Abstract
Gender influences clinical presentations, duration and severity of symptoms, and therapy outcome in coronavirus disease 2019 (COVID-19) infection. Whether the immune response to Tα1 treatment for SARS-CoV-2 differs between the sexes, and whether this difference explains the male susceptibility to COVID-19, is unclear. This study aimed to investigate the efficiency and safety of Tα1 treatment and provide a basis for practically identifying gender differences characteristics and features of COVID-19. One hundred twenty-seven patients had COVID-19 symptoms and tested COVID19-positive (female 42.52%) in Wuhan union hospital were enrolled for medication. They were randomly divided into groups Control and Tα1 intervention. Seventy-eight patients received a subcutaneous injection of 1.6 mg Tα1, based on supportive treatment for 15 days. The control group included untreated 49 COVID19 patients closely matched for gender and age and received regular supportive treatment. In this retrospective analysis, we found that COVID-19-infected males reported more symptoms than COVID-19-infected females. A high degree of gender differences-related variability was observed in CRP and PCT levels and the cell counts of many lymphocyte subpopulations in the COVID-19 patients after Tα1 intervention. Levels of CRP and IL-6 were higher in Tα1-treated male group than Tα1-treated female group, while the level of PCT was significantly lower in Tα1-treated male group. Gender differences may be a factor in sustaining COVID-19 immunity responded to Tα1, male and female show statistically significant differences in relevance to cytokine production associated with the development of a more significant number of symptoms. This leaves the question of identifying gender-specific risk factors to explain these differences.
Keywords: COVID-19, Gender, Cytokine, Thymosin-alpha 1
1. Introduction
In December 2019, an alarmingly contagious and newly discovered primary atypical viral pneumonia broke out in Wuhan, China. It has been identified as a zoonotic coronavirus, similar to SARS and MERS coronavirus and named SARS-CoV-2 [1], [2]. As of May 11, 2020, coronavirus disease 2019 (COVID-19) has been confirmed in 4,196,972 people worldwide, carrying mortality of approximately 6.77%, compared with a mortality rate of <1% from influenza [3]. Currently, no medications or vaccines have been verified for the treatment or prevention of COVID-19.
National Health Commission of the People’s Republic of China and State Administration of Traditional Chinese Medicine issued a Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) in March 2020, which including suggestions of clinical characteristics, case definitions, clinical classification, differential diagnosis, and treatment, to promote the establishment of a therapeutic regimen [4]. Based on nutritional support, antiviral treatments should be useful in fighting COVID‐19. Interferons, intravenous gamma globulin, thymosin-α1, thymopentin, levamisole, cyclosporine A and traditional Chinese medicine are also used for clinical intervention [5].
Tα1 is a thymic peptide that demonstrates a peculiar ability to restore immune system homeostasis in different physiological and pathological conditions acting as multitasking protein depending on the host state of inflammation or immune dysfunction[6]. It is a heat-stable highly acidic molecule composed of 28 amino acid residues, it that regulates the immune system by enhancing the function of T cell [7]. Tα1 affect thymocytes by stimulating thymocytes differentiation or converting them into active T cells [8]. It has been documented that Tα1 can enhance SARS patients' immune responses and help inhibit SARS spreading [9]. A retrospective study also found that Tα1 intervention significantly reduces the mortality of severe COVID-19 patients [10].
Currently, there are no specific therapies or human vaccines available to treat and prevent COVID-19 infection and hardly understand about the host factors affecting the immune response to COVID-19. The therapeutic strategies to deal with the infection are only supportive, and respiratory failure from acute respiratory distress syndrome (ARDS) is the leading cause of mortality [11]. Cytokine storm (CS) is one of ARDS's main mechanisms, its deadly uncontrolled systemic inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines and chemokines by immune effector cells [12], [13], [14]. Accumulating evidence suggests that patients with severe COVID-19 might have a cytokine storm syndrome [15], [16]. SARS-CoV-2 invades through the respiratory mucosa and infects other cells, systemically inducing cytokine storm [17]. In severe cases of SARS-CoV-2 infection, CS will trigger an intense attack by the immune system to the body, cause ARDS and multiple organ failure, and finally lead to death [18]. In the severe patients, the inflammatory factors (IL-6, IL-10, TNF-α), neutrophil count, D-dimer, blood urea and creatinine levels were higher significantly, and the lymphocyte counts continued to decrease [19].
In the study of immunological, gender is a biological variable that should be considered. A growing body of evidence indicates gender difference in the clinical outcomes of COVID-19 [20], [21], [22], [23]. Numerous studies have shown that females have higher innate and adaptive immune responses than males, leading to faster clearance of viruses and contributes to increased development of immunopathology [24], [25], [26]. After virus infection, females have been observed to mount more robust humoral and adaptive immune responses than males [27]. As a result of heightened immunity to viruses, both the intensity and prevalence of viral infections are often lower for females than males [28]. However, reports on the gender difference regulation of the human cytokine response to COVID-19 in significant shortage. Tα1 has been recommended for some patients to enhance cellular immunity for the resistance of viral infection [29]. Although Tα1 intervention has been recommended for adjuvant immunoregulation therapy in COVID-19 patients, the efficiency and security of Tα1 cannot be determined due to the influence of many factors on curative effect. To establish a scientific and rigorous Tα1 intervention therapeutic regimen, the retrospective study of clinical outcomes is incredibly essential. The effect of Tα1 intervention on patients with COVID-19, patients were divided into Tα1 intervention group and control group (without Tα1 intervention) and compared T lymphocyte subsets, cytokine and other laboratory examinations levels.
2. Methods
2.1. Subject enrollment
After careful medical chart review, we compiled the clinical data of laboratory-confirmed hospitalized cases, from the Union Hospital of Huazhong University of Science and Technology in Wuhan between January 30th, 2020, and April 2nd, 2020. Patients diagnosed with COVID-19 based on the World Health Organization interim guidance were enrolled. This study was approved by the Ethics Committee of Union Hospital and registered at the Chinese Clinical Trial Registry (ChiCTR2000030803). The diagnosis of COVID-19 was according to ‘Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia’ Released by National Health Commission & State Administration of Traditional Chinese Medicine and confirmed by RNA detection of the SARS-CoV-2 in the clinical laboratory of Union Hospital. Confirmed cases denoted the patients whose real-time reverse-transcription polymerase-chain-reaction (RT-PCR) assay findings for nasal and pharyngeal swab specimens were positive.
2.2. Clinical evaluations at Wuhan Union Hospital
Clinical data, including recent exposure history, clinical symptoms and signs, comorbidities, and laboratory results at admission, were reviewed and abstracted by senior medical practitioners and entered into a computerized database for further verification. According to Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), patients are classified as mild cases, ordinary cases, severe cases and critical illness COVID-19. Medical history was collected based on the patient’s self-report at the time of admission. Comorbidities were initially treated as a categorical variable (Yes vs. No) and were subsequently classified according to single and multiple numbers. Besides, comorbidities were classified according to the organ system, including the respiratory system, cardiovascular system, and endocrine system. The endpoint of our study was a synthetic measure, including the intensive-care unit (ICU), invasive ventilation, or death.
2.3. Design and patients
A total of 127 COVID-19 patients [54 female (42.52%) with a mean age of 62.08 years (S.D. 12.11)] were identified. The researchers systematically collected the following data from medical records: demographic characteristics (gender, age), disease condition, respiratory support, clinical characteristics(fever, cough, expectoration, hypodynamia, headache, chest distress, muscle soreness, nausea, emesis and diarrhea), therapeutic medication, Lymphocyte subsets(CD3+ T cells, CD4+ T cells, CD8+ T cells, B lymphocytes and NK lymphocytes), cytokines(IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ), D-dimer, C-reactive protein, homocysteine, procalcitonin and prognosis.
2.4. Statistical analysis
One hundred twenty-seven patients were divided into Tα1 intervention group (78 patients) and control group (49 patients) according to whether Tα1 was used in clinical treatment. Continuous variables were expressed as Mean value (S.D.) and compared using the T-test. Using χ2 tests, we compared lymphocytes, cytokines, D-dimer, C-reactive protein, homocysteine and procalcitonin between females and males in each group. We used multiple logistic regression analysis to calculate unadjusted OR and 95% CI for T lymphocyte subsets and cytokines in all patients and within two age groups and determine whether there was a significant interaction between gender and Tα1 intervention concerning the level of Lymphocyte subsets and cytokines. All comparisons were 2-tailed, with a p value < 0.05 considered statistically significant. Data processing using Statistical Product and Service Solutions 25 software (SPSS 25) and GraphPad Prism 8.
3. Results
3.1. Baseline characteristics
Among the 127 patients, 1 had moderate disease, 107 had severe disease, 19 had critical disease. All the patients were released from quarantine. The average duration from symptom onset to discharge from the hospital was 45 ± 13 days. The average duration from symptom onset to hospitalization was 11 ± 7 days. The average age was 62.08 ± 12.11 years and 92(72.44%) of cases were community-acquired infection. Respiratory support was provided to 108 patients (71 patients in Tα1 intervention group, 37 patients in control group), of which 85.04% were used nasal cannula or face mask and 7.87% used high flow nasal cannula or non-invasive mechanical ventilation, with only one patient used invasive mechanical ventilation. The main symptoms were fever (81.10%), cough (77.95%), Chest distress (43.31%), fatigue (40.94%). The white blood cell count was decreased by 9.45% of patients, and the lymphocyte count was decreased by 62.99% of the patients. On admission, 81.89% of patients showed pneumonia on chest CT scans. In the control group, the average age was 61.08 ± 13.57 years and the average duration from symptom onset to hospitalization was 13 ± 7 days. Compared with the Tα1 intervention group, these patients were older and the duration was longer (Table 1 ). When the 46 patients were released from quarantine, the white blood cell count of 13.04% of the patients was < 3.5 G/L, the lymphocyte count of 60.87% of the patients was < 1.1 G/L, and the absolute counts of white blood cells and lymphocytes were 6.96 ± 3.18 G/L and 0.97 ± 0.46 G/L.
Table 1.
Patient groups and basic clinical information.
| Characteristics | All patients (n = 127) | Tα1 intervention group (n = 78) | Control group (n = 49) |
|---|---|---|---|
| Demographic | |||
| Female (%) | 42.52 | 44.87 | 38.78 |
| Age [Mean value (S.D.)] | 62.08(12.11) | 62.71(11.13) | 61.08(13.57) |
| Symptoms and Conditions (%) | |||
| Disease Condition | severe cases (84.25) | severe cases (83.33) | severe cases (87.76) |
| critical illness (14.96) | critical illness (16.67) | critical illness (12.24) | |
| Respiratory support | 85.04 | 91.03 | 75.51 |
| Fever | 81.10 | 80.77 | 81.63 |
| Cough | 77.95 | 78.20 | 77.55 |
| Expectoration | 29.92 | 26.92 | 34.69 |
| Fatigue | 40.94 | 44.87 | 34.69 |
| Headache | 8.61 | 7.69 | 10.20 |
| Chest distress | 43.31 | 38.46 | 51.02 |
| Muscle soreness | 22.83 | 19.23 | 28.57 |
| Nausea | 6.30 | 5.13 | 8.16 |
| Emesis | 8.61 | 6.41 | 12.24 |
| Diarrhea | 16.54 | 20.51 | 10.20 |
| Prognosis (%) | |||
| Cure Rate | 94.49 | 94.87 | 93.88 |
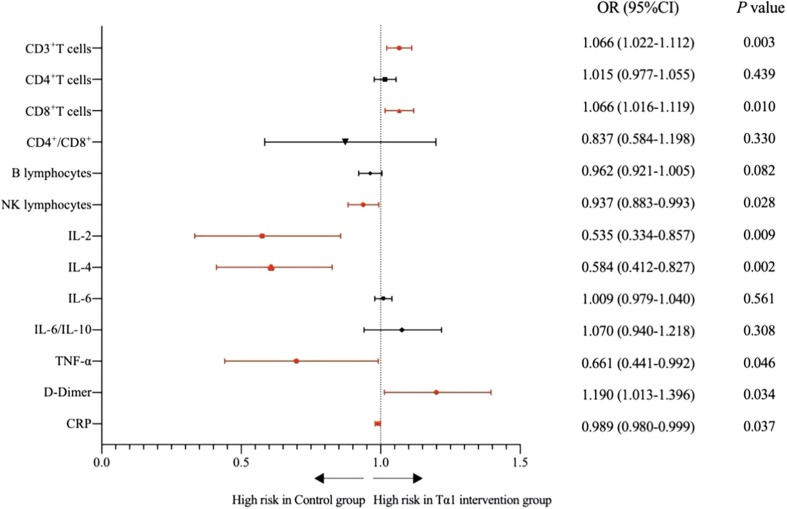
Average levels of CD4+ T cells and IL-6 in 127 patients were higher than the normal range. The average levels of CD3+ T cells, CD8+ T cells and D-Dimer in Tα1 intervention group were significantly higher than those in control group (CD3+ T cells P = 0.003 ; CD8+ T cells P = 0.010; D-dimer P = 0.034), and the average levels of NK lymphocytes, IL-2, IL-4, TNF-α and C-reactive protein were significantly lower than those in control group(NK lymphocytes P = 0.028; IL-2P = 0.009; IL-4P = 0.002; TNF-α P = 0.046; CRP P = 0.037). The Tα1 intervention group's cure rate was higher than that of the control group, and the average level of CRP in Tα1 group was significantly lower than control group. There was no significant difference in PCT and IL-6 between the two groups, perhaps Tα1 intervention is not the essential condition for influencing these two indicators (see Table 2, Table 3 and Fig. 1 ).
Table 2.
The level of T lymphocyte subsets and cytokines in patients.
| All patients (n = 127) |
Tα1 intervention group (n = 78) |
Control group (n = 49) |
|
|---|---|---|---|
| [Mean value (S.D.)] | [Mean value (S.D.)] | [Mean value (S.D.)] | |
| CD3+ T cells (%) | 71.96 (9.29) | 73.99 (8.53) | 68.74 (9.65) |
| CD4+ T cells (%) | 43.41 (9.40) | 43.92 (9.92) | 42.60 (8.53) |
| CD8+ T cells (%) | 24.80 (8.55) | 26.41 (9.28) | 22.26 (6.57) |
| CD4+/CD8+ | 2.03 (0.99) | 1.97 (1.05) | 2.14 (0.90) |
| B lymphocytes (%) | 13.27 (8.33) | 12.23 (7.26) | 14.92 (9.64) |
| NK lymphocytes (%) | 9.15 (6.30) | 8.15 (5.56) | 10.73 (7.11) |
| IL-2 (pg/ml) | 2.73 (0.81) | 2.57 (0.62) | 2.98 (1.00) |
| IL-4 (pg/ml) | 2.38 (1.54) | 2.03 (0.83) | 2.93 (2.16) |
| IL-6 (pg/ml) | 9.66 (12.61) | 10.18 (13.62) | 8.84 (10.88) |
| IL-10 (pg/ml) | 3.94 (3.09) | 3.74 (3.01) | 4.27 (3.21) |
| TNF-α(pg/ml) | 2.26 (0.93) | 2.12 (0.73) | 2.47 (1.16) |
| IFN-γ(pg/ml) | 3.79 (17.43) | 4.66 (22.23) | 2.40 (1.28) |
| IL-6/IL-10 | 2.73 (3.62) | 3.00 (4.36) | 2.28 (2.35) |
| TNF-α/IL-10 | 0.65 (0.21) | 0.65 (0.20) | 0.65 (0.22) |
Table 3.
The level of D-dimer, C-reactive protein, procalcitonin and homocysteine in patients.
| Cases | Mean value | S.D. | |
|---|---|---|---|
| D-dimer(μg/ml) | |||
| All patients | 125 | 2.25 | 2.59 |
| Tα1 intervention group | 76 | 2.66 | 2.71 |
| Control group | 49 | 1.62 | 2.29 |
| CRP (mg/L) | |||
| All patients | 127 | 34.94 | 36.71 |
| Tα1 intervention group | 78 | 29.47 | 31.15 |
| Control group | 49 | 43.66 | 43.07 |
| HCY(μmol/L) | |||
| All patients | 113 | 10.37 | 4.27 |
| Tα1 intervention group | 71 | 10.75 | 4.77 |
| Control group | 42 | 9.72 | 3.21 |
| PCT (%) | |||
| All patients | 125 | 0.23 | 0.08 |
| Tα1 intervention group | 76 | 0.22 | 0.08 |
| Control group | 49 | 0.23 | 0.07 |
Fig. 1.
Unadjusted risk of the level of T lymphocyte subsets, cytokines, D-dimer, C-reactive protein, procalcitonin and homocysteine were comparing Tα1 intervention group with control group. Red markers are significant statistical difference indexes.
3.2. Gender differentiation of Tα1 intervention
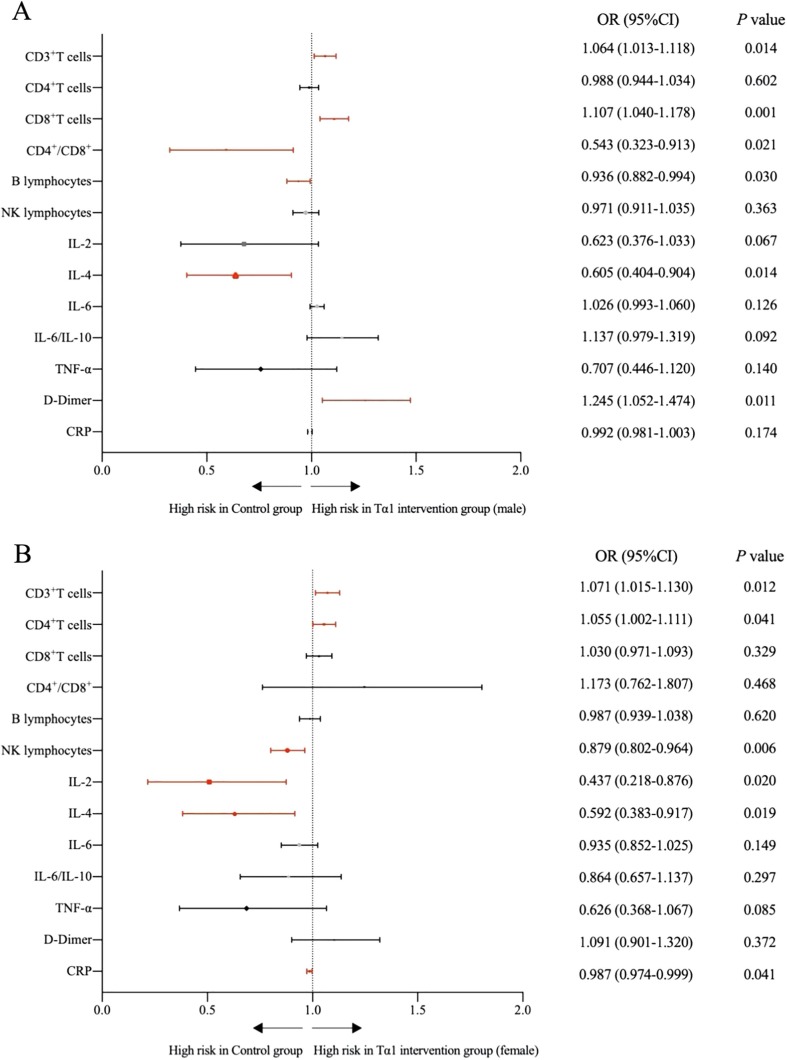
We first compared male and female differences of Tα1 intervention group and control group. A high degree of gender differences-related variability was observed in the cell counts of many lymphocyte subpopulations in the COVID-19 patients’ group after Tα1 intervention. Compared to the control group, these levels of CD3 + T cells (73.75 ± 8.51% vs 68.74 ± 9.65%), CD8 + T cells (28.47 ± 9.46% vs 22.26 ± 6.57%) and D-dimer (3.12 ± 2.95 μg/ml vs 1.62 ± 2.29 μg/ml) were significantly higher in Tα1-treated male group. Meanwhile, levels of B lymphocytes (14.92 ± 9.64% vs 10.83 ± 6.62%), IL-4 (2.93 ± 2.16 pg/ml vs 2.04 ± 0.85 pg/ml) and CD4 + T cells to CD8 + T cell ratio (2.14 ± 0.90 vs 1.69 ± 0.87) were significantly lower in Tα1-treated male group (Fig. 2 A). Compared to the control group, levels of CD3 + T cells (68.74 ± 9.65% vs 74.29 ± 8.66%) and CD4 + T cells (42.60 ± 8.53% vs 46.77 ± 9.31%) were significantly higher in Tα1-treated female group. While levels of NK lymphocytes (10.73 ± 7.11% vs 6.52 ± 4.54%), IL-2 (2.98 ± 1.00 pg/ml vs 2.51 ± 0.44 pg/m), IL-4 (2.93 ± 2.16 pg/ml vs 2.02 ± 0.83 pg/m) and CRP (43.66 ± 43.07 mg/L vs 25.53 ± 30.18 mg/L) were significantly lower in Tα1-treated female group (Fig. 2B).
Fig. 2.
Unadjusted risk of the level of T lymphocyte subsets, cytokines, D-dimer, C-reactive protein, procalcitonin and homocysteine were comparing males (or females) in Tα1 intervention group with control group. Red markers are significant statistical difference indexes. A: Comparison of Tα1-treated male group with control group; B: Comparison of Tα1-treated female group with control group.
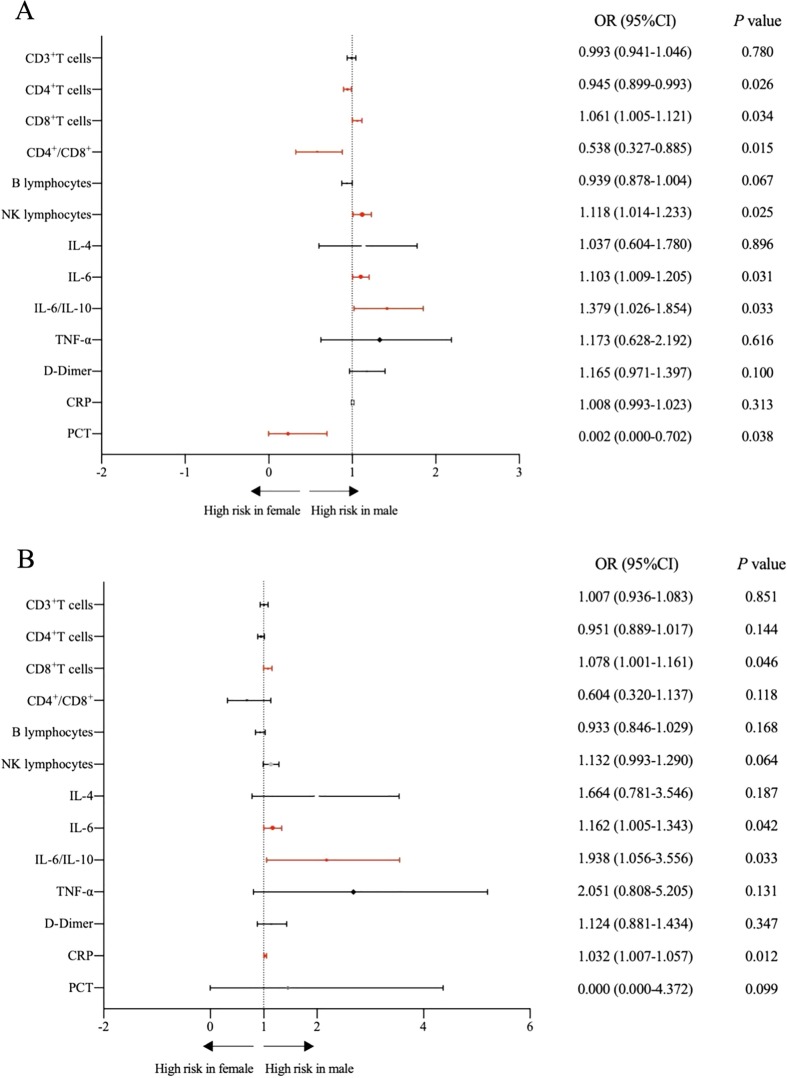
Next, we analyzed the effect of Tα1 intervention and age on gender differences-related. CD8 + T cells (28.47 ± 9.46% vs 23.86 ± 8.51%), NK lymphocytes (9.48 ± 5.99% vs 6.52 ± 4.54%), IL-6 (13.70 ± 17.43 pg/ml vs 5.85 ± 3.19 pg/ml) and IL-6 to IL-10 ratios (3.96 ± 5.66 vs 1.83 ± 0.98) were significantly higher in males than females with Tα1 intervention group. While CD4 + T cells (41.61 ± 9.90% vs 46.77 ± 9.31%), PCT (0.20 ± 0.08% vs 0.25 ± 0.09%) and CD4 + T cells to CD8 + T cells ratios (1.69 ± 0.87 vs 2.31 ± 1.16) were significantly lower in Tα1-treated male group (Fig. 3 A). The CRP level decreased after the Tα1 intervention both males and females, and Tα1-treated female group significantly lower than control group. The level of IL-6 decreased in Tα1-treated female group compared to control group, but the difference was not significant. The level of IL-6 was higher in Tα1-treated male group than control group and significantly higher than Tα1-treated female group.
Fig. 3.
Unadjusted risk of the level of T lymphocyte subsets, cytokines, D-dimer, C-reactive protein, procalcitonin and homocysteine were comparing males with females. Red markers are significant statistical difference indexes. A: In the Tα1 intervention group, comparison of males with females; B: Patients older than 65 age in the Tα1 intervention group, comparison of males with females.
This is how we discovered that age was a factor influencing CRP and IL-6 elevation in males. Levels of CD8 + T cells (30.66 ± 12.23% vs 23.04 ± 8.4%), IL-6 (17.86 ± 19.29 pg/ml vs 6.15 ± 3.32 pg/ml), CRP (0.19 ± 0.08 mg/L vs 0.24 ± 0.09 mg/L) and IL-6 to IL-10 ratios (4.71 ± 4.72 vs 1.77 ± 0.72) were significantly higher in males than females with Tα1 intervention group over 65 years of age (Fig. 3B).
4. Discussion
The SARS-CoV-2 virus is one of the seven coronaviruses that cause infections in humans, it became an epidemic in a brief period and had a considerable impact on a global scale [30]. Whenever a new infectious disease emerges, knowledge regarding clinical features, diagnostic tools and treatment options is critical [31]. Tα1 intervention has been recommended for adjuvant immunoregulation therapy in COVID-19 patients, but the efficiency and security of Tα1 cannot be determined due to the influence of many factors on curative effect. CRP, PCT and IL-6 play important roles in anti-infective immune response, they often serve as biomarkers useful in the differential diagnosis of disease condition and the prediction of prognosis [32], [33], [34]. However, in the current clinical diagnosis and treatment of COVID-19, the criteria of CRP, PCT and IL-6 as biomarkers are still controversial, and the relevant clinical data are urgently needed to provide support.
Tα1 is a polypeptide biological response modifier that plays a significant role in activating and regulating various cells' immune system. Tα1 ability to activate the tolerogenic pathway of tryptophan catabolism, through the immunoregulatory enzyme indoleamine 2,3-dioxygenase. It potentiates immune tolerance mechanisms, breaking the vicious circle that perpetuates chronic inflammation in response to various infectious noxae [35]. Several clinical have been carried out with Tα1 for treatment or prevention of many infectious diseases, such as hepatitis B, hepatitis C, sepsis and Aspergillosis [36], [37]. Existing studies observed that rhIFN-α nasal drops combined Tα1 may effectively prevent COVID-19 in medical staff [38]. This work observed the efficiency and safety of thymosin-α1 treatment in patients with COVID-19 infection and revealed gender differences in the T lymphocyte subsets, cytokine, and other laboratory examinations levels of COVID19 patients.
Our findings suggest that for the COVID-19 patients with severe disease, IL-6, D-dimer and CRP level was markedly elevated. In this study, prominent laboratory abnormalities agreed with that reported clinical features in COVID-19 cases [20], [21], [22]. Previous studies showed that many clinical cases have shown that severe COVID-19 patients were prone to immune system disorders, and growing evidence that male gender is a risk factor for more severe diseases [23]. On this basis, we found that gender differences in levels of CRP, PCT and IL-6 in COVID-19 patients. After Tα1 intervention, the levels of CRP and IL-6 in females showed a decreasing trend, but the decrease of CRP in males was not significant, and the level of IL-6 was significantly higher than that in females. In particular, patients older than 65 age in Tα1 intervention, the levels of IL-6, CRP and the ratio of IL-6 to IL-10 in males were significantly higher than females.
This study is the first to identify the gender differences of CRP, PCT and IL-6 levels in COVID-19 patients after Tα1 intervention. This retrospective analysis found that COVID-19 infected males reported more symptoms than COVID-19 infected females, despite similar initial medication and age. As the infection progressed, males exhibited a protracted cytokine response marked by higher IL-6, C-reactive protein and D-dimer than females. These analyses provide a potential rationale for a gender-differentiated approach to the prevention, prognosis, treatment, and care of patients with COVID-19. Although this study's results can not be confirmed the efficacy of Tα1 intervention in COVID-19 patients, our data suggest that clinical indicators of gender differences in Tα1 interventions must be closely monitored and treatment regimens adjusted accordingly.
Acknowledgments
This research was financially supported by the Faculty Research Grants of Macau University of Science and Technology (FRG-20-004-SKL). The content is solely the responsibility of the authors. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of, V. (2020). The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. [DOI] [PMC free article] [PubMed]
- 2.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27(2):1–4. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Health Commission, State Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 4).3(2020).
- 5.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matteucci C., Grelli S., Balestrieri E., Minutolo A., Argaw-Denboba A., Macchi B., Sinibaldi-Vallebona P., Perno C.F., Mastino A., Garaci E. Thymosin alpha 1 and HIV-1: recent advances and future perspectives. Future Microbiol. 2017;12(2):141–155. doi: 10.2217/fmb-2016-0125. [DOI] [PubMed] [Google Scholar]
- 7.Yan-Rong Guo, Qing-Dong Cao, Zhong-Si Hong, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research volume, 11 (2020). [DOI] [PMC free article] [PubMed]
- 8.Goldstein A.L., Low T.L., McAdoo M., McClure J., et al. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. PNAS. 1977;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David Ancell C., Pharm D., Phipps Jerry, Pharm D., Young Linda, Pharm D. Thymosin alpha-1. American J. Health-Syst. Pharm. 2001;58(15):879–885. doi: 10.1093/ajhp/58.10.886. [DOI] [PubMed] [Google Scholar]
- 10.Yueping Liu, Yue Pang, Zhenhong Hu, Ming Wu, Chenhui Wang, Zeqing Feng, , et al., Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clinical Infectious Diseases, 2020, dio: https://doi.org/10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed]
- 11.Ruan Q., Yang K., Wang W., et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 13.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti P., Ronconi G., Caraffa A., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2: anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19: The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun P., Qie S., Liu Z., et al. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L.i., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Z.C., Zhu J.H., Sun Y., et al. Clinical investigation of outbreak of nosocomial severe acute respiratory syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15(6) [PubMed] [Google Scholar]
- 20.Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C., Wang F.-S. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020;112 doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Wang, Hongyan Hou, Ying Luo, Guoxing Tang, et al. (2020). The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. [DOI] [PMC free article] [PubMed]
- 22.Castelli R., Gidaro A. Abnormal Hemostatic Parameters and Risk of Thromboembolism Among Patients With COVID-19 Infection. J. Hematol. 2020;9(1-2):1–4. doi: 10.14740/jh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jian-Min Jin, Peng Bai, Wei He, Fei Wu, Xiao-Fang Liu, et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health, 2020. [DOI] [PMC free article] [PubMed]
- 24.Boissier J., Chlichlia K., Digon Y., Ruppel A., Mone H. Preliminary study on sex-related inflammatory reactions in mice infected with Schistosoma mansoni. Parasitol Res. 2003;91:144–150. doi: 10.1007/s00436-003-0943-1. [DOI] [PubMed] [Google Scholar]
- 25.Xia H.J., Zhang G.H., Wang R.R., Zheng Y.T. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques. Cell Mol. Immunol. 2009;6:433–440. doi: 10.1038/cmi.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melgert B.N., Oriss T.B., Qi Z., Dixon-McCarthy B., Geerlings M., et al. Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol. 2010;42:595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein S.L., Huber S. In: Sex Hormones and Immunity to Infection. Klein S., Roberts C., editors. Springer; Berlin Heidelberg: 2010. Sex Differences in Susceptibility to Viral Infection. [Google Scholar]
- 28.Klein Sabra L. Sex influences immune responses to viruses, and efficacy of prophylaxis and therapeutic treatments for viral diseases. BioEssays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Li R., Wu Z., et al. Therapeutic strategies for critically ill patients with COVID-19. Ann. Intensive Care. 2020;10:45. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lake Mary A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. (Lond) 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddamreddy Suman, Thotakura Ramakrishna, corresponding author, Dandu Vasuki, Kanuru Sruthi, Meegada Sreenath. Corona Virus Disease 2019 (COVID-19) Presenting as Acute ST Elevation Myocardial Infarction. Cureus. 2020;12(4) doi: 10.7759/cureus.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junyan Qu., Lü Xiaoju, Liu Yanbin, Wang Xiaohui. Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J. Med. Res. 2015;141(3):315–321. doi: 10.4103/0971-5916.156617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ríos-Toro Juan-Jesús, Márquez-Coello Mercedes, García-Álvarez José-María, Martín-Aspas Andrés, et al. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LiMa HuiZhang, Yan-lingYina Wen-zhiGuo, et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Stincardini Claudia, Renga Giorgia, Villella Valeria, Pariano Marilena, Oikonomou Vasilis, Borghi Monica, Bellet Marina M., Sforna Luigi, Costantini Claudio, Goldstein Allan L., Garaci Enrico, Romani Luigina. Cellular proteostasis: a new twist in the action of thymosin α1. Expert Opin. Biol. Ther. 2018;18(S1):43–48. doi: 10.1080/14712598.2018.1484103. [DOI] [PubMed] [Google Scholar]
- 36.Yang Na, Ke Lu, Tong Zhihui, Li Weiqin. The effect of thymosin α1 for prevention of infection in patients with severe acute pancreatitis. Expert Opin. Biol. Ther. 2018;18(sup1):53–60. doi: 10.1080/14712598.2018.1481207. [DOI] [PubMed] [Google Scholar]
- 37.Camerini Roberto, Garaci Enrico. Historical review of thymosin α 1 in infectious diseases. Expert Opin. Biol. Ther. 2015;15(sup1):117–127. doi: 10.1517/14712598.2015.1033393. [DOI] [PubMed] [Google Scholar]
- 38.Zhongji Meng, Tongyu Wang, Li Chen, Xinhe Chen, et al. (2020). An experimental trial of recombinant human interferon alpha nasal drops to prevent COVID-19 in medical staff in an epidemic area. doi: https://doi.org/10.1101/2020.04.11.20061473. [DOI] [PubMed]