Abstract
Purpose of review
Technological advances have allowed dried blood spots (DBS) to be utilized for various measurements, helpful in population-based studies. The following is a review of the literature highlighting the advantages and disadvantages of DBS and describing their use in multiple areas of research.
Recent findings
DBS can track pollutant exposure to understand their impact on health. DBS can also be used for (epi-)genetic studies, to measure clinical biomarkers, and to monitor drug adherence. Advantages of DBS include being minimally invasive, requiring low blood volume, and being cost-effective to collect, transport, and store. Disadvantages of DBS include the hematocrit effect, which is related to the viscosity of the blood affecting its spread on to the filter paper, causing a major source of error when assessing concentrations, and the possibility of low DNA volume.
Summary
Numerous uses for DBS makes them an important source of biomaterial but they require additional validation for accuracy and reproducibility.
Keywords: blood spots, genetic, environmental, biomarkers, pharmacologic
Introduction
The history of the public health use of dried blood spots (DBS) for newborn screening is of note as it forms the foundation of DBS use more widely in research studies. DBS are collected by pricking the skin and depositing the blood onto filter paper within 24–48 hours of a child’s birth. Five 10-mm-diameter circles are filled with blood drops and DBS are then dried and stored at room temperature for days to weeks, and/or frozen for much longer [1]. DBS were first collected in newborns by two pediatricians, Dr. Robert Guthrie and Dr. Ada Susi in 1961 to detect the metabolic disease phenylketonuria (PKU) [2]. For his role in pioneering this work, the filter paper cards commonly used for DBS collection became called Guthrie cards. Early diagnosis of conditions such as PKU allows physicians to intervene before harsh symptoms or developmental problems occur [3]. By 1963, newborn screening programs in the United States (US) were using DBS in 29 states and Puerto Rico [4]. DBS are now used worldwide in many newborn screening programs [5]. However, many developing countries still do not have national programs, and/or poor coverage where programs exist [6]. In addition, not all screening programs are equal. Despite the fact that more than 70 metabolic diseases can be diagnosed early and treated using newborn screening [7], some programs may only screen for a limited number owing to few resources [6]. In the US, state-by-state differences also exist as new tests are introduced over time. In 2018, for example, all states began screening for severe combined immune deficiency (SCID) as part of the Recommended Universal Screening Panel [8]. As DBS tests in newborn screening expanded, so has their use in research settings (Table 1).
Table 1.
Summary of research on analytes in DBS
| Analytes | Validity/Reliability | Limitations | References |
|---|---|---|---|
| Cotinine | Plasma vs. DBS cotinine (Spearman r= 0.9); Linear correlation between the repeated measures of DBS cotinine measured 11–26 months apart | Half-life of cotinine (∼9h), hematocrit effect, contamination | [21] |
| Perfluorinated compounds (PFC) | Accuracy of spiked DBS were acceptable (53–146% for low and 60–116% for high concentrations) and intraday precesion from 5 repeated samples ranged 2.3–12.6% [28] | Small volume, stability under different storage conditions | [28] |
| PCBs, PBDE, DDE | Correlated with whole blood measures (r=0.80) | Small volume leading to lower limits of detection, background contamination from filter paper manufacturing, processing, transport, etc making lower levels potentially biased | [33] |
| Heavy metals | DBS measures of Hg, Cd, As, Pb had correlations of 0.02–0.50 vs. whole blood levels [36] Mg, K, Fe, Cu, Zn, As, and Se were found to have good correlations between DBS and whole blood samples, but Co, As, Cd and Pb were only accurate and precise at harmful and heightened levels [37] | Background contamination an issue; pre-treatment of filter paper may limit prospective use | [36, 37] |
| Genetics | High call rates for SNPs (PMID: 19371215) [42]; DBS measured methylation levels were highly correlated (r=0.9907) with methylation levels from buffy coat of the same participants | DNA degradation over time; utility for gene expression unclear | [42, 51] |
| Metabolomics | Repeated samples show low variability (<2.5% for most metabolites although there is diurnal variability) [57]; −15% analytes lost for finger-prick spotted DBS vs. plasma [57] | Lack of temperature control may lead to deteriation; Loss of metabolites may be due to air drying; Participants may not always follow protocols for collection, drying and shipping; lack of “referent” concentrations for most speciest of analytes also make it difficult to compare validity and reliability | [57] |
| Infections | Infection specific Example validity: Human immunodeficiency virus analysis from DBS had 100% sensitivity and specificity. Hepatitis C virus analysis revealed a sensitivity of 92.6%, specificity of 100%, positive predicted value of 100%, and negative predicted value of 79.5%. |
Blood must be spotted during viremic period, storage conditions | [83] |
| Inflammation | Validity: 25 inflammatory markers and neurotrophins were elevated in children with known inflammatory conditions relative to healthy controls. | Low blood volume | [67] |
| Pharmacologic | Medication specific Example validity: 10 cardiovascular drugs were measured in DBS to monitor adherence. Drugs were quantifiable in DBS, even when multiple drugs were taken by a single person. Example reliability: cardiovascular drug analytes were stable at room temperature >= 10 weeks, and across different volumes and hematocrit values. |
Individual differences in drug metabolism; requires timing the blood spot collection to active hours of medication | [82] |
Advantages and Disadvantages of Dried Blood Spots
DBS can be a favorable method to collect a blood sample in research settings for multiple reasons and which is why they have been used across various disciplines [9]. First, they are minimally invasive and require a low volume of blood [10]. For instances where it is unethical to obtain more than 1 mL of blood (e.g., underweight or small for gestational age newborns [11]), the low blood volume obtained for DBS remains within such ethical guidelines. In addition to being minimally invasive, other benefits of DBS include being cost-effective to collect, transport, and store [12]. DBS alleviate the burden of having to visit a phlebotomist, which can lower medical expenses. International guidelines for safely collecting quality DBS samples for health screenings [1] are available and can also be used in research. Blood collection by venipuncture requires timely processing by centrifugation and separation into useable aliquots, and long-term storage of cryovials in below freezing temperatures to maintain their quality can be costly. DBS can alleviate the costly burden of storing and maintaining biospecimens in temperate locations. In addition to DBS being used for newborn screening, they can also be used for persons of all age groups to assess hormone levels, drug therapy tracking, and other environmental factors. Analytes that may shift day to day (e.g., vitamin D) and have larger variability could potentially be addressed by frequent sampling [13]. To understand diurnal variation, multiple samples collected at different times of day from the same individual may also be necessary. Frequent blood sampling may be more feasible with DBS than venipuncture.
Balancing these advantages are several disadvantages. Accuracy of tests must be thoroughly proven and may take substantial resources to develop. False positives, whether in early detection or for research studies, can waste resources. Hence, ideally the sensitivity and specificity of tests should always be compared to current tests using blood sampling by venipuncture. Due to the small volume, there may be higher limits of detection and reproducibility may be untested if there is insufficient material to conduct measures in duplicate. Reliability within and between laboratories should be evaluated if its measurement is to be conducted by multiple sites. It is also of note that the DBS serves as a source of whole blood, and thus not directly comparable to either serum or plasma, which are subfractions of whole blood. Thus, clinical cut points based on whole blood may be more comparable than for serum/plasma and may be a disadvantage if gold standard measures require a subfraction (e.g., fasting plasma glucose). Refrigeration is another factor that can affect the accuracy of analytes measured in DBS; some analytes can withstand non-refrigerated storage and still be viable for months, if not longer [14]; however other analytes may only remain viable non-refrigerated for a short amount of time before degradation occurs [14]. There is also the possibility of contamination which is why it is important for researchers to void the first drop prior to collection and wipe it with a sterile gauze. If chemicals are on the gauze or other instruments of collection (e.g., capillary tubes) then there is a chance for contamination. Studies should prepare appropriate collection materials to avoid contamination, especially if environmental chemicals are of interest. Sources of contamination may also stem from the manufacturing, transportation and other factors related to the DBS card itself [14]. Background measures can be made by punching unused portions of cards and then subtracted from measured values.
Another disadvantage of DBS is the hematocrit effect, which occurs due to different ratios of red blood cells to total blood volume. The hematocrit effect affects blood viscosity and may cause uneven spreading throughout the filter paper, leading to a major source of error in biomarker measurement both within and between people, as blood volume is not consistent within a given DBS [15]. A drop of blood with high hematocrit will not travel as far on the filter paper as a drop of blood with low hematocrit [16]. However, analyses typically assume that the same size punch of DBS contains the same amount of blood in order to estimate concentrations. But the punch of a DBS of someone with high hematocrit will come from a higher blood volume than the punch of someone with low hematocrit. The hematocrit ratio can change due to race/ethnicity, pregnancy status, and residential elevation heights among other factors and thereby change the concentration and distribution of what is being measured in the DBS [15]. Proposed solutions to the hematocrit effect include utilizing a correction factor which accounts for the DBS area, calibrating pipettes so that whole blood is evenly distributed, and measurement by experienced personnel who can manipulate the DBS sampling to better ensure even spreading [15]. Using the full circle collected should also eliminate this issue compared to using punches [16]. Another potential issue is a chromatographic effect, which is how a specific analyte travels across the filter paper [16]. If a biomarker moves with the leading edge of the blood as it is absorbed, punches in the center of the spot may be erroneously low [16]. Testing multiple punches from the same DBS can help decipher if this is a major source of variability. The hematocrit effect affects measurements of biomarkers and chemical concentrations which require a known blood volume to be interpretable whereas as a source of DNA, it should be minimally impacted. In other research utilizing nucleic acids, it is important to consider that enzyme activity ceases after drying which can interfere with how RNases interact with RNA [17]. Finally, it can be costly to analyze DBS in specialized labs, as processing requires an additional elution step and can lengthen turnaround time.
Uses of Dried Blood Spots for Research
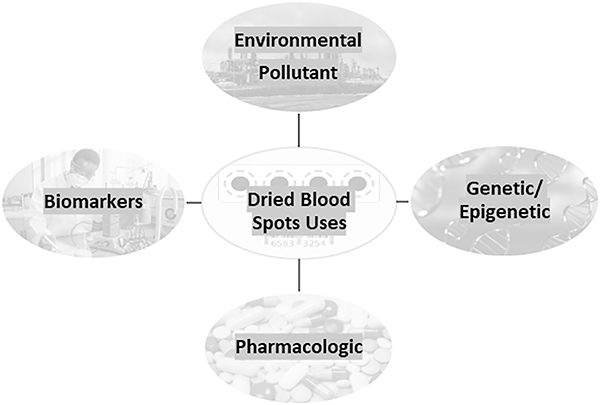
With the advantages and disadvantages of DBS in mind, the remaining review compiles information that explores the use of DBS for research that involves environmental chemicals, biomarkers, genetics/epigenetics, and pharmacologic agents as depicted in Figure 1. The utility of using DBS for research purposes varies; for example, in the US typically five 10-mm-diameter circles are routinely collected, but the amount of time these samples are stored for, and the laws allowing for research use vary by state. Since the primary purpose of DBS is for mandatory newborn screening, the use of DBS for research purposes is limited to the sample which is not used for screening. Researchers can request the use of the whole blood spot or 3 mm punches.
Figure 1:
The various uses of dried blood spots in scientific research
Environmental Pollutants
Environmental pollutant tracking and detection are top priorities for public health professionals because of their population level exposure, especially among pregnant women, the elderly, and children [18]. Unborn children are exposed to chemicals able to cross the placental barrier [19]. Examples of environmental pollutants measured in DBS include tobacco smoke, perfluoroalkyl compounds, pesticides, lead and other heavy metals. Environmental chemicals can cause imbalances in the hormonal and homeostatic system which can affect metabolism, growth, and even fetal development [20]. Obtaining chemical measurements during developmentally relevant time periods for exposure can be difficult which is why researchers have turned to DBS.
Tobacco Smoke
Biomarkers related to tobacco smoke can cross the placental barrier [19]. A well-known tobacco biomarker is cotinine, which is a metabolite of nicotine [21]. As self-reported smoking data can be biased, using cotinine levels can be a better alternative to determine tobacco exposure [22]. Multiple research studies have used cotinine as an indicator for maternal tobacco smoke/product exposure in DBS [23–25] One study quantified cotinine levels in 50 DBS from infants, children, and young adults for identifying tobacco smoke exposure [24], finding 100% sensitivity and 94% specificity compared to plasma levels [24]. Another study used cotinine levels in DBS to estimate mother’s smoking exposure close to the delivery date of the child [25]. The researchers chose to focus on the time period closer to the delivery date because cotinine has a half-life of 9 hours [25]. They also found that DBS are a valid source to quantify active maternal smoking closer to the time of delivery; with 92.3% sensitivity and 99.7% specificity [25].
Organic Compounds
DBS can be used for biomonitoring organic compounds within various populations [26]. A study in New York explored the temporal trends in perfluorinated compound (PFC) exposure in New York State using DBS samples collected as part of Newborn Screening from 1997 through 2007 [27]. This study found an increase in perflurorooctanesulfonate (PFOS), perfluorooctanesulfonamide, perfluorohexanesulfonate, and perfluorooctanoate (PFOA) in newborn DBS samples that were taken between December 1997 and June 2001 compared to samples taken between June 2005 and January 2007 [27]. A separate study measured PFCs using DBS from infants born in Texas, after similarly finding that their methods had acceptable accuracy and precision [28]. They also found that DBS storage at room temperature for up to 61 days had little impact on concentrations [28]. While they did not have any samples collected by venipuncture from the same infants to compare to, the researchers noted that the concentrations were lower than those previously reported from analyses using cord sera [28]. However, DBS represents whole blood from infants so the differences may be due to the blood processing or to maternal sources of contamination [28]. PFCs measured using DBS was used in etiologic research into the determinants of health and behavior. The Upstate New York Infant Development Screening Program (Upstate KIDS) study requested consent from parents to use residual DBS from the state’s Newborn Screening Program to measure chemicals and biomarkers [29]. The study found that the majority of parents consented to their use [29]. Almost all newborns had available DBS, although for chemical analyses, requiring a full DBS (and not only 3mm punches), more newborns had insufficient numbers [29]. Still, 84% of infants had full circles available [29]. Using these DBS samples, researchers subsequently assessed the association between levels of PFOA and PFOS at delivery and later child behavior in 788 children [30].
One weakness in using DBS for the measurement of other organic pollutants is that limits of detection may require multiple spots to be used, which in turn could require more complicated methods involving pooling of samples [31]. Researchers have had to pool 5 DBS from different infants in order to measure levels of 10 polychlorinated biphenyl congeners (PCBs), polybrominated diphenyl ether-47 (PBDE-47), and p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) [31, 32]. Pooling samples has the advantage of increasing available blood volume but disadvantage of insufficient analytic methods to accommodate range of interested outcomes. Similar issues with detection limits by use of DBS were observed among 21 participants averaging 48 years of age [14]. A single 12cm DBS punch was collected and compared against plasma and whole blood levels of 54 persistent organic pollutants (including PCBs, PBDEs, hexachlorobenzene, among others). Among the 54 target compounds, 33 and 32 compounds had at least 25% of samples above detection limits using mass spectrometry for plasma and whole blood, respectively but this dropped to only 14 compounds in DBS samples due to the low volume to work with [14]. The researchers published the exact limits of detection which were 2 to even >100 times higher for DBS than for plasma/whole blood (e.g., PCBs 0.1–0.9 ng/L in plasma/whole blood vs. 5–22 ng/L in DBS) [14]. However, the detectable levels of the 14 compounds in DBS were correlated with the measures in whole blood providing evidence of their validity even if limited to detection of high exposure [14].
Contamination from manufacturing of DBS cards is of particular concern and can also add to additional background noise that may be difficult to remove if levels are low [14]. Another study utilized DBS from different countries to quantify the amounts of polychlorinated biphenyls, brominated flame retardants, and chlorinated pesticides.[33]. The study found that DBS are effective for quantifying exposures to these three chemical groups, including reliability even after long-term storage of up to a year if refrigerated or frozen [33]. DBS measurements were correlated to whole blood concentrations, (r=0.80) but were lower and relative differences exaggerated at lower concentrations due to detection limit issues in DBS. In addition, similar to previous studies trace levels of background contamination with certain POPs such as PCBs and PBDEs were observed [33]. Another study in Texas took DBS measurements of p,p’-DDE from 2-day old infants who had never been breastfed [34]. The researchers were able to obtain measurable amounts of DDE in the each of the samples and found that if one uses better cleaning techniques and a more efficient determinative step their detection rate can be increased [34]. In summary, measurements of POPs, aside from PFCs, using DBS remain a challenge due to issues of background contamination and sample volume which impact limits of detection.
Heavy Metals / Trace Elements
DBS have been used to measure heavy metals and trace elements. Some heavy metals such as mercury, cadmium, arsenic, and lead are toxic, while others such as iron and zinc are essential nutrients. Heavy metals can, in turn, interact with trace elements to affect their absorption and metabolism. Methylmercury (MeHg) is a neurodevelopmental toxicant which can also compromise the cardiovascular and the immune systems. Researchers used DBS from 675 newborns from Michigan as their source of biomaterial because of their applicability of use to children [35]. The methylmercury levels from this study using measures from DBS was similar to previous studies that used cord blood, and the assay’s accuracy ranged from 96%−115% which met performance guidelines [35]. Their findings confirmed that DBS could be used as a new method for quantifying MeHg levels [35]. Another study tested several methodologies including a one-batch method to avoid contamination and analyte loss, normalized dried blood mass to better quantify blood volumes, and using paired-filter paper blanks to track any background contamination for quantifying mercury, cadmium, arsenic, and lead using DBS through Inductively Coupled Plasma Mass Spectrometry (ICP-MS; Thermo Fisher) [36]. While these heavy metals had significant correlations between the DBS measured levels and venous whole blood levels, they were not high (R2 of 0.02–0.50) unless filter paper cards were pre-treated with an acid wash to remove all possible contamination of heavy metals (R2 of 0.66–0.99) [36]. Even paired filter paper blanks subtracting out background levels due to contamination were insufficient to correct for the random way cards are contaminated [36].Other researchers sought to measure a range of trace elements in DBS using ICP-MS with two, rather than a single 3.2mm DBS punch [37]. Trace elements including Mg, K, Fe, Cu, Zn, As, and Se were found to have good correlations between DBS and whole blood samples, but Co, As, Cd and Pb were only accurate and precise at harmful and heightened levels due to issues of background contamination [37]. Methods continue to require development and care should be taken especially for retrospective use of DBS cards collected without pre-treatment for contamination removal. [36]
Genetic / Epigenetic
Utilizing DBS for genetic studies grants researchers access to larger portions of their target population and makes it feasible to include a diverse cohort of people. Genetic studies often lack diversity in their samples which limits their generalizability. This sampling bias can have adverse effects on underrepresented populations when the results from studies that are not diverse in background, culture, or sex are extrapolated. There have been successful studies that used DBS to analyze genomic contributions of various diseases and disorders, to measure genome-wide DNA methylation, and to find more efficient methods to analyze genomic data.
Genetic Disorders
Extraction of genetic material from DBS has enabled researchers to prospectively test for genetic conditions and to retrospectively understand genetic etiologies of diseases in case-control study designs. Prospective tests prior to disease onset or evidence of complications include use of DBS to screen for the CTFR gene in development of cystic fibrosis [38], gene variants associated with glucose-6-phosphate dehydrogenase deficiency [39], and Rh incompatibility between mother and child by noninvasive fetal genotyping [40], to mention a few. Microarrays have also allowed for use of DNA derived from DBS for genome-wide association studies (GWAS) measuring large numbers of single nucleotide polymorphisms (SNPs). One study examined the impact of storage conditions at the Michigan Neonatal Biobank on genotyping, finding that uncontrolled storage conditions in earlier years (1990s) may have affected DNA quality [41]. The call rates from DBS were high with almost all samples collected between 2000–2008 having >99% call rates [41]. Another study had noted similar observations regarding storage conditions of more dated specimens as compared to more recent in impacting genotyping [42]. Other than gene mutations or SNPs, DBS can also be used for measurement of copy-number variants (CNVs) [43]. A range of birth defects have successfully used DBS in evaluating their relations to CNVs in this way [43]. For example, a study explored the presence of CNVs in a rare heart condition, hypoplastic right heart syndrome (HRHS) [44]. Researchers utilized the Illumina HumanOmni2.5–8 array to genotype 42 HRHS cases and identified 14 CNVs in 33% of the cases [44]. A similar study was done on CNVs and Ebstein anomaly (EA) [45]. Researchers genotyped 60 EA cases and concluded that there are genetic factors that contribute to EA [45].
DBS may also be used for purposes of measuring gene expression. Ho and colleagues utilized DBS to understand how gene expression could reflect pathophysiological disturbances characteristic in the development of cerebral palsy [17]. The researchers used 53 archived newborn DBS and found 4 gene sets that were dysregulated in newborns who later developed cerebral palsy [17]. The DBS used in this project retained a useable amount of mRNA to perform the analyses, which served as proof of concept that DBS are a suitable source of biomaterial for similar gene expression studies [17]. They had previously demonstrated that the reproducibility of microarray gene expression data was high (R>0.90) [46]. In another example, researchers examined the genetic contribution of hearing loss by utilizing DBS [47]. They found roughly 107 genes associated with nonsyndromic hearing loss [47]. The total mapped reads found in the samples of DBS were not significantly different from those found in whole blood and they surpassed quality thresholds for variant analysis[47].
Epigenetics
DBS have also been used to assess DNA methylation. Methylation involves the addition of a methyl group covalently bound to the cytosine of the DNA backbone, affecting DNA transcription [48]. Bisulfite conversion changes the cytosines unprotected by the methyl group into uracils, leading to a simple way for identifying where in the genome methyl groups occurred [49]. However, bisulfite conversion can also damage DNA resulting in insufficient amounts of DNA from DBS [49]. A research study utilized DBS to assess epigenome-wide DNA methylation levels by [50] Researchers used a method to purify DNA after extraction from DBS and also found a method of bisulfite modification that was less damaging to DNA was needed [50]. When they compared the quality of DNA stored in DBS to that found in blood samples frozen in Ethylenediaminetetraacetic acid (EDTA) tubes [50], they demonstrated that it is possible to obtain high quality DNA methylation profiles from DBS that were comparable with those found in the frozen blood samples [50]]. Similar to the previous study, researchers found that DBS samples are a quality resource for genome-wide DNA methylation profiling when using the Infinium Human Methylation 450 array [51]. DBS measured methylation levels were highly correlated (r=0.9907) with methylation levels from buffy coat of the same participants [51]. Given the reliability of using DBS for DNA methylation, several studies have successfully used archived newborn DBS has a source of DNA for evaluating associations with prenatal exposures in population health studies [52–54].
Measurement in DBS of microRNA (miRNA), another epigenetic regulation of gene transcription, is also beginning to be explored [55]. One small study (n=20) found differential miRNA concentrations in association with both low birth weight and macrosomia [56]. However, more studies are needed in this area.
Biomarkers
Biomarkers can provide insight for disease risk and serve as indicators for toxic exposures [9]. Medical professionals can use biomarkers to distinguish between normal or pathogenic levels of hormones, growth factors, and immune markers. Biomaterials widely used in the past to quantify biomarkers include saliva and blood by venipuncture. In recent years, researchers have begun to utilize DBS to better understand disease pathology and make diagnoses. DBS have lower risk of infection than venipuncture [9] which may make them a suitable alternative for research in children. DBS can be used to assess biomarkers of several different biological processes including metabolism, immune health, and endocrinology.
Metabolism
Metabolomics measurements using DBS has been explored by a research team which formulated a protocol for utilizing DBS and dried urine strips to analyze a person’s blood and urine metabolome [57]. A similar study utilized DBS and dried urine samples (DUS) to show that some metabolites have increased stability in DBS and DUS as compared to whole blood and urine at certain storage temperatures and times [58]; for example, DBS and DUS showed good stability when stored at −20 °C for 1 year based on the amount of metabolites recovered after assays[58]. One study rigorously compared four types of filter paper used on samples that were taken from the same person and stored at the same temperature for analyses of metabolomics by mass spectrometry, finding some differences in two types of paper but overall consistent results, which shows promise for applying these methods in various studies [59]. DBS can be used to monitor metabolomic errors in children. A study conducted by Han et. al. used DBS to understand the stabilities of 21 amino acids throughout simulated global health travel flow conditions. They found that lower temperature and low humidity storage is a contributing factor for maintaining stable amino acids on DBS [60]. Another study used DBS from the national children’s study to measure the levels of 25 amino acids in healthy children to create a range that can be used for future studies [61]. They found that small statistically significant differences of amino acid concentrations based on sex, birthweight, and geography and concluded they have little implications for diagnosing of metabolic diseases [61]. Finally, DBS have been used to detect neonatal diabetes at 5 days after birth based on glucose concentration [62].
Infections / Immune Health
DBS can be used to assess the presence of infectious diseases, especially in low-income settings where storage and shipment of biomaterials at temperatures low enough to preserve antibody stability is infeasible. For example, in 2009, researchers used DBS to detect HIV-1 and hepatitis C virus (HCV) in adults [63]. They detected at least 2500 copies/ml for HCV and 400 copies/ml for HIV-1 on DBS using a multiplex real-time RT-PCR [63]. In a study of leishmaniasis, an infection caused by parasites spread by phlebotomine sand flies, researchers identified certain cytokines/chemokines that resulted from leishmaniasis infection in DBS which allowed identification of asymptomatic individuals [64]. This screening tool could be used to help decrease the risk of further infections and assist researchers in identifying individuals with leishmaniasis while conducting clinical trials [64]. Another study utilized DBS to find a new method to detect herpes simplex virus (HSV) DNA, using two different extraction methods [65, 66]. Minimal essential medium extraction method was the better method, and helped the researchers discover the usefulness of DBS as a diagnostic tool for detecting HSV DNA [66].
In addition to enhancing detection of diseases across populations, immune biomarkers from DBS can be used in epidemiologic research. Tracking the concentration of inflammatory markers present at birth could help doctors understand and possibly prevent the onset of diseases like cerebral palsy, autism, and diabetes. Scientists utilized DBS to detect 25 inflammatory markers and neurotrophins in newborn DBS, finding different concentrations in 8 children known to have inflammatory conditions at birth compared to 7 healthy controls [67]. A similar study quantified the concentrations of interleukin-6 and inflammatory cytokine in DBS but found it difficult to detect at low concentrations [68]. In Upstate KIDS, using newborn DBS to measure various immune markers at delivery, researchers generally found no associations when the children were between 0–3 years of age with failing the Ages and Stages Questionnaire, a screening tool for developmental delay [69]. The role of inflammatory cytokines in neurodevelopment is complicated regardless of how they are measured as there is evidence of pleiotropic effects, with potential for cytokines to be protective as well as putative [70].
Endocrinology
The ability to quantify hormone levels in DBS could provide a convenient tool for practitioners and researchers. For example, a study conducted on healthy adults tested the accuracy of utilizing DBS to analyze the amount of insulin and insulin analogs [71]. DBS measures were limited by small sample volumes and difficulty purifying the target peptides from the DBS matrix. While utilizing urine and blood were still better choices due to their sensitivity and simplicity of measurements, DBS collected during non-fasting periods provided reliable results [71]. Similarly, adiponectin is a hormone involved in regulating glycemia, lipidemia, and proinflammatory mechanisms, which has an inverse relationship with obesity and insulin resistance. A group of researchers used DBS from 13,879 children to verify if DBS were a reliable source for measuring adiponectin by an ELISA assay [3]. The correlation coefficient between the 50 bloodspots and plasma samples was high (0.87) [3]. They concluded that DBS contain stable adiponectin that can be utilized for research on diabetes and cardiovascular risk in population-based studies [3].
Measuring endocrine biomarkers in DBS can also assist in prospectively identifying individuals at risk for disease. A large population-based case-control study in Denmark was designed to evaluate type 1 diabetes risk (n=2476 cases, 4172 controls) using DBS measurements of genetic variants, inflammatory cytokines, mannose binding lectin, leptin, and other biomarkers [72]. Initial pilot studies demonstrated their feasibility of measurement from stored DBS samples [72]. They subsequently identified two autoantibodies which predict type 1 diabetes risk at birth using DBS measures [73]. In another study, researchers collaborated with 94 hospitals to use DBS to conduct mass screening on newborns for congenital hypothyroidism by quantifying free thyroxine (FT4) and thyroid stimulating hormone (TSH) [74]. They concluded that FT4 and TSH levels could be used for diagnosing congenital hypothyroidism, but more work needs to be done to determine a better cutoff value for FT4 [74].
Pharmacologic
DBS have become a top commodity in pharmacologic studies because they are a good source of biomaterial, are easy to collect, and grant patients the autonomy of collecting their own biological specimens in the comfort of their home. Hence DBS may be used to assess compliance with treatment regimens in randomized controlled trials or screen for use of illicit or addictive drugs. Tracking a patient’s adherence to therapeutic drugs can assist physicians to understand and ensure they are providing their patient with a proper dosage that is effective enough to correct the imbalance in the patient’s body without causing further disruptions to the patient’s social and physical experiences.
Drug Monitoring
As with other biomarkers, the first step in determining if DBS can be used for drug monitoring is to assess the validity of measurement in DBS versus biomaterial resources like urine and blood. Such validation has been done for several drugs. For example, a study explored the applicability of using DBS versus plasma to monitor drug usage of antiepileptic drugs in children with epilepsy [75]. The researchers concluded that DBS would be a valuable tool to aid epilepsy drug monitoring in children [75]. In adults, a study assessed the ability to utilize DBS to facilitate therapeutic drug monitoring (TDM) of adalimumab and anti-adalimumab in patients with various inflammatory diseases [76]. The researchers found that DBS simplify the process of TDM, create a method for more personalized dosage schemes, and provide accurate concentration predictions of adalimumab [76]. Another study utilized DBS to monitor vemurafenib treatment in melanoma patients [77]. After conducting a validity study, researchers concluded that there were differences in composition between DBS and blood plasma samples, so they developed a conversion factor that could be useful in predicting the plasma concentration from the DBS samples [77]. DBS have also been used to track aspirin use (acetylsalicylic acid), through monitoring concentrations of salicylic acid, the main metabolite of acetylsalicylic acid. Researchers used desorption electrospray ionization mass spectrometry (DESI-Ms) to analyze the DBS [78]. In this work, the researchers discovered that enhancing the filter paper or Guthrie card with other reagents and utilizing DESI-Ms greatly improved detection and quantification on the filter paper [78]. Another study that used therapeutic drug monitoring and DBS explored their use to measure adherence to HIV Pre-exposure Prophylaxis (Pr-EP), a preventative measure for people at high risk of HIV infection The scientists found strong efficacy of utilizing DBS for tracking Pr-EP [79, 80]. Kidney function can be assessed with measurements of creatinine from filter paper [81]. The study confirmed that their methodology provides insight for analyzing metformin and sitagliptin concentrations in relation to creatinine [81]. Finally, another study found that DBS were a good source of biomaterial to detect ten different cardiovascular drugs and determine adherence to medication regimens [82]. In all these cases, utilizing larger blood samples to track adherence would be more invasive, while DBS are faster, less invasive, and minimize the risk of infection while maintaining the ability to accurately measure drug metabolites.
Conclusion
Dried blood spots are a good source of biomaterial to analyze biomarker presence, track environmental pollutants, sequence DNA for genomic/epigenetic purposes, and assess adherence to pharmaceutical drugs. DBS are cost efficient, easier to store, and one does not need to be a phlebotomist to obtain the biomaterial. Disadvantages of utilizing DBS in scientific studies include the limited volume of blood available to make accurate analyses, the low levels of biomaterial that may be captured on the DBS, and the hematocrit effect [15]. Indeed, there is still research needed to completely implement utilizing DBS instead of other resources, but this compilation of data from various papers indicates that DBS have multiple promising applications for measuring environmental pollutants, genetics and epigenetics, and biomarkers.
Acknowledgements
Funding: Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Zakaria R, Allen KJ, Koplin JJ, Roche P, Greaves RF. Advantages and Challenges of Dried Blood Spot Analysis by Mass Spectrometry Across the Total Testing Process. EJIFCC. 2016;27(4):288–317. [PMC free article] [PubMed] [Google Scholar]
- 2.Scriver CC. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants, by Robert Guthrie and Ada Susi, Pediatrics, 1963;32:318–343. Pediatrics. 1998;102(1 Pt 2):236–7. [PubMed] [Google Scholar]
- 3.Martin RM, Patel R, Oken E, Thompson J, Zinovik A, Kramer MS et al. Filter paper blood spot enzyme linked immunoassay for adiponectin and application in the evaluation of determinants of child insulin sensitivity. PLoS One. 2013;8(8):e71315. doi: 10.1371/journal.pone.0071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya K, Wotton T, Wiley V. The evolution of blood-spot newborn screening. Transl Pediatr. 2014;3(2):63–70. doi: 10.3978/j.issn.2224-4336.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJ et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39(3):171–87. doi: 10.1053/j.semperi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Therrell BL, Jr., Padilla CD. Newborn screening in the developing countries. Curr Opin Pediatr. 2018;30(6):734–9. doi: 10.1097/MOP.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 7.Denes J, Szabo E, Robinette SL, Szatmari I, Szonyi L, Kreuder JG et al. Metabonomics of newborn screening dried blood spot samples: a novel approach in the screening and diagnostics of inborn errors of metabolism. Analytical chemistry. 2012;84(22):10113–20. doi: 10.1021/ac302527m. [DOI] [PubMed] [Google Scholar]
- 8.Kanungo S, Patel DR, Neelakantan M, Ryali B. Newborn screening and changing face of inborn errors of metabolism in the United States. Ann Transl Med. 2018;6(24):468. doi: 10.21037/atm.2018.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. J Vis Exp. 2014(83):e50973. doi: 10.3791/50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin NJ, Cooper HJ. Challenges and opportunities in mass spectrometric analysis of proteins from dried blood spots. Expert Rev Proteomics. 2014;11(6):685–95. doi: 10.1586/14789450.2014.965158. [DOI] [PubMed] [Google Scholar]
- 11.Rajatileka S, Luyt K, El-Bokle M, Williams M, Kemp H, Molnar E et al. Isolation of human genomic DNA for genetic analysis from premature neonates: a comparison between newborn dried blood spots, whole blood and umbilical cord tissue. BMC Genet. 2013;14:105. doi: 10.1186/1471-2156-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massaro AN, Wu YW, Bammler TK, MacDonald JW, Mathur A, Chang T et al. Dried blood spot compared to plasma measurements of blood-based biomarkers of brain injury in neonatal encephalopathy. Pediatr Res. 2019;85(5):655–61. doi: 10.1038/s41390-019-0298-7. [DOI] [PubMed] [Google Scholar]
- 13.Jensen BP, Saraf R, Ma J, Berry S, Grant CC, Camargo CA, Jr. et al. Quantitation of 25-hydroxyvitamin D in dried blood spots by 2D LC-MS/MS without derivatization and correlation with serum in adult and pediatric studies. Clin Chim Acta. 2018;481:61–8. doi: 10.1016/j.cca.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Lu D, Wang D, Ip HS, Barley F, Ramage R, She J. Measurements of polybrominated diphenyl ethers and polychlorinated biphenyls in a single drop of blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;891–892: 36–43. doi: 10.1016/j.jchromb.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Daousani C, Karalis V, Malenovic A, Dotsikas Y. Hematocrit effect on dried blood spots in adults: a computational study and theoretical considerations. Scand J Clin Lab Invest. 2019;79(5):325–33. doi: 10.1080/00365513.2019.1622033. [DOI] [PubMed] [Google Scholar]
- 16.Fan L, Lee JA. Managing the effect of hematocrit on DBS analysis in a regulated environment. Bioanalysis. 2012;4(4):345–7. doi: 10.4155/bio.11.337. [DOI] [PubMed] [Google Scholar]
- 17.Ho NT, Furge K, Fu W, Busik J, Khoo SK, Lu Q et al. Gene expression in archived newborn blood spots distinguishes infants who will later develop cerebral palsy from matched controls. Pediatr Res. 2013;73(4 Pt 1):450–6. doi: 10.1038/pr.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhuri SN, Butala SJ, Ball RW, Braniff CT, Rocky Mountain Biomonitoring C. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J Expo Sci Environ Epidemiol. 2009;19(3):298–316. doi: 10.1038/jesee.2008.19. [DOI] [PubMed] [Google Scholar]
- 19.Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1902–5. doi: 10.1158/1055-9965.EPI-07-0230. [DOI] [PubMed] [Google Scholar]
- 20.Kolatorova L, Duskova M, Vitku J, Starka L. Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol Res. 2017;66(Supplementum 3):S305–S15. [DOI] [PubMed] [Google Scholar]
- 21.Murphy SE, Wickham KM, Lindgren BR, Spector LG, Joseph A. Cotinine and trans 3’-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J Expo Sci Environ Epidemiol. 2013;23(5):513–8. doi: 10.1038/jes.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector LG, Murphy SE, Wickham KM, Lindgren B, Joseph AM. Prenatal tobacco exposure and cotinine in newborn dried blood spots. Pediatrics. 2014;133(6):e1632–8. doi: 10.1542/peds.2013-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosnoff CS, Bernert JT. Analysis of cotinine in dried blood spots by LC APCI tandem mass spectrometry. Clin Chim Acta. 2008;388(1–2):228–9. doi: 10.1016/j.cca.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Ladror D, Pitt B, Funk W. Quantification of cotinine in dried blood spots as a biomarker of exposure to tobacco smoke. Biomarkers. 2018;23(1):44–50. doi: 10.1080/1354750X.2017.1375558. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Pearl M, Jacob P, 3rd, DeLorenze GN, Benowitz NL, Yu L et al. Levels of cotinine in dried blood specimens from newborns as a biomarker of maternal smoking close to the time of delivery. Am J Epidemiol. 2013;178(11):1648–54. doi: 10.1093/aje/kwt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poothong S, Papadopoulou E, Lundanes E, Padilla-Sanchez JA, Thomsen C, Haug LS. Dried blood spots for reliable biomonitoring of poly- and perfluoroalkyl substances (PFASs). Sci Total Environ. 2019;655:1420–6. doi: 10.1016/j.scitotenv.2018.11.214. [DOI] [PubMed] [Google Scholar]
- 27.Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K et al. Use of newborn screening program blood spots for exposure assessment: declining levels of perluorinated compounds in New York State infants. Environ Sci Technol. 2008;42(14):5361–7. doi: 10.1021/es8006244. [DOI] [PubMed] [Google Scholar]
- 28.Kato K, Wanigatunga AA, Needham LL, Calafat AM. Analysis of blood spots for polyfluoroalkyl chemicals. Anal Chim Acta. 2009;656(1–2):51–5. doi: 10.1016/j.aca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Yeung EH, Louis GB, Lawrence D, Kannan K, McLain AC, Caggana M et al. Eliciting parental support for the use of newborn blood spots for pediatric research. BMC Med Res Methodol. 2016;16:14. doi: 10.1186/s12874-016-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghassabian A, Bell EM, Ma WL, Sundaram R, Kannan K, Buck Louis GM et al. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut. 2018;243(Pt B):1629–36. doi: 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell GA, Perkins N, Buck Louis GM, Kannan K, Bell EM, Gao C et al. Exposure to Persistent Organic Pollutants and Birth Characteristics: The Upstate KIDS Study. Epidemiology. 2019;30 Suppl 2:S94–S100. doi: 10.1097/EDE.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma WL, Yun S, Bell EM, Druschel CM, Caggana M, Aldous KM et al. Temporal trends of polybrominated diphenyl ethers (PBDEs) in the blood of newborns from New York State during 1997 through 2011: analysis of dried blood spots from the newborn screening program. Environ Sci Technol. 2013;47(14):8015–21. doi: 10.1021/es401857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batterman S, Chernyak S. Performance and storage integrity of dried blood spots for PCB, BFR and pesticide measurements. Sci Total Environ. 2014;494–495: 252–60. doi: 10.1016/j.scitotenv.2014.06.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burse VW, DeGuzman MR, Korver MP, Najam AR, Williams CC, Hannon WH et al. Preliminary investigation of the use of dried-blood spots for the assessment of in utero exposure to environmental pollutants. Biochem Mol Med. 1997;61(2):236–9. doi: 10.1006/bmme.1997.2603. [DOI] [PubMed] [Google Scholar]
- 35.Basu N, Eng JWL, Perkins M, Santa-Rios A, Martincevic G, Carlson K et al. Development and application of a novel method to characterize methylmercury exposure in newborns using dried blood spots. Environ Res. 2017;159:276–82. doi: 10.1016/j.envres.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funk WE, McGee JK, Olshan AF, Ghio AJ. Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers. 2013;18(2):174–7. doi: 10.3109/1354750X.2012.750379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen L, Andersen-Ranberg K, Hollergaard M, Nybo M. Quantification of multiple elements in dried blood spot samples. Clin Biochem. 2017;50(12):703–9. doi: 10.1016/j.clinbiochem.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Lefterova MI, Shen P, Odegaard JI, Fung E, Chiang T, Peng G et al. Next-Generation Molecular Testing of Newborn Dried Blood Spots for Cystic Fibrosis. J Mol Diagn. 2016;18(2):267–82. doi: 10.1016/j.jmoldx.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Zhou J, Zhao Y, Cheng Z, Song W, Sun Y et al. High-Throughput, Multiplex Genotyping Directly from Blood or Dried Blood Spot without DNA Extraction for the Screening of Multiple G6PD Gene Variants at Risk for Drug-Induced Hemolysis. J Mol Diagn. 2017;19(5):638–50. doi: 10.1016/j.jmoldx.2017.05.007.This study utilized a novel method to understand G6PD gene variants and how they are affected by certain malaria therapeutic drugs.
- 40.Xiong Y, Jeronis S, Hoffman B, Liebermann DA, Geifman-Holtzman O. First trimester noninvasive fetal RHD genotyping using maternal dried blood spots. Prenat Diagn. 2017;37(4):311–7. doi: 10.1002/pd.5006.This study highlights a novel use of DBS for RHD genotyping which can help prevent complications when maternal and fetal RHD are not identical.
- 41.Sok P, Lupo PJ, Richard MA, Rabin KR, Ehli EA, Kallsen NA et al. Utilization of archived neonatal dried blood spots for genome-wide genotyping. PLoS One. 2020;15(2):e0229352. doi: 10.1371/journal.pone.0229352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollegaard MV, Grove J, Thorsen P, Norgaard-Pedersen B, Hougaard DM. High-throughput genotyping on archived dried blood spot samples. Genet Test Mol Biomarkers. 2009;13(2):173–9. doi: 10.1089/gtmb.2008.0073. [DOI] [PubMed] [Google Scholar]
- 43.Carter TC, Sicko RJ, Kay DM, Browne ML, Romitti PA, Edmunds ZL et al. Copy-number variants and candidate gene mutations in isolated split hand/foot malformation. J Hum Genet. 2017;62(10):877–84. doi: 10.1038/jhg.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannakou A, Sicko RJ, Kay DM, Zhang W, Romitti PA, Caggana M et al. Copy number variants in hypoplastic right heart syndrome. Am J Med Genet A. 2018;176(12):2760–7. doi: 10.1002/ajmg.a.40527. [DOI] [PubMed] [Google Scholar]
- 45.Giannakou A, Sicko RJ, Zhang W, Romitti P, Browne ML, Caggana M et al. Copy number variants in Ebstein anomaly. PLoS One. 2017;12(12):e0188168. doi: 10.1371/journal.pone.0188168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haak PT, Busik JV, Kort EJ, Tikhonenko M, Paneth N, Resau JH. Archived unfrozen neonatal blood spots are amenable to quantitative gene expression analysis. Neonatology. 2009;95(3):210–6. doi: 10.1159/000155652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shearer AE, Frees K, Kolbe DL, Smith RJH. Comprehensive Genetic Testing for Deafness from Fresh and Archived Dried Blood Spots. Otolaryngol Head Neck Surg. 2018:194599818797291. doi: 10.1177/0194599818797291. [DOI] [PubMed] [Google Scholar]
- 48.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leti F, Llaci L, Malenica I, DiStefano JK. Methods for CpG Methylation Array Profiling Via Bisulfite Conversion. Methods Mol Biol. 2018;1706:233–54. doi: 10.1007/978-1-4939-7471-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker RM, MacGillivray L, McCafferty S, Wrobel N, Murphy L, Kerr SM et al. Assessment of dried blood spots for DNA methylation profiling. Wellcome Open Res. 2019;4:44. doi: 10.12688/wellcomeopenres.15136.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joo JE, Wong EM, Baglietto L, Jung CH, Tsimiklis H, Park DJ et al. The use of DNA from archival dried blood spots with the Infinium HumanMethylation450 array. BMC Biotechnol. 2013;13:23. doi: 10.1186/1472-6750-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novakovic B, Lewis S, Halliday J, Kennedy J, Burgner DP, Czajko A et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat Commun. 2019;10(1):3922. doi: 10.1038/s41467-019-11929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Wong EM, Bui M, Nguyen TL, Joo JE, Stone J et al. Causal effect of smoking on DNA methylation in peripheral blood: a twin and family study. Clin Epigenetics. 2018;10:18. doi: 10.1186/s13148-018-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonseth S, Roy R, Houseman EA, de Smith AJ, Zhou M, Lee ST et al. Periconceptional folate consumption is associated with neonatal DNA methylation modifications in neural crest regulatory and cancer development genes. Epigenetics. 2015;10(12):1166–76. doi: 10.1080/15592294.2015.1117889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diener C, Galata V, Keller A, Meese E. MicroRNA profiling from dried blood samples. Crit Rev Clin Lab Sci. 2019;56(2):111–7. doi: 10.1080/10408363.2018.1561641. [DOI] [PubMed] [Google Scholar]
- 56.Otero-Santos SM, Delinsky AD, Valentin-Blasini L, Schiffer J, Blount BC. Analysis of perchlorate in dried blood spots using ion chromatography and tandem mass spectrometry. Analytical chemistry. 2009;81(5):1931–6. doi: 10.1021/ac802419n. [DOI] [PubMed] [Google Scholar]
- 57.Drolet J, Tolstikov V, Williams BA, Greenwood BP, Hill C, Vishnudas VK et al. Integrated Metabolomics Assessment of Human Dried Blood Spots and Urine Strips. Metabolites. 2017;7(3). doi: 10.3390/metabo7030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer EA, Cooper HJ, Dunn WB. Investigation of the 12-Month Stability of Dried Blood and Urine Spots Applying Untargeted UHPLC-MS Metabolomic Assays. Analytical chemistry. 2019;91(22):14306–13. doi: 10.1021/acs.analchem.9b02577. [DOI] [PubMed] [Google Scholar]
- 59.Trifonova OP, Maslov DL, Balashova EE, Lokhov PG. Evaluation of Dried Blood Spot Sampling for Clinical Metabolomics: Effects of Different Papers and Sample Storage Stability. Metabolites. 2019;9(11). doi: 10.3390/metabo9110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han J, Higgins R, Lim MD, Lin K, Yang J, Borchers CH. Short-Term Stabilities of 21 Amino Acids in Dried Blood Spots. Clin Chem. 2018;64(2):400–2. doi: 10.1373/clinchem.2017.278457. [DOI] [PubMed] [Google Scholar]
- 61.Dietzen DJ, Bennett MJ, Lo SF, Grey VL, Jones PM. Dried Blood Spot Reference Intervals for Steroids and Amino Acids in a Neonatal Cohort of the National Children’s Study. Clin Chem. 2016;62(12):1658–67. doi: 10.1373/clinchem.2016.263434. [DOI] [PubMed] [Google Scholar]
- 62.McDonald TJ, Besser RE, Perry M, Babiker T, Knight BA, Shepherd MH et al. Screening for neonatal diabetes at day 5 of life using dried blood spot glucose measurement. Diabetologia. 2017;60(11):2168–73. doi: 10.1007/s00125-017-4383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Crignis E, Re MC, Cimatti L, Zecchi L, Gibellini D. HIV-1 and HCV detection in dried blood spots by SYBR Green multiplex real-time RT-PCR. J Virol Methods. 2010;165(1):51–6. doi: 10.1016/j.jviromet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Ibarra-Meneses AV, Mondal D, Alvar J, Moreno J, Carrillo E. Cytokines and chemokines measured in dried SLA-stimulated whole blood spots for asymptomatic Leishmania infantum and Leishmania donovani infection. Sci Rep. 2017;7(1):17266. doi: 10.1038/s41598-017-17315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandes ND, Badri T. Congenital Herpes Simplex. StatPearls. Treasure Island (FL) 2019. [Google Scholar]
- 66.Lewensohn-Fuchs I, Osterwall P, Forsgren M, Malm G. Detection of herpes simplex virus DNA in dried blood spots making a retrospective diagnosis possible. J Clin Virol. 2003;26(1):39–48. [DOI] [PubMed] [Google Scholar]
- 67.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51(10):1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 68.Miller EM, McDade TW. A highly sensitive immunoassay for interleukin-6 in dried blood spots. Am J Hum Biol. 2012;24(6):863–5. doi: 10.1002/ajhb.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghassabian A, Sundaram R, Chahal N, McLain AC, Bell EM, Lawrence DA et al. Concentrations of immune marker in newborn dried blood spots and early childhood development: Results from the Upstate KIDS Study. Paediatr Perinat Epidemiol. 2018;32(4):337–45. doi: 10.1111/ppe.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghassabian A, Albert PS, Hornig M, Yeung E, Cherkerzian S, Goldstein RB et al. Gestational cytokine concentrations and neurocognitive development at 7 years. Transl Psychiatry. 2018;8(1):64. doi: 10.1038/s41398-018-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas A, Thevis M. Analysis of insulin and insulin analogs from dried blood spots by means of liquid chromatography-high resolution mass spectrometry. Drug Test Anal. 2018;10(11–12):1761–8. doi: 10.1002/dta.2518. [DOI] [PubMed] [Google Scholar]
- 72.Eising S, Svensson J, Skogstrand K, Nilsson A, Lynch K, Andersen PS et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: design and feasibility of an unselected case-control study. Paediatr Perinat Epidemiol. 2007;21(6):507–17. doi: 10.1111/j.1365-3016.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- 73.Eising S, Nilsson A, Carstensen B, Hougaard DM, Norgaard-Pedersen B, Nerup J et al. Danish children born with glutamic acid decarboxylase-65 and islet antigen-2 autoantibodies at birth had an increased risk to develop type 1 diabetes. Eur J Endocrinol. 2011;164(2):247–52. doi: 10.1530/EJE-10-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. Eur J Endocrinol. 2012;166(5):829–38. doi: 10.1530/EJE-11-0653. [DOI] [PubMed] [Google Scholar]
- 75.Linder C, Wide K, Walander M, Beck O, Gustafsson LL, Pohanka A. Comparison between dried blood spot and plasma sampling for therapeutic drug monitoring of antiepileptic drugs in children with epilepsy: A step towards home sampling. Clin Biochem. 2017;50(7–8):418–24. doi: 10.1016/j.clinbiochem.2016.12.008.This study highlighted the benefits of DBS for homesampling, therapeutic drug monitoring in children, and how it could relieve cost burdens.
- 76.Kneepkens EL, Pouw MF, Wolbink GJ, Schaap T, Nurmohamed MT, de Vries A et al. Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol. 2017;83(11):2474–84. doi: 10.1111/bcp.13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nijenhuis CM, Huitema AD, Marchetti S, Blank C, Haanen JB, van Thienen JV et al. The Use of Dried Blood Spots for Pharmacokinetic Monitoring of Vemurafenib Treatment in Melanoma Patients. J Clin Pharmacol. 2016;56(10):1307–12. doi: 10.1002/jcph.728. [DOI] [PubMed] [Google Scholar]
- 78.Siebenhaar M, Kullmer K, Fernandes NM, Hullen V, Hopf C. Personalized monitoring of therapeutic salicylic acid in dried blood spots using a three-layer setup and desorption electrospray ionization mass spectrometry. Anal Bioanal Chem. 2015;407(23):7229–38. doi: 10.1007/s00216-015-8887-8. [DOI] [PubMed] [Google Scholar]
- 79.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar VS, Webster M. Measuring Adherence to HIV Pre-Exposure Prophylaxis through Dried Blood Spots. Clin Chem. 2016;62(7):1041–3. doi: 10.1373/clinchem.2015.253179. [DOI] [PubMed] [Google Scholar]
- 81.Scherf-Clavel M, Hogger P. Analysis of metformin, sitagliptin and creatinine in human dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;997:218–28. doi: 10.1016/j.jchromb.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Bernieh D, Lawson G, Tanna S. Quantitative LC-HRMS determination of selected cardiovascular drugs, in dried blood spots, as an indicator of adherence to medication. J Pharm Biomed Anal. 2017;142:232–43. doi: 10.1016/j.jpba.2017.04.045. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez-Moron S, Ryan P, Ardizone-Jimenez B, Martin D, Troya J, Cuevas G et al. Evaluation of dried blood spot samples for screening of hepatitis C and human immunodeficiency virus in a real-world setting. Sci Rep. 2018;8(1):1858. doi: 10.1038/s41598-018-20312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]