Abstract
Background:
Focal Electrically-Administered Seizure Therapy (FEAST) is a form of electroconvulsive therapy (ECT) that spatially focuses the electrical stimulus to initiate seizure activity in right prefrontal cortex. Two open-label non-comparative studies suggested that FEAST has reduced cognitive side effects when compared to historical data from other forms of ECT. In two different ECT clinics, we compared the efficacy and cognitive side effects of FEAST and Right Unilateral Ultrabrief Pulse (RUL-UBP) ECT.
Methods:
Using a non-randomized, open-label design, 39 depressed adults were recruited after referral for ECT. Twenty patients received FEAST (14 women; age 45.2±12.7), and 19 received RUL-UBP ECT (16 women; age 43.2±16.4). Key cognitive outcome measures were the postictal time to reorientation and the Columbia University Autobiographical Memory Interview: Short-Form (CUAMI-SF). Antidepressant effects were assessed using the Hamilton Rating Scale for Depression (HRSD24).
Results:
In the Intent-to-treat sample, a repeated measures mixed model suggested no between group difference in HRSD24 score over time (F1,35 = 0.82, p=0.37), while the response rate favored FEAST (FEAST: 65%; RUL-UBP ECT: 57.9%), and the remission rate favored RUL-UBP ECT (FEAST: 35%; RUL-UBP ECT: 47.4%). The FEAST group had numeric superiority in average time to reorientation (FEAST: 6.6±5.0 minutes; RUL-UBP ECT: 8.8±5.8 minutes; Cohens d = 0.41), and CUAMI-SF consistency score (FEAST: 69.2±14.2%; RUL-UBP ECT: 63.9±9.9%; Cohens d = 0.43); findings that failed to meet statistical significance.
Conclusions:
FEAST exerts similar efficacy relative to an optimal form of conventional ECT and may have milder cognitive side effects. A blinded, randomized, non-inferiority trial is needed.
Keywords: Focal Electrically-Administered Seizure Therapy, FEAST, Electroconvulsive Therapy, ECT, Depression, Treatment Resistant Depression, TRD
Introduction
Major depression is common and costly worldwide [1, 2]. The suicide rate in the United States has risen steadily since 1999, increasing by 33% [3], with major depression the most common diagnosis, by far, among those who commit suicide [4]. Treatment-resistant depression (TRD) disproportionately contributes to the overall burden of major depression, and to the suicide rate, in particular [5, 6]. TRD, which is defined as persistent depressive symptoms despite adequate trials of antidepressant treatment, characterizes approximately 30% of patients with major depression [7, 8]. Electroconvulsive therapy (ECT) remains the most effective treatment for TRD [9-11]. ECT has anti-suicidal properties, in addition to its antidepressant effect [12-14]. Despite its marked efficacy, ECT utilization remains low, with many considering it a treatment of “last-resort” [15-17]. The reasons for this status are multifactorial, but it is likely that the amnestic effects of ECT are an important factor.
A main focus of ECT research has been the effort to preserve clinical efficacy, while reducing and eliminating its characteristic amnestic effects. There has been substantial progress in this regard, including changes in electrode positioning (from bitemporal to right unilateral) [18-20], electrical waveform (from sine wave to brief pulse) [21-23], electrical dosing (from high invariant dosing to dosing determined by empirical titration) [22-24], and pulse width (from brief to ultrabrief pulse width) [25-28]. These innovations have resulted in reduced amnestic effects and preserved efficacy in most, but not all, investigations [29, 30]. These efforts to improve ECT technique have been guided by the mechanistic hypothesis that distinct circuits subserve the antidepressant and amnestic effects of the treatment [31]. This view is supported by evidence linking seizure initiation in prefrontal cortical regions to antidepressant efficacy, and seizure initiation in medial temporal lobe structures to amnestic side effects [32-34].
Both Focal Electrically-Administered Seizure Therapy (FEAST) and Magnetic Seizure Therapy (MST) [35] are focal stimulation techniques that offer superior control over the sites of seizure initiation compared to traditional ECT seizure induction methods. FEAST was developed to concentrate electrical stimulation in right-sided fronto-orbital regions, and achieves enhanced focality by three design features: a) asymmetric electrode geometry — a large oblong posterior electrode, and a small anterior circular electrode; b) novel placement — the anterior electrode is placed above the right eyebrow and the posterior electrode is placed anterior to the vertex; and c) unidirectional current — the direction of current flow is from the posterior electrode to the anterior electrode.
Electric field modeling has confirmed that this novel design results in a more focal stimulation than traditional ECT electrode placements, including RUL ECT [36, 37]. Research with nonhuman primates demonstrated the feasibility of inducing generalized seizures with FEAST, suggested that unidirectional stimulation is more efficient in seizure induction than traditional bidirectional stimulation, and that there is greater postictal suppression when current flows from the posterior to anterior electrode [38]. Early human imaging research supported the hypothesis that FEAST selectively initiates seizure activity in right-sided prefrontal cortex [39]. To date, our group has completed two proof-of-concept, prospective, open-label clinical trials. The first study was a methods development investigation with 20 participants. The geometry of stimulating electrodes and pulse amplitude (current) were optimized [40], with encouraging clinical results. In the second study, we delivered the optimized treatment technique to a new cohort of 20 patients in a major depressive episode (MDE). The changes in depressive symptoms following FEAST were of a similar magnitude as obtained with conventional ECT, and there were suggestions that FEAST had especially rapid recovery of orientation in the postictal period and reduced amnestic effects following the treatment course [41].
This early work on FEAST was limited in important respects, and the efficacy and cognitive effects of FEAST were compared to other interventions only using historical data from other published trials. Prior to initiating a large scale, definitive, randomized controlled trial, we thought it prudent to perform a bridging study, comparing, over the same time frame and at the same facilities, the outcomes of patients treated with FEAST and with a form of traditional ECT. We chose right unilateral ultrabrief pulse (RUL-UBP) ECT as the comparator, since this form of ECT has established efficacy and is well documented to result in the least severe and persistent cognitive side effects [25, 42-44]. We used an open-label, naturalistic comparative design rather than conducting a randomized double-blind trial to expedite study completion and to determine the necessary sample size and other features of a definitive double-blind, randomized trial, if indicated. This study was an intermediary trial, as we sought to confirm and extend the promising findings of the earlier research, address some of the limitations in this work, and quantify effect sizes for potential differences in efficacy and cognitive effects to power a larger more definitive trial.
Methods
Overview
This was an open-label, non-randomized, parallel group, clinical trial performed at two enrolling sites, Medical University of South Carolina (MUSC), in Charleston, SC and Augusta University (AU), in Augusta, GA. The trial was designed in accordance with the Declaration of Helsinki, approved by the Institutional Review Boards of both enrolling sites, conducted under an Investigational Device Exemption from the Food and Drug Administration (PI: Dr. George), and pre-registered with clinicaltrials.gov (NCT02535572). Participants were recruited from a pool of inpatients and outpatients evaluated for treatment-resistant MDE who elected to be treated with ECT. These patients were offered enrollment in the FEAST arm of the trial with the understanding that this was an experimental treatment that may have reduced cognitive side effects. Those who declined receiving FEAST were offered entry into the RUL-UBP ECT study arm, with the understanding that this technique was the current standard of care at each facility, and that study participation would entail participation in additional evaluations.
Participant enrollment, screening, and clinical assessment
All participants provided signed informed consent after study description. The baseline assessment included medical exam, structured interview (SCID-5) to apply Diagnostic and Statistical Manual, 5th Edition criteria [45], 24-item Hamilton Rating Scale for Depression (HRSD24) [46], Inventory of Depressive Symptoms: Self-Report (IDS-SR) [47], Beck Scale for Suicidal Ideation (SSI) [48], Clinical Global Impression Scales (CGI) [49], Antidepressant History Treatment Form: Short Form (ATHF-SF) [50], Mini-Mental Status Exam [51], Columbia University Autobiographical Memory Interview: Short Form (CUAMI-SF) [52], Buschke Selective Reminding Test (BSRT) [53], Medical Outcomes Study, Health-Related Quality of Life: Short Form 36 (SF-36) [54, 55], and ECT Attitudes and Expectancy Inventory [56, 57]. Participants were included if they currently met DSM-5 criteria for a major depressive episode (unipolar or bipolar), had a baseline HRSD24 score of 21 or greater, and were between the ages of 18 and 90 years old. Participants were excluded if they had a history of dementia, non-mood related psychosis, or rapid-cycling bipolar disorder, or greater than mild alcohol/substance use disorder within the past three months. Participants were also excluded if they were currently enrolled in another study using an investigational device, had ECT in the past 6 months, were unable to discontinue lithium, anticonvulsant, or stimulant medications, or had active and unstable neurologic or medical conditions. We elected to include a pregnant woman in our comparator RUL-UBP ECT group given that the standard of care is to recommend ECT to pregnant women with severe TRD, and pregnancy, per se, does not appear to impact on ECT outcomes. The RUL-UBP ECT arm of this study mainly involved additional assessments beyond routine clinical care. However, we would not have included pregnant women in the FEAST condition due to its experimental status.
HRSD24 scores provided the primary efficacy outcome measure. The HRSD24 was administered at preECT baseline, prior to every other ECT treatment, and within 48 hours of the final treatment. Response was defined as a 50% or greater improvement in scores at postECT evaluation relative to baseline, and remission was defined as a HRSD24 score 10 or less at postECT assessment. For those participants (N = 7; FEAST N = 2 and RUL-UBP N = 5) lost to follow-up before their final assessment, the last observation was carried forward (assuming at least one post-baseline assessment). Secondary outcomes included CGI-S severity scores (assessed at the same interval as the HRSD24), and IDS-SR scores (assessed at baseline and end-of-acute treatment). Findings regarding the SSI and SF36 will be reported separately.
The primary outcomes for cognitive effects were the CUAMI-SF consistency score and the postictal time to recovery of orientation. The CUAMI-SF has been sensitive to long-term effects of ECT and to differences among forms of ECT in the severity and persistence of retrograde amnesia for autobiographical information [20, 22, 58]. The CUAMI-SF was administered at baseline and between 24 and 48 hours of the final ECT treatment. Time to recovery of orientation in the postictal period is also highly sensitive to variations in ECT technique [59, 60] and is a predictor of the magnitude and persistence of long-term retrograde amnesia [35, 61]. Orientation recovery was assessed continuously at each treatment session, starting with eyes opening upon command until correct response to four of five orientation items (name, day of the week, date, location, and date of birth) following the methods described by Sobin et al.[61]. The average re-orientation time was computed across all treatment sessions, excluding the first session which involved titration of electrical dose to seizure threshold. Alternative versions of the BSRT were administered at baseline and end-of-acute assessments (12 unrelated words, 6 trials), and these scores (total recall over 6 trials) and MMSE scores were secondary cognitive outcomes.
At pre-treatment, patients rated their global expectation regarding the effects of the intervention on their mood and memory, using 7-point Likert scales, with scores between 1-3 indicating an expectation of a negative effect, 4 indicating no change, and scores between 5-7 indicating a positive effect. Identical ratings made following the treatment course provided global subjective assessment of the impact of the treatment on mood and memory. These post-treatment scores provided additional secondary measures of antidepressant and cognitive effects [56, 57].
Adverse Events (AE’s) were assessed by one of the study physicians at each visit and at the end of the acute treatment course. Each AE was coded based on its seriousness and likely relatedness to treatment.
Treatment procedures
RUL-UBP ECT was administered with a standard MECTA spECTrum 5000Q device (MECTA Corporation, Tualatin, OR), and circular stainless steel electrodes (2-inch diameter) positioned according to the d’Elia placement [62]. With FEAST, a small (1.25-in diameter) circular electrode was placed with the lower boundary just above the center of the right eyebrow, and a large oblong (2 x 3 in curved) posterior electrode was placed with the medial boundary tangential to the nasion-to-inion line, the posterior boundary 1-inch anterior to the vertex, and the lateral portion extending over the right hemisphere [41]. FEAST was delivered by a custom-modified MECTA spECTrum 5000Q device identical to the standard device, with the exception that a unidirectional current was delivered with current flow from the posterior to anterior electrode. Traditional ECT uses a bidirectional stimulus, with the direction of current flow reversing with each pulse. With both FEAST and RUL-UBP ECT, hand-held electrode assemblies were used to keep the stainless-steel stimulating electrodes in place. An adhesive, disposable circular locator was used to mark the position of the smaller anterior FEAST electrode and to prevent spread of the electrolyte beyond the boundaries of the stimulating electrode.
Participants were oxygenated by mask (100% O2) prior to anesthesia induction and until the resumption of spontaneous respiration. Methohexital (0.75–1.0 mg/kg) and succinylcholine (0.75–1.0 mg/kg) served as the anesthetic agent and the muscle relaxant, respectively. Glycopyrrolate (0.2–0.4 mg IV) was administered at each titration session before anesthesia induction. Seizure threshold was quantified at the first treatment session using the empirical titration procedure [63], and the same titration schedule was used for FEAST and RUL-UBP ECT, with identical stimulus parameters at each titration step. Electroencephalography (EEG) was monitored with left and right prefrontal leads each referenced to a lead over the ipsilateral mastoid process. The distribution of the muscle relaxant was blocked in the right foot to aid in assessment of the duration of the motor ictal response. A motor or EEG seizure duration of at least 20 seconds was the criterion for an adequate treatment. Seizures of inadequate duration were followed 60 seconds later by re-stimulation at the next step of titration. After the initial seizure titration session, treatments were delivered at an electrical charge that was six times the initial seizure threshold (6 x ST), which is the routine dosing when administering RUL-UBP ECT [25, 42, 64]. For patients failing to achieve 40% or greater improvement following six treatments, those in the FEAST arm were offered the option of increasing their dosage to 9 x ST or switching to RUL-UBP ECT. Participants in the RUL-UBP ECT arm who did not reach this milestone were offered a dosage increase to 9 x ST.
Following the current standard care during ECT, participants were withdrawn from specific classes of medications, specifically lithium, stimulants, and anticonvulsants. Withdrawn medications were tapered with their last dose given 24 hours or more prior to the first ECT treatment session. Other psychotropic medications were continued, including antidepressant and antipsychotic medications. Benzodiazepine medications were limited to the equivalent of lorazepam 3 mg/d, with the last dose of medication administered no later than 9 pm on the evening prior to each treatment session.
Statistical Analyses
Our primary intent-to-treat (ITT) analysis included all participants who received at least one treatment and had at least one subsequent assessment (N = 20 FEAST; N = 19 RUL). We also defined a Completer sample that included all participants either achieving remission or having had at least 8 study treatments in their assigned group (N = 17 FEAST; N = 17 RUL). All analyses were conducted in both the ITT and Completer samples using SAS version 9.4 (Cary, North Carolina, USA).
The treatment conditions (FEAST vs. RUL-UBP ECT) and sites (AU vs. MUSC) were compared in baseline characteristics using t-tests for continuous variables, and chi-square tests (or Fisher’s exact test) for categorical outcomes. Analyses of covariance (ANCOVAs) were conducted on electrical dosing and seizure duration treatment parameters. Each model included the main effects and interaction term involving treatment group and site, with age as a covariate. Log (base 10) transformations were applied to the electrical charge and seizure duration measures prior to analysis.
The primary analysis of clinical outcome contrasted the treatment groups, sites, and their interaction in HRSD24 scores over time. Longitudinal mixed models were applied to the serial HRSD24 scores from baseline to following the end of acute treatment, with fixed effects for treatment condition, site, and their interaction, and patient as a random effect. Various covariance structures were examined for best fit during construction of the mixed models, and the final covariance structure was selected based on corrected Akaike information (AICC) and Bayesian information (BIC) criteria.
All secondary analyses of antidepressant outcomes included treatment condition, site, and their interaction as terms, as well as the covariate age. An ANCOVA was conducted on the percentage change in HRSD24 scores from baseline to last observation. Logistic regressions were conducted on response and remission rates. ANCOVAs were conducted on post-treatment HRSD24, CGI-S, and IDS-SR scores, with the baseline scores also serving as covariates. A similar ANCOVA was conducted on the post-treatment subjective evaluation of the impact of treatment on mood, with the pre-treatment expectancy score included as a covariate.
Similar ANCOVAs were also performed on the subjective and objective cognitive outcomes. The primary cognitive outcomes, which included, postECT percent consistency on the CUAMI-SF, and mean time to reorientation each required transformation prior to analysis (arcsine in the case of CUAMI-SF and log10 in the case of time to reorientation). Secondary cognitive measures were also evaluated using ANCOVA including post-treatment MMSE, BSRT, and the subjective evaluation of the treatment effect on memory. Each of these secondary outcomes were modeled as described above.
Results
Participant Characteristics
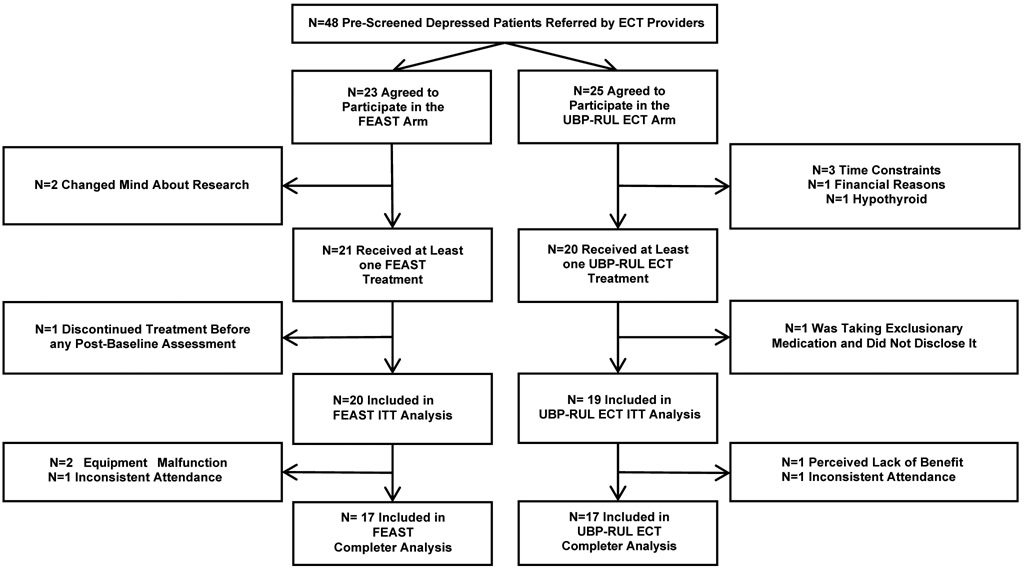
A total of 48 prescreened participants assessed for eligibility agreed to treatment with FEAST (N = 23) or RUL-UBP ECT (N = 25) during the study period (see the CONSORT diagram in Figure 1). Two participants in the FEAST condition and five in the RUL-UBP ECT condition withdrew prior to their first treatment session. One FEAST participant discontinued treatment following the initial titration session with no subsequent evaluation. These participants were excluded from further analysis, as they did not have a post-baseline assessment. One participant in the RUL-UBP ECT condition was surreptitiously taking an exclusionary medication (anticonvulsant) and was also excluded from further analysis. The remaining 39 participants (20 FEAST, 19 RUL-UBP ECT) comprised the ITT sample. Three participants in the FEAST group, and two in the RUL-UBP ECT group discontinued treatment before completing eight sessions or reaching remission and were thus excluded from the Completer sample. Two of the three non-completers in the FEAST group (one at each site) were discontinued due to equipment malfunction. In both cases, the complete electrical stimulus could not be delivered at one or more sessions due to excessive impedance in the circuit. This was determined to be due to an oxidative residue on the electrode surface. The malfunction did not recur after removing the residue and instituting more thorough electrode cleaning. Two participants (one FEAST, one RUL-UBP ECT) discontinued treatment due to inability to consistently attend outpatient treatment sessions. The final non-completer (RUL-UBP ECT) discontinued treatment before the eighth session due to a perceived lack of efficacy. Seventeen participants in the FEAST group and fourteen participants in the RUL-UBP group completed all endpoint assessments including a final HRSD24 and the cognitive battery. Two participants in the FEAST group and five in the RUL-UBP group were lost to follow-up prior to their final assessment. Cognitive data were not imputed, while the last observed HRSD24 recording was carried forward and served as the endpoint for efficacy.
Figure 1. Consort Diagram of Study Enrollment, Treatment, and Assessment.
Demographic and baseline descriptive characteristics
The ITT sample was predominantly comprised of women (N=77%), diagnosed with nonpsychotic (90%), major depressive disorder (72%) (see Table 1). The majority of participants were treated with one or more concurrent antidepressant medications (85%; 72% at adequate dose). Eleven participants were treated with a selective serotonin reuptake inhibitor (SSRI), 14 participants were treated with a serotonin and norepinephrine reuptake inhibitor (SNRI), 20 participants were treated with a second-generation antipsychotic medication (SGA), 5 participants were treated with bupropion, 1 participant received mirtazapine, and 1 participant was treated with a tricyclic antidepressant (TCA). The ITT sample had failed on average 2.69 ± 1.82 (Mean ± SD) adequate antidepressant trials in the current episode. The FEAST and RUL-UBP ECT groups differed in two baseline characteristics. Specifically, the RUL-UBP ECT group had greater representation of bipolar MDE and was more likely to begin ECT as an inpatient. A similar pattern was observed in the Completer sample (See Supplemental Table 1). Baseline characteristics were generally well matched between the two sites AU and MUSC, but differed significantly in number of failed adequate medication trials and racial composition (see Supplemental Tables 6a and 6b).
Table 1:
Demographics, and Baseline Characteristics: Intent-to-Treat Sample
| Total Sample | FEAST | RUL-UBP | Significance | |
|---|---|---|---|---|
| Sex | 30F (76.92%) | 14F (70.00%) | 16F (84.21%) | p=0.45 |
| Age | 44.17 ± 14.47SD | 45.15 ± 12.68 | 43.16 ± 16.44 | p=0.67 |
| Race C = Caucasian; AA = African American |
35C (89.74%) 4AA (10.26%) |
17C (85.00%) 3AA (15.00%) |
18C (94.74%) 1AA (5.26%) |
p=0.99 |
| ≥Bachelors | 14 (36.84%) | 7 (36.84%) | 7 (36.84%) | p= 0.99 |
| Employed | 7 (18.92%) | 5 (26.32%) | 2 (11.11%) | p= 0.41 |
| Married | 21 (53.85%) | 10 (50.00%) | 11 (57.89%) | p=0.75 |
| Diagnosis (Bipolar Affective Disorder)* | 11 BPAD (28.21%) | 1 BPAD (5.00%) | 10 BPAD (52.63%) | p=0.001 |
| Depression with Psychosis | 4 (10.26%) | 2 (10.00%) | 2 (10.53%) | p=0.99 |
| Inpatient* | 15 (38.46%) | 4 (20.00%) | 11 (57.89%) | p=0.02 |
| Duration (weeks) | 85.71 ± 119.60 | 76.94 ± 138.20 | 97.85 ± 91.79 | p=0.64 |
| Adequate antidepressant trials (current episode) |
2.69 ± 1.82 | 2.70 ± 1.92 | 2.68 ± 1.77 | p=0.98 |
| Concurrent antidepressant | 33 (84.62%) | 17 (85.00%) | 16 (84.21%) | p=0.99 |
| Concurrent Adequate antidepressant | 28 (71.79%) | 15 (75.00%) | 13 (68.42%) | p=0.73 |
All values expressed in means ± standard deviations.
Denotes significant baseline difference between condition at p<0.05.
ECT Treatment Characteristics
In the ITT sample, the ANCOVA on initial seizure threshold yielded main effects of treatment group, F(1, 34) = 4.71, P = 0.04, and enrolling site, F(1, 34) = 8.06, P = 0.008, without a significant interaction. Age was modestly associated with initial seizure threshold, F(1, 34) = 3.50, P = 0.07 (see Table 2). A similar pattern emerged for the average charge per treatment across all sessions, with main effects of treatment group, F(1, 34) = 6.29, P = 0.02, and site, F(1, 34) = 12.89, P = 0.001, without a significant interaction. Age was positively associated with average charge, F(1, 34) = 7.08, P = 0.01. Initial seizure threshold and average charge were higher with FEAST compared to RUL-UBP ECT and higher at AU than MUSC. In contrast, the ANCOVAs on average motor seizure and EEG seizure duration did not yield any effects involving treatment group or site. In both cases, age was negatively associated with seizure duration (both P’s < 0.002). The findings were unchanged in the Completer sample (see Supplemental Table 2).
Table 2:
Electrical and Seizure Characteristics: Intent-to-Treat Sample.
| Total Sample | FEAST | RUL-UBP | |
|---|---|---|---|
| Seizure Threshold (Millicoulombs) | 31.49 ± 31.62 | 41.26 ± 41.59 | 21.22 ± 8.21 |
| Mean Charge delivered (Millicoulombs) | 201.52 ± 147.23 | 253.90 ± 178.80 | 146.40 ± 75.84 |
| Mean Motor Seizure duration (Threshold; Seconds) | 43.51 ± 35.43 | 42.84 ± 42.27 | 44.22 ± 27.70 |
| Mean EEG Seizure duration (Threshold; Seconds) | 78.35 ± 49.48 | 84.42 ± 47.32 | 71.94 ± 52.25 |
| Mean Motor Seizure duration (Subsequent; Seconds) | 30.68 ± 16.70 | 31.12 ± 19.80 | 30.22 ± 13.21 |
| Mean EEG Seizure duration (Subsequent; Seconds) | 54.67 ± 29.15 | 53.02 ± 31.28 | 56.41 ± 27.47 |
All values expressed as mean ± standard deviation.
Clinical Efficacy
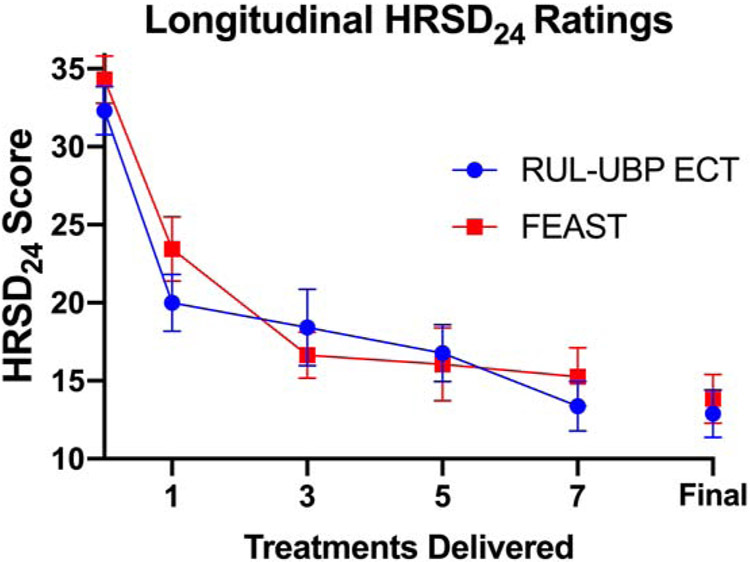
Based on AICC and BIC criteria, the optimal covariance structure for the longitudinal mixed model on HRSD24 scores were compound symmetry for the ITT analysis and Type-1 autoregressive for the Completer analysis. Each of the two models showed a significant time effect, ITT: F(13, 191) = 22.17, P < 0.0001; Completer: F(13, 166) = 18.37, P < 0.0001. Neither the treatment groups nor sites differed in change over time in HRSD24 scores, and there were no treatment group or site interactions with time (see Figure 2).
Figure 2. Hamilton Rating Scale for Depression (HRSD) Scores Over Time in the Intent-to-Treat Sample.
This figure presents longitudinal scores for HRSD24 ratings ± standard error of the mean (SEM) through the first seven FEAST or RUL-UBP ECT treatments. Final scores for all participants are also displayed (treatment range = 3-15).
Secondary efficacy analyses were conducted on all the clinical outcome variables in Table 3 for the ITT sample and in Supplemental Table 3 for the Completer group. Neither ITT nor Completer analyses yielded a main effect of treatment group or site for any clinical outcome variable. In both the ITT and Completer analyses there was a significant interaction between treatment group and site on the participant’s postECT subjective rating of mood (both P’s ≤ 0.03). At AU, FEAST resulted in superior post-treatment subjective mood ratings compared to RUL-UBP ECT, an effect not observed at MUSC. Otherwise, there were no indications that the two treatment conditions differed in antidepressant effects. Both FEAST and RUL-UBP ECT exerted marked antidepressant properties. In the total sample, more than 60% of participants were classified as responders and more than 40% were classified as remitters.
Table 3:
Efficacy Measures: Intent-to-Treat Sample
| Total Sample | FEAST | RUL-UBP | |
|---|---|---|---|
| Hamilton Rating Scale for Depression (HRSD24) Pre | 33.26 ± 6.69 | 34.25 ± 6.76 | 32.21 ± 6.63 |
| Hamilton Rating Scale for Depression (HRSD24) Post | 13.00 ± 6.67 | 13.20 ± 6.89 | 12.79 ± 6.61 |
| Hamilton Rating Scale for Depression (HRSD24) %-Change | 59.94 ± 19.71 | 60.67 ± 18.46 | 59.18 ± 21.42 |
| Hamilton Rating Scale for Depression (HRSD24) Response | 24/39 (61.54%) | 13/20 (65.00%) | 11/19 (57.89%) |
| Hamilton Rating Scale for Depression (HRSD24) Remission | 16/39 (41.03%) | 7/20 (35.00%) | 9/19 (47.37%) |
| Inventory of Depressive Symptomatology (IDS-SR) Pre | 51.64 ± 10.92 | 50.75 ± 11.06 | 52.58 ± 11.00 |
| Inventory of Depressive Symptomatology (IDS-SR) Post | 26.22 ± 13.81 | 27.41 ± 14.20 | 24.79 ± 13.71 |
| Clinician Global Impression (CGI) Severity Pre | 5.49 ± 0.60 | 5.45 ± 0.60 | 5.53 ± 0.61 |
| Clinician Global Impression (CGI) Severity Post | 3.21 ± 1.13 | 3.30 ± 1.26 | 3.10 ± 1.00 |
| ECT Expectancy Mood | 5.58 ± 1.31 | 5.50 ± 1.10 | 5.67 ± 1.53 |
| ECT Attitudes Mood | 5.71 ± 1.24 | 5.59 ± 1.33 | 5.86 ± 1.17 |
All values expressed as mean ± standard deviation.
Table 4 (ITT) and Supplemental Table 4 (Completer) present information on the number of ECT treatments administered in each treatment group, as well as the number of treatments to first meet response and remission criteria. There were no main effects of treatment group on any of these measures in either the ITT or Completer samples. Similarly, there was no difference between the conditions in terms of the percentage of patients who experienced a dosage escalation or change in electrode placement. Of the 20 FEAST participants, 12 completed the course with a dosage 6 x ST, 7 were increased in dosage to 9 x ST, and one patient was switched to bifrontal electrode placement. Among the 19 RUL-UBP ECT participants, 4 were increased in dosage to 9 x ST.
Table 4:
Total Treatments and Treatments to Improvement Thresholds: Intent-to-Treat Sample
| Total Sample | FEAST | RUL-UBP | |
|---|---|---|---|
| Total Treatments Received | 9.36 ± 2.98 | 9.55 ± 3.15 | 9.16 ± 2.85 |
| Treatments Received at Last Efficacy Observation | 9.10 ± 3.05 | 9.40 ± 3.17 | 8.79 ± 2.97 |
| Treatments to Meet Response Criteria | 3.54 ± 2.34 | 3.54 ± 1.94 | 3.55 ± 2.84 |
| Treatments to Meet Remission Criteria | 6.00 ± 4.18 | 5.14 ± 3.08 | 6.67 ± 4.95 |
| Number of Patients Increased in Dosage to 9 x ST | 11/39 (28.21%) | 7/20 (35.00%) | 4/19 (21.05%) |
All values expressed as mean ± standard deviation.
Cognitive Effects
There was no significant baseline difference in any cognitive measure in the ITT (Table 5) or Completer sample (Supplemental Table 5). There were no significant effects in the ANCOVAs on the primary post-treatment cognitive measures (CUAMI-SF and time to reorientation). However, in each case the numerical differences favored4 the FEAST group. Indeed, the main effect of treatment condition in time to reorientation approached significance in the Completer sample, F (1, 29) = 3.36, P = 0.08, Cohens d = 0.41, where recovery time was approximately 33% faster with FEAST compared to RUL-UBP ECT. Likewise, while not statistically significant, the FEAST group also had substantially higher CUAMI-SF consistency scores (Cohens d = 0.43), indicating less retrograde amnesia for autobiographical information.
Table 5.
Cognitive Measures: Intent-to-Treat Sample
| Sample | FEAST | RUL-UBP | |
|---|---|---|---|
| Time to Postictal Orientation Recovery (Minutes) | 7.64 ± 5.48 | 6.56 ± 5.03 | 8.79 ± 5.83 |
| Columbia University Autobiographical Memory Index (CUAMI) Pre | 51.36 ± 5.64 | 52.65 ± 4.08 | 50.00 ± 6.77 |
| Columbia University Autobiographical Memory Index (CUAMI) Consistency (%) |
66.81 ± 12.53 | 69.21 ± 14.21 | 63.90 ± 9.86 |
| Buschke Selective Reminding Test (BSRT) Pre | 38.14 ± 12.27 | 38.58 ± 11.05 | 37.67 ± 13.75 |
| Buschke Selective Reminding Test (BSRT) Post | 42.04 ± 14.31 | 44.87 ± 14.75 | 38.50 ± 13.51 |
| Mini Mental State Examination (MMSE) Pre | 28.50 ± 2.24 | 28.95 ± 1.43 | 28.05 ± 2.80 |
| Mini Mental State Examination (MMSE) Post | 28.73 ± 2.66 | 29.65 ± 0.86 | 27.54 ± 3.67 |
| ECT Expectancy: Memory | 4.03 ± 1.24 | 3.75 ± 1.29 | 4.33 ± 1.14 |
| ECT Attitudes: Memory | 4.48 ± 1.43 | 3.94 ± 1.25 | 5.14 ± 1.41 |
All values expressed as mean ± standard deviation
A similar pattern was obtained with the BSRT and MMSE secondary cognitive measures where advantages for FEAST relative to RUL-UBP ECT approached significance. For the BSRT, there were trends for a main effect of treatment condition in the ITT sample, F (1, 21) = 3.35, P = 0.08, Cohens d = 0.69, and the Completer sample, F (1, 19) = 4.13, P = 0.06. Patients treated with FEAST showed significant improvement in BSRT scores at post-treatment relative to pre-ECT in both the ITT and Completer samples (both P’s < 0.04, while there was no change in patients treated with RUL-UBP ECT (both P’s > 0.72). There were also trends for a main effect of treatment condition in the analysis of post-treatment MMSE scores in both the ITT, F(1, 24) = 3.36, P = 0.08, Cohens d = 0.92, and Completer, F(1, 22) = 3.11, P = 0.09, samples. Post-treatment MMSE scores tended to be higher with FEAST than RUL UBP ECT. However, the interaction between treatment group and site also approached significance in these analyses and post hoc comparisons (using Tukey HSD) demonstrated that there was a significant advantage for FEAST over RUL-UBP ECT at MUSC and no effect at AU. In the ITT and Completer samples, MMSE scores increased significantly from baseline in the FEAST condition (both P’s < 0.03) and tended to decrease in the RUL-UBP ECT group (both P’s ≤ 0.08).
The analyses of the global subjective assessment of the impact on memory yielded main effects of site (both P’s ≤ 0.01), with this assessment considerably more positive at MUSC than AU. While not significant, and in contrast to the objective cognitive measures, there was a numerical advantage to RUL-UBP ECT in post-treatment subjective ratings.
Adverse Events
Treatment was generally well tolerated in both groups, and the majority of participants (87%) met study completion criteria. There were, however, two serious AEs including a suicide attempt (early in treatment), and a spontaneous abortion in a first trimester pregnant patient. Both serious AEs occurred in the RUL-UBP ECT group, and in both cases the participants completed the treatment course. There were two moderate AEs in the FEAST condition (midazolam was required to stop a prolonged seizure in one participant, and there was an instance of postictal agitation), and one moderate AE in the RUL-UBP ECT group (a brief episode of postictal delirium). Mild AEs, such as postictal headache, nausea, or arthralgia were relatively common and did not distinguish the two treatment conditions.
Discussion
In this two-site, non-randomized, open-label trial, FEAST displayed comparable antidepressant efficacy, with numerically reduced cognitive side effects, compared to RUL-UBP ECT. Given the small sample size many of the findings expectedly failed to reach statistical significance, but provide effect sizes for powering larger trials. We contextualize the main findings below.
Clinical efficacy
We found near equivalency in the magnitude of clinical improvement with FEAST and RUL-UBP ECT, and a high degree of efficacy in both groups. When comparing the findings to recent investigations using RUL-UBP ECT, our categorical rates of response and remission were in line with other trials. The finding of 65% response and 35% remission in the FEAST group, and 57.9% response and 47.4% remission in the RUL-UBP ECT group are consistent with other investigations of RUL-UBP ECT which range from 48 to 78% for response, and 29 to 77% for remission [25, 26, 28, 30, 42, 65-68]. Though the remission rates were on the lower range for reported trials, it is noteworthy that the current sample had a relatively high degree of treatment resistance, with an average of 2.7 adequate failed treatment trials in both treatment groups. In addition, the overall efficacy observed here compares quite favorably to that reported in the largest MST trial [35].
Cognitive effects
There was a general trend for reduced adverse cognitive effects with FEAST compared to RUL-UBP ECT. Neither of the differences in the primary outcome measures (time to reorientation; CUAMI consistency score) attained statistical significance. The average time to reorientation with FEAST was shorter than all other reported recovery times for RUL-UBP ECT, of a similar magnitude for those reported with MST, and slightly longer than in our two previous FEAST investigations. With RUL-UBP ECT, the observed average time to recover orientation was on the lower end of that reported in other trials.
Consistency scores on the AMI-SF were numerically higher among the FEAST participants compared to those treated with RUL-UBP ECT. The consistency score on the CUAMI-SF for both the FEAST and UBP-RUL ECT groups were on the lower side compared to other studies using RUL-UBP ECT [25, 26, 28, 44] and MST [35], a finding that may relate, in part, to this trials inclusivity of participants receiving a variety of concomitant medications. Similarly, there were trends in favor of the FEAST group when compared to the RUL-UBP ECT group to have higher post-treatment scores on the BSRT, a measure of verbal anterograde learning and memory; and the MMSE, a measure of global cognitive status. Thus, FEAST showed numerical advantages on each of the four objective cognitive measures, with the differences with RUL-UBP ECT reflecting moderate effect sizes.
Unexpectedly, those treated with FEAST reported less subjective cognitive benefit from ECT compared to those treated with RUL-UBP ECT. Participants treated with FEAST on average reported no change in cognition (4 out of 7 on a Likert scale); while participants treated with RUL-UBP ECT reported some improvement (5 out 7). This finding is discordant with the objective markers of cognition that showed an advantage in the FEAST group. It is unclear why participants in the FEAST group had less objective adverse cognitive effects but reported less cognitive benefit than the group receiving RUL-UBP ECT. It is possible that the members of the FEAST group were more focused on amnestic effects and were subsequently more subjectively sensitive to memory effects. The remission rate was also numerically higher in the RUL-UBP ECT group and this may have resulted in a larger perceived cognitive benefit in this group.
Treatment parameters
Generalized seizure induction with FEAST required higher stimulus intensity (charge) than with RUL-UBP ECT. This finding was consistent with the previous FEAST investigation which reported an average seizure threshold of 33.1 ± 33.7mC [41], which is higher than the average seizure thresholds reported in published trials of RUL-UBP ECT which range from 22 to 36 mC [26, 28, 42, 66, 67]. Of note, one of the other RUL-UBP ECT trials recruited exclusively older adults [42], a group known to have higher seizure thresholds, and others used anesthetic agents thought to result in higher seizure thresholds relative to the anesthetic used in this trial [26, 28, 67].
The use of a unidirectional stimulus waveform is linked to reduced seizure threshold relative to traditional bidirectional stimulation [38, 69]. However, FEAST differs from RUL-UBP ECT by having a smaller interelectrode distance, and putative seizure initiation in prefrontal cortex rather than seizure initiation in the motor strip which is likely the case in RUL-UBP ECT. A smaller interelectrode distance results in more of the current shunted through the scalp and a lower intracerebral current density and, thus, a higher threshold [70, 71]. The motor cortex and hippocampus are thought to have especially low seizure thresholds [31, 72-74]. Basic [38], modeling [36, 37], and imaging [39] research indicate that generalized seizures induced by FEAST are more likely to initiate in prefrontal cortex. This may also account for its higher threshold values.
Limitations
This study had several limitations. The trial was designed to gather preliminary data for a larger more definitive trial. The small sample size and, especially, the non-randomized design, limit inferences. As with previous investigations of FEAST, it is possible that there was biased sampling. Those participants most concerned about amnestic effects may have elected treatment with this new modality, while those participants with more severe illness might have elected treatment with standard RUL-UBP ECT. The baseline differences between the two groups (with more inpatients and bipolar depressed patients in the RUL-UBP ECT condition) suggest this may have been at least partly the case. However, of note, the groups did not differ on other baseline characteristics, and baseline levels of depression severity were numerically higher in the FEAST group.
Both inpatient status and bipolarity are typically associated with more severe/acute illness, and so the RUL-UBP ECT group may have had more severe illness at study start. Though important imbalances in this study, it is not clear that either variable had an impact on our findings. There has been much work trying to examine the differential efficacy of ECT based on clinical variables including age, illness severity, bipolarity, and treatment resistance. Two recent and complimentary meta-analyses suggested that increased symptom severity may be a positive predictor of response [75, 76]. Bipolarity has neither been associated with increased or decreased rates of response following ECT [76], but studies have consistently found that patients with bipolar depression require fewer treatments with ECT than unipolar depressed patients [77-79]. The impact of illness severity and bipolarity on amnestic effects following ECT are less well established, but at least one study reported that patients with bipolar depression have worse cognitive outcomes than those with unipolar depression [80].
The relatively small sample size in this trial limited our statistical power to control for more than the key variables. Though statistical controlling for the bipolar diagnosis and inpatient status may have been desirable, we instead chose to control for age which has consistently shown importance in clinical efficacy [42, 75, 76], seizure threshold [81], and amnestic effects [80, 82]. Future studies with larger, randomized samples will provide an opportunity to examine the contributions of additional covariates, if indicated.
Conclusions
The findings from this bridging trial were encouraging, as the results were consistent with the goal of developing a new focal form of ECT that has reduced cognitive effects, while retaining ECT’s remarkable efficacy. The results, however, are preliminary given the constraints of the study design and sample sizes. A larger randomized and blinded comparative trial is needed to determine whether, in fact, FEAST has reduced cognitive effects with preserved antidepressant efficacy.
Supplementary Material
Highlights:
This trial compared FEAST and RUL-UBP ECT to determine preliminary differences
Using an open-label, non-randomized design, we assessed efficacy and amnestic effects
There were no significant differences between the two groups in any primary outcome
Our preliminary findings, however, suggest a larger comparative study is indicated
Acknowledgements:
We acknowledge the many technical contributions of the MECTA Corporation, including those made by Robin Nicol, Adrienne Kettering, Burt Thompson, and John Shaw. In addition to their technical contributions, the MECTA corporation also loaned and supported custom-modified Spectrum 5000Q devices. However, the MECTA Corporation was not involved in study design, data analysis, or data interpretation. We also acknowledge the many contributions of the nursing teams at MUSC and AU, including Carol Burns, Al Lopez, and Christine Bowie. Finally, we posthumously acknowledge Cecile Mazingue, who was a key member of the MUSC team while this project was being developed and implemented.
Funding Source: This work was also supported by the National Institutes of Health, grant number: K23DA043628 (PI: Sahlem, NIH/NIDA).
Declaration of Interests: This study was supported in part by an unrestricted educational grant from the MECTA Corporation. The MECTA Corporation also provided custom-modified MECTA spECTrum 5000Q devices. Dr. Sackeim is the inventor on a patent for FEAST (US8712532 B2) and serves as a consultant to the MECTA Corporation, Neuronetics Inc, and LivaNova LPC. Dr. McCall receives research support from MECTA, Vistagen, and Merck. Dr. McCall receives royalties from Wolters Kluwer, and is a scientific adviser for Jazz, Sage, and Janssen Pharmaceuticals. None of the other authors have any conflicts. Dr. Youssef discloses that he receives research support (but not salary support) from the U.S. Department of Veterans Affairs, Augusta Biomedical Research Corporation, Merck & Co., VistaGen Therapeutics, Inc., and MECTA Corporation.
Disclosures: This study was supported in part by an unrestricted educational grant from the MECTA Corporation. The MECTA Corporation also provided custom-modified MECTA spECTrum 5000Q devices for the delivery of FEAST. MECTA was not involved in study design, data analysis, or data interpretation. Dr. Sackeim is the inventor on a patent for FEAST (US8712532 B2) and serves as a consultant to the MECTA Corporation, Neuronetics Inc, and LivaNova LPC. Dr. McCall receives research support from MECTA, Vistagen, and Merck. Dr. McCall receives royalties from Wolters Kluwer, and is a scientific adviser for Jazz, Sage, and Janssen Pharmaceuticals. Dr. Youssef discloses that he receives research support (but not salary support) from the U.S. Department of Veterans Affairs, Augusta Biomedical Research Corporation, Merck & Co., VistaGen Therapeutics, Inc., and MECTA Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Collaborators USBoD, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, et al. The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA : the journal of the American Medical Association 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hedegaard H CS, Warner M. Suicide Mortality in the United States, 1999–2017. NCHS Data Brief 2018;330. [PubMed] [Google Scholar]
- [4].Prevention CfDCa. Vital Signs: Trends in State Suicide Rates — United States, 1999–2016 and Circumstances Contributing to Suicide — 27 States, 2015. Morbidity and Mortality Weekly Report 2018;67(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatric services 2014;65(8):977–87. [DOI] [PubMed] [Google Scholar]
- [6].Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. Journal of affective disorders 2018;235:362–7. [DOI] [PubMed] [Google Scholar]
- [7].Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007;17(11):696–707. [DOI] [PubMed] [Google Scholar]
- [8].Cepeda MS, Reps J, Fife D, Blacketer C, Stang P, Ryan P. Finding treatment-resistant depression in real-world data: How a data-driven approach compares with expert-based heuristics. Depress Anxiety 2018;35(3):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol 2010;30(5):616–9. [DOI] [PubMed] [Google Scholar]
- [10].Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. American Journal of Psychiatry 1996;153(8): 985–92. [DOI] [PubMed] [Google Scholar]
- [11].Rasmussen KG, Mueller M, Knapp RG, Husain MM, Rummans TA, Sampson SM, et al. Antidepressant medication treatment failure does not predict lower remission with ECT for major depressive disorder: a report from the consortium for research in electroconvulsive therapy. Journal of Clinical Psychiatry 2007;68(11):1701–36. [DOI] [PubMed] [Google Scholar]
- [12].Liang CS, Chung CH, Ho PS, Tsai CK, Chien WC. Superior anti-suicidal effects of electroconvulsive therapy in unipolar disorder and bipolar depression. Bipolar disorders 2018;20(6):539–46. [DOI] [PubMed] [Google Scholar]
- [13].Prudic J, Sackeim HA. Electroconvulsive therapy and suicide risk. The Journal of clinical psychiatry 1999;60 Suppl 2:104–10; discussion 11-6. [PubMed] [Google Scholar]
- [14].Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. The journal of ECT 2014;30(1):5–9. [DOI] [PubMed] [Google Scholar]
- [15].Wilkinson ST, Agbese E, Leslie DL, Rosenheck RA. Identifying Recipients of Electroconvulsive Therapy: Data From Privately Insured Americans. Psychiatric services 2018;69(5):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sackeim HA. Modern Electroconvulsive Therapy: Vastly Improved yet Greatly Underused. JAMA psychiatry 2017;74(8):779–80. [DOI] [PubMed] [Google Scholar]
- [17].Slade EP, Jahn DR, Regenold WT, Case BG. Association of Electroconvulsive Therapy With Psychiatric Readmissions in US Hospitals. JAMA psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives of general psychiatry 2000;57(5):425–34. [DOI] [PubMed] [Google Scholar]
- [19].Sackeim HDEP, J; Cooper T; McCall VW; Rosenquist P; Isenberg K; Garcia K; Mulsant BH; Haskett RF. Effect of Concomitant Pharmacotherapy on Electroconvulsive Therapy Outcomes. Archives of general psychiatry 2009;66(7):729–37. [DOI] [PubMed] [Google Scholar]
- [20].Semkovska M, Landau S, Dunne R, Kolshus E, Kavanagh A, Jelovac A, et al. Bitemporal Versus High-Dose Unilateral Twice-Weekly Electroconvulsive Therapy for Depression (EFFECT-Dep): A Pragmatic, Randomized, Non-Inferiority Trial. The American journal of psychiatry 2016;173(4):408–17. [DOI] [PubMed] [Google Scholar]
- [21].Weiner RD RH, Davidson JRT, Squire LR. Effects of Stimulus Parameters on Cognitive Side Effects. Annals New York Academy of Sciences 1986. [DOI] [PubMed] [Google Scholar]
- [22].Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2007;32(1):244–54. [DOI] [PubMed] [Google Scholar]
- [23].Prudic J, Olfson M, Marcus SC, Fuller RB, Sackeim HA. Effectiveness of electroconvulsive therapy in community settings. Biological psychiatry 2004;55(3):301–12. [DOI] [PubMed] [Google Scholar]
- [24].McCall WV RD, Weiner RD, Sackeim HA. Titrated Moderately Suprathreshold vs Fixed High-Dose Right Unilateral Electroconvulsive Therapy. Archives of general psychiatry 2000;57. [DOI] [PubMed] [Google Scholar]
- [25].Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain stimulation 2008;1(2):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loo CK, Sainsbury K, Sheehan P, Lyndon B. A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 2008;11(7):883–90. [DOI] [PubMed] [Google Scholar]
- [27].Mayur P, Byth K, Harris A. Autobiographical and subjective memory with right unilateral high-dose 0.3-millisecond ultrabrief-pulse and 1-millisecond brief-pulse electroconvulsive therapy: a double-blind, randomized controlled trial. The journal of ECT 2013;29(4):277–82. [DOI] [PubMed] [Google Scholar]
- [28].Loo CK, Katalinic N, Smith DJ, Ingram A, Dowling N, Martin D, et al. A randomized controlled trial of brief and ultrabrief pulse right unilateral electroconvulsive therapy. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 2015; 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. The British journal of psychiatry : the journal of mental science 2010;196(3):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: cognitive side-effects. Journal of affective disorders 2010;122(1-2):60–7. [DOI] [PubMed] [Google Scholar]
- [31].Sackeim HA. The convulsant and anticonvulsant properties of electroconvulsive therapy: towards a focal form of brain stimulation. Clinical Neuroscience Review 2004;4:39–57. [Google Scholar]
- [32].Sackeim HA, Luber B, Katzman GP, Moeller JR, Prudic J, Devanand DP, et al. The effects of electroconvulsive therapy on quantitative electroencephalograms. Relationship to clinical outcome. Archives of general psychiatry 1996;53(9):814–24. [DOI] [PubMed] [Google Scholar]
- [33].Sackeim HA, Luber B, Moeller JR, Prudic J, Devanand DP, Nobler MS. Electrophysiological correlates of the adverse cognitive effects of electroconvulsive therapy. The journal of ECT 2000;16(2):110–20. [DOI] [PubMed] [Google Scholar]
- [34].Nobler MS, Sackeim HA, Prohovnik I, Moeller JR, Mukherjee S, Schnur DB, et al. Regional cerebral blood flow in mood disorders, III. Treatment and clinical response. Arch Gen Psychiatry 1994;51(11):884–97. [DOI] [PubMed] [Google Scholar]
- [35].Daskalakis ZJ, Dimitrova J, McClintock SM, Sun Y, Voineskos D, Rajji TK, et al. Magnetic seizure therapy (MST) for major depressive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee WH, Lisanby SH, Laine AF, Peterchev AV. Comparison of electric field strength and spatial distribution of electroconvulsive therapy and magnetic seizure therapy in a realistic human head model. European psychiatry : the journal of the Association of European Psychiatrists 2016;36:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bai S, Loo C, Al Abed A, Dokos S. A computational model of direct brain excitation induced by electroconvulsive therapy: comparison among three conventional electrode placements. Brain Stimul 2012;5(3):408–21. [DOI] [PubMed] [Google Scholar]
- [38].Spellman T, Peterchev AV, Lisanby SH. Focal Electrically Administered Seizure Therapy: A Novel form of ECT Illustrates the Roles of Current Directionality, Polarity, and Electrode Configuration in Seizure Induction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2009;34(8):2002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chahine G, Short B, Spicer K, Schmidt M, Burns C, Atoui M, et al. Regional Cerebral Blood Flow Changes Associated With Focal Electrically Administered Seizure Therapy (FEAST). Brain Stimul 2014;7(3):483–5. [DOI] [PubMed] [Google Scholar]
- [40].Nahas Z, Short B, Burns C, Archer M, Schmidt M, Prudic J, et al. A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy [FEAST]. Brain stimulation 2013;6(3):403–8. [DOI] [PubMed] [Google Scholar]
- [41].Sahlem GL, Short EB, Kerns S, Snipes J, DeVries W, Fox JB, et al. Expanded Safety and Efficacy Data for a New Method of Performing Electroconvulsive Therapy: Focal Electrically Administered Seizure Therapy. The journal of ECT 2016;32(3):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kellner CH, Husain MM, Knapp RG. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. 2016;173(11):1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tor PC, Bautovich A, Wang MJ, Martin D, Harvey SB, Loo C. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. The Journal of clinical psychiatry 2015. [DOI] [PubMed] [Google Scholar]
- [44].Lisanby SH, McClintock SM, Alexopoulos G, Bailine SH, Bernhardt E, Briggs MC, et al. Neurocognitive Effects of Combined Electroconvulsive Therapy (ECT) and Venlafaxine in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Geriatr Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].First MW, JB; Karg RS; and Spitzer RL. Structured Clinical Interview for DSM-5® Disorders—Clinician Version (SCID-5-CV). New York; 2016. [Google Scholar]
- [46].Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26(3):477–86. [DOI] [PubMed] [Google Scholar]
- [48].Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology 1979;47(2):343–52. [DOI] [PubMed] [Google Scholar]
- [49].Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- [50].Sackeim HA, Aaronson ST, Bunker MT, Conway CR, Demitrack MA, George MS, et al. The assessment of resistance to antidepressant treatment: Rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). Journal of psychiatric research 2019;113:125–36. [DOI] [PubMed] [Google Scholar]
- [51].Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- [52].McElhiney MC MB, and Sackeim HA. Manual for Administration and Scoring of the Columbia Autobiographical Memory Interview (AMI)-Short form. 2000.
- [53].Buschke H. Selective Reminding for Analysis of Memory and Learning. Journal of Verbal Learning and Verbal Behavior 1973;12(5). [Google Scholar]
- [54].Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21(2):271–92. [DOI] [PubMed] [Google Scholar]
- [55].Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [56].Berman RM, Prudic J, Brakemeier EL, Olfson M, Sackeim HA. Subjective evaluation of the therapeutic and cognitive effects of electroconvulsive therapy. Brain Stimul 2008;1(1):16–26. [DOI] [PubMed] [Google Scholar]
- [57].Brakemeier EL, Berman R, Prudic J, Zwillenberg K, Sackeim HA. Self-evaluation of the cognitive effects of electroconvulsive therapy. Journal of ECT 2011;27(1):59–66. [DOI] [PubMed] [Google Scholar]
- [58].Sackeim HA. Autobiographical Memory and Electroconvulsive Therapy: Do Not Throw Out the Baby. J ECT 2014;30:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sackeim HA. The cognitive effects of electroconvulsive therapy In: Moos WH, Gamzu ER, Thal LJ, editors. Cognitive Disorders: Pathophysiology and Treatment, New York: Marcel Dekker; 1992, p. 183–228. [Google Scholar]
- [60].Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. New England Journal of Medicine 1993;328(12):839–46. [DOI] [PubMed] [Google Scholar]
- [61].Sobin C, Sackeim HA, Prudic J, Devanand DP, Moody BJ, McElhiney MC. Predictors of retrograde amnesia following ECT. The American journal of psychiatry 1995;152(7):995–1001. [DOI] [PubMed] [Google Scholar]
- [62].d'Elia G. Unilateral electroconvulsive therapy. Acta Psychiatr Scand Suppl 1970;215:1–98. [PubMed] [Google Scholar]
- [63].Sackeim H, Decina P, Prohovnik I, Malitz S. Seizure threshold in electroconvulsive therapy. Effects of sex, age, electrode placement, and number of treatments. Archives of general psychiatry 1987;44(4):355–60. [DOI] [PubMed] [Google Scholar]
- [64].Weiss A, Hussain S, Ng B, Sarma S, Tiller J, Waite S, et al. Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy. Aust N Z J Psychiatry 2019;53(7):609–23. [DOI] [PubMed] [Google Scholar]
- [65].Galletly C, Clarke P, Paterson T, Rigby A, Gill S. Practical considerations in the use of ultrabrief ECT in clinical practice. The journal of ECT 2014;30(1):10–4. [DOI] [PubMed] [Google Scholar]
- [66].Spaans HP, Verwijk E, Comijs HC, Kok RM, Sienaert P, Bouckaert F, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. The Journal of clinical psychiatry 2013;74(11):e1029–36. [DOI] [PubMed] [Google Scholar]
- [67].Mayur P, Byth K, Harris A. Acute antidepressant effects of right unilateral ultra-brief ECT: a double-blind randomised controlled trial. Journal of affective disorders 2013;149(1-3):426–9. [DOI] [PubMed] [Google Scholar]
- [68].Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: clinical efficacy. Journal of affective disorders 2009;116(1-2):106–12. [DOI] [PubMed] [Google Scholar]
- [69].Wilcox PH. Electroshock therapy: a review of over 23,000 treatments using unidirectional currents. The American journal of psychiatry 1947;104(2):100–12. [DOI] [PubMed] [Google Scholar]
- [70].Sackeim HA, Long J, Luber B, Moeller JR, Prohovnik I, Devanand DP, et al. Physical properties and quantification of the ECT stimulus: I. Basic principles. Convulsive Therapy 1994;10(2):93–123. [PubMed] [Google Scholar]
- [71].Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. The journal of ECT 2010;26(3):159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Engel JJ. Seizures and Epilepsy. Philadelphia: F. A. Davis; 1989. [Google Scholar]
- [73].Abdelmalik PA, Burnham WM, Carlen PL. Increased seizure susceptibility of the hippocampus compared with the neocortex of the immature mouse brain in vitro. Epilepsia 2005;46(3):356–66. [DOI] [PubMed] [Google Scholar]
- [74].Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 2016;139(Pt 4):1079–93. [DOI] [PubMed] [Google Scholar]
- [75].van Diermen L, van den Ameele S, Kamperman AM, Sabbe BCG, Vermeulen T, Schrijvers D, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. The British journal of psychiatry : the journal of mental science 2018;212(2):71–80. [DOI] [PubMed] [Google Scholar]
- [76].Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. The Journal of clinical psychiatry 2015;76(10):1374–84. [DOI] [PubMed] [Google Scholar]
- [77].Daly JJ, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord 2001;3(2):95–104. [DOI] [PubMed] [Google Scholar]
- [78].Sackeim HA, Prudic J. Length of the ECT course in bipolar and unipolar depression. Journal of ECT 2005;21(3):195–7. [DOI] [PubMed] [Google Scholar]
- [79].Sienaert P, Vansteelandt K, Demyttenaere K, Peuskens J. Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disord 2009;11(4):418–24. [DOI] [PubMed] [Google Scholar]
- [80].Martin DM, Katalinic N, Ingram A, Schweitzer I, Smith DJ, Hadzi-Pavlovic D, et al. A new early cognitive screening measure to detect cognitive side-effects of electroconvulsive therapy? Journal of psychiatric research 2013;47(12):1967–74. [DOI] [PubMed] [Google Scholar]
- [81].Galvez V, Hadzi-Pavlovic D, Smith D, Loo CK. Predictors of Seizure Threshold in Right Unilateral Ultrabrief Electroconvulsive Therapy: Role of Concomitant Medications and Anaesthesia Used. Brain stimulation 2015;8(3):486–92. [DOI] [PubMed] [Google Scholar]
- [82].Martin DM, Galvez V, Loo CK. Predicting Retrograde Autobiographical Memory Changes Following Electroconvulsive Therapy: Relationships between Individual, Treatment, and Early Clinical Factors. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.