Abstract
Endoscopic stenting of the colorectum has emerged as a viable alternative to surgical interventions in a selected group of patients. The main indication for stenting is bowel obstruction. As such stenting can be used to palliate patients with metastatic disease or bridge patients to surgical intervention. The main advantages of stenting in the emergency setting include lower morbidity and mortality, lower incidence of stoma formation, shorter hospitalization, and better quality of life. For patients with unresectable disease and short life expectancy, stenting can be considered. However, for patients with longer life expectancy, the potential long-term complications of a metal stent such as erosion, migration, or obstruction have engendered debate whether such patients are better served by operative intervention. Stenting as a bridge to surgery is an alternative to surgery in patients who are high risk for emergency surgery but concerns remain regarding its impact on oncologic outcome in potentially curable patients.
Keywords: colorectal, obstruction, endoscopic, decompression, self-expanding metallic stent
Across the world and in most countries, colorectal cancer is the second to third most common malignancy in both men and women. 1 2 Recent data demonstrate an increasing risk in patients younger than 50 years, prompting the recommendations to screen patients with average risk starting at the age of 45 years instead of 50. 3 Approximately 20% of patients are diagnosed at presentation with stage 4 metastatic disease. 4 Patients with colorectal cancer can be asymptomatic or can present with symptoms such as bleeding and obstruction. Obstruction is the presenting symptom in 20 to 30% of patients with colorectal cancer. 5 This subgroup of patients with acute malignant obstruction poses significant challenges to the surgeon. A significant proportion of patients presenting with obstructing colorectal cancer have advanced stage disease, are elderly patients, and often have medical comorbidities. In addition to these factors that can adversely affect the outcomes of surgery, emergency surgery on an unprepped and dilated large bowel can be technically challenging and is associated with increased morbidity. 6 Emergency colorectal surgery is associated with significant morbidity, some mortality especially in the elderly patients, and a high rate of stoma formation. 7 These factors have triggered significant interest in exploring alternative interventions for patients with malignant obstruction. It is important to note that while colon cancer accounts for ∼80% of cases of malignant obstruction, patients with other types of malignancy such as hepatobiliary, gynecologic, and urologic can present with colonic obstruction secondary to extrinsic compression. Patients with acute malignant colonic obstruction have a 5-year survival rate of less than 20%, a less favorable prognosis compared with patients with colonic malignancy without obstruction. Without urgent decompression of the obstruction, the patient can suffer severe complications such as bowel ischemia, necrosis, and/or perforation with sepsis. Emergency surgery is associated with high morbidity (40–50%) and mortality rates (15–20%) compared with elective surgery where the mortality rate ranges between 0.9 and 6%. 8
The application of metal stents for the treatment of acute malignant colonic obstruction was first reported by Dohmoto in 1991. 9 Since its introduction, a significant experience has been accumulated worldwide and hundreds of scientific publications have reported the short- and long-term results of endoscopic stenting for malignant obstruction of the upper and lower gastrointestinal tract. 10 11 12 As such, endoscopic stent placement has emerged as a viable alternative to surgical intervention in a selected group of patients. While early studies have focused primarily on technical feasibility of stent placement, recent reports have investigated long-term clinical success, predictors of technical and clinical outcomes, and impact on oncologic outcome. 13 14 15 16
The purpose of this article is to review the role and results of endoscopic stent placement for palliation of patients with unresectable disease and for potentially curable patients who are bridged to elective surgical resection.
Indications for Colorectal Stenting
The main role of endoscopic colorectal stenting is to relieve malignant obstruction. The bulk of scientific literature available on colorectal stenting pertains to cancer. Although not the focus of this review, it is important to mention that colorectal stenting has been used to treat benign disease such as complex colonic fistula, strictures, and anastomotic complications. 17 18 19 Patients who present with acute malignant obstruction secondary to a primary colon cancer or an extracolonic malignancy can be considered for stenting. The short-term success rate and long-term patency of stent are highest when treating patients with primary carcinoma of the colon with intrinsic luminal lesions. 19 Patients with extracolonic malignancy who present with extrinsic compression of the large bowel carry a lower success rate for both short- and long-term results of stenting. 20 While a stent procedure can be considered in patients with extracolonic compression of the colon, it is best to avoid it for patients with long strictures or those associated with diffuse carcinomatosis because of higher failure rate. 19 Although there has been past discussion about the use of prophylactic stent placement in patients with metastatic disease to prevent potential obstruction, we do not recommend such practice. The main goal of stent placement is to relieve obstruction and avoid emergency surgery. In patients with metastatic unresectable disease, stenting is done with palliative intent in patients with limited life expectancy. Patients with potentially resectable disease can undergo stenting with the intent to bridge them to elective surgical intervention. Later, we will discuss the importance of patient selection when a stent is used as bridge due to potential adverse impact on oncologic outcome.
It is important to note the contraindications to colorectal stenting. Absolute contraindications include perforation with free intraperitoneal gas and disseminated peritoneal carcinomatosis with multifocal colonic strictures. Relative contraindications consist of early bowel ischemia and coagulopathy.
Stricture Evaluation, Patient Preparation, Technical Consideration, and Postprocedural Care
To ensure the highest technical and clinical success rates for endoscopic stenting, patient selection and preparation are key factors in outcome. The first step is determination of the location of the lesion and its morphology. Although colonic obstruction in any area of the colon can be tackled with endoscopic stenting, patients with tumor obstruction at the ileocecal valve or low rectum are not candidates for this procedure. Obstructing lesions at the ileocecal valve can be difficult to cannulate without much proximal margin of unaffected bowel segment to ensure proper stent deployment. In addition, rectal tumors that are readily palpable on digital examination should not be stented as stent placement is associated with significant discomfort to the patient due to the distal aspect of the stent touching the anorectal junction and upper anus. Furthermore, stenting in that location is associated with a high stent migration rate. Ideally, 4 cm of normal rectum above the anorectal junction is needed for successful stent placement. We recommend assessment of the lesion location and morphology with a Gastrografin enema or alternatively computed tomography scan with rectal contrast injection ( Fig. 1 ). Determination of the stricture location, length, and degree of obstruction can be helpful in determining the stent specification and type of delivery devices. Technical difficulties can be anticipated with completely obstructing lesions (no demonstrable lumen on Gastrografin enema) and lesions at a sharp turn/angulation such as splenic flexure location. From personal experience of the senior author, splenic flexure lesions are associated with an increased risk of perforation and proximal or distal stent migration after deployment.
Fig. 1.
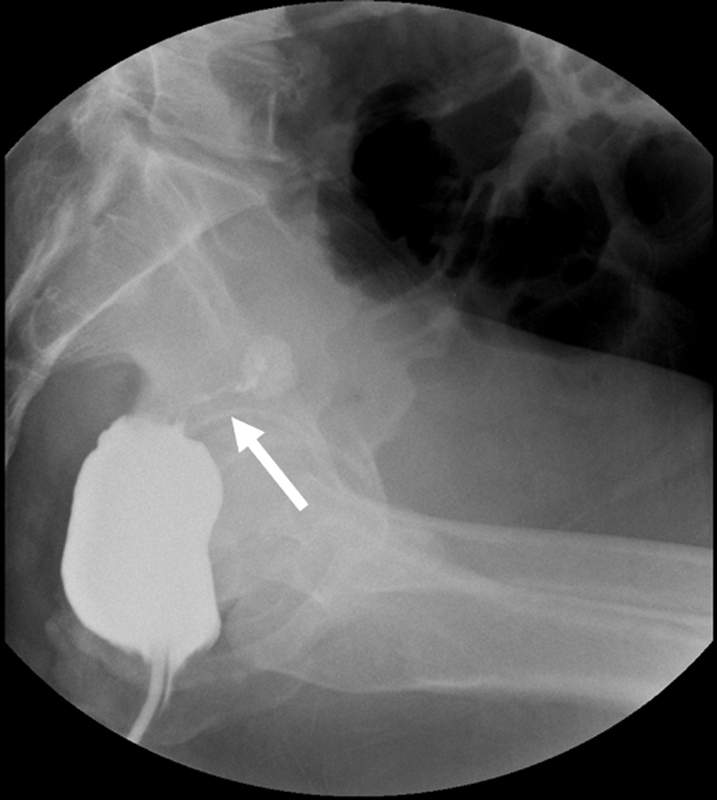
Gastrografin enema demonstrates an obstructing rectal cancer in a patient with stage 4 disease. A string like lumen is noted (arrow).
Preprocedural patient preparation includes counseling regarding the risks and benefits of stenting. Informed consent is obtained for the stent procedure and for potential surgical intervention in case of technical failure or complication. For patients with complete colorectal obstruction and dilated colon, we administer prophylactic intravenous antibiotics. For left-sided lesions, two rectal enemas are administered 1 hour prior to the procedure. We routinely perform the stent procedure in the endoscopy suite under endoscopic and fluoroscopic guidance. Intravenous sedation with propofol and fentanyl is administered by an endoscopy nurse for patient comfort. For patients with severe abdominal distention and potential airway compromise, we prefer to perform the procedure in the operation with an anesthesiologist to ensure airway protection as needed.
In a previous publication, we have described all the technical steps and tips for successful stenting. 21 We refer the reader to this literature for a more comprehensive understanding of the technical details. At the completion of the procedure, a plain abdominal radiograph is obtained to assess for the stent position, degree of expansion, and to rule out free air from perforation. The abdominal film is routinely repeated at 24 to 48 hours and on as needed based in the future depending on the clinical course. The majority of patients will experience the passage of many bowel movements within the first few hours of the procedure. If a nasogastric tube has been inserted before the procedure, it is typically removed within 24 to 48 hours of the procedure and a liquid diet is started. Although there is immediate onset of decompression following stent placement, most patients with complete or near complete obstruction require a few days for the dilated colon to return to its normal size. Patients who are stented as a bridge to surgery can undergo elective surgery 1 to 2 weeks later following medical and nutritional optimization and bowel preparation. Complications of stenting include perforation, erosion, bleeding, stent migration, and tumor ingrowth with obstruction. No current guidelines are available to guide long-term surveillance of palliative stents in patients with advanced disease. In our practice, we see the patient every 4 months and perform flexible sigmoidoscopy for left-sided stents. For more proximal stents, a Gastrografin enema or colonoscopy can be performed if indicated by clinical symptoms. Tumor ingrowth can be treated with endoscopic fulguration ( Fig. 2A, B ) or by deployment of a stent through a stent in terminally ill patients. Long-term survivors with tumors responsive to chemotherapy (stable burden of disease or very slow progression) are best treated by resection of the segment containing the metal stent. No objective data exist to guide the care of such patients, but in our current practice, we would consider resection of the stented segment after 1 year to avoid the potential long-term complications of metal stents. It is important to note that surgical intervention in patients with long-term indwelling stent can be very challenging due to gradual erosion of the metal stent through the bowel wall and/or significant inflammatory response to the metal ( Fig. 3 ). Such inflammatory reaction can be intense especially in patients who receive radiation therapy following stent placement ( Fig. 4 ). Under such circumstances, operative intervention should be carried by surgeons with extensive experience with difficult colorectal resection especially in cases of pelvic stents in male patients.
Fig. 2.
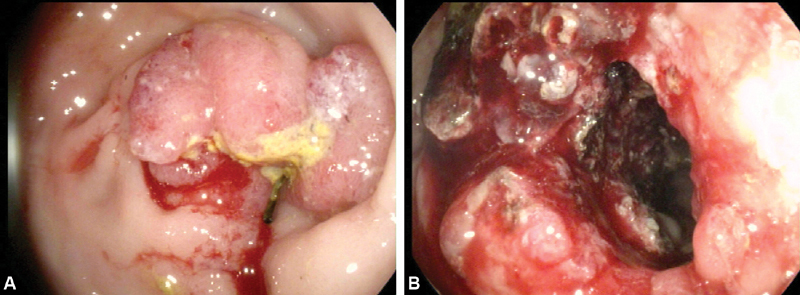
( A ) Tumor ingrowth 6 months after stent deployment. ( B ) Successful tumor fulguration using argon plasma coagulation.
Fig. 3.
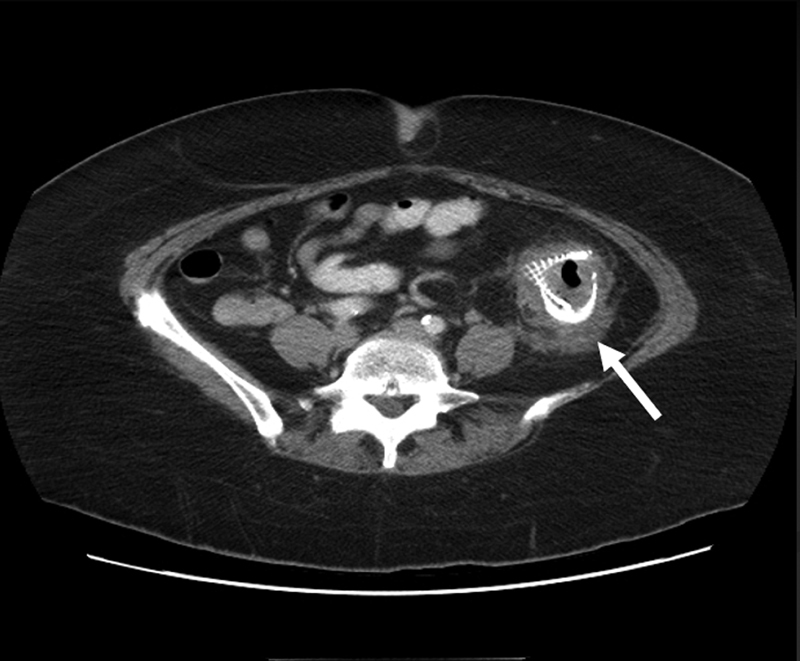
Computed tomography scan shows severe inflammatory and scarring response (arrow) surrounding a stent in the descending colon.
Fig. 4.
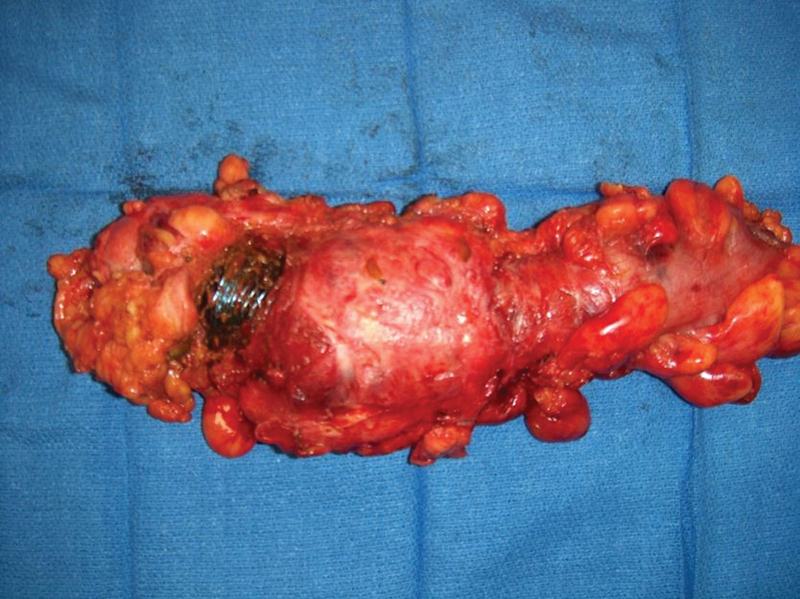
Severe inflammatory response with erosion of stent through rectal wall following radiation therapy.
Patient Selection and Outcome of Stent Placement for Palliation
This is considered in patients who are medically unfit for surgery and those who have advanced unresectable disease with symptomatic colonic obstruction. In both cases, the patient's life expectancy can be limited due to significant medical comorbidities or extensive disease burden. From an oncologic standpoint, patients with stage 4 colorectal cancer with malignant obstruction are good candidates. However, no current guidelines exist to stratify patients with stage 4 disease into those with short life expectancy versus those with potentially longer life expectancy. Stratification of such patients is very complex due to the wide spectrum of burden of disease (i.e., stage 4 with limited resectable liver or lung disease vs. diffuse metastatic disease), tumor biology, response to chemotherapeutic agent, and overall patient condition (i.e., weight loss, cachexia). Until such stratification becomes available in the future, an accurate determination of life expectancy is not possible and often it is a judgment call. In our practice, patients with stage 4 disease and an anticipated life expectancy of less than 1 year are considered for an endoscopic stenting procedure. Patients with limited disease burden, a good functional status, should be considered for surgical resection due to potential long-term risks of stents such as erosion and tumor ingrowth with obstruction. A personalized approach to each patient with a multidisciplinary discussion involving the patient, family, oncologist, and surgeon is helpful in determining the best course of action. While in the past, the focus of discussion was on technical feasibility and short-term benefits of stenting compared with surgery, it is important to assess the suitability of a patient for the procedure within an overall comprehensive approach.
Taking into consideration a personalized approach, a patient who undergoes a stent placement with palliative intent can reap many short-term benefits such as shorter hospitalization, less morbidity and mortality, potential avoidance of a diverting stoma, and earlier resumption of chemotherapy. 22 Karoui et al found that time to chemotherapy initiation was shorter in patients who underwent stent placement compared with those treated with surgical intervention (14 vs. 28.5 days, respectively). Two patients (6%) with stent placement who underwent subsequent chemotherapy experienced tumor perforation and required emergency surgery. Median survival was not different in the two groups (13.7 months for stent vs. 11.4 months for surgery). However, with continued evolution of more effective chemotherapeutic agents in the future, the impact on long-term survival may be more evident.
Palliative stenting of advanced colorectal cancer currently does not appear to affect the long-term survival but is associated with improved quality of life. 23 Endoscopic stenting seems to be equally as effective as surgical intervention in achieving bowel decompression. Lee et al found similar clinical success rate when comparing patients with unresectable metastatic colorectal cancer treated with emergency surgery to those who underwent emergency colonic stenting. 24 Their retrospective study compared 71 patients who received stent intervention with 73 patients who underwent emergency surgery. Stent intervention was associated with significantly fewer early postoperative complications (15.5 vs. 32.9%) and shorter hospital stay (13.3 vs. 24.4 days). However, the rate of late complications was significantly higher in the stent group (33.8 vs. 17.8%). Similarly, Vemulapalli et al reported the clinical and technical success rates, duration of hospital stay, early and long-term complications, and overall survival rate in 123 patients who underwent palliation of unresectable colonic malignancy. 25 Patients in the stent group ( n = 53) had significantly lower early complication rate and shorter hospital stay compared with patients in the emergency surgery group (70 patients) (8 vs. 30% and 2 vs. 8 days, respectively). The rate of late complications was higher in the stent group, although the difference was not statistically significant. No difference was noted in median survival (24 weeks for stent group vs. 23 weeks for the emergency surgery group). Small et al from the Mayo Clinic reported their long-term results with endoscopic stenting. 26 Similarly, long-term complications occurred more frequently in patients with stents. Perforation, stent occlusion, and/or migration accounted for an overall complication rate of 24.4%. Another study demonstrated a long-term clinical failure rate of 51% with a mean stent patency duration of 145 days. 27 Albeit some of these long-term complications can be avoided with the newer generation of metal stents (more tapered edges and no sharp protruding metal and better delivery devices), we believe that the risks associated with metal stents will remain a concern. Therefore, patient selection as previously discussed is of paramount importance to minimize these risks. An individualized risk versus benefit assessment needs to be done to balance the short-term advantages of stent with its potential long-term complications. 28 With the future introduction of more effective chemotherapeutic agents and the potential for more prolonged survival, the significance of this issue will become even more apparent.
Patient Selection and Outcome of Stenting as a Bridge to Future Surgical Intervention
The concept of stenting as a bridge to surgery was introduced to convert from an emergency surgical intervention to an elective one. The rationale for such an approach was to allow for medical and nutritional optimization of the patient and provide an opportunity for bowel cleansing. The advantages of such a strategy included lower morbidity and mortality, and decreased need for stoma formation. It is important to note that while numerous studies have advocated the use of stent as bridge to surgery, their major limitations are small number of patients and their retrospective nature. However, two clinical trials are worthy of discussion. Pirlet et al from France conducted a prospective, randomized, controlled clinical trial to compare metal stent intervention to surgical procedure for acute left-sided malignant colonic obstruction. 29 This multicenter trial involved nine centers. The primary end point of the trial was the need for a stoma, either temporary or permanent, and secondary end points included mortality, morbidity, and length of hospital stay. Unfortunately, the trial was stopped prematurely without full accrual due to a high rate of technical failure in the stent arm (54%) and a high perforation rate (7%). The Dutch Stent-In study group conducted a prospective randomized controlled trial in 25 hospitals in the Netherlands. 30 Patients with acute obstructive left-sided colorectal cancer were randomized into a group of bridge to elective surgery versus a group of emergency surgery. The study was suspended after two successive interim analyses due to an increased 30-day morbidity in the stent. Brehant et al conducted a small prospective study to investigate the effectiveness of stent placement in bridging patients with acute colorectal obstruction to elective surgery. 31 Technical success was noted in 25 out of 30 patients (83%) who underwent stent placement and 77% of the patients were successfully bridged to elective surgery without the need for a stoma. Out of the many studies published in the last decade on the use of stent for malignant colorectal obstructions, three meta-analysis worthy of discussion compared stent intervention as a bridge to surgery with emergency surgical intervention. 32 33 34 Tilney et al reported the results of 451 patients included in 10 studies. The stent technical success rate was 92.6%. Patients who received a stent had a shorter hospital stay, lower stoma formation rate, and lower mortality. 32 In a second meta-analysis by Tan et al, four randomized controlled trials of stent insertion in obstructing left-sided colonic cancer were included. 33 The technical and clinical success rates for stent were lower than expected (70.7 and 69%). Stent intervention was associated with a high incidence of clinical and silent perforation (6.9 and 14%, respectively). Successful primary anastomosis rate was higher and overall stoma rate was lower in the stent group compared with the emergency surgery group. Furthermore, there was no difference in permanent stoma, in-hospital mortality, anastomotic leak, 30-day reoperation, and surgical site infection between the two groups. Ye et al reported no advantages of stent placement compared with emergency surgery for left-sided malignant colonic obstruction in a meta-analysis of eight studies, involving 444 patients (219 with stent and 225 with emergency surgery). 34
With such mixed short-term results for stent intervention as a bridge to elective surgery, it is important to assess the long-term impact of stent placement on the oncologic outcome of potentially curable patients. Of particular concern is tumor spread in the bloodstream during endoscopic manipulation of the lesions and the potential intraperitoneal cavity spread in case of a silent or clinical perforation. Kim et al compared the long-term oncologic outcomes of elective laparoscopic surgery following stent insertion with one-stage open emergency surgical treatment of obstructive left-sided colon and rectal cancers. 35 Ninety-five consecutive patients with left-sided obstructive colorectal cancers were reviewed: 25 underwent stent decompression and elective laparoscopic surgery, while 70 underwent emergency open surgery with intraoperative on-table colonic lavage. The median follow-up was 51 months. The 5-year overall survival was 67.2% in the stent group compared with 61.6% in the surgical group. Similarly, no difference was noted in the 5-year recurrence-free survival (61.2% in the stent group vs. 60% in the surgical group). However, the perineural invasion of the primary tumor was more frequent in the stent group (76 vs. 51.4%, p = 0.033). Sabbagh et al showed worse overall survival in patients with left-sided malignant colonic obstruction who underwent stent insertion compared with those who received emergency surgery. 36 The 5-year cancer-specific mortality was significantly higher in the stent group (48 vs. 21%, p = 0.02). Stent perforation can lead to peritoneal seeding, upstaging of the patient's malignancy, and converting a potentially curable patient into an incurable one. In the recent guidelines published by the European Society of Gastrointestinal Endoscopy and endorsed by the Governing Board of the American Society for Gastrointestinal Endoscopy, the use of self-expanding metallic stent as bridge to surgery is not recommended because of the concerns about its oncological safety. 37 We believe that stent as a bridge to surgery should be avoided in patients with potentially curable colorectal malignancy and good functional status. It can be considered in special circumstances when immediate surgery carries significant medical risks such as in severely malnourished patients, recent cardiovascular or cerebrovascular event, or significant active pulmonary disease such as pneumonia.
Role of Stenting in Patients with Extracolonic Malignant Obstruction
Endoscopic endoluminal stent placement is most effective in decompressing intrinsic compression from a colorectal primary malignancy. However, large bowel obstruction can occur from extracolonic malignancy. Intra-abdominal malignancy such as esophageal, gastric, hepatobiliary and pancreatic, gynecologic, or urologic can present with colonic obstruction. Nonabdominal malignancies such as breast can also spread to the abdomen. While such extracolonic malignant obstruction can involve a short segment of large bowel, often they can be multifocal, can affect a long segment of the colon, and are often associated with carcinomatosis. Furthermore, many patients with recurrent extracolonic malignancy have a history of prior abdominopelvic surgery, intra-abdominal adhesions and fibrosis, and some cases, radiotherapy. Such factors can limit proper stent expansion. Although stenting of a long stricture is technically feasible, the lack of colonic propulsive waves due to the presence of metal in a long bowel segment often leads to a patent but nonfunctional lumen ( Fig. 5 ). In cases of significant fibrosis, the pliable metal stent may kink leading to persistent anatomical obstruction ( Fig. 6 ). All these factors lead to a lower success rate. Several studies have demonstrated this finding. Luigiano et al showed a lower patency rate in patients with extrinsic obstruction compared with those with intrinsic malignancies. 38 Another study reported 12 stent procedures performed in 11 patients with colonic obstruction from extracolonic cancers. 39 The underlying malignancies were ovarian, urinary bladder, kidney, prostatic, breast, cholangiocarcinoma, and carcinoid. The technical and clinical success rates were 42 and 25%, respectively. Both colostomy formation rate (45%) and the 30-day mortality rate (36%) were high. Stent placement in patients with extracolonic malignant obstruction seems less favorable compared with colorectal patients. 15 While it is reasonable to consider it under such circumstances as often the patient is terminally ill with a limited life expectancy, proper counseling of the patient and the family and setting the right expectation are important so that they are aware of the higher failure rate and potential need for operative intervention.
Fig. 5.
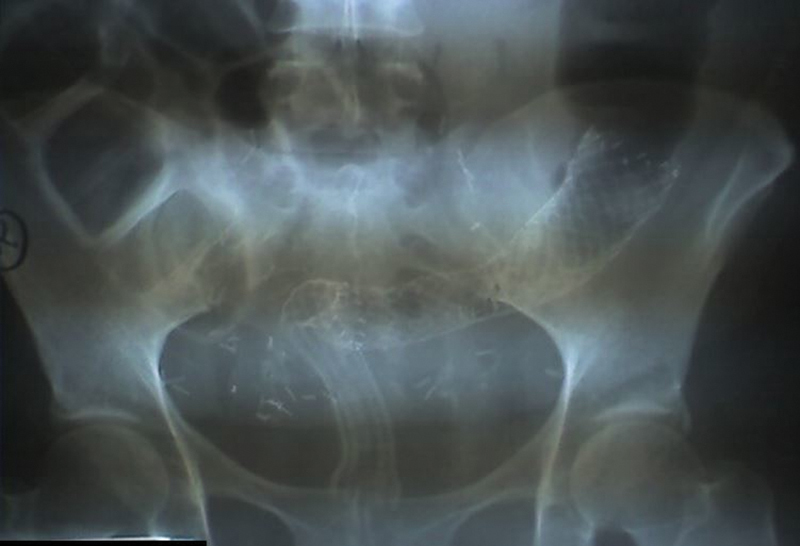
A patient with recurrent cervical cancer and a long rectosigmoid stricture. Despite the accurate deployment of two stents in tandem to relieve the blockage, the patient remained obstructed due to inability to propel stool through the long metal segment.
Fig. 6.
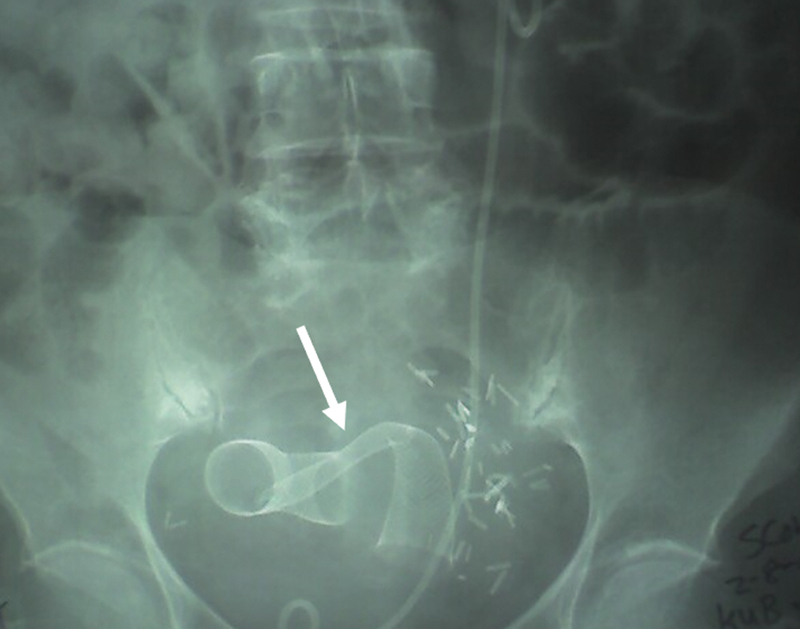
A patient with recurrent ovarian cancer and prior extensive pelvic surgery. Two-stent placement was performed to relieve the blockage. Due to the extensive nature of pelvic fibrosis and scarring, inadequate stent expansion and kinking was noted (arrow).
Conclusion
Malignant colorectal obstruction is a complex issue. When facing a patient in such situation, the surgeon needs to balance the immediate necessity to decompress the obstruction with a broader perspective of oncologic outcome. Short-term issues include morbidity and mortality of any therapeutic intervention, stoma formation rate, recovery, and time to initiation of adjuvant therapy. Long-term issues revolve around complications of a metal stent and oncologic outcome. Undoubtedly, the future will yield new endoscopic endoluminal technologies including a new generation of devices and stents. Coupled with the prospect of novel chemotherapeutic agents with a potential for a more prolonged survival for many patients, the management of large bowel obstruction will continue to evolve. For now, our current recommendations are summarized in Flowchart 1 . Such approach is based on the senior author with 15-year experience in therapeutic endoscopy and endoluminal stenting as a surgeon and the insight provided by the results of a growing body of literature.
Flowchart 1.
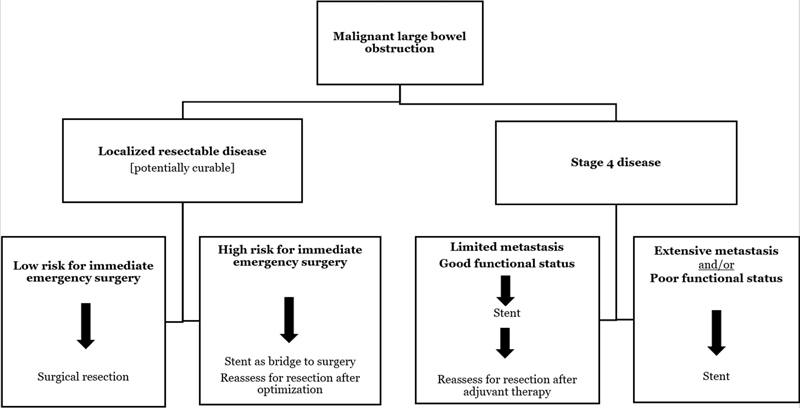
Algorithm for management of patients presenting with acute malignant colorectal obstruction.
Footnotes
Conflict of Interest None.
References
- 1.Arnold M, Sierra M S, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(04):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Colorectal Cancer Statistics May 29, 2018. May 29 2018 at:www.cdc.gov/cancer/colorectal/statistics/index.htm
- 3.Wolf A MD, Fontham E TH, Church T R. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(04):250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 4.Rosen S A, Buell J F, Yoshida A.Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg 200013505530–534., discussion 534–535 [DOI] [PubMed] [Google Scholar]
- 5.Aslam M I, Kelkar A, Sharpe D, Jameson J S. Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg. 2010;8(04):305–313. doi: 10.1016/j.ijsu.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Serpell J W, McDermott F T, Katrivessis H, Hughes E S. Obstructing carcinomas of the colon. Br J Surg. 1989;76(09):965–969. doi: 10.1002/bjs.1800760932. [DOI] [PubMed] [Google Scholar]
- 7.Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. 2003;46(01):24–30. doi: 10.1007/s10350-004-6492-6. [DOI] [PubMed] [Google Scholar]
- 8.Breitenstein S, Rickenbacher A, Berdajs D, Puhan M, Clavien P A, Demartines N. Systematic evaluation of surgical strategies for acute malignant left-sided colonic obstruction. Br J Surg. 2007;94(12):1451–1460. doi: 10.1002/bjs.6007. [DOI] [PubMed] [Google Scholar]
- 9.Dohmoto M. New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507–1512. [Google Scholar]
- 10.Davids P H, Groen A K, Rauws E A, Tytgat G N, Huibregtse K.Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction Lancet 1992340(8834-8835):1488–1492. [DOI] [PubMed] [Google Scholar]
- 11.Bethge N, Sommer A, Vakil N. Palliation of malignant esophageal obstruction due to intrinsic and extrinsic lesions with expandable metal stents. Am J Gastroenterol. 1998;93(10):1829–1832. doi: 10.1111/j.1572-0241.1998.00528.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y S, Li M H, Chen W X, Zhuang Q X, Chen N W, Shang K Z. Follow-up evaluation for benign stricture of upper gastrointestinal tract with stent insertion. World J Gastroenterol. 2003;9(11):2609–2611. doi: 10.3748/wjg.v9.i11.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui A, Cosgrove N, Yan L H. Long-term outcomes of palliative colonic stenting versus emergency surgery for acute proximal malignant colonic obstruction: a multicenter trial. Endosc Int Open. 2017;5(04):E232–E238. doi: 10.1055/s-0043-102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai M, Kamimura K, Takahashi Y. The factors influencing long-term outcomes of stenting for malignant colorectal obstruction in elderly group in community medicine. Int J Colorectal Dis. 2018;33(02):189–197. doi: 10.1007/s00384-017-2946-x. [DOI] [PubMed] [Google Scholar]
- 15.Abbas M A, Kharabadze G, Ross E M, Abbass M A. Predictors of outcome for endoscopic colorectal stenting: a decade experience. Int J Colorectal Dis. 2017;32(03):375–382. doi: 10.1007/s00384-016-2696-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda A, Miyashita M, Matsumoto S. Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22(02):497–504. doi: 10.1245/s10434-014-3997-7. [DOI] [PubMed] [Google Scholar]
- 17.Abbas M A. Endoscopic management of acute colorectal anastomotic complications with temporary stent. JSLS. 2009;13(03):420–424. [PMC free article] [PubMed] [Google Scholar]
- 18.Rayhanabad J A, Abbas M A. Long-term outcome of endoscopic stenting for benign and malignant colorectal disorders. Am Surg. 2009;75(10):897–900. [PubMed] [Google Scholar]
- 19.Abbas M A, Falls G N. Endoscopic stenting of colovaginal fistula: the transanal and transvaginal “kissing” wire technique. JSLS. 2008;12(01):88–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E J, Kim Y J. Stents for colorectal obstruction: past, present, and future. World J Gastroenterol. 2016;22(02):842–852. doi: 10.3748/wjg.v22.i2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackberg Z, Abbas M A. Colonic stenting: when and how. Semin Colon Rectal Surg. 2017;28(01):34–40. [Google Scholar]
- 22.Karoui M, Charachon A, Delbaldo C.Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration Arch Surg 200714207619–623., discussion 623 [DOI] [PubMed] [Google Scholar]
- 23.Nagula S, Ishill N, Nash C. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg. 2010;210(01):45–53. doi: 10.1016/j.jamcollsurg.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Lee H J, Hong S P, Cheon J H. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73(03):535–542. doi: 10.1016/j.gie.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Vemulapalli R, Lara L F, Sreenarasimhaiah J, Harford W V, Siddiqui A A. A comparison of palliative stenting or emergent surgery for obstructing incurable colon cancer. Dig Dis Sci. 2010;55(06):1732–1737. doi: 10.1007/s10620-009-0945-7. [DOI] [PubMed] [Google Scholar]
- 26.Small A J, Coelho-Prabhu N, Baron T H. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71(03):560–572. doi: 10.1016/j.gie.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Esparrach G, Bordas J M, Giráldez M D. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105(05):1087–1093. doi: 10.1038/ajg.2009.660. [DOI] [PubMed] [Google Scholar]
- 28.Súarez J, Jiménez J, Vera R. Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis. 2010;25(01):91–96. doi: 10.1007/s00384-009-0814-z. [DOI] [PubMed] [Google Scholar]
- 29.Pirlet I A, Slim K, Kwiatkowski F, Michot F, Millat B L. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25(06):1814–1821. doi: 10.1007/s00464-010-1471-6. [DOI] [PubMed] [Google Scholar]
- 30.van Hooft J E, Bemelman W A, Oldenburg B et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12(04):344–352. doi: 10.1016/S1470-2045(11)70035-3. [DOI] [PubMed] [Google Scholar]
- 31.Brehant O, Fuks D, Bartoli E, Yzet T, Verhaeghe P, Regimbeau J M. Elective (planned) colectomy in patients with colorectal obstruction after placement of a self-expanding metallic stent as a bridge to surgery: the results of a prospective study. Colorectal Dis. 2009;11(02):178–183. doi: 10.1111/j.1463-1318.2008.01578.x. [DOI] [PubMed] [Google Scholar]
- 32.Tilney H S, Lovegrove R E, Purkayastha S. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21(02):225–233. doi: 10.1007/s00464-005-0644-1. [DOI] [PubMed] [Google Scholar]
- 33.Tan C J, Dasari B V, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99(04):469–476. doi: 10.1002/bjs.8689. [DOI] [PubMed] [Google Scholar]
- 34.Ye G Y, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012;18(39):5608–5615. doi: 10.3748/wjg.v18.i39.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H J, Choi G S, Park J S, Park S Y, Jun S H. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28(03):407–414. doi: 10.1007/s00384-012-1556-x. [DOI] [PubMed] [Google Scholar]
- 36.Sabbagh C, Browet F, Diouf M. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258(01):107–115. doi: 10.1097/SLA.0b013e31827e30ce. [DOI] [PubMed] [Google Scholar]
- 37.van Hooft J E, van Halsema E E, Vanbiervliet G et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46(11):990–1053. doi: 10.1055/s-0034-1390700. [DOI] [PubMed] [Google Scholar]
- 38.Luigiano C, Ferrara F, Fabbri C. Through-the-scope large diameter self-expanding metal stent placement as a safe and effective technique for palliation of malignant colorectal obstruction: a single center experience with a long-term follow-up. Scand J Gastroenterol. 2011;46(05):591–596. doi: 10.3109/00365521.2011.551886. [DOI] [PubMed] [Google Scholar]
- 39.Trompetas V, Saunders M, Gossage J, Anderson H. Shortcomings in colonic stenting to palliate large bowel obstruction from extracolonic malignancies. Int J Colorectal Dis. 2010;25(07):851–854. doi: 10.1007/s00384-010-0941-6. [DOI] [PubMed] [Google Scholar]


