ABSTRACT.
It is estimated that by 2030 there will be 82 million people in the world with dementia.
Objective:
To evaluate the effect of dietary interventions on the cognitive performance of individuals with Alzheimer’s disease (AD).
Methods:
A systematic review of randomized controlled trials (RCT) was conducted in the Scopus, PubMed, and Cochrane databases. Thirty-two RCT were included.
Results:
Omega-3 fatty acid showed positive effects at different doses. Fortasyn Connect seemed to be effective in the early stages of the disease. Probiotic, Ginseng, Inositol and specialized nutritional formulas seemed to have a positive effect on cognition. Most of the primary studies presented poor methodological quality, included patients with mild AD, small samples, and did not obtain significative results for all the cognitive outcomes.
Conclusions:
The effect of most dietary interventions on cognition in AD patients remains inconclusive, however, several nutrients, isolated or not, show potential to improve cognitive function in AD, especially in its early stages.
Keywords: Alzheimer’s disease, diet, nutrients, dietary supplements, cognition
RESUMO.
Estima-se que até 2030 haverá 82 milhões de pessoas no mundo com demência.
Objetivo:
Avaliar o efeito de intervenções dietéticas no desempenho cognitivo de indivíduos com DA.
Métodos:
Foi realizada uma revisão sistemática de ensaios clínicos randomizados (ECR) nas bases de dados Scopus, PubMed e Cochrane. Trinta e dois ECRs foram incluídos.
Resultados:
Ácidos graxos ômega-3 apresentaram efeitos positivos em diferentes doses. O Fortasyn Connect mostrou-se efetivo em estágios iniciais. Probióticos, Ginseng, Inositol e fórmulas nutricionais especializadas também demonstraram efeito positivo sobre a cognição. A maioria dos estudos primários apresentou baixa qualidade metodológica, incluiu pacientes com DA leve e amostras pequenas e não obteve resultados significativos para todos os desfechos cognitivos.
Conclusões:
O efeito da maioria das intervenções dietéticas em pacientes com DA permanece inconclusivo; entretanto, vários nutrientes, isolados ou não, apresentam potencial para melhorar a função cognitiva na DA, principalmente em seu estágio inicial.
Palavras-chave: doença de Alzheimer, dieta, nutrientes, suplementos nutricionais, cognição
INTRODUCTION
It is estimated that by 2030 there will be 82 million people in the world with dementia. Most of these cases occur in middle- and low-income countries that currently hold 66% of all people with the disease. 1 Alzheimer’s disease (AD) is the leading cause of dementia, representing 50 to 70% of dementia diagnoses in the population over 65 years of age. 2 , 3 In Brazil, the epidemiological data on AD are still restricted to the most developed regions of the country, where the prevalence of dementia in the elderly ranges from 7.1 to 12.9%, being AD the responsible for 55.1‒59.8% of cases. 4 , 5
Categorized as a progressive neurodegenerative disorder, AD has as its main physical and anatomopathological marker the abnormal accumulation of β-amyloid peptide (Aβ) in senile plaques (SP) and hyperphosphorylated tau protein (TP) in neurofibrillary tangles (NFT), resulting in diffuse cerebral atrophy in areas of the hippocampus and frontal, parietal and temporal cortex. 2 , 6 , 7 , 8 The development of the disease includes a long preclinical period, with amyloid pathology being present 15 to 20 years before the onset of cognitive decline symptoms. 2 , 6 , 9 Risk factors for AD include advanced age, presence of Apoliprotein E4 allele genes, family history of AD and brain injury. Increasing evidence also suggests that lifestyle-related modifiable risk factors such as inadequate diet, physical and intellectual inactivity, diabetes, obesity, depression, smoking, and low education have an important role in AD due to their relationship with mechanisms involving inflammation, oxidative stress, and mitochondrial dysfunction. 3 , 6 , 7
To date, the therapeutic resources available for AD are limited to symptom management and cannot prevent cognitive decline and disease progression. Thus, there is a growing interest in strategies that can intervene in their pathophysiological mechanisms, targeting modifiable risk factors for the disease. 6 , 8 , 10 , 11 , 12 In the field of AD prevention, scientific evidence on the role of diet is more robust. Prospective cohort studies with the Mediterranean Diet (MeDi), Dietary Approach to Stop Hypertension (DASH), and Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) in healthy subjects demonstrated a lower incidence of AD, 13 lower rates of cognitive decline, 14 , 15 , 16 , 17 cerebral atrophy 18 and Aβ deposition 19 in the highest adherence scores to these diets. In two other studies, both MeDi and folate and vitamin B6 consumption were inversely associated with disease incidence. 20 , 21
In the field of intervention, folate, B6 vitamin and other nutrients and dietary components have been studied for their neuroprotective properties and potential positive effect on cognition. Positive results in cognitive performance have been found in experimental studies and clinical trials using omega-3 fatty acids, 8 , 11 , 12 , 22 alpha-lipoic acid, 8 , 11 , 12 polyphenols, 12 , 22 , 23 Q10 coenzyme, 8 , 11 , 12 vitamin supplements, 8 , 11 , 12 , 22 , 23 selenium, 8 and phytochemicals. 8 , 11 , 12 , 22 , 23 However, several questions are still present regarding the reproducibility of the results found in animal models and the effectiveness of such interventions in individuals with AD in the various stages of the disease.
Therefore, considering the potential relationship between diet, cognition and AD, the present systematic review aimed to evaluate the existing evidence in controlled randomized controlled trials for the use of specific dietary interventions in the management of cognitive decline in AD patients.
METHODS
It was a systematic review of randomized controlled trials conducted according to the protocol proposed by the Cochrane Collaboration 24 and structured as proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA). 25
Literature search strategy
The search for randomized controlled trials (RCT) was conducted in the PubMed, Cochrane Central Register of Controlled Trials, and Scopus databases, in September 2016, using a combination of the Medical Subject Heading (MeSH) terms related to the factor under study (dietary interventions in Patients with AD), outcome (cognitive performance), and design (randomized controlled trials) (Table 1). Additionally, manual searches were performed in the reference list of studies relevant to this review.
Table 1. Pubmed search strategy.
| Descriptions of search terms used |
|---|
| (((((diet[Title/Abstract]) OR Dietary therapy[Title/Abstract]) OR Food habits[Title/Abstract]) OR Food formulations[Title/Abstract]) OR Food formulations[Title/Abstract]))) AND ((((Randomized Controlled Trials as Topic/) OR randomized controlled trial/) OR Random Allocation/) OR Double Blind Method/) OR Single Blind Method/) OR clinical trial/) OR clinical trial, phase i.pt) OR clinical trial, phase ii.pt) OR clinical trial, phase iii.pt) OR clinical trial, phase iv.pt) OR controlled clinical trial.pt) OR randomized controlled trial.pt) OR multicenter study.pt) OR clinical trial.pt) OR exp Clinical Trials as topic/))))) AND Alzheimer disease[Title/Abstract] |
Selection of eligible studies
The studies identified in the search in the three databases were stored in the reference organizer program Endnote Web, after deleting duplicates. Two reviewers (AKJ and SHCM) independently assessed the titles and abstracts and the discrepancies were resolved by a third reviewer (FMS). After the selection of potentially eligible articles, a reviewer (SHCM) read the full texts for confirmation of inclusion in this systematic review and collected the data from a standardized form.
The following criteria were considered eligible for the two stages of study selection:
A randomized clinical trial design.
Including participants with a prior diagnosis of AD.
Having assessed cognition as primary or secondary endpoint.
Having had interventions such as diet or food or specific supplement.
Studies that included participants with different types of dementia and did not present stratified results for AD were excluded, as were those whose presented open-label phase results from an included study and whose full text could not be accessed. Only studies whose manuscript were published in English were selected.
Data extraction and methodological quality assessment of eligible studies
The data extracted from the articles were:
Authorship.
Date of publication.
Country where the study was conducted.
Study design.
Method of randomization.
Blinding of participants, researchers, and evaluators.
Sample size and proportion of subjects completing the study.
Inclusion and exclusion criteria.
Criteria used to diagnose ad.
Mean age of participants.
Proportion of male subjects.
Dietary intervention protocol.
Cognitive outcomes analyzed.
Main results.
To evaluate the methodological quality, the criteria proposed by Cochrane were evaluated from the six domains: randomization method, allocation concealment, blinding scheme (participants, professionals, and outcome assessors), intention-to-treat analysis (ITT), follow-up losses and selection of outcomes. 24
RESULTS
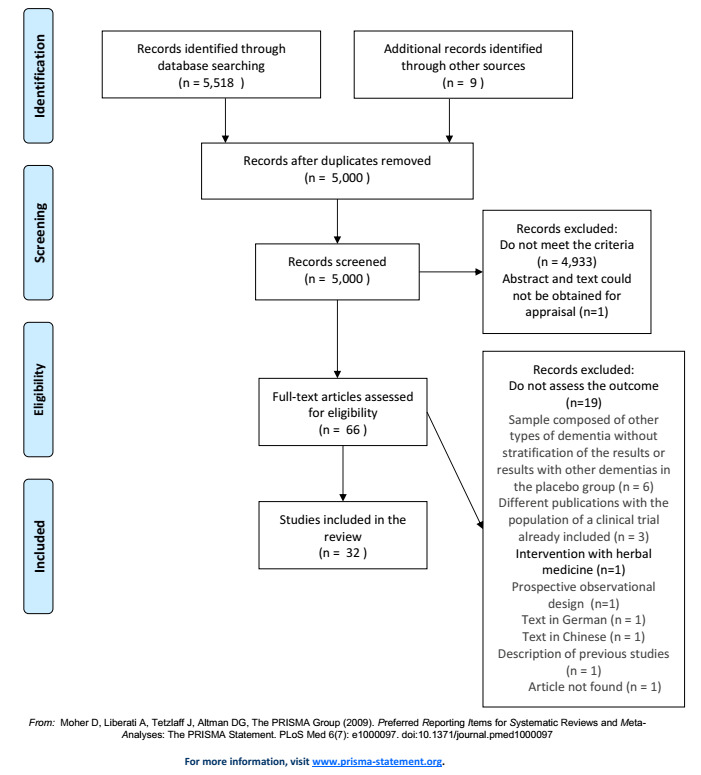
From the search in the three databases, 5,000 articles were identified, after duplicate exclusion. Additionally, nine articles considered relevant to the study were included. Thirty-two studies met the eligibility criteria and were included in this review. The study flowchart is presented in Figure 1.
Figure 1. Prisma flow diagram.
General characteristics of studies
The overall characteristics of the selected studies are presented in Table 2. The majority were conducted in the United States (41.0%) and in European countries (34.0%). The sample size ranged from 12 to 613 individuals per test with a follow-up period of 25 weeks in men, ranging from three weeks to thirty months. The mean age of participants ranged from 66.1 to 84.8 years and in seven studies (22%), more than half of the sample consisted of men.
Table 2. Characteristics and results of randomized clinical trials evaluating dietary interventions in Alzheimer’s disease.
| Study (Year)Ref |
|
|
Interventions | Cognitive outcomes | Results and main findings |
|---|---|---|---|---|---|
| ORAL NUTRITIONAL FORMULATIONS | |||||
| Lauque et al. (2004) 26 |
|
|
|
MMSE | There was no significant difference between groups in the change in MMSE scores after 3 and 6 months of intervention compared to the baseline. |
| Planas et al. (2004) 27 |
|
|
|
MEC and Set | There was no significant difference between groups in the change in MMSE scores after 3 and 6 months of intervention compared to the baseline. |
| Salas-Salvadó et al. (2005) 28 |
|
|
|
GDS scale and Pfeiffer test | No statistically significant difference was found between the groups in the change in Mini-cog and Set scores after 6 months of treatment. |
| Scheltens et al. (2010) 29 |
|
|
|
WMS-r, ADAS-cog and MMSE |
|
| De Sousa et al. (2012) 30 |
|
|
|
MMSE and CLOX-1 | There was no significant difference between the groups in the MMSE and CLOX-1 scores. Differences in scores relative to the baseline were not computed in both tests. |
| Scheltens et al. (2012) 31 |
|
|
|
NTB (z-score): memory function, executive function and total score. |
|
| Shah et al. (2013) 32 |
|
|
|
ADAS-cog, CDR-SOB and cognitive test battery | No significant difference was found between the groups in the rates of change of all cognitive outcomes after 24weeks of treatment. |
| Soininen et al. (2017)33 |
|
|
|
|
|
| OMEGA-3 FATTY ACIDS ISOLATED OR IN ASSOCIATION WITH OTHER NUTRIENTS | |||||
| Yehuda et al. (1996) 34 |
|
|
|
12-item questionnaire, completed by caregivers, including areas of spatial orientation; cooperation; humor; appetite; organization; short-term memory; long-term memory; sleep disorders; alertness during the day; hallucinations; capacity for expression; and bladder control. |
|
| Freud-Levi et al. (2006) 35 |
|
|
|
MMSE, ADAS-cog, CDR and CDR-SOB. |
|
| Quinn et al. (2010) 36 |
|
|
|
ADAS-cog, MMSE, CDR-SOB. |
|
| Shinto et al. (2014) 37 |
|
|
|
MMSE and ADAS-cog |
|
| MICRONUTRIENTS ISOLATED OR IN ASSOCIATION | |||||
| Sano et al. (1997) 38 |
|
|
|
ADAS-cog and MMSE | There was no significative difference among the groups in both outcomes. |
| Sun et al. (2007) 39 |
|
|
|
ADAS-Cog/11, MMSE and CASI | There was no significative difference among the groups in all outcomes. |
| Kessler et al. (2008) 40 |
|
|
|
ADAS-cog and MMSE | There was no significant difference between the groups in the analysis of time x treatment interaction for both ADAS-cog and MMSE. |
| Aisen et al. (2008) 41 |
|
|
|
ADAS-cog, MMSE, CDR-SOB | There was no significant difference between groups in the rate of decline of ADAS-cog, MMSE, CDR-SOB during treatment. |
| Lloret et al. (2009) 42 |
|
|
|
MMSE, CLOX-1 and Blessed-Dementia Scale, |
|
| Remington et al. (2009) 43 |
|
|
|
DRS-2 and CLOX-1 | There was no significant difference between the groups in the comparison of the total DRS-2 and CLOX-1 scores after treatment. |
| Galasko et al. (2012) 44 |
|
|
|
MMSE |
|
| Dysken et al. (2014) 45 |
|
|
|
ADAS-cog and MMSE | There were no significant differences between the groups in the MMSE and ADAS-cog scores. |
| Nolan et al. (2015) 46 |
|
|
|
MMSE | There was no statistically significant difference in MMSE after 6 months of treatment in the two branches of the study (individuals with AD and individuals without AD). |
| Remington et al. (2015) 47 |
|
|
|
CLOX-1 and DRS-AEMSS |
|
| GINSENG | |||||
| Lee et al. (2008) 48 |
|
|
|
ADAS-cog and MMSE |
|
| Heo et al. (2012) 49 |
|
|
|
ADAS-cog and MMSE |
|
| PHYTOCHEMICALS | |||||
| Baumet al. (2008) 50 |
|
|
|
MMSE | There was no significative difference among the groups in changes of MMSE score after 6 months. |
| Ringman et al. (2012) 51 |
|
|
|
ADAS-cog, MMSE. | There was no significant difference between groups in the changes presented in all cognitive parameters after 24 weeks of treatment. |
| Farokhnia et al. (2014) 52 |
|
|
|
MMSE and SCIRS | There was no difference among the groups in the changes of scores of MMSE and SCIR after 12 months of treatment. |
| Gleason et al. (2015) 53 |
|
|
|
MMSE and Battery of Neuropsychological Tests. | There was no difference among the groups in the MMSE and tests of verbal memory, executive function, executive function and language, visual memory and visuomotor function after 6 months of treatment. |
| Turner et al. (2015) 54 |
|
|
|
CDR-SOB, ADAS-cog, and MMSE | There was no significative difference among the groups in the scores of CDR-SOB, ADAS-cog, and MMSE (data not provided by the authors). |
| COCCONUT OIL | |||||
| Chan et al. (2017) 55 |
|
|
|
MMSE and CLOX-1 |
|
| PROBIOTIC | |||||
| Akbari et al. (2016) 56 |
|
|
|
MMSE |
|
| INOSITOL | |||||
| Barak et al. (1996) 57 |
|
|
|
CAMCOG |
|
ALA: α-lipoic Acid; AD: Alzheimer’s disease; ADAS-cog: Alzheimer's Disease Assessment Scale-Cognitive Subscale; ADAS-cog/11: Alzheimer's Disease Assessment Scale 11-item Cognitive Subscale; CAMCOG: Cognitive Subscale of Cambridge Mental Disorder of the Elderly Examination; CASI: Cognitive Abilities Screening Instrument; CDR-SOB: Clinicial Dementia Rating Scale - Sum of Boxes; CG: control group; CLOX-1: Clock Drawning Test; CoQ: Q Coenzyme; DHA: docosaexaenoic acid; RCT: Randomized Controled Trial; EPA: Eicosapentaenoic Acid; GDS: Global Deterioration Scale; IG: Intervention Group; IWG-1=International Working Group; MEC: Mini Examen Cognitivo; MMSE: Mini Mental State Examination; MRI: magnetic resonance imaging; NIA-AA: National Institute of Aging - Alzheimer Association; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NTB: Neuropsychological Test Battery; ONS: oral nutritional supplement; SCIRS: Severe Cognitive Impairment Rating Scale; Set: Isaacs Set Test; SG: Sun Ginseng, WMS-r: Wichsler Memory Scale - revised.
Of the 32 RCT included, one (3%) had a cross-over design, 57 26 (87%) 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 ,, 41 , 42 , 43 , 44 , 45 , 46 , 47 , 50 , 51 , 53 , 54 , 55 , 56 , 57 were placebo-controlled, five (16%) 26 , 28 , 30 , 48 , 49 used a conventional control treatment, and one (3%) 52 compared dietary intervention with pharmacological treatment. Regarding the blinding scheme, 26 (81%) trials were double-blind, 27 , 29 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 47 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 four (13%) were open, 28 , 30 , 48 , 49 and two (6%) were single blind. 26 , 43
In most trials (59%), the diagnosis of AD was based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-III and DSM-IV). In one study, 33 the International Working Group/National Institute of Aging-Alzheimer's Association (IWG/NIA-AA) criteria were used for the diagnosis of prodromal Alzheimer's.
The intervention period in the studies ranged from three weeks to three and a half years. For the assessment of cognitive performance, different instruments were used, being the Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog), and Clinical Dementia Rating Scale - Sum of Boxes (CDR-SOB) the most frequent tests adopted.
Effect of dietary interventions on the cognition of patients with Alzheimer's disease
The effect of different dietary interventions on the cognition of AD patients evaluated by the studies is presented in Table 2, grouped by the type of intervention. Dietary interventions were grouped as oral nutritional formulations, fatty acids (alone or in combination with other nutrients), micronutrients (alone or in combination with other nutrients), ginseng, phytochemicals, coconut oil, probiotics, and inositol. Details of the effects of each intervention are detailed below.
Oral nutritional formulations
Eight parallel RCT 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 evaluated the effect of intervention with ONF on the cognitive performance of patients with AD. Sample sizes ranged from 37 to 528 subjects, most of whom had mild cognitive decline (MMSE>15 at baseline). Supplements tested included either liquid or semi-solid formulations, offered one to three times a day.
There was heterogeneity among the studies regarding the macro and micronutrient composition of the supplements, trial design, intervention duration (21 days to 24 months), and cognitive outcomes evaluated.
Three RCT tested the same supplement. In these, the use of omega-3, phospholipids, choline, uridine monophosphate, vitamin E, vitamin C, selenium, vitamin B12, vitamin B6, and folic acid enriched formula resulted in a lower decline in the Wechsler Memory Scale - revised immediate recall 29 and better memory domain performance in the Neuropsychological Test Battery (NTB) 31 in patients with mild AD and less worsening in the CDR-SOB in patients with prodromal AD. 33 Among the other studies, one evaluated the same previous formulation in patients with mild to moderate AD, 32 three 26 , 27 , 30 evaluated hypercaloric and hyperprotein supplements with different micronutrient compositions and one analyzed a complete dietary formula based on lyophilized foods. 28 None of them, however, found statistically significant effects on the cognitive outcomes evaluated.
The randomization method used to allocate participants was described in six studies (Table 3), two of which used computer-generated block randomization, 29 , 33 two had central randomization from codes, 31 , 32 one used sequential numbers that were kept in sealed envelopes 26 and a centralized randomization stratified by the initial body mass index. 28 Of the eight RCT, two were opened 28 , 30 and five reported blinding of researchers and outcome evaluators. 27 , 29 , 31 , 32 , 33 The groups studied were comparable at baseline in seven trials. 26 , 27 , 28 , 29 , 30 , 31 , 32 Regarding the evaluated outcomes, only one study did not present the results of all pre-established outcomes. 26 Seven of the trials reported follow-up losses, 26 , 28 , 29 , 30 , 31 , 32 which was greater than 10% in five studies. 26 , 28 , 29 , 32 , 33 Among those with losses, 26 , 29 , 30 , 31 , 32 six performed ITT and one 28 did not report the type of analysis performed.
Table 3. Methodological characteristics of included studies.
| Study (year)ref | Randomization method | Similar groups in the baseline | Blinding of participants | Blinding of outcome assessors | Blinding of researchers | Follow-up losses (%) | IITT | Selection of outcomes. | Other sources of bias |
|---|---|---|---|---|---|---|---|---|---|
| ORAL NUTRITION SUPPLEMENT | |||||||||
| Lauque et al. (2004) 26 | Sequential numbers stored in sealed envelopes | Yes | Yes | No | No | 12% | Yes | Yes | Individuals who received an OS prescription during the intervention were not excluded from the CG. |
| Planas et al. (2004) 27 | NR | Yes | Yes | Yes | Yes | NR | NR | No | |
| Salas-Salvadó et al. (2005) 28 | Centralized randomization stratified by initial BMI | Yes | No | No | No | 21% | NR | No | Acceptance and quantity of dietary formula consumed was not presented. |
| Scheltens et al. (2010) 29 | Computer-generated block randomization | Yes | Yes | Yes | Yes | 12% | Yes | No | |
| De Sousa et al. (2012) 30 | NR | Yes | No | No | No | 5% | Yes | No | |
| Scheltens et al. (2012) 31 | Central randomization done from codes | Yes | Yes | Yes | Yes | 8% | Yes | No | Daily acceptance of the supplement bottle was not reported |
| Shah et al. (2013) 32 | Central randomization done from codes | Yes | Yes | Yes | Yes | 14% | Yes | No | |
| Soininen et al. (2017) 33 | Computer-generated block randomization | No | Yes | Yes | Yes | 21% | Yes | No | |
| OMEGA-3 FATTY ACIDS ISOLATED OR IN ASSOCIATION WITH OTHER NUTRIENTS | |||||||||
| Yehuda et al. (1996) 34 | NR | NR | Yes | Yes | NC | 0% | Yes | No | |
| Freud-Levi et al. (2006) 35 | NR | Yes | Yes | NC | NC | 15% | No | Yes | |
| Quinn et al. (2010) 36 | Centralized block randomization using interactive voice response system | No | Yes | Yes | Yes | 27% | Yes | No | |
| Shinto et al. (2014) 37 | Computer-generated randomization scheme stratified by smoking status (current smoker versus nonsmoker) | No | Yes | Yes | Yes | 13% | NR | No | |
| MICRONUTRIENTS ISOLATED OR IN ASSOCIATION | |||||||||
| Sano et al. (1997) 38 | Permuted-block randomization | Yes | Yes | NC | NC | 7% | Yes | No | |
| Sun et al. (2007) 39 | Computer generated random number list | Yes | Yes | Yes | Yes | 29% | Yes | No | |
| Kessler et al. (2008) 40 | NR | Yes | Yes | NC | NC | 16% | NR | No | |
| Aisen et al. (2008) 41 | Permuted-block randomization | Yes | Yes | NC | Yes | 16% | Yes | No | |
| Lloret et al. (2009) 42 | Randomized list of numbers | Yes | Yes | NC | NC | 56% | No | No | |
| Remington et al. (2009) 43 | NR | NR | Yes | NC | NC | 58% | Yes | No | |
| Galasko et al.(2012) 44 | Permuted-block randomization | Yes | Yes | NC | Yes | 20% | No | No | Patients were allowed to continue using antioxidant supplements for daily use, as long as within the following limits: <100 IU/day of α-tocopherol; <200 mg/day of C vitamin; 60 mg/day of CoQ; <100 IU/day of ALA |
| Dyskenet al. (2014) 45 | Central permuted-block randomization | Yes | Yes | NC | Yes | 42% | Yes | No | Dose adjustments were allowed based on the participants' tolerance |
| Nolan et al. (2015) 46 | Computer generated block randomization | No | Yes | NC | NC | 15% | NR | Yes | |
| Remington et al. (2015) 47 | Randomization done from codes | No | Yes | Yes | Yes | 78% | Yes | Yes | |
| GINSENG | |||||||||
| Lee et al. (2008) 48 | NR | Yes | No | No | No | 15% | Yes | No | |
| Heo et al. (2012) 49 | NR | Yes | No | No | No | NR | NR | No | |
| PHYTOCHEMICALS | |||||||||
| Baum et al. (2008) 50 | NR | Yes | Yes | NC | NC | 21% | NR | No | |
| Ringman et al. (2012) 51 | Block randomization | Yes | Yes | Yes | Yes | 17% | No | No | |
| Farokhnia et al. (2014) 52 | Randomization done from codes | Yes | Yes | Yes | Yes | 12% | Yes | No | |
| Gleason et al. (2015) 53 | NR | Yes | Yes | NC | NC | 9% | Yes | No | |
| Turner et al. (2015) 54 | Permuted-block randomization | No | Yes | NC | Yes | 13% | No | Yes | |
| COCONUT OIL | |||||||||
| Chan et al. (2017) 55 | Block randomization | Yes | Yes | NC | Yes | 45% (most in IG) | No | Yes | |
| PROBIOTIC | |||||||||
| Akbari et al. (2016) 56 | Computer generated random number list | Yes | Yes | NC | Yes | 10% | Yes | No | |
| INOSITOL | |||||||||
| Barak et al. (1996) 57 | NR | NR | Yes | NC | NC | 8% | No | No | |
ALA: α-lipoic acid; ITT: intention-to-treat analysis; CG: control group; IG: intervention group; BMI: body mass index; NC: not clear; NR: not reported; OS: oral supplementation.
Fatty acids
Four parallel RCT 34 , 35 , 36 , 37 tested the effect of fatty acid supplementation alone or associated with other nutrients on cognitive outcomes in individuals with AD. The duration of interventions varied between 4 weeks and 18 months and the trials were heterogeneous in relation to the sample size (39‒582 participants per trial), dose used, presence of vitamin E and α-lipoic acid (ALA), composition and fatty acid content (isolated docosahexaenoic acid (DHA), DHA+eicosapentaenoic acid (EPA) and mixture of α-linolenic acid with linoleic acid).
In all four studies, fatty acid supplementation resulted in significant improvement in part of the cognitive parameters analyzed. Twelve-month supplementation with 5,800 mg per day of omega-3 associated with vitamin E in patients with mild AD was effective in promoting a lower rate of cognitive decline on MMSE, but only in the first six months of treatment. 35 Smaller decline on MMSE was also observed with the use of omega-3 associated with ALA in mild AD (3,000 mg of omega-3+600 mg of ALA per day) 37 and the use of seaweed-derived DHA (2,000 mg per day, 45‒55% of DHA) has shown to be beneficial for patients with negative Apoliprotein E (APOE) ε4 allele, being able to reduce the cognitive decline assessed by ADAS-cog. 36
In patients with advanced AD (baseline MMSE=7.8+3.8), the use of 2 mL per day of a formulation containing a mixture of α-linolenic and linoleic in a ratio of 1:4.5 combined with α-tocopherol resulted in more reports by caregivers of improvement in the patients’ general condition. 34
Regarding methodological quality, one of the trials used a block randomization method based on an interactive voice response system with stratification by center, 36 one had computer-generated randomization with stratification by smoking, 37 and the others did not describe the randomization method used to allocate participants. 34 , 35 The groups studied were comparable at baseline in only one 35 of the four RCT, and one of the remaining RCT this data was not reported. 34 Three trials 35 , 36 , 37 showed follow-up losses, all greater than 10%. Of these, 36 performed the ITT and one did not report the type of analysis performed. 34 Regarding the evaluated outcomes, only one study 37 did not present the results of all pre-established outcomes.
Micronutrients
Ten parallel RCT evaluated micronutrient supplementation alone or in combination compared to placebo 39 , 40 , 41 , 42 , 43 , 44 , 46 , 47 or pharmacological treatment. 38 , 45 There was heterogeneity in relation to blinding (nine double-blind and one-blind), sample size (12-613 participants per trial), duration of intervention (16 weeks to 4 years), assessed cognitive outcomes and nutrients used (α-tocopherol alone, 38 , 42 , 45 B 41 vitamins association, carotenoid association, 46 copper, 58 coenzyme Q, 44 multivitamin and mineral supplement, 39 vitamins associated with ALA 44 and nutraceutical formulation containing vitamins, minerals, and peptides). 43 , 47
In patients with mild AD (MMSE=22.2+5.1), the use of a nutraceutical formula containing folic acid, vitamin B12, α-tocopherol, S-adenosyl methionine, N-acetyl cysteine, and acetyl-L-carnitine resulted in significant improvement in the Clock Drawing Test (CLOX-1) and the Age- and Education-adjusted Dementia Rating Scale (DRS-AEMSS) scores compared to baseline. 47 Such result differs from the pilot RCT performed with the same formulation, where no significant differences were found between groups in DRS-2 and CLOX-1. 43
Copper orotate supplementation was also evaluated in a group of patients with mild AD (MMSE=20‒25). Both the intervention and control groups showed an increase in the ADAS-cog score and a reduction in MMSE performance in relation to the baseline. These results were statistically significant. In the comparison between groups, the increase in ADAS-cog was smaller in the supplemented group, indicating lower cognitive decline. There was no difference between the groups regarding MMSE performance. 58
In patients with mild to severe AD, supplementation with vitamin E alone (800 IU/day) proved to be ineffective and, for some participants, harmful. Comparing intervention and control groups, supplement use did not alter cognitive performance in the MMSE, CLOX-1, and Blessed Dementia Scale. However, patients that at the end of the intervention did not obtain a reduction on serum oxidized glutathione levels, considered “unresponsive” to treatment with vitamin E, had a significant decline in MMSE when compared to “responsive” ones. 42
Similar results in MMSE performance were also found with vitamin E supplementation (800 IU/day) associated with vitamin C (500 mg/day) and ALA (900 mg/day). 44 Compared to the control, the supplemented group showed a significant reduction in MMSE performance. Paradoxically, in the same group, there was a decline in levels of F2-isoprostane relative to baseline, a biomarker of oxidative damage.
Of the five other studies, two compared α-tocopherol supplementation with pharmacological treatment (Selegiline 38 and Memantine 45 ), one analyzed the effect of methylcobalamin supplementation in combination with multivitamin, 39 one used a combination of folic acid, vitamin B12 and pyridoxine 41 and one a carotenoid supplement containing mezo-zeaxanthin, lutein, and zeaxanthin. 46 In none of these trials, however, the results found were significant for the cognitive parameters evaluated.
The randomization method used to allocate participants was described in eight of the ten studies. Of these, five 16 , 38 , 41 , 44 used permuted block randomization, two 39 , 42 were randomized using a list of random numbers, and one 47 used code randomization.
The studied groups were comparable at baseline in seven 38 , 39 , 40 , 41 , 42 , 44 , 45 RCT, and in two 43 , 37 of the remainder these data were not reported. All trials showed follow-up losses greater than 10% for the most part of the studies. 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 Five studies 38 , 41 , 43 , 45 , 47 performed the ITT and two did not report the type of analysis performed. 58 , 46 Regarding the evaluated outcomes, two studies 46 , 47 did not present the results of all pre-established outcomes.
Ginseng
Two open parallel trials evaluated ginseng supplementation in individuals with AD. 48 , 49 The duration of interventions varied between 12 and 24 weeks and the studies were heterogeneous in terms of sample size (58‒97 subjects), doses, and types of ginseng used.
In both trials, ginseng supplementation resulted in significant improvement in the cognitive outcomes evaluated. Patients with moderate to severe AD (MMSE<20 and CDR score>1) treated with 4.5 g/d of Sun Ginseng (SG-135) showed significant improvement in the ADAS-cog and MMSE after 12 and 24 weeks of supplementation. 49 Similar results were found with the use of 4.5 and 9.0 g/d of Korean white ginseng in a sample of patients with mild to moderate AD. Compared to control, both doses resulted in improvement in the MMSE and ADAS-cog scores after 12 weeks of supplementation and such effect was eliminated 12 weeks after discontinuation of treatment. 49
The groups studied were comparable at baseline in both RCT. A follow-up loss of 15% was reported by one of the trials, which performed an ITT analysis. 48 In the other trial, information about follow-up losses and data analysis method were not reported. 49 In both trials, the results of all predetermined outcomes were presented.
Phytochemicals
Five parallel double-blind RCT 50 , 51 , 52 , 53 , 54 evaluated the effect of supplementation of different phytochemicals extracted from foods and condiments compared with placebo 50 , 51 , 53 , 54 or pharmacological treatment. 52 Of the five trials, two evaluated the effect of turmeric at different doses and concentrations of curcuminoids, 50 , 51 one used dried turmeric extract, 52 one purified soy isoflavone, 53 and another, steady doses of Resveratrol. 54 In none of the trials, however, the results found were significant for the cognitive parameters evaluated.
Heterogeneity among studies was observed in relation to sample size (34‒119 subjects per trial), degree of cognitive decline (mild to severe), duration of the intervention (6 to 13 months), and cognitive outcomes (ADAS-cog, CDR-SOB, MMSE, and Severe Cognitive Impairment Rating Scale - SCIRS).
The randomization method used to allocate participants was described in three of the five studies. Of these, two 51 , 54 used block randomization and one 52 had randomization carried out from codes that were kept in opaque, sealed, and sequentially numbered envelopes.
The groups studied were comparable at baseline in four RCT. 50 , 51 , 52 , 53 All trials had follow-up losses greater than 10% in most studies. 50 , 51 , 52 , 54 Of the five trials, two 52 , 53 performed ITT and one did not report the type of analysis performed. 50 As for the outcomes evaluated, four of the five studies 50 , 51 , 52 , 53 presented the results of all pre-established outcomes.
Coconut oil
A parallel, double-blind, placebo-controlled RCT 55 evaluated the effect of coconut oil on the cognitive performance of subjects with mild to moderate AD. Using the block randomization method, 58 subjects were randomized to receive coconut oil or placebo. The characteristics of the groups were similar at baseline. After 6 months of intervention, no change was observed in the cognitive performance assessed by MMSE and CLOX-1.
Regarding the methodological quality, the study showed a 45% loss of follow-up, which was higher in the intervention group due to side effects such as diarrhea and abdominal discomfort. Data analysis was performed per protocol and the results of all pre-established outcomes were not presented.
Probiotics
A parallel, double-blind, placebo-controlled RCT 56 assessed the effect of probiotic supplementation onIN 60 patients with advanced AD. Using a computer program to generate a random list, participants were randomly allocated to receive milk with probiotics (L. Acidophilus, L. Casei. B. Bifidum, and L. Fermentum) or placebo. After 12 weeks of intervention, the probiotic supplemented group had a significant improvement in MMSE performance.
Regarding methodological quality, the groups were different at baseline in relation to some metabolic parameters (triglyceride levels, high density lipoprotein -HDL, and very low density lipoprotein - VLDL), but had similar cognitive characteristics. The follow-up loss was 10% and data were analyzed by ITT. The blinding of participants and researchers was maintained until the analysis conclusion and all the pre-established outcomes were reported.
Inositol
The effect of inositol supplementation was evaluated on a double-blind, placebo-controlled crossover RCT. 57 Twelve women with mild to severe AD were randomly allocated to receive inositol or dextrose (placebo). After 4 weeks of treatment, supplementation resulted in a significant improvement in the orientation and language domains assessed by the Cognitive Subscale of Cambridge Mental Disorder of the Elderly Examination (CAMCOG).
Regarding the methodological quality, the randomization method used was not reported in the study, there was a loss of 8% follow-up and data analysis was performed per protocol.
DISCUSSION
This systematic review aimed to evaluate the effect of different dietary interventions on the management of cognitive decline in AD patients.
Our study indicates the effects of improving or delaying cognitive decline with the use of specialized nutritional formulas, fatty acid supplements, ginseng, inositol probiotics. However, it is noteworthy that such results were mostly obtained in patients with mild AD, limited to only one part of the cognitive outcomes evaluated and resulted from the use of associated and not isolated nutrients in most trials, which suggests that the observed effects may (or may not) be due to the association of rather than a single target nutrient.
Regarding specialized oral nutritional formulations, in the prodromal phase and in the early stages of the disease, Fortasyn Connect (Souvenaid®), an oral supplement that includes a combination of EPA, DHA, phospholipids, uridine monophosphate, choline, selenium, and vitamins B6, B12, B9, C, and E, showed good results in the patients’ cognitive performance.
Suggested mechanisms for the effects of Fortasyn Connect include increased bioavailability of precursors and co-factors required for neuronal formation, maintenance and function, increased acetylcholine levels and cholinergic receptors with consequent stimulation of synaptogenesis and reduction of Aβ production and neurotoxicity. 58 However, despite the promising therapeutic effect of prodromal AD, the effects of supplementation with Fortsasyn Connect are still divergent in patients with mild to moderate disease, and further studies are needed to elucidate differences in the outcomes and to confirm the existence of therapeutic benefits on cognition.
The neuroprotective action of omega-3 fatty acids, especially DHA, has been demonstrated in several in vitro experiments and in animal models of AD, reinforcing the idea that supplementation of these nutrients could help reduce neuroinflammation and cognitive decline. 59 In mouse models of AD, DHA treatment resulted in reduced brain levels of Aβ, particularly β-Amyloid 42 (Aβ-42), the main component of amyloid plaques that contributes to irreversible neuronal death and rapid disease progression. Other mechanisms involved in the action of DHA include anti-inflammatory, antioxidant, and anti-apoptotic effects. 59
Neuroinflammation, chronic activation of glial cells and increased production of reactive oxygen species are involved in the pathogenesis and progression of AD. In in vitro studies and in patients with AD, DHA administration was able to induce microglial phagocytosis of Aβ-42, decrease IL-1beta, IL-6 production and the activation of proinflammatory transcription of the nuclear factor Kappa B (NFκB). 59 In animal models of AD, treatment with DHA has also been shown to increase levels of antioxidant enzymes catalase and glutathione peroxidase, as well as to reduce oxidative damage to the cerebral cortex and hippocampal cells. 59
Oxidative stress-induced by Aβ deposition is primarily responsible for TP hyperphosphorylation, which is associated with neuronal damage and induction of an apoptotic cascade. 59 In addition to the antioxidant effects already mentioned, DHA performance includes the regulation of the apoptotic cascade induced by Aβ at the level of lipid peroxides, conferring neuroprotection to neuronal cells. However, the effects of DHA on cognitive function and AD progression are absent in individuals who possess the APOE ε4 allele, which is associated with lower DHA uptake in the brain. 59
In the three studies that evaluated the use of omega-3 in AD, 35 , 36 , 37 supplementation was effective in delaying cognitive decline in individuals with AD, but there was a significant variation between studies in relation to the doses, proportion of EPA and DHA and association of antioxidants (only one study used DHA alone). Adverse effects were also reported in the studies, including changes in the International Normalized Ratio (INR) in subjects taking warfarin 36 and with diarrhea. 35 , 37
Improvement in several cognitive domains was also observed in a study of patients with advanced stages of the disease who were supplemented with a mixture of α-Linolenic fatty acids and Linoleic acid. 34 However, some methodological flaws of the study should be considered, including the use of an invalidated instrument for cognitive assessment and the absence of statistical treatment adjusting for confounding factors. Thus, other studies with greater methodological rigor are necessary to confirm the efficacy in the cognitive improvement of patients with AD.
Among micronutrient supplements, only supplementation with formula containing folic acid, vitamin B12, α-tocopherol, S-adenosyl methionine, N-acetyl cysteine, and acetyl-L-carnitine showed positive results in the cognitive performance of AD patients. 47 In in vitro experiments and in animal models of AD, the administration of such components has been associated with reduced oxidative stress and decreased Aβ production and TP phosphorylation. 43 However, such results should be interpreted with caution due to limitations and methodological flaws of the study.
Ginsenoside administration in AD animal models has been shown to be associated with a neuroprotective effect and better memory performance. 60 In the two studies in this review that evaluated Ginseng supplementation, 48 , 49 the treated groups demonstrated better cognitive performance in ADAS-cog and MMSE. However, limitations in the methodological quality of the trials do not allow us to draw a conclusion about the benefits found. Therefore, further studies with better methodological quality are necessary to evaluate the use of Ginseng supplementation in individuals with AD.
In individuals with advanced AD, supplementation for three months of a probiotic milk containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacteriumbifidum, and Lactobacillus fermentum resulted in improved performance in MMSE, but the mechanisms for this finding still need clarification. 56 Thus, further studies are needed to confirm this therapeutic potential and to elucidate the mechanism by which probiotics interfere with the neurodegenerative process of AD.
The use of inositol resulted in improved performance of AD patients in orientation and language cognitive domains. 57 However, this effect should be interpreted with caution once it was only a small trial and the sample was heterogeneous in relation to educational level, time of diagnosis and phase of dementia, factors that are known to influence cognitive performance. Therefore, further studies are needed to confirm a possible benefit with inositol supplementation.
Other interventions included the use of B-complex vitamins, Copper Orate, α-tocopherol, carotenoids, turmeric, soy isoflavones, resveratrol, and coconut oil, but there was no evidence of benefit in cognition in the studies.
The limitations of this review should be considered and include the limitation of articles in English and the impossibility of performing a meta-analysis due to methodological differences and the limited extent of available literature.
The strengths of this paper should also be highlighted and include the search in three databases, reading of titles and abstracts by two researchers and unlimited search for the date of publication of the articles.
The present systematic review points out that the effect of most dietary interventions on cognition in AD patients is inconclusive due to limited scientific evidence due to the poor methodological quality of the primary studies and the reduced number of studies. However, several nutrients associated and isolated DHA derived from algae show potential to improve cognitive function in AD, especially in its early stages. Thus, in a challenging scenario with a significant increase in the number of diagnoses of AD, better quality studies are urgently needed to confirm the therapeutic potential of the diet so that a dietary recommendation in AD that contributes to the quality of life of patients and relatives can be established.
Footnotes
This study was conducted at the Universidade Federal de Minas Gerais, Ringgold Standard Institution, Belo Horizonte, MG, Brazil.
REFERENCES
- 1.Petterson C. World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. London: Alzheimer’s Disease International; 2018. [Google Scholar]
- 2.Cohen RM. Epidemiology and clinical diagnosis; Alzheimer disease. PET Clin. 2013;8(4):391–405. doi: 10.1016/j.cpet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors andbiomarkers. Biochem Pharmacol. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera E, Jr, Caramelli P, Silveira AS, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16(2):103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bottino CM, Azevedo DJ, Tatsch M, Hototian SR, Moscoso MA, Folquitto AZ, et al. Estimate of dementia prevalence in a community sample from São Paulo, Brazil. Dement Geriatr Cogn Disord. 2008;26(4):291–299. doi: 10.1159/000161053. [DOI] [PubMed] [Google Scholar]
- 6.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcanti JLS, Engelhardt E. Aspectos da fisiopatologia da doença de Alzheimer esporádica. Rev Bras Neurol. 2012;48(4):21–29. [Google Scholar]
- 8.Kumar A, Singh A, Ekavali A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Dubois B, Hampel H, Feldman HH, Sheltens P, Aisen P, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honig LS, Boyd CD. Treatment of Alzheimer’s disease: current management and experimental therapeutics. Curr Transl Geriatr Exp Gerontol Rep. 2013;2(3):174–181. doi: 10.1007/s13670-013-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Li Y, Shi X, Ma C. An overview on therapeutics attenuating amyloid β level in Alzheimer’s disease: targeting neurotransmission, inflammation, oxidative stress and enhanced cholesterol levels. Am J Transl Res. 2016;8(2):246–269. [PMC free article] [PubMed] [Google Scholar]
- 13.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, Guillén-Grima F, Martínez-González MA. Mediterranean diet and cognitive function: The SUN project. J Nutr Health Aging. 2015;19(3):305–312. doi: 10.1007/s12603-015-0441-z. [DOI] [PubMed] [Google Scholar]
- 15.Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. 2015;54(8):1311–1321. doi: 10.1007/s00394-014-0811-z. [DOI] [PubMed] [Google Scholar]
- 16.Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurol. 2014;83(16):1410–1416. doi: 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diets lows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier A, Barul C, Féart C, Helmer C, Bernard C, Periot O, et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement. 2015;11(9):1023–1031. doi: 10.1016/j.jalz.2015.06.1888. [DOI] [PubMed] [Google Scholar]
- 19.Berti V, Walters M, Sterling J, Quinn CG, Logue M, Andrews R, et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90(20):e1789–e1798. doi: 10.1212/WNL.0000000000005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusufov M, Weyandt LL, Piryatinsky I. Alzheimer’s disease and diet: a systematic review. Int J Neurosci. 2017;127(2):161–175. doi: 10.3109/00207454.2016.1155572. [DOI] [PubMed] [Google Scholar]
- 21.Dangour AD, Whitehouse PJ, Rafferty K, Mitchell SA, Smith L, Hawkesworth S, et al. B-vitamins and fatty acids in the prevention and treatment of Alzheimer's disease and dementia: a systematic review. J Alzheimers Dis. 2010;22(1):205–224. doi: 10.3233/jad-2010-090940. [DOI] [PubMed] [Google Scholar]
- 22.Ramesh BN, Sathyanarayana Rao TS, Prakasam A, Sambamurti K, Jagannatha Rao KS. Neuronutrition and Alzheimer's disease. J Alzheimers Dis. 2010;19(4):1123–1139. doi: 10.3233/jad-2010-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essa MM, Vijayan RK, Castellano-Gonzales G, Memon MA, Braidy N, Guillemin GJ. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res. 2012;37(9):1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 24.Higgnis JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration; Sep, 2008. [Jan 10, 2017]. Available at: http://www.cochrane-handbook.org. [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Lauque S, Arnaud-Battandier F, Gillette S, Plaze JM, Andrieu S, Cantet C, et al. Improvement of weight and fat-free mass with oral nutritional supplementation in patients with Alzheimer's disease at risk of malnutrition: A prospective randomized study. J Am Geriatr Soc. 2004;52(10):1702–1707. doi: 10.1111/j.1532-5415.2004.52464.x. [DOI] [PubMed] [Google Scholar]
- 27.Planas M, Conde M, Audivert S, Pérez-Portabella C, Burgos R, Chacón P, et al. Micronutrient supplementation in mild Alzheimer disease patients. Clin Nutr. 2004;23(2):265–272. doi: 10.1016/s0261-5614(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 28.Salas-Salvadó J, Torres M, Planas M, Altimir S, Pagan C, Gonzalez ME, et al. Effect of oral administration of a whole formula diet on nutritional and cognitive status in patients with Alzheimer's disease. Clin Nutr. 2005;24(3):390–397. doi: 10.1016/j.clnu.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikkert MG, Wurtman RJ, Wilkinson D, et al. Efficacy of a medical food in mild Alzheimer’s disease: A randomized controlled trial. Alzheimers Dement. 2010;6(1):1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.De Sousa OL, Amaral TF. Three-week nutritional supplementation effect on long-term nutritional status of patients with mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26(2):119–123. doi: 10.1097/wad.0b013e31822c5bb3. [DOI] [PubMed] [Google Scholar]
- 31.Scheltens P, Twisk JWR, Blesa R, Scarpini E, Von Arnim CAF, Bongers A, et al. Efficacy of souvenaid in mild Alzheimer's disease: Results from a randomized, controlled trial. J Alzheimers Dis. 2012;31(1):225–236. doi: 10.3233/jad-2012-121189. [DOI] [PubMed] [Google Scholar]
- 32.Shah RC, Kamphuis PJ, Leurgans S, Swinkels SH, Sadowsky CH, Bongers A. The S-Connect study: results from a randomized, controlled trial of souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther. 2013;5(6):59–59. doi: 10.1186/alzrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(12):965‐75–965‐75. doi: 10.1016/S1474-4422(17)30332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yehuda S, Rabinovtz S, Carasso RL, Mostofsky DI. Essential fatty acids preparation (SR-3) improves Alzheimer’s patients quality of life. Intern J Neurosci. 1996;87(3-4):141–149. doi: 10.3109/00207459609070833. [DOI] [PubMed] [Google Scholar]
- 35.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 36.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, van Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's disease. J Alzheimers Dis. 2014;38(1):111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano M, Ernesto C, Thomas RG, Klauber MR, Shafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med. 1997;336(17):1216–1222. doi: 10.1056/nejm199704243361704. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Lu CJ, Chien KL, Chen ST, Che RC. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007;29(10):2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Kessler H, Bayer TA, Bach D, Schneider-Axmann T, Supprian T, Herrmann W, et al. Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: a pilot phase 2 clinical trial. J Neural Transm (Vienna) 2008;115(8):1181–1187. doi: 10.1007/s00702-008-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloret A, Badía M, Mora NJ, Pallardó FV, Alonso M, Viña J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17(1):143–149. doi: 10.3233/jad-2009-1033. [DOI] [PubMed] [Google Scholar]
- 43.Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer's disease: a placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24(1):27–33. doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69(7):836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan JM, Loskutova E, Howard A, Mulcahy R, Moran R, Stack J, et al. The impact of supplemental macular carotenoids in Alzheimer’s disease: a randomized clinical trial. J Alzheimers Dis. 2015;44(4):1157–1169. doi: 10.3233/jad-142265. [DOI] [PubMed] [Google Scholar]
- 47.Remington R, Bechtelb C, Larsenc D, Samard A, Doshanjhf L, Fishman P, et al. A phase ii randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. J Alzheimers Dis. 2015;45(2):395–405. doi: 10.3233/jad-142499. [DOI] [PubMed] [Google Scholar]
- 48.Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 49.Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, Shim JY, et al. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer's disease. Nutr Neurosci. 2012;15(6):278–282. doi: 10.1179/1476830512y.0000000027. [DOI] [PubMed] [Google Scholar]
- 50.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 51.Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4(5):43–43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: A double-blind randomized clinical trial. Hum Psychopharmacol. 2014;29(4):351–359. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- 53.Gleason CE, Fischer BL, Dowling NM, Setchell KD, Atwood CS, Carlsson CM, et al. Cognitive effects of soy isoflavones in patients with Alzheimer's disease. J Alzheimers Dis. 2015;47(4):1009–1019. doi: 10.3233/jad-142958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan SC, Esther GE, Yip HL, Sugathan S, Chin PS.Effect of cold pressed coconut oil on cognition and behavior among patients with Alzheimer's disease - A pilot intervention study Natl J Physiol Pharm Pharmacol 20177121432–1435. 10.5455/njppp.2017.0829311082017 [DOI] [Google Scholar]
- 56.Akbari E, Asemi Z, Kakhaki RD, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;10(8):256–256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barak Y, Levine J, Glasman A, Elizur A, Belmaker RH. Inositol treatment of alzheimer’s disease. a double blind, cross-over placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatr. 1996;20(4):729–735. doi: 10.1016/0278-5846(96)00043-7. [DOI] [PubMed] [Google Scholar]
- 58.van Wijk N, Broersen LM, de Wilde MC, Hageman RJ, Groenendijk M, Sijben JW, et al. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38(3):459–479. doi: 10.3233/jad-130998. [DOI] [PubMed] [Google Scholar]
- 59.Belkouch M, Hachem M, Elgot A, Van AL, Picq M, Guichardant M, et al. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer's disease. J Nutr Biochem. 2016;38:1–11. doi: 10.1016/j.jnutbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Sheng C, Peng W, Xia ZA, Wang Y, Chen Z, Su N, et al. The impact of ginsenosides on cognitive deficits in experimental animal studies of Alzheimer's disease: a systematic review. BMC Complement Altern Med. 2015;15:1–15. doi: 10.1186/s12906-015-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


