Highlights
-
•
D-dimer levels can discriminate between COVID 19 patients with and without worse clinical outcomes (all-cause mortality, ICU admission or ARDS).
-
•
Elevated D-dimer levels are associated with increased risk of adverse clinical outcomes in patients hospitalized with COVID 19 infection.
-
•
Patients who died had higher D-dimer levels than patients who survived.
-
•
A substantial elevation in D-dimers may be an indicator of progressive disease.
Keywords: D-dimer, All-cause mortality, ICU admission, ARDS, SARS-CoV-2
Abstract
Aim
To determine if D-dimers are elevated in individuals with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who have adverse clinical outcomes including all-cause mortality, intensive care unit (ICU) admission or acute respiratory distress syndrome (ARDS).
Methods
We conducted a systematic review and meta-analysis of the published literature in PubMed, Embase and Cochrane databases through April 9, 2020 for studies evaluating D-dimer levels in SARS-COV-2 infected patients with and without a composite clinical endpoint, defined as the presence of all-cause of mortality, Intensive care unit (ICU) admission or acute respiratory distress syndrome (ARDS). A total of six studies were included in the meta-analysis.
Results
D-dimers were significantly increased in patients with the composite clinical end point than in those without (SMD, 1.67 ug/ml (95% CI, 0.72-2.62 ug/ml). The SMD of the studies (Tang et al, Zhou et al, Chen et al), which used only mortality as an outcome measure was 2.5 ug/mL (95% CI, 0.62-4.41 ug/ml).
Conclusion
We conclude that SARS-CoV-2 infected patients with elevated D-dimers have worse clinical outcomes (all-cause mortality, ICU admission or ARDS) and thus measurement of D-dimers can guide in clinical decision making.
Introduction
The 2019 novel coronavirus (2019-nCoV) or the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) first identified in Wuhan district in China has spread rapidly to more than 177 countries and was declared as a global pandemic on March 11th, 2020.1 As of March 28th, there were 640,589 confirmed cases and 29,848 deaths globally.2 In up to 5% of infected patients, the disease may manifest as hypoxic respiratory failure, multi organ dysfunction or shock and around 2.5% patients die from the infection.3 Laboratory predictors of clinical deterioration can aid in escalating the care of the patients with this infection and assist in appropriate triaging and resource utilization. Studies have reported an association of D-dimer >1 ug/ml with increased mortality in patients with COVID 19 infection.4 We systematically reviewed the current scientific literature to understand whether D-dimer is associated with an increased risk of all-cause mortality, Intensive care unit (ICU) admission or acute respiratory distress syndrome (ARDS) in patients hospitalized with SARS-CoV-2 infection.
Methods
Literature search
We carried out an electronic search in Medline (PubMed), Embase, and Cochrane database using the keywords “D-dimer” AND “Coronavirus 2019” OR “COVID 19” OR “SARS-CoV-2” OR “severe acute respiratory syndrome coronavirus 2” OR “2019-nCoV” between 2019 and current date (9th April, 2020). Only the articles published in peer-reviewed journals were included in the analysis. Articles were limited to English language publications.
Selection of studies
We applied the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) to the methods for this study5 (Fig. 1 ). After duplications were removed, the title and abstracts were independently screened by two reviewers (AB and VJ). The studies reporting the mean or the median D-dimer values in COVID 19 patients with and without a composite end point defined as all-cause mortality, ICU admission or ARDS were included in the study. All-cause mortality was analyzed as a separate outcome in addition to the composite end-point. We excluded case reports, studies involving pediatric patient population and those not reporting the above-mentioned composite end points. We cross-referenced the research papers to identify additional studies meeting the inclusion criteria. Full texts of the included studies were then reviewed by two independent reviewers (AB and VJ) and data was extracted. Any conflicts were settled by a third author (ADS).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses statement (PRISMA) flow chart for this study
Data extraction and study quality appraisal
The following data variables were collected: author name, year published, country where the study was performed, type of study, number of patients, composite end point definition, and mean D-dimer values in patients with and without outcome of interest (all-cause mortality, ICU admission and ARDS).
Two authors (AB and VJ) independently assessed the risk of bias in the included studies using the validated Newcastle-Ottawa Scale.
Statistical analysis
The meta-analysis was conducted with the calculation of standardized mean difference (SMD) and 95% confidence interval (95% CI) of D-dimers in coronavirus 2019 patients with and without a composite clinical end point. D-dimers were entered as a continuous variable. The mean and the standard deviation were extrapolated from the sample size, median and interquartile range (Q1-Q3) as per Hozo et al.6 I2 statistic was used to assess the heterogeneity between studies with values 0–30%, more than 30–60%, and more than 60% corresponding to low, moderate, and high degree of heterogeneity, respectively. DerSimonian and Laird random effects model was used for pooling the studies. The statistical analysis was performed using Stata 12 software (Stata Corp, College Station, Texas).
Results
Our systematic electronic search resulted in 21 publications after the initial screening of titles and abstracts. Subsequently, 16 studies were excluded, yielding 5 studies that met the inclusion criteria for systematic review. Cross-referencing of full-text articles resulted in 1 additional study. Therefore, 6 studies were included in the final meta-analysis for association of mean/median D-dimer values with all-cause mortality, ICU admission or ARDS. Table 1 elucidates the baseline characteristics and outcomes of the included studies.
Table 1.
Characteristics of the studies (n=6) included in the meta-analysis
| Study | Zhou et al | Chen et al | Tang et al | Wang et al | Huang et al | Wu et al |
|---|---|---|---|---|---|---|
| Study year | 2020 | 2020 | 2020 | 2020 | 2020 | 2020 |
| Study location | Wuhan, China | Wuhan, China | Wuhan, China | Wuhan, China | Wuhan, China | Wuhan, China |
| Study type | Retrospective cohort | Retrospective cohort | Cross-sectional study | Retrospective cohort | Prospective Cohort | Retrospective Cohort |
| Sample size | 191 (Cases 54, Controls 137) | 274 (Cases 113, Controls 161) | 183 (Cases 21, Controls 162) | 138 (Cases 36, controls 102) | 41 (Cases 13, Controls 28) | 201 (Cases 84, Controls 117) |
| Median age | 56 (46-67) | 62 (44-70) | 54 (44-62) | 56 (42-68) | 49 (41-58) | 51 (43-60) |
| Female | 72 (38%) | 103(38%) | 85 (46.44%) | 63 (45.7%) | 11 (27%) | 73 (36.3%) |
| Composite end point | All-cause mortality (in-hospital) | All-cause mortality (in-hospital) | All-cause mortality (in-hospital) | ICU admission | ICU admission | ARDS (WHO definition) |
| Median D-dimer level, case and control | Cases- 5.2 (1.5-21.1) Controls- 0.6 (0.3-1.0) | Cases- 4.6 (1.3-21.0) Controls- 0.6 (0.3-1.3) | Cases- 2.12 (0.77-5.27) Controls- 0.61 (0.35-1.29) | Cases- 4.14 (1.91-13.24) Controls- 1.66 (1.01-2.85) | Cases- 2.4 (0.6-14.4) Controls- 0.5 (0.3-0.8) | Cases- 1.16 (0.46-5.37) Controls- 0.52 (0.21-0.94) |
There were a total of 1329 patients with 434 (32.65%) patients having a composite clinical end point. The composite end point was defined as defined as mortality in 3 studies,4 , 8 , 10 ICU admission in 2 studies7 , 11 and onset of ARDS in another study.9 Zhou et al4 showed the clinical and laboratory data of 191 hospitalized patients and observed that D-dimer levels were about 8-9 times higher in patients who died (median D-dimer 5.2 ug/ml, IQR:1.5-21.1 ug/ml) than those who survived (median D-dimer 0.6 ug/ml, IQR 0.3-1.0 ug/ml). Similarly, Chen et al10 also observed an approximate seven-fold increase in D-dimer values in patients who had in-hospital all-cause mortality (median 4.6 ug/ml, IQR: 1.3-21.0 ug/ml) compared to patients who did not have the outcome (median 0.6 ug/ml, IQR: 0.3-1.3 ug/ml). Tang et al8 showed a 3-4 times greater levels of D-dimer levels in patients who had in-hospital mortality compared to those who did not. Wang et al and Huang et al7 , 11 showed that D-dimers were significantly elevated in patients who required ICU admission. Furthermore, D-dimers were also significantly higher in patients having ARDS during the admission than those not having the outcome.9
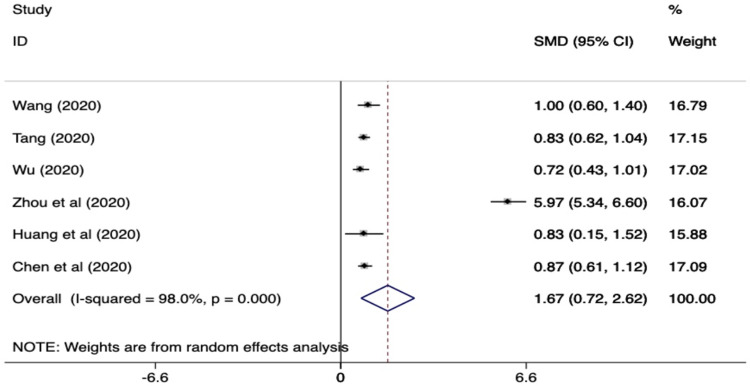
The standardized mean difference (SMD) for the six studies is summarized in Fig. 2 . The values of D-dimer were found to be significantly increased in patients with the composite clinical end point than in those without (SMD, 1.67 ug/ml (95% CI, 0.72-2.62 ug/ml). The SMD of the studies (Tang et al,8 Zhou et al,4 Chen et al10), which used only mortality as an outcome measure was 2.5 ug/mL (95% CI, 0.62-4.41). The heterogeneity of the studies was found to be relatively high (i.e. I2 statistic 98%).
Fig. 2.
Standardized mean difference (SMD) and 95% confidence interval (CI) for predicting composite clinical end point (ARDS, ICU admission and mortality) in patients with COVID 19 infection
There were two additional studies which reported higher d-dimer levels in patients with worse outcomes. However, they were not included in our meta-analysis as they did not report the median/mean D-dimer levels. Zhang et al12 described the characteristics of 95 patients and found that out of the 25 patients having an outcome (ICU admission, mechanical ventilation or death), 23 (92%) had D-dimer values ≥ 1 ug/ml. Similarly, another study13 showed around 70% of the patients with worse outcome (death, mechanical ventilation or ICU admission) having D-dimers ≥ 0.5 ug/ml.
Discussion
We performed a systematic review and meta-analysis of studies to assess whether the D-dimer levels were associated with a composite end point defined as the presence of all-cause mortality, ICU admission or ARDS in patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We found that 1) D-dimers were significantly elevated in patients having a composite end point compared to those not having the outcome, 2) the level of D-dimers was higher in studies having mortality as an outcome in comparison to other end-points.
There are several plausible reasons for elevated D-dimer over the normal value of < 0.5 ug/ml in patients hospitalized with SARS-CoV-2 infection having worse clinical outcomes. First, patients with severe SARS-CoV-2 infection can have disseminated intravascular coagulation (DIC) secondary to sepsis. Severe acute lung injury or ARDS by itself has also been associated with increased incidence of DIC. Tang et al8 mentioned in their study that the vast majority of patients who died during admission fulfilled the criteria for DIC (71.6% vs 0.6% in survivors). Second, prior studies have shown that severe acute respiratory infection can cause injury to the endothelial cells and increase the levels of hemostatic factors such as d-dimers and vWF.14 Third, respiratory infections have been associated with deep vein thrombosis and pulmonary embolism. Wang et al15 postulated about the possible formation of pulmonary microthrombus in patients infected with H1N1 infection and a consequent elevation in D-dimer. There have been 2 cases reported of pulmonary embolism in SARS-CoV-2 infected patients.16 Fourth, the SARS-CoV-2 infected patients with critical form of the disease are more likely to have additional complications including acute kidney injury, acute cardiac injury, congestive heart failure, all of which can cause increase the levels of D-dimers. Finally, the elderly patients are at an increased risk of having worse clinical outcomes from SARS-CoV-2 infection and D-dimer levels are higher in elderly patient population.17
In severe cases of SARS-CoV-2 infection, there is an uncontrolled release of pro-inflammatory cytokines (IL-2, IL-6, IL-8, IL-17, and TNF-a) which lead to upregulation of tissue factor expression on the endothelial cells, resulting in an increased pro-coagulant state. There is increasing evidence that SARS-CoV-2 infection is associated with increased risk of venous thromboembolism (VTE) and in-situ microvascular thrombosis which has been linked to worse clinical outcomes.18
The major limitation of the studies included was lack of information on the timing of the D-dimer measurements relative to admission. In addition, there was a significant heterogeneity in the reported results. This was likely due to differences in study size, selection bias, and different stages at which the D-dimer values were measured. Also, since all the studies included have been performed in China, the external validity is lacking.
The results of this concise meta-analysis suggest that D-dimer is significantly increased in patients having a worse clinical outcome (all-cause mortality, ICU admission or ARDS). Further studies are required to assess if the serial measurement of D-dimer plays any role in predicting evolution towards a more critical form of disease. Finding a threshold D-dimer level, above which SARS-CoV-2 infected patients are at an increased risk of having worse clinical outcomes can assist in following a proactive approach and aid in clinical decision making. Also, it will be imperative to know if anticoagulation therapies are of use in patients with severe SARS-CoV-2 infection.
Declaration of Competing Interest
There were NO conflicts of interest
Acknowledgments
Acknowledgements
None
Source of funding
None
References
- 1.World Health Organization . WHO; 2020. The Coronavirus Disease 2019 (COVID-19):Situation report-36. [Google Scholar]
- 2.Johns Hopkins University & Medicine. Coronavirus resource center. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU).
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(28 (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., Atkins D., Barbour V., Barrowman N., Berlin J.A., Clark J., Clarke M., Cook D., D'Amico R., Deeks J.J., Devereaux P.J., Dickersin K., Egger M., Ernst E., Gøtzsche P.C., Grimshaw J., Guyatt G., Higgins J., Ioannidis J.P.A., Kleijnen J., Lang T., Magrini N., McNamee D., Moja L., Mulrow C., Napoli M., Oxman A., Pham B., Rennie D., Sampson M., Schulz K.F., Shekelle P.G., Tovey D., Tugwell P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(Apr 20):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(Mar 17 (11)):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(Apr (4)):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;(Mar 13) doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;(Mar 26) doi: 10.1136/bmj.m1091. m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(Feb (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(Mar 26(1)):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;(Feb 28) doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Wissen M., Keller T.T., van Gorp E.C.M., Gerdes V.E.A., Meijers J.C.M., van Doornum G.J.J. Acute respiratory tract infection leads to procoagulant changes in human subjects. J Thromb Haemost. 2011;9(Jul (7)):1432–1434. doi: 10.1111/j.1538-7836.2011.04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z.F., Su F., Lin X.J., Dai B., Kong L.F., Zhao H.W. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1) Thromb Res. 2011;127(Mar (3)):198–201. doi: 10.1016/j.thromres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol: Cardiothorac Image. 2020;2(Apr 1(2)) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tita-Nwa F., Bos A., Adjei A., Ershler W.B., Longo D.L., Ferrucci L. Correlates of D-dimer in older persons. Age Clin Exp Res. 2010;22(Feb (1)):20–23. doi: 10.1007/bf03324810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]