Abstract
Although chimeric antigen receptor (CAR) T cells have demonstrated signs of antitumor activity against glioblastoma (GBM), tumor heterogeneity remains a critical challenge. To achieve broader and more effective GBM targeting, we developed a peptide-bearing CAR exploiting the GBM-binding potential of chlorotoxin (CLTX). We find that CLTX peptide binds a great proportion of tumors and constituent tumor cells. CAR T cells using CLTX as the targeting domain (CLTX-CAR T cells) mediate potent anti-GBM activity, and efficiently target tumors lacking expression of other GBM-associated antigens. Treatment with CLTX-CAR T cells resulted in tumor regression in orthotopic xenograft GBM tumor models. Importantly, CLTX-CAR T cells do not exhibit observable off-target effector activity against normal cells, or following adoptive transfer into mice. Effective targeting by CLTX-CAR T cells requires cell surface expression of matrix metalloproteinase-2 (MMP-2). Our results pioneer a peptide toxin in CAR design, expanding the repertoire of tumor-selective CAR T cells with the potential to reduce antigen escape.
One sentence summary:
CAR T cells using chlorotoxin as the tumor-targeting domain recognize and kill glioblastoma with high specificity and potency.
Introduction
Glioblastoma (GBM) is the most common type of primary brain tumor. Despite increasingly aggressive treatments incorporating surgery, chemotherapy and radiotherapy, survival of patients with GBM has only modestly improved over the last several decades (1). Such poor prognosis has prompted the development of advanced therapies, among which is immunotherapy using T cells engineered to express chimeric antigen receptors (CARs) (2, 3). CAR T cell therapy redirects the cytotoxic activity of T lymphocytes independent of MHC restriction and without need for antigen priming. This cellular therapy, therefore, provides a strategy to generate de novo antitumor immunity, which may help overcome the challenges of highly heterogeneous expression of targetable tumor antigens, as well as the lack of intrinsic immunogenicity for tumors such as GBMs with low mutational burdens (4, 5). We and others have demonstrated that CAR T cell therapy can be successfully translated for the treatment of GBM (6-9), demonstrating safety, evidence for antitumor activity, and in one case, the potential for mediating complete tumor remission (7).
Despite encouraging evidence of clinical safety and bioactivity for GBM-targeted CAR T cells, the overall response rates have been unsatisfyingly low, especially as compared to the remarkable clinical responses achieved against B cell malignancies (10, 11). One of the major obstacles limiting CAR T cell therapeutic efficacy has been tumor heterogeneity, which is particularly substantial in GBMs. The classification of GBM subtypes has illustrated the heterogeneity across patients, and more recent studies using single cell sequencing also revealed considerable genetic variations among intratumoral subpopulations, as well as plasticity between different cellular states (12, 13). Efforts to develop CAR T cell immunotherapy must contend with this high diversity of potential target antigen expression. For example, CAR T cells targeting IL13 receptor α2 (IL13Rα2) are under active clinical development (7, 14), as we and others have reported that expression of IL13Rα2 is frequently found on GBM tumors, and on a high proportion of cells within these tumors (15). However, after treating patients with IL13Rα2-targeted CAR T cells, instances of tumor recurrence with loss and/or reduced expression of IL13Rα2 has been observed (7, 14). Similar results have been reported following EGFR variant III (EGFRvIII)-targeted immunotherapies, with lower EGFRvIII expressions in recurrent tumors post-therapy (9, 16). In general, tumors are able to rapidly adapt to the selection pressures imposed by immunotherapies, resulting in relapsed tumors with distinct intratumoral cellular profiles (17), so-called antigen escape. The clinical performance of CAR T cell therapy against B cell malignancies is greatly aided by the homogenous expression of CD19 as a target antigen on all B cell lineages and malignancies (18). Therapeutic outcomes for GBM-targeting CAR T cell designs would thus be expected to benefit from immunotherapies with broader tumor recognition, but progress has been limited by the scarcity of brain tumor antigen candidates that are both widely expressed and highly specific.
An opportunity to extend the repertoire of target antigens amenable to CAR T cell therapy is presented by the tumor-binding potential of some naturally-derived molecules (19). One example is chlorotoxin (CLTX), a 36-amino acid peptide isolated from the venom of the death stalker scorpion Leiurus quinquestriatus (20). The GBM-binding potential of CLTX was first identified through conjugation with the radioisotope 131I (21). Subsequently, CLTX has been established to bind broadly and specifically to GBM and other neuroectodermal tumors, while showing minimal cross-reactivity with non-malignant cells in brain and elsewhere (22). Although the precise cell surface receptor for CLTX on GBM cells remains unclear, CLTX binding impairs GBM cell migration and invasiveness (23, 24). CLTX itself is non-cytotoxic to tumor and normal tissues (25, 26). Consequently, efforts have focused on using CLTX for tumor-specific delivery of cytotoxic agents (27). Preclinically, CLTX has been used to coat a variety of vehicles for delivery of chemotherapeutics and small interfering RNAs (siRNAs) (28, 29). Further, an early clinical study in patients with GBM demonstrated that 131I-conjugated CLTX radiotherapy delivered into the post-resection cavity containing residual tumor was well-tolerated and without major neurotoxicity (25). More recently, the utility of fluorescently-labeled CLTX to specifically mark tumor cells during surgery has been demonstrated in preclinical models, and confirmed in a clinical study of glial tumors showing safety of intravenously delivered CLTX and tumor-specific uptake (26, 30-32). These studies indicate that CLTX can be safely employed for tumor-specific targeting.
Here we sought to develop a CAR T cell using CLTX peptide as the tumor-targeting domain. We optimized the design of CLTX-CAR T cells, and tested their antitumor activity against a panel of patient-derived GBM lines as well as in orthotopic mouse xenograft models. We also evaluated potential off-tumor targeting and toxicity against normal human cells, tissues, and in CLTX cross-reactive mouse models. These studies aim to expand the repertoire of GBM-targeting CAR T cells and address the challenge of tumor heterogeneity by achieving broad tumor cell coverage.
Results
CLTX binds to a broad spectrum of GBM cells.
Previous studies have documented the GBM-selective binding properties of CLTX (21, 22). However, CTLX binding, in relation to other tumor associated antigens or to GBM subpopulations, has not been specifically examined. Therefore, we first evaluated CLTX binding to freshly-dissociated tumor cells from surgical resection specimens. These primary brain tumor (PBT) cells were examined by flow cytometry for binding of Cy5.5-conjugated CLTX peptide (CLTX-Cy5.5) and compared with expression of IL13Rα2, HER2 and EGFR, three receptors being clinically evaluated as CAR T cell immunotherapy targets for GBM (9, 14, 33). Strong CLTX-Cy5.5 binding was observed for almost all patient tumors, with greater than 80% of cells binding CLTX (Fig. 1A and 1B, left panel). Across 23 tumor samples from 15 different patients, only samples from two patients with GBM (PBT114 and PBT131) showed CLTX-Cy5.5 binding in less than 40% of total cells. At the same time, expression of immunotherapy targets IL13Rα2, HER2 and EGFR varied widely between patient tumors. CLTX-Cy5.5 binding appeared to be independent of other antigens, and was observed on tumors with both high and low expression of IL13Rα2, HER2 and EGFR (see representative flow cytometry histograms in Fig. 1A).
Fig. 1. CLTX binds broadly to freshly-dispersed primary GBM cells and to cultured tumor lines.
(A) Freshly dispersed viable (DAPI-) patient brain tumor (PBT) GBM cells (CD45- CD31-) were immunostained for expression of IL13Rα2, HER2 and EGFR, or binding by CLTX-Cy5.5. Percentages of stained cells (blue) above isotype control (grey) are indicated in each histogram. (B) Summary of results [% positive as in (A)] for 23 freshly-dispersed (primary) PBTs (left) and 22 cultured PBT-tumor spheres (TS) (right). nd, not done. (C) Representative phenotype of a GBM xenograft established by stereotactic injection and engraftment of 1 × 105 PBT106-TS cells in the right forebrain of an NSG mouse. Tumor-bearing mouse brain was harvested 97 days after cell injection, and paraffin sections were stained with antibodies against IL13Rα2 and EGFR, and with biotin-conjugated CLTX. Staining was visualized by fluorochrome-conjugated secondary antibodies or streptavidin, and DAPI to identify nuclei. Scale bars: 20 μm. (D) Freshly-dispersed GBM samples separated into CD44+ and CD44− (left, n=11) or CD133+ and CD133− (right, n=15) subpopulations, and examined for differences in CLTX-Cy5.5 staining (measured as mean fluorescence intensity, MFI). Only GBM samples with > 20% CD44+ or CD133+ fractions were analyzed. (E) PBT-TS lines (n=7), maintained in neural stem cell medium (TS) or differentiation medium (Dif) for 14 days, were evaluated for CLTX-Cy5.5 staining and CD133 expression (MFI). (D, E) p values evaluated by paired two-way Student’s t-test.
We also examined CLTX-Cy5.5 binding to low-passage patient-derived GBM tumor sphere (PBT-TS) lines, expanded under conditions favoring cancer stem cell-like phenotypes (34). Similar to dissociated patient tumor samples, 21 of 22 PBT-TS lines (95.5%) showed greater than 70% CLTX-Cy5.5 binding (Fig. 1B right panel and Fig. S1A), displaying a wider coverage compared to IL13Rα2, HER2 and EGFR, as well as the relatively widely-expressed GBM-associated antigen EphA2 (35, 36). To evaluate CLTX binding in engrafted tumors, GBM orthotopic xenografts were examined by fluorescence microscopy using biotin-conjugated CLTX peptide. Consistent with the analyses of freshly resected patient tumors, CLTX displayed consistent binding to all engrafted GBM xenograft tumors established using five different patient-derived PBT-TS lines, and marked a greater proportion of tumor cells as compared to the expression of IL13Rα2 and EGFR (Fig. 1C and Fig. S1B). Taken together, these studies confirmed the capacity of CLTX to bind to a high percentage of patient GBM tumors, as well as to the majority of GBM cells within each tumor.
GBM tumors are highly heterogeneous, composed of phenotypically distinct cellular subpopulations. Within GBM tumors, stem-like cells (GSCs) display self-renewal and tumor-initiation capacity (37), identifying this population as a therapeutically important component of a GBM-targeted CAR T cell therapy (33, 38, 39). Hence, we examined CLTX-Cy5.5 binding with respect to this stem cell-like population. In one approach, using freshly-dissociated primary PBTs, we distinguished two (often non-overlapping) GSC populations by expression of surface markers CD133 and CD44 (Fig. 1B left) (40, 41). We observed CLTX-Cy5.5 binding to both GSC populations as well as non-GSCs, with slightly higher fluorescence intensity in stem cell-like (CD44+ or CD133+) versus differentiated (CD44- or CD133-) cells (Fig. 1D). In another approach, we used PBT-TS lines and varied culture conditions to favor growth of GSCs or, alternatively, to promote differentiation (38). Although in vitro differentiation led to reduced expression of the GSC-marker CD133, it did not reduce CLTX-Cy5.5 binding (Fig. 1E). These studies demonstrated that CLTX- binding, while showing some preference for CD133+ GSCs in freshly dispersed tumor samples, remains robust on both stem-like and more differentiated GBM cells. Together, these studies confirm the broad GBM binding potential of CLTX, providing rationale for investigating its use for CAR T cell immunotherapy.
CLTX-CAR T cell design can be optimized through modifying non-targeting domains
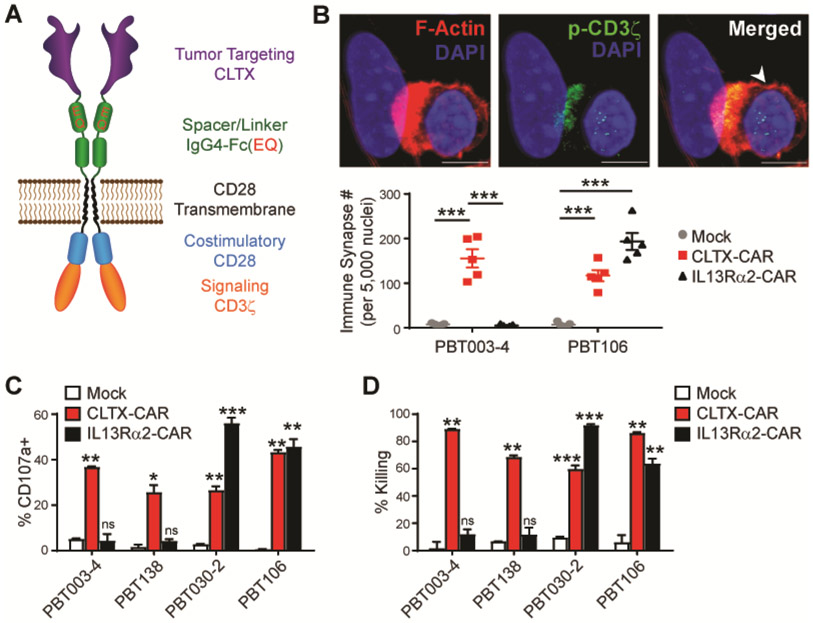
We next sought to design an optimized CAR incorporating the CLTX peptide as the tumor targeting domain. The initial CLTX-CAR was generated using the backbone of our CD19-targeted CAR that has shown safety and clinical activity against B-cell malignancies (42). This CLTX-CAR construct is comprised of the CLTX peptide, an IgG4Fc(EQ) spacer, and a CD28 costimulatory domain (Fig. 2A), and is referred to as CLTX-EQ-28ζ. Cytotoxicity of CLTX-EQ-28ζ CAR T cells was evaluated against a panel of GBM PBT-TS lines. CLTX-EQ-28ζ CAR T cells formed immunological synapse-like structures with tumor cells within 2 hours (Fig. 2B). The number of CLTX-CAR T cell-tumor cell conjugates was equivalent to IL13Rα2-targeted CAR T cells between 2.5 and 5 hours, although the formation rate appeared to be slower with CLTX-CAR T cells (0.5 to 1.5 hours) (Fig. S2A). Co-culture with GBM cells stimulated CLTX-EQ-28ζ CAR T cells to up-regulate T cell activation markers CD69 and 4-1BB (CD137), and to degranulate as measured by cell-surface CD107a expression (Fig. 2C and Fig. S2B). Further, CLTX-EQ-28ζ CAR T cells efficiently killed GBM cells in 48-hour co-culture assays at an effector to target (E:T) ratio of 1:4 (Fig. 2D). Of note, CLTX-EQ-28ζ CAR T cells were effective against PBT-TS lines from four different patients that displayed distinct IL13Rα2, HER2 and EGFR expression profiles. In particular, while IL13Rα2-targeted CAR T cells failed to respond to the PBT-TS lines with low-to-negative IL13Rα2 expression (PBT003-4-TS, PBT138-TS), CLTX-EQ-28ζ CAR T cells recognized and responded to all four TS lines (Fig. 2, B-D). Further, utilizing PBT003-4-TS cells, which show negligible expression of IL13Rα2, HER2 or EGFR, we demonstrated that co-culture with CLTX-EQ-28ζ, but not the combination of CAR T cells targeting all three other antigens, mediated tumor cell elimination (Videos S1 and S2). Lentiviral transduction of PBT003-4-TS to over-express IL13Rα2, HER2 or EGFR did not reduce CLTX-EQ-28ζ cytotoxic activity (Fig. S2, C and D). We thus conclude that CLTX-EQ-28ζ can recognize and target GBM cells, and its cytotoxicity is consistent with the broad GBM-binding potential of the CLTX peptide while independent of other GBM-associated antigens.
Fig. 2. Effector activity of CLTX-CAR T cells.
(A) Diagram of a CAR incorporating a CLTX tumor targeting domain, an IgG4-Fc spacer domain with EQ mutations, a CD4 transmembrane domain, and intracellular costimulatory and signaling domains (CD28 and CD3ζ). (B) Immunological synapse formation at 2 hr after adding CLTX-CAR T cells to dissociated PBT003-4-TS or PBT106-TS GBM cells (E:T = 1:1). Top, a representative image of an immunological synapse indicated by co-localization of phosphorylated-CD3ζ (p-CD3ζ) and polarized F-actin accumulation at the interface between the CLTX-EQ-28ζ CAR T cell (arrowhead) and PBT003-4 tumor cell. Scale bars: 5 μm. Bottom, Number of immunological synapses per 5,000 tumor nuclei, for mock-transduced (mock), CLTX-CAR, or IL13Rα2-CAR T cells, co-cultured with dissociated PBT003-4-TS (IL13Rα2-negative) or PBT106-TS (IL13Rα2-positive) GBM cells. Five fields were counted for each group. Shown is mean ± SEM; *** denotes p < 0.001. (C) Degranulation of mock, CLTX-CAR or IL13Rα2-CAR T cells (% CD107a) after 5 hr co-culture with either IL13Rα2-negative (PBT003-4-TS, PBT138-TS) or IL13Rα2-positive (PBT030-2-TS, PBT106-TS) GBM cells (E:T ratio = 1:1). CAR T cells (gated as CD3+ CD19t+) were analyzed by flow cytometry for surface CD107a expression as a marker of degranulation. Shown are mean ± SEM of % CD107a+ cells in duplicate wells. (D) Percent total target cell killing of different PBT-TS cells co-cultured with mock, CLTX-CAR, or IL13Rα2-CAR T cells (E:T ratio = 1:4; 48 hr). Shown are mean ± SEM of % cell killing in duplicate wells. (C, D) ns denotes not significant (p>0.05), * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001 comparing CAR vs Mock, by one-way ANOVA with Bonferroni’s multiple comparison correction.
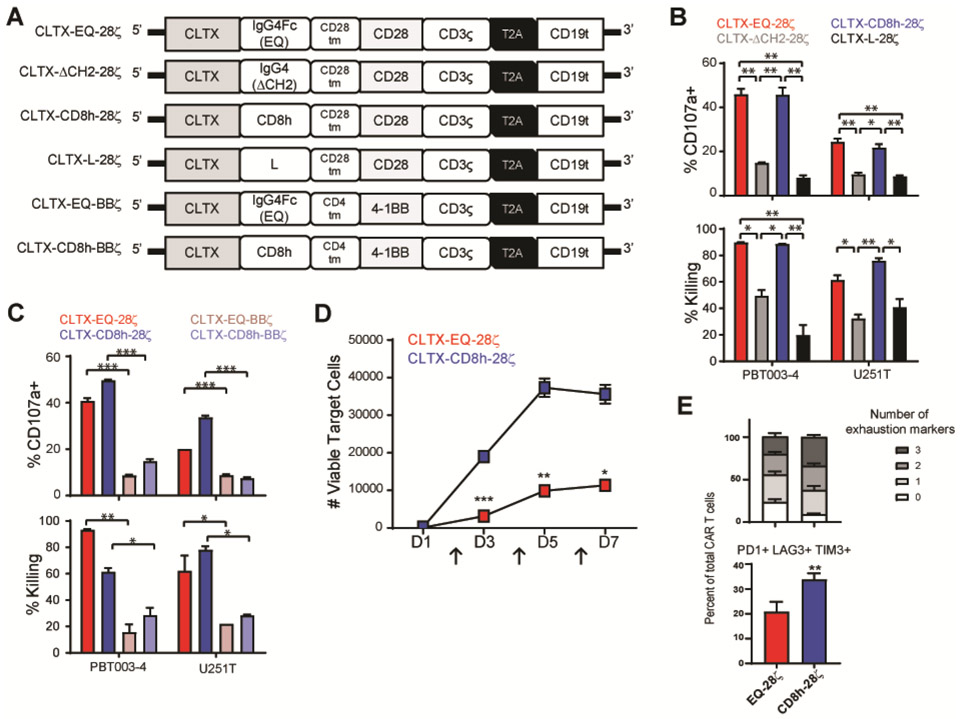
The cytolytic activity of CAR T cells is greatly influenced by regions outside of the antigen-targeting domain, including the spacer (43) and the co-stimulatory domains (44). Using the CLTX-EQ-28ζ CAR as a reference, we first addressed the impact of spacer length by generating CAR constructs to compare IgG4Fc(EQ) (239 amino acids) with three shorter spacers: IgG4-Fc with the CH2-domain deleted (∆CH2) (129 amino acids), CD8 hinge (CD8h) (44 amino acids), and a short synthetic linker (L) (10 amino acids) (Fig. 3A and data file. S2). We observed that CAR T cell-mediated tumor killing was greatly reduced with ∆CH2 or L spacers, while the CD8h spacer retained CAR function similar to that of IgG4Fc(EQ) (Fig. 3B). We next evaluated contributions of co-stimulatory signals by generating CARs bearing CD28 or 4-1BB co-stimulatory domains in the context of the more efficacious spacers IgG4Fc(EQ) and CD8h (Fig. 3A). Intriguingly, CAR T cells incorporating a CD28 co-stimulation domain consistently showed higher killing potency as compared with CARs incorporating a 4-1BB co-stimulation domain (Fig. 3C).
Fig. 3. Optimization of CLTX-CAR design for effector potency.
(A) Schemas of CLTX-CARs incorporating different spacers [IgG4Fc(EQ), IgG4(ΔCH2), L, CD8h] and co-stimulatory domains (CD28, 4-1BB). (B) Evaluation of alternative spacers for CLTX-CAR T cells with shared CD28 costimulatory domain, tested against PBT003-4-TS or U251T GBM cells. Indices of efficacy were degranulation (% CD107a+; top) and cytotoxicity (% target cell killing; bottom). (C) Evaluation of alternative costimulatory domains for CLTX-CAR T cells with IgG4Fc(EQ) or CD8h spacers, tested against PBT003-4-TS or U251T GBM cells. Measured were degranulation (top) and cytotoxicity (bottom). (B, C) Shown are mean ± SEM of duplicate wells; * denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001 by one-way ANOVA with Bonferroni’s multiple comparison correction. (D) Evaluation in a repetitive re-challenge assay of CLTX-CAR T cells with alternative spacers [IgG4Fc(EQ) or CD8h] and shared CD28 costimulatory domain. CAR T cells were co-cultured with PBT003-4-TS cells (4,000 CAR+ T cells, 16,000 tumor cells), repetitively challenged with 32,000 tumor cells every 48 hours (arrows at D2, D4 and D6), and numbers of remaining viable tumor cells were quantified at the indicated time points (D1, D3, D5 and D7). (E) Evaluation of alternative spacer domains by comparison of co-expression of inhibitory receptors PD-1, LAG-3 and TIM-3 by CLTX-EQ28ζ and CLTX-CD8h28ζ CAR T cells at day 4 of re-challenge with PBT003-4-TS tumor cells. (D, E) Shown are mean ± SEM of triplicate wells;** denotes p < 0.01 by unpaired Student’s t-test.
We then considered possible mechanisms underlying functional differences across CLTX-CAR constructs. Notably, for the six CLTX-CAR constructs evaluated, measures of tumor-dependent activation for each construct were consistent across multiple assays: killing potency (% Killing), extent of degranulation (% CD107a+), and secretion of cytokine IFNγ (Fig.3, B and C, and Fig. S3A). Moreover, consistent with the stronger cytotoxic effector functions of CLTX-EQ-28ζ and CLTX-CD8h-28ζ CARs, both also showed more frequent expression of T cell activation markers 4-1BB and CD69 (Fig. S3B), and inhibitory molecule PD-1 (which is linked to T cell activation) (Fig. S3C) after tumor cell exposure. These results therefore suggest that differences in initial activation were the major contributor to variations in effector function between various CLTX-CAR designs.
We next returned to the comparison of effector potency between CLTX-EQ-28ζ and CLTX-CD8h-28ζ CAR T cells. First, screening degranulation and cytokine-production against a panel of 10 PBT-TS lines, we observed that CLTX-EQ-28ζ CAR T cells displayed higher degranulation, but lower IFNγ production as compared to CLTX-CD8h-28ζ CAR T cells (Fig.S3, D and E). Next, the capacities of CLTX-EQ-28ζ and CLTX-CD8h28ζ CAR T cells to maintain long-term antitumor activity were evaluated in a repetitive tumor challenge assay in vitro (45, 46). We observed that CLTX-EQ-28ζ, but not CLTX-CD8h-28ζ, CAR T cells retained activity through multiple rounds of tumor challenge (Fig. 3D and S3F). Since both CLTX-EQ-28ζ and CLTX-CD8h-28ζ CAR T cells upregulated PD-1 expression upon tumor stimulation, we then assessed co-expression of three T cell inhibitory receptors (PD-1, LAG-3 and TIM-3), which together mark exhausted T cells (45, 47), and observed that the absence of durable effector function in CLTX-CD8h-28ζ CAR T cells was associated with an exhausted phenotype featuring co-expression of all three inhibitory receptors (p=0.009) (Fig. 3E). Considering together these observations about effector potency, we adopted the CLTX-EQ-28ζ CAR as the optimal design for subsequent evaluation.
CLTX-CAR T cells mediate antitumor activity against established GBM xenografts
To test the in vivo antitumor activity of CLTX-EQ-28ζ CAR T cells, xenograft tumors were established using two patient-derived PBT-TS lines with distinct antigen expression patterns: PBT003-4-TS (<15% expression of IL13Rα2, HER2 or EGFR) and PBT106-TS(>65% expression of IL13Rα2, HER2 and EGFR). Against subcutaneously engrafted GBMs, intratumoral administration of CLTX-EQ-28ζ CAR T cells resulted in tumor regression, whereas tumors injected with mock-transduced T cells displayed growth kinetics similar to tumor-only controls (Fig. S4A). Next, the antitumor function of CLTX-EQ-28ζ CAR T cells was evaluated in orthotopic GBM models (48). Consistent with the subcutaneous tumor model, intracranial-tumor (ICT) administered CLTX-EQ-28ζ CAR T cells, but not mock T cells, controlled tumor growth and prolonged the survival of mice bearing PBT003-4-TS or PBT106-TS tumors (Fig. 4, A-D). We then examined the contribution of delivery routes to CLTX-CAR T cell therapeutic efficacy (Fig. S4C), and observed that the intracerebroventricular (ICV) route also mediated potent antitumor activity, but required slightly higher CAR T cell doses to obtain similar efficacy compared with ICT delivery (Fig. S4, C-E). By contrast, intravenous (IV) delivery yielded minor therapeutic benefit (Fig. S4, C-E), consistent with our previous results observed with other CAR T cells (49, 50). The in vivo antitumor potency of CLTX-CAR T cells was similar compared with IL13Rα2-CAR T cells (Fig. S4F).
Fig. 4. In vivo antitumor activity of CLTX-CAR T cells.
ffLuc+ PBT106-TS (A,B) or PBT003-4-TS (C,D) GBM cells were stereotactically implanted into the right forebrain of NSG mice (1 × 105 cells/mouse). On day 8 post tumor implantation, mice received either no treatment (Tumor only, n = 5-6); intracranial-tumor (ICT) treatment with 1 × 106 mock-transduced T cells (Mock, n = 6) or CLTX-EQ-28ζ CAR T cells (CLTX-CAR, n = 6-8). (A,C) Kaplan Meier survival analysis with Log-rank (Mantel Cox) test comparing CLTX-EQ28ζ CAR T cell and Mock T cell treated groups. (B,D) Tumor volumes over time monitored using bioluminescent imaging. (E) Tumors harvested from NSG mice bearing PBT003-4-TS GBM xenografts that were either untreated (n = 5) or relapsed from those treated with CLTX-CAR T cells (n = 3), were dissociated into single cell suspensions and stained with CLTX-Cy5.5. Lines indicate mean MFI ± SEM. (F) Immunochemical staining for CD3 and granzyme B on mouse brain sections at D14 post T cell injection and 7 days after tumor clearance (top), or on the relapsed tumor (bottom). (G) PD-L1 staining on untreated (top) or relapsed (bottom) PBT003-4-TS tumors. (H) Expression of IFNγ Receptor A (IFNγRA) on GBM cells dissociated from PBT003-4-TS and PBT106-TS. Percentages of immunoreactive cells (blue) above that of isotype control staining (grey) are indicated in each histogram. (I) PBT003-4-TS and PBT106-TS GBM cells were co-cultured with mock or CLTX-CAR T cells for 24 hr, and condition media were transferred to fresh GBM cells and cultured for 24 hr. Numbers indicate PD-L1 MFI above isotype control. Shown are mean ± SEM of triplicate wells; ** denotes p < 0.01 by unpaired Student’s t-test.
Of note, although CLTX-CAR T cells had potently targeted both PBT003-4-TS and PBT106-TS in vitro, the responses of mice with engrafted tumors were not equivalent. After ICT CLTX-CAR T cell administration, all mice previously bearing PBT106-TS tumors remained tumor-free for over 170 days, whereas only a subset of PBT003-4-TS tumor-bearing mice achieved similar long-term tumor eradication (Fig. 4A-D and Fig. S4B). To investigate mechanisms underlying this variation in anti-tumor response, we examined recurrent PBT003-4-TS tumors post CLTX-EQ-28ζ CAR T cell therapy. We first observed that CLTX-Cy5.5 binding to recurrent tumors was similar to untreated tumors (Fig. 4E), suggesting that antigen escape did not account for tumor relapse. Examining the CAR T cells within these orthotopic tumors, we found that during the primary antitumor response, granzyme B-expressing CAR T cells were detected 14 days following adoptive transfer (Fig. 4F top row). By comparison, the persisting CLTX-EQ-28ζ CAR T cells in the recurrent tumors only weakly stained for granzyme B (Fig. 4F bottom row). At the same time, these relapsed PBT003-4-TS tumors displayed increased expression of PD-L1 as compared to untreated tumors (Fig. 4G). It is well-established that PD-L1 expression can be induced by IFNγ signaling leading to tumor adaptive resistance to immunotherapy (51). Consistent with this, PD-L1 induction on PBT003-4-TS tumors post CAR treatment was associated with higher IFNγ receptor A (IFNγRA) expression before implantation (Fig. 4H). Further, we observed greater induction of PD-L1 on PBT003-4-TS cells, as compared to PBT106-TS, when incubated with conditioned media from CLTX-CAR T cells co-cultured with each tumor line (Fig. 4I). This difference was also evident in response to recombinant IFNγ treatment (Fig. S4G). Although other immunosuppressive mechanisms may be involved, these observations suggest that immunostimulatory cytokines produced by activated CAR T cells can induce GBM adaptive resistance pathways, such as PD-L1 induction, and hinder tumor eradication by CAR T cells.
CLTX-CAR T cells exhibit minimal off-target effects
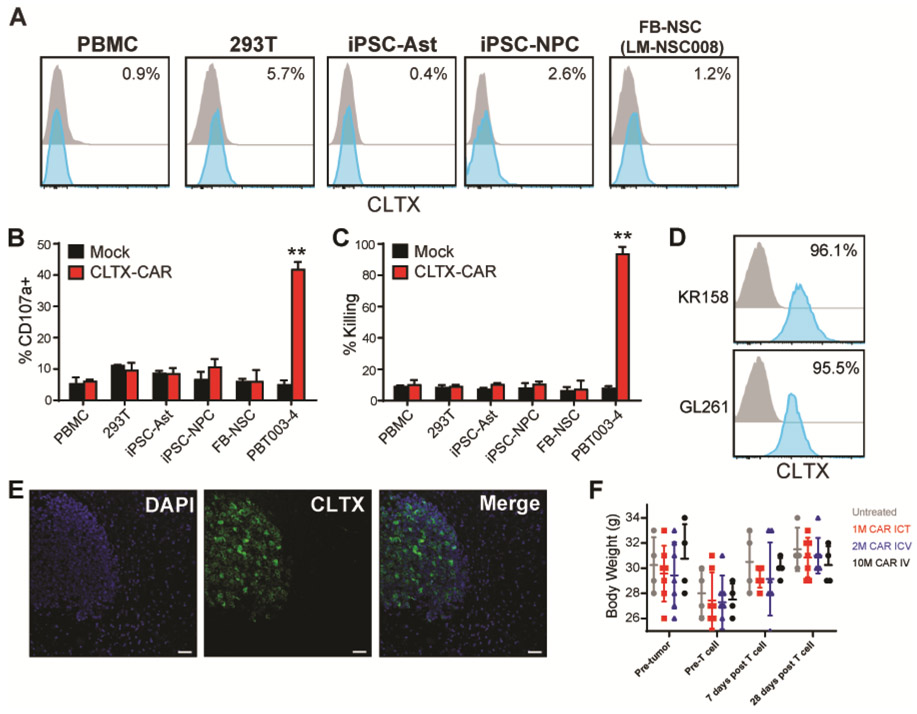
Prior clinical studies using CLTX to deliver radiation therapy and imaging reagents to tumor sites for patients with GBM have not reported significant adverse events (25, 32). Consistent with these reports, we observed limited to undetectable CLTX-Cy5.5 binding to a panel of human non-tumor cells, including peripheral blood mononuclear cells (PBMCs), human embryonic kidney 293T cells, as well as induced pluripotent stem cell-differentiated astrocytes (iPSC-Ast), neural progenitor cells (iPSC-NPCs) and immortalized fetal brain-derived neural stem cell (FB-NSC) line LM-NSC008 (Fig. 5A). Further, these normal cells were insufficient to trigger activation of CLTX-EQ-28ζ CAR T cells during co-culture. As indicated by degranulation and in vitro cytotoxicity assays (Fig. 5B and C), CLTX-EQ-28ζ CAR T cells did not target PBMC or 293T cells, or, in particular, neural lineage cells iPSC-Ast, iPSC-NPC and FB-NSC, exhibiting in all cases effector activity comparable to the mock T cell negative controls.
Fig. 5. Off-target evaluation of CLTX-CAR T cells.
(A) CLTX-Cy5.5 staining on viable peripheral blood mononuclear cells (PBMC), human embryonic kidney 293T cells, iPSC-derived astrocytes (iPSC-Ast) and neural progenitor cells (iPSC-NPC) or human fetal brain neural stem cells (FB-NSC line LM-NSC008), evaluated by flow cytometry. Percentages of stained cells (blue) above control (grey) are indicated in each histogram. (B-C) Degranulation (B) and cytotoxicity (C) of mock-transduced (Mock) or CLTX-EQ-28ζ CAR+ (CLTX-CAR) T cells against non-malignant cell lines, with PBT003-4-TS GBM cells as a positive control. Data are mean ± SEM of duplicate wells; **, p < 0.01 when compared with mock-transduced T cells using unpaired Student’s t-test. (D) CLTX-Cy5.5 staining on mouse GBM cell lines. Percentages of positively-stained cells (blue) above that of isotype control (grey) are indicated in each histogram. (E) Representative immunofluorescent phenotype of a GBM xenograft established by stereotactic injection of 1 × 105 PBT106-TS cells into the right forebrain of an NSG mouse. The tumor-bearing mouse brain was harvested 93 days after cell injection. Paraffin sections were stained with DAPI to identify nuclei, and CLTX-Cy5.5 to depict the border between xenograft and normal mouse brain. Scale bars: 20μm. (F) PBT106-TS GBM cells were implanted in NSG mice (2 × 105 cells/mouse), and after 8 days received ICT administration of 1 × 106, intracerebroventricular (ICV) administration of 2 × 106, or intravenous (IV) administration of 1 × 107, CLTX-CAR T cells (n = 4, 7, 7 and 4, respectively). Body weights were measured before tumor injection, before CAR T cell injection, and 7 days and 28 days after CAR T cell injection, and compared with untreated NSG mice. Lines indicate mean ± SEM.
The potential for toxicity was further assessed in mouse models, an approach justified by the observation that CLTX-Cy5.5 also binds to mouse GBM cells, as shown for the mouse KR158 and GL261 GBM lines (Fig. 5D). First, in mouse brains bearing GBM xenograft tumors, CLTX-Cy5.5 bound only to tumor cells but not surrounding normal brain tissue, thereby delineating the xenograft-tumor border (Fig. 5E). In addition, ICT and ICV regional administration of CLTX-EQ-28ζ CAR T cells in GBM-bearing mice in the experiments described above showed no evidence of adverse reaction, such as neurological symptoms and/or loss of body weight (Fig. 5F). After ICT administration of CLTX-EQ-28ζ CAR T cells to orthotopic GBM-bearing mice, no morphological alterations were evident in close examinations of multiple organs including brain, kidney, liver, spleen, intestine, colon, lung and bladder (Fig. S5A). The potential for systemic toxicity was first assessed by intravenous injection of 50 × 106 CLTX-EQ-28ζ CAR T cells into unmanipulated NSG mice. Even at this high dose, CLTX-CAR T cells were well-tolerated; all animals remained alert and active, and did not show changes in body weight (Fig. S5B). Further, while both mock and CLTX-CAR T cells were detected in lung 7 days after systemic IV injection, neither expressed granzyme B (Fig. S5, C-D). Additionally, no T cells were detected at day 14 in the lung, or at days 7 and 14 in intestine or bladder (Fig. S5, C-D). Together these results indicate the absence of CAR T cell activation or persistence in these organs. These observations are consistent with other preclinical studies of CLTX-redirected agents (26, 31), and strongly support our hypothesis that CLTX-EQ-28ζ CAR T cells specifically target GBM cells without causing observable toxicities.
MMP2-expression is required for CLTX-CAR targeting
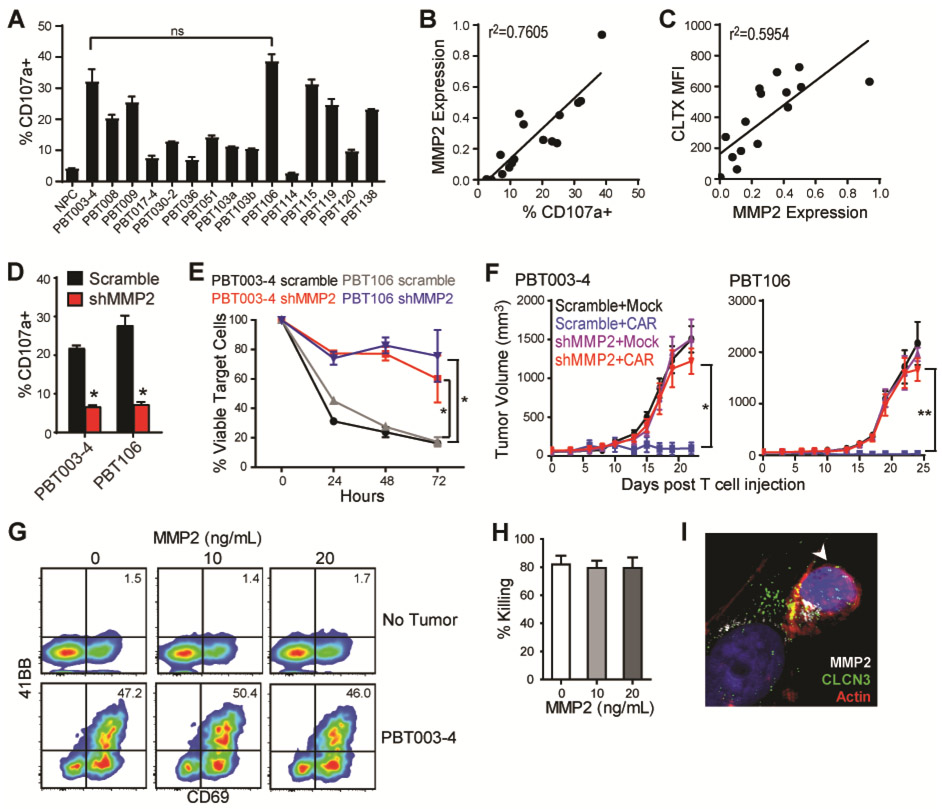
CLTX binding has been reported to associate with multiple membrane proteins, including membrane-bound matrix metalloproteinase 2 (MMP2), chloride channel CLCN3, and phospholipid protein annexin A2 (ANXA2) (23, 24, 52). To better understand target recognition, we used 15 different PBT-TS lines and iPSC-NPCs (negative control) as CLTX-EQ-28ζ CAR T cell targets, and assessed the correlations of MMP2, CCLN3, and ANXA2 expression on target cells with CLTX-CAR T cell activity (CD107a degranulation). We found that CLTX-EQ-28ζ CAR T cell activity varied between these different GBM target cells (Fig. 6A), and observed a strong correlation between CLTX-CAR T cell degranulation and target cell MMP2 expression (Fig. 6B), but no correlation with expression of CLCN3 or ANXA2 (Fig. S6A). Consistent with this pattern, CLTX-Cy5.5 binding correlated with MMP2 expression on target cells (Fig. 6C). Differences in CLTX-CAR cytotoxic potential against different GBM cells were most evident at a low E:T ratio of 1:8 (Fig. S6B), and correlated well with degranulation (Fig. S6C). Importantly, the extent of degranulation did not correlate with IFNγRA (CD119) expression on PBT-TS lines (Fig. S6D), which was previously related to low in vivo CLTX-CAR T cell function through induction of PD-L1. These results suggest that for GBM cells, MMP2 is the primary mediator of CLTX-Cy5.5 binding as well as the activation of CLTX-CAR T cells.
Fig. 6. CLTX-CAR T cell effector activity requires MMP2 expression on target cells.
(A) Degranulation of CLTX-EQ-28ζ CAR T cells against iPSC-NPCs and GBM cells from indicated TS lines. Shown are mean ± SEM of duplicate wells. ns denotes not significant between PBT003-4 and PBT106 using an unpaired Student’s t-test. (B-C) MMP2 mRNA expression (2−ΔCt compared with actin) in the PBT-TS lines were correlated to their corresponding stimulation of CLTX-CAR T cell degranulation (B) and CLTX-Cy5.5 staining (C); with correlation coefficients (r2) indicated in each graph (n = 16). (D) Degranulation of CLTX-CAR T cells tested against PBT003-4-TS or PBT106-TS lines that had been transduced with scrambled shRNA or shRNA targeting MMP2 (shMMP2). (E) Cytotolytic activity of CLTX-CAR T cells against scrambled shRNA- or shMMP2-transduced GBM cells over 72 hr co-culture (E:T=1:4). (D, E) * denotes p < 0.05 compared with scrambled shRNA-transduced targets using an unpaired Student’s t-test. (F) Scrambled shRNA- or shMMP2-transduced GBM cells (5 × 106) were injected subcutaneously into the right flank of NSG mice. Mock-transduced or CLTX-EQ-28ζ CAR T cells (3 × 106) were then injected into the tumors at day 14 (tumor diameter ~5 mm, n = 4 in each group), and tumor size was monitored over time with caliper measurements. * denotes p < 0.05; ** denotes p < 0.01 using one-way ANOVA with Bonferroni’s multiple comparison tests. (G) CLTX-CAR T cells were cultured with the indicated concentrations of soluble MMP2 in the absence (top row) or presence (bottom row) of PBT003-4-TS cells (E:T=1:4) for 24 hr. CAR T cell activation was determined by flow cytometric analysis of 4-1BB and CD69 surface expression. Quadrants were drawn based on control staining, and percentages of double-stained cells are indicated in each histogram. (H) Elimination (% Killing) of PBT003-4-TS cells by CLTX-CAR T cells after 48 hr of co-culture (E:T=1:4) in the presence of different concentrations of soluble MMP2. Percentages of tumor killing were calculated against the numbers of viable tumor cells when cultured in the absence of CLTX-CAR T cells; mean ± SEM of duplicate wells are shown. (I) Immunofluorescence staining of MMP2, CLCN3 and actin at 2 hr after initiating co-culture of CLTX-CAR T cells and PBT003-4-TS cells (E:T=1:1); arrowhead, T cell.
To further verify the dependence of CLTX-CAR activation on MMP2, we knocked down MMP2 expression in GBM cells using lentiviral shRNA (shMMP2). The knockdown efficiently decreased MMP2 mRNA and soluble MMP2 secretion, while resulting in only modest decreases in CLCN3 expression and minimal changes in ANXA2 expression (Fig. S6E). We found that MMP2 knockdown in GBM cells substantially reduced CLTX-EQ-28ζ CAR T cell activation and cytotoxicity (Fig.6, D and E, Fig. S6F). Further, shMMP2 significantly reduced in vivo antitumor activity of CLTX-EQ-28ζ CAR T cells against established PBT003-4-TS and PBT106-TS tumors (p=0.01 and p=0.003, respectively) (Fig. 6F). Together, these results demonstrate that MMP2 is required for CLTX-CAR recognition and activation against tumor targets. Importantly, MMP2 shows low to negligible expression in various human normal tissues in comparison to GBM (Fig. S5E), which is consistent with our findings and other reports showing low CLTX-Cy5.5 binding against normal human cells and tissues (22).
MMP2 is a secreted matrix metalloproteinase that can either associate with the membrane through interaction with αvβ3 integrin and MMP14 [also known as membrane-type 1 (MT1)-MMP], or exist as a soluble enzyme (23, 53). Although our data and others indicate that CLTX binds to the membrane-bound form of MMP2 (23), we further investigated whether CLTX-CAR T cells may also respond to soluble MMP2. In the presence of soluble MMP2, we observed no activation of CLTX-CAR T cells (Fig. 6G) or reduction in CLTX-CAR T cell cytotoxicity against GBM cells (Fig. 6, G and H); nor did adding soluble MMP2 restore CLTX-CAR activity after MMP2 knockdown in GBM target cells (Fig. S6G). Consistent with CLTX-CAR recognition of MMP2, we observed that MMP2 was recruited to the CLTX-CAR T cell-GBM cell immunological synapse, and this interaction also co-localized with CLCN3 in PBT003-4 cells (Figure 6I). The recruitment of MMP2 and CLCN3 to the immunological synapse did not occur when co-culturing IL13Rα2-CAR T cells and GBM cells (Fig. S6H). Moreover, we also observed co-localization of MMP2 and CLCN3 when CLTX peptide was applied to GBM cells (Fig. S6I). These results suggest that interaction between CLTX-CAR T cells and GBM cells requires surface expression of MMP2, and involves CLCN3. Recruitment of CLCN3 following MMP2 interaction with CLTX peptide or CLTX-CAR T cells was consistent with another study using CLTX-coated liposomes (54). Together, our results suggest that membrane-bound MMP2 is necessary for CLTX-CAR T cell activation, and that soluble MMP2 neither activates nor disrupts CLTX-CAR function.
Discussion
This study demonstrates that CLTX, a peptide component of scorpion venom, can be successfully incorporated into a CAR construct to redirect cytotoxic T cells to target GBMs. The antitumor effects of CLTX-CAR T cells were robust and specific. Moreover, CLTX binding extended across a wider range of freshly-dissociated patient tumor cells and patient-derived GBM cell lines than expression of IL13Rα2, HER2, EPHA2 and EGFR, antigens currently under consideration as CAR immunotherapy targets. Our study thus establishes that the intrinsic binding properties of a natural toxin peptide can be exploited in generating targeted CAR T cells.
The criteria for selection of GBM-associated immunotherapy targets are particularly stringent because of the sensitivity of the brain to off-target activity or reactions to the therapy (2). For the target antigens currently under clinical investigation, one selection criterion is their specific expression on GBM tumors. IL13Rα2 has negligible expression in normal brain and elsewhere (except as a cancer-testis antigen) (55, 56). EGFRvIII, the most common mutated form of EGFR, is restricted to multiple cancers including GBM (57). Other antigen targets such as EGFR are over-expressed by tumor GBM cells as compared to normal cells, and HER2, while moderately expressed in some normal tissues, shows only minimal expression on postnatal neurons or glial cells (58). Development of CLTX-CAR T cells was justified by previous reports of the safety of CLTX itself, as demonstrated in preclinical and clinical studies (25, 26, 31, 32). Specifically, no toxicity was observed in a previous clinical study using CLTX to deliver 131I to tumor sites (25), or in on-going clinical studies evaluating IV delivery of fluorophore-conjugated CLTX as a real-time imaging agent during surgery (32).
For the results presented here, we utilized mouse models to investigate potential toxicities of CLTX-CAR T cells. Safety evaluation in mouse models was supported by our observation that CLTX efficiently binds to mouse glioma cells, and the fact that the putative target MMP2 is conserved between human and mouse. Therefore, although these murine models may only represent a surrogate for human safety, they provide relevant information about off-tumor targeting. Using these preclinical models, we determined that systemic (IV) and regional (ICT and ICV) delivery of CLTX-CAR T cells into both healthy and tumor-bearing mice did not show systemic toxicity. Further, the organ distribution and persistence of systemically administered CAR T cells in tumor-bearing mice were similar to those of mock T cells. Overall, these results from preclinical models provide strong evidence for efficacy together with negligible off-tumor toxicity, and support future clinical evaluation in patients with GBM. However, despite the clinical utilization of CLTX peptide, the potential immunogenicity of CLTX-CAR T cells may represent another safety consideration, which was not specifically addressed in these preclinical models.
Tumor recurrence remains a barrier to successful immunotherapy for GBM. Recurrence is commonly associated with the presence of cell subpopulation(s) resistant to radiotherapy and chemotherapy, and characterized by high tumor-initiating potential, GSCs (37). The virulence of GSCs has made them a critical consideration of GBM-targeted immunotherapy (59), and previous studies have demonstrated that GSCs can be as responsive to CAR T cell-mediated cytotoxicity as more differentiated GBM cells (33, 38, 39), despite their intrinsic immunosuppressive properties (60). Functional evaluations of CLTX-CAR T cells throughout this study have utilized patient-derived TS lines, which maintain stem cell-like properties under appropriate culture conditions (34). Further, since the identification of GSCs using single surface markers may confer bias towards certain GBM molecular subtypes (61), we used two different markers, CD133 and CD44, to identify GSC-like subsets in primary tumor samples, and showed that CLTX-Cy5.5 binding was similar in both GSC subsets and in non-GSC populations. Our results indicate that CLTX-CAR T cells inherit the binding properties of CLTX peptide, and thus will be active against both GSC and non-GSC populations in patient tumors, suggesting the potential to target the “seeds” of GBM recurrence.
The choice of co-stimulatory signal has proven to be a critical element of CAR design. CAR T cells with CD28 (62) or 4-1BB (7, 63) costimulatory signals have been introduced to patients with hematological and solid tumors. In general, compared with CD28, 4-1BB co-stimulation has resulted in slower but more long-lasting T cell activation, as indicated by differential metabolic profiling (64), and consistent with CAR T cell expansion dynamics when co-infused into B-ALL patients (65). Therefore, it was surprising that in this study we found that CLTX-CAR T cells displayed degranulation and killing antitumor responses only when incorporating CD28 co-stimulation, whereas 4-1BB co-stimulation reduced both short-term and long-term effector activity. One possible explanation may be the nature of CLTX’s interaction with its receptors. Although the binding affinity between CLTX and membrane bound MMP-2 is not known, an early study identified a low-affinity CLTX receptor with high abundance on GBM cell membranes (21). Since CD28 has been reported to lower the affinity threshold for TCR activation (66), it is possible that interaction between the CLTX tumor-recognition domain and low-affinity GBM receptors requires CD28 co-stimulation to reach a threshold for initiating CLTX-CAR T cell activation. We noted that along with higher effector function, CLTX-EQ28ζ and CLTX-CD8h28ζ CAR T cells also more frequently expressed the exhaustion marker PD-1 compared to the other CLTX-CARs evaluated. This observation would be consistent with incomplete activation by other non-CD28-bearing CLTX-CAR T cells upon tumor binding, as T cell exhaustion markers are also induced upon antigen engagement and serve as indicators of T cell activation (67). Further optimization of CLTX-CAR design may be achieved by protein design strategies to modulate its binding affinity through single amino acid mutations or replacements, which may be facilitated by the small size and helix-dominated structure of CLTX.
Our studies also provide important insights into the clarification of the interaction between CLTX and its receptor, which previously has not been well-defined (27). The well-characterized inhibition of GBM cell migration and invasion by CLTX (23, 68) has been attributed to either the chloride channel CLCN3 through inhibiting the Cl- flux required for cell shape changes during invasion (24), or to MMP2 thereby decreasing extracellular matrix cleavage (23). Interpretation of immunoprecipitation-based assays showing interaction between CLTX and its receptor(s) has been complicated by difficulties distinguishing between direct versus indirect association. Using genetic manipulations to knockdown MMP2, we determined that MMP2 expression is required for CLTX-CAR T cell activation. Together with previous discoveries that overexpressing MMP2 facilitates the binding of CLTX (30), our data suggest that membrane-associated MMP2 serves as the CLTX receptor or a critical component within a receptor complex. Importantly, CLTX-CAR T cells did not respond to secreted MMP2, thus preserving their activation potential even in a soluble MMP2-rich microenvironment such as tumors (69). The development of CLTX-CAR T cells shows that CAR T cells are able to selectively target membrane-bound forms of secreted enzymes, in addition to the previously-reported CAR designs against soluble proteins (70, 71). Since CAR T cell activation requires direct antigen engagement, our results suggest MMP2 as a direct target for CLTX, and this interaction may also recruit other associated proteins such as CLCN3. Similar patterns of CLTX’s association with tumor cells have been inferred by previous studies using CLTX-coated nanoparticles and fusion proteins (54, 72). Although CLCN3 expression appeared unrelated to CLTX-CAR activity, it might still participate in CLTX’s interaction with target cells. Of note, we also observed correlations between MMP2 expression, degranulation, in vivo CLTX-CAR efficacy, and CLTX-Cy5.5 staining intensity, suggesting that CLTX-Cy5.5 staining serves as an efficient strategy for identifying the potential responsiveness of GBM and other tumors to CLTX-CAR T cell therapy.
A generalizable finding arising from our in vivo CLTX-CAR studies is the observation that antitumor function of GBM-targeted CARs may be inhibited by adaptive resistance mechanisms of GBM tumors, an observation also reported in a clinical study showing upregulated immunosuppressive pathways in patients with GBMs after treating with EGFRvIII-targeted CAR T cells (9). Although PD-L1 is expressed in a subset of GBMs (73), we found that PD-L1 could be strongly induced in GBM xenografts and TS lines after CAR T cell therapy. This induction was associated with IFNγ receptor expression, consistent with the mechanism of adaptive resistance characterized in metastatic melanomas (51). The inhibitory effect of PD-L1 on CLTX-CAR T cells is mostly on long-term in vivo function, as initial CLTX-CAR activation (degranulation and in vitro killing) was not well correlated with the expression of tumor IFNγRA (CD119), which mediates PD-L1 induction. CLTX-CAR T cells displayed similar initial activation despite the differential IFNγRA expression of the two PBT-TS lines we used for in vivo studies. Our results lead to the potential of correlating tumor IFNγ receptor expression with CLTX-CAR T cell therapeutic effect, as well as the possibility of combining CLTX-CAR T cells with checkpoint inhibitors for more effective GBM clearance.s
Overall, we were able to observe broad GBM-targeting capability of CLTX-CAR T cells, consistent with the wide expression of its receptor, membrane-bound MMP2, in GBM samples (74). Of particular importance, CLTX-CAR T cells elicited potent cytotoxic responses against tumor cells with no or little expression of other targetable antigens (IL13Rα2, HER2 and EGFR). Further, CLTX-CAR T cells efficiently eradicated GBM tumors in vivo with no observed toxicity, with the limitation that the GBM xenograft models may not fully recapitulate the invasive nature of GBM tumors in patients. We believe that CLTX-CAR T cells address two major hurdles to effective immunotherapy for GBM: reduction of antigen escape while maintaining tumor cell restriction. We suggest that CLTX-CAR T cells present a strategic combination of selective yet ubiquitous tumor targeting, and are a candidate for clinical development as anti-GBM immunotherapy, mitigating antigen escape either as a single agent, or in combination with other CAR T cells or immunotherapy strategies.
Materials and Methods
Study design
In this study we evaluated the antitumor potency of CLTX-CAR T cells against GBM. First, CLTX binding was verified in various GBM samples. Primary brain tumor (PBT) cells were obtained from GBM resections at COH under protocols approved by the City of Hope Internal Review Board. For experiments on functional evaluation, CAR T cells were generated from three different healthy donors, and tested in vivo and in vitro against at least two independent GBM models. To ensure statistical power, for in vitro assays, 2-5 replicates within each condition were used to sufficiently represent intra-group variations and allow for defining statistical significance. In all in vivo experiments, 6-8 week-old NOD/SCID/IL2R−/− (NSG) mice were used, and 4-8 mice were included within each group to ensure statistical power, which enabled us to statistically distinguish tumor sizes and survival rates across groups. Before CAR T cell treatment, mice were randomized based on bioluminescent imaging to ensure similar average tumor sizes across groups. The health condition of mice was monitored on a daily basis by the Department of Comparative Medicine at City of Hope, with euthanasia applied according to the American Veterinary Medical Association Guidelines. Investigators were not blinded when monitoring mice survival. For every mouse euthanized, the brain was collected to confirm the presence of GBM tumors. If a mouse died during imaging processes with no prior sign of tumor progression, it was considered anesthesia-related death and the mouse was excluded from survival analysis. The pathological conditions of mouse organs were determined by a mouse pathologist from the Veterinary Pathology Program at City of Hope. All primary data are reported in data file S1.
Isolation of primary brain tumor cells, establishment of neurospheres and other cell lines
Resected brain tumor specimens were digested using a human tumor dissociation kit (Miltenyi Biotech Inc) to generate PBT cells. TS lines were subsequently established from PBTs and maintained as described previously (38, 75). To generate cells for in vivo biophotonic imaging, these cells were engineered to express the ffLuc reporter gene as previously described (38). Differentiation of TS lines was performed by withdrawal of epidermal growth factor (EGF) and fibroblast growth factor (FGF) in the TS culture media and supplement with 10% fetal calf serum (FCS), as described previously (38). FB-NSCs were established and characterized as reported previously (76). Astrocytes and NPCs were differentiated from health donor-derived iPSCs based on established protocols (77).
DNA constructs
All CLTX-CAR constructs contain a CLTX peptide and the cytoplasmic domain of human CD3 zeta, with different spacers including: IgG4EQ [IgG4 with two point mutations (L235E, N297Q) (6)]; ∆CH2: IgG4-Fc with the CH2-domain deleted; CD8h: CD8 hinge; L: a synthetic 10 amino acids short linker (data file S2). CAR constructs also contain CD4 or CD28 transmembrane domains, and CD28 or 4-1BB costimulatory domains. All domains have been previously described (42, 44, 49). A truncated CD19 was also introduced in the construct to allow for potential enrichment and tracking of transduced cells. The firefly luciferase (ffLuc)-GFP construct for tumor biophotonic imaging was generated as described previously (38).
CAR T cell production
Blood products were obtained from healthy donors under protocols approved by the City of Hope Internal Review Board, and naïve/memory T cell (Tn/mem) isolation followed the procedures described in previous studies (49). In brief, PBMCs were isolated by density gradient centrifugation over Ficoll-Paque (GE Healthcare) and then underwent sequential rounds of CliniMACS/AutoMACS depletion to remove CD14- and CD25-expressing cells, followed by a CD62L positive selection for Tn/mem cells. To generate CAR T cell products, T cells were stimulated with Dynabeads Human T expander CD3/CD28 (Invitrogen) at a 1:3 ratio (T cell:bead), and transduced with lentivirus to express CAR (MOI=2) in X-VIVO 15 (Lonza) containing 10% FCS with 5 μg/mL protamine sulfate (APP Pharmaceuticals), 50 U/mL rhIL-2 and 0.5 ng/mL rhIL-15. Cultures were then maintained at 37°C, 5% CO2 under the same condition of media and cytokines (cytokines were replenished every other day). On day 7 post transduction, the CD3/CD28 Dynabeads were removed from cultures using the DynaMag-50 magnet (Invitrogen). CAR-transduced T cells were enriched by positive selection using anti-CD19 magnetic beads (Stem Cell Technologies). Cultures were propagated for 14-16 days before applying to assays or cryo-preservation. Mock-transduced T cells were generated by stimulating and culturing Tn/mem cells from the same donors as described above, without lentivirus addition.
GBM xenograft studies
All mouse experiments were approved by the COH Institutional Animal Care and Use Committee (IACUC protocol #18059). Orthotopic GBM models were generated using NSG mice as previously described (48). Briefly, on day 0, ffLuc+ GBM cells (1 ×105 or 2 × 105) were stereotactically implanted into the right forebrain. After 8 (PBT106-TS) or 12 (PBT003-4-TS) days, mice were then treated by intracranial-tumor (ICT) application of 0.2, 0 5 or 1 × 106 CAR T cells, intracerebroventricular (ICV) with 1-2 × 106 CAR T cells, or intravenous (IV) routes with 1-10 × 106 CAR T cells (indicated in figure legends). Tumor volumes were determined by in vivo non-invasive optical biophotonic imaging using a Xenogen IVIS 100 as previously described (38). For subcutaneous tumor xenografts, GBM cells (5 × 106) were mixed with Matrigel (Corning) and injected into the right flank of NSG mice, and tumors were allowed to grow for 16-21 days until tumor sizes reached 5 mm × 5 mm. CAR T cells (2 × 106) were injected intratumorally, and tumors sizes were monitored until animal euthanasia when tumor sizes reached 15 mm × 15 mm. To acquire single cells for flow cytometric analysis, xenograft tumors were cut into pieces, physically dissociated and filtered. The pathological conditions of mouse organs were determined by the Veterinary Pathology Program at City of Hope.
Statistical analysis
Data analysis was performed using Prism v6.0 (GraphPad Software) and presented as stated in individual figure legends. Comparisons were determined using Student’s t-test (two groups) or one-way ANOVA (three or more groups). For comparisons between three or more groups, Bonferroni’s Multiple Comparison Tests were used to compare all or selected pairs of data (95% confidence intervals). Comparison of Kaplan-Meier survival data was performed using the Log-rank (Mantel-Cox) test. Detailed comparisons in each experiment are described in figure legends.
Supplementary Material
Fig. S1. Antigen expression and CLTX-Cy5.5 binding on GBMs
Fig. S2. Activation of CLTX-CAR T cells after GBM stimulation.
Fig. S3. CLTX-EQ-28ζ and CLTX-CD8h-28ζ CARs initiate stronger T cell activity than other CLTX-CAR constructs.
Fig. S4. CLTX-CAR T cell targeting of GBM xenografts.
Fig. S5. CLTX-CAR T cells do not elicit off-tumor targeting in mouse models.
Fig. S6. MMP2 is necessary for CLTX-CAR T cell activation.
Video.S1. Killing of PBT003-4-TS-derived GBMs during a 72h co-culture with the mixture of IL13Rα2-, HER2- and EGFRvIII-targeted CAR T cells
Data File S1. Primary data
Data File S2. Amino acid sequence of spacers
Video.S2. Killing of PBT003-4-TS-derived GBMs during a 72h co-culture with CLTX-EQ-28ζ CAR T cells
Acknowledgements:
We thank the Department of Comparative Medicine, and the cores of Synthetic and Biopolymer Chemistry, Small Animal Imaging, Light Microscopy, Mouse Pathology, Solid Tumor Pathology, as well as Brenda Chang, Juan Ruiz-Delgado and Dr. Lihong Weng, for their technical assistance. We thank Dr. James M. Olson for interactive discussions and intellectual feedback on this work.
Funding: This work was supported by grants from the Ben and Catherine Ivy Foundation and NIH grant P30CA33572 (cores). D.W. is supported by NCI fellowship 5F99CA234923-02.
Footnotes
Competing interests: S.J.F. and C.E.B. receive royalty payments from Mustang Bio; all other authors declare no competing interests. A patent associated with this study covering the CLTX-CAR has been held and submitted by City of Hope (WO2017066481A8) with M.E.B., C.E.B., S.J.F. and D.W. as inventors.
Data and materials availability: All data associated with this study are present in the paper or Supplementary Materials. PBT-TS lines are available from C.E.B. under a material transfer agreement with City of Hope.
References and Notes
- 1.Alexander BM, Cloughesy TF, Adult Glioblastoma. J Clin Oncol 35, 2402–2409 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Sampson JH, Maus MV, June CH, Immunotherapy for Brain Tumors. Journal of Clinical Oncology 35, 2450-+ (2017). [DOI] [PubMed] [Google Scholar]
- 3.Choi BD, Curry WT, Carter BS, Maus MV, Chimeric antigen receptor T-cell immunotherapy for glioblastoma: practical insights for neurosurgeons. Neurosurg Focus 44, (2018). [DOI] [PubMed] [Google Scholar]
- 4.Priceman SJ, Forman SJ, Brown CE, Smart CARs engineered for cancer immunotherapy. Current opinion in oncology 27, 466–474 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fesnak AD, June CH, Levine BL, Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16, 566–581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S, Ostberg JR, Forman SJ, Brown CE, Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Molecular therapy : the journal of the American Society of Gene Therapy 23, 757–768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B, Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med 375, 2561–2569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, Ghazi A, Gerken C, Yi Z, Ashoori A, Wu MF, Liu H, Rooney C, Dotti G, Gee A, Su J, Kew Y, Baskin D, Zhang YJ, New P, Grilley B, Stojakovic M, Hicks J, Powell SZ, Brenner MK, Heslop HE, Grossman R, Wels WS, Gottschalk S, HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol 3, 1094–1101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV, A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarfo I, Maus MV, Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. Journal for immunotherapy of cancer 5, 28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill S, June CH, Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunological reviews 263, 68–89 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE, Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science (New York, N.Y.) 344, 1396–1401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, Dewitt J, Gritsch S, Perez EM, Gonzalez Castro LN, Lan X, Druck N, Rodman C, Dionne D, Kaplan A, Bertalan MS, Small J, Pelton K, Becker S, Bonal D, Nguyen QD, Servis RL, Fung JM, Mylvaganam R, Mayr L, Gojo J, Haberler C, Geyeregger R, Czech T, Slavc I, Nahed BV, Curry WT, Carter BS, Wakimoto H, Brastianos PK, Batchelor TT, Stemmer-Rachamimov A, Martinez-Lage M, Frosch MP, Stamenkovic I, Riggi N, Rheinbay E, Monje M, Rozenblatt-Rosen O, Cahill DP, Patel AP, Hunter T, Verma IM, Ligon KL, Louis DN, Regev A, Bernstein BE, Tirosh I, Suva ML, An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178, 835–849 e821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, Zhai Y, Bading JR, Ressler JA, Portnow J, D’Apuzzo M, Forman SJ, Jensen MC, Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res 21, 4062–4072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CE, Warden CD, Starr R, Deng X, Badie B, Yuan YC, Forman SJ, Barish ME, Glioma IL13Ralpha2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS One 8, e77769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD, Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28, 4722–4729 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majzner RG, Mackall CL, Tumor Antigen Escape from CAR T-cell Therapy. Cancer discovery 8, 1219–1226 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Lim WA, June CH, The Principles of Engineering Immune Cells to Treat Cancer. Cell 168, 724–740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajesh E, Sankari LS, Malathi L, Krupaa JR, Naturally occurring products in cancer therapy. Journal of pharmacy & bioallied sciences 7, S181–183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBin JA, Maggio JE, Strichartz GR, Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. The American journal of physiology 264, C361–369 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H, Use of chlorotoxin for targeting of primary brain tumors. Cancer Research 58, 4871–4879 (1998). [PubMed] [Google Scholar]
- 22.Lyons SA, O’Neal J, Sontheimer H, Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 39, 162–173 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Deshane J, Garner CC, Sontheimer H, Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. The Journal of biological chemistry 278, 4135–4144 (2003). [DOI] [PubMed] [Google Scholar]
- 24.McFerrin MB, Sontheimer H, A role for ion channels in glioma cell invasion. Neuron glia biology 2, 39–49 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamelak AN, Rosenfeld S, Bucholz R, Raubitschek A, Nabors LB, Fiveash JB, Shen S, Khazaeli MB, Colcher D, Liu A, Osman M, Guthrie B, Schade-Bijur S, Hablitz DM, Alvarez VL, Gonda MA, Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol 24, 3644–3650 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Parrish-Novak J, Byrnes-Blake K, Lalayeva N, Burleson S, Fidel J, Gilmore R, Gayheart-Walsten P, Bricker GA, Crumb WJ Jr., Tarlo KS, Hansen S, Wiss V, Malta E, Dernell WS, Olson JM, Miller DM, Nonclinical Profile of BLZ-100, a Tumor-Targeting Fluorescent Imaging Agent. International journal of toxicology 36, 104–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dardevet L, Rani D, Aziz TA, Bazin I, Sabatier JM, Fadl M, Brambilla E, De Waard M, Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 7, 1079–1101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Shi X, Zhao J, Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma. Current topics in medicinal chemistry 15, 1196–1208 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, Mok H, Ellenbogen RG, Park JO, Zhang M, Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 31, 8032–8042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM, Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res 67, 6882–6888 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Fidel J, Kennedy KC, Dernell WS, Hansen S, Wiss V, Stroud MR, Molho JI, Knoblaugh SE, Meganck J, Olson JM, Rice B, Parrish-Novak J, Preclinical Validation of the Utility of BLZ-100 in Providing Fluorescence Contrast for Imaging Spontaneous Solid Tumors. Cancer Res 75, 4283–4291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil CG, Walker DG, Miller DM, Butte P, Morrison B, Kittle DS, Hansen SJ, Nufer KL, Byrnes-Blake KA, Yamada M, Lin LL, Pham K, Perry J, Parrish-Novak J, Ishak L, Prow T, Black K, Mamelak AN, Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery, (2019). [DOI] [PubMed] [Google Scholar]
- 33.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S, HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 16, 474–485 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI, Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences of the United States of America 100, 15178–15183 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wykosky J, Gibo DM, Stanton C, Debinski W, EphA2 as a novel molecular marker and target in glioblastoma multiforme. Molecular Cancer Research 3, 541–551 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, Grossman RG, Powell S, Lee D, Ahmed N, Gottschalk S, T cells redirected to EphA2 for the immunotherapy of glioblastoma. Molecular therapy : the journal of the American Society of Gene Therapy 21, 629–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN, Cancer stem cells in glioblastoma. Genes & development 29, 1203–1217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, Barish ME, Forman SJ, Jensen MC, Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res 18, 2199–2209 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, Zhang W, Fine HA, Rosenberg SA, Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther 23, 1043–1053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB, Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Pietras A, Katz AM, Ekstrom EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC, Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell 14, 357–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, Khaled SK, Siddiqi T, Budde LE, Xu J, Chang B, Gidwaney N, Thomas SH, Cooper LJ, Riddell SR, Brown CE, Jensen MC, Forman SJ, Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 127, 2980–2990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudecek M, Silva A, Kosasih PL, Chen YY, Turtle CJ, Jensen MC, Riddell SR, The Non-Signaling Extracellular Spacer Domain of CD19-Specific Chimeric Antigen Receptors Is Decisive for in Vivo Anti-Tumor Activity. Blood 120, (2012). [Google Scholar]
- 44.Priceman SJ, Gerdts EA, Tilakawardane D, Kennewick KT, Murad JP, Park AK, Jeang B, Yamaguchi Y, Yang X, Urak R, Weng L, Chang WC, Wright S, Pal S, Reiter RE, Wu AM, Brown CE, Forman SJ, Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology 7, e1380764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, Ostberg JR, Forman SJ, Brown CE, Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Starr R, Alizadeh D, Yang X, Forman SJ, Brown CE, In Vitro Tumor Cell Rechallenge For Predictive Evaluation of Chimeric Antigen Receptor T Cell Antitumor Function. Journal of visualized experiments : JoVE, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M, Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC, Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res 64, 9160–9166 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Brown CE, Aguilar B, Starr R, Yang X, Chang WC, Weng L, Chang B, Sarkissian A, Brito A, Sanchez JF, Ostberg JR, D’Apuzzo M, Badie B, Barish ME, Forman SJ, Optimization of IL13Ralpha2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Molecular therapy : the journal of the American Society of Gene Therapy, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, Chang WC, Ostberg JR, Neman J, Jandial R, Portnow J, Forman SJ, Brown CE, Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2(+) Breast Cancer Metastasis to the Brain. Clin Cancer Res 24, 95–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A, Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell reports 19, 1189–1201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatenhorst L, Rescher U, Gerke V, Paulus W, Knockdown of annexin 2 decreases migration of human glioma cells in vitro. Neuropathology and applied neurobiology 32, 271–277 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Egeblad M, Werb Z, New functions for the matrix metalloproteinases in cancer progression. Nature reviews. Cancer 2, 161–174 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Qin C, He B, Dai W, Lin Z, Zhang H, Wang X, Wang J, Zhang X, Wang G, Yin L, Zhang Q, The impact of a chlorotoxin-modified liposome system on receptor MMP-2 and the receptor-associated protein ClC-3. Biomaterials 35, 5908–5920 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY, Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clinical Cancer Research 5, 985–990 (1999). [PubMed] [Google Scholar]
- 56.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK, Expression of interleukin-13 receptor alpha 2 in glioblastoma multiforme: Implications for targeted therapies. Cancer Research 67, 7983–7986 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Padfield E, Ellis HP, Kurian KM, Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front Oncol 5, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang JG, Kruse CA, Driggers L, Hoa N, Wisoff J, Allen JC, Zagzag D, Newcomb EW, Jadus MR, Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. Journal of neuro-oncology 88, 65–76 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prager BC, Xie Q, Bao S, Rich JN, Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 24, 41–53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF, Heimberger AB, Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Molecular cancer therapeutics 9, 67–78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown DV, Daniel PM, D’Abaco GM, Gogos A, Ng W, Morokoff AP, Mantamadiotis T, Coexpression analysis of CD133 and CD44 identifies Proneural and Mesenchymal subtypes of glioblastoma multiforme. Oncotarget 6, 6267–6280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL, T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH, Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7, 303ra139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr., Patel PR, Guedan S, Scholler J, Keith B, Snyder NW, Blair IA, Milone MC, June CH, Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity 44, 380–390 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Cheng Z, Wei R, Ma Q, Shi L, He F, Shi Z, Jin T, Xie R, Wei B, Chen J, Fang H, Han X, Rohrs JA, Bryson P, Liu Y, Li QJ, Zhu B, Wang P, In Vivo Expansion and Antitumor Activity of Coinfused CD28- and 4–1BB-Engineered CAR-T Cells in Patients with B Cell Leukemia. Molecular therapy : the journal of the American Society of Gene Therapy 26, 976–985 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A, Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. The Journal of experimental medicine 179, 709–713 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson AC, Joller N, Kuchroo VK, Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44, 989–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soroceanu L, Manning TJ, Sontheimer H, Modulation of glioma cell migration and invasion using Cl- and K+ ion channel blockers. J Neurosci 19, 5942–5954 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egeblad M, Werb Z, New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer 2, 161–174 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Chang ZL, Lorenzini MH, Chen X, Tran U, Bangayan NJ, Chen YY, Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nature chemical biology 14, 317–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou AJ, Chang ZL, Lorenzini MH, Zah E, Chen YY, TGF-beta-responsive CAR-T cells promote anti-tumor immune function. Bioengineering & translational medicine 3, 75–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Ghlban S, Kasai T, Shigehiro T, Yin HX, Sekhar S, Ida M, Sanchez A, Mizutani A, Kudoh T, Murakami H, Seno M, Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. Biomed Res Int 2014, 152659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, Burks JK, Fuller GN, Calin GA, Conrad CA, Creasy C, Ritthipichai K, Radvanyi L, Heimberger AB, PD-L1 expression and prognostic impact in glioblastoma. Neuro-oncology 18, 195–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beliveau R, Delbecchi L, Beaulieu E, Mousseau N, Kachra Z, Berthelet F, Moumdjian R, Del Maestro R, Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Ann Ny Acad Sci 886, 236–239 (1999). [DOI] [PubMed] [Google Scholar]
- 75.Brown CE, Starr R, Martinez C, Aguilar B, D’Apuzzo M, Todorov I, Shih CC, Badie B, Hudecek M, Riddell SR, Jensen MC, Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res 69, 8886–8893 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Oganesyan D, Mooney R, Rong X, Christensen MJ, Shahmanyan D, Perrigue PM, Benetatos J, Tsaturyan L, Aramburo S, Annala AJ, Lu Y, Najbauer J, Wu X, Barish ME, Brody DL, Aboody KS, Gutova M, L-MYC Expression Maintains Self-Renewal and Prolongs Multipotency of Primary Human Neural Stem Cells. Stem cell reports 7, 483–495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Tian E, Chen X, Chao J, Klein J, Qu Q, Sun G, Sun G, Huang Y, Warden CD, Ye P, Feng L, Li X, Cui Q, Sultan A, Douvaras P, Fossati V, Sanjana NE, Riggs AD, Shi Y, GFAP Mutations in Astrocytes Impair Oligodendrocyte Progenitor Proliferation and Myelination in an hiPSC Model of Alexander Disease. Cell Stem Cell 23, 239–251 e236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Antigen expression and CLTX-Cy5.5 binding on GBMs
Fig. S2. Activation of CLTX-CAR T cells after GBM stimulation.
Fig. S3. CLTX-EQ-28ζ and CLTX-CD8h-28ζ CARs initiate stronger T cell activity than other CLTX-CAR constructs.
Fig. S4. CLTX-CAR T cell targeting of GBM xenografts.
Fig. S5. CLTX-CAR T cells do not elicit off-tumor targeting in mouse models.
Fig. S6. MMP2 is necessary for CLTX-CAR T cell activation.
Video.S1. Killing of PBT003-4-TS-derived GBMs during a 72h co-culture with the mixture of IL13Rα2-, HER2- and EGFRvIII-targeted CAR T cells
Data File S1. Primary data
Data File S2. Amino acid sequence of spacers
Video.S2. Killing of PBT003-4-TS-derived GBMs during a 72h co-culture with CLTX-EQ-28ζ CAR T cells