Abstract
Breast implant-associated anaplastic large cell lymphoma (BI-ALCL) is a rare T-cell lymphoma that arises around breast implants. Most patients manifest with periprosthetic effusion, whereas a subset of patients develops a tumor mass or lymph node involvement (LNI). The aim of this study is to describe the pathologic features of lymph nodes from patients with BI-ALCL and assess the prognostic impact of LNI. Clinical findings and histopathologic features of lymph nodes were assessed in 70 patients with BI-ALCL. LNI was defined by the histologic demonstration of ALCL in lymph nodes. Fourteen (20%) patients with BI-ALCL had LNI, all lymph nodes involved were regional, the most frequent were axillary (93%). The pattern of involvement was sinusoidal in 13 (92.9%) cases, often associated with perifollicular, interfollicular, and diffuse patterns. Two cases had Hodgkin-like patterns. The 5-year overall survival was 75% for patients with LNI and 97.9% for patients without LNI at presentation (P = 0.003). Six of 49 (12.2%) of patients with tumor confined by the capsule had LNI, compared with LNI in 8/21 (38%) patients with tumor beyond the capsule. Most patients with LNI achieved complete remission after various therapeutic approaches. Two of 14 (14.3%) patients with LNI died of disease compared with 0/56 (0%) patients without LNI. Twenty percent of patients with BI-ALCL had LNI by lymphoma, most often in a sinusoidal pattern. We conclude that BI-ALCL beyond capsule is associated with a higher risk of LNI. Involvement of lymph nodes was associated with decreased overall survival. Misdiagnosis as Hodgkin lymphoma is a pitfall.
Keywords: breast implant, ALCL, T-cell lymphoma, CD30, Hodgkin lymphoma, anaplastic large cell lymphoma, lymph node involvement
Breast implant-associated anaplastic large cell lymphoma (BI-ALCL) is a T-cell lymphoma that arises around breast implants. This rare neoplasm was first reported by Keech and Creech1 in 1997 and currently over 380 cases have been reported in the literature and in various international reports.2–4 This lymphoma can arise following placement of breast implants for either cosmetic reasons or for reconstruction after breast cancer surgery. Textured rather than smooth implants have been strongly associated with BI-ALCL.4 Most patients present initially with an effusion around the breast implant and the lymphoma cells are confined by the fibrous capsule; these patients are usually cured by removal of the implants and complete excision of the capsule in line with diagnosis and treatment guidelines established by the National Comprehensive Cancer Network.5,6 A subset of patients presents with a tumor mass, with lymphoma throughout and beyond the capsule, which is associated with decreased overall survival (OS) and with a lower event-free survival.7 BI-ALCL has been recognized as a provisional entity in the revised 4th edition of the World Health Organization classification of lymphoid neoplasms.8
Lymphadenopathy, usually of regional lymph nodes, in patients with BI-ALCL has received relatively little attention in the literature. Particularly in patients with implants placed for cosmetic reasons, lymphadenopathy is often clinically attributed to silicone granulomas, and therefore pathologic examination may even not be performed. Two relatively large studies have described benign changes in regional lymph nodes from patients with breast implants. Katzin et al,9 in their study of 96 patients with silicone breast implants, but no BI-ALCL, reported benign reactive changes with foamy macrophages in the enlarged lymph nodes from these patients. Foreign material including silicone and polyurethane were commonly seen on microspectroscopy.9 Story et al10 in a review article reported that 7/31 (23%) patients with BI-ALCL had lymphadenopathy, although they did not elaborate on pathologic findings or the clinical significance of lymphadenopathy.
In this study, we focused on patients with documented lymph node involvement (LNI) by BI-ALCL. Our aims were to: (1) describe the histopathologic features of lymph nodes involved by BI-ALCL, (2) determine the relationship between the depth of tumor infiltration into the fibrous capsule and LNI, and (3) assess the prognostic importance of LNI by lymphoma in these patients.
MATERIALS AND METHODS
Case Selection
We searched the database of the Department of Hematopathology at The University of Texas MD Anderson Cancer Center for cases diagnosed with BI-ALCL from January 1, 1997 through December 31, 2016. We also included cases accessioned at collaborative institutions over the same period. All patients who underwent evaluation of lymph nodes by clinical or radiologic methods and pathologic assessment were included in this study.
Inclusion criteria were cases of confirmed BI-ALCL based on review of pathology, radiology, and clinical examination. All patients with LNI had clinical or radiologic evidence of lymph node enlargement, prompting lymph node biopsy or excision that proved BI-ALCL involvement. Patients without LNI (control group) included: (1) those who underwent lymph node biopsy and pathologic evaluation was negative for lymphoma and (2) patients who had no evidence of lymphadenopathy by clinical or radiologic studies. Criteria for exclusion were patients with BI-ALCL with enlarged lymph nodes by physical examination or imaging studies, who did not undergo lymph node biopsy.
Extracted information included demographic data, reason for undergoing breast implants, implant filling (silicone or saline), implant surface (textured or smooth), time interval from implant to diagnosis of BI-ALCL, clinical presentation, Ann Arbor clinical stage, clinical stage as proposed by Clemens et al5, and clinical follow-up. This study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Pathologic Features
All patients were diagnosed with BI-ALCL using previously defined criteria: patients had breast implants and the presence of lymphoma cells that were CD30+, anaplastic lymphoma kinase (ALK) negative and T-cell lineage+.2,11,12 For all cases, we assessed routine hematoxylin and eosin-stained sections, as well as CD30 and other immunohistochemical studies, as available. The following features were systematically assessed in involved lymph nodes: maximum diameter, tumor burden (percentage of tumor cells highlighted by CD30 divided by total cells in tissue sections), and pattern of infiltration. The latter was classified as sinusoidal if lymphoma cells were identified within subcapsular or interfollicular sinusoids; perifollicular when lymphoma cells surrounded reactive follicles; interfollicular if lymphoma cells were located between hyperplastic follicles; or diffuse when sheets of lymphoma cells were seen throughout the lymph node. A classic Hodgkin lymphoma (CHL)-like pattern was recorded when the infiltrates were similar to those of nodular sclerosis Hodgkin lymphoma (HL), characterized by nodules of lymphoma cells admixed with inflammatory cells and surrounded by birefringent fibrosclerotic bands, whereas an interfollicular HL-like pattern was considered when the lymphoma cells were admixed with abundant inflammatory infiltrate, but without fibrous bands. Cyto-morphologic features assessed included the nuclear shape (round, oval, lobated, or anaplastic) and the presence of horseshoe-shaped nuclei (“hallmark” cells). Other features assessed were reactive features of lymph nodes and included presence of follicular hyperplasia, sinus histiocytosis (presence of histiocytes expanding the sinus, without presence of tumor cells), and paracortical hyperplasia, as well as the presence of silicone-laden (refringent material), or foamy histiocytes.
The breast implant capsule of these cases was also reviewed. The term “capsule” is defined as the fibrous tissue that develops around a breast implant. The depth of infiltration by lymphoma cells was designated as within or beyond the capsule. Tumor within the capsule indicated that the lymphoma was confined to the space surrounding the implant or superficially extended into the fibrous capsule. Tumor beyond the capsule was defined as lymphoma detected beyond the outlines of the capsule, infiltrating into surrounding fibroadipose tissue or breast parenchyma. The tumor cell distribution was usually best highlighted with immunohistochemistry for CD30.
Immunohistochemical analysis was performed using formalin-fixed, paraffin-embedded tissue sections in our laboratory or at the referring institutions at the time of initial diagnosis. At our institution, we used 4μm thick tissue sections, heat-induced epitope retrieval, and an avidin-biotin complex detection method.13 3,3’-Dia-minobenzidine was used as the chromogen, and staining was performed in an automated immunostainer (Leica Biosystems, Buffalo Grove, IL). Antibodies specific for the following antigens were used: CD2, CD3, CD4, CD5, CD7, CD8, CD15, CD20, CD30, CD45, ALK, EMA, and PAX5 (Dako, Carpinteria, CA), as well as CD43 (Becton-Dickinson Biosciences, San Jose, CA). In situ hybridization analysis for Epstein-Barr virus encoded small RNA was performed as described previously.14 Clonality studies at our institution were based on DNA extracted from fixed, paraffin-embedded tissue using a QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA). TRG and TRB gene rearrangement analysis was performed using a 4-color polymerase chain reaction coupled with capillary electrophoresis as described previously.15
Clinical Follow-up and Outcomes
Follow-up information, therapy, and outcomes were obtained from medical records, or from contributing collaborators, including the extent of sampling such as needle core biopsy, excisional biopsy, or node dissection. Clinical stage and outcome as well as cause of death were annotated when applicable.
Statistical Analysis
All statistical analyses were performed using Graphpad software. The OS curves of different groups were analyzed by using the Kaplan-Meier survival curves and differences were compared using the logrank (Mantel-Cox) test. All differences with P-value <0.05 were considered statistically significant.
RESULTS
Clinical Features
The clinical features of the 14 patients with BI-ALCL and LNI are detailed in Table 1 and summarized in Table 2. Partial information of 12 patients was included in earlier publications3,5,11,16–21 and 2 patients have not been reported previously (cases 9 and 14). All patients were women with a median age of 60 years (range, 40 to 83 y). The right breast was involved in 9 (64.3%) patients, the left in 3 (21.4%), and bilateral involvement was present in 2 (14.3%). Breast implants were placed for cosmetic reasons in 6 (42.9%) patients and for reconstructive surgery for breast carcinoma in 8 (57.1%). The median interval from implant to diagnosis of BI-ALCL was 12 years (range, 5 to 34 y). The implant filling was known in 11 patients and was silicone in 8 (73%), saline in 2 (18%), and silicone as well as saline implants in 1 (7%). All 6 patients for whom implant surface information was available had textured implants. Ten of 14 (71.4%) patients had a tumor mass; 5 of these also had an effusion around the implant. Three (21.4%) patients had effusion only. Four (14.3%) patients presented with lymphadenopathy proved to be LNI, 2 of which did not have an accompanying mass or effusion at presentation. The most common location for lymphadenopathy was the axilla in 13 (92.9%) patients. Three patients had enlargement of multiple lymph nodes in axillary, supraclavicular, mediastinal, and internal mammary groups. Clinical staging using the Ann Arbor system was available for all 14 patients at the time of presentation, and was stage I in 6 (42.9%), stage II in 7 (50%), and stage IV in 1 (7.1%). Clinical staging according to the system of Clemens et al5 was: stage IB in 3 (21.4%), stage IIA in 2 (14.3%), stage IIB in 3 (21.4%), stage III in 5 (35.7%), and stage IV in 1 (7.1%) patient.
TABLE 1.
Clinical Characteristics of Patients With BI-ALCL and LNI (n = 14)
| Case No. | References | Age (y) | Reason For Implants | Clinical Presentation | Extent of Periimplant Capsule Involvement | Timing of LNI* |
|---|---|---|---|---|---|---|
| 1 | Aladily11 | 63 | Cancer | Effusion | Within | (+) 37 |
| 2 | Alobeid et al16 | 68 | Cancer | L axillary node | Within | (−) 5 |
| 3 | Aladily11 | 57 | Cosmetic | Effusion, mass | Beyond | Synchronous |
| 4 | Clemens et al5 | 52 | Cosmetic | Mass | Beyond | Synchronous |
| 5 | Miranda et al3 | 40 | Cosmetic | L axillary node | Beyond | (−) 36 |
| 6 | George17 | 67 | Cancer | Effusion | Within | (+) 6 |
| 7 | Estes et al18 | 77 | Cosmetic | Effusion, mass | Within | (+) 13 |
| 8 | Miranda et al3 | 41 | Cosmetic | Effusion, mass | Beyond | (+) 21 |
| 9 | Unpublished | 72 | Cancer | Effusion, mass | Beyond | (+) 36 |
| 10 | Clemens et al5 | 54 | Cancer | Effusion, mass | Within | (+) 22 |
| 11 | Acevedo-Banez et al19 | 50 | Cancer | Mass | Beyond | Synchronous |
| 12 | Tardio and Granados20 | 51 | Cancer | L axillary node, mass | Within | Synchronous |
| 13 | Laurent et al21 | 83 | Cancer | Mass | Beyond | Synchronous |
| 14 | Unpublished | 51 | Cosmetic | R axillary node, mass | Beyond | Synchronous |
| Location of LNI | Clinical Stage Ann Arbor on Presentation | Clinical Stage MDACC† on Presentation | Management | Time Follow-up (mo) | Outcome | Cause of Death |
| Axilla | I | IB | Surgery, chemotherapy | 100 | CR | NA |
| Axilla bilateral | II | IIB | Surgery, chemotherapy | 71 | CR | NA |
| Supraclavicular, internal mammary | II | III | Surgery, chemotherapy, radiotherapy | 100 | CR | NA |
| Axilla, infraclavicular | II | III | Surgery, chemotherapy, radiotherapy | 7 | DOD | Mediastinal mass with bronchial compression |
| Axilla, supraclavicular, internal mammary, mediastinal, infraclavicular | II | III | Surgery, chemotherapy, radiotherapy, ASCT | 100 | CR | NA |
| Axilla, mediastinal, internal mammary | I | IB | Surgery, chemotherapy | 14 | CR | NA |
| Axilla | I | IB | Surgery, chemotherapy, radiotherapy | 41 | CR | NA |
| Axilla | I | IIA | Surgery, chemotherapy, radiotherapy | 47 | CR | NA |
| Axilla | I | IIA | Surgery | 38 | AWD | NA |
| Axilla | I | IIB | Surgery, chemotherapy | 30 | CR | NA |
| Axilla contralateral | IV | IV | Surgery, chemotherapy, radiotherapy | 20 | CR | NA |
| Axilla | II | IIB | Surgery, chemotherapy, radiotherapy | 29 | CR | NA |
| Axilla | II | III | Surgery, chemotherapy, immunotherapy | 13 | DOD | ALCL (manner of death not determined) |
| Axilla | II | III | Surgery, chemotherapy, radiotherapy | 60 | CR | NA |
Surgical therapy encompasses partial surgery, complete surgery.
Timing of LNI:synchronous: LNI and periimplant capsule BI-ALCL diagnoses at the same time; (+ value): months after periimplant capsule diagnosis; (−value): months before periimplant capsule diagnosis.
Clinical staging according to Clemens et al5.
ASCT indicates autologous stem cell transplant; CR, complete remission; NA, not available.
TABLE 2.
Clinical Characteristics of Patients With BI-ALCL and LNI (n = 14)
| Age (y) | |
| Median | 60 |
| Range | 40-83 |
| N (%) | |
| Reason for implant (n = 14) | |
| Reconstructive for cancer | 8 (57.1) |
| Cosmetic | 6 (42.9) |
| Implant filling (n = 11) | |
| Silicone | 8 (72.7) |
| Saline | 2 (18.2) |
| Silicone/saline | 1 (9.1) |
| Implant texture (n = 6) | |
| Yes | 6 (100) |
| No | 0 |
| Time from implantation to diagnosis | |
| Median (y) | 12 |
| Range (y) | 5-34 |
| Side involved (n = 14) | |
| Left | 3 (21.4) |
| Right | 9 (64.3) |
| Bilateral | 2 (14.3) |
| Clinical presentation (n = 14) | |
| Mass | 10 (71.4) |
| Mass only | 3 (21.4) |
| Mass+effusion | 5 (35.7) |
| Mass+LNI | 2 (14.3) |
| Effusion only | 2 (14.3) |
| Enlarged lymph node | 4 (28.6) |
| LNI+mass | 2 (14.3) |
| Lymph node location (n = 14) | |
| Axillary | 13 (92.9) |
| Axillary only | 10 (71.4) |
| Axillary+infraclavicular | 1 (7.1) |
| Axillary+mediastinal+internal mammary | 1 (7.1) |
| Axillary+* | 1 (7.1) |
| Supraclavicular, internal mammary | 1 (7.1) |
| Timing of LNI (n = 14) | |
| At presentation | 8 (57.1) |
| Metachronous | 6 (42.9) |
| Ann Arbor stage at presentation (n = 14) | |
| I | 6 (42.9) |
| II | 7 (50.0) |
| III | 0 |
| IV | 1 (7.1) |
| MDACC clinical stage at diagnosis (n = 14) | |
| IA | 0 |
| IB | 3 (21.4) |
| IIA | 2 (14.3) |
| IIB | 3 (21.4) |
| III | 5 (35.7) |
| IV | 1 (7.1) |
| Extent of capsular involvement (n = 14) | |
| Within capsule | 6 (42.9) |
| Beyond capsule | 8 (57.1) |
| Outcome (n = 14) | |
| Complete remission | 11 (78.6) |
| Dead of disease | 2 (14.3) |
| Alive with disease | 1 (7.1) |
| Cause of death (n = 2) | |
| ALCL related death | 2 |
| Bronchial compression | 1 (50) |
| No details | 1 (50) |
Axillary + supraclavicular, infraclavicular, internal mammary, and mediastinal.
We identified 56 patients without LNI (control group), of whom partial information of 53 patients was included in earlier publications;3,5,21–32 3 patients were previously unreported. The median age of these patients was 56 years (range, 35 to 77 y). Twenty-two (39%) patients had breast implants for reconstruction after breast cancer surgery, 33 (59%) were cosmetic, and the indication for 1 (2%) patient was not known. Clinical staging using the Ann Arbor system was available for all patients: 55 (98%) were stage I, and 1 case (2%) was stage IV; the patient with stage IV had disease involving the T7 vertebra. The clinical stage according to the system of Clemens et al5 was: stage IA in 29 (52%), stage IB in 7 (12%), stage IC in 6 (11%), stage IIA in 13 (23%), and stage IV in 1 (2%) case. Two patients in this group died; 1 due to BI-ALCL and 1 from unrelated disease. Two patients are alive with disease and 52 have achieved complete remission.
Pathologic Features Lymph Nodes
The lymph node specimens were obtained by excisional biopsy in 7 (50%) patients, lymph node dissection in 6 (42.9%), and needle biopsy in 1 (7.1%) (Tables 3, 4). The maximum dimension of the lymph nodes was determined in 13 cases with a median of 23.6 mm, and a range of 8.9 to 51 mm. The pattern of infiltration was determined in all cases and multiple patterns were often identified. The pattern was sinusoidal in 13 (92.9%) cases (Figs. 1A, B), perifollicular in 7 (50%) (Figs. 1C, D), interfollicular in 12 (85.7%), and diffuse in 4 (28.6%) cases (Figs. 1E, F). These patterns were best assessed by using immunohistochemistry to detect CD30 expression by the lymphoma cells (Figs. 1B, D, F). The median tumor burden was semi-quantitatively estimated at 25% (range, 1% to 95%). Two cases with minimal infiltration are illustrated in Figure 2: Case 2 had 5% and case 9 had 1% tumor infiltration. A nodular sclerosis HL-like pattern was noted in 1 (7.1%) case (case 8) (Fig. 3); and 1 (7.1%) case (case 5) showed an interfollicular HL-like pattern. The predominant nuclear shape of neoplastic cells was oval in 13 (92.9%) (Fig. 4A), lobated in 9 (64.3%) (Fig. 4B), and anaplastic in 5 (35.7%) (Fig. 4C) cases, however 10 (71.4%) cases had significant number of cells with > 1 type of nuclear shape. Hallmark cells (Fig. 4D) were identified in 7 (50%) cases. Tumor necrosis was noted in 5 (39%) lymph node specimens. A variable number of eosinophils, occasionally frequent, was noted in 13 (92.9%) cases (Fig. 4A). Associated non-neoplastic changes included follicular hyperplasia in 13 (92.9%), paracortical hyperplasia in 5 (35.7%), and sinus histiocytosis in 8 (57.1%) cases. A minimal amount of refractile material consistent with silicone was found in 1 (7.1%) case, and foamy histiocytes were identified in another case.
TABLE 3.
Pathologic Features of Patients With BI-ALCL and LNI (n = 14)
| Case No. | References | Specimen Type | LN maximum Dimension (mm) | Tumor Burden (%) | Infiltration Pattern |
|---|---|---|---|---|---|
| 1 | Aladily11 | Excision | 20 | 70 | S, P, I, D |
| 2 | Alobeid et al16 | Excision | 11.8 | 5 | S, P, I |
| 3 | Aladily11 | Excision | 25 | 5 | S, P, I |
| 4 | Clemens et al5 | Dissection | 40 | 60 | S, P, I, D |
| 5 | Miranda et al3 | Excision | 17 | 5 | HL-like |
| 6 | George17 | Excision | 8.9 | 30 | S, P, I |
| 7 | Estes et al18 | Excision | 51 | 10 | S |
| 8 | Miranda et al3 | Dissection | 13 | 30 | S, I, D, NSHL-like |
| 9 | Unpublished | Excision | NA | 1 | S |
| 10 | Clemens et al5 | Dissection | 21 | 95 | S, P, I, D |
| 11 | Acevedo-Banez et al19 | Needle bx | 12 | 20 | S, I |
| 12 | Tardio and Granados20 | Dissection | 29 | 5 | S, P, I |
| 13 | Laurent et al21 | Dissection | 25 | 10 | S, I |
| 14 | Unpublished | Dissection | 12 | 5 | S, I |
| Reactive features |
|||||
| Cytomorphology | Hallmark Cells | Follicular Hyperplasia | Sinus Histiocytosis | Paracortical Hyperplasia | Eosinophils |
| Oval, lobated, anaplastic | Yes | Yes | Yes | Yes | Yes |
| Oval | No | Yes | No | Yes | Yes |
| Oval, lobated | Yes | Yes | No | No | Yes |
| Oval | No | Yes | Yes | No | Yes |
| Oval | No | Yes | Yes | Yes | Yes |
| Oval, anaplastic | Yes | Yes | Yes | No | Yes |
| Oval, lobated | Yes | Yes | Yes | No | Yes |
| Oval, lobated | No | Yes | Yes | Yes | Yes |
| Lobated | No | Yes | Yes | No | No |
| Oval, lobated | No | Yes | No | No | Yes |
| Oval, lobated | Yes | No | No | No | Yes |
| Lobated, anaplastic | Yes | Yes | Yes | Yes | No |
| Lobated | Yes | Yes | No | No | Yes |
| Oval | No | Yes | Yes | Yes | Yes |
D indicates diffuse; I, interfollicular; NSHL-like, nodular sclerosis Hodgkin lymphoma-like; HL-like, Hodgkin lymphoma-like (excludes NSHL-like); LN, Lymph node; P, perifollicular; S, sinusoidal.
TABLE 4.
Pathologic Features of LNI of Patients With BI-ALCL (n = 14)
| N (%) | |
|---|---|
| Specimen type | |
| Excisional biopsy | 7 (50.0) |
| Lymph node dissection | 6 (42.9) |
| Needle biopsy | 1 (7.1) |
| Lymph node maximum dimension (n = 13) | |
| Median (mm) | 23.6 |
| Range (mm) | 8.9-51 |
| Lymphoma features | |
| Pattern of infiltration | |
| Sinusoidal | 13 (92.9) |
| Perifollicular | 7 (50.0) |
| Interfollicular | 12 (85.7) |
| Diffuse | 4 (28.6) |
| Hodgkin lymphoma like* | 2 (14.3) |
| Tumor burden | |
| Median (%) | 25 |
| Range (%) | 1-95 |
| Cytomorphology | |
| Oval | 13 (92.9) |
| Lobated | 9 (64.3) |
| Anaplastic | 5 (35.7) |
| Hallmark cells | 7 (50.0) |
| Lymph node necrosis | 5 (35.7) |
| Non-neoplastic features | |
| Follicular hyperplasia | 13 (92.9) |
| Paracortical hyperplasia | 5 (35.7) |
| Sinus histiocytosis | 8 (57.1) |
| Eosinophils | 13 (92.9) |
| Refringent material | 1 (7.1) |
| Foamy histiocytes | 1 (7.1) |
Hodgkin lymphoma-like: 1 nodular sclerosis HL-like, 1 mixed cellularity HL-like.
FIGURE 1.
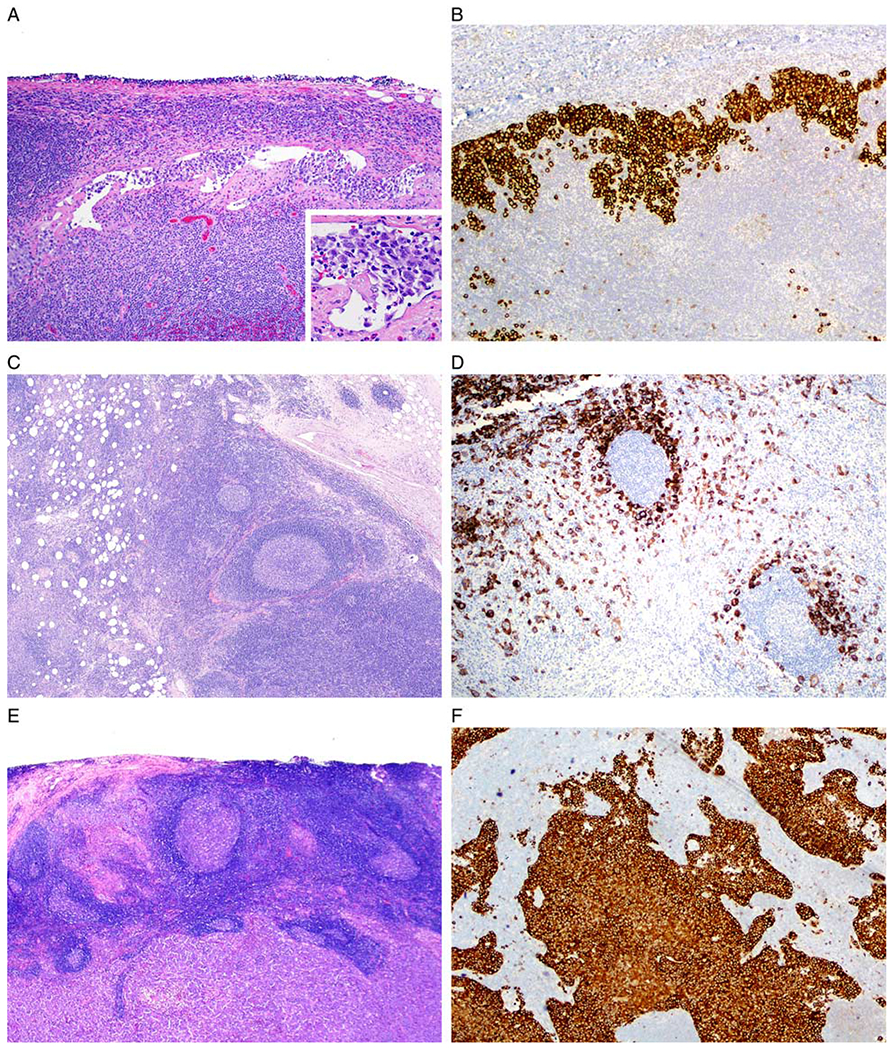
BI-ALCL: patterns of lymph node infiltration. A and B Sinusoidal pattern (case 7). A, A dilated subcapsular sinus of axillary lymph node contains clusters of lymphoma cells. Inset shows high magnification of cells within an open sinus (hematoxylin and eosin). B, Anti-CD30 immunohistochemistry with hematoxylin counterstain highlights the lymphoma cells within a dilated sinus. C and D, Perifollicular pattern (case 1). C, Lymph node displays hyperplastic lymphoid follicles partially surrounded by large lymphoma cells. D, The anti-CD30 immunohistochemistry highlights lymphoma cells around hyperplastic follicles (C, hematoxylin and eosin; D, anti-CD30 immunohistochemistry with hematoxylin counterstain). E and F Diffuse pattern (case 10). E, The lymph node architecture is effaced by sheets of lymphoma cells (hematoxylin and eosin). F, Sheets of lymphoma cells are highlighted with anti-CD30 (anti-CD30 immunohistochemistry with hematoxylin counterstain).
FIGURE 2.
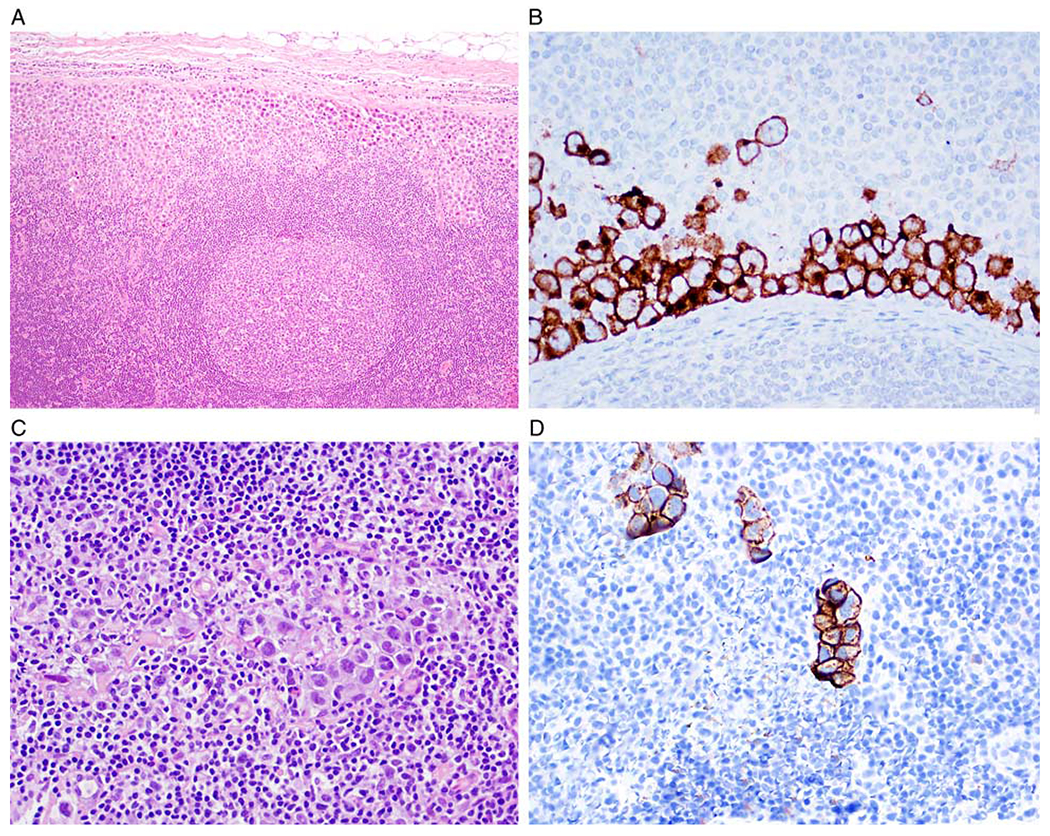
Cases of BI-ALCL with LNI with low tumor burden. A and B, Case 2. A, Lymph node with ~5% tumor burden involving the subcapsular sinus (hematoxylin and eosin). B, CD30 immunohistochemistry highlights lymphoma cells in the subcapsular sinus (anti-CD30 with hematoxylin counterstain). C and D, Case 9. C, 1% tumor burden in a sinusoidal pattern;only rare large cells are identified (hematoxylin and eosin). D, CD30 immunohistochemistry highlights scattered lymphoma cells with a sinusoidal pattern (anti-CD30 with hematoxylin counterstain).
FIGURE 3.
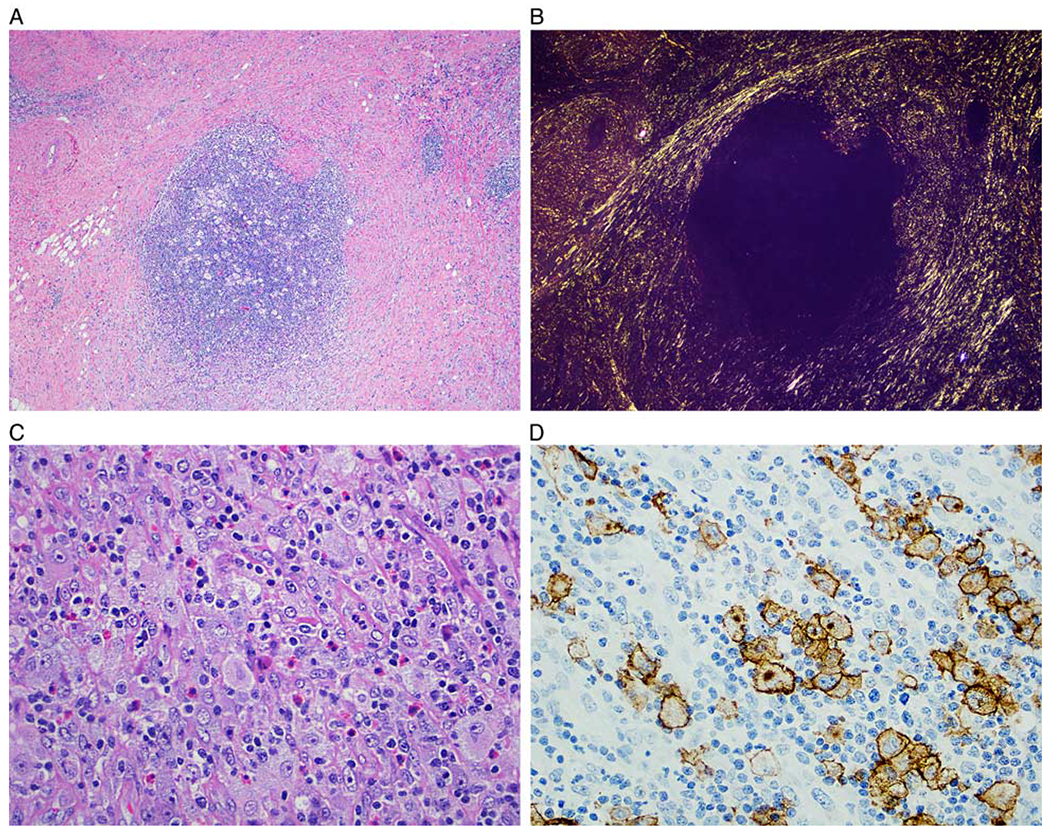
Nodular sclerosis Hodgkin lymphoma-like (NSHL-like) pattern (case 4). A, Lymphomatous nodule surrounded by sclerotic bands resembling NSHL (hematoxylin and eosin). B, The birefringent collagen is highlighted with polarized light. C, The cellular nodule is composed of a polymorphic infiltrate of small lymphocytes, histiocytes, eosinophils, and scattered large Hodgkin and Reed-Sternberg-like cells (hematoxylin and eosin). D, Immunohistochemistry for CD30 highlights the large neoplastic cells, mimicking CHL (anti-CD30 immunohistochemistry with hematoxylin counterstain).
FIGURE 4.
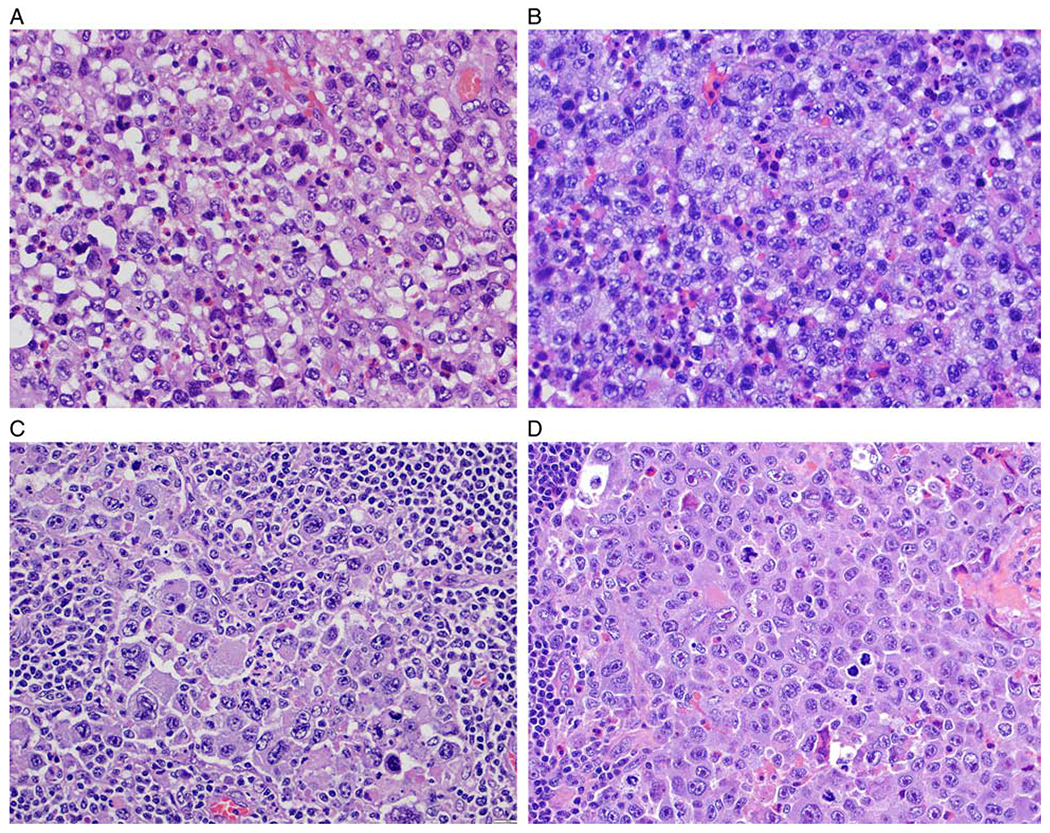
BI-ALCL cytomorphology. A, This case displays a predominance of cells with round to oval nuclei, vesicular chromatin and distinct nucleoli admixed with scattered eosinophils. B, This case displays a predominance of cells with lobulated nuclei, vesicular chromatin and irregular nuclear membrane with indentations. C, Most of the neoplastic cells are large and pleomorphic and have vesicular or hyperchromatic nuclei. D, This case illustrates a subset of lymphoma cells with cytomorphology of “hallmark cells” with nuclear indentations, abundant cytoplasm, and distinct paranuclear clearing (all figures stained with hematoxylin and eosin).
In all positive lymph nodes and periimplant capsules, the neoplastic cells were strongly positive for CD30 (Figs. 1–4) and were negative for ALK. All cases expressed at least 1 T-cell antigen including CD2, CD4, CD5, or CD43, but were negative for CD3. All tested cases were negative for CD20 (n = 11), PAX5 (n = 6), and Epstein-Barr virus encoded RNA (n = 11) (Table 5). Five of 14 cases were tested for TRG or TRB rearrangements: 3 were monoclonal for TRG, 1 was for TRB, and 1 case was polyclonal.
TABLE 5.
Summary of the immunohistochemical and EBER Profile of BI-ALCL Cases With LNI (n = 14)
| (+) Cases (n [%]) | |
|---|---|
| CD2 (n = 7) | 3 (42.9) |
| CD3 (n = 14) | 0 |
| CD4 (n = 13) | 11 (84.6) |
| CD5 (n = 11) | 2 (18) |
| CD7 (n = 9) | 0 |
| CD8 (n = 12) | 1 (8.3) |
| CD15 (n = 9) | 3 (33.3) |
| CD20 (n = 11) | 0 |
| CD30 (n=14) | 14 (100) |
| CD43 (n = 9) | 8 (88.9) |
| CD45 (n = 11) | 10 (90.9) |
| ALK (n=14) | 0 |
| EBER (n=9) | 0 |
| EMA (n = 7) | 5 (71.4) |
| PAX5 (n = 6) | 0 |
ALK indicates anaplastic lymphoma kinase; EBER, Epstein-Barr virus encoded RNA; EMA, epithelial membrane antigen; PAX5, paired-box protein 5.
Breast Capsule
Capsulectomy specimens were available for evaluation in all cases. In patients with LNI, the lymphoma in the breast capsule specimen was confined to the luminal surface of the capsule in 6 (42.9%) patients (Figs. 5A, B) and infiltrated beyond the capsule in 8 (57.1%) patients (Figs. 5C, D). Overall, 6/49 (12.2%) patients with tumor confined by the capsule had LNI, compared with 8/21 (38%) patients with tumor beyond the capsule who had LNI.
FIGURE 5.
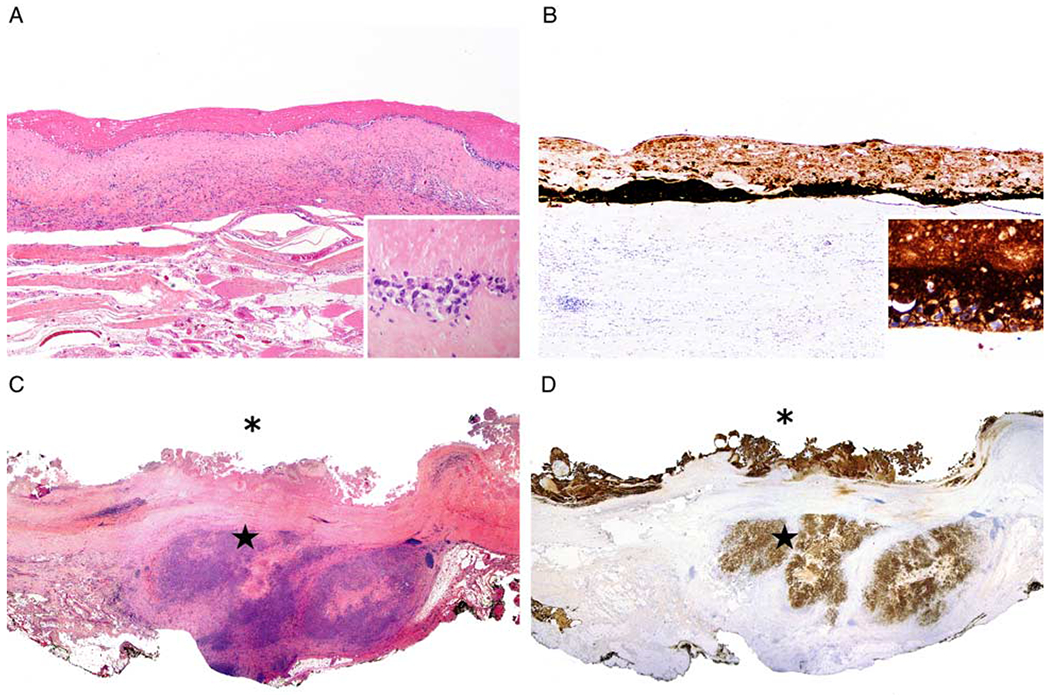
Extent of lymphoma infiltration into the periimplant capsule. A, Lymphoma confined to the luminal side of the capsule surrounding an implant. Large lymphoma cells are noted on the luminal surface of the capsule. Note in the inset that only rare lymphoma cells are viable, while most of the pink material corresponds to necrotic or ghost cells (hematoxylin and eosin). B, Anti-Cd30 highlights the lymphoma cells on the luminal side of the capsule. Note in the inset that only rare lymphoma cells are viable and strongly reactive with CD30, while most of the CD30 reactivity corresponds to necrotic or ghost cells (anti-CD30 immunohistochemistry with hematoxylin counterstain). C, This illustration is a panoramic view of well-oriented capsulectomy specimen that displays lymphoma on the luminal surface of the capsule (asterisk), as well as lymphoma growing beyond the capsule (star) (hematoxylin and eosin). D, CD30 immunohistochemistry highlights abundant reactivity of viable as well as necrotic cells or ghost cells both at the luminal side (asterix) of the capsule as well as at the extracapsular extension (star) of BI-ALCL into surrounding soft tissue (anti-CD30 immunohistochemistry with hematoxylin counterstain).
Clinical Management, Therapy, and Survival
The therapy used was available for all patients with LNI, and included chemotherapy in 13/14 (92.3%), and radiation therapy in 8/13 (61.5%) patients. All patients were surgically managed. One of 12 (8.3%) patients received autologous stem cell transplant (Table 6). The total number of therapeutic procedures exceeds the number of patients with LNI because several patients received multiple modalities of treatment along the course of their disease. The median time of follow-up was 48 months (range, 7 to 100 mo). Outcome was available for all 14 patients: 11 (78.6%) achieved complete remission, 1 (7.1%) patient is alive with disease, and 2 (14.2%) died of BI-ALCL. The cause of death of 1 patient (case 4) was bronchial compression by tumor mass, and the cause of death of the other patient (case 13) was lymphoma, with the manner of death unavailable.
TABLE 6.
Clinical Management and Therapy of Patients With BI-ALCL and LNI (n = 14)
| N (%) | |
|---|---|
| Chemotherapy (n = 14) | 13 (92.8) |
| Radiotherapy (n = 13) | 8 (61.5) |
| Surgery (n = 14) | 14 (100) |
| ASCT (n = 12) | 1 (8.3) |
| Salvage chemotherapy (n = 10) | 4 (40) |
ASCT indicates autologous stem cell transplant.
The 5-year OS for the entire cohort of 70 patients with BI-ALCL was 95.1%, and the median OS was not reached (Fig. 6A). The 5-year OS for the 14 patients with LNI was 85.7.6%, and the median OS was not reached (Fig. 6B).
FIGURE 6.
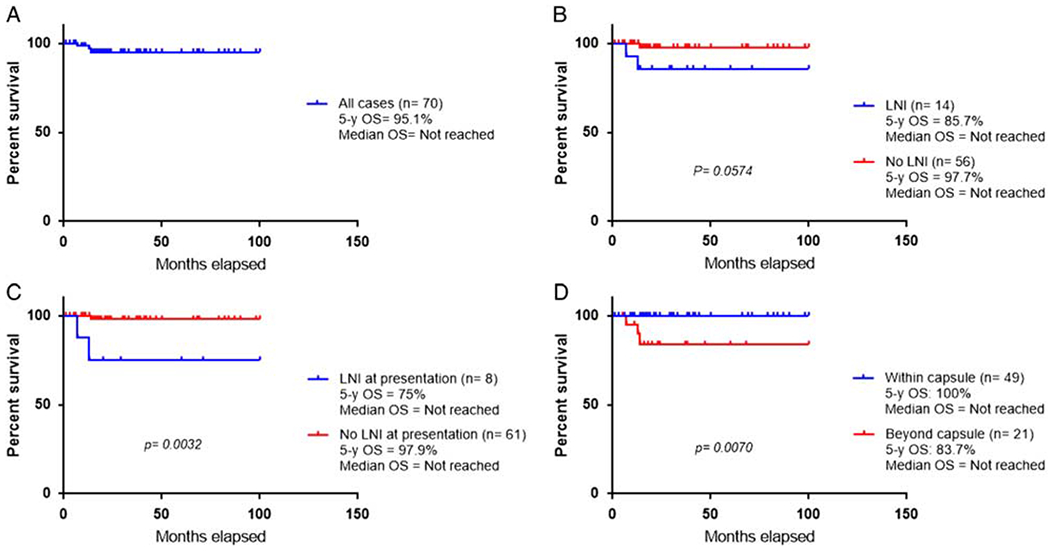
Survival curves. A, Five-year OS for the cohort of 70 patients with BI-ALCL, with and without LNI. B, Comparison of 5-year OS between patients with BI-ALCL, with (n = 14) and without LNI (n = 56). C, Comparison of 5-year OS between patients with BI-ALCL with (n = 8) and without (n = 61) LNI at presentation D, Comparison of 5-year OS of patients with BI-ALCL within (n = 49) and beyond the capsule (n = 21).
For comparison, we assessed OS for 56 patients with BI-ALCL without LNI (Table 7). The 5-year OS was 97.7%, and the median OS was not reached. The difference in OS between patients with LNI and those without LNI both at presentation or on follow-up was not significant (P = 0.0574) (Fig. 6B), however, the difference was significant when we compared the 5-year OS between patients with and without LNI at presentation (P = 0.0032) (Fig. 6C). For all 70 patients, the 5-year OS of patients with tumor confined by the fibrous capsule surrounding the implant (with or without LNI) was 100% versus 83.7% for patients with tumor beyond the capsule (P = 0.007); the median OS for both groups was not reached (Fig. 6D).
TABLE 7.
Five-year OS of BI-ALCL Patients With and Without LNI
| 5-Year OS (%) | P | |
|---|---|---|
| BI-ALCL with and without LNI (n = 70) (Fig. 6A) |
95.1 | |
| BI-ALCL with and without LNI (Fig. 6B) | 0.0574 | |
| With LNI (n=14) | 85.7 | |
| Without LNI (n=56) | 97.7 | |
| BI-ALCL with and without LNI at presentation (n=69) (Fig. 6C) | 0.0032 | |
| LNI at presentation (n= 8) | 75 | |
| No LNI at presentation (n = 61) | 97.9 | |
| BI-ALCL within vs. beyond capsule (Fig. 6D) | 0.007 | |
| Within capsule (n = 49) | 100 | |
| Beyond capsule (n = 21) | 83.7 |
Significant P-values are shown in bold.
DISCUSSION
We evaluated the histopathologic features of lymph nodes involved by BI-ALCL in 14 patients. This subgroup represents ~20% of patients with BI-ALCL available for review. The main pattern of LNI was sinusoidal, followed by interfollicular, and perifollicular patterns; about 30% of patients had a diffuse pattern of involvement. The median tumor burden was about 30% in lymph node specimens, consistent with focal or partial involvement.
Our study provides further support for assertions other that LNI indicates either disease progression11 or clinically aggressive disease,7,16,33 but that the most important finding appears to be the degree of fibrous capsule (around the implant) involvement. LNI in our study was associated with a reduced survival of 75% versus 97.9% in patients with LNI at presentation (P = 0.003). We confirmed previous observation that patients with capsule confined tumor (ie, only luminal side of capsule involvement) demonstrated a higher survival rate relative to those with tumor extending beyond the capsule (P = 0.007). Six of 49 (12.2%) patients with tumor confined by the capsule had LNI, compared with 8/21 (38%) patients with tumor beyond the capsule suggesting that the degree of infiltration of tumor through the capsule determines the risk of LNI.
We and others believe that BI-ALCL arises initially around the breast implant, suspended in an effusion, then as a layer of lymphoma cells deposited on the luminal surface of the fibrous capsule, and subsequently infiltrates first into the capsule, then beyond where the tumor may form a tumor mass that may be detected clinically or by imaging studies. That said, the presence of LNI in 6 patients with tumor confined by the capsule is intriguing, and the possibility that microscopic foci of extracapsular extension were perhaps missed on sampling, or evaluation, cannot be entirely excluded. The answer to this puzzle warrants further analysis. LNI by lymphoma was therefore more frequent among patients with disease beyond the capsule. We recently proposed a clinical staging system for patients with BI-ALCL based on the American Joint Committee on Cancer solid tumor staging systems and suggested that LNI confers clinical stage IIB and is associated with decreased survival when compared with patients with stage I disease.5 The findings in this study support the use of this system.
Pathologists need to be particularly alert to not misdiagnosing nodal involvement by BI-ALCL as CHL. In our series, 2 patients were originally misdiagnosed as CHL on nodal evaluation. These patients received multiple chemotherapy regimens for CHL and their correct diagnosis and management were delayed by 8 and 36 months. Case 5 is illustrative: the patient presented with left axillary lymphadenopathy and was diagnosed as interfollicular CHL, for which she received doxorubicin (Adriamycin), bleomycin, vinblastine and dacarbazine. At disease recurrence in supraclavicular lymph node, the patient received cyclophosphamide, doxorubicin, vincristine, and prednisone as well as etoposide, solumedrol, cytarabine, and cisplatin regimens, achieving complete remission. She was finally diagnosed with BI-ALCL 36 months after initial presentation with tumor beyond the breast implant capsule whereas imaging studies revealed internal mammary enlarged lymph nodes. She achieved complete remission after complete capsulectomy with tumor resection, and received 2/6 cycles of recommended chemotherapy, and was disease free 5 years after surgery. This illustrative case also supports the recommendation of Clemens et al5 that complete surgical excision of the capsule and tumor is a cornerstone of therapy for this lymphoma, although it is worth emphasizing that therapy and management are beyond the scope of this manuscript.
The similarity to HL is heightened by the presence of scattered CD30-positive large cells in lymph nodes that can be misleading to the pathologist if the history of breast implants is unknown and the possibility of BI-ALCL is not suspected. The similarity to HL is further enhanced by the presence of eosinophilia, sclerotic bands, and anaplastic cells that can resemble Hodgkin or Reed-Sternberg cells in both diseases (Fig. 3). The immunohistochemical studies also may be misleading, as both HL and BI-ALCL contain large cells positive for CD30 that can be negative for CD3 and CD20. However, important clues to the diagnosis of BI-ALCL are the expression of CD45, positive in 90% of cases in this study, as well as the absence of PAX5. In contrast, CHL cells are CD45− and express PAX5 in most cases.
We would like to acknowledge the limitations of our study. Although we did review the original specimens of all the cases of BI-ALCL with LNI, we acknowledge that there is unavoidable variation in reporting the clinical findings of LNI, the timing of LNI and the outcomes and OS considering the many sources of information of multiple authors from multiple institutions. The limited number of cases and the retrospective nature of this study may be additional drawbacks. However, it is important to realize that a review of all cumulative data in a retrospective analysis, all biopsy proven, may have enhanced the accuracy of this report.
In summary, we describe the clinical and histopathologic features of lymph nodes involved by lymphoma in patients with BI-ALCL. Lymph nodes typically show low tumor burden with a sinusoidal or perifollicular pattern. The low tumor burden strongly argues for excisional biopsy over limited sampling of core biopsies for accurate diagnosis. Misdiagnosis as CHL is a particular pitfall of which pathologists should be aware. Patients with tumor beyond the capsule surrounding the breast implant appear to have a higher risk of LNI than patients with tumor confined to the capsule. Patients with LNI have a lower 5-year OS compared with patients without LNI at presentation, however, most patients still achieve complete remission after therapy.
Acknowledgments
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. [DOI] [PubMed] [Google Scholar]
- 2.Miranda RM, Medeiros LJ. Breast implant-associated anaplastic large cell lymphoma In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Salt Lake City, UT: Elsevier; 2018:634–643. [Google Scholar]
- 3.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doren EL, Miranda RN, Selber JC, et al. US epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. [DOI] [PubMed] [Google Scholar]
- 5.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016; 34:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens MW, Horwitz SM. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017;37:285–289. [DOI] [PubMed] [Google Scholar]
- 7.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–118e. [DOI] [PubMed] [Google Scholar]
- 8.Feldman AL, Harris NL, Stein H, et al. Breast implant-associated anaplastic large cell lymphoma In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). Lyon: IARC; 2017:421–422. [Google Scholar]
- 9.Katzin WE, Centeno JA, Feng LJ, et al. Pathology of lymph nodes from patients with breast implants: a histologic and spectroscopic evaluation. Am J Surg Pathol. 2005;29:506–511. [DOI] [PubMed] [Google Scholar]
- 10.Story SK, Schowalter MK, Geskin LJ. Breast implant-associated ALCL: a unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist. 2013;18:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aladily T Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. [DOI] [PubMed] [Google Scholar]
- 12.Miranda RN, Lin L, Talwalkar SS, et al. Anaplastic large cell lymphoma involving the breast: a clinicopathologic study of 6 cases and review of the literature. Arch Pathol Lab Med. 2009;133: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 13.Admirand JH, Rassidakis GZ, Abruzzo LV, et al. Immunohistochemical detection of ZAP-70 in 341 cases of non-Hodgkin and Hodgkin lymphoma. Mod Pathol. 2004;17:954–961. [DOI] [PubMed] [Google Scholar]
- 14.Kanungo A, Medeiros LJ, Abruzzo LV, et al. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol. 2006;19:25–33. [DOI] [PubMed] [Google Scholar]
- 15.Vega F, Medeiros LJ, Jones D, et al. A novel four-color PCR assay to assess T-cell receptor gamma gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol. 2001;116:17–24. [DOI] [PubMed] [Google Scholar]
- 16.Alobeid B, Sevilla DW, El-Tamer MB, et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma. 2009;50:831–833. [DOI] [PubMed] [Google Scholar]
- 17.George E Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Path. 2013;6:1631–1642. [PMC free article] [PubMed] [Google Scholar]
- 18.Estes CF, Zhang D, Reyes R, et al. Locally advanced breast implant-associated anaplastic large-cell lymphoma: a case report of successful treatment with radiation and chemotherapy. Front Oncol. 2015;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acevedo-Banez I, Garcia-Gomez FJ, Jimenez-Granero P, et al. 18F-FDG-PET/CT in implant-associated anaplastic large cell lymphoma of the breast. Br J Haematol. 2015;169:1. [DOI] [PubMed] [Google Scholar]
- 20.Tardio JC, Granados R. Axillary lymphadenopathy: an outstanding presentation for breast implant-associated alk-negative anaplastic large cell lymphoma. Int J Surg Pathol. 2015;23:424–428. [DOI] [PubMed] [Google Scholar]
- 21.Laurent C, Delas A, Gaulard P, et al. Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol. 2016;27:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olack B, Gupta R, Brooks GS. Anaplastic large cell lymphoma arising in a saline breast implant capsule after tissue expander breast reconstruction. Ann Plast Surg. 2007;59:56–57. [DOI] [PubMed] [Google Scholar]
- 23.Farkash EA, Ferry JA, Harris NL, et al. Rare lymphoid malignancies of the breast: a report of two cases illustrating potential diagnostic pitfalls. J Hematop. 2009;2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor kO Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: five Australian cases. Plast Reconstr Surg. 2012;129:610e–617e. [DOI] [PubMed] [Google Scholar]
- 25.Popplewell L, Thomas SH, Huang Q, et al. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk Lymphoma. 2011;52:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talagas M, Uguen A, Charles-Petillon F, et al. Breast implant-associated anaplastic large-cell lymphoma can be a diagnostic challenge for pathologists. Acta Cytol. 2014;58:103–107. [DOI] [PubMed] [Google Scholar]
- 27.Ivaldi C, Perchenet AS, Jallut Y, et al. Two cases of lymphoma in an implant capsule: a difficult diagnosis, an unknown pathology. Ann Chir Plast Esthet. 2013;58:688–693. [DOI] [PubMed] [Google Scholar]
- 28.Bautista-Quach MA, Nademanee A, Weisenburger DD, et al. Implant-associated primary anaplastic large-cell lymphoma with simultaneous involvement of bilateral breast capsules. Clin Breast Cancer. 2013;13:492–495. [DOI] [PubMed] [Google Scholar]
- 29.Weathers WM, Wolfswinkel EM, Hatef DA, et al. Implant-associated anaplastic large cell lymphoma of the breast: insight into a poorly understood disease. Can J Plast Surg. 2013;21:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai SM, Kavangh S, Ooi SS, et al. Anaplastic large-cell lymphoma associated with breast implants: a unique entity within the spectrum of peri-implant effusions. Diagn Cytopathol. 2014;42:929–938. [DOI] [PubMed] [Google Scholar]
- 31.Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Breast implant-associated anaplastic large cell lymphoma: proposal for a monitoring protocol. Plast Reconstr Surg. 2015;136:144e–151e. [DOI] [PubMed] [Google Scholar]
- 32.Locke MB, Lofts J. Variable presentation of anaplastic large-cell lymphoma in patients with breast implants. ANZ J Surg. 2017;87: 789–794. [DOI] [PubMed] [Google Scholar]
- 33.Thompson PA, Prince HM. Breast implant-associated anaplastic large cell lymphoma: a systematic review of the literature and minimeta analysis. Curr Hematol Malig Rep. 2013;8:196–210. [DOI] [PubMed] [Google Scholar]


