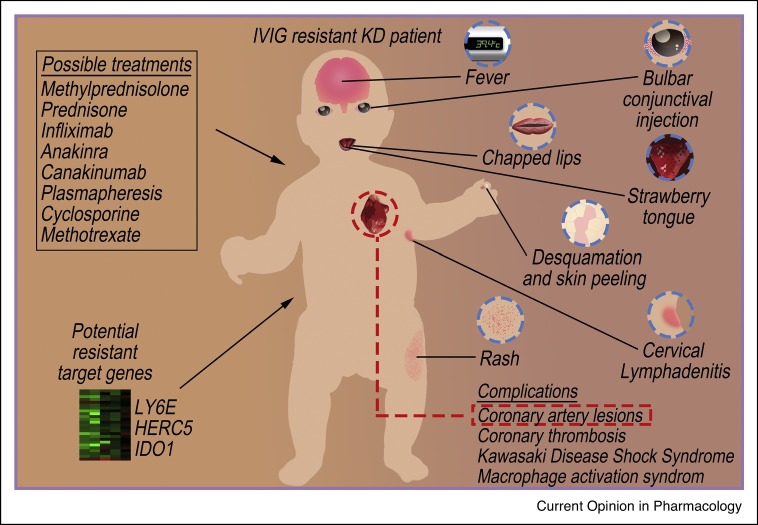
Graphical abstract
Schematic diagram of the complications, possible treatments, and potential resistant target genes of IVIG resistant KD patient.
Abstract
Kawasaki disease is an acute childhood self-limited vasculitis, causing the swelling or inflammation of medium-sized arteries, eventually leading to cardiovascular problems such as coronary artery aneurysms. Acetylsalicylic acid combined with intravenous immunoglobulin (IVIG) is the standard treatment of Kawasaki disease (KD). However, a rising number of IVIG resistant cases were reported with severe disease complications such as the KD Shock Syndrome or KD-Macrophage activation syndrome. Recent reports have depicted the overlapped number of children with SARS-CoV-2 and KD, which was called multisystem inflammatory syndrome. Simultaneously, the incidence rate of KD-like diseases are increased after the outbreak of COVID-19, suggesting the virus may be associated with KD. New intervention is important to overcome the problem of IVIG treatment resistance. This review aims to introduce the current pharmacological intervention and possible resistance genes for the discovery of new drug for IVIG resistant KD.
Current Opinion in Pharmacology 2020, 54:72–81
This review comes from a themed issue on Drug resistance
Edited by Vincent Kam Wai Wong
For a complete overview see the Issue and the Editorial
Available online 18th September 2020
https://doi.org/10.1016/j.coph.2020.08.008
1471-4892/© 2020 Elsevier Ltd. All rights reserved.
Introduction
Kawasaki Disease (KD) was first described as the mucocutaneous lymph node syndrome in 1967 [1]. It is an acute systemic vasculitis and a multi-system disease that can lead to severe complications such as stenosis, thrombosis or coronary artery aneurysms (CAA) which can lead to sudden death [2]. According to the American Heart Association (AHA) guideline, typical diagnostic symptoms and signs for children are fever, bulbar conjunctival injection, chapped lips, strawberry tongue, desquamation and skin peeling, rash, and cervical lymphadenitis [3,4]. Single Intravenous immunoglobulin (IVIG) therapy can reduce the risk of bulging of the artery wall and CAA which can lead to a heart attack [5•]. Up to now, combined use of high dose of IVIG and aspirin remains the standard treatment for KD. In fact, about 10–20% of patients did not respond to standard primary treatment and developed resistance to IVIG. Unresponsiveness such as persistent fever after the standard IVIG treatment was considered as resistant KD [6] with increased risk of life-threatening complications such as the Kawasaki Disease Shock Syndrome (KDSS) or KD-MAS (KD-Macrophage activation syndrome) (Figure 1 ).
Figure 1.
Symptoms, complications, possible treatments, and potential resistant target genes of IVIG resistant KD patient.
KD patients commonly shared symptoms including fever, bulbar conjunctival injection, chapped lips, strawberry tongue, desquamation and skin peeling, rash and cervical lymphadenitis (in blue dotted circles). List of major complications for example, the coronary artery lesions (in red dotted rectangle) were presented. Other complications included coronary thrombosis, KDSS and MAC. Possible treatments for IVIG resistant KD patients including methylprednisolone, prednisone, infliximab, anakinra, canakinumab, cyclosporine, methotrexate, and plasmapheresis are listed. Potential KD resistant targeted genes predicted by bioinformatics analysis included LY6E, HERC5 and IDO1.
In December 2019, a new infectious disease named Coronavirus disease 2019 (COVID-19) pneumonia infected by the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared and spread rapidly worldwide. On January 30th 2020, the World Health Organization (WHO) has announced SARS-CoV-2 as a health emergency of international concern. By March 11th 2020, the WHO recognized COVID19 as a pandemic. As of June 2020, the number of confirmed cases in the world has exceeded 10 000 000. Recently, many literatures reported the number of SARS-CoV-2 infected children with KD multisystem inflammatory syndrome (MIS-C) has increased sharply. While KD and MIS-C shared some common symptoms such as lymphadenopathy, skin rash, prolonged fever, diarrhea and upregulation of inflammation related protein markers [7,8], recent statistics have shown that the incidence rate of KD-like diseases increased by 30 times after the popularity of COVID-19 [9••]. Although without known pathophysiology, the recent increased cases of KD symptoms occurrence in COVID-19 patients have drawn researchers’ attention to the identification of alternate therapeutic drugs and molecular gene targets for both typical or resistant KD [10].
Standard treatment of classical and refractory Kawasaki disease
Intravenous immunoglobulin treatment and resistance in Kawasaki disease
IVIG treatment generally provide its anti-inflammatory effects via neutralization of the infectious antigens or pathogenic autoantibodies, inhibition of tumor necrosis factors (TNF)-α and the release of inflammatory cytokines, and regulation on the function of both B and T cells [11, 12, 13]. According to AHA guidelines, patients with persistent fever lasting for 36 hours to a maximum of 7 days after first IVIG infusion is defined as IVIG resistant [4]. Although optimal therapeutic response can be observed when IVIG therapy is administered within 10 days since the symptom existed, or when a high dose of 2 g/kg was administered within 12 hours of symptom onset [4], around 5% of patients who received an appropriate IVIG treatment at an early stage still developed CAA [14].
Aspirin treatment and resistance in Kawasaki disease
Single Acetylsalicylic acid (ASA) treatment shows significant anti-inflammatory activity (at a high dose of 30−100 mg/kg/day), anti-platelet activity (at a low dose of 3−5 mg/kg day), and may reduce the risk of vascular thrombosis. ASA combined with IVIG was reported to possess anti-inflammatory and anti-platelet role during different phases of KD [15, 16, 17]. However, KD patients who are concurrently affected by influenza or varicella are more likely to develop Reye syndrome if they have taken aspirin for a long time [4]. Aspirin is usually prescribed to reduce the rate of myocardial infarction, stroke, thrombosis or death in patient with a high risk of cardiovascular disease [18•,19•]. However, aspirin resistance was also reported with poor anti-platelet aggregation ability [20]. In fact, aspirin resistance can be defined as two categories. Clinical aspirin resistance usually refers to the failure of aspirin in protecting patients from ischemic cardiovascular events, while in the laboratory, aspirin resistance implies the incomplete inhibition of platelet reactivity [21,22]. Possible causes for clinical aspirin resistance include the activation of alternative pathway for platelet activation, incomplete suppression of thromboxane, inappropriate dosage, and the drug–drug adverse interactions with other medicines such as ibuprofen [4]. Of noted, there is a continuing debate regarding the dosage and side effects of ASA. It was reported that no significant differences in the incidence of CAA and IVIG resistance, and the duration of fever between low-dose and high-dose of ASA [23,24] were observed, therefore, whether high-dose ASA should be used during the acute phase of KD requires further evaluation.
Treatment of complications of Kawasaki disease
Cardiovascular complications and treatments
Coronary artery disease is the most prominent feature which can further induce CAA, coronary artery stenosis, myocardial infarction, sudden death or even ventricular arrhythmias in KD patients [25]. Chronic use of antiplatelet low-dose ASA (3−5 mg/kg/day) for inhibiting platelet activation was required in KD patients with persistent CAA [15]. Coronary thrombosis and acute coronary syndrome are more likely to occur in patients with middle sized aneurysms and giant aneurysms [26]. In fact, increasing number of patients with coronary aneurysms associated with KD are entering adulthood, which increase the risk of heart attack caused by progressive arterial stenosis due to aneurysm thrombosis or vascular reconstruction [26]. It is known that endothelial cell damage and inflammation are the two essential processes involved in the coronary endothelial dysfunction of KD. Patients with KD were demonstrated with an increased serum level of pyroptosis-related proteins, including ASC, caspase-1, interleukin (IL)-1β, IL-18, gasdermin D and lactic dehydrogenase when compared with healthy controls [27]. Besides, IL-10 was identified as a negative regulator of cardiac inflammation in a murine model of KD induced by Candida albicans water-soluble fraction [28]. These findings demonstrated that IL-10 supplementation may help to prevent coronary vasculitis and aneurysm formation. Furthermore, atorvastatin therapy which can restore the expression of Krüppel-like factor 4-miR-483, was tested in clinical trial of KD patients and was shown to ultimately suppress the level of connective tissue growth factor and endothelial-mesenchymal transition in endothelial cells which contributed to the coronary artery abnormalities in KD patients [29].
Kawasaki disease-macrophage activation syndrome and treatments
Macrophage activation syndrome (MAS), also known as secondary or reactive hemophagocytic lymphohistiocytosis, is characterized by the overactivation and proliferation of T-lymphocyte and well-differentiated macrophages, leading to high mortality rate. It is commonly found in systemic juvenile idiopathic arthritis [30]. The clinical symptoms of MAS were persistent fever, lymphadenopathy, hepatosplenomegaly, hypocythemia (anemia, leucopenia, thrombocytopenia), increased C-reactive protein (CRP) expression, low erythrocyte sedimentation rate, lower level of fibrinogenemia, high triglyceridemia expression and significant elevation of serum ferritin leading to multiple organ dysfunction [31,32]. MAS led to an overexpression of proinflammatory cytokines such as interferon (IFN)-γ, IL-1, IL-6, IL-18 and TNF-α, resulting in systemic immune injury [33]. MAS may occur when KD patients showed persistent fever, splenomegaly, thrombocytopenia, hyperferritinemia or IVIG resistance. Early diagnosis and control to the level of cytokine are important to reduce mortality rate of KD-MAS patients [34]. According to the clinical evaluation on patients, corticosteroid, IVIG, cyclosporine and monoclonal anti-TNF are the first-line therapy [35]. Plasmapheresis (PE) are effective alternatives for severe refractory cases [36].
Kawasaki disease shock syndrome and treatments
Kawasaki Disease Shock Syndrome (KDSS) is defined as the phenomenon of KD with hemodynamic instability. KDSS usually occurs in the acute phase of KD and is more prevalent in children with atypical KD. The exact mechanism and pathogenesis of KDSS remains unknown [37]. The clinical therapy of KDSS is based on symptomatic treatment. For example, with the early anti-inflammation strategy and maintenance of hemodynamic stability to control systemic inflammatory response, viscera and coronary artery damage, the prevention on the deterioration of multiple organ damage was achieved by using high-dose IVIG therapy combined with glucocorticoid anti-inflammatory therapy as the primary treatment [38]. IVIG plays an essential role in the modulation of KD and KDSS by decreasing the level of inflammatory factors, inhibiting the production of antibody and alleviating the immune response. Although there is no conclusive evidence that children with KDSS are more likely to develop IVIG resistance, based on the IVIG unresponsiveness risk prediction score and the characteristics of an intensified inflammatory response observed in KDSS children, caution should be taken with IVIG resistance in KDSS children [37,39]. The anti-inflammatory effect of glucocorticoid can stabilize lysosomal membrane and strengthen myocardial contractility. Methylprednisolone (MPL), a systemic synthetic corticosteroid [40], can be injected at 20−30 mg/kg for 1–3 days [41,42•]. Although only a few cases of vascular and capillary leak were reported in KDSS patients [43], the use of combined clinical therapy of IVIG and vasoactive agents were also reported [44,45]. While noradrenaline is the first choice for vasoactive agents, epinephrine, dopamine and dobutamine can be used according to the state of the disease [46,47]. The strong inflammatory reaction of KDSS may cause IVIG resistance in some cases, consequently increase the risk of coronary artery disease and lead to a massive coronary aneurysm [48].
Therapies for targeting intravenous immunoglobulin resistance and its complications in Kawasaki disease
Although the lack of a widely adopted scoring system to predict IVIG resistance and limited available treatments remains the major problems in clinical practice [4], several drug alternatives (Table 1 ) have been reported [49•].
Table 1.
Alternate therapies for IVIG resistance in Kawasaki disease
| Medicine | Dosage | Mechanistic action | Reference |
|---|---|---|---|
| Methylprednisolone | 20−30 mg/kg, maximum 1 g/day (oral administration) | Blocked inflammatory cytokines which resulted in the immunosuppressive effect | [42•] |
| Prednisone/prednisolone | 1−2 mg/kg/day (intravenous injection, oral administration after symptom relief) | Inhibited the transcription of different inflammatory cytokines and promoted the transcription of anti-inflammatory cytokines and proteins | [4,55] |
| Infliximab | 5 mg/kg (intravenous injection) | Binded and inhibited TNF-α, prevented the release of proinflammatory cytokine and interleukin | [4] |
| Anakinra | 2−6 mg/kg/day (oral administration) | Downregulated various IL-1ß-mediated inflammatory responses, and as a receptor antagonist competitively inhibit the binding between IL-1 and the receptor | [4] |
| Canakinumab | 4 mg/kg (body weight <40 kg) (oral administration) | Suppressed the inflammation through the neutralization of IL-1β | [63] |
| Plasmapheresis (PE) | – | Replaced the plasma harboring the inflammatory cytokines by another colloid including plasma or albumin from donor | [66] |
| Cyclosporine | 4−8 mg/kg/day (intravenous injection or oral administration) | Inhibited the calcineurin-NFAT signaling pathway and increased the activity of T cells | [70] |
| Methotrexate | 10 mg/m2/week (oral administration) | Inhibited lymphocyte proliferation and as a folic acid antagonist | [70] |
Corticosteroids
Corticosteroids are one of the main treatments for various vasculitis and IVIG non-responsive KD patients. Because of its immunosuppressive and anti-inflammatory effect, corticosteroids are usually administered for lowering the risk of CAA in KD. Although the optimal steroid regimen is unclear, intravenous injection and long-term corticosteroids therapy remain one of the treatment choices for resistant KD [50].
Methylprednisone
MPL is the most commonly used corticosteroids to treat KDSS by blocking the rapid immunosuppressive effect from inflammatory cytokines and reducing the risk of electrolytes imbalance. Single intravenous injection of 30 mg/kg can be used in combination with IVIG as a first-line treatment for high-risk KD patients [51]. The AHA recommended using steroids in patients who have failed to response to 2 or more IVIG injections [52]. It was reported that the combined IVIG and steroid therapy for KD was more effective in attenuating the risk of CAA [53]. The use of IVIG combined with MPL on resistant KD patients improved prognosis, and shortened the duration of fever [54].
Prednisone
Prednisone has a strong effect on lowering the transcription level of multiple inflammatory cytokine genes, and simultaneously upregulated the level of various anti-inflammatory related proteins [55]. Combined treatment of prednisone with IVIG was reported to reduce the risk of coronary artery abnormalities [56], and is suggested as the treatment of IVIG resistant patients in Japan [57].
The biologic drugs
Biologic drugs are important agents for regulating TNF-α or IL-1 which triggered KD-related vasculitis. Owing to the role of TNF-α in the pathogenesis of coronary artery dilation and KD, using TNF-α inhibitors in the treatment of KD is possible [58••]. However, without consolidated evidence about its safety and efficacy on KD treatment, evaluations can only be performed according to the experience of non- KD patients with other diseases using anti-TNF or anti-IL-1 biologic drugs.
Infliximab
KD patients demonstrated an increase in TNF-α expression. Infliximab (IFX), a TNF-α monoclonal antibody, exert its anti-inflammatory role via inhibition of TNF-α or soluble TNF-α receptor, lowering the levels of IL-6 or CRP to reduce the severity of vasculitis [4]. It has been reported in many studies to treat refractory KD, and considered as a replacement for the second line treatment in IVIG resistant patients [59••,60]. A phase III clinical trial result showed that the combinational treatment of IFX with IVIG can shorten the duration of fever and decrease the level of TNF-α in KD patients [61].
Anakinra
Anakinra is a recombinant IL-1 antagonist that competitively inhibits IL-1 and its receptors, thereby downregulating various IL-1 mediated inflammatory responses and IL-1 biological activity [4]. Anakinra may possess anti-inflammatory effects on systemic and coronary artery inflammation in a hypothetical KD model [62]. Of note, there are only a few reports of anakinra being used to treat KD in children.
Canakinumab
Canakinumab is a monoclonal antibody that reacts with IL-1β [63]. First phrase clinical trials are currently undergoing for its efficacy in various diseases including gout, chronic obstructive pulmonary and coronary heart disease [64]. Unfortunately, a phase II clinical trial on the treatment of canakinumab to KD was withdrawn before recruitment of KD patients, and therefore further investigation is still required.
Plasmapheresis
PE can directly reduce the level of inflammatory cytokines and chemokines activated from the bloodstream, and prevent the occurrence of CAA in KD patients [65]. Colloid was used for the replacement of plasma during PE [66]. Clinical study has reported PE as a treatment for both IVIG and IFX non-responsive KD patients with their fever symptoms relieved, and body temperature restored to normal after treatments [67].
Cyclosporine
As a calcineurin inhibitor, cyclosporine was suggested as a potential treatment to refractory KD via intervening the inositol 1,4,5-trisphosphate 3-kinase C/calcineurin pathway [68]. Besides, cyclosporine can also block the release of cytokine signaling molecule IL-2 and inhibit the differentiation of T cells, therefore attenuating inflammatory status of KD through cell-mediated immunity regulation. Although the efficacy of cyclosporine in the treatment of KD is still controversial, a phase III clinical trial in Japan using both IVIG and cyclosporine was reported effective in treating severe KD [69••].
Methotrexate
Methotrexate (MTX) is used primarily as an anti-cancer and anti-metabolic drug that blocked the synthesis and replication of DNA. Methotrexate is reported to reduce the level of CRP, erythrocyte sedimentation rate and decrease the level of IL-1 and IL-6 in KD [70,71]. In clinical studies, low dose of MTX was reported to improve clinical KD symptoms in resistant KD patients [72,73], suggesting oral administration of MTX might be an effective treatment to refractory KD.
Analysis of possible drug resistance gene of Kawasaki disease
Methodology on bioinformatics analysis
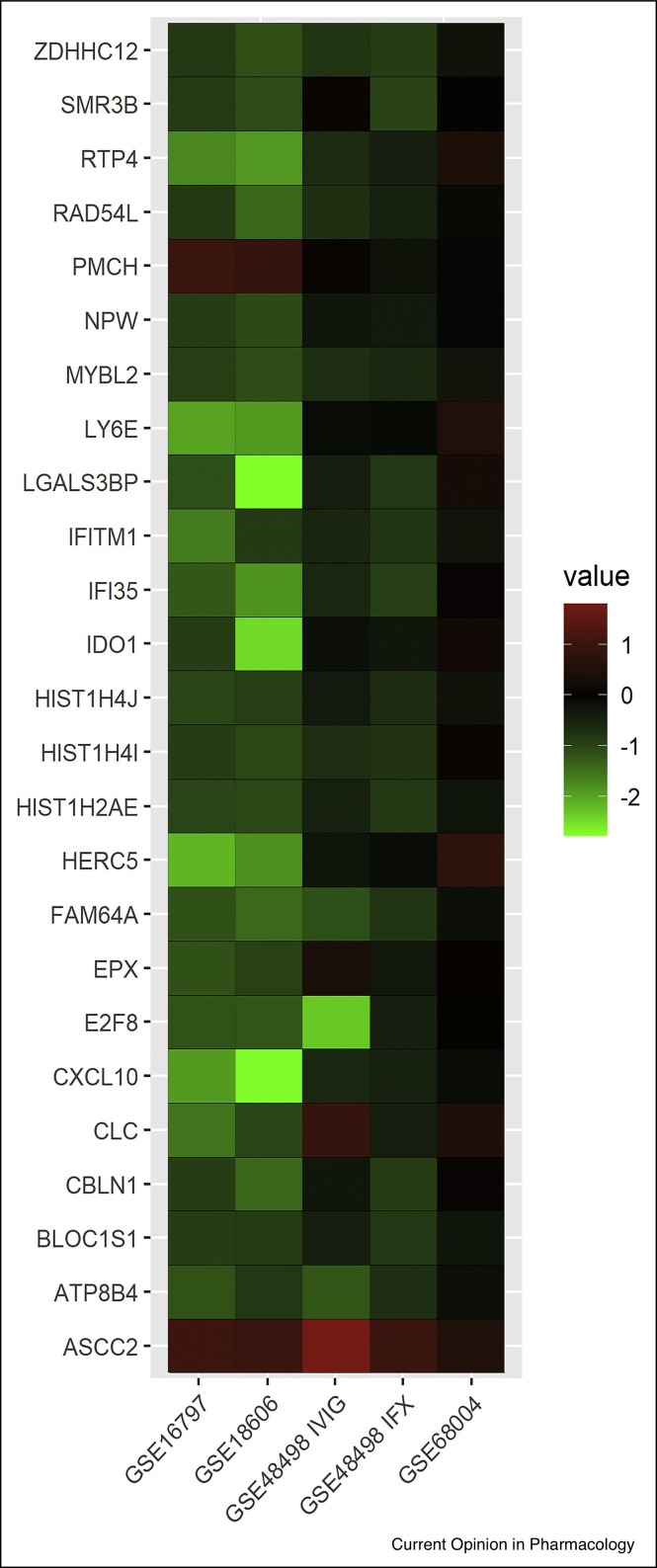
Analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement. Key words are [‘Kawasaki disease’] and [‘resistance’ OR ‘drug resistance’] to access the potential related data in the NCBI Gene Expression Omnibus DataSets database until May 2020. Relevant articles were also cited in this review. Statistical analyses were performed by using the R 3.5.0 software with Biobase and GEOquery package provided by Bioconductor (http://www.bioconductor.org/). 1221 datasets were retrieved in the primary search using the keywords. Consequently, 4 datasets with IVIG responder patient’s data were obtained. In these 4 datasets, 43 cases were involved (14 in the IVIG-responsive group, 21 in the IVIG-resistant patients’ group, 8 in the IFX group). Besides, the data of 46 healthy individuals and 86 KD patients were involved as control. Among the 4 datasets, 2 of them were single IVIG treatment group, and with the gene expression data recorded before and after IVIG therapy in both IVIG resistant and responsive KD patient. While the 3rd dataset included the gene expression level of IVIG-resistant patients after IFX rescue or secondary IVIG treatment, the last dataset included the gene expression profile for KD patients and with healthy individuals as control. A selection was performed according to these 2 criteria: 1. Gene expression data was found in all these 4 studies with no NA (which represents null in r language) value. 2. Expression of genes changes only in IVIG-resistant patients who received IVIG treatments (but not in IFX treatments or KD patients who did not receive treatments). After analysis, out of a total of 197 581 genes data contained in the 4 datasets, 25 genes appeared to be the potential IVIG resistant gene targets (Figure 2 ) for further investigation.
Figure 2.
Potential resistant target genes of IVIG resistant KD patients.
Heatmap of 25 genes which matched the selection criteria were identified from 4 datasets. GSE16797 and GSE18606 columns represents the difference of average gene expression before and after IVIG treatments in IVIG non-responsive patients, and in IVIG responsive patients (GSE16797 and GSE18606 datasets). GSE48498 IVIG column represents the difference of average gene expression before and after second IVIG treatments in IVIG non-responsive patients (GSE48498 dataset), while GSE48498 IFX column represents the difference of average gene expression before and after IFX treatments in IVIG non-responsive patients who showed failure in initial IVIG treatments (GSE48498 dataset). GSE68004 column represents the gene expression difference of KD patients with healthy control used as the baseline from GSE68004 dataset.
Potential intravenous immunoglobulin resistance genes
Lymphocyte Antigen 6 Family Member E (LY6E) is a member of human Ly-6 gene family [74]. It was identified as an encoder for stem cell protein markers which are related to the resistance of radiotherapy and the promotion of tumor metastasis in animal models [75]. Knockdown of LY6E led to significant reduction to a series of gene expressions includes ATP binding cassette subfamily G member 2, fibroblast growth factor 7, Nanog homeobox, CD34 molecule, and prostate stem cell antigen which are related to chemotherapy drug resistance [75]. Hence, the high expression of LY6E maybe one of the causes for IVIG resistance in patients. HECT and RLD Domain Containing E3 Ubiquitin Protein Ligase 5 (HERC5), also known as CEB1, participates in the ubiquitin-like interferon-stimulated gene 15/Ubiquitin-specific proteases 18 pathways. This pathway is closely related to the infection of hepatitis C virus and resistance to IFN treatment [76]. Since interferon-stimulated gene 15 can stimulate the production of IFN-γ [76,77], which participates in the activation of cellular immune response of IVIG treatments [78], this may further explain the potential relationship between HERC5 and IVIG resistance. In human tumor cells, constitutive indoleamine 2,3-Dioxygenase 1 (IDO1) expression depends on cyclooxygenase-2 upon autocrine signaling [79], while cyclooxygenase-2 was found to be associated with the susceptibility of KD [80]. This may suggest the possible correlation between the IDO1 and IVIG drug resistance in KD patients. With the limitation on the inadequate available data set on resistant KD genes, it is with great anticipation that more precise and accurate targets could be identified if the sample number can be increased. Although the above genes expression matched with the required criteria, more clinical and experimental evidence are required to characterize their roles and effects in resistant KD patients. Based on the meta analysis results on the possible drug resistance genes of KD, the anti-LY6E antibody which binds to LY6E could be a solution to high LY6E expression [81]. HZ-6d, a 7, 11-disubstituted quinazoline derivative that downregulated HERC5 [82], and inhibitors and antibodies targeting IDO1 including indoximod, 4-PI, N3-benzyl derivative, ortho-hydroxyl modifications and navoximod [83] are also suggested as potential therapeutic agents for resistant KD in further pharmacological evaluation.
Adjuvant therapies for treating resistant Kawasaki disease
Current studies have shown that traditional Chinese medicines (TCMs) or natural compounds can be used as an adjuvant therapy for KD [84]. For example, combined IVIG treatment with triptolide reduced the level of intracellular cell adhesion molecule-1 and TNF-α in KD mouse models [85]. In additional to the single use of the IVIG, many clinical reports have described the combinational use of traditional herbal decoction or natural compounds to treat KD and resistant cases. For example, Jiang et al. summarized a list of TCMs prescribed for KD from 1990 to now, and concluded that Gypsum fibrosum, Radix Rehmanniae, Lonicera japonica Thunb., Forsythia suspense and Cornu Bubali are the effective herbal medicines commonly prescribed for the treatment of KD [86]. Dan-Shen-Yin is widely used to treat coronary heart disease in clinical practice [87], studies have found that Dan-Shen-Yin reduced infarction size, the level of serum CRP, IL-6, TNF-α and malondialdehyde, and increased superoxide dismutase activity [87], which are closely correlated to the inflammatory level of KD. ‘Qingre Liangxue Decoction’ composed of Gypsum fibrosum, Rhizoma Anemarrhenae, Lonicera japonica Thunb. and other TCMs reduced the level of serum IL-33, TNF-α and platelet count, thus alleviated inflammation and hypercoagulability of KD patients, suggesting that Chinese herbs may play its protective role via reducing inflammatory reaction to protect the myocardium in KD patients [88]. Consistently, many clinical observations have reported the high efficacy of treating KD patients with the modified ‘Qingre Liangxue Decoction’ [89,90]. Furthermore, several traditional anti-inflammatory decoctions including ‘Danshen Shengmaiyin Jiawei’ [91], ‘Baihu Decoction’ [92], ‘Qingying Decoction’ and ‘Zhuye Shigao Decoction’ [93] were reported with clinical efficacy in relieving the inflammatory level and clinical symptoms of KD patients. All these clinical application of TCMs in both classical and IVIG-resistance cases of KD still worth further investigation.
Conclusion
Since the first case of KD was reported in 1967, although modification on several major diagnostic criteria of KD have been made, combined IVIG and AAS therapy remains the standard major therapy for KD. With the limitations and side effects of the current combined therapy, such as the prognosis of IVIG resistance and Reye’s syndrome, other factors such as regional and ethnical variation in the epidemiology of KD, pathogenesis and immunological regulation of KD, ambient environment, dietary and genetic factors contributing to KD remains un-elucidated. To this end, many recent works have been focused on the identification of novel susceptibility gene and pharmacological therapy to both typical and resistant KD. On the other hand, consistent with the reported high incidence rate (90%) of new KD cases which were also diagnosed with COVID-19 [94], IgG antibodies of SARS-CoV-2 in KD patients were detected, indicating that the virus was associated with KD. However, MIS-C also has some unique characteristics, such as a higher age of onset with more teenager’s patients, prevalence of abdominal symptom, more signs of heart involvement and macrophage activation syndrome. Although recent study has reported the recovery of a five-year-old child with positive COVID-19 and KD after intravenous IVIG [95], new diagnostic methods to avoid missed or delayed cases of KD during the COVID-19 pandemic is important [96]. With very few studies on COVID-19 and KD, although they may share many similarities, there is insufficient evidence to show the causal relationship between them. In the future, it is anticipated that the relationship between COVID-19 and KD, and also the new pharmacological treatment and adjuvant therapy to KD and MIS-C could be identified.
Author contributions
BYKL and JC conceived and designed the review content; BYKL, RLZ and HHL drafted the manuscript; HHL performed the bioinformatics analysis, designed and drafted the figures and graphical abstract; BYKL, CL and NI edited and proofread the whole manuscript. RLZ and HHL performed literature search and table preparation. RLZ and HHL are co-first authors and contributed equally to the work. Dr Betty Yuen-Kwan Law and Prof Juan Chen are co-corresponding authors.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by grants from the Macao Science and Technology Development Fund (Project Code: 0060/2018/A2 and 0036/2018/AFJ), National Natural Science Foundation of China (Grant No. 81861168035 to JC), and Foshan Medicine Dengfeng Project (2019–2021).
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Dietz S.M., van Stijn D., Burgner D., Levin M., Kuipers I.M., Hutten B.A., Kuijpers T.W. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176:995–1009. doi: 10.1007/s00431-017-2937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S., Jindal A.K., Pilania R.K. Diagnosis of Kawasaki disease. Int J Rheum Dis. 2018;21:36–44. doi: 10.1111/1756-185X.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 5•.Dionne A., Burns J.C., Dahdah N., Tremoulet A.H., Gauvreau K., de Ferranti S.D., Baker A.L., Son M.B., Gould P., Fournier A., et al. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. 2019;143 doi: 10.1542/peds.2018-3341. [DOI] [PubMed] [Google Scholar]; The Meta-Analysis including children who had CAA with a z score ≥2.5 and <10 at the time of diagnosis and received primary therapy with IVIG alone or in combination with either corticosteroids or infliximab within 10 days of onset of fever. The findings suggested that among the Kawasaki disease patients with CAA, those treated with corticosteroids or infliximab in addition to IVIG had less progression in CAA size when compared with IVIG treatment only.
- 6.Phuong L.K., Curtis N., Gowdie P., Akikusa J., Burgner D. Treatment options for resistant Kawasaki disease. Paediatr Drugs. 2018;20:59–80. doi: 10.1007/s40272-017-0269-6. [DOI] [PubMed] [Google Scholar]
- 7.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., Schnuriger A., Lorrot M., Guedj R., Ducou le Pointe H. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020:202288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowley A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020:1–2. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]; This observational study article evaluated the incidence and characteristics of patients with Kawasaki-like disease diagnosed during the SARS-CoV-2 epidemic. The study found a significant increase in the incidence of Kawasaki-like disease during the SARS-CoV-2 epidemic and predicted similar outbreaks of Kawasaki-like disease in SARS-CoV-2 endemic countries.
- 10.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuercher A.W., Spirig R., Baz Morelli A., Käsermann F. IVIG in autoimmune disease - potential next generation biologics. Autoimmun Rev. 2016;15:781–785. doi: 10.1016/j.autrev.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Burns J.C., Franco A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev Clin Immunol. 2015;11:819–825. doi: 10.1586/1744666X.2015.1044980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong P.H., White K.M. Impact of immunoglobulin therapy in pediatric disease: a review of immune mechanisms. Clin Rev Allergy Immunol. 2016;51:303–314. doi: 10.1007/s12016-015-8499-2. [DOI] [PubMed] [Google Scholar]
- 14.Kuwabara M., Yashiro M., Ae R., Yanagawa H., Nakamura Y. The effects of early intravenous immunoglobulin therapy for Kawasaki disease: the 22nd nationwide survey in Japan. Int J Cardiol. 2018;269:334–338. doi: 10.1016/j.ijcard.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 15.Eleftheriou D., Levin M., Shingadia D., Tulloh R., Klein N.J., Brogan P.A. Management of Kawasaki disease. Arch Dis Child. 2014;99:74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallaire F., Fortier-Morissette Z., Blais S., Dhanrajani A., Basodan D., Renaud C., Mathew M., De Souza A.M., Dionne A., Blanchard J., et al. Aspirin dose and prevention of coronary abnormalities in Kawasaki disease. Pediatrics. 2017;139 doi: 10.1542/peds.2017-0098. [DOI] [PubMed] [Google Scholar]
- 17.Sosa T., Brower L., Divanovic A. Diagnosis and management of Kawasaki disease. JAMA Pediatr. 2019;173:278–279. doi: 10.1001/jamapediatrics.2018.3307. [DOI] [PubMed] [Google Scholar]
- 18•.Zheng S.L., Roddick A.J. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321:277–287. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]; This Meta-Analysis evaluated the association of aspirin with cardiovascular disease and bleeding when used for primary disease prevention. Conclusions indicated that the use of aspirin in individuals without cardiovascular disease was associated with a lower risk of cardiovascular events but an increased risk of bleeding.
- 19•.Patrono C., Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16:675–686. doi: 10.1038/s41569-019-0225-y. [DOI] [PubMed] [Google Scholar]; This review discussed the mechanistic and pharmacological action of aspirin and summarized clinical evidence, benefit and possible side effects of using aspirin for primary or secondary prevention of cardiovascular diseases.
- 20.Azmin S., Sahathevan R., Rabani R., Nafisah W.Y., Tan H.J., Raymond A.A., Hamidon B.B., Shamsul A.S., Norlinah M.I. Biochemical aspirin resistance in stroke patients - a cross-sectional single centre study. EXCLI J. 2013;12:907–915. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuliczkowski W., Witkowski A., Polonski L., Watala C., Filipiak K., Budaj A., Golanski J., Sitkiewicz D., Pregowski J., Gorski J., et al. Interindividual variability in the response to oral antiplatelet drugs: a position paper of the working group on antiplatelet drugs resistance appointed by the section of cardiovascular interventions of the polish cardiac society, endorsed by the working group on thrombosis of the European Society of Cardiology. Eur Heart J. 2009;30:426–435. doi: 10.1093/eurheartj/ehn562. [DOI] [PubMed] [Google Scholar]
- 22.Mehta J.L., Mohandas B. Aspirin resistance: fact or fiction? A point of view. World J Cardiol. 2010;2:280–288. doi: 10.4330/wjc.v2.i9.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai G., Zhou W., Lu Y., Chen P., Lu Z., Fu Y. Aspirin resistance and other aspirin-related concerns. Neurol Sci. 2016;37:181–189. doi: 10.1007/s10072-015-2412-x. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X., Yue P., Liu L., Tang C., Ma F., Zhang Y., Wang C., Duan H., Zhou K., Hua Y., et al. Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: current evidence based on a meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S., Dong Y., Kiuchi M.G., Wang J., Li R., Ling Z., Zhou T., Wang Z., Martinek M., Pürerfellner H., et al. Coronary artery complication in Kawasaki disease and the importance of early intervention : a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1156–1163. doi: 10.1001/jamapediatrics.2016.2055. [DOI] [PubMed] [Google Scholar]
- 26.Denby K.J., Clark D.E., Markham L.W. Management of Kawasaki disease in adults. Heart. 2017;103:1760–1769. doi: 10.1136/heartjnl-2017-311774. [DOI] [PubMed] [Google Scholar]
- 27.Jia C., Zhang J., Chen H., Zhuge Y., Chen H., Qian F., Zhou K., Niu C., Wang F., Qiu H., et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019;10:778. doi: 10.1038/s41419-019-2021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura J., Watanabe S., Kimura H., Kobayashi M., Karasawa T., Kamata R., Usui Kawanishi F., Sadatomo A., Mizukami H., Nagi-Miura N., et al. Adeno-associated virus vector-mediated interleukin-10 induction prevents vascular inflammation in a murine model of Kawasaki disease. Sci Rep. 2018;8:7601. doi: 10.1038/s41598-018-25856-0. PMID: 29765083; PMCID: PMC5953966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M., Chen Z., Martin M., Zhang J., Sangwung P., Woo B., Tremoulet A.H., Shimizu C., Jain M.K., Burns J.C., et al. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: therapeutic implications in Kawasaki disease. Circ Res. 2017;120:354–365. doi: 10.1161/CIRCRESAHA.116.310233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grom A.A., Horne A.C., De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravelli A., Davì S., Minoia F., Martini A., Cron R.Q. Macrophage activation syndrome. Hematol Oncol Clin North Am. 2015;29:927–941. doi: 10.1016/j.hoc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Ravelli A., Minoia F., Davì S., Horne A., Bovis F., Pistorio A., Aricò M., Avcin T., Behrens E.M., De Benedetti F., et al. Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75:481–489. doi: 10.1136/annrheumdis-2015-208982. [DOI] [PubMed] [Google Scholar]
- 33.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han S.B., Lee S.Y. Macrophage activation syndrome in children with Kawasaki disease: diagnostic and therapeutic approaches. World J Pediatr. 2020 doi: 10.1007/s12519-020-00360-6. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Pavon S., Yamazaki-Nakashimada M.A., Baez M., Borjas-Aguilar K.L., Murata C. Kawasaki disease complicated with macrophage activation syndrome: a systematic review. J Pediatr Hematol Oncol. 2017;39:445–451. doi: 10.1097/MPH.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 36.Kinjo N., Hamada K., Hirayama C., Shimizu M. Role of plasma exchange, leukocytapheresis, and plasma diafiltration in management of refractory macrophage activation syndrome. J Clin Apher. 2018;33:117–120. doi: 10.1002/jca.21570. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Zheng Q., Zou L., Wu J., Guo L., Teng L., Zheng R., Jung L.K.L., Lu M. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17:1. doi: 10.1186/s12969-018-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tissandier C., Lang M., Lusson J.R., Bœuf B., Merlin E., Dauphin C. Kawasaki shock syndrome complicating a recurrence of Kawasaki disease. Pediatrics. 2014;134:e1695–e1699. doi: 10.1542/peds.2014-0004. [DOI] [PubMed] [Google Scholar]
- 39.Liang Y.C., Chang C.H., Lin M.T., Kao F.Y., Huang S.K., Wu M.H. Shock and unresponsiveness to repeated courses of intravenous immunoglobulin in Kawasaki disease: a nationwide database study. Pediatr Res. 2020;87:961–966. doi: 10.1038/s41390-019-0668-1. [DOI] [PubMed] [Google Scholar]
- 40.Miura M. Role of glucocorticoids in Kawasaki disease. Int J Rheum Dis. 2018;21:70–75. doi: 10.1111/1756-185X.13209. [DOI] [PubMed] [Google Scholar]
- 41.Okubo Y., Michihata N., Morisaki N., Sundel R.P., Matsui H., Fushimi K., Yasunaga H. Association between dose of glucocorticoids and coronary artery lesions in Kawasaki disease. Arthritis Care Res (Hoboken) 2018;70:1052–1057. doi: 10.1002/acr.23456. [DOI] [PubMed] [Google Scholar]
- 42•.de Graeff N., Groot N., Ozen S., Eleftheriou D., Avcin T., Bader-Meunier B., Dolezalova P., Feldman B.M., Kone-Paut I., Lahdenne P., et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease - the SHARE initiative. Rheumatology (Oxford) 2019;58:672–682. doi: 10.1093/rheumatology/key344. [DOI] [PubMed] [Google Scholar]; This guide is part of the SHARE program (Single Hub and Access point for pediatric Rheumatology in Europe) and provided international evidence-based recommendations for diagnosing and treating KD, aimed at facilitating improvement and uniformity of care.
- 43.Natterer J., Perez M.H., Di Bernardo S. Capillary leak leading to shock in Kawasaki disease without myocardial dysfunction. Cardiol Young. 2012;22:349–352. doi: 10.1017/S1047951111001314. [DOI] [PubMed] [Google Scholar]
- 44.Chen P.S., Chi H., Huang F.Y., Peng C.C., Chen M.R., Chiu N.C. Clinical manifestations of Kawasaki disease shock syndrome: a case-control study. J Microbiol Immunol Infect. 2015;48:43–50. doi: 10.1016/j.jmii.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M.-M., Shi L., Li X.-H., Lin Y., Liu Y. Clinical analysis of Kawasaki disease shock syndrome. Chin Med J. 2017;130:2891–2892. doi: 10.4103/0366-6999.219151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 47.Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M., Sevransky J.E., Sprung C.L., Douglas I.S., Jaeschke R., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y.J., Cheng M.C., Lo M.H., Chien S.J. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. 2015;34:1163–1167. doi: 10.1097/INF.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 49•.Miura M., Kobayashi T., Kaneko T., Ayusawa M., Fukazawa R., Fukushima N., Fuse S., Hamaoka K., Hirono K., Kato T., et al. Association of severity of coronary artery aneurysms in patients with Kawasaki disease and risk of later coronary events. JAMA Pediatr. 2018;172 doi: 10.1001/jamapediatrics.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article using multicenter, collaborative retrospective cohort study of 44 participating institutions including 1006 patients who received a coronary angiography between 1992 and 2011. The findings suggested that using the internal diameter z score is useful for assessing the severity of CAA in patients with KD.
- 50.Wardle A.J., Connolly G.M., Seager M.J., Tulloh R.M. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD011188.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furukawa T., Kishiro M., Akimoto K., Nagata S., Shimizu T., Yamashiro Y. Effects of steroid pulse therapy on immunoglobulin-resistant Kawasaki disease. Arch Dis Child. 2008;93:142–146. doi: 10.1136/adc.2007.126144. [DOI] [PubMed] [Google Scholar]
- 52.Newburger J.W., Takahashi M., Gerber M.A., Gewitz M.H., Tani L.Y., Burns J.C., Shulman S.T., Bolger A.F., Ferrieri P., Baltimore R.S., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 53.Chen S., Dong Y., Yin Y., Krucoff M.W. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013;99:76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 54.Ogata S., Ogihara Y., Honda T., Kon S., Akiyama K., Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129:e17–23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 55.Jusko W.J. Pharmacokinetics and receptor-mediated pharmacodynamics of corticosteroids. Toxicology. 1995;102:189–196. doi: 10.1016/0300-483x(95)03047-j. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi T., Saji T., Otani T., Takeuchi K., Nakamura T., Arakawa H., Kato T., Hara T., Hamaoka K., Ogawa S., et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379:1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 57.Cardiology RCotJSoP, Disease CSCfDoGfMToAK Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version) Pediatr Int. 2014;56:135–158. doi: 10.1111/ped.12317. [DOI] [PubMed] [Google Scholar]
- 58••.Yamaji N., da Silva Lopes K., Shoda T., Ishitsuka K., Kobayashi T., Ota E., Mori R. TNF-α blockers for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2019;8 doi: 10.1002/14651858.CD012448.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article included randomized controlled trials that compared the effect of TNF-α blockers such as infliximab and etanercept, to placebo or other drugs including IVIG, in children with KD. The findings suggested that TNF-α blockers have beneficial effects on the treatment of resistant KD.
- 59••.Mori M., Hara T., Kikuchi M., Shimizu H., Miyamoto T., Iwashima S., Oonishi T., Hashimoto K., Kobayashi N., Waki K., et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8:1994. doi: 10.1038/s41598-017-18387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This clinical trial compared the efficacy and safety of infliximab with standard intravenous immunoglobulin (IVIG) therapy, in a phase III trial for Japanese patients with Kawasaki disease (KD) and persistent fever after initial IVIG treatment. The experimental results showed that infliximab was well tolerated in patients with IVIG-refractory KD.
- 60.Crayne C.B., Mitchell C., Beukelman T. Comparison of second-line therapy in IVIg-refractory Kawasaki disease: a systematic review. Pediatr Rheumatol Online J. 2019;17:77. doi: 10.1186/s12969-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tremoulet A.H., Jain S., Jaggi P., Jimenez-Fernandez S., Pancheri J.M., Sun X., Kanegaye J.T., Kovalchin J.P., Printz B.F., Ramilo O., et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–1738. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 62.Gorelik M., Lee Y., Abe M., Andrews T., Davis L., Patterson J., Chen S., Crother T.R., Aune G.J., Noval Rivas M., et al. IL-1 receptor antagonist, anakinra, prevents myocardial dysfunction in a mouse model of Kawasaki disease vasculitis and myocarditis. Clin Exp Immunol. 2019;198:101–110. doi: 10.1111/cei.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhimolea E. Canakinumab. mAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 65.Ebato T., Ogata S., Ogihara Y., Fujimoto M., Kitagawa A., Takanashi M., Ishii M. The clinical utility and safety of a new strategy for the treatment of refractory Kawasaki disease. J Pediatr. 2017;191:140–144. doi: 10.1016/j.jpeds.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 66.Takagi N., Kihara M., Yamaguchi S., Tamura K., Yabana M., Tokita Y., Ishii M. Plasma exchange in Kawasaki disease. Lancet. 1995;346:1307. doi: 10.1016/s0140-6736(95)91916-3. [DOI] [PubMed] [Google Scholar]
- 67.Sonoda K., Mori M., Hokosaki T., Yokota S. Infliximab plus plasma exchange rescue therapy in Kawasaki disease. J Pediatr. 2014;164:1128–1132.e1121. doi: 10.1016/j.jpeds.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Tremoulet A.H., Pancoast P., Franco A., Bujold M., Shimizu C., Onouchi Y., Tamamoto A., Erdem G., Dodd D., Burns J.C. Calcineurin inhibitor treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2012;161:506–512 e501. doi: 10.1016/j.jpeds.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Hamada H., Suzuki H., Onouchi Y., Ebata R., Terai M., Fuse S., Okajima Y., Kurotobi S., Hirai K., Soga T., et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. 2019;393:1128–1137. doi: 10.1016/S0140-6736(18)32003-8. [DOI] [PubMed] [Google Scholar]; This clinical trial article evaluated the safety and efficacy of ciclosporin in the prevention of coronary artery anomalies in patients with Kawasaki disease. Conclusions indicated that IVIG in combination with ciclosporin is safe and effective in patients with Kawasaki disease who did not respond to IVIG.
- 70.Marchesi A., Tarissi de Jacobis I., Rigante D., Rimini A., Malorni W., Corsello G., Bossi G., Buonuomo S., Cardinale F., Cortis E., et al. Kawasaki disease: guidelines of Italian society of pediatrics, part II - treatment of resistant forms and cardiovascular complications, follow-up, lifestyle and prevention of cardiovascular risks. Ital J Pediatr. 2018;44:103. doi: 10.1186/s13052-018-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jang H., Kim K.Y., Kim D.S. Clinical outcomes of low-dose methotrexate therapy as a second-line drug for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2018;59:113–118. doi: 10.3349/ymj.2018.59.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahn S.Y., Kim D.S. Treatment of intravenous immunoglobulin-resistant Kawasaki disease with methotrexate. Scand J Rheumatol. 2005;34:136–139. doi: 10.1080/03009740510026328. [DOI] [PubMed] [Google Scholar]
- 73.Lee T.J., Kim K.H., Chun J.K., Kim D.S. Low-dose methotrexate therapy for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2008;49:714–718. doi: 10.3349/ymj.2008.49.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu J., Liu S.-L. Emerging role of LY6E in virus-host interactions. Viruses. 2019;11:1020. doi: 10.3390/v11111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.AlHossiny M., Luo L., Frazier W.R., Steiner N., Gusev Y., Kallakury B., Glasgow E., Creswell K., Madhavan S., Kumar R., et al. Ly6E/K signaling to TGFβ promotes breast cancer progression, immune escape, and drug resistance. Cancer Res. 2016;76:3376. doi: 10.1158/0008-5472.CAN-15-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L., Li S., McGilvray I. The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in hepatitis C virus infection and resistance to interferon therapy. Int J Biochem Cell Biol. 2011;43:1427–1431. doi: 10.1016/j.biocel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Osiak A., Utermöhlen O., Niendorf S., Horak I., Knobeloch K.-P. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol. 2005;25:6338–6345. doi: 10.1128/MCB.25.15.6338-6345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park-Min K.H., Serbina N.V., Yang W., Ma X., Krystal G., Neel B.G., Nutt S.L., Hu X., Ivashkiv L.B. FcγRIII-dependent inhibition of interferon-γ responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Hennequart M., Pilotte L., Cane S., Hoffmann D., Stroobant V., Plaen E.D., Eynde B.J.V.D. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res. 2017;5:695. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 80.Li S., Shi R., Tian L., Chen J., Li X., Huang L., Yang Z. The relationship of COX-2 gene polymorphisms and susceptibility to Kawasaki disease in Chinese population. Immunol Invest. 2019;48:181–189. doi: 10.1080/08820139.2018.1529790. [DOI] [PubMed] [Google Scholar]
- 81.Sakanaka C, Chang P: Anti-Ly6E Antibodies and Immunoconjugates and Methods of Use. Edited by: Google Patents US9290578B2; 2017.
- 82.Wang Y., Ding Q., Xu T., Li C.Y., Zhou D.D., Zhang L. HZ-6d targeted HERC5 to regulate p53 ISGylation in human hepatocellular carcinoma. Toxicol Appl Pharmacol. 2017;334:180–191. doi: 10.1016/j.taap.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Prendergast G.C., Malachowski W.P., DuHadaway J.B., Muller A.J. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77:6795–6811. doi: 10.1158/0008-5472.CAN-17-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang B., Lo H.H., Lei C., Ka In U., Hsiao W.W., Guo X., Bai J., Wong V.K., Law B.Y. Adjuvant herbal therapy for targeting susceptibility genes to Kawasaki disease: an overview of epidemiology, pathogenesis, diagnosis and pharmacological treatment of Kawasaki disease. Phytomedicine. 2020;70 doi: 10.1016/j.phymed.2020.153208. 153208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Z.T., Zou J.W. Triptolide as an alternative to IVIG therapy for Kawasaki disease in a mouse model. Balkan Med J. 2013;30:225–228. doi: 10.5152/balkanmedj.2013.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang W.W., Wong W.Z., Xu Y.J., Yang J.H., Huang T., Xu K.S., Pan J.K., Zhao W. Analysis on composition principles of prescriptions for Kawasaki disease based on data mining methods. J Emerg Tradit Chin Med. 2015;024:1755–1757. [Google Scholar]
- 87.Yan K.-P., Guo Y., Xing Z., Huang X., Dai S., Duan M., Sun X., Huang W., Peng W. Dan-Shen-Yin protects the heart against inflammation and oxidative stress induced by acute ischemic myocardial injury in rats. Exp Ther Med. 2012;3:314–318. doi: 10.3892/etm.2011.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen J.Y., Yin J.M., Du Z.D., Hao J., Yan H.M. Qing Re Liang Xue decoction alleviates hypercoagulability in Kawasaki disease. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/864597. 864597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang L., Xie J.D. Clinical observation on combine therapy of γ-Immunoglobulin and Chinese decoction in 68 cases of treatments of Kawasaki Diseases. Chin J Mod Drug Appl. 2014:138–139. [Google Scholar]
- 90.Tan J. Clinical research on combine therapy of γ-immunoglobulin and Chinese decoction in treatments of Kawasaki diseases. Asia Pac Tradit Med. 2014;010:98–99. [Google Scholar]
- 91.Wang J.X., Qiu Z.Z. Clinical research of Danshen Shengmai decoction addition in treatment of convalescent phase of Kawasaki syndrome in 60 cases. Lishizhen Med Mater Med Res. 2009;020:2073–2074. [Google Scholar]
- 92.Wang Y. Treatment of 32 cases of Kawasaki disease with Baihu Decoction. Shaanxi J Tradit Chin Med. 2011:1458. [Google Scholar]
- 93.Liao R.S., Du S.J. Effect of heat-clearing and blood-activating herbs on platelet parameters in children with Kawasaki disease. J Guangzhou Univ Tradit Chin Med. 2008:492–494. [Google Scholar]
- 94.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., Debray A., Basmaci R., Salvador E., Biscardi S., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivera-Figueroa E.I., Santos R., Simpson S., Garg P. Incomplete Kawasaki disease in a child with Covid-19. Indian Pediatr. 2020;57:680–681. doi: 10.1007/s13312-020-1900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harahsheh A.S., Dahdah N., Newburger J.W., Portman M.A., Piram M., Tulloh R., McCrindle B.W., de Ferranti S.D., Cimaz R., Truong D.T., et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]