Abstract
Background & aims
Nutritional knowledge in patients with SARS-Cov2 infection (COVID-19) is limited. Our objectives were: i) to assess malnutrition in hospitalized COVID-19 patients, ii) to investigate the links between malnutrition and disease severity at admission, iii) to study the impact of malnutrition on clinical outcomes such as transfer to an intensive care unit (ICU) or death.
Methods
Consecutive patients hospitalized in a medicine ward at a university hospital were included from March 21st to April 24th 2020 (n = 114, 60.5% males, age: 59.9 ± 15.9 years). Nutritional status was defined using Global Leadership Initiative on Malnutrition (GLIM) criteria. Clinical, radiological and biological characteristics of COVID-19 patients were compared according to the presence of malnutrition. Logistic regression was used to assess associations between nutritional parameters and unfavourable outcomes such as transfer to intensive care unit (ICU) or death.
Results
The overall prevalence of malnutrition was 42.1% (moderate: 23.7%, severe: 18.4%). The prevalence of malnutrition reached 66.7% in patients admitted from ICU. No significant association was found between nutritional status and clinical signs of COVID-19. Lower albumin levels were associated with a higher risk of transfer to ICU (for 10 g/l of albumin, OR [95%CI]: 0.31 [0.1; 0.7]; p < 0.01) and this association was independent of age and CRP levels.
Conclusions
COVID-19 in medical units dedicated to non-intensive care is associated with a high prevalence of malnutrition, especially for patients transferred from ICU. These data emphasize the importance of early nutritional screening in these patients to adapt management accordingly.
Keywords: COVID-19, Malnutrition, Pneumonia, SARS-Cov2, Albumin
1. Introduction
Malnutrition is known as a risk factor for severity of and mortality from viral pneumonia since the times of the 1918 influenza pandemic [1]. Similarly, the recently described SARS-Cov2 infection (COVID-19) and related pneumonia could be closely associated with malnutrition [2,3]. Indeed, a number of features observed in COVID-19 patients are likely to lead to body weight loss and malnutrition [4,5]. These include: symptoms potentially resulting in decreased food intake such as dyspnea, dysgueusia, anosmia, anorexia, dysphagia, nausea, vomiting, diarrhea; hyper-metabolism and increased energy requirements, as observed in various types of severe infection [6]; older age with frailty and various comorbidities [4]; prolonged hospital stay in conventional or intensive care units [2].
Currently, very little is known about the prevalence of malnutrition in COVID-19 patients and its potential effect on disease severity and mortality. In one study from Wuhan, China, using the widely used questionnaire-based Mini Nutritional Assessment (MNA) tool, Li et al. reported that 52.7% of hospitalized COVID-19 patients over the age of 65 years were malnourished and 27.5% of them were at risk of malnutrition [7]. In another study, Du et al. identified a composite malnutrition score, based on albumin and cholesterol levels and lymphocyte count (Controlling Nutritional Status and Prognostic Nutritional Index), as an independent predictor of mortality in COVID-19 patients [8]. There is also some evidence in recent literature of an association between low albumin levels and COVID-19 severity [2,3]. Importantly, major phenotypic criteria of malnutrition such as low body mass index (BMI) or recent weight loss have not been described in this setting.
The objectives of the present study were to assess the prevalence and severity of malnutrition in hospitalized adult patients with COVID-19 admitted in a (non-intensive) medical unit. We first assessed malnutrition according to the phenotypical and biological criteria defined by the Global Leadership Initiative on Malnutrition (GLIM) [9], a consensus scheme for diagnosing malnutrition in adults in clinical settings. We then investigated the links between malnutrition and disease severity at admission. Finally, we studied the impact of malnutrition parameters on specific clinical outcomes such as transfer to an intensive care unit (ICU) or death.
2. Materials & methods
2.1. Study population
This observational longitudinal study included all COVID-19 adult patients admitted to the E3M Institute in Pitié-Salpétrière hospital from March 21st 2020 to April 24th 2020. All patients were admitted from the emergency department, medical units or intensive care units. Confirmed SARS-Cov2 infection was defined by a positive result of real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens and/or evocative CT-scan lesions. All data were collected in the context of care, thus, in accordance with the French law, including the GPRD, an informed consent of the patient was not sought. Patients were informed that data collected in medical records might be used for research study in accordance to privacy rules and that they could express their refusal.
2.2. Demographics, comorbidities, laboratory values
Data on characteristics such as patient age, sex, weight and smoking status and comorbidities including diabetes and hypertension, known to have a potential impact on the prognosis of COVID-19 [2,3], were extracted from the medical charts. Blood samples were collected in all patients at admission for determination of albumin, transthyretin, C- reactive protein (CRP), D-Dimers, lactate dehydrogenase and lymphocyte blood count to assess nutritional status, magnitude of inflammatory response and prognostic markers from earlier COVID-19 studies [2,3].
2.3. Nutritional assessment
Height and last known weight (i.e. weight before COVID-19 within the past 6 months) data were collected by questioning patients during or shortly after their stay in hospital. Weight was measured on calibrated scales during their medical stay allowing to calculate percentage of weight loss defined as 100∗(last known weight - measured weight)/last known weight. BMI was calculated as measured weight (kg) divided by height (meter) squared.
Diagnosis of malnutrition according to the GLIM criteria requires at least one etiologic criterion among reduced food intake or assimilation, inflammation or disease burden and at least one phenotypic criterion among non-volitional weight loss, low BMI and reduced muscle mass [9]. In our setting, we considered that an etiologic criterion of malnutrition was always present due to COVID-19 induced inflammation. Additionally, in most cases, reduced food intake or assimilation, the other etiologic criterion was present due to COVID-19 functional signs (anosmia, dysgueusia, nausea, vomiting, diarrhea). Phenotypic criteria were assessed using BMI and % weight loss. We also used the GLIM criteria to categorize the severity of malnutrition. We distinguished three groups of patients: i) no malnutrition; ii) moderate malnutrition defined by weight loss between 5 and 10% within the past 6 months or 10–20% beyond 6 months and/or BMI <20 kg/m2 (if age <70 years) or BMI < 22 kg/m2 (if age ≥ 70); iii) severe malnutrition defined by weight loss over 10% within the past 6 months or over 20% beyond 6 months and/or BMI < 18.5 kg/m2 (if age <70 years) or BMI < 20 kg/m2 (if age ≥ 70).
2.4. Assessment of severity of COVID-19
Initial common symptoms of COVID-19 were screened including fever at home, high body temperature at admission (defined by body temperature > 37.5 °C) and gastrointestinal symptoms (nausea, vomiting, diarrhea), anosmia, dysgueusia and anorexia. Oxygen intake (L/min) at admission and during hospitalization, chest CT scan grade were also recorded to assess COVID-19 respiratory severity. Transfer to an intensive care unit (ICU) from our medical unit and death were considered as the two main unfavorable clinical outcomes.
2.5. Statistical analyses
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as absolute values and percentages. Continuous variable with a non-parametric distribution were log-transformed before analysis. Linear regression with analysis of variance (ANOVA) for continuous variables and Pearson's chi-square (χ2) test or Fisher's exact test for discrete variables were used to compare the different characteristics according to nutrition status. When significant overall differences were found, Tukey's post hoc test or pairwise chi-square test with Bonferroni correction were performed for continuous or discrete variables, respectively. The links between nutritional parameters and negative outcomes of COVID-19 (transfer to ICU or death) were investigated using logistic regression in a univariate model after exclusion from the analysis of patients initially admitted from ICU. Small sample-adjusted unconditional maximal likelihood estimation (UMLE) was used to determine odds ratio and confidence interval when the number of patients was too low in the contingency table. To find out if the observed associations were independent of age and inflammation (known to be prognostic factors of COVID-19), a multivariate logistic regression analysis was performed using age and CRP as covariates. Statistical tests were considered significant if P < 0.05. All statistical analyses were conducted using R studio software version 1.2.1335 (http://www.r-project.org).
3. Results
3.1. Prevalence of malnutrition in COVID-19 hospitalized patients
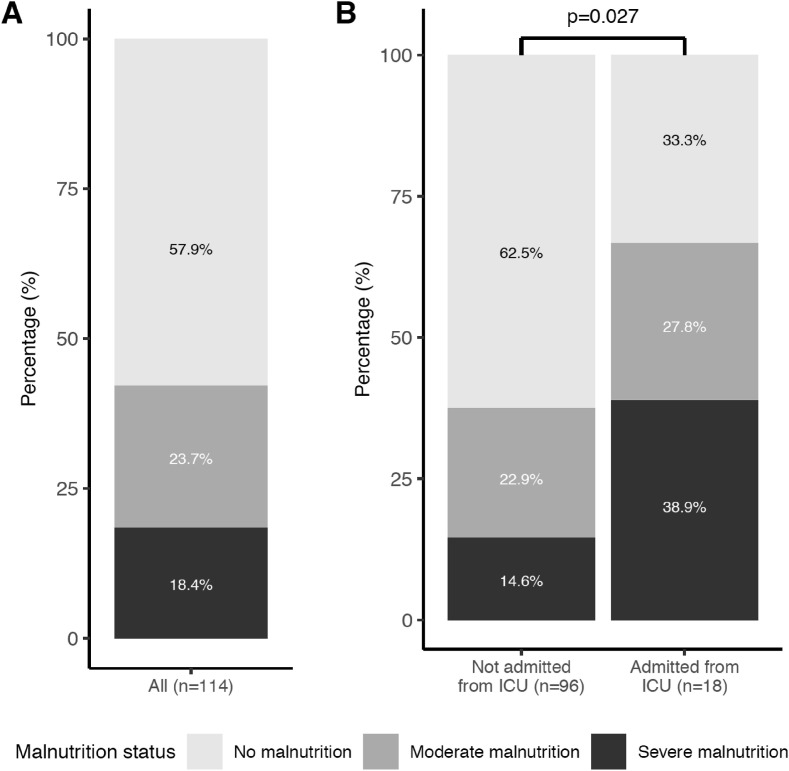
A total of 160 patients were admitted during the study period and complete nutritional data were available for 114 subjects. Subjects without complete nutritional data were older (67.5 y ± 15.5 vs. 59.9 ± 15.9, p = 0.006) but there was no significant difference in the prevalence of hypertension, diabetes, COPD, chronic kidney disease, coronary heart disease and active neoplasia (data not shown). Among these, 18 were transferred to our medical unit from ICU whereas 96 were admitted as a first line of care. The main characteristics of the cohort are shown in Table 1 . In this sample, 47 (42.1%) patients had malnutrition according to the GLIM criteria with a prevalence of moderate and severe malnutrition of 23.7% and 18.4% respectively (Fig. 1 A). Prevalence of malnutrition was significantly higher in patients admitted from ICU than in the other patients (66.7% vs. 37.5% respectively, p < 0.05) (Fig. 1B). Age, presence of diabetes, hypertension, obesity, COPD, chronic kidney disease, coronary heart diseases and active neoplasia, were not significantly different according to nutritional status. The main nutritional parameters that differed between the 3 groups were BMI and % weight loss (p < 0.01) while there was no significant difference in albumin or transthyretin levels (Table 1).
Table 1.
General and nutritional characteristics.
| All | No malnutrition | Moderate malnutrition | Severe malnutrition | P value | |
|---|---|---|---|---|---|
| Number of patients | 114 | 66 | 27 | 21 | |
| General | |||||
| Age (years) | 59.9 (15.9) | 58.4 (15.2) | 61.1 (16.6) | 63.0 (17.1) | 0.47 |
| Male, n (%) | 69 (60.5) | 38 (57.6) | 20 (74.1) | 11 (52.4) | 0.23 |
| Hypertension, n (%) | 60 (52.6) | 35 (53.0) | 16 (59.3) | 9 (42.9) | 0.53 |
| Diabetes, n (%) | 44 (38.6) | 28 (42.4) | 7 (25.9) | 9 (42.9) | 0.30 |
| COPD, n (%) | 8 (7) | 5 (7.6) | 1 (3.7) | 2 (9.5) | 0.69 |
| CKD, n (%) | 23 (20.2) | 13 (19.7) | 5 (18.5) | 5 (23.8) | 0.90 |
| CHD, n (%) | 23 (20.4) | 13 (20) | 4 (14.8) | 6 (28.6) | 0.53 |
| Active neoplasia, n (%) | 9 (8.3) | 5 (7.9) | 2 (7.7) | 2 (10) | 0.90 |
| Active smoker, n (%) | 7 (6.7) | 4 (6.8) | 1 (3.9) | 2 (10.5) | 0.75 |
| Anthropometrics | |||||
| BMI (kg/m2) | 26.6 (5.1) | 27.9 (4.9) | 26.3 (4.1) | 23.3 (5.3) | <0.01 |
| Obesity, n (%) | 28 (25.0) | 20 (31.2) | 6 (22.2) | 2 (9.5) | 0.13 |
| Low BMI, n (%) | 13 (11.6) | 2 (3.1) | 3 (11.1) | 8 (38.1) | <0.01 |
| Very low BMI, n (%) | 7 (6.2) | 0 (0.0) | 0 (0.0) | 7 (33.3) | <0.01 |
| Weight loss (%) | 4.41 (5.6) | 1.07 (3.9) | 6.54 (1.9) | 12.2 (4.4) | <0.01 |
| Weight loss >5%, n (%) | 47 (41.2) | 2 (3.0) | 25 (92.6) | 20 (95.2) | <0.01 |
| Weight loss >10%, n (%) | 18 (15.8) | 0 (0.00%) | 0 (0.0) | 18 (85.7) | <0.01 |
| Biological markers | |||||
| Albumin (g/l) | 30.1 (5.5) | 30.4 (6.0) | 30.0 (4.8) | 29.3 (4.9) | 0.87 |
| Albumin <35 g/l, n (%) | 91 (84.3) | 49 (81.7) | 23 (85.2) | 19 (90.5) | 0.68 |
| Albumin <30 g/l, n (%) | 46 (42.6) | 24 (40.0) | 11 (40.7) | 11 (52.4) | 0.60 |
| Transthyretin (g/l) | 0.15 (0.08) | 0.16 (0.07) | 0.14 (0.09) | 0.17 (0.08) | 0.55 |
Results are expressed as mean (SD) for continuous data and n (%) for categorical data.
P values shown result from analysis of variance (ANOVA) for continuous data and Chi2 or Fisher's exact test for categorical data between the 3 categories of nutritional status.
COPD: Chronic Obstuctive Pulmonary Disease.
CKD: Chronic Kidney Disease.
CHD: Coronary Heart Disease.
BMI: Body Mass Index; Low BMI: BMI <22 kg/m2 and age <70 or <20 kg/m2 and age ≥70; Very low BMI: BMI<20 kg/m2 and age <70 or <18.5 kg/m2 and age ≥70; Obesity: BMI ≥30 kg/m2.
Fig. 1.
Prevalence of malnutrition in hospitalized COVID-19 patients. A All patients with complete nutritional data. B All patients stratified according to their mode of admission (not from ICU or from ICU). Chi-square test was performed to compare nutritional status according to mode of admission. ICU: Intensive Care Unit.
3.2. Presentation of COVID-19 and malnutrition status
Fever, one of the most common symptoms of COVID-19, was less frequently reported as an initial symptom in patients with severe malnutrition compared to those without malnutrition (61.9% vs 89.4% respectively, p < 0.05). A similar trend was observed for high body temperature at admission which was present in 23.8% of severely malnourished patients vs. 55.6% and 46.2% in the moderate malnutrition and the group without malnutrition, respectively (p = 0.08). There was no significant difference in any other common symptoms of COVID-19 including anosmia or dysgueusia, nausea, diarrhea, vomiting and respiratory symptoms. There was no significant difference in oxygen intake at day one or in the severity of the initial chest CT scan between the three groups. Pneumonia severity was also not different between the three groups. Lymphocytes count was significantly higher in the severe malnutrition group than compared with moderate malnutrition group and group without malnutrition (p < 0.05); there was no difference in levels of CRP and D-dimer between the three groups (see Table 2 ).
Table 2.
Clinical presentations of COVID-19 in the three groups of patients.
| No malnutrition n = 66 |
Moderate malnutrition n = 27 |
Severe malnutrition n = 21 |
P value | |
|---|---|---|---|---|
| Functional signs | ||||
| Fever at home, n (%) | 59 (89.4) | 24 (88.9) | 13 (61.9) | 0.02 |
| Gastrointestinal symptoms, n (%) | 22 (33.3) | 7 (25.9) | 3 (14.3) | 0.23 |
| Anosmia or dysgueusia, n (%) | 25 (37.9) | 9 (33.3) | 7 (33.3) | 0.88 |
| Anorexia, n (%) | 45 (69.2) | 14 (53.8) | 12 (57.1) | 0.31 |
| Respiratory symptoms, n (%) | 57 (86.4) | 25 (92.6) | 16 (76.2) | 0.25 |
| Respiratory severity | ||||
| O2 intake day one (L/min) | 1.45 (1.2) | 1.96 (3.3) | 1.31 (1.3) | 0.42 |
| Severe Chest CT Scan, n (%) | 5 (8.3) | 4 (16.0) | 1 (7.7) | 0.63 |
| Pneumonia severity, n (%) | 0.78 | |||
| O2 < 3L/min | 42 (64.6) | 14 (53.8) | 13 (61.9) | |
| O2 3–5 L/min | 6 (9.2) | 3 (11.5) | 1 (4.8) | |
| O2 > 5L/min and/or respiratory rate >30/min | 9 (13.8) | 3 (11.5) | 2 (9.5) | |
| Biological severity markers | ||||
| Lymphocytes (G/L) | 1.07 (0.52) | 0.91 (0.45) | 1.45 (0.62) | 0.04 |
| CRP (mg/L) | 81.5 (77.7) | 127 (95.7) | 73.5 (73.9) | 0.06 |
| D-Dimer (μg/L) | 1619 (2252) | 3002 (5715) | 2087 (2213) | 0.57 |
Results are expressed as mean (SD) for continuous data and n (%) for categorical data.
P values shown result from analysis of variance (ANOVA) for continuous data and Chi square or Fisher's exact test for categorical data between the 3 categories of nutritional status.
CRP: C-Reactive Protein.
3.3. Factors associated with negative outcomes of COVID-19
As summarized in Table 3 , a lower albumin level at admission was significantly associated with a higher risk of transfer to ICU (for 10 g/l of albumin, OR: 0.31 [0.1; 0.7]; p < 0.01). Importantly, in a multivariate analysis adjusted for age and initial CRP levels, this association remained significant (OR: 0.38 [0.14, 0.96]; p < 0.05). Although not significant, there was a trend for a higher risk of mortality in patients with weight loss above 5% of initial weight (OR: 3.7 [1.0; 26.5], p = 0.09). Nutritional status was not associated with the risk of transfer to ICU or death.
Table 3.
Factors associated with transfer to intensive care unit or death.
| N = 96 | Transfer to Intensive Care Unit |
Death |
||
|---|---|---|---|---|
| OR [95% CI] | P value | OR [95%CI] | P value | |
| Moderate malnutrition | 0.58 [0.1; 1.9] | 0.39 | 1.90 [0.2; 12.3] | 0.50 |
| Severe malnutrition | 0.72 [0.1; 2.7] | 0.64 | 1.46 [0.1; 12.5] | 0.75 |
| Albumin (per 10 g/l) | 0.31 [0.1; 0.7] | <0.01 | 1.30 [0.3; 6.2] | 0.76 |
| Albumin <35 g/la | 5.33 [0.6; 213] | 0.06 | 0.38 [0.01; 10.1] | 0.51 |
| Albumin <30 g/la | 9.9 [1.2; 393] | <0.01 | 0.27 [0.3; 41.0] | 0.55 |
| Weight loss (%) | 1.0 [0.9; 1.1] | 0.44 | 1.0 [0.9; 1.2] | 0.91 |
| Weight loss >5% | 1.1 [0.3; 3.1;] | 0.9 | 3.7 [1.0; 26.5] | 0.09 |
| Weight loss > 10% | 1.2 [0.2; 4.8] | 0.8 | 0.0 [0.0; 23] | 0.99 |
Univariate logistic regression.
OR: Odds Ratio.
95% CI: 95% Confidence Interval.
Small sample-adjusted UMLE was used to determine Odds ratio and confidence interval because of low n in contingency table (cf methods for explanation).
4. Discussion
In this study, in adult patients hospitalized for documented COVID-19 in a non-intensive medical unit from a university hospital, we found that 42.1% of patients were malnourished and 18.4% of them had severe malnutrition. When stratifying our sample based on mode of admission to our unit, as much as two thirds (66.7%) of patients admitted from ICU were malnourished and more than a third (38.9%) displayed severe malnutrition. Low albumin levels were associated with serious adverse outcomes such as transfer to ICU, independently of age and CRP.
The overall prevalence of malnutrition in the present study is very high. Poor nutritional status is an established risk factor for community-acquired pneumonia [10] and in an Italian study by Russo et al., malnutrition was an independent risk factor for hospitalization in residents of long-term care facilities with such home-acquired pneumonia [11]. Prevalence of malnutrition in these hospitalized elderly patients (mean age 81.5 y) was 40.4% which is comparable to our results despite a much younger age in our population. This high prevalence of malnutrition in COVID-19 patients is in the same order of magnitude as the one reported by Li et al. in hospitalized COVID-19 patients in Wuhan (52.7%) [7]. However, to compare these two results is difficult since Li. et al. studied elderly subjects and they only assessed malnutrition using the MNA tool. In addition, using MNA does not allow to specifically assess the severity of malnutrition. Moreover, although the prevalence of malnutrition in COVID-19 patients in our study is very high with about two thirds of malnourished patients among those admitted from ICU, this number could still be underestimated. Indeed, we were not able to assess muscular strength which is one of the phenotypic criteria of malnutrition according to GLIM [9]. Given the marked deterioration of general condition or performance status associated with COVID-19, it is likely that muscle strength would be decreased in this setting. In addition, prolonged stay in ICU is known to be associated with muscle wasting, also resulting in reduced muscular strength [12].
We found a significant association between hypoalbuminemia at admission and worsening of COVID-19, reflected by a necessity of transfer to the ICU. A difference of 10 g/l of albumin at admission was associated with a 3-fold increased risk of transfer to ICU. Importantly, the relationship we found between low albumin and transfer to ICU was independent of CRP level. Indeed, it is well known that albumin is an important inflammation marker and is not exclusively dependent on nutritional status [13]. In addition, high CRP has been shown to be associated with the progression of COVID-19, as expected in a context of severe infection [2,3]. Moreover, we performed a complementary analysis showing that there is a significant correlation between albuminemia and recent weight loss (r = −0.19, p = 0.05) (data not shown). Altogether, this suggests that even in this highly inflammatory context, lower albumin remains a marker of weight loss and malnutrition.
The predictive role of low albumin levels regarding transfer to ICU is in line with previous findings by Liu et al. [3]. These authors observed in COVID-19 patients that a difference of 10 g/l in baseline albumin concentration was associated with a 5-fold increased risk of acute respiratory distress syndrome and a 2-fold increased risk of mortality [3]. It can be hypothesized that poor nutritional status would weaken immune response of the host, as observed in other settings [14,15]. Furthermore, experimental studies with the influenza virus suggest that not only does malnutrition suppress immune response to the virus but it could actually facilitate the emergence of novel variants, which may display increased pathogenicity relative to the original virus strain [16]. Altogether, our data reinforce the importance of routine assessment of nutritional markers in COVID-19 patients as recommended by a recent statement of ESPEN [4].
Our data show that patients with severe malnutrition had less reported fever as part of initial symptoms and there was a trend for less frequent high body temperature at admission in severely malnourished patients. Although this result was unexpected and requires confirmation in additional studies, it was demonstrated by Kauffman et al. that severe protein-calorie malnutrition could lead to altered thermoregulation and poor febrile response [17]. Since higher initial body temperature has been described as a pejorative factor of COVID-19 evolution in previous reports [2,3] it is important to note that fever may be underestimated in severely malnourished patients.
An important strength of our study in COVID-19 patients is that we used the specific metrics defined by GLIM [9] for the diagnosis of malnutrition, yet difficult to collect owing to the lack of scales and in consideration of the hygienic precautions required [18]. In this series of consecutive adult patients with a mean age under 60 years and with various comorbidities, we were able to collect a range of phenotypical (BMI, weight loss) and biological (albumin, CRP) parameters to describe nutritional status concurrently with clinical signs related to COVID-19. Beyond the baseline information we collected, we were able to obtain follow-up data, specifically regarding transfer to ICU or death. Our study therefore provides new nutritional knowledge in the setting of the COVID-19 and an improved description of malnutrition using objective phenotypic criteria and not limited to questionnaire data [7,8].
Some limitations need to be mentioned. Our sample size possibly led to insufficient power when assessing relationships between severity of malnutrition and evolution of COVID-19. Although we assessed in our patients a relatively large set of clinical and biological variables of interest, difficulties in obtaining such simple measures as body weight in this acute or post-acute setting should not be overlooked, as emphasized recently [4,[18], [19], [20]]. Selection bias cannot be ruled out. Patients admitted to our hospital through the emergency room were either immediately transferred to intensive care when in an acute condition or transferred to our department for specific, but non-intensive, care. Finally, relationship between malnutrition and COVID-19 could be bidirectional, as the infection can potentially lead to severe malnutrition but malnutrition may negatively impact prognosis in infected patients.
In conclusion, we observed a prevalence of malnutrition in COVID-19 patients hospitalized in non-intensive medical units as high as 42% and even higher (67%) when patients are admitted from an ICU. Low albumin levels at admission is a predictive marker of more severe outcome of the disease (transfer to ICU). These results reinforce the value of nutritional risk screening and the need of early nutritional management in COVID-19 patients, as recently emphasized by ESPEN [4] and others [[18], [19], [20]]. Further research is needed to assess the impact of nutritional care on longer term prognosis in these patients.
Authorship statement
D. Bedock, P. Bel Lassen and P. Faucher conceptualized the study; D. Bedock, P. Bel Lassen A. Mathian, P. Moreau and J. Fadlallah collected the data; P. Bel Lassen and P. Moreau analysed the data; D. Bedock, P. Bel Lassen and P. Faucher drafted the original manuscript; J.-M. Oppert supervised the study; all authors reviewed and edited the final manuscript.
Funding sources
None.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors express their thanks to all doctors, nurses, dieticians and all other staff of E3M institute at Pitié-Salpêtrière university hospital (AP-HP) that participated to the collection of data and patient care.
References
- 1.Short K.R., Kedzierska K., van de Sandt C.E. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8 doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W., Tao Z.-W., Wang L., Yuan M.-L., Liu K., Zhou L. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr Edinb Scotl. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller R., Englund K. Clinical presentation and course of COVID-19. Cleve Clin J Med. 2020;87:384–388. doi: 10.3949/ccjm.87a.ccc013. [DOI] [PubMed] [Google Scholar]
- 6.Wischmeyer P.E. Nutrition therapy in sepsis. Crit Care Clin. 2018;34:107–125. doi: 10.1016/j.ccc.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Du X., Liu Y., Chen J., Peng L., Cheng Z. Comparison of the clinical implications among two different nutritional indices in hospitalized patients with COVID-19. MedRxiv. 2020 doi: 10.1101/2020.04.28.20082644. 2020.04.28.20082644. [DOI] [Google Scholar]
- 9.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr Edinb Scotl. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Risk Factors for Community-Acquired Pneumonia in Adults: A Systematic Review of Observational Studies. NCBI; 2017. https://www.ncbi.nlm.nih.gov/pubmed/28738364 [DOI] [PubMed] [Google Scholar]
- 11.Russo A., Picciarella A., Russo R., Sabetta F. Clinical features, therapy and outcome of patients hospitalized or not for nursing-home acquired pneumonia. J Infect Chemother Off J Jpn Soc Chemother. 2020;26:807–812. doi: 10.1016/j.jiac.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Flower L., Puthucheary Z. Muscle wasting in the critically ill patient: how to minimise subsequent disability. Br J Hosp Med Lond Engl. 2020;81:1–9. doi: 10.12968/hmed.2020.0045. 2005. [DOI] [PubMed] [Google Scholar]
- 13.Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8 doi: 10.3390/jcm8060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomax A.R., Calder P.C. Prebiotics, immune function, infection and inflammation: a review of the evidence. Br J Nutr. 2009;101:633–658. doi: 10.1017/S0007114508055608. [DOI] [PubMed] [Google Scholar]
- 15.Yaqoob P. Ageing alters the impact of nutrition on immune function. Proc Nutr Soc. 2017;76:347–351. doi: 10.1017/S0029665116000781. [DOI] [PubMed] [Google Scholar]
- 16.Beck M.A., Handy J., Levander O.A. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauffman C.A., Jones P.G., Kluger M.J. Fever and malnutrition: endogenous pyrogen/interleukin-1 in malnourished patients. Am J Clin Nutr. 1986;44:449–452. doi: 10.1093/ajcn/44.4.449. [DOI] [PubMed] [Google Scholar]
- 18.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutr Burbank Los Angel Cty Calif. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arkin N., Krishnan K., Chang M.G., Bittner E.A. Nutrition in critically ill patients with COVID-19: challenges and special considerations. Clin Nutr Edinb Scotl. 2020;39:2327–2328. doi: 10.1016/j.clnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krznarić Ž., Bender D.V., Laviano A., Cuerda C., Landi F., Monteiro R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr Edinb Scotl. 2020;39:1983–1987. doi: 10.1016/j.clnu.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]