Abstract
Patients with inflammatory bowel disease (IBD) are at an increased risk for developing colorectal cancer (CRC). However, the incidence has declined over the past 30 years, which is probably attributed to raise awareness, successful CRC surveillance programs and improved control of mucosal inflammation through chemoprevention. The risk factors for IBD-related CRC include more severe disease (as reflected by the extent of disease and the duration of poorly controlled disease), family history of CRC, pseudo polyps, primary sclerosing cholangitis, and male sex. The molecular pathogenesis of inflammatory epithelium might play a critical role in the development of CRC. IBD-related CRC is characterized by fewer rectal tumors, more synchronous and poorly differentiated tumors compared with sporadic cancers. There is no significant difference in sex distribution, stage at presentation, or survival. Surveillance is vital for the detection and subsequently management of dysplasia. Most guidelines recommend initiation of surveillance colonoscopy at 8 to 10 years after IBD diagnosis, followed by subsequent surveillance of 1 to 2 yearly intervals. Traditionally, surveillance colonoscopies with random colonic biopsies were used. However, recent data suggest that high definition and chromoendoscopy are better methods of surveillance by improving sensitivity to previously “invisible” flat dysplastic lesions. Management of dysplasia, timing of surveillance, chemoprevention, and the surgical approaches are all areas that stimulate various discussions. The aim of this review is to provide an up-to-date focus on CRC in IBD, from laboratory to bedside.
Keywords: colorectal cancer, Crohn's disease, ulcerative colitis, dysplasia, surveillance, surgery, IBD
Colorectal cancer (CRC) is the third most common cancer and cause of cancer deaths in the developed world. In Europe, 412,000 people are diagnosed with CRC, and 207,000 patients died of the disease. 1 Similarly, in the United States, an estimated 140,250 new cases of CRC and approximately 50,630 deaths will be attributable to CRC in 2018, accounting for 8% of all cancer deaths. The lifetime risk of CRC for an average American is approximately 5%. 2
Inflammatory bowel disease (IBD) is a chronic condition affecting people of all ages. Crohn's disease (CD) and ulcerative colitis (UC) are the two main forms of IBD. Taking the United Kingdom as an example, it is known that IBD affects more than 300,000 people. The annual incidence of CD is between 0.1 and 16/100,000 in Europe. The incidence is increasing, especially in newly diagnosed children and also in developing countries. Children account for 20% of all newly diagnosed patients with CD. Similarly, with UC, the incidence is rising, with current estimates suggesting a range from 0.5 to 24.5/100,000 in Europe. One of the complications of IBD is the development of CRC. 3
The incidence of CRC in IBD was recognized many years ago with initial reports dating back to more than 80 years ago. Burrill Crohn first identified the incidence of CRC in IBD in 1925. This remains a problem, with CRC accounting for 10 to 15% of deaths in patients with IBD. 4
IBD-related CRC is estimated to account for less than 2% of all CRCs identified annually 5 and ranks as the third commonest condition associated with a high risk of developing CRC, with familial adenomatous polyposis and hereditary nonpolyposis CRC syndrome being the first and second. 6
Although a challenging topic with a great impact on patients with IBD, recent epidemiological studies have suggested a lower incidence of IBD-related CRC. Perhaps this change from historic data to current might be due to greater awareness and improvement in IBD surveillance programs. 7 In this article, we will discuss the etiopathogenesis and management options of IBD-related CRC.
Epidemiology of IBD-Related CRC
The epidemiological, clinical, and pathological features of IBD-related cancers differ from sporadic CRC. Cancers that occur in patients with IBD, and particularly UC, tend to be localized more evenly throughout the colon, are more likely to be synchronous, and have a higher histological grade than sporadic carcinomas. 8 Additionally, there is a higher prevalence of mucinous carcinomas in IBD. 9 Recently, there has been an increase in incidence of early-stage tumors (stages I–II) compared with IBD-related cancers from previous decades. Studies have shown that 50 to 60% of newly diagnosed IBD-related cancers are stage I or II. This is highly likely to be due to an increased level of awareness and early detection due to colonoscopic surveillance. 8
Incidence of CRC in UC
The risk of CRC increases after 8 to 10 years from establishing the diagnosis of UC. 10 11 Depending on the study and country, the risk of developing CRC in patients with UC fluctuates between 0.9- and 8.8-fold and between 0.8- and 23-fold in patients with pancolitis. The probability of developing CRC 10 years after diagnosis is reported to be 2%, which increases to 8% after 20 years and 18% after 30 years. 12 Recent population-based studies have shown a decreasing risk of CRC in IBD. 13
In 2015, Choi et al reported on a 40-year analysis of colonoscopic surveillance program for neoplasia in UC. The cumulative incidence of CRC in this cohort was 21.2% at 5 years and 35.8% at 10 years of disease. 14 This change in incidence may be explained with the widespread use of surveillance colonoscopy, 15 a chemoprotective effect attributable to the more use of maintenance therapy with 5-ASA (5-ASA) and immunosuppressive drugs, 16 17 more aggressive surgical intervention for high-grade dysplasia (HGD) and environmental factors ( Fig. 1 ). 18
Fig. 1.
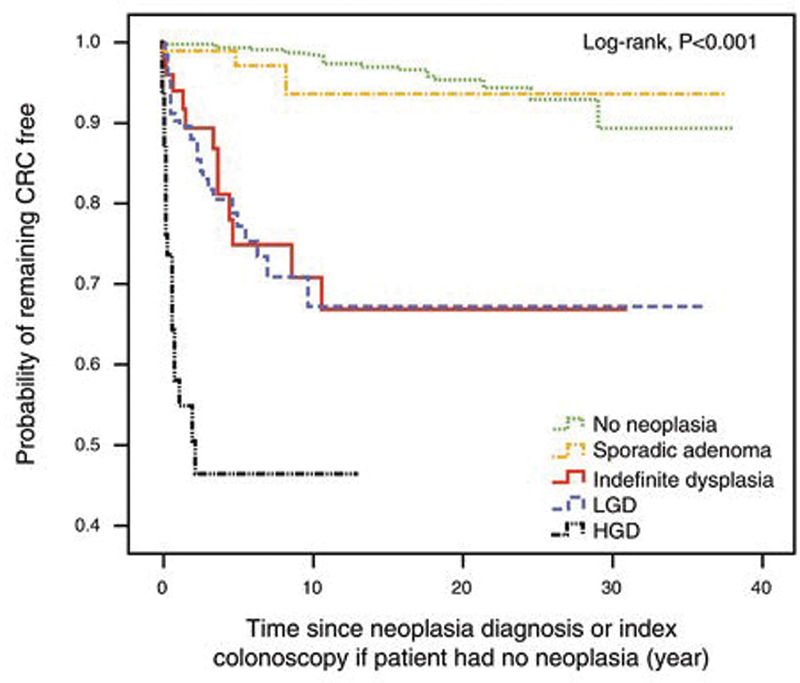
Kaplan–Meier plot and a life table showing cumulative incidence of CRC for each type of neoplasia grade. CRC, colorectal cancer; HGD, high-grade dysplasia; LGD, low-grade dysplasia. (Reproduced with permission from Am J Gastroenterol .)
Incidence of CRC in Crohn's Disease
While the relationship between UC and CRC has long been established, the association between Crohn's colitis and CRC was recently discovered. The risks remain similar and are attributed to the colonic extent of inflammation. 19 20 21
The relative risk of CRC in Crohn's colitis was been reported to be 23.8, whereas the risk was 4.3 in the general Crohn's population. 22 It has also been suggested that the relative risk of developing CRC in isolated colonic Crohn's was 6.9. 23 A study from Sweden demonstrated a relative risk of CRC of 5.6 for those with exclusively colonic involvement, as compared with a relative risk of 3.2 for patients with ileocolitis and 1.0 for patients with ileal involvement only. 24
Patients whose IBD was diagnosed prior to the age of 30 years have a higher relative risk of developing CRC than those diagnosed at an older age. A meta-analysis of 12 hospital and population-based studies of CRC risk in CD revealed an overall relative risk of 2.5. 25 In the subset of patients with colonic disease, this risk rose to 4.5. Patients with ileal disease only have the same risks as the general population. The cumulative risk of CRC for all patients with CD, regardless of disease distribution, was 2.9% after 10 years, 5.6% after 20 years, and 8.3% after 30 years of disease.
Patients who have only had small intestinal CD without colonic involvement are not considered to be at elevated risk for CRC. Patients with CD of the colon are at increased risk for dysplasia and CRC, and similar to UC, this risk is related to cumulative effect of colonic inflammation. 26
Risk Factors
Several factors increase the risk of CRC in patients with IBD, including age at the disease onset, extent, duration and severity of the disease, inflammatory complications, family history of CRC, and having primary sclerosing cholangitis (PSC). 27
The most important risk factor is the duration of the disease, with the incidence of CRC being relatively rare within the first 8 years of the diagnosis.
In a global meta-analysis by Eaden et al, the risk of developing IBD-CRC was reported to be 0.3% per year, or three cases of CRC per 1,000 patient-years of follow-up. The cumulative incidence of IBD-CRC in patients with UC was 2% at 10 years, 8% at 20 years, and 18% after 30 years of disease. The mean duration from IBD diagnosis to the development of IBD-CRC diagnosis was 16.3 years. 12
Söderlund et al 28 examined the risk of CRC according to extent of colitis in their population-based study, and reported the relative risks of CRC to be 2.7 for all patients with UC, 5.6 for pancolitis, 2.1 for Crohn's colitis, and 1.7 for proctitis. Patients with no significant colonic inflammation and patients with UC limited to the rectum are not at increased risk of CRC. 4 24
Inflammation is a determinant factor for the development of CRC, and severity of inflammation has been directly linked to CRC risk. 29 There is a strong association between endoscopic or histologic score of inflammation and CRC or dysplasia. The presence of postinflammatory polyps and strictures have also been associated with an augmented risk, while the macroscopically normal colon is not associated with a neoplastic risk. 30 Moreover, the development of colonic strictures is an important marker of disease severity. Persistence of inflammation may be the reason behind reports quoting that 2 to 3.5% of colonic strictures harbor dysplasia or CRC. 31
Predictors of malignant strictures include the development of strictures after 20 years of disease, location proximal to the splenic flexure, and symptomatic obstruction. 32
Both family history of CRC (whether IBD related or sporadic) and the presence of PSC significantly increase the risk of IBD-CRC by two- to threefold for family history. 33 A meta-analysis by Molodecky et al 34 reported that the pooled proportion of IBD in PSC cases was 68%. Among IBD subtypes, PSC was more common in UC than CD (80 vs. 10% of cases). In patients with pancolitis, the prevalence of PSC was about 6%, in contrast to 1% in those with only distal colitis. 35 Therefore, the more extensive the colonic involvement, the stronger association with IBD-CRC.
Pathogenesis and Molecular Biology
Colonic carcinogenesis in IBD is thought to be similar to the adenoma-carcinoma sequence found in sporadic CRC. The development of IBD-CRC progresses through a sequence from early/indefinite to low-grade dysplasia (LGD), and then to HGD prior to conversion to invasive adenocarcinoma. However, unlike sporadic CRC, which develops from dysplasia in one or two foci of the colon, cancer arising in colitis mucosa usually develops from multifocal dysplasia, indicating a “field change effect.” 6 10
The molecular pathway involves alterations in key regulatory genes in the colonic epithelium that are also found in sporadic CRC. However, the timing of these changes is different, probably because of chronic inflammation that characterizes long-standing IBD. 36
There are similarities of the genetic pathway between sporadic colon cancer and colitis-associated CRC, including microsatellite instability (MSI), DNA methylation, and mutation and loss of heterozygosity (LOH) of p53. However, the distinguishing features of IBD-CRC are differences in the timing and frequency of these alterations.
The two major types of genomic instability found in CRCs are chromosomal instability (CIN) and MSI. CIN and MSI in colitis-associated CRC have the same frequency (85% CIN and 15% MSI) as seen in sporadic CRC, but they differed in the timing and frequency from the pattern seen with sporadic CRC. 37
Loss of p53 tumor suppressor function and MSI are early events in IBD-associated CRC, while adenomatous polyposis coli (APC) loss of function, considered to be a very common early event in the development of sporadic CRC, is less frequent in IBD and appears to occur late.
In addition, MSI is an early event and K-Ras mutation is uncommmon in IBD-CRC in contrast to sporadic CRC. 38 Moreover, the oxidative stress is associated with p53 loss of functional mutations, hypermethylation of the MLH1 gene, and MSI. P53 LOH correlates with malignant transformation. 39
Aneuploidy is a marker of genomic instability and is found in 20 to 50% of dysplastic lesions and 50 to 90% of cancers; it is also detected in long-standing UC. As aneuploidy is often more widespread than dysplasia in IBD, substantial genomic alterations must occur in colonic mucosa without disturbing the morphology. 40
Chronic colitis-associated dysplasia may also be associated with aberrations in both innate and adaptive immune responses that are themselves proneoplastic. TNF is not only the fundamental mediator of mucosal inflammation in IBD but also plays a role in carcinogenesis, inducing activation of nuclear factor (NF)-κβ in the intestinal epithelium, subsequently upregulating antiapoptotic signals mediated by netrin-1 and Stat3 (via interleukin [IL]-6). 41
The adaptive immune response also appears to play a role in IBD-related carcinogenesis through the interaction between chronic inflammatory cytokines and the resident intestinal microbiome. Regulatory T-cells, stimulated by IL-10, dampen intestinal inflammation and induce epithelial apoptosis. 42 The global antiapoptotic effects resulting from the inflammatory cytokine milieu may serve to induce resistance against acute epithelial damage. 10 43 However, when inflammation persists, the antiapoptotic response in the setting of accumulating genomic damage culminates in mucosal dysplasia. Additionally, the development of dysplasia within chronically damaged de-epithelialized mucosa could also explain the nonpolypoid morphologies often observed in IBD-associated colonic dysplasia.
The frequent k-Ras mutations in sporadic CRC are believed to be responsible for the typical polypoid growth pattern, so their relative infrequency in IBD-associated CRC offers a biologically plausible explanation for the development of flat, nonpolypoid dysplasia. 44
As for CD-associated CRC, the data are limited; however, it has been noted that mutations in CARD15/NOD2 gene that activate NF-kβ might be associated with the pathogenic mechanism of CD. 45 Associations have also been found between CD and SNP in the Toll-like receptor 4 or IL-23 receptor. 46 K-Ras and p53 alterations seems to occur early during inflammatory tumor development, while APC and other mutations are rare in CD-associated CRC. 47
Dysplasia in IBD
Most CRCs develop from a dysplastic precursor lesion and patients with IBD develop dysplastic lesions that can be polypoid, flat, localized, or multifocal. 48
Dysplasia is defined as an unequivocal neoplastic alteration of the intestinal epithelium that remains restricted within the basement membrane, without invasion into the lamina propria. 49
Historically, three major classifications of dysplasia associated with IBD have been used. In 1983, Riddell et al 50 established a classification for dysplasia in IBD that has stood the test of time and incorporates four main categories (no dysplasia, indefinite for dysplasia, LGD, and HGD). 50 A second classification was proposed when Schlemper et al in 2000 headed a group of gastrointestinal pathologists from Europe, Japan, and North America to define a new (Vienna) classification to unify the terminologies. 51 This classification was further modified to be applicable to all luminal gastrointestinal tract cancers.
The distinction between LGD and HGD depends on the distribution of nuclei within the mucosa, with LGD usually defined by crowded, hyperchromatic nuclei localized in the basal half of the cells compared with HGD demonstrating nuclear stratification and loss of cellular polarity ( Fig. 2 ). 52
Fig. 2.

(A) Low-grade dysplasia. (B) High-grade dysplasia.
Pathogenesis of CRC in IBD can follow the standard pathway of progression from no dysplasia to indefinite dysplasia to dysplasia (LGD and HGD) and ultimately to CRC. In IBD, however, CRC appears to be able to develop directly from any of the dysplastic lesions (indefinite, LGD, or HGD) without following the standard pathway. The frequency of progression of LGD to HGD or CRC is low. When IBD patients with LGD where followed up for 3 years, 4.9% (five patients) progressed to either HGD (three patients) or CRC (two patients). The risk of malignant evolution, however, is higher in flat dysplasia in the distal colon. 26 The most important predictor for the development of HGD and CRC from LGD is a nonpolypoid, or plaque-like (Paris type 0-IIa—flat elevated height less than 2.5 mm above mucosal surface or type 0-IIb—completely flat) appearance of the lesion. The other predictors are macroscopically invisible dysplasia, lesion >1 cm and previously identified indefinite dysplasia. The higher the number of these predictive factors, the greater the risk of development of HGD or CRC in a LGD lesion ( Table 1 ). 53
Table 1. Definition of different types of dysplasia.
| Term | Definition |
|---|---|
| Visible dysplasia | Dysplasia discovered on targeted biopsies |
| Polypoid | Lesion protruding > 2.5 mm into the lumen |
| Pedunculated | Attached to the mucosa by a stalk |
| Sessile | Base of the lesion is contiguous with the mucosa |
| Nonpolypoid | Lesion protruding < 2.5 m into the lumen |
| Elevated | Protrudes less than the height of a closed cup of biopsy forceps |
| Flat | At the level of the mucosa |
| Depressed | At least a portion of the lesion depressed below the level of surrounding normal mucosa |
| Invisible dysplasia | Discovered on nontarget biopsy |
Surveillance in IBD
The aim of surveillance is to identify any dysplasia prior to the development of CRC, or cancer at an earlier stage to allow an appropriate management, improving quality of life and survival rates.
Even if randomized controlled trials have not been performed to verify that surveillance colonoscopy is effective, indirect evidence supports an efficacy of surveillance in reducing the risk of death from IBD-associated CRC and that surveillance may be acceptably cost-effective, although no evidence supports that surveillance colonoscopy improved survival in patients with extensive colitis. 54
Surveillance Guidelines
Several guidelines are available for recommending a specific surveillance program in IBD patients.
The Cochrane review suggests that surveillance is effective at reducing the risk of death from CRC detecting cancer at earlier stage and thus have a better prognosis, even if there is no strong evidence that surveillance colonoscopy prolongs survival in extensive colitis. 55 This was supported by a nation-wide Dutch study of 149 patients with CRC over a period of 15 years. This study found that after 5 years of CRC diagnosis, survival was 100% in the surveillance group compared with 74% in the nonsurveillance group, and CRC was detected at earlier stage in the surveillance group. 56
Most of these studies agree that screening colonoscopy should be performed on patients during clinical remission of the disease in order to avoid bias of inflammatory changes with dysplasia that surveillance colonoscopy should be started 8 to 10 years after the onset of symptoms for patients with left-sided or extensive colitis and that regular surveillance need to be followed up after the initial colonoscopy.
Timing of Surveillance
Current guidelines recommend initiating CRC surveillance at 8 to 10 years after onset of IBD symptoms for all UC patients (excluding those with isolated proctitis) and for CD patients with at least one-third colonic involvement ( Fig. 3 ). 10 57
Fig. 3.
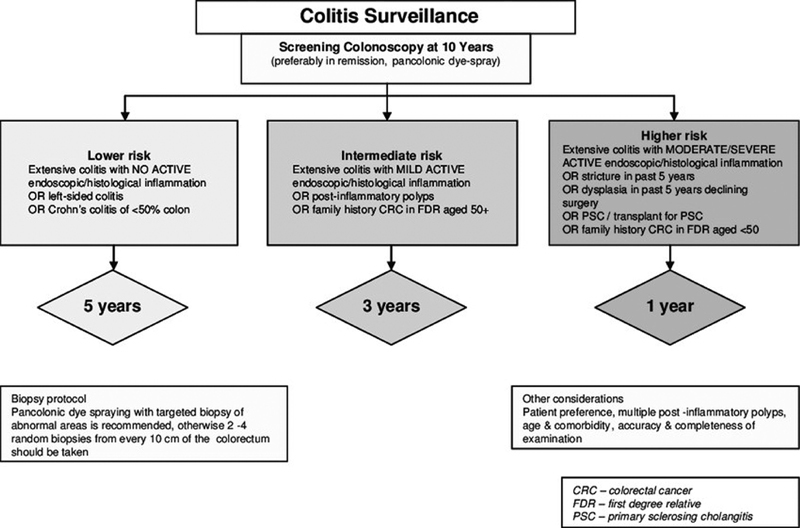
Algorithm for colitis surveillance ( www.bsg.org.uk ).
The American Cancer Society recommends screening colonoscopy 8 years after the onset of pancolitis (both CD and UC) and 12 to 15 years after the onset of left-sided colitis, while the British Society of Gastroenterology recommends screening colonoscopy at 10 years after the onset of symptoms in both UC and CD. The European Crohn's and Colitis Organisation (ECCO) guidelines are similar, apart from that they recommend screening colonoscopy at 8 years after symptom onset in patients with distal UC and at 6 to 8 years in patients with CD. 58 Furthermore, the Australian Cancer Council recommends screening colonoscopy at 8 years after symptoms onset in patients with at least distal UC and CD involving at least one-third of the colon or complicated anorectal disease.
Methods of Surveillance
Traditionally, surveillance colonoscopy with standard white light endoscopy (WLE) was used in screening programs. The technique was to undertake random colonic four quadrantic biopsies every 10 cm of colon in order to screen for dysplasia, providing about 33 biopsies. However, a random biopsy only samples less than 1% of the total colon surface area and has a detection rate of < 2 per 1,000 biopsies. 59
The use of high-definition endoscopic equipment leads to an enhanced visualization of mucosal details that dramatically improved dysplasia detection in IBD. A retrospective observational study in 357 patients with IBD found high-definition colonoscopy detected over twice as many dysplastic lesions compared with standard definition WLE. Moreover, retrospective studies have reported that dysplasia detected on random biopsies was visible in 90 to 94% of cases using high-definition endoscopic equipment. 60
The random-biopsy protocol is now increasingly criticized, and focus is being placed on target biopsies supported by chromoendoscopy (CE) or other newer endoscopic techniques, such as equipment-based image-enhanced endoscopy and narrow-band imaging (NBI). 59
CE involves spraying a dye, such as methylene blue or indigo carmine, on colonic mucosa to enhance the visualization of the border, surface topography, and crypt pattern of any lesion present during surveillance endoscopy. The benefit of CE is that it can highlight mucosal irregularities, leading to higher detection rates with a sensitivity of 93 to 97% and a specificity of 93%.
An early prospective, randomized, controlled trial demonstrated superiority of CE using methylene blue to a random-biopsy approach. 61
The limitations of CE include its operator dependence, need for an adequate bowel preparation, and the concern that it might be too time-consuming. 62
The SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations) guidelines supported CE over WLE, whether using standard or high-definition colonoscopy. 11
CE can be improved adding confocal laser endomicroscopy, which, in combination with CE, has shown an increase in diagnostic yield for CRC compared with CE or standard colonoscopy alone. 63
Equipment-Based Image-Enhanced Endoscopy
This technique relies on NBI, flexible spectral imaging color enhancement, blue laser image, autofluorescence imaging, and i-scan, of which clinical trials are only available for NBI and AFI. All these techniques, however, follow the same principle which is the intensification of the superficial structural and vascular aspects of the mucosa by filtering the white light images. 64
NBI uses filters to provide narrow bands of blue and green light wavelengths to accentuate the intestinal mucosal surface.
Further attempts to study the use of other equipment-based image enhanced endoscopy have been pursued, but no convincing evidence is available to currently recommend their use in routine practice for IBD surveillance. 65
Noninvasive Surveillance
Noninvasive surveillance methods, such as stool DNA and RNA test, have not been widely used or studied for IBD surveillance specifically, but studies are currently underway.
Special Conditions
Patients with PSC have a higher risk to develop CRC. A 33% of incidence of CRC at 20 years of disease duration is found in patients with concomitant PSC. 66
Deoxycholic acid may play a role in carcinogenesis in PSC. Current recommendations suggest starting surveillance colonoscopy immediately after diagnosis and following with annual follow-up. 67
Ursodeoxycholic acid (UDCA) has shown some benefit in reducing colonic dysplasia in this population: this effect can be related to effect in reducing reduced colonic concentration of deoxycholic acid. 68 However, the CRC risk persists after orthotopic liver transplantation. 69
Ileal Pouch–Anal Anastomosis and Pouch Surveillance
Development of dysplasia in patients who have undergone colectomy with ileal pouch–anal anastomosis (IPAA) is quite uncommon, either in ileal pouch mucosa and anorectal mucosa. 66
Derikx et al 67 suggested that 1.8% of patients develop pouch neoplasia, with 1.3% developing adenocarcinoma. Risk factors for developing dysplasia after IPAA surgery include history of dysplasia or CRC, history of PSC, refractory pouchitis, and atrophic pouch mucosa with severe inflammation. 67 68 Considering these factors, patients with a history of prior CRC, PSC, or refractory pouchitis should undergo annual surveillance, with biopsies being obtained in the pouch as well as distally within the anal transition zone. 34 The ideal timing for surveillance in patients with pouch and without risk factors following an IPAA is still unknown. Currently, many studies suggest an interval of every 3 years in the setting of a pouch and long-standing history of IBD.
Medical Management and Chemoprevention
Chemoprevention of cancer is defined as the administration of a synthetic, natural, or biological agent able to reduce, reverse, or delay the occurrence of malignancy. 70 A chemopreventive agent should be effective for eliminating or reducing the risk, safe, acceptable to patients, and inexpensive.
In UC-associated CRC, chronic inflammation is one of the most important risk factors, and it is the only one potentially modifiable: reducing inflammation means reducing the risk of developing CRC.
Pinczowski et al in 1994 71 first reported a chemopreventive effect of sulfasalazine in IBD, then several agents with chemopreventive potential have been identified, including 5-ASA compounds, immunomodulators, UDCA, and folic acid. 72
Mesalazine (5-ASA) is a derivative of aspirin without gastrointestinal toxicity, antineoplastic properties, and it activates the peroxisome proliferator activated receptor which helps maintain mucosal integrity and can suppress carcinogenesis by interfering with the Wnt/β-catenin signaling pathway. It also has an antioxidant and free radical scavenger properties, which help reduce DNA oxidative stress and the development of MSI. Thanks to the propriety of reducing beta-catenin signaling, a key regulator in proliferative mechanism, 5-ASA may also reduce colitis-induced dysplasia. It have been shown that mesalazine can also downregulate the expression of c-myc oncogene, which is noted to be overexpressed in the presence of dysplasia. 73
Purine analogs azathioprine and mercaptopurine are increasingly used as maintenance treatment in IBD because these can induce sustained remission by reducing colonic inflammation and promoting mucosal healing.
In the CESAME National French prospective observational cohort study, the use of thiopurine was associated with a reduced hazard of CRC among those with long-standing extensive colitis. 74
Immunomodulators also have anti-inflammatory properties and are used as maintenance therapy in IBD patients. Vedolizumab, for example, is an antiadhesion biologic therapy approved for UC and CD in 2014, capable to reduce intestinal inflammation by retarding leukocyte migration. While immunomodulators anti-inflammatory properties are well established, only limited evidence exists for a chemopreventive role for immunomodulators in IBD. A recent population-based study using a database linked to a nationwide pathology network from the Netherlands demonstrated a significant chemopreventive effect of thiopurine in patients with IBD. However, the majority of studies have not shown a chemopreventive effect for immunomodulators. 49
The role of UDCA in chemoprevention is well supported from studies of patients with concomitant IBD and PSC. The colonic concentration of additional bile acids has been implicated as a carcinogen, as this is cytotoxic to colonic epithelial cells and induces hyperproliferation. 56 An analysis of a randomized, placebo-controlled trial of patients with UC and PSC demonstrated a significant chemoprotective effect for UDCA in these patients, with a 74% reduction seen in the risk for dysplasia or cancer in those assigned to the UDCA group. 75
Tung et al 76 reported that UDCA use was strongly associated with a decreased prevalence of colonic dysplasia. However, a retrospective analysis of data from a randomized controlled trial using a high-dose UDCA (28–30 mg/kg body weight) showed that long-term use of high-dose UDCA was associated with an augmented risk of colorectal neoplasia in patients with UC and PSC. 75 The chemoprotective effect of using UDCA for patients with UC but without PSC has not been explored.
Folate deficiency may have a role in colon carcinogenesis as it may lead to aberrant DNA synthesis and repair, hypomethylation, and decreased apoptosis. IBD patients can be deficient in nutrients due to poor oral intake and decreased absorption, particularly in active disease. Observational studies investigating the benefit of folate replacement in chemoprevention for dysplasia and CRC in IBD patients, however, did not reach statistical significance, and thus, recommendation of folate supplementation in routine practice is not undertaken for CRC prevention. 77
The role of statins in chemoprevention of CRC was studied due to their ability to competitively inhibit 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an enzyme overexpressed in cancer cells and statins can also induce apoptosis in cancer cell lines in vitro. In a meta-analysis of 42 studies, chemopreventive effect of statins on sporadic CRC was modest. 78
Management of Dysplasia in IBD
Discernible dysplastic lesions in areas of the colon uninvolved by colitis should be treated with standard polypectomy techniques, and surveillance should continue based on the patient's IBD risk.
Polypoid Visible Lesion
For polypoid visible lesions, it is critical to establish if the polypoid lesion can be fully removed. Endoscopic resection is indicated only if complete resection is possible. Depending on the size of the polypoid lesion, endoscopic mucosal resection (EMR) may be considered, even if only few, small studies have demonstrated success with this technique. 79 80 The current standard of care is to obtain additional biopsies of the flat mucosa around the polypectomy site to verify absence of any surrounding dysplasia. 10 81 82 However, Lahiff et al and Ten Hove et al in two recent studies criticize this approach because further biopsies rarely provide any additional information. 83 84
Follow-up of patients with completely resected dysplastic polypoid lesions depends on the type of lesion: if polypoid visible dysplasia is present but there is no history of underlying colitis in the area, routine IBD-specific surveillance should be considered. However, in areas where colitis is present, a closer surveillance monitoring with colonoscopy in 6 to 12 months is recommended. Patients with larger sessile lesions that are removed via EMR or piecemeal resection should repeat surveillance in 3 to 6 months, followed by annual controls if the initial follow-up shows no evidence of residual polyp. 11 When lesions are not endoscopically resectable or there is evidence of endoscopically invisible multifocal LGD or invisible HGD, total proctocolectomy (TPC) should be recommended. 10 82
Nonpolypoid Visible Lesions
Nonpolypoid visible lesions should be evaluated for safety and efficacy of endoscopic resection. The presence of submucosal invasion or underlying malignancy is associated with a lack of success of endoscopic resection and includes depressed or ulcerated lesions, unclear margins, inability to lift the lesion with submucosal injection, and flat malignant changes next to the lesion. 85 In addition, biopsies should be obtained adjacent to the resection site, and a tattoo should be placed to aid in future surveillance. 86
Only few data regarding the risk of CRC after resection of nonpolypoid dysplastic lesions are available. Recent studies suggest early surveillance colonoscopy at 3 to 6 months for resected nonpolypoid dysplastic lesions. 11 For unresectable nonpolypoid lesions, proctocolectomy should be considered after biopsies confirm the presence of dysplasia.
Endoscopically Invisible Dysplasia
Endoscopically invisible dysplasia detected through random biopsies should be confirmed from pathologist experienced in IBD because there is a significant interobserver variability in the diagnosis of IBD-associated dysplasia. 10 87 Invisible dysplasia is associated with the presence of synchronous CRC. In fact, synchronous CRC is present in 22% of patients with invisible LGD, while the estimated rates of CRC in invisible HGD range from 45 to 67%. 30 88
Recent guidelines recommend referral to an experienced center for CE with high-definition endoscopy and repeat random biopsies after detection of invisible dysplasia. 11 If visible lesions are present on CE, resection and further follow-up should be recommended. In the case that LGD and no presence of dysplasia are present on CE, it is necessary to consider risks and benefits of surveillance versus proctocolectomy.
In a 2003 study of 46 patients with LGD, 7 (15.2%) patients developed CRC and 4 of 17 (23.5%) patients who underwent colectomy for LGD were found to have either HGD or CRC. 89 In a recent study, Navaneethan et al 90 analyzed 102 patients with LGD and found that with a median follow-up of 36 months, only 4.9% of patients progressed from LGD to either HGD or CRC.
Surgical Management Options
The diagnosis should be confirmed by an external second pathologist because there is a significant interobserver variability in the interpretation of the presence and the grade of dysplasia even among experienced gastrointestinal pathologists given the chronic inflammatory changes in the mucosa and the effect of concomitant medical therapies. 58
Indications for Surgery
High-Grade Dysplasia
According to the ECCO statement, this condition represents an indication for surgery because of the risk of a concomitant or future CRC. This is supported by three studies, including a limited number of cases with HGD, which have found association with syncronous concomitant CR in 42 to 67% or cases. 89 91 92 In a review of collected data from prospective surveillance trials, 15 of 47 patients (32%) with HGD developed CRC upon further follow-up. 91 Data from the St Mark's surveillance program confirmed the risk, since two of eight patients (25%) with HGD without colectomy progressed to CRC. 30 Overall, the immediate and subsequent risk of CRC in patients with flat HDG is large enough to warrant a recommendation for surgery. 93
Nonadenoma-Like HGD/LGD Lesions
Patients with nonadenoma-like dysplastic raised lesions should undergo colectomy, regardless of the grade of dysplasia detected on biopsy analysis because of the high association with metachronous or synchronous carcinoma.
All raised lesions should be completely endoscopically resected if it is possible and the surrounding area should undergo biopsy: surgery should be considered if dysplasia is present in the adjacent mucosa or if the mass cannot be completely resected. 93 If polyps in colonic segments proximal to UC involvement are present, they should be treated as sporadic adenomas.
Adenoma-Like Lesions
Adenoma-like raised lesions can be adequately treated by polypectomy provided that the lesion can be completely excised, no dysplasia at the margins of the specimen and no evidence of flat dysplasia elsewhere in the colon, the raised lesion. There is no surgical indication here. 58
Low-Grade Dysplasia
The current data are insufficient to assess the balance of risks and benefits of colectomy for flat LGD. The decision to suggest colectomy or continued surveillance should be tailored to the single patient after careful discussion, from the moment that the reported variability in the risk of progression to HGD or cancer (0–50%). 94 95
Surgical Options
Although differing in patterns of inflammation, UC and CD colitis are often grouped together for both surveillance and treatment recommendations, with TPC being the only definitive procedure to treat all synchronous and metachronous colonic and rectal lesions, the recommended surgical procedure in case of dysplasia in UC or cancer is proctocolectomy with IPAA, considering oncologic principles. There are no data supporting an oncologic advantage of mucosectomy and handsewn anastomosis over stapled anastomosis in this setting. Colectomy with ileorectal anastomosis (IRA) could be considered in selected patients. 58 96
In patients with a preoperative diagnosis of dysplasia/cancer, proctocolectomy should include oncologic lymphadenectomy with ligation of the vessels at their origins. Restorative surgery is feasible in most patients, while an abdominoperineal excision with end ileostomy should be performed in patients with very low rectal cancer where adequate distal clearance cannot be obtained or in whom the anal sphincter is damaged. 92
CD is a surgical decision-making challenge due to the segmental nature of inflammation, the presence of perianal disease, malignant transformation of anal fistulae, and risk of small bowel carcinoma. Most surgeons support TPC when cancer or dysplasia is discovered, but this usually results in a permanent ileostomy. Both total abdominal colectomy (TAC) and segmental colectomy in patients with CD (as well UC) with comorbidities are possible alternatives but require careful preoperative patient counseling regarding cancer risk and compulsive postoperative surveillance of any remaining colorectum. IPAA is not recommended for patients with CD.
TPC and IPAA versus end ileostomy . The risk of development of cancer in the ileal pouch or at the anal anastomosis is still present and patients should follow regular surveillance. Incidences of neoplasia in patients with the IPAA are approximately 1% at 5 years and up to 5% at 25 years, with higher rates in patients who undergone previous resections for colonic cancer. 97 Most of these tumors are found in retained rectal mucosa at the IPAA. However, a TPC with ileostomy abolish the risk of subsequent cancer development by virtual removal of the anus. A permanent ileostomy, instead of an IPAA, is usually performed in the patient who has sphincter weakness or involvement with tumor, wants to avoid multiple operations (as would be necessary for the IPAA), or has comorbidities that prevent living with the expected augmented bowel movements ( Fig. 4 ).
Fig. 4.
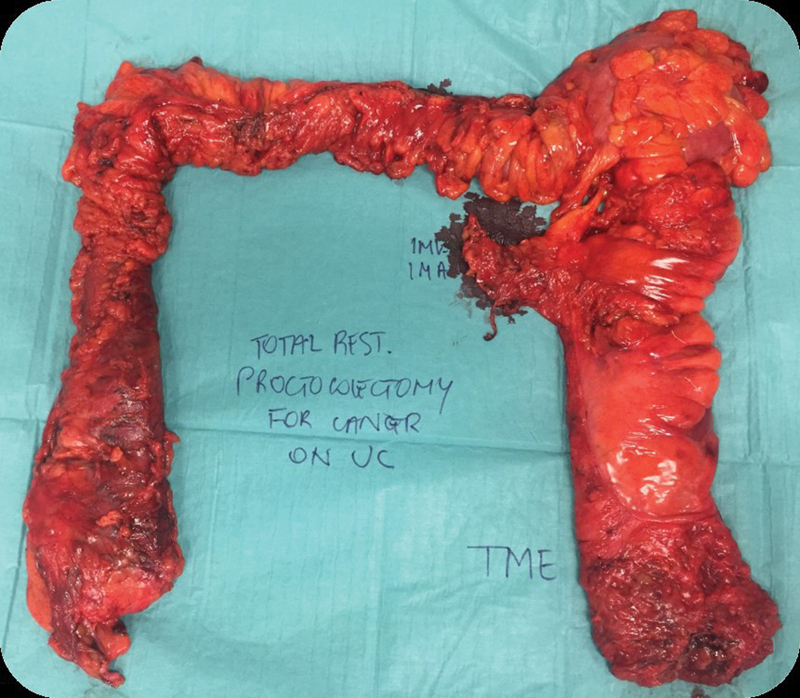
Total proctocolectomy with ileal pouch–anal anastomosis and TME (Colorectal Surgery Unit, Humanitas Research Hospital, Milan). TME, total mesorectal excision.
Contraindications to IPAA
IPAA is contraindicated in patients with poor anal sphincter function, for example, in the elderly postpartum female population. Patients with function warrant physiological studies, such as manometry as part of a preoperative workup. For these patients, TPC with end ileostomy is most often the more appropriate choice. 98
Complications of IPAA
Pouchitis is the most common postoperative complication in patients undergoing pouch surgery. Reported risk factors for pouchitis include younger age at colectomy, smoking status, regular use of nonsteroidal anti-inflammatory drugs, extensive UC, the presence of backwash ileitis, and extraintestinal manifestations, particularly PSC. 99
TAC with IRA versus End Ileostomy
In the few patients who have no evidence of inflammation or dysplasia in the rectum, a TAC/ileostomy with a blind Hartmann's rectal stump or TAC with IRA may be offered as an alternative to the TPC. Leaving the rectum has the advantage of a significant decrease in the morbidity of the operation by avoiding pelvic dissection. Before the advent of the IPAA, the TAC with IRA was the main continence-sparing procedure for IBD-related dysplasia. The TAC has many advantages over the TPC; most importantly, the avoidance of pelvic dissection and possible subsequent pelvic nerve damage that compromises sexual or urinary function or adhesions that worsen female fertility. Patients who have undergone TAC/IRA should undergo completion proctectomy if rectal dysplasia is subsequently discovered ( Fig. 5 ). 96
Fig. 5.

Laparoscopic colectomy with ileorectal anastomosis (Colorectal Surgery Unit, Humanitas Research Hospital, Milan).
Surgical Options in CD
Currently, the choice of the appropriate surgical procedure in all IBD-related dysplasia is influenced by both lesion morphology and patient fitness for surgery. CD presents distinct features to UC in this regard. In CD, once dysplasia is identified, segmental resection is a more feasible option than in UC, especially if there is no active inflammation in other parts of the colon. However, extended colectomy or even TPC with end ileostomy is often more practical choices due to the significant risk of synchronous dysplasia or cancer. Moreover, in CD patients with severe perianal disease or incontinence, TPC with end ileostomy offers treatment to resolve the perianal morbidity. IPAA reconstruction is a very rare option in CD patients with or without dysplasia, as it requires an entirely normal small bowel and no evidence of anal disease. 100
Total Abdominal Colectomy
If the patient is not an appropriate candidate for a TPC due to comorbidity issues or refusal of an ileostomy, the segmental distribution of CD may suggest the consideration of a lesser resection. If rectal biopsies show no dysplasia, a TAC with either an end ileostomy or reconstruction with an IRA is possible in CD, particularly. An IRA would require a relatively healthy rectum, where a Hartmann's procedure would be done if rectal inflammation was present. This may offer a middle ground between TPC and segmental resection, decreasing the risk of synchronous and metachronous colonic lesions but preserving continence. A Hartmann's procedure does not address the increased risk of metachronous rectal or small bowel cancer in CD. Therefore, close postoperative follow-up is still necessary, although rates of rectal cancer (including that in the retained stump post-colectomy) in CD is lower than that in UC, presumably due to the relative lack of inflammation in the rectum in CD versus UC patients. 101 102
The Fate of the Rectum
Patients having a subtotal colectomy for UC or less frequently for CD need to have an in-depth discussion with their surgeon with regard to the fate of the rectal stump. Persistent proctitis may continue to be problematic even with the discontinuation of luminal flow. There is also an associated cancer risk of 3.1% in the retained rectal stump. Patients with UC are usually advised to undergo either a restorative proctocolectomy as a two- or three-staged procedure, or in those who do not feel they wish to have an ileoanal pouch constructed, then a completion proctectomy would be the other option. For patients with CD who wish to retain the rectum which has no active inflammation, an IRA is a potential surgical option, provided a clear plan for rectal stump surveillance is discussed and agreed. 103 104
Overall Survival
Several different recent studies, including meta-analysis, case–control studies, and large retrospective review, show that the prognosis of IBD-associated CRC is similar to sporadic cancer.
Reynolds et al 105 reported on survival data for 243,186 patients with IBD-associated CRC. IBD-associated CRC had no effect on 5-year overall patient survival; however, patients with IBD have higher rates of synchronous and poorly differentiated tumors with lower rates of rectal tumors.
Similarly, Thicoïpé et al 106 reported on patients with IBD-CRC matched to patients with sporadic CRC on gender, UICC stage, tumor localization, and date of surgery. The study found disease-free and overall survivals to be similar in both groups despite older age in the sporadic control group and more frequent multiple cancer location in the IBD cases.
Summary
This overview was a whistle stop tour on the pertinent issues surrounding IBD-related CRC. The associated molecular biology and pathogenesis, the risk factors, surveillance, and management options including chemoprevention and surgery were all discussed. The take-home message is the importance of regular surveillance in order to identify and deal with dysplasia promptly and prevent progression into CRC.
Footnotes
Conflict of Interest None.
References
- 1.Zavoral M, Suchanek S, Zavada F. Colorectal cancer screening in Europe. World J Gastroenterol. 2009;15(47):5907–5915. doi: 10.3748/wjg.15.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R L, Miller K D, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(01):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan G G, Ng S C. Understanding and preventing the global Increase of inflammatory bowel disease. Gastroenterology. 2017;152(02):313–32100. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Dyson J K, Rutter M D. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18(29):3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triantafillidis J K, Nasioulas G, Kosmidis P A. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29(07):2727–2737. [PubMed] [Google Scholar]
- 6.Kulaylat M N, Dayton M T. Ulcerative colitis and cancer. J Surg Oncol. 2010;101(08):706–712. doi: 10.1002/jso.21505. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan A N, Cagan A, Cai T. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2015;13(02):322–3290. doi: 10.1016/j.cgh.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaunoit T, Limburg P J, Goldberg R M, Lymp J F, Loftus E V., Jr Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(03):335–342. doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Fogt F, Vortmeyer A O, Goldman H, Giordano T J, Merino M J, Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29(02):131–136. doi: 10.1016/s0046-8177(98)90222-2. [DOI] [PubMed] [Google Scholar]
- 10.Farraye F A, Odze R D, Eaden J et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(02):738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Kaltenbach T, Barkun A, McQuaid K R, Subramanian V, Soetikno R; SCENIC Guideline Development Panel.SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease Gastroenterology 201514803639–6.51E30. [DOI] [PubMed] [Google Scholar]
- 12.Eaden J A, Abrams K R, Mayberry J F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(04):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jess T, Simonsen J, Jørgensen K T, Pedersen B V, Nielsen N M, Frisch M.Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years Gastroenterology 201214302375–810., quiz e13–e14 [DOI] [PubMed] [Google Scholar]
- 14.Choi C H, Rutter M D, Askari A. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol. 2015;110(07):1022–1034. doi: 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus E V., Jr Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35(03):517–531. doi: 10.1016/j.gtc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Velayos F S, Terdiman J P, Walsh J M. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100(06):1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 17.Baars J E, Looman C W, Steyerberg E W. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106(02):319–328. doi: 10.1038/ajg.2010.428. [DOI] [PubMed] [Google Scholar]
- 18.Rubin D T, Rothe J A, Hetzel J T, Cohen R D, Hanauer S B. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65(07):998–1004. doi: 10.1016/j.gie.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Baker K T, Salk J J, Brentnall T A, Risques R A. Precancer in ulcerative colitis: the role of the field effect and its clinical implications. Carcinogenesis. 2018;39(01):11–20. doi: 10.1093/carcin/bgx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman S, Rubin P H, Bodian C, Harpaz N, Present D H.Screening and surveillance colonoscopy in chronic Crohn's colitis: results of a surveillance program spanning 25 years Clin Gastroenterol Hepatol 2008609993–998., quiz 953–954 [DOI] [PubMed] [Google Scholar]
- 21.Gillen C D, Walmsley R S, Prior P, Andrews H A, Allan R N. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35(11):1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camarillo D B, Krummel T M, Salisbury J K., JrRobotic technology in surgery: past, present, and future Am J Surg 2004188(4A, Suppl):2S–15S. [DOI] [PubMed] [Google Scholar]
- 23.Greenstein A J, Sachar D B, Smith H, Janowitz H D, Aufses A H., Jr A comparison of cancer risk in Crohn's disease and ulcerative colitis. Cancer. 1981;48(12):2742–2745. doi: 10.1002/1097-0142(19811215)48:12<2742::aid-cncr2820481231>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Ekbom A, Helmick C, Zack M, Adami H O.Increased risk of large-bowel cancer in Crohn's disease with colonic involvement Lancet 1990336(8711):357–359. [DOI] [PubMed] [Google Scholar]
- 25.Canavan C, Abrams K R, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23(08):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 26.Zisman T L, Rubin D T. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14(17):2662–2669. doi: 10.3748/wjg.14.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulai P S, Sandborn W J, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Phila) 2016;9(12):887–894. doi: 10.1158/1940-6207.CAPR-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Söderlund S, Brandt L, Lapidus A.Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease Gastroenterology 2009136051561–1567., quiz 1818–1819 [DOI] [PubMed] [Google Scholar]
- 29.Itzkowitz S H, Present D H; Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group.Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease Inflamm Bowel Dis 20051103314–321. [DOI] [PubMed] [Google Scholar]
- 30.Rutter M D, Saunders B P, Wilkinson K H. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130(04):1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Roy D, Steyer G J, Gargesha M, Stone M E, Wilson L. 3D cryo-imaging: a very high-resolution view of the whole mouse. Anat Rec (Hoboken) 2009;292(03):342–351. doi: 10.1002/ar.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron T H. New York: John Wiley & Sons; 2009. Benign and malignant colorectal strictures; pp. 689–702. [Google Scholar]
- 33.Askling J, Dickman P W, Karlén P. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120(06):1356–1362. doi: 10.1053/gast.2001.24052. [DOI] [PubMed] [Google Scholar]
- 34.Molodecky N A, Kareemi H, Parab R. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(05):1590–1599. doi: 10.1002/hep.24247. [DOI] [PubMed] [Google Scholar]
- 35.Loftus E V, Jr, Harewood G C, Loftus C G. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54(01):91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(04):405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Azer S A. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25(03):271–281. doi: 10.1097/MEG.0b013e32835b5803. [DOI] [PubMed] [Google Scholar]
- 38.Aghagolzadeh P, Radpour R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J Gastroenterol. 2016;22(25):5678–5693. doi: 10.3748/wjg.v22.i25.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burmer G C, Rabinovitch P S, Haggitt R C. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103(05):1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 40.Tsai J H, Rabinovitch P S, Huang D. Association of aneuploidy and flat dysplasia with development of high-grade dysplasia or colorectal cancer in patients with inflammatory bowel disease. Gastroenterology. 2017;153(06):1492–1.495E7. doi: 10.1053/j.gastro.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 41.Bhalla A, Zulfiqar M, Bluth M H. Molecular diagnostics in colorectal carcinoma: advances and applications for 2018. Clin Lab Med. 2018;38(02):311–342. doi: 10.1016/j.cll.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Johdi N A, Ait-Tahar K, Sagap I, Jamal R.Molecular signatures of human regulatory T cells in colorectal cancer and polyps Front Immunol 20178(MAY):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie J, Itzkowitz S H. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14(03):378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattar M C, Lough D, Pishvaian M J, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4(02):53–61. [PMC free article] [PubMed] [Google Scholar]
- 45.Philpott D J, Viala J. Towards an understanding of the role of NOD2/CARD15 in the pathogenesis of Crohn's disease. Best Pract Res Clin Gastroenterol. 2004;18(03):555–568. doi: 10.1016/j.bpg.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Rioux J D, Xavier R J, Taylor K D. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39(05):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaeger R, Shah M A, Miller V A. Genomic alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology. 2016;151(02):278–2.87E8. doi: 10.1053/j.gastro.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakatos P-L, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14(25):3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jess T, Loftus E V, Jr, Velayos F S. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted county, Minnesota. Gastroenterology. 2006;130(04):1039–1046. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 50.
- 51.Schlemper R J, Riddell R H, Kato Y. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47(02):251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itzkowitz S H, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126(06):1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Heuschen U A, Hinz U, Allemeyer E H. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120(04):841–847. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 54.Collins P D. Strategies for detecting colon cancer and dysplasia in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(04):860–863. doi: 10.1097/MIB.0b013e3182802c6a. [DOI] [PubMed] [Google Scholar]
- 55.Collins P D, Mpofu C, Watson A J, Rhodes J M. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(02):CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 56.Lutgens M WMD, Oldenburg B, Siersema P D. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009;101(10):1671–1675. doi: 10.1038/sj.bjc.6605359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eaden J, Mayberry J. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51 05:v10–v12. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Øresland T, Bemelman W A, Sampietro G M et al. European evidence based consensus on surgery for ulcerative colitis. J Crohn's Colitis. 2015;9(01):4–25. doi: 10.1016/j.crohns.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Kim E R, Chang D K. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20(29):9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulusu S SR, Lawrance I C. Dysplasia and colorectal cancer surveillance in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2017;11(08):711–722. doi: 10.1080/17474124.2017.1327347. [DOI] [PubMed] [Google Scholar]
- 61.Kiesslich R, Fritsch J, Holtmann M. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124(04):880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 62.Sinangil A, Celik V, Barlas S, Akin E B, Ecder T. Should transplant ureter be stented routinely or not? Eur Rev Med Pharmacol Sci. 2014;18(23):3551–3556. [PubMed] [Google Scholar]
- 63.Nguyen D L, Lee J G, Parekh N K, Samarasena J, Bechtold M L, Chang K. The current and future role of endomicroscopy in the management of inflammatory bowel disease. Ann Gastroenterol. 2015;28(03):331–336. [PMC free article] [PubMed] [Google Scholar]
- 64.Koo J S. Equipment-based image-enhanced endoscopy for differentiating colorectal polyps. Clin Endosc. 2014;47(04):330–333. doi: 10.5946/ce.2014.47.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumoto T, Nakamura S, Moriyama T, Hirahashi M, Iida M.Autofluorescence imaging colonoscopy for the detection of dysplastic lesions in ulcerative colitis: a pilot study Colorectal Dis 201012(10 Online):e291–e297. [DOI] [PubMed] [Google Scholar]
- 66.Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41(04):522–525. doi: 10.1136/gut.41.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derikx LAAP, Nissen LHC, Smits LJT, Shen B, Hoentjen F. Risk of neoplasia After colectomy in patients with inflammatory bowel disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14(06):798–806.e20 [DOI] [PubMed]
- 68.Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar D B, Colombel J-F. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(05):497–508. doi: 10.1111/j.1365-2036.2011.04753.x. [DOI] [PubMed] [Google Scholar]
- 69.Singh S, Edakkanambeth Varayil J, Loftus EV Jr, Talwalkar JA. Incidence of colorectal cancer after liver transplantation for primarysclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 2013;19(12):1361–1369 [DOI] [PubMed]
- 70.Steward W P, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109(01):1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinczowski D, Ekbom A, Baron J, Yuen J, Adami H O. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994;107(01):117–120. doi: 10.1016/0016-5085(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 72.Subramanian V, Logan R F.Chemoprevention of colorectal cancer in inflammatory bowel disease Best Pract Res Clin Gastroenterol 201125(4-5):593–606. [DOI] [PubMed] [Google Scholar]
- 73.Chu E C, Chai J, Ahluwalia A, Tarnawski A S. Mesalazine downregulates c-Myc in human colon cancer cells. A key to its chemopreventive action? Aliment Pharmacol Ther. 2007;25(12):1443–1449. doi: 10.1111/j.1365-2036.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 74.
- 75.Eaton J E, Silveira M G, Pardi D S. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106(09):1638–1645. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tung B Y, Emond M J, Haggitt R C. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134(02):89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 77.Kawakita D, Lee Y A, Gren L H, Buys S S, La Vecchia C, Hashibe M. The impact of folate intake on the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian cancer screening trial (PLCO) cohort. Br J Cancer. 2018;118(02):299–306. doi: 10.1038/bjc.2017.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Tang W, Wang J. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014;25(02):237–249. doi: 10.1007/s10552-013-0326-6. [DOI] [PubMed] [Google Scholar]
- 79.Smith L A, Baraza W, Tiffin N, Cross S S, Hurlstone D P. Endoscopic resection of adenoma-like mass in chronic ulcerative colitis using a combined endoscopic mucosal resection and cap assisted submucosal dissection technique. Inflamm Bowel Dis. 2008;14(10):1380–1386. doi: 10.1002/ibd.20497. [DOI] [PubMed] [Google Scholar]
- 80.Hurlstone D P, Sanders D S, Atkinson R. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut. 2007;56(06):838–846. doi: 10.1136/gut.2006.106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shergill A K, Farraye F A. Toward a consensus on endoscopic surveillance of patients with colonic inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24(03):469–481. doi: 10.1016/j.giec.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 82.Annese V, Daperno M, Rutter M D et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn's Colitis. 2013;7(12):982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Lahiff C, Mun Wang L, Travis S PL, East J E. Diagnostic yield of dysplasia in polyp-adjacent biopsies for patients with inflammatory bowel disease: a cross-sectional study. J Crohn's Colitis. 2018;12(06):670–676. doi: 10.1093/ecco-jcc/jjy007. [DOI] [PubMed] [Google Scholar]
- 84.Ten Hove J R, Mooiweer E, Dekker E. Low rate of dysplasia detection in mucosa surrounding dysplastic lesions in patients undergoing surveillance for inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2017;15(02):222–22800. doi: 10.1016/j.cgh.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 85.
- 86.Moss A, Bourke M J, Williams S J. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140(07):1909–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 87.Feakins R M; British Society of Gastroenterology.Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines J Clin Pathol 201366121005–1026. [DOI] [PubMed] [Google Scholar]
- 88.Thomas T, Abrams K A, Robinson R J, Mayberry J F. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25(06):657–668. doi: 10.1111/j.1365-2036.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- 89.Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125(05):1311–1319. doi: 10.1016/j.gastro.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 90.Navaneethan U, Jegadeesan R, Gutierrez N G. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohn's Colitis. 2013;7(12):e684–e691. doi: 10.1016/j.crohns.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein C N, Shanahan F, Weinstein W M.Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994343(8889):71–74. [DOI] [PubMed] [Google Scholar]
- 92.O'Connell J B, Maggard M A, Livingston E H, Yo C K. Colorectal cancer in the young. Am J Surg. 2004;187(03):343–348. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 93.Dignass A, Eliakim R, Magro F. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohn's Colitis. 2012;6(10):965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Frøslie K F, Jahnsen J, Moum B A, Vatn M H; IBSEN Group.Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort Gastroenterology 200713302412–422. [DOI] [PubMed] [Google Scholar]
- 95.Carbonnel F, Gargouri D, Lémann M. Predictive factors of outcome of intensive intravenous treatment for attacks of ulcerative colitis. Aliment Pharmacol Ther. 2000;14(03):273–279. doi: 10.1046/j.1365-2036.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 96.Connelly T M, Koltun W A.The surgical treatment of inflammatory bowel disease-associated dysplasia Expert Rev Gastroenterol Hepatol 2013704307–321., quiz 322 [DOI] [PubMed] [Google Scholar]
- 97.Kariv R, Remzi F H, Lian L.Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy Gastroenterology 201013903806–812., 812.e1–812.e2 [DOI] [PubMed] [Google Scholar]
- 98.Delaney C P, Fazio V W, Remzi F H. Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch-anal anastomosis. Ann Surg. 2003;238(02):221–228. doi: 10.1097/01.sla.0000080825.95166.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashavia E, Dotan I, Rabau M, Klausner J M, Halpern Z, Tulchinsky H. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14(11):1365–1371. doi: 10.1111/j.1463-1318.2012.02993.x. [DOI] [PubMed] [Google Scholar]
- 100.Kiran R P, Nisar P J, Goldblum J R. Dysplasia associated with Crohn's colitis: segmental colectomy or more extended resection? Ann Surg. 2012;256(02):221–226. doi: 10.1097/SLA.0b013e31825f0709. [DOI] [PubMed] [Google Scholar]
- 101.Svrcek M, Cosnes J, Beaugerie L. Colorectal neoplasia in Crohn's colitis: a retrospective comparative study with ulcerative colitis. Histopathology. 2007;50(05):574–583. doi: 10.1111/j.1365-2559.2007.02663.x. [DOI] [PubMed] [Google Scholar]
- 102.Freeman H-J. Colorectal cancer risk in Crohn's disease. World J Gastroenterol. 2008;14(12):1810–1811. doi: 10.3748/wjg.14.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Munie S, Hyman N, Osler T. Fate of the rectal stump after subtotal colectomy for ulcerative colitis in the era of ileal pouch-anal anastomosis. JAMA Surg. 2013;148(05):408–411. doi: 10.1001/jamasurg.2013.177. [DOI] [PubMed] [Google Scholar]
- 104.Yamamoto T, Keighley M RB. Fate of the rectum and ileal recurrence rates after total colectomy for Crohn's disease. World J Surg. 2000;24(01):125–129. doi: 10.1007/s002689910023. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds I S, O'Toole A, Deasy J, McNamara D A, Burke J P. A meta-analysis of the clinicopathological characteristics and survival outcomes of inflammatory bowel disease associated colorectal cancer. Int J Colorectal Dis. 2017;32(04):443–451. doi: 10.1007/s00384-017-2754-3. [DOI] [PubMed] [Google Scholar]
- 106.Thicoïpé A, Laharie D, Smith D. Oncological outcomes of IBD-associated versus sporadic colorectal cancer in modern era: a matched case-control study. Int J Colorectal Dis. 2018;33(07):963–966. doi: 10.1007/s00384-018-3049-z. [DOI] [PubMed] [Google Scholar]


