Abstract
COVID-19 is a pandemic that has affected not only the United States, but the entire world. The impact it has had has overwhelmed the entire healthcare system, from the unknown carrier status, poor testing capabilities to hospitals running out of ventilators for severely ill patients. There has been a variety of potential treatment modalities for the various forms of illness ranging from asymptomatic carriers to the ventilated ICU patients. These include anti-inflammatory medications, antibiotics, immune-modulators, convalescent plasma, and others. The cytokine storm that inflicts some patients can be devastating to the vital organs of the human body in the form of acute respiratory distress syndrome (ARDS), renal failure, coagulopathy, and death. Cytosorbents® cytokine filter is a potential treatment methodology aimed at reducing the cytokine storm, thus serving as a bridge for therapy in the acutely ill patients infected with COVID-19. The following case report demonstrates the utility in a critically ill patient who survived the cytokine storm after receiving the cytokine filter via continuous renal replacement therapy bridging him to further definitive therapy.
Keywords: Covid-19, SARS-CoV-2, Cytososorb, Cytokine storm
Introduction
SARS-CoV-2 or COVID-19 is a coronavirus that has caused a pandemic worldwide, leaving no region of the world untouched, as announced by the World Health Organization (WHO) on March 10, 2020. This rapidly spreading disease results in significant morbidity and mortality. We are still learning the broad range of presenting signs and symptoms, organ dysfunction, treatment responses and patient outcomes.1 As of September 29, 2020, there have been over 33 million cases and over 1 million deaths worldwide.5
Understanding the virus pathway, immune response, and affected organ systems specific to COVID-19 is important to identify the pathophysiology of the disease process. This particular strain was first identified in Wuhan, Hubei Province, China, in December 2019. It was isolated in China by bronchoalveolar lavage in three infected patients allowing study of the virus. A zoonotic origin is suspected to be from bats; however, humans are considered the main source of infection. Presentations of infected individuals range from asymptomatic to critical life-threatening illness. It is suspected that the severe cases have a higher viral load than the mild or asymptomatic carriers.4
Coronavirus is an enveloped single-stranded RNA virus. It enters the cell by binding to the cell spike protein and host cell protease priming by protein S to Angiotensin Converting Enzyme (ACE2) receptor in order to enter the cell.8 the viral particles can cause respiratory, enteric, hepatic, and neurologic presentations due to the presence of the ACE2 receptor. ACE2 is broadly expressed in nasal mucosa, bronchus, lung, heart, esophagus, kidney, stomach, bladder, and ileum.4
The most common presentation is respiratory in nature. Fever, cough and myalgias are the most common symptoms and about half the patients have dyspnea. Leukopenia and lymphopenia seem to be a mainstay for laboratory findings. CT findings, if obtained, can be consistent with bilateral ground glass opacities in non-ICU patients, however multilobar consolidation is evident in critically ill patients.7
Severe cases of COVID-19 have a variety of systemic signs however, the most critical cases demonstrate the development of ARDS (Acute Respiratory Distress Syndrome), followed by an acute kidney injury (AKI) and then liver dysfunction. ARDS is an inflammatory response mediated by innumerable factors including ACE2, interleukin 10 (IL-10), tumor necrosis factor (TNF), and vascular endothelial growth factor (VEGF) This type of lung injury is characterized by bilateral pulmonary infiltrates, hypoxemia, however without cardiogenic pulmonary edema being the etiology. The exact etiology is not yet well understood; however, the pathophysiology involves alveolar capillary damage, endothelial cellular damage leading to inflammation, necrosis, and apoptosis. This leads to alveolar edema. The diagnosis is made by a clinical presentation of respiratory failure, chest x-ray revealing bilateral infiltrates, hypoxemia as defined by PaO2/FiO2 ratio of ≤200 mmHg.
NIH sponsored a multicenter randomized control trial in the treatment of patients with ARDS. The patients randomized to the low tidal volume arm had a survival benefit. The mortality rate was reduced from 40% in the conventional arm to the 31% in the low tidal volume arm. It is speculated that an associated decrease in IL-6 concentrations may be a protective factor in the multisystem organ failure that is incurred in patients with ARDS. This protocol became known as ARDS Net protocol with decreased tidal volumes (4–6 ml/kg of predicted body weight), maintaining plateau pressure between 25 and 30 cmH20 and lower levels of PEEP.3 Additional therapies in severe ARDS include prone positioning to improve ventilation perfusion matching and alveolar recruitment, high frequency oscillatory ventilation at high respiratory frequencies, and extracorporeal lung support.
With regards to ARDS in the COVID-19 patient, the demographic for severe illness is unclear. It has affected immunocompromised patients, the elderly population, those with comorbidities, as well as young, and healthy patients. Theories include the initial viral load, rate of replication, amount of ACE2 deregulation, etc. The initial onset of rapid viral replication may cause massive epithelial and endothelial cell death and vascular leakage, triggering the production of exuberant pro-inflammatory cytokines. The extensive inflammatory response of cytokines may lead to Cytokine Release Syndrome or the “cytokine storm,” which occurs when white blood cells are activated and release inflammatory signals (cytokines) that further activate more white blood cells. IL-6, IL-8, ferritin, TNF-alpha are some of the pro-inflammatory markers released in this cascade. This cytokine storm has been seen in a variety of medical conditions such as post-operative state after a major surgery, sepsis, shock, among others.6 The disproportionate release beyond that of a controlled immune response appears to play a crucial role in the significant morbidity and mortality of COVID-19 disease process. The imbalance and pro-inflammatory state causes endovascular or capillary leakage, hemodynamic collapse, and severe end organ dysfunction.2
Cytosorbents® is a filter device approved in Europe in 58 different countries and being used as a trial basis in the United States to treat patients in a cytokine storm. The hemadsorption filter is inserted in the device medium used for patients receiving continuous renal replacement therapy (CRRT) or extracorporeal membrane oxygenation (ECMO) therapy. Inflammatory labs such as ferritin, CRP, procalcitonin, and IL-6 have been measured throughout the course of the filter therapy to monitor responsiveness.2 It has been successfully used in cytokine storm patients previously with septic shock, severe pro-inflammatory state in post-operative conditions. Not only has it been shown to reduce the measured inflammatory markers, but also improves hemodynamics including MAP and overall short-term survival as measured. 7 Given the limited time since the COVID-19 outbreak, there is not a significant amount of published research data available on COVID-19 patients and their responsiveness to this therapy. Currently, medications have been considered that target specific inflammatory markers such as tocilizumab, which targets IL-6. This cytokine filter essentially targets multiple different cytokines at a larger scale. However, applying the concept of the cytokine storm and the previous indication for cytokine filter, it became a promising consideration for refractory cases. It has been used in Europe for treating these refractory cases of COVID-19 who are being treated with mechanical ventilation with severe ARDS, failing the standard treatment of Azithromycin and Hydroxychloroquine. Currently there are open trials in Germany and Italy enrolling patients and we hope to soon have more information on a global scale that may be applied to more patients. It has been used in over 500 critically ill patients infected with COVID-19 in Italy, China, Germany, and France with preliminary data that is very positive in reduction of cytokine levels.1 In fact, the Italy Brescia Renal COVID Task Force has made a formal recommendation to specifically use Cytosorb in severe COVID −19 patients with Stage 3 AKI and receiving CRRT.
The following is a case report on a patient encounter and management through the course of illness in which the Cytosorbents® filter was used for his presentation of COVID-19 with severe ARDS, worsening renal dysfunction and evidence of evolving cytokine storm.
History & physical examination
Patient is a 51-year-old male who presented to ED on March 28, 2020 for complaint of dyspnea, lethargy, myalgias and fever. He presumably came in contact with a COVID-19 positive patient at a birthday party two weeks prior. His-past medical history was significant for diabetes, and the patient denied cough, chest pain, vomiting, or diarrhea. Initial vital signs revealed heart rate 98, respiratory rate 18, blood pressure 102/66, pulse oximeter of 70% on room air, and fever with a temperature of 100.4F. The patient's oxygen saturation improved to 90% on 6 liters of nasal cannula oxygen but progressively needed 10 L via a non-rebreather mask to maintain that saturation during his four-hour ED stay. Physical examination revealed no signs of acute distress. His-breath sounds were coarse, but no focal wheezing or rhonchi. His-heart tones were regular and he had no lower extremity edema. Overall, there were no remarkable findings on his physical exam.
Laboratory work-up was obtained in the emergency department(ED). There was no leukocytosis or anemia. Hyponatremia, hyperglycemia were notable. Given his glucose of 334, Hgb A1C was evaluated and found to be 12.7 (reference range 4.3 - 5.6) indicating poorly controlled diabetes mellitus. This sodium level was then corrected to 137 (within normal range) to account for the hyperglycemia. His-LDH was significantly elevated to 547. He also had evidence of AKI with creatinine of 1.29. Initial laboratory results are demonstrated in Table 1 .
Table 2.
Daily ventilator settings and ABG results.
| Hospital Day | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Filter Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Tidal Volume | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 450 | 500 | 500 | 500 | 500 |
| Respiratory Rate | 30 | 30 | 30 | 26 | 26 | 26 | 26 | 35 | 35 | 35 | 28 | 28 |
| PEEP | 14 | 12 | 8 | 8 | 8 | 8 | 8 | 8 | 5 | 5 | 5 | 5 |
| FiO2 | 50% | 50% | 40% | 40% | 50% | 40% | 40% | 50% | 50% | 50% | 40% | 40% |
| pH | 7.25 | 7.39 | 7.36 | 7.31 | 7.26 | 7.2 | 7.24 | 7.09 | 7.31 | 7.373 | 7.4 | 7.41 |
| PCO2 | 52 | 39 | 46.9 | 47.6 | 47.8 | 46 | 49 | 64 | 43.3 | 42.3 | 39.5 | 40.6 |
| PaO2 | 80 | 81 | 75.7 | 92.3 | 98 | 71 | 79.7 | 111 | 70.6 | 158 | 101 | 113 |
| O2 Sat | 94% | 94 | 91% | 94 | 94 | 88 | 91 | 93.9 | 91.5 | 96.8 | 94.9 | 95.5 |
| HCO3 | 23 | 23 | 24 | 23.8 | 20 | 17.7 | 20.5 | 18.9 | 22.8 | 24 | 24.2 | 25.3 |
| Sedation/Paralytic | Y/Y | Y/Y | Y/Y | Y/N | Y/N | Y/N | Y/Y | Y/Y | Y/N | Y/N | Y/N | Y/N |
Table 1.
Laboratory results at initial presentation.
 |
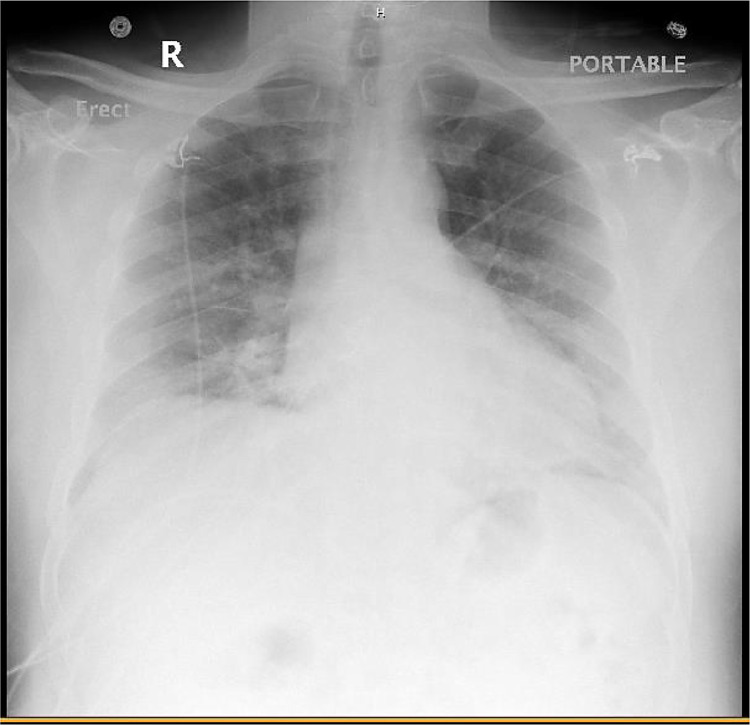
His ED chest x-ray revealed bilateral infiltrates (Fig. 1 ). Per hospital protocol, influenza and respiratory syncytial virus swabs were ordered first and were negative. Those negative results allowed for the Respiratory viral panel and SARS-CoV-2 test to be sent out to the state lab.
Fig. 1.
Emergency Department chest x-ray.
Given his hypoxia, he was admitted to the general pulmonary floor to a primary care team with a pulmonary consultation. The pulmonary team empirically treated him for community acquired pneumonia with ceftriaxone and azithromycin as well as hydroxychloroquine on his first day of admission for presumed COVID-19 infection. His-respiratory viral panel came back negative. On hospital day 2, virology report resulted positive for COVID-19 via nasopharyngeal swab.
Hospital course
His first few days in the hospital were relatively benign. At this time, the rest of his laboratory work was grossly unremarkable with the exception of elevated lactate dehydrogenase (LDH) of 622 and AKI. He also had one positive blood culture of gram-positive rods, diphtheroids likely due to contamination. He had no leukocytosis (however, had leukopenia), anemia, electrolyte abnormalities, or liver dysfunction.
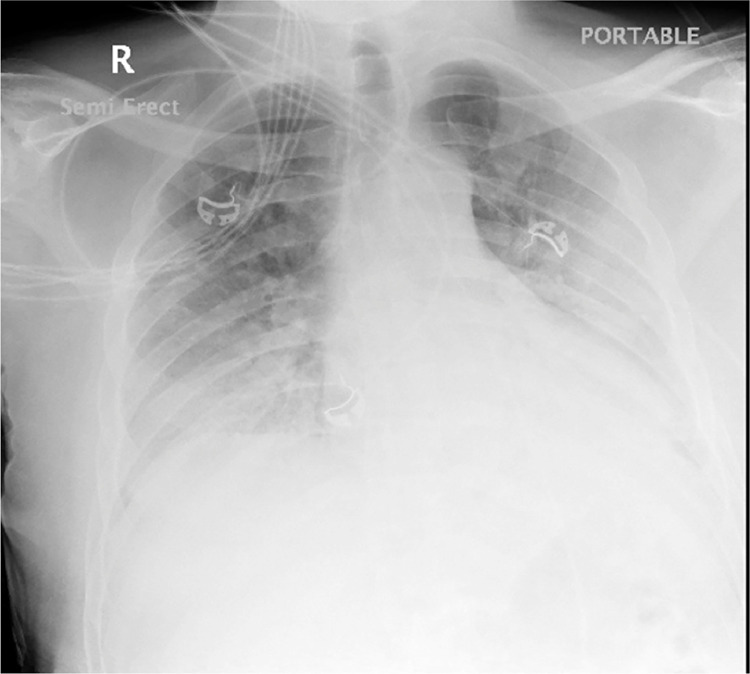
The patient's hypoxemia initially responded to high flow nasal cannula and NRB, but on hospital day 4 he required intubation for respiratory distress and arterial blood gas PaO2 of 54 mmHg and oxygen saturation of 86%. Day 4 pre-intubation xray is demonstrated in Fig. 2 . He developed ARDS as noted by his PaO2/FiO2 ratio < 200. He was placed on ARDS net protocol, as described above of, high PEEP, low tidal volume (4–6 ml/kg IBW), and goal plateau pressure less than 30 mmHg.
Fig. 2.
Chest x-ray on hospital day 4 showing worsening bilateral infiltrates prior to intubation.
On hospital day #6, the patient's clinical status worsened and was persistently hypoxic and became hypotensive. He also developed a fever of 103.2F. Repeat sepsis work-up was initiated including urine cultures, blood culture. He was previously on ceftriaxone and azithromycin per pulmonary team from initial admission. Once his COVID-19 swab resulted positive, the ceftriaxone was discontinued, and he was only on azithromycin. Efforts were made to improve ventilation with paralysis, ARDS Net ventilator settings and prone positioning for alveolar recruitment. These maneuvers with ventilator settings of TV 450, RR 32, PEEP 16, FiO2 60% temporarily improved oxygenation and blood cultures from peripheral sites showed cleared diphtheroids contaminant. Sputum revealed minimal candida. However, the team noticed that his creatinine was starting to elevate slightly from 0.93 to 1.32.
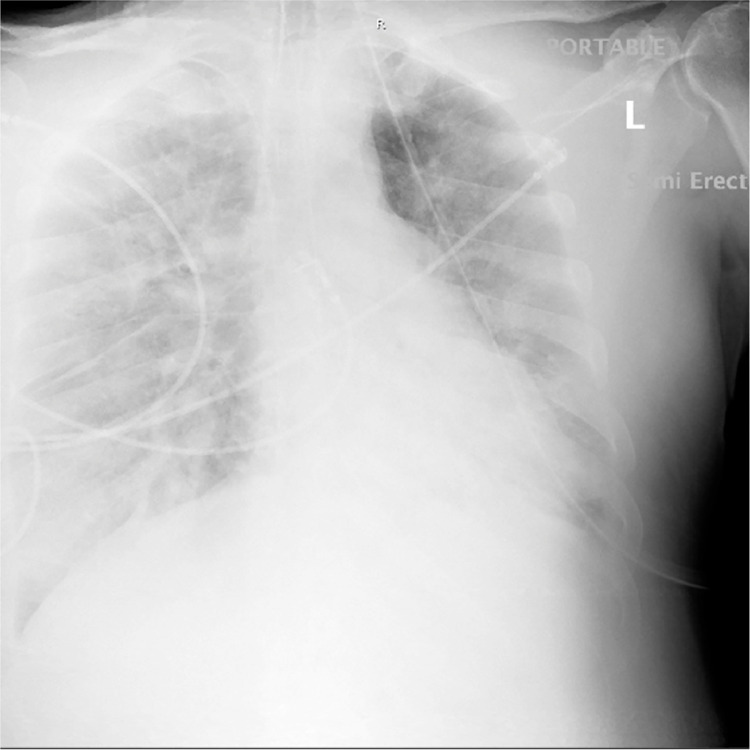
The patient continued to exhibit worsening oxygenation with significant bilateral pulmonary infiltrates (Fig. 3 ), worse in the right lung. Antibiotic therapy was broadened to cover hospital acquired pneumonia with piperacillin 4g/tazobactam 500mg every 8 h, vancomycin to be dosed by pharmacy for fluctuating renal function, and methylprednisolone 40 mg every 8 h was added for pulmonary inflammation. As renal function worsened nephrology was consulted, and a right femoral Quinton catheter was placed for CRRT.
Fig. 3.
Chest x-ray on hospital day 6 with persistent hypoxia and fever. The patient continued to demonstrate bilateral lung infiltrates, but there is a new appearing consolidation predominantly in the right lung base.
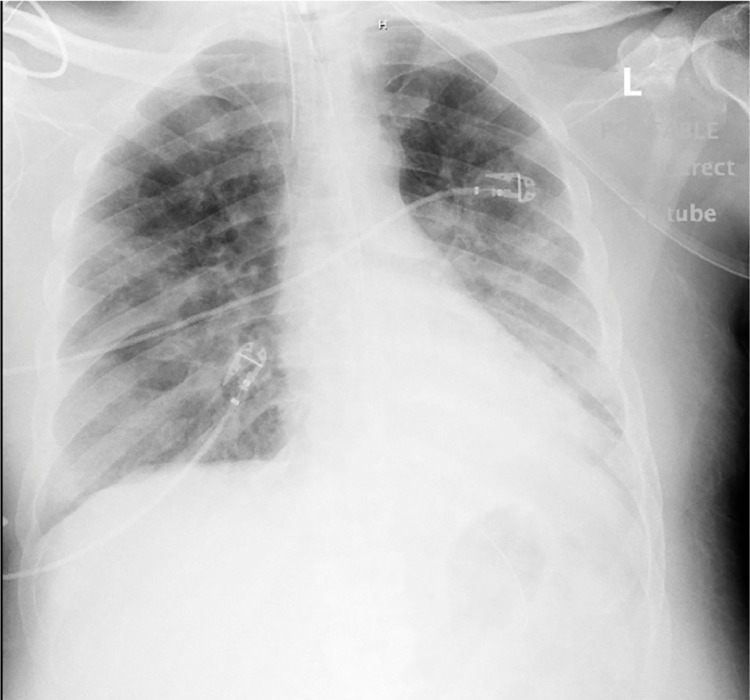
The progression of the patient's COVID disease began to mimic other patterns the team had seen that often resulted in cytokine storm and eventual mortality, so the team considered applying a cytokine filter before the cytokine storm became insurmountable. In combination with nephrology we sought to obtain emergency Internal Review Board(IRB) approval for compassionate use to protect the lungs and kidneys from further damage secondary to cytokine storm. Approval from the IRB and family occurred on Hospital day 11 and the cytokine filter was shipped to our location. Initiation of the Cytosorbents® filter included a live educational webinar to the ICU team and live video assistance from the manufacturer of installation of the device to the standard CRRT machine. Chest xray prior to initation of the filter showed improved aeration (Fig. 4 ).
Fig. 4.
Chest x-ray on hospital day 11 which coincided with the first day of cytokine filter therapy showing persistence of infiltrates, improved aeration and improved right lower lobe consolidation.
We obtained the filters from Cytosorbents® (Seven Deerpark Drive, Suite K., Monmouth Junction, NJ 08,852). The initial protocol required filter change every 12 h for the first 48 h followed by every 24 h. The flow rate of the filter was consistent at 200 cc/minute blood flow throughout the therapy. A total of 8 devices were used and thus the expected protocol of changing the filter every 12 h was extended to 24 h. He received the cytokine filter therapy and CRRT spanning twelve days lest one due to hemodynamic instability requiring temporary halt four days into the treatment. That day the patient went into shock and required pressor support for seven more days. The length of treatment was based on recommendations by Cytosorbents® with the number of devices sent for this patient's treatment.
Upon initiating cytokine filter therapy, his respiratory status, hemodynamics, inflammatory markers, and labs were monitored. Table 1 describes the lung parameters measured during the filter therapy with trends including ventilator settings and ABG results on a daily basis. Between day 2–3 in which the oxygen requirements began to decrease, the patient did develop hemodynamic instability which is discussed below. His PEEP was decreased from 14 to 5 through the span of the therapy. His-ABG did show persistent hypoxia on hospital day 16 which resulted in the patient being paralyzed with vecuronium for two days after which his oxygenation was above 100 at minimal PEEP (5) and FiO2 of 40%.
After twenty-four hours on CRRT with cytokine filter (hospital day #12), the patient was noted to be hypothermic requiring warm blankets and fluids. The subsequent day the patient went into shock with persistent tachycardia, hypotension, and hemodynamic instability. Previously, mean arterial pressures) Backspace (MAPS) were between 85 and 90 mmHg however now dropped persistently to MAPS of 45 mmHg. Bedside echocardiogram was done to assess for potential causes of shock. He did have a reduced ejection fraction without focal area of hypokinesis. There was no evidence of right ventricular strain and he was already adequately anticoagulated with IV heparin therapy. Furthermore, there was no evidence of active bleeding (i.e. blood in foley catheter, stool, pulmonary secretions, or expanding hematoma in femoral central access site) to suggest hemorrhagic etiology. He was responsive to fluid boluses and pressor support suggesting hypovolemia. He was started on norepinephrine drip and subsequently epinephrine drip which improved his hemodynamic instability, and this improved his ejection fraction on bedside echocardiography. Discussion was made with nephrology to halt CRRT at this time and fluid resuscitate the patient. It was apparent that there was some difficulty in compatibility of the Cytosorbents® filter with the conventional CRRT hollow fiber filter. Subsequently, the filter was placed pre-CRRT filter and it appeared to have resolved this issue. The following day, CRRT with cytokine filter was reinstated. His ABG showed persistant hypoxia on hospital day #16, after which he was paralyzed with vecuronium until hospital day #18 when his oxygenation requirement was PEEP of 5 and FIO2 of 40%.
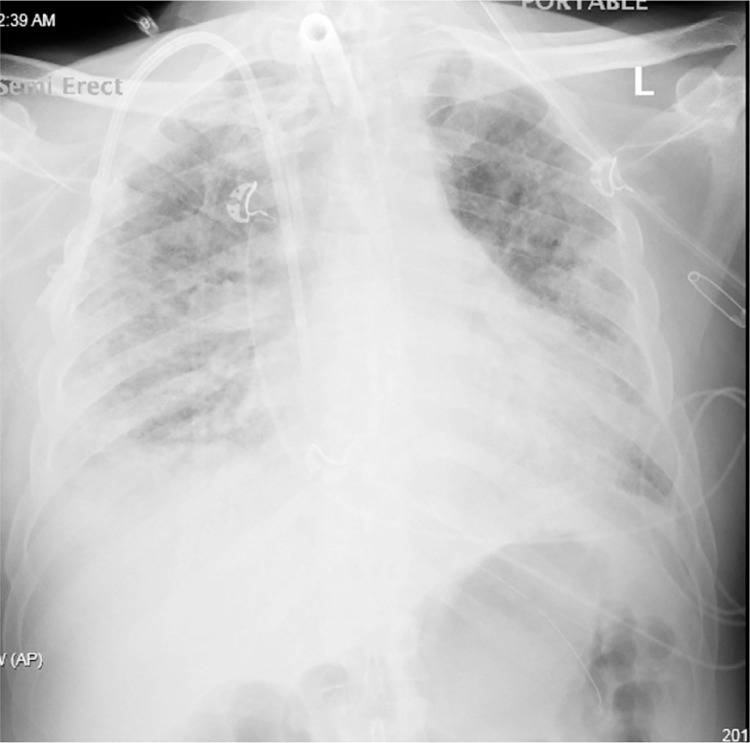
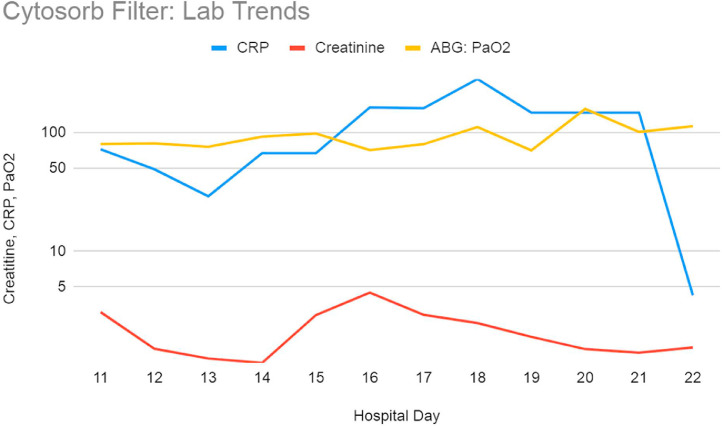
Laboratory results were monitored daily as well. Standard testing including acute phase reactants were also periodically evaluated to determine if they were trending in the right direction. These included standard blood counts and chemistry, ferritin, C-reactive protein, d-dimer, and IL-6 level. The IL-6 level was obtained twice and remained stable at 5 on day 0 and 2 of filter therapy with a reference range of 0–16.4 pg/mL. While unable to monitor IL-6 as frequently as desired, the patient's clinical picture resembled that of cytokine storm and because the filter was in place, we were able to maintain hemodynamic stability with pressors and volume support. In other cases when we have witnessed cytokine storm, the patients were non responsive to pressors and volume and developed severe acidosis. Once he was weaned off of pressors (approximately seven days of pressor support total), he began tolerating higher volume removal via CRRT. His-hemoglobins initially remained stable but decreased on day 15 without any active signs of bleeding. Although at this point he had received IV heparin therapy for a total of 5 days. His-D-dimer remained persistently elevated to beyond the highest range coinciding with hypercoagulable state with this illness. His-renal function on the day of initiation was poor with Creatinine of 3.07 which improved daily with therapy. On hospital day 15, along with the significant hemoglobin drop as mentioned above, his renal function also worsened from 2.9 to 4.48. Additionally, chest xray showed worsening infiltrates (Fig. 5 ). His-CRP which was initially downtrending also went up significantly higher than the value on the day of initiation. However, after the setback on this day his status began to stabilize and by the last day of therapy, his CRP was within normal range, renal function had improved, and hemoglobin remained low but stable. Table 3 discusses the daily hemodynamics, pressor requirements (with dose, if applicable) and relevant laboratory results. Fig. 6 is a graphic representation of the patient's laboratory trends while on the cytokine filter.
Fig. 5.
Chest x-ray hospital day 16, 5 days after the initiation of cytokine filter when the patient developed worsening hypoxia and increased oxygen requirements as indicated in Table 2 below. Compared with prior x-ray there is worsening of bilateral pneumonia with interstitial and alveolar opacities.
Table 3.
Daily hemodynamic monitoring, pressor support requirement and laboratory results.
| Hospital Day | Ref Range | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Filter Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| MAP | 89 | 90 | 70–90 | 60–80 | 60–70 | 60–80 | 60–70 | 60–70 | 60–70 | 70–80 | 70–80 | 75–80 | |
| Pressors? Y/N | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | |
| Norepinephrine (mcg/kg/min) | 0.06 | 0.07 | |||||||||||
| Phenylephrine (mcg/min) | 70 | 55 | |||||||||||
| Epinephrine (mcg/kg/min) | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||||
| Volume In | ml | 2540 | 4873 | 5945 | 6401 | 6001 | 1912 | 6474 | 7923 | 7012 | 6752 | 6737 | 6280 |
| Volume Out | ml | 1070 | 4653 | 6391 | 5798 | 1133 | 532 | 1446 | 3884 | 2514 | 7754 | 7968 | 7656 |
| NET | 1470 | 220 | −446 | 603 | 4868 | 1380 | 5028 | 4039 | 4498 | −1002 | −1231 | −1376 | |
| Lactate | 0.5–2.2 mmol/L | 1.73 | 1.49 | 0.86 | 0.79 | ||||||||
| Hgb | 12–17.3 g/dL | 9.3 | 11.4 | 13 | 11.8 | 7.1 | 8.2 | 8.9 | 7.8 | 7.7 | 7.9 | 7.6 | 7.9 |
| WBC | 4.5–11 K/cmm | 8.8 | 8.3 | 14 | 16.5 | 10.6 | 10.7 | 8.3 | 3 | 2.6 | 3.2 | 3.7 | 7.9 |
| PLT | 140–440 K/cmm | 177 | 177 | 231 | 278 | 249 | 284 | 246 | 199 | 194 | 198 | 227 | 257 |
| D dimer | 0–500 ng/ml | >7650 | >7650 | >7650 | >7650 | >7650 | >7650 | >7650 | >7650 | 7277 | >7650 | ||
| Albumin | 3.5–5 g/dL | 2.7 | 2.4 | 2.4 | 2.9 | 2.8 | 2.3 | 2.2 | 2.2 | 2.2 | 2.2 | 2.4 | |
| CRP | 0–5 mg/L | 72.22 | 48.9 | 29 | 66.89 | 162.7 | 160.23 | 282 | 146.78 | 4.25 | |||
| Ferritin | 24–336 ng/mL | 2000 | 3277 | 3818 | 4004 | 2748 | 2785 | 2135 | |||||
| Creatinine | 0.61–1.25 mg/dL | 3.07 | 1.51 | 1.25 | 1.15 | 2.9 | 4.48 | 2.91 | 2.48 | 1.9 | 1.5 | 1.4 | 1.55 |
| GFR | >60 ml/min/1.73 sqm | 26 | 59 | 74 | 81 | 38 | 17 | 38 | 33 | 45 | 60 | 65 | 48 |
Fig. 6.
Graphic representation of lab trend during cytokine filter therapy as indicated by hospital day 11 coinciding with the first day of therapy and completion of therapy on hospital day 22.
On hospital day 18, he developed DVT in his right upper and lower extremity, consistent with the laterality that included his femoral quinton and picc line for intravenous access. Despite adequate anticoagulation initially with heparin drip when the two blood clots developed, he was switched to argatroban drip due to persistent clotting and still developed DVT in all four extremities by day twenty-two, or the last day of the filter therapy. When the CRRT and cytokine filter therapy was discontinued, the patient was switched over to hemodialysis for renal replacement.
Follow up and outcome
Having survived the cytokine storm and sepsis associated with COVID-19 the patient was able to tolerate further novel treatment modalities. The patient received tocilizumab 400 mg on hospital day 18 and 19. He also received one unit of convalescent plasma on hospital day 28. After 21 days on the ventilator the patient was extubated, however, had to be reintubated within 24 h. A tracheotomy was performed for respiratory management and the patient tolerated that procedure well and began to communicate with hand motions and facial gestures. The next several days of his admission were uneventful and he was receiving supportive care and PEG tube for nutritional support.
On hospital day #39 the patient suffered a PEA arrest that resulted in return of spontaneous circulation after three rounds of compressions and 1 mg of epinephrine. This was almost three weeks after the cytokine filter therapy was discontinued. It was suspected due to hypoxia as he disconnected himself from the ventilator just prior to the code blue. He had complete neurological recovery from this code blue once oxygenation had improved.
Behavioral health was consulted and recommended sertraline 100 mg daily and quetiapine 25 mg daily for treatment of severe depression and anxiety secondary to extensive hospital stay. On hospital day #47 blood was noted from his nasogastric tube and he had worsening anemia requiring blood transfusion (hemoglobin of 6.7). Gastroenterology team was consulted and an urgent esophagogastroduodenoscopy (EGD) revealed that he developed gastric ulcers. This made anticoagulation complicated since he has a thrombogenic disease process resistant to heparin requiring him to be on argatroban for several weeks. The patient is still alive today and was discharge to a subacute rehab facility after 60 days in the hospital. He is motivated to continue with therapy and improve over a long period of time given the active lifestyle he held prior to hospitalization.
Conclusion & discussion
FDA requirements for emergency use criteria include (1) the patient has a life threatening or serious disease or condition that needs immediate treatment; (2) no generally acceptable alternative treatment for the condition exists; and (3) because of the immediate need to use the device, there is no time to use existing procedures to obtain FDA approval for the use. Cytosorbents® required that a patient's lung disease progressed to a severe state and evidence of multiorgan dysfunction was present. It can be speculated that the substantial lung damage may have already occurred prior to the initiation of the therapy and may not resolve quickly or even completely. We believe that earlier application of blood filtering could potentially mitigate cytokine storm before the possibly irreversible damage occurs. This may allow for better recovery. The application of a cytokine filter therapy has shown great potential benefit and it would be worth-while to obtain more studies in regard to this therapy for future use.
This patient was a young 51-year-old male with a past medical history of diabetes that had COVID-19 exposure, respiratory symptoms, and hypoxia. Throughout his hospitalization, he developed ventilator dependent respiratory failure, received hydroxychloroquine, azithromycin as well as other supportive measures including vitamin C and zinc. Despite this he continued toward cytokine storm, AKI and was selected for cytokine filter via CRRT. He has had a very long hospital course and was discharged to a subacute rehab facility on hospital day 60. Upon discharge, he was tracheostomy dependent with pressure support only at night. He required enteral feedings due to persistent dysphagia and aspiration risk. He will still require hemodialysis three times per week. Overall, the cytokine filter was by no means a curative therapy. We believe it was a bridge for the patient to prevent imminent death due to severe inflammatory response and extend his life. The patient was most critically ill in terms of ventilator requirements and pressor support just prior to each of the two courses of the cytokine filter therapy and seemed to stabilize correlating to the initiation of blood filtration with oxygen requirements, pressor support and inflammatory markers. He received Tocilizumab IV and convalescent plasma. His-clinical course was complicated further by a hypoxic cardiopulmonary arrest, upper GI bleed and severe depression and anxiety. Given the severity of his illness to begin with, the rapid progression toward cytokine storm and the subsequent medical complications he endured, I believe that the cytokine filter successfully completed the goal of prolonging his life to bridge definitive therapy in cytokine storm.
Declaration of Competing Interest
Authors have no conflicts of interest to declare.
References
- 1.Anderson Mark. Brita Filter for Blood’ Aims to Remove Harmful Cytokines for COVID-19 Patients. IEEE Spectrum: Technology, Engineering, and Science News. 26 Mar. 2020 spectrum.ieee.org/the-human-os/biomedical/devices/blood-filtration-tech-removes-harmful-cytokines-covid19-patients. [Google Scholar]
- 2.Bruenger Frank. First Successful Combination of ECMO with Cytokine Removal Therapy in Cardiogenic Septic Shock: a Case Report. Int. J. Artif. Organs. 2015;38(2):113–116. doi: 10.5301/ijao.5000382. [DOI] [PubMed] [Google Scholar]
- 3.Fanelli Vito, Vlachou Aikaterini. Acute Respiratory Distress Syndrome: new Definition, Current and Future Therapeutic Options. Journal of Thoracic Disease. June 2013;5(3):326–334. doi: 10.3978/j.issn.2072-1439.2013.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Yuefei. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 27 Mar. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John Hopkins, University. “CORONAVIRUS RESOURCE CENTER.” Johns Hopkins University & Medicine, coronavirus.jhu.edu/. Domain record activated: 19-Mar-1987. Accessed 31 May 2020.
- 6.Kivela Paul. Paradigm Shift for COVID-19 Response: identifying High-Risk Individuals and Treating Inflammation. WestJEM 21.3 May Issue Western Journal of Emergency Medicine. 2020;21(3) doi: 10.5811/westjem.2020.3.47520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolia Vaishal. Preliminary Results of Initial Testing for Coronavirus (COVID-19) in the Emergency Department. WestJEM 21.3 May Issue Western Journal of Emergency Medicine. 2020;21(3) doi: 10.5811/westjem.2020.3.47348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]