Picual) showed strong antioxidant activity in ABTS, DPPH, and ORAC assays. Furthermore, all cultivars of VOO bioaccessible fractions facilitated decreased ROS levels in Caco‐2 cells.
6. OP AND HYPERTENSION
Hypertension, which is also known as high blood pressure (HBP), is one of the main risk factors for cardiovascular stroke and myocardial infarction. Hypertension makes structural alternation in the arterial walls of the heart, brain, and kidney. The endothelial dysfunction (decrease in endothelium nitric oxide synthase expression) due to less availability of functional components, oxidative stress (increase in ROS level), inflammation (increase in cytokines production, etc.), and vascular remodeling are the key factors that lead to hypertension. The prevalence of HBP is increasing day by day, and almost one billion people are suffering from this silent killer disease. Therefore, medicines such as angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, thiazide‐type diuretics, and calcium channel blockers are used to balance the HBP but all caused numerous adverse effects and are out of range due to the expensive cost. Plant‐based medicines are considerable alternative to overcome the cost and less side effect (Armstrong & Joint National Committee, 2014; Turnbull, 2003).
The prophylactic properties of olive (OO, VOO, EVOO, OL, pomace, etc.) are directly associated with the high amount of biologically active compounds such as polyphenols and MUFAs. Recently numerous researchers reported outstrip benefits of consumption of OP toward handling blood pressure (BP), endothelial function in the initial level of hypertension (Ghibu et al., 2015; Lockyer, et al., 2017; Romero et al., 2016; Schwingshackl, Christoph, & Hoffmann, 2015; Valero‐Muñoz et al., 2014). OO and VOO contain around 70%–80% oleic acid, which plays a significant role in the management of BP (Rodriguez‐Rodriguez, Herrera, de Sotomayor, & Ruiz‐Gutierrez, 2007). OO and VOO increase the concentration of oleic acid in the membrane, which makes structure alternations in membrane lipid (HII phase propensity) in a way to handle G protein‐mediated signaling, which regulates phospholipase C and adenylyl cyclase and results in a decrease in BP (Tere´s et al., 2008). A similar mechanism was reported by Rodriguez‐Rodriguez et al. (2008). They reported that OO components (oleanolic acid) decrease smooth muscle cell Ca by NO release from dependent endothelium and result in relaxation. Furthermore, this NO release takes part in PI3K‐dependent phosphorylation of Akt‐Ser473 followed by phosphorylation of eNOS at Ser1177. In another study, they found that OO improved endothelial function in small mesenteric resistance arteries (SMRA) in spontaneously hypertensive (SHR) rats by modulating an agonist‐mediated EDHF/NO response, which resulted from repair dysfunctional endothelium with hypertension (Rodriguez‐Rodriguez, Herrera, de Sotomayor, & Ruiz‐Gutierrez, 2009). A double‐blind randomized crossover study conducted by Lockyer et al. (2017) reported that consumption of OLE polyphenols (6 mg hydroxytyrosol, 136 mg OL) for 6 weeks reduced BP without the alternation in inflammation, glucose metabolism, and vascular function biomarkers in 60 experimental participants. In another randomized, single‐blinded placebo control study, Rozati et al. (2015) observed that adding EVOO in the American diet (corn, soybean oil, and butter) reduced the BP after 3‐month consumption of EVOO in old overweight/obese (age > 65) contributor (41n). Earlier, Ferrara et al. (2000) also reported that daily consumption of OO for 6 months reduced the BP significantly in (23n) participants present in the study trial.
Oleuropein is a key biphenol compound present in OL reported to have a strong antihypertensive effect. Oleuropein reduced systolic blood pressure (SBP) in male SHR Sprague Dawley rats (Ghibu et al., 2015). The supplementation of OL extract EFLA®943 (500–1,000 mg) tablets to hypertensive monozygotic twins (40n, age: 16–60) showed significant reduction in BP after 8 weeks (Perrinjaquet‐Moccetti et al., 2008). In another comparative study conducted by Susalit et al. (2011), the authors also found similar effects of OL extract EFLA®943 (500–1,000 mg twice daily) tablets and captopril (12.5 mg twice daily) to stage 1 hypertension patients (aged 25–60 year) after 8 weeks. A decrease in BP was also observed in stage 1 essential hypertension young women (24n), when they consumed OO (34 mg/day) (Moreno‐Luna et al., 2012). In another study, oral administration of pomace olive oil (POO) (10 mg/kg/day bw) to the SHR for 8 weeks showed antihypertensive effects by modulating endothelial NO (Valero‐Muñoz et al., 2014). Earlier, Rodriguez‐Rodriguez et al. (2007) also figured out that administration (800 ppm, 12 weeks) of POO to SHR rats enhanced eNOS expression and improved endothelial dysfunction. Administration of triterpenic components (oleanolic acid, erythrodiol, maslinic acid, and uvaol) present in POO to SHR rats enhanced eNOS expression and improved the endothelial dysfunction (Rodriguez‐Rodriguez, Perona, Herrera, & Ruiz‐Gutierrez, 2006). A meta‐analysis and systematic review reported that OO consumption (1–50 mg) increased flow‐mediated dilatation and is very effective for the endothelial function (Schwingshackl et al., 2015). Recent study conducted by Romero et al. (2016) also claimed that oral administration of OL extract (30 mg/kg/day bw 5 weeks) to SHR reduces SBP by modulating the pro‐oxidative and pro‐inflammatory status and improves vascular function.
7. OP AND INFLAMMATION
Inflammation is responsible for the development of many diseases including CVDs, because release of pro‐inflammatory cytokines such as tumor necrosis factor and interleukin‐1 (IL‐1) in vascular wall directly affects endothelial function by modulating the adhesion molecules and results in endothelial dysfunction, which is a predictor of atherosclerosis and CVDs (Cook‐Mills, Marchese, & Abdala‐Valencia, 2011; Inaba, Chen, & Bergmann, 2010; Roos & Sperber, 1997) . Inflammation and oxidative stress are entangling processes. The bioactive compounds present in OO, EVOO, and by‐products protect from inflammation and endothelial activation (Aparicio‐Soto, Sanchez‐Hidalgo, Rosillo, Castejón, & Alarcon‐de‐la‐Lastra, 2016; Schwingshackl et al., 2015). Regular consumption of OO, butter, or walnut has important effects on the plasma level of pro‐inflammatory cytokines (Jiménez‐Gómez et al., 2009). OOP reduces the eicosanoid inflammatory mediators derived from arachidonic acids, such as thromboxane B2 and 6‐keto‐prostaglandin F1a and other inflammatory markers, such as high‐sensibility C‐reactive protein (hs‐CRP) or IL‐6 (Bogani, Galli, Villa, & Visioli, 2007; Fito et al., 2008). Contradictory results have been obtained concerning the effects of OOP on cell adhesion molecules. A decrease in ICAM‐1 and vascular cell adhesion molecule‐1 serum levels at postprandial state after VOO when compared to refined OO ingestion has been reported (Pacheco et al., 2007). However, no differences in ICAM‐1 levels were reported after sustained virgin or refined OO consumption (Fito et al., 2008). In vitro study conducted by Talhaoui et al. (2016) observed that Andalusian OL extract inhibited pro‐inflammatory mediator NO in LPS‐stimulated RAW264.7 cells and showed anti‐inflammatory properties. EVOO polyphenols (hydroxytyrosol and tyrosol) inhibited mitogen‐activated protein kinase (MAPK) phosphorylation, ROS production, and reduced cytokine secretion induced by the oxysterols in peripheral blood mononuclear cells (Serra, Deiana, Spencer, & Corona, 2017). A double‐blind, randomized, crossover study conducted by Lockyer, Corona, Yaqoob, Spencer, and Rowland (2015) reported that consumption of OLP (olive leaves polyphenols) (10 mg HT, 51 mg OL) for 4 weeks modulated the IL‐8 production in 18 healthy volunteers (nine females and nine males). In another study, Cárdeno, (2014) claimed that EVOO exhibited inflammatory activity by decreasing NO and ROS production. Furthermore, EVOO also modulated COX‐2, iNOS, and mPGES‐1 protein expressions, reduced MAPK phosphorylation, and prevented the nuclear NFkB translocation in LPS‐stimulated murine macrophages. A recent meta‐analysis and systematic review figured out that OO consumption (1–50 mg) decreased in C‐reactive protein and interleukin‐6 (Schwingshackl et al., 2015). A double‐blind randomized crossover study conducted by Lockyer et al. (2017) observed that consumption of olive polyphenols (6 mg hydroxytyrosol, 136 mg OL) for 6 weeks reduced the interleukin‐8 in 60 experimental members.
8. OP AND HYPERLIPIDEMIA
Hyperlipidemia is another CVDs referring to an elevated level of fasting and total cholesterol level in the blood. Postprandial lipemia and hyperglycemia are the key risk factors for causing atherosclerosis and other CVDs (Roche & Gibney, 2000). Types and concentration of fat intake also influenced postprandial lipemia. It is reported that the daily intake of OO (25 ml) did not promote postprandial lipemia (Weinbrenner, Fitó, et al., 2004). Because, OO is rich in MUFA, which decreases LDL level and increases HDL. Numerous, in vitro, animal, human, and meta‐analysis reported that OO and its components are helpful in lowering blood lipid profile (Ferna´ndez‐Castillejo et al., 2016; Filip et al., 2015; Fonolla et al., 2010; Hernáez et al., 2014; López, 2008; Lockyer et al. 2017). A double‐blind, randomized, placebo‐controlled study revealed that 12‐month consumption of OP‐enriched extract (250–1,000 mg) by the osteopenic female patients (64n, age: 49–68 years) significantly reduced total and LDL cholesterol level (Filip et al., 2015). Another randomized, crossover, controlled study also elucidated that consumption of OP significantly increased the HDL level in 47 healthy European male volunteers (Hernáez et al., 2014). A recently double‐blind, randomized, crossover, controlled trial also claimed that consumption of VOO (25 ml/day) reduced LDL/HDL particles, HDL cholesterol/HDL‐P ratios, small HDL/large HDL, and lipoprotein insulin resistance index (LP‐IR) in 33 hypercholesterolemic individuals (Fernández‐Castillejo et al., 2016). Lockyer et al. (2017) observed that consumption of OLP‐enriched extract (136 mg OL; 6 mg hydroxytyrosol) for 6 weeks significantly eliminated the blood lipid profile in prehypertensive man (60n, aged: 45 years). Earlier, a double‐blind, randomized, controlled, longitudinal study reported that consumption of OL extract (1,200 mg/day for 28 days) also eroded total cholesterol (−7%), LDL cholesterol (−12%), total cholesterol/HDL cholesterol ratio (8%), oxidized LDL (−13%), and gamma‐ GT (−28%) in Granada, Spain hypercholesterolemic subjects (39n, aged 45.0 ± 8.8 years) (Fonolla et al., 2010).
9. OP AND PLATELET AGGREGATION
During the early stage of endothelial disruption, platelets are the first responders of vascular injury and amplify inflammatory process resultant atherosclerotic disease (Nording, Seizer, & Langer, 2015; Rondina, Weyrich, & Zimmerman, 2013). Several facilitator components are responsible for platelet aggregation such as platelet 12‐LOX‐derived 12‐HETE, COX‐derived TXB2, and 15‐LOX‐derived 15‐HETE (Tourdot, Ahmed, & Holinstat, 2013). To prevent platelet aggregation, it is necessary to limit the platelet activation via inhibiting phosphodiesterase, platelet–platelet interactions through glycoprotein IIb/IIIa, cyclooxygenase (COX), and adenosine diphosphate (ADP) receptors (Yousuf & Bhatt, 2011). OO, VOO, EVOO, and their bioactive compounds prevent the platelet aggregation by various pathways. Hydroxytyrosol and OL are potent antiplatelet compounds that are widely distributed in OO inhibit collagen‐induced platelet activation or ADP (Petroni et al., 1995), whereas oleocanthal and oleacein possess antiplatelet activity by inhibiting COX and a 5‐LOX inhibitor (Beauchamp et al., 2005; Vougogiannopoulou et al., 2014). Earlier, an in vitro study conducted by Singh, Mok, Christensen, Turner, and Hawley (2008) observed that OLP inhibited the platelet function in blood taken from 11 healthy males. In another study, OOP inhibited platelet aggregation by cAMP‐PDE inhibition (Dell’Agli et al., 2008). A recently randomized crossover study conducted by Agrawal et al. (2017) reported that weekly consumption of EVOO (40 ml), which is rich in oleocanthal, resulted in the stoppage of the platelet aggregation.
10. OP AND ATHEROSCLEROSIS
Atherosclerosis is a systemic lipid‐driven inflammatory condition associated with endothelial dysfunction that results in accumulation and subsequent oxidation of lipids in the vessel wall or plaque development. These abnormalities trigger inflammatory cell infiltration and macrophage foam cell formation, leading to apoptosis and secondary necrosis and plaque advancement (Tabas, 2010). Montserrat‐de la Paz et al. (2016) reported that diets enrich with OO‐protected atherosclerosis as compared to saturated fatty acid‐rich diet in niacin‐treated mice. Another in vivo study conducted by Claro, Ogalla, Rodriguez‐Rodriguez, Herrera, and de Sotomayor (2015) also claimed that EVOO polyphenols lowered the protein expression and macrophage accumulation of iNOS, VCAM‐1, ICAM‐1, NFκB, TNF‐α, and superoxide anion production. Furthermore, EVOO polyphenols improved endothelial function and lowered lipid accumulation within the atherosclerotic lesion of Apo E‐deficient mice. A randomized, double‐blind, crossover, controlled trial, conducted by Fernández‐Castillejo et al. (2016), reported that incorporation of thyme into VOO improved lipoprotein particle atherogenic ratios in 33 hypercholesterolemic individuals after 3 weeks. In the recent past, the research work of Valls et al. (2017) also stated that consumption of thyme and VOO 25 ml/day for three weeks improved endothelial function in 12 healthy subjects. Administration of squalene to atherosclerotic rabbits reversed endothelial activation and lowered cellularity in gingival mucosa (Bullon et al., 2009). In rabbits, administration of hydroxytyrosol at 4 mg/kg body weight in the presence of saturated fat and cholesterol reduced the size of atherosclerotic lesions when compared to animals receiving this diet without hydroxytyrosol (Gonzalez‐Santiago, Martin‐Bautista, Carrero, & Fonolla, 2006). Similarly, many earlier studies reported that consumption of OO, VOO, EVOO, and their bioactive compounds prevented from atherosclerosis (Buus et al., 2011; Graham et al., 2012; Lou‐Bonafonte, Arnal, Navarro, & Osada., 2012; Murie‐Fernandez et al., 2011; Zrelli, Matsuoka, Kitazaki, Zarrouk, & Miyazaki, 2011).
11. SAFETY OF OP
Olive and its components do not possess toxicity to humans. An animal model study conducted by Lee‐Huang, Zhang, and Huang (2003) reported that an administration of 1 g/kg bw for 7 days did not cause toxicity to the rats. In another in vitro human cell toxicity study, it was also confirmed that OL extract (1 mg/ml) was not toxic to human cell lines (Petkov & Manolov, 1972). Other studies conducted by various researchers reported that OO and its components were not toxic. OO polyphenols enriched with hydroxytyrosol is safe at the limit of 20 mg/kg daily (Christian et al., 2004; Soni, Burdock, Christian, & Botler, 2006).
12. CONCLUSION
Over the past few decades, the use of olive oil and its products in everyday life has a long history of nutrition and therapeutical actions. A large number of study results confirmed that OO and its metabolites are very beneficial for the handling of CVDs through several mechanisms and provide some important nutrimental bioactive compounds besides energy and fat‐soluble vitamins. In the recent past, there was a growing trend to conduct a lot of research work on olive oil polyphenols and results found that olive oil polyphenols increase the HDL level, prevent from oxidative stress, reduce thrombogenic, endothelial dysfunction, BP, and inflammation, and alter gene expression responsible for atherosclerosis process. Furthermore, olive oil still needs clinical efficacy to elucidate the understanding of molecular mechanisms. From this point of view, the ingestion of olive oil and its products needs to be recommended not only due to its beneficial fatty acid profile but also in order to gain advantages from its very important bioactive components that have constructive effects on human health.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This article is for conveying up‐to‐date information regarding olive and its cardioprotective role. There were no experiments conducted in this paper because this is the review article. All the data used in this study are properly cited.
Mehmood A, Usman M, Patil P, Zhao L, Wang C. A review on management of cardiovascular diseases by olive polyphenols. Food Sci Nutr. 2020;8:4639–4655. 10.1002/fsn3.1668
Contributor Information
Arshad Mehmood, Email: arshadfst@yahoo.com.
Lei Zhao, Email: zhaolei@th.btbu.edu.cn.
Chengtao Wang, Email: wctbtbu@hotmail.com.
REFERENCES
- Aggoun, M. , Arhab, R. , Cornu, A. , Portelli, J. , Barkat, M. , & Graulet, B. (2016). Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food chemistry, 209, 72‐80. [DOI] [PubMed] [Google Scholar]
- Agrawal, K. , Melliou, E. , Li, X. , Pedersen, T. L. , Wang, S. C. , Magiatis, P. , … Holt, R. R. (2017). Oleocanthal‐rich extra virgin olive oil demonstrates acute anti‐platelet effects in healthy men in a randomized trial. Journal of Functional Foods, 36, 84–93. 10.1016/j.jff.2017.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aludatt, M. H. , Alli, I. , Ereifej, K. , Alhamad, M. , Al‐Tawaha, A. R. , & Rababah, T. (2010). Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chemistry, 123, 117‐122. [Google Scholar]
- Ambra, R. , Natella, F. , Lucchetti, S. , Forte, V. , & Pastore, G. (2017). α‐Tocopherol, β‐carotene, lutein, squalene and secoiridoids in seven monocultivar Italian extra‐virgin olive oils. International Journal of Food Sciences and Nutrition, 68(5), 538–545. 10.1080/09637486.2016.1265099 [DOI] [PubMed] [Google Scholar]
- Aparicio‐Soto, M. , Sanchez‐Hidalgo, M. , Rosillo, M. A. , Castejón, M. L. , & Alarcon‐de‐la‐Lastra, C. (2016). Extra virgin olive oil: A key functional food for prevention of immune‐inflammatory diseases. Food and Function, 7, 4492–4505. 10.1039/C6FO01094F [DOI] [PubMed] [Google Scholar]
- Armstrong, C. and Joint National Committee , (2014). JNC8 guidelines for the management of hypertension in adults. American family physician, 90(7), 503‐504. [PubMed] [Google Scholar]
- Arslan, D. (2012). Physico‐chemical characteristics of olive fruits of Turkish varieties from the province of Hatay. Grasas y aceites, 63, 158‐166. [Google Scholar]
- Beauchamp, G. K. , Keast, R. S. J. , Morel, D. , Lin, J. , Pika, J. , Han, Q. , … Breslin, P. A. S. (2005). Phytochemistry: Ibuprofen like activity in extra virgin olive oil. Nature, 437, 45–46. 10.1038/437045a [DOI] [PubMed] [Google Scholar]
- Blumenthal, R. S. , Merz, C. N. , Bittner, V. , & Gluckman, T. J. (2008). Task force 10: Training in preventive cardiovascular medicine. Journal of the American College of Cardiology 51, 393–398. [DOI] [PubMed] [Google Scholar]
- Bogani, P. , Galli, C. , Villa, M. , & Visioli, F. (2007). Postprandial anti‐inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis, 190, 181–186. 10.1016/j.atherosclerosis.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Borges, T. H. , Alberto, J. , Cabrera‐Vique, C. , Lara, L. , Oliveira, A. F. , & Seiquer, I. (2017). Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chemistry, 215, 454–462. 10.1016/j.foodchem.2016.07.162 [DOI] [PubMed] [Google Scholar]
- Borges, T. H. , Cabrera‐Viqueb, C. , & Seiquer, I. (2015). Antioxidant properties of chemical extracts and bioaccessible fractions obtained from six Spanish monovarietal extra virgin olive oils: Assays in Caco‐2 cells. Food and Function, 6, 2375–2383. 10.1039/C5FO00529A [DOI] [PubMed] [Google Scholar]
- Borges, T. H. , Carlos, L. C. , Alberto, J. A. , Vique‐Cabrera, C. , & Seiquer, I. (2017). Comparative analysis of minor bioactive constituents (CoQ10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. Journal of Food Composition and Analysis, 63, 47–54. 10.1016/j.jfca.2017.07.036 [DOI] [Google Scholar]
- Brahim, S. B. , Kelebek, H. , Ammar, S. , Abichou, M. , & Bouaziz, M. (2017). LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food chemistry, 229, 9‐19. [DOI] [PubMed] [Google Scholar]
- Buckland, G. , & Gonzalez, C. A. (2015). The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. British Journal of Nutrition, 113(Suppl. 2), S94–S101. [DOI] [PubMed] [Google Scholar]
- Bullon, P. , Quiles, J. L. , Morillo, J. M. , Rubini, C. , Goteri, G. , Granados‐Principal, S. , … Ramirez‐Tortosa, M. C. (2009). Gingival vascular damage in atherosclerotic rabbits: Hydroxytyrosol and squalene benefits. Food and Chemical Toxicology, 47, 2327–2331. 10.1016/j.fct.2009.06.026 [DOI] [PubMed] [Google Scholar]
- Buus, N. H. , Hansson, N. C. , Rodriguez‐Rodriguez, R. , Stankevicius, E. , Andersen, M. R. , & Simonsen, U. (2011). Antiatherogenic effects of oleanolic acid in apolipoprotein E knockout mice. European Journal of Pharmacology, 670, 519–526. 10.1016/j.ejphar.2011.09.037 [DOI] [PubMed] [Google Scholar]
- Cárdeno, A. , Sánchez‐Hidalgo, M. , Aparicio‐Soto, M. , Sánchez‐Fidalgo, S. , & Alarcón‐de‐la‐Lastra, C. (2014). Extra virgin olive oil polyphenolic extracts downregulate inflammatory responses in LPS‐activated murine peritoneal macrophages suppressing NFkB and MAPK signalling pathways. Food and Function, 5, 1270–1277. [DOI] [PubMed] [Google Scholar]
- Christian, M. S. , Sharper, V. A. , Hoberman, A. M. , Seng, J. E. , Fu, L. J. , Covell, D. , … Crea, R. (2004). The toxicity profile of hydrolyzed aqueous olive pulp extract. Pharmacology and Toxicology, 27, 309–330. 10.1081/DCT-200039714 [DOI] [PubMed] [Google Scholar]
- Cioffi, G. , Pesca, M. S. , De Caprariis, P. , Braca, A. , Severino, L. , & De Tommasi, N. (2010). Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chemistry, 121(1), 105‐111. [Google Scholar]
- Claro, C. , Ogalla, E. , Rodriguez‐Rodriguez, R. , Herrera, M. D. , & de Sotomayor, M. A. (2015). Phenolic content of extra virgin olive oil is essential to restore endothelial dysfunction but not to prevent vascular inflammation in atherosclerotic lesions of Apo E deficient mice. Journal of Functional Foods, 15, 126–136. 10.1016/j.jff.2015.03.008 [DOI] [Google Scholar]
- Cook‐Mills, J. M. , Marchese, M. E. , & Abdala‐Valencia, H. (2011). Vascular cell adhesion molecule‐1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxidants and Redox Signaling, 15, 1607–1638. 10.1089/ars.2010.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas, M.‐I. , Nyyssönen, K. , Poulsen, H. E. , Kaikkonen, J. , Zunft, H.‐J. , Kiesewetter, H. , … Marrugat, J. (2006). The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Annals of Internal Medicine, 145, 333–341. 10.7326/0003-4819-145-5-200609050-00006 [DOI] [PubMed] [Google Scholar]
- Covas, M.‐I. , Ruiz‐Gutiérrez, V. , Torre, R. , Kafatos, A. , Lamuela‐Raventós, R. M. , Osada, J. , … Visioli, F. (2006). Minor components of olive oil: Evidence to date of health benefits in humans. Nutrition Reviews, 64, 20–30. 10.1111/j.1753-4887.2006.tb00260.x [DOI] [Google Scholar]
- de la Torre, R. (2008). Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology, 16(5), 245‐247. [DOI] [PubMed] [Google Scholar]
- Dell’Agli, M. , Maschi, O. , Galli, G. V. , Fagnani, R. , Dal Cero, E. , Caruso, D. , & Bosisio, E. (2008). Inhibition of platelet aggregation by olive oil phenols via cAMP‐phosphodiesterase. British Journal of Nutrition, 99, 945–951. 10.1017/S0007114507837470 [DOI] [PubMed] [Google Scholar]
- Dias, M. G. , Olmedilla‐Alonso, B. , Hornero‐Méndez, D. , Mercadante, A. Z. , Osorio, C. , Vargas‐Murga, L. , & Meléndez‐Martínez, A. J. (2018). Comprehensive database of carotenoid contents in Ibero‐American foods. A valuable tool in the context of functional foods and the establishment of recommended intakes of bioactives. Journal of Agricultural and Food Chemistry, 66(20), 5055–5107. [DOI] [PubMed] [Google Scholar]
- Difonzo, G. , Russo, A. , Trani, A. , Paradiso, V. M. , Ranieri, M. , Pasqualone, A. , … Caponio, F. (2017). Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. Journal of Functional Foods, 31, 63–70. [Google Scholar]
- Eggersdorfer, M. , & Wyss, A. (2018). Carotenoids in human nutrition and health. Archives of Biochemistry and Biophysics, 652, 18–26. 10.1016/j.abb.2018.06.001 [DOI] [PubMed] [Google Scholar]
- El‐Abbassi, A. , Kiai, H. , & Hafidi, A. (2012). Phenolic profile and antioxidant activities of olive mill wastewater. Food Chemistry, 132(1), 406‐412. [DOI] [PubMed] [Google Scholar]
- Fernández‐Castillejo, S. , Valls, R.‐M. , Castañer, O. , Rubió, L. , Catalán, Ú. , Pedret, A. , … Solà, R. (2016). Polyphenol rich olive oils improve lipoprotein particle atherogenic ratios and subclasses profile: A randomized, crossover, controlled trial. Molecular Nutrition and Food Research, 60, 1544–1554. 10.1002/mnfr.201501068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara, L. A. , Raimondi, A. S. , Episcopo, L. , Guida, L. , Russo, A. D. , & Marotta, T. (2000). Olive oil and reduced need for antihypertensive medications. Archives of Internal Medicine, 160, 837–842. 10.1001/archinte.160.6.837 [DOI] [PubMed] [Google Scholar]
- Filip, R. , Possemiers, S. , Heyerick, A. , Pinheiro, I. , Raszewski, G. , Davicco, M. J. , & Coxam, V. (2015). Twelve‐month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. Journal of Nutrition, Health and Aging, 19I, 77–86. [DOI] [PubMed] [Google Scholar]
- Fitó, M. , Cladellas, M. , de la Torre, R. , Martí, J. , Muñoz, D. , Schröder, H. , … Covas, M. I. (2008). Anti‐inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. European Journal of Clinical Nutrition, 62, 570–574. 10.1038/sj.ejcn.1602724 [DOI] [PubMed] [Google Scholar]
- Fonolla, J. , Diaz‐Ropero, P. , de la Fuente, E. , & Quintela, J.C. (2010). MS358 One‐month consumption of an olive leaf extract enhances cardiovascular status in hypercholesterolemic subjects. Atherosclerosis Supplements, 11(2), 182. [Google Scholar]
- Franco, M. N. , Galeano‐Díaz, T. , López, Ó. , Fernández‐Bolaños, J. G. , Sánchez, J. , De Miguel, C. , … Martín‐Vertedor, D. (2014). Phenolic compounds and antioxidant capacity of virgin olive oil. Food chemistry, 163, 289‐298. 10.1016/j.foodchem.2014.04.091 [DOI] [PubMed] [Google Scholar]
- Ghibu, S. , Morgovan, C. , Vostinaru, O. , Olah, N. , Mogosan, C. , & Muresan, A. (2015). Diuretic, antihypertensive and antioxidant effect of Olea europaea leaves extract, in rats. Archives of Cardiovascular Diseases, 7, 184. [Google Scholar]
- Ghorbel, I. , Khemakhem, M. , Boudawara, O. , Marrekchi, R. , Jamoussi, K. , Amar, R. B. , … Kamoun, N. G. (2015). Effects of dietary extra virgin olive oil and its fractions on antioxidant status and DNA damage in the heart of rats co‐exposed to aluminum and acrylamide. Food and Function, 6, 3098–3108. 10.1039/C5FO00342C [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Santiago, M. , Fonolla, J. , & Lopez‐Huertas, E. (2010). Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low‐density lipoproteins. Pharmacological Research, 61, 364–370. 10.1016/j.phrs.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Santiago, M. , Martin‐Bautista, E. , Carrero, J. J. , & Fonolla, J. (2006). One‐month administration of hydroxytyrosol, a phenolic antioxidant presents in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis, 188, 35–42. [DOI] [PubMed] [Google Scholar]
- Graham, V. S. , Lawson, C. , Wheeler‐Jones, C. P. D. , Perona, J. S. , Ruiz‐Gutierrez, V. , & Botham, K. M. (2012). Triacylglycerol‐rich lipoproteins derived from healthy donors fed different olive oils modulate cytokine secretion and cyclooxygenase‐2 expression in macrophages: The potential role of oleanolic acid. European Journal of Nutrition, 51, 301–309. 10.1007/s00394-011-0215-2 [DOI] [PubMed] [Google Scholar]
- Hernáez, A. , Fernández‐Castillejo, S. , Farràs, M. , Catalán, Ú. , Subirana, I. , Montes, R. , … Fitó, M. (2014). Olive oil polyphenols enhance high‐density lipoprotein function in humans a randomized controlled trial. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 2115–2119. 10.1161/ATVBAHA.114.303374 [DOI] [PubMed] [Google Scholar]
- Inaba, Y. , Chen, J. A. , & Bergmann, J. A. S. R. (2010). Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: A meta‐analysis. International Journal of Cardiovascular Imaging, 26, 631–640. [DOI] [PubMed] [Google Scholar]
- IOC (2012). International Olive Council. Retrieved from http://www.internationaloliveoil.org/estaticos/view/136‐country‐profiles. [Google Scholar]
- IOC (2014). International Olive Council. Retrieved from http://www.internationaloliveoil.org/estaticos/view/131‐world‐olive‐oil‐figures. [Google Scholar]
- Jiménez‐Gómez, Y. , López‐Miranda, J. , Blanco‐Colio, L. M. , Marín, C. , Pérez‐Martínez, P. , Ruano, J. , … Pérez‐Jiménez, F. (2009). Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis, 204, e70–e76. 10.1016/j.atherosclerosis.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Kouka, P. , Priftis, A. , Stagos, D. , Angelis, A. , Stathopoulos, P. , Xinos, N. , … Kouretas, D. (2017). Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. International Journal of Molecular Medicine, 40, 703–712. 10.3892/ijmm.2017.3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier, J. B. , Bury, D. C. , & Richardson, S. W. (2016). Diet and physical activity for cardiovascular disease prevention. American Family Physician, 93, 919–924. [PubMed] [Google Scholar]
- Lee‐Huang, S. , Zhang, L. , & Huang, P. L. (2003). Anti‐HIV. Activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV‐1 infection and OLE treatment. Biochemical and Biophysical Research Communications, 307, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Leopold, J. A. , & Loscalzo, J. (2009). Oxidative risk for atherothrombotic cardiovascular disease. Free Radical Biology and Medicine, 47, 1673–1706. 10.1016/j.freeradbiomed.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leouifoudi, I. , Zyad, A. , Amechrouq, A. , Oukerrou, M. A. , Mouse, H. A. , & Mbarki, M. (2014). Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Science and Technology, 34(2), 249‐257. [Google Scholar]
- Lockyer, S. , Corona, G. , Yaqoob, P. , Spencer, J. P. E. , & Rowland, I. (2015). Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double‐blind, placebo‐controlled, cross‐over trial. British Journal of Nutrition, 114, 75–83. 10.1017/S0007114515001269 [DOI] [PubMed] [Google Scholar]
- Lockyer, S. , Rowland, I. , Spencer, J. P. E. , Yaqoob, P. , & Stonehouse, W. (2017). Impact of phenolic‐rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomized controlled trial. European Journal of Nutrition, 56, 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo, L. , Grasso, F. , Lanciano, F. , Loria, S. , & Monetti, E. (2018). Broad spectrum health protection of extra virgin olive oil compounds. Studies in Natural Products Chemistry, 57, 41–77. [Google Scholar]
- Longo, V. D. , & Mattson, M. P. (2014). Fasting: Molecular mechanisms and clinical applications. Cell Metabolism 19(2): 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, S. , Bermúdez, B. , Pacheco, Y. M. , Villar, J. , Abia, R. , & Muriana, F. J. (2008). Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: Monounsaturated compared with saturated fatty acids. American Journal of Clinical Nutrition, 88, 638–644. [DOI] [PubMed] [Google Scholar]
- Lou‐Bonafonte, J. M. , Arnal, C. , Navarro, M. A. , & Osada, J. (2012). Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Molecular Nutrition and Food Research, 56, 1043–1057. [DOI] [PubMed] [Google Scholar]
- Lubos, E. , Loscalzo, J. , & Handy, D. (2011). Glutathione peroxidase‐1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxidants and Redox Signaling, 15, 1957–1997. 10.1089/ars.2010.3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos, R. , Martínez‐López, S. , Baeza Arévalo, G. , Amigo‐Benavent, M. , Sarriá, B. , & Bravo‐Clemente, L. (2016). Hydroxytyrosol in functional hydroxytyrosol‐enriched biscuits is highly bioavailable and decreases oxidized low density lipoprotein levels in humans. Food Chemistry, 205, 248–256. [DOI] [PubMed] [Google Scholar]
- Meléndez‐Martínez, A. J. , Pérez‐Gálvez, A. , Roca, M. , Estévez‐Santiago, R. , Olmedilla‐Alonso, B. , Mercadante, A. Z. , & Ornelas‐Paz, J. J. (2017). Biodisponibilidad de carotenoides, factores que la determinan y métodos de estimación In Meléndez‐Martínez A. J. (Ed.), Carotenoides En Agroalimentación Y Salud (pp. 574–608). Ciudad de México, México: Editorial Terracota. [Google Scholar]
- Mendis, S. , Armstrong, T. , Bettcher, D. , Branca, F. , Lauer, J. , Mace, C. , … Tang, K. C. (2015). Report No.: ISBN 978 92 4 156485 4, Contract No.: Document Number. Geneva: World Health Organization; 2014. Global status report on non‐communicable diseases 2014. [Google Scholar]
- Miro Casas, E. , Albadalejo, M. F. , Covas Planells, M. I. , Fitó Colomer, M. , Lamuela Raventós, R. M. , De la Torre, R. , & Fornell, R. (2001). Tyrosol bioavailability in humans after ingestion of virgin olive oil. Clinical Chemistry, 47, 341–343. 10.1093/clinchem/47.2.341 [DOI] [PubMed] [Google Scholar]
- Miro‐Casas, E. , Covas, M. I. , Farre, M. , Fito, M. , Ortuño, J. , Weinbrenner, T. , … de la Torre, R. (2003). Hydroxytyrosol disposition in humans. Clinical chemistry, 49(6), 945‐952. [DOI] [PubMed] [Google Scholar]
- Montserrat‐de la Paz, S. , Naranjo, M. C. , Lopez, S. , Abia, R. , Muriana, F. J. G. , & Bermudez, B. (2016). Olive oil, compared to a saturated dietary fat, has a protective role on atherosclerosis in niacin treated mice with metabolic syndrome. Journal of Functional Foods, 26, 557–564. 10.1016/j.jff.2016.08.028 [DOI] [Google Scholar]
- Moreno‐Luna, R. , Munoz‐Hernandez, R. , Miranda, M. L. , Costa, A. F. , Jimenez‐Jimenez, L. , Vallejo‐Vaz, A. J. , … Stiefel, P. (2012). Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. American Journal of Hypertension, 25, 1299–1304. 10.1038/ajh.2012.128 [DOI] [PubMed] [Google Scholar]
- Mosele, J. I. , Martín‐Peláez, S. , Macià, A. , Farràs, M. , Valls, R. M. , Catalán, Ú. , & Motilva, M. J. (2014). Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Molecular nutrition & food research, 58(9), 1809‐1819. [DOI] [PubMed] [Google Scholar]
- Murie‐Fernandez, M. , Irimia, P. , Toledo, E. , Martínez‐Vila, E. , Buil‐Cosiales, P. , Serrano‐Martínez, M. , … Martínez‐González, M. Á. (2011). Carotid intima‐media thickness changes with Mediterranean diet: A randomized trial (PREDIMED‐Navarra). Atherosclerosis, 219, 158–162. 10.1016/j.atherosclerosis.2011.06.050 [DOI] [PubMed] [Google Scholar]
- Nording, H. M. , Seizer, P. , & Langer, H. F. (2015). Platelets in inflammation and atherogenesis. Frontiers in Immunology, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obied, H. K. , Prenzler, P. D. , Omar, S. H. , Ismael, R. , Servili, M. , Esposto, S. , … Urbani, S. (2012). Pharmacology of olive biophenols In Fishbein J. C. (Ed.). Advances in molecular toxicology (Vol. 6, pp. 195–242). Amsterdam, The Netherlands: Elsevier; [Google Scholar]
- Oliveras‐López, M. J. , Berná, G. , Jurado‐Ruiz, E. , de la Serrana, H. L. G. , & Martín, F. (2014). Consumption of extra‐virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. Journal of Functional Foods, 10, 475–484. 10.1016/j.jff.2014.07.013 [DOI] [Google Scholar]
- Pacheco, Y. M. , Bermúdez, B. , López, S. , Abia, R. , Villar, J. , & Muriana, F. J. (2007). Minor compounds of olive oil have postprandial anti‐inflammatory effects. British Journal of Nutrition, 98, 260–263. 10.1017/S0007114507701666 [DOI] [PubMed] [Google Scholar]
- Paiva‐Martins, F. , Barbosa, S. , Silva, M. , Monteiro, D. , Pinheiro, V. , Mourão, J. L. , … Santos‐Silva, A. (2014). The effect of olive leaf supplementation on the constituents of blood and oxidative stability of red blood cells. Journal of Functional Foods, 9, 271–279. 10.1016/j.jff.2014.04.027 [DOI] [Google Scholar]
- Paiva‐Martins, F. , Gonçalves, P. , Borges, J. E. , Przybylska, D. , Ibba, F. , Fernandes, J. , & Santos‐Silva, A. (2015). Effects of the olive oil phenol metabolite 3, 4‐DHPEA‐EDAH 2 on human erythrocyte oxidative damage. Food and Function, 6, 2350–2356. [DOI] [PubMed] [Google Scholar]
- Peralbo‐Molina, Á. , & Luque de Castro, M. D. (2013). Potential of residues from the Mediterranean agriculture and agrifood industry. Trends in Food Science and Technology, 32, 16–24. 10.1016/j.tifs.2013.03.007 [DOI] [Google Scholar]
- Perrinjaquet‐Moccetti, T. , Busjahn, A. , Schmidlin, C. , Schmidt, A. , Bradl, B. , & Aydogan, C. (2008). Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytotherapy Research, 22, 1239–1242. [DOI] [PubMed] [Google Scholar]
- Petkov, V. , & Manolov, P. (1972). Pharmacological analysis of the iridoid oleuropein. Arzneimittel‐Forschung, 22, 1476–1486. [PubMed] [Google Scholar]
- Petroni, A. , Blasevich, M. , Salami, M. , Papini, N. , Montedoro, G. F. , & Galli, C. (1995). Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thrombosis Research, 78, 151–160. 10.1016/0049-3848(95)00043-7 [DOI] [PubMed] [Google Scholar]
- Pinto, J. , Paiva‐Martins, F. , Corona, G. , Debnam, E. S. , Jose Oruna‐Concha, M. , Vauzour, D. , … Spencer, J .P. (2011). Absorption and metabolism of olive oil secoiridoids in the small intestine. British Journal of Nutrition, 105, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Quintero‐Florez, A. , Pereira‐Caro, G. , Sanchez‐Quezada, C. , Moreno‐Rojas, J. M. , Gaforio, J. J. , & Beltrán, A. J. G. (2017). Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. European Journal of Nutrition, 5, 1–22. [DOI] [PubMed] [Google Scholar]
- Quirantes‐Piné, R. , Lozano‐Sánchez, J. , Herrero, M. , Ibáñez, E. , Segura‐Carretero, A. , & Fernández‐Gutiérrez, A. (2013). HPLC–ESI–QTOF–MS as a powerful analytical tool for characterising phenolic compounds in olive‐leaf extracts. Phytochemical Analysis, 24(3), 213‐223. [DOI] [PubMed] [Google Scholar]
- Raederstorff, D. (2009). Antioxidant activity of olive polyphenols in humans: A Review. International Journal for Vitamin and Nutrition Research, 79, 152–165. 10.1024/0300-9831.79.3.152 [DOI] [PubMed] [Google Scholar]
- Ramírez‐Tortosa, M. C. , Granados, S. , & Quiles, J. L. (2006). Chemical composition. Types and characteristics of olive oil. Olive Oil & Health. Oxfordshire, UK: CAB International. [Google Scholar]
- Robles‐Almazan, M. , Pulido‐Moran, M. , Moreno‐Fernandez, J. , Ramirez‐Tortosa, C. , Rodriguez‐Garcia, C. , Quiles, J. L. , & Ramirez‐Tortosa, M. (2018). Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Research International, 105, 654–667. 10.1016/j.foodres.2017.11.053 [DOI] [PubMed] [Google Scholar]
- Roche, H. M. , & Gibney, M. J. (2000). The impact of postprandial lipemia in accelerating atherothrombosis. Journal of Cardiovascular Risk, 7, 317–324. 10.1177/204748730000700504 [DOI] [PubMed] [Google Scholar]
- Rodrigues, N. , Casal, S. , Peres, A. M. , Baptista, P. , Bento, A. , Martín, H. , … Pereira, J. A. (2018). Effect of olive trees density on the quality and composition of olive oil from cv. Arbequina. Scientia Horticulturae, 238, 222–233. 10.1016/j.scienta.2018.04.059 [DOI] [Google Scholar]
- Rodriguez‐Concepcion, M. , Avalos, J. , Bonet, M. L. , Boronat, A. , Gomez‐Gomez, L. , Hornero‐Mendez, D. , … Zhu, C. (2018). A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research, 70, 62–93. 10.1016/j.plipres.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Rodriguez, R. , Herrera, M. D. , de Sotomayor, M. A. , & Ruiz‐Gutierrez, V. (2007). Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. American Journal of Hypertension, 20, 728–734. 10.1016/j.amjhyper.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Rodriguez, R. , Herrera, M. D. , de Sotomayor, M. A. , & Ruiz‐Gutierrez, V. (2009). Effects of pomace olive oil‐enriched diets on endothelial function of small mesenteric arteries from spontaneously hypertensive rats. British Journal of Nutrition, 102, 1435–1444. 10.1017/S0007114509990754 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Rodriguez, R. , Perona, J. S. , Herrera, M. D. , & Ruiz‐Gutierrez, V. (2006). Triterpenic compounds from “Orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. Journal of Agriculture and Food Chemistry, 54, 2096–2102. 10.1021/jf0528512 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Rodriguez, R. , Stankevicius, E. , Herrera, M. D. , Østergaard, L. , Andersen, M. R. , Ruiz‐Gutierrez, V. , & Simonsen, U. (2008). Oleanolic acid induces relaxation and calcium‐independent release of endothelium‐derived nitric oxide. British Journal of Pharmacology, 155, 535–546. 10.1038/bjp.2008.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, M. , Toral, M. , Gómez‐Guzmán, M. , Jiménez, R. , Galindo, P. , Sánchez, M. , … Duarte, J. (2016). Antihypertensive effects of oleuropein‐enriched olive leaf extract in spontaneously hypertensive rats. Food and Function, 7, 584–593. 10.1039/C5FO01101A [DOI] [PubMed] [Google Scholar]
- Rondina, M. T. , Weyrich, A. S. , & Zimmerman, G. A. (2013). Platelets as cellular effectors of inflammation in vascular diseases. Circulation Research, 112, 1506–1519. 10.1161/CIRCRESAHA.113.300512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, M. W. , & Sperber, G. O. (1997). A diffusion model of cerebral microischemia. Experimental Neurology, 147, 142–150. 10.1006/exnr.1997.6572 [DOI] [PubMed] [Google Scholar]
- Rosignoli, P. , Fuccelli, R. , Sepporta, M. V. , & Fabiani, R. (2016). In vitro chemo‐preventive activities of hydroxytyrosol: The main phenolic compound present in extra‐virgin olive oil. Food and Function, 7, 301–307. 10.1039/C5FO00932D [DOI] [PubMed] [Google Scholar]
- Roth, G. A. , Johnson, C. , Abajobir, A. , Abd‐Allah, F. , Abera, S. F. , Abyu, G. , … Murray, C. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology, 70(1), 1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozati, M. , Barnett, J. , Wu, D. , Handelman, G. , Saltzman, E. , Wilson, T. , … Meydani, S. N. (2015). Cardio‐metabolic and immunological impacts of extra virgin olive oil consumption in overweight and obese older adults: A randomized controlled trial. Nutrition and Metabolism, 12, 28 10.1186/s12986-015-0022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano, J. , López‐Miranda, J. , de la Torre, R. , Delgado‐Lista, J. , Fernández, J. , Caballero, J. , … Pérez‐Jiménez, F. (2007). Intake of phenol‐rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. American Journal of Clinical Nutrition, 86, 341–346. 10.1093/ajcn/86.2.341 [DOI] [PubMed] [Google Scholar]
- Rubió, L. , Serra, A. , Chen, C.‐Y. , Macià, A. , Romero, M.‐P. , Covas, M.‐I. , … Motilva, M.‐J. (2014). Effect of the co‐occurring components from olive oil and thyme extracts on the antioxidant status and its bioavailability in an acute ingestion in rats. Food and Function, 5, 740 10.1039/c3fo60446b [DOI] [PubMed] [Google Scholar]
- Salvini, S. , Sera, F. , Caruso, D. , Giovannelli, L. , Visioli, F. , Saieva, C. , … Palli, D. (2006). Daily consumption of a high‐phenol extra‐virgin olive oil reduces oxidative DNA damage in postmenopausal women. British Journal of Nutrition, 95, 742–751. 10.1079/BJN20051674 [DOI] [PubMed] [Google Scholar]
- Schwingshackl, L. , Christoph, M. , & Hoffmann, G. (2015). Effects of olive oil on markers of inflammation and endothelial function‐a systematic review and meta‐analysis. Nutrients, 7, 7651–7675. 10.3390/nu7095356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, G. , Deiana, M. , Spencer, J. P. E. , & Corona, G. (2017). Olive oil phenolics prevent oxysterol‐induced pro‐inflammatory cytokine secretion and ROS production in human PBMCs, through modulation of p38 and JNK pathways. Molecular Nutrition and Food Research, 61, 1–7. 10.1002/mnfr.201700283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, I. , Mok, M. , Christensen, A. M. , Turner, A. H. , & Hawley, J. A. (2008). The effect of polyphenols in olive leaves on platelet function. Nutrition, Metabolism and Cardiovascular Diseases, 18, 127–132. [DOI] [PubMed] [Google Scholar]
- Soler, A. , Romero, M. P. , Macia, A. , Saha, S. , Furniss, C. S. , Kroon, P. A. , & Motilva, M. J. (2010). Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small‐intestinal epithelial Caco‐ 2/TC7 cell line. Food Chemistry, 119, 703–714. [Google Scholar]
- Soni, M. G. , Burdock, G. A. , Christian, M. S. , & Botler, C. M. (2006). Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food and Chemical Toxicology, 44, 903–915. [DOI] [PubMed] [Google Scholar]
- Squeo, G. , Silletti, R. , Summo, C. , Paradiso, V. M. , Pasqualone, A. , & Caponio, F. (2016). Influence of calcium carbonate on extraction yield and quality of extra virgin oil from olive (Olea europaea L. cv. Coratina). Food Chemistry, 209, 65–71. [DOI] [PubMed] [Google Scholar]
- Srigley, C. T. , Oles, C. J. , Reza, A. , Kia, F. , & Mossoba, M. M. (2016). Authenticity assessment of extra virgin olive oil: Evaluation of dimethyl sterols and triterpene dialcohols. Journal of the American Oil Chemists’ Society, 93, 171–181. [Google Scholar]
- Susalit, E. , Agus, N. , Effendi, I. , Tjandrawinata, R. R. , Nofiarny, D. , Perrinjaquet‐Moccetti, T. , & Verbruggen, M. (2011). Olive (Olea europaea) leaf extract effective in patients with stage‐1 hypertension: Comparison with Captopril. Phytomedicine, 18, 251–258. 10.1016/j.phymed.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Tabas, I. (2010). Macrophage death and defective inflammation resolution in atherosclerosis. Nature Reviews Immunology, 10, 36–46. 10.1038/nri2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhaoui, N. , Vezza, T. , Gómez‐Caravaca, A. M. , Fernández‐Gutiérrez, A. , Gálvez, J. , & Segura‐Carretero, A. (2016). Phenolic compounds and in vitro immunomodulatory properties of three Andalusian olive leaf extracts. Journal of Functional Foods, 22, 270–277. 10.1016/j.jff.2016.01.037 [DOI] [Google Scholar]
- Teres, S. , Barcelo‐Coblijn, G. , Benet, M. , Alvarez, R. , Bressani, R. , Halver, J. E. , & Escriba, P. V. (2008). Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proceedings of the National Academy of Sciences, 105, 13811–13816. 10.1073/pnas.0807500105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourdot, B. E. , Ahmed, I. , & Holinstat, M. (2013). The emerging role of oxylipins in thrombosis and diabetes. Frontiers in Pharmacology, 4, 176 10.3389/fphar.2013.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, N. , Wilson, L. , Bhatnagar, P. , Wickramasinghe, K. , Rayner, M. , & Nichols, M. (2016). Cardiovascular disease in Europe: Epidemiological update 2016. European Heart Journal, 37, 3232–3245. 10.1093/eurheartj/ehw334 [DOI] [PubMed] [Google Scholar]
- Turnbull, F. (2003). Blood Pressure Lowering Treatment Trialists' Collaboration: Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: Results of prospectively‐designed overviews of randomized trials. Lancet, 362, 1527–1535. [DOI] [PubMed] [Google Scholar]
- Valero‐Muñoz, M. , Martín‐Fernández, B. , Ballesteros, S. , de la Fuente, E. , Quintela, J. C. , Lahera, V. , & de las Heras, N. (2014). Protective effect of a pomace olive oil concentrated in triterpenic acids in alterations related to hypertension in rats: Mechanisms involved. Molecular Nutrition and Food Research, 58, 376–383. 10.1002/mnfr.201300256 [DOI] [PubMed] [Google Scholar]
- Valls, R. M. , Farràs, M. , Pedret, A. , Fernández‐Castillejo, S. , Catalán, Ú. , Romeu, M. , … Rubió, L. (2017). Virgin olive oil enriched with its own phenolic compounds or complemented with thyme improves endothelial function: The potential role of plasmatic fat‐soluble vitamins. A double blind, randomized, controlled, cross‐over clinical trial. Journal of Functional Foods, 28, 285–292. 10.1016/j.jff.2016.10.032 [DOI] [Google Scholar]
- Vissers, M. N. , Zock, P. L. , & Katan, M. B. (2004). Bioavailability and antioxidant effects of olive oil phenols in humans: A review. European Journal of Clinical Nutrition, 58, 955–965. 10.1038/sj.ejcn.1601917 [DOI] [PubMed] [Google Scholar]
- Vougogiannopoulou, K. , Lemus, C. , Halabalaki, M. , Pergola, C. , Werz, O. , Smith, A. B. , … Deguin, B. (2014). One‐step semisynthesis of oleacein and the determination as a 5‐lipoxygenase inhibitor. Journal of Natural Products, 77, 441–445. 10.1021/np401010x [DOI] [PubMed] [Google Scholar]
- Weinbrenner, T. , Fitó, M. , Farré Albaladejo, M. , Saez, G. T. , Rijken, P. , Tormos, C. , … Covas, M. I. (2004). Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs under Experimental and Clinical Research, 30, 207–214. [PubMed] [Google Scholar]
- Weinbrenner, T. , Fitó, M. , Torre, R. D. L. , Saez, G. T. , Rijken, P. , Tormos, C. , … Covas, M.‐I. (2004). Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. Journal of Nutrition, 134, 2314–2321. 10.1093/jn/134.9.2314 [DOI] [PubMed] [Google Scholar]
- Xie, P. J. , Huang, L. X. , Zhang, C. H. , & Zhang, Y. L. (2015). Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. Journal of Functional Foods, 16, 460‐471. [Google Scholar]
- Yousuf, O. , & Bhatt, D. L. (2011). The evolution of antiplatelet therapy in cardiovascular disease. Nature Reviews Cardiology, 8, 547–559. 10.1038/nrcardio.2011.96 [DOI] [PubMed] [Google Scholar]
- Zamora‐Ros, R. , Knaze, V. , & González, C. A. (2012). Response to ‘Is virgin olive oil a main food source of lignans in Mediterranean countries?’. European journal of clinical nutrition, 66(12), 1376 10.1038/ejcn.2012.150 [DOI] [PubMed] [Google Scholar]
- Zrelli, H. , Matsuoka, M. , Kitazaki, S. , Zarrouk, M. , & Miyazaki, H. (2011). Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK‐FOXO3a pathway. European Journal of Pharmacology, 660, 275–282. 10.1016/j.ejphar.2011.03.045 [DOI] [PubMed] [Google Scholar]


