Abstract
Objective
We conducted a phenotype-wide association study (PheWAS) to compare diagnoses among Blacks with those of Whites in one health center in Tennessee using data from 1,883,369 patients.
Methods
We used our deidentified EHR, the Synthetic Derivative, to assess risk of diagnoses associated with Black as compared with White race using Firth logistic regression with covariates including age, sex, and density of clinical encounters.
Results
There were anchoring associations in both directions, including the highest increased risk for Blacks of having sickle cell anemia, and strongest decreased risk of basal cell carcinoma. Results included established areas of disparity and many novel associations.
Conclusions
PheWAS is a viable tool for calculating risk associated with any biomarker. The current analysis provide a new approach to generating hypotheses and understanding the breadth of health disparities. Future analyses will further explore causality, risk factors, and potential confounders not accounted for here.
Keywords: Health disparities, Racial disparities, Phenome-wide association study
Introduction
Health and healthcare disparities, and their myriad influences on the wellbeing of individuals in affected groups, are a major focus of initiatives in the United States. The National Institute on Minority Health and Health Disparities designated health disparity populations include “racial/ethnic minorities, socioeconomically disadvantaged populations, underserved rural populations, and sexual and gender minorities.”1 Health disparities are complex, and we know in particular that racial health disparities are multifactorial and that the ‘variable’ of race is often correlated with other factors (e.g., socioeconomic level, experience of discrimination, habits, health system interactions), each of which can independently and interdependently influence health.
Thoughtful design of strategies to mitigate the untoward effects of disparities requires a sound understanding of both the scope and magnitude of health disparities affecting a group. The phenome-wide association study (PheWAS) provides a powerful and validated methodology for visualizing the effect of an exposure on the relative risk of all diagnoses documented in the electronic health record (EHR).2 , 3 While most commonly used to explore effects of genetic variation, PheWAS is readily adaptable to explore effects of other exposures such as race.
To aid in estimating the breadth of racial health disparities, we conducted a PheWAS to compare diagnoses among Blacks with those of Whites at one health center in Tennessee. The analysis was not undertaken to ignore the importance of other factors. Rather, it was intended to assess variance in disease risk holistically, across many diseases, to: 1) visualize and obtain insight into the overall phenome-wide burden, 2) evaluate concordance between individual disease risk in the PheWAS analysis compared to established disparity to demonstrate utility; and 3) identify disparities among rarer diseases that might be overlooked in public health literature.
Materials and methods
We extracted diagnoses for all individuals with documented White or Black race from our Derivative (SD), a deidentified version of the entire EHR at our medical center.4 Our EHR currently includes more than 3 million patients of all ages; the current study extracted data from 1990 to 2019. We employed PheWAS to analyze variability in risk of all documented diagnoses associated with Black as compared with White race using demographic data from the SD (99% accuracy compared to genetic ancestry5). International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10-CM codes were converted to phenotype codes (phecodes).6 , 7 For each phecode, a case was defined as having a minimum of two phecodes on different dates; controls were those having no related phecodes as is standard. Firth logistic regression was performed using R with covariates including age, sex, and the number of ages with a clinical encounter recorded. We report associations using the Bonferroni corrected p value of 2.7 × 10−5 (minimum detectable bound p = 5 × 10−324). For comparisons with the published literature, we extracted published odds ratios (OR) or calculated relative risk comparing Blacks and Whites.
Results and discussion
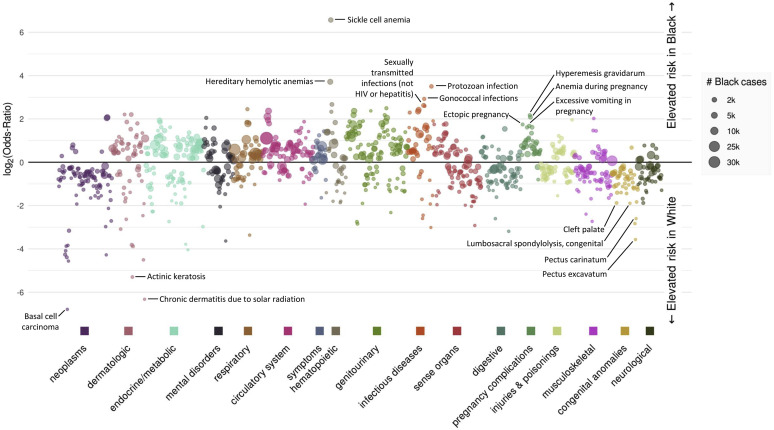
Figure 1 illustrates the phenome wide results, including phenotypes with increased (top section) or decreased risk (bottom section) among Blacks as compared with Whites, representing a diverse range of disease types and affected organ systems. A dynamic version of the PheWAS results, with hover-over labels for all phenotypes, is available online at https://prod.tbilab.org/phewas_race.
Figure 1.
Manhattan plot of PheWAS results comparing phenotype risk between Blacks and Whites. The plot illustrates represents log odds ratio for phenotypes with increased or decreased odds among Blacks as compared with Whites; significance is set at the Bonferroni-corrected threshold of p < 2.7 × 10−5.
Dataset characteristics and anchoring
Our analysis included 1,883,369 patients, including 269,872 Blacks and 1,613,497 Whites. Mean age at last encounter was 37.8 years (range 0-90 years). Approximately 52.8% (n = 994,930) were females and 47.2% (n = 888,439) were males. We found anchoring associations as the absolute strongest OR in both directions in the data, including the highest increased risk for Blacks of sickle cell anemia8 (OR 94.7; 95% CI 79.14, 114.51; p < 5 x 10–324), and strongest decreased risk of basal cell carcinoma9 (OR 0.009; 95% CI 0.005, 0.01; p < 5 x 10–324). Agreement with previous research estimating risk magnitude was also apparent (Table 1 ). Notably, almost all pregnancy complications were higher risk in Black women whereas many congenital anomalies carried higher risk in Whites. There were some apparent areas of discordance. For example, the risk of low birth weight among Blacks was lower in our data (OR 1.30; 95% CI 1.25, 1.35; p < 5 x 10–324) as compared with the literature, while the odds of end stage renal disease was greater (OR 5.2; 95% CI 4.9-5.4; p < 5 x 10–324). Further, though the odds of diabetes or cerebrovascular disease were similar between our results and the literature, the odds of downstream sequelae including diabetic retinopathy and end stage renal disease were larger among Blacks in our data than estimates reported elsewhere.
Table 1.
Odd ratios (ORs) of selected diagnoses among blacks compared with whites.
| Phecode description | PheWAS OR | Relative risk estimate from the literature | Source, relative risk data |
|---|---|---|---|
| Morbid obesity | 1.9 | 2.0 | Sturm and Hattori, 201315 |
| Preeclampsia and eclampsia | 2.2 | 1.6 | Fingar et al., 201716 |
| Hypertension | 2.2 | 1.4 | Office of Minority Health17 |
| Diabetes | 1.7 | 1.6 | Office of Minority Health18 |
| Systemic lupus erythematosus | 1.8 | 3a | CDC19 |
| Senile dementia | 3.0 | 2.6 | Chen and Zissimopoulos, 201820 |
| Cerebrovascular disease | 1.5 | 1.5 | Office of Minority Health21 |
| Low birth weighta | 1.3 | 2.4 | Ratnasiri et al., 201822 |
| End stage renal disease | 5.2 | 3.5 | Office of Minority Health18 |
| Diabetic retinopathy | 2.7 | 1.6 | Zhang et al., 201023 |
This OR estimate is based on comparison of risk among women, consistent with the predominance of this disease among females.
Greatest risk among Blacks across the phenome
The disease categories with the greatest racial disparity (as indicated by the highest ORs) include HIV, end stage renal disease, hypertension, uterine fibroids, diabetes, sarcoidosis, asthma, atherosclerosis, and glaucoma (Tables 1 and 2). PheWAS recapitulated widely established areas of disparity, but also identified several diseases for which significant disparities do not appear to be as well studied or understood.
Table 2.
Risk of additional anchoring, immune-related, mental health, or rare disease diagnoses among blacks compared with whites.
| Category | Phecode | Phenotype | Odds ratio | Confidence interval | P-value |
|---|---|---|---|---|---|
| Additional anchoring diagnoses | 071 |
HIV |
5.08 |
(4.84, 5.32) |
<5 x 10–324 |
| 218.1 | Uterine leiomyoma | 4.21 | (4.03, 4.39) | <5 x 10–324 | |
| 697 | Sarcoidosis | 3.62 | (3.32, 3.95) | <5 x 10–324 | |
| 495 | Asthma | 2.06 | (2.02, 2.10) | <5 x 10–324 | |
| 440 | Atherosclerosis | 1.40 | (1.32, 1.48) | <5 x 10–324 | |
| 365 | Glaucoma | 2.41 | (2.33, 2.50) | <5 x 10–324 | |
| Immune-related diseases | 695.4 |
Lupus |
1.87 |
(1.75, 2.00) |
<5 x 10–324 |
| 704.11 | Alopecia areata | 1.66 | (1.43, 1,92) | <1.56 x 10–10 | |
| 709.4 | Polymyositis | 2.55 | (2.04, 3.15) | <5.66 x 10–15 | |
| 250.1 | Type 1 diabetes | 1.30 | (1.25, 2.35) | <5 x 10–324 | |
| 242.1 | Graves' disease | 1.30 | (1.20, 1.41) | <7.04 x 10–10 | |
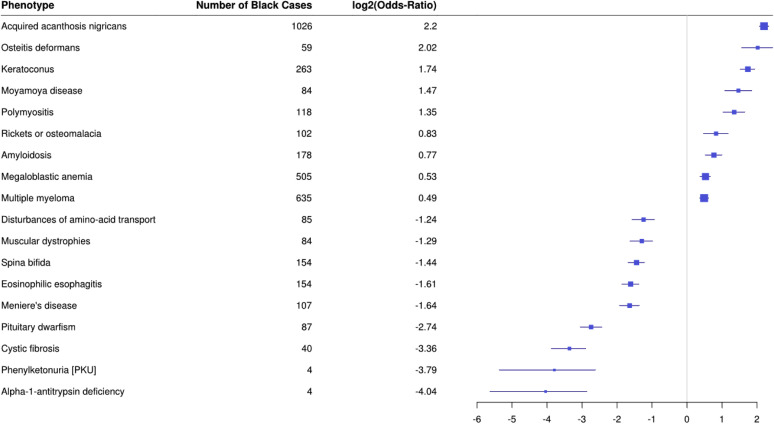
| Rare diseases | 364.41 |
Keratoconus |
3.34 |
(2.87, 3.88) |
<5 x 10–324 |
| 731.1 | Paget's disease | 4.05 | (2.96, 5.48) | <1.89 x 10–15 | |
| 709.4 | Polymyositis | 2.55 | (2.04, 3.15) | <5.66 x 10–15 | |
| 433.32 | Moyamoya disease | 2.77 | (2.11, 3.61) | <2.81 x 10–12 | |
| 204.4 | Multiple myeloma | 1.40 | (1.28, 1.54) | <2.02 x 10–12 | |
| 281.1 | Megaloblastic anemia | 1.44 | (1.30, 1.59) | <3.03 x 10–11 | |
| 270.33 | Amyloidosis | 1.70 | (1.43, 2.00) | <3.61 x 10–9 | |
| 261.41 | Rickets | 1.78 | (1.39, 2.26) | <8.53 x 10–6 | |
| 270.34 | Alpha-1-antitrypsin deficiency | 0.06 | (0.02, 0.14) | <5 x 10–324 | |
| 499 | Cystic fibrosis | 0.10 | (0.07, 0.13) | <5 x 10–324 | |
| 270.12 | Phenylketonuria | 0.07 | (0.02, 0.16) | <5 x 10–324 | |
| 253.5 | Pituitary dwarfism | 0.15 | (0.12, 0.19) | <5 x 10–324 | |
| 386.1 | Meniere's disease | 0.32 | (0.26, 0.39) | <5 x 10–324 | |
| 530.15 | Eosinophilic esophagitis | 0.33 | (0.28, 0.39) | <5 x 10–324 | |
| 752.11 | Spina bifida | 0.37 | (0.31, 0.43) | <5 x 10–324 | |
| 359.1 | Muscular dystrophies | 0.41 | (0.32, 0.51) | <5 x 10–324 | |
| 270.1 | Disturbances of amino-acid transport | 0.42 | (0.34, 0.53) | <5 x 10–324 | |
| Mental health diseases | 295.1 | Schizophrenia | 2.99 | (2.82, 3.17) | <5 x 10–324 |
| 290.1 |
Dementias |
2.06 |
(1.95, 2.18) |
<5 x 10–324 |
|
| 312 | Conduct disorders | 1.89 | (1.81, 1.99) | <5 x 10–324 | |
| 295.3 | Psychosis | 1.84 | (1.72, 1.96) | <5 x 10–324 | |
| 292.6 | Hallucinations | 1.76 | (1.58, 1.96) | <5 x 10–324 | |
| 305.21 | Anorexia nervosa | 0.08 | (0.05, 0.12) | <5 x 10–324 | |
| 300.3 | Obsessive-compulsive disorders | 0.24 | (0.20, 0.28) | <5 x 10–324 | |
| 300 | Anxiety disorders | 0.59 | (0.58, 0.61) | <5 x 10–324 | |
| 313.2 | Tics and stuttering | 0.49 | (0.42, 0.58) | <5 x 10–324 | |
| 301 | Personality disorders | 0.58 | (0.52, 0.65) | <5 x 10–324 | |
| 296.1 | Bipolar | 0.73 | (0.70, 0.77) | <5 x 10–324 | |
| 313.3 | Autism | 0.74 | (0.70, 0.78) | <5 x 10–324 | |
| 296 | Mood disorders | 0.78 | (0.77, 0.80) | <5 x 10–324 | |
| 313 | Pervasive developmental disorders | 0.86 | (0.84, 0.89) | <5 x 10–324 |
The neoplasm category of phecodes showed the lowest relative volume of phenotypes among Blacks, with seven phenotypes with higher risk among Blacks and 89 neoplasm-related phenotypes with higher risk among Whites. High risk disease categories with the largest number of patients (shown by the size of the circle in Figure 1) generally conform to known prevalence among Blacks (although PheWAS represents health system data, not the general public) include those in Table 2 . In addition, the risk of readily remediable high health disparity conditions, such as vitamin D deficiency (OR 1.54; 95% CI 1.49, 1.58; p < 5 x 10–324, remains apparent.
Other less well reported phenotypes with sizable populations included dermatophytosis (OR 2.00; 95% CI 1.89, 2.12; p < 5 x 10–324, iron deficiency anemia (OR 2.61; 95% CI 2.53, 2.71; p < 5 x 10–324), and fever of unknown origin (OR 1.67; 95% CI 1.64, 1.70; p < 5 x 10–324). While these are likely related to underlying disease such as diabetes, other immune dysfunction, or sickle cell, their appearance in the data might be reflecting the known ripple effect of disparities; that is, that individual health disparities are compounded, producing new, incremental increases in comorbidities over time in the Black population.
Immune-related, rare, and mental health diseases among blacks
Phenotypes carrying risks of immunocompromise, which may be particularly relevant in times of community outbreaks of communicable disease, are also notable. In addition to HIV and type 2 diabetes, we also see increased risk of various autoimmune diseases conferring risk of immune compromise due to the disease process and/or need for immunosuppressing treatment regimens (Table 2). Less reported in the public health literature than common diseases, Blacks have an increased risk of many rare diseases (Table 2 and Figure 2 ). Blacks in this analysis also have an increased risk of many psychiatric diagnoses (Table 2).
Figure 2.
Forest plot of PheWAS results comparing phenotype risk between Blacks and Whites among rare diseases. The plot illustrates represents log odds ratio for rare disease phenotypes with increased or decreased odds among Blacks as compared with Whites; significance is set at the Bonferroni-corrected threshold of p < 2.7 × 10−5.
Utility of PheWAS in assessing relative risk
Using a large disease-agnostic, real world database of diagnoses, we applied PheWAS which can calculate risk associated with any biomarker (here, we used race as the social construct). The data are credible, recapitulating known relative risk. Appropriate disease complexity is reflected (such as, a cluster of pregnancy-related complications). Long-term consequences of risk factors (e.g., cerebral atherosclerosis) are also present in the data (e.g., dementia).
The spectrum of risks noted above are concordant with those inducing increased risk of COVID-19 infection: hypertension, diabetes, heart disease, asthma, obesity, and immune compromising conditions are likely playing a significant role in the increased COVID-19 disease severity and mortality experienced by Blacks in communities across the United States. The implications of these issues are potentially further worsened by delayed or cancelled health visits among those who cannot access telehealth formats.
Limitations
All of the limitations of the PheWAS method apply to this work, and have been described.2 We note several of particular relevance to the current report. First, these codes do not separate biologic risk from risks associated with systematic differences in health system factors such as utilization or diagnostic biases; for example, the differences in mental health conditions are also concordant with previous literature on systemic biases in diagnoses among Blacks as compared with Whites.10, 11, 12, 13
Other important factors also affect health and healthcare disparities and may lead to selection bias, including access to care, trust in the health system, and insurance status. For example, we observed many fewer diagnostic codes indicating neoplasms among Blacks, in contradiction with the published literature. This discrepancy is perhaps explained at least in part by insurance characteristics; many of our cancer clinics do not accept Medicaid; further, cancer incidence, as estimated in the current study, and mortality are different issues. Incorporation of data representing additional key exposures and outcomes such as these into future modeling will further inform our discussion of the breadth and implications of health and healthcare disparities. Despite these limitations, PheWAS represents a useful complement to existing approaches to visualizing health disparities, can highlight diseases of particular relevance to various audiences, and aid in decision making regarding high priority health disparities research and other programs.
Implications
As stated, any given disease can have many individual (but not independent) risk factors such as genetics, socioeconomics, lifestyle, healthcare access, stressors, environmental exposures, and many others. But these factors converge in the Black population to produce drastically poorer health. All of the multifactorial risks that correlate with race and contribute to poorer health are implicitly included within the aggregate results described above, experienced in the real world in their composite by the individuals whose diagnoses comprise these data. As health systems charged with maintaining the health of the public, we need to better understand and recognize the overwhelming disparity that exists among Black patients, both a single disease at a time, and in their totality. Indeed, the preponderance of health risk in Blacks culminates in variable longevity; Whites live on average 4 years longer than Blacks.14 Poorer health is an important driver of that loss of life, with socioeconomic and other factors being principal underlying components.
Funding
The project described was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Conflict of interest
None.
Acknowledgements
The authors express their sincere gratitude to Siwei Zhang for data analysis and graphics development, and to Ingrid Mayer, Tuya Pal, and Xiao-ou Shu for sharing clinical insights.
References
- 1.Alvidrez J., Castille D., Laude-Sharp M., Rosario A., Tabor D. The national Institute on minority health and health disparities research framework. Am J Public Health. 2019;109(Suppl 1):S16–S20. doi: 10.2105/AJPH.2018.304883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denny J.C., Ritchie M.D., Basford M.A. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny J.C., Bastarache L., Ritchie M.D. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31(12):1102–1110. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danciu I., Cowan J.D., Basford M. Secondary use of clinical data: the Vanderbilt approach. J Biomed Inform. 2014;52:28–35. doi: 10.1016/j.jbi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall J.B., Dumitrescu L., Dilks H.H., Crawford D.C., Bush W.S. Accuracy of administratively-assigned ancestry for diverse populations in an electronic medical record-linked biobank. PLoS One. 2014;9(6):e99161. doi: 10.1371/journal.pone.0099161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei W.-Q., Bastarache L.A., Carroll R.J. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS One. 2017;12(7):e0175508. doi: 10.1371/journal.pone.0175508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu P., Gifford A., Meng X. Mapping ICD-10 and ICD-10-CM codes to phecodes: workflow development and initial evaluation. JMIR Med Inform. 2019;7(4):e14325. doi: 10.2196/14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC . Centers for Disease Control and Prevention; 2016. Data & Statistics on Sickle Cell Disease | CDC.https://www.cdc.gov/ncbddd/sicklecell/data.html [Google Scholar]
- 9.Guy G.P., Thomas C.C., Thompson T. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–596. [PMC free article] [PubMed] [Google Scholar]
- 10.Akinhanmi M.O., Biernacka J.M., Strakowski S.M. Racial disparities in bipolar disorder treatment and research: a call to action. Bipolar Disord. 2018;20(6):506–514. doi: 10.1111/bdi.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medlock M., Weissman A., Wong S.S. Racism as a unique social determinant of mental health: development of a didactic curriculum for psychiatry residents. MedEdPORTAL. 2017;13 doi: 10.15766/mep_2374-8265.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCoux Hampton M. The role of treatment setting and high acuity in the overdiagnosis of schizophrenia in African Americans. Arch Psychiatr Nurs. 2007;21(6):327–335. doi: 10.1016/j.apnu.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz R.C., Blankenship D.M. Racial disparities in psychotic disorder diagnosis: a review of empirical literature. World J Psychiatry. 2014;4(4):133–140. doi: 10.5498/wjp.v4.i4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bond M.J., Herman A.A. Lagging life expectancy for black men: a public health imperative. Am J Public Health. 2016;106(7):1167–1169. doi: 10.2105/AJPH.2016.303251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm R., Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes. 2013;37(6):889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingar K., Mabry-Hernandez I., Ngo-Metzger Q., Wolff T., Steiner C., Elixhauser A. Agency for Healthcare Research and Quality; 2017. Delivery Hospitalizations Involving Preeclampsia and Eclampsia, 2005-2014 #222.https://hcup-us.ahrq.gov/reports/statbriefs/sb222-Preeclampsia-Eclampsia-Delivery-Trends.jsp?utm_source=ahrq&utm_medium=en-1&utm_term=&utm_content=1&utm_campaign=ahrq_en4_25_2017 [PubMed] [Google Scholar]
- 17.Heart disease and African Americans - the office of minority health. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=19
- 18.Diabetes and African Americans - the office of minority health. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=18
- 19.Lupus in women | CDC. https://www.cdc.gov/lupus/basics/women.htm
- 20.Chen C., Zissimopoulos J.M. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y) 2018;4:510–520. doi: 10.1016/j.trci.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroke and African Americans - the office of minority health. https://www.minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=28
- 22.Ratnasiri A.W.G., Parry S.S., Arief V.N. Recent trends, risk factors, and disparities in low birth weight in California, 2005–2014: a retrospective study. Maternal Health Neonatol Perinatol. 2018;4(1):15. doi: 10.1186/s40748-018-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X., Saaddine J.B., Chou C.-F. Prevalence of diabetic retinopathy in the United States, 2005-2008. J Am Med Assoc. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]