Abstract
Background Intravenous leiomyomatosis is a rare disease. Histologically, intravenous leiomyomatosis is a benign tumor, but its biological behavior can be malignant. The development of intracardiac extensions leads to congestive heart failure and occasionally sudden fatalities.
Case Description The cases of three patients treated at our university between 2017 and 2018 were studied retrospectively. Intravenous tumors extending into the right heart system were fully removed without perioperative complications or death. Only one tumor recurrence was observed during the followed-up period.
Conclusion The gold standard for the treatment of intravenous leiomyomatosis with intracardiac extension is complete and successful surgical resection.
Keyword: cardiovascular surgery, heart disease, tumor
Introduction
Intravenous leiomyomatosis (IVL) is a benign smooth muscle tumor that grows in the venous system of the uterus and occasionally extends into the inferior vena cava (IVC). IVL with intracardiac extension (ICE) occurs when the mass spreads into the cardiac chambers. 1 IVL with ICE causes serious cardiovascular symptoms in patients, including sudden syncope, dyspnea, and right heart failure, which eventually lead to sudden death if untreated. 2 Because the occurrence of ICE is rare and no symptoms appear in patients with IVL until the tumor extends into the heart, early diagnosis is difficult. 3 Currently, complete surgical excision is the only effective treatment. Herein, we report three cases of IVL with ICE. The tumors were completely resected. A detailed description of preoperative assessment methods and surgical strategies to ensure a complete and long-term cure is provided.
Case Description
We retrospectively reviewed the cases of three patients diagnosed with IVL with ICE. The patients were treated at our university between 2017 and 2018. After surgical treatment, patients were followed up every 3 months in the first year. Subsequent follow-ups were every 6 months for the next 3 years and then every year thereafter.
Case A was a 49-year-old woman who was gravida (G) 5 para (P) 2, with initial symptoms of chest tightness, palpitations, and lower limb swelling. The patient had a previous history of uterine fibroids, without a notable family history. Echocardiography showed a mobile, solid mass occupying the IVC and right atrium (RA). The serum levels for tumor markers were 78.8 U/mL (reference values: 0–35 U/mL) for carbohydrate antigen (CA) 125, 35.3 U/mL (reference values: 0–27 U/mL) for CA199, 0.5 ng/mL (reference values: 0–4.7 ng/mL) for carcinoembryonic antigen (CEA), and 58.9 pmol/L (reference values: 0–76.2 pmol/L) for human epididymis protein (HE) 4. All other blood and biochemical markers were within the normal range. Thoracic and abdominal computed tomography (CT) scans were performed with and without contrast media. On the CT scans, a filling defect was observed in the right ovarian vein, IVC, and RA with a soft tissue density ( Fig. 1A ). Magnetic resonance imaging (MRI) was not performed.
Fig. 1.
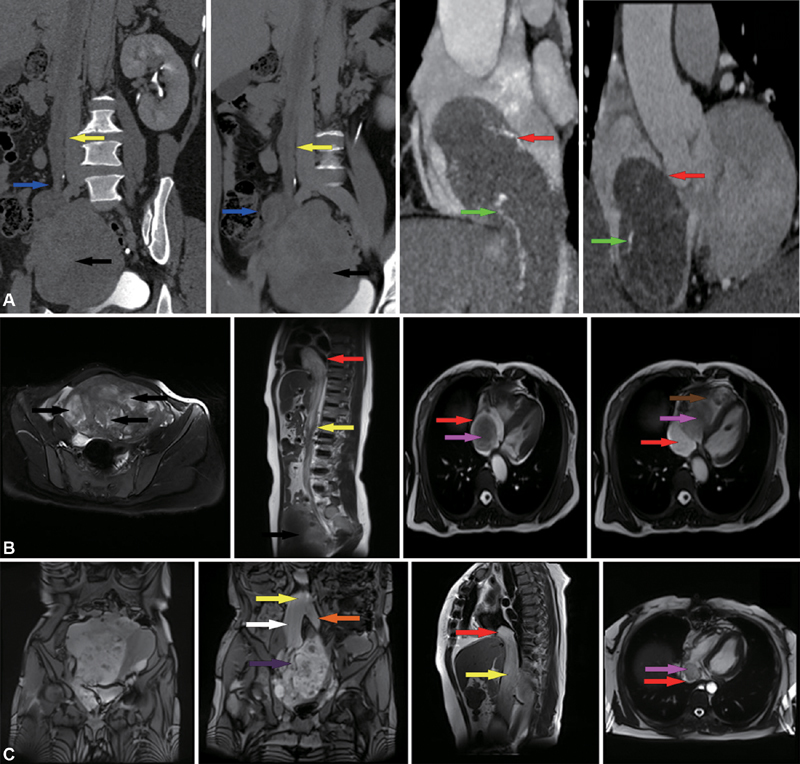
( A ) A CT (computed tomography) scan from case A is shown. The volume of the uterus is significantly enlarged and complicated with multiple uterine fibroids. Multiple masses are extending into the right ovarian vein and the inferior vena cava (IVC) and right atrium (RA). An enhanced vascular shadow can be seen in the lesion ( green arrow ). ( B ) A magnetic resonance imaging (MRI) from case B shows multiple uterine fibroids. The tumor extends from the IVC to the RA. The tumor could be observed moving with the movement of the RA. The distal portion of the tumor traverses the tricuspid valve into the RV during diastole. ( C ) The MRI from case C indicates a large, multilobulated, complex pelvic mass closely related to the uterus. The uterus deviates left due to pressure. The mass extends from the right internal iliac vein and the common iliac vein and extends into the IVC and RA ( red arrow ); RV (brown arrow); mass (pink arrow); IVC (yellow arrow); Right ovarian vein (blue arrow); Right common iliac vein (white arrow); Left common iliac vein (orange arrow); Right internal iliac vein (purple arrow); Uterine fibroids (black arrow).
Case B was a 49-year-old woman who was G2 P2 with initial symptoms of intermittent syncope. The patient had a previous history of uterine fibroids, without a notable family history. Echocardiography showed a mobile, solid mass occupying the IVC, RA, and right ventricle (RV). The serum levels for tumor markers were 15.8 U/mL for CA125, 12.3 U/mL for CA199, 1.1 ng/mL for CEA, and 52.4 pmol/L for HE4. All other blood and biochemical markers were within the normal range. MRI of the chest and abdomen revealed a large streak-shaped filling defect in the IVC, RA, and RV ( Fig. 1B ). A CT scan was not performed.
Case C was a 48-year-old woman who was G2 P2 with initial symptoms of chest tightness, dyspnea, and lower abdominal mass. The patient had no relevant medical history and no notable family history. An echocardiography showed a mobile, solid mass occupying the RA only. The serum levels for tumor markers were 29 U/mL for CA125, 14.5 U/mL for CA199, 1.8 ng/mL for CEA, and 127.9 pmol/L for HE4. All other blood and biochemical markers were within the normal range. MRI of the chest and abdomen revealed a cordlike mass extending from the right internal iliac vein into right the common iliac vein, then the IVC, and, finally, the RA ( Fig. 1C ).
All three patients underwent surgical resection of the tumor using a multidisciplinary approach. The body temperature of all three patients was measured through an anorectal probe during the procedure. Two patients (cases A and B) underwent a single-stage surgery and one patient (case C) underwent a two-stage surgery because her preoperative condition was poor. The ovarian uterine vein that was first invaded was ligated in all three patients.
In the two patients who underwent a single-stage surgery, a total hysterectomy (TH) and a bilateral salpingo-oophorectomy (BSO) were performed through an abdominal incision. Serious adhesions were found when separating the IVL and the vein. Cardiopulmonary bypass (CPB) was established through a central thoracotomy under mild hypothermia (33°C–34.5°C) conditions with the heart beating. Arterial perfusion along the ascending aorta with venous return through the superior vena cava and right femoral vein was established. The tumor was pushed through the IVC into the RA. The tumor was removed through an RA incision. A tricuspid valvuloplasty was performed along with reparation of the vein and RA.
For the patient (case C) who underwent a two-stage surgery, as much of the intracardiac tumor as possible was removed and the damaged tricuspid valve was repaired during the first surgery to relieve the severe heart failure. The first surgery was performed with mild hypothermia and CPB. After 19 days of recovery and reevaluation, the patient underwent a second-stage surgery to remove the IVC tumor. A TH and BSO were performed during the second surgery. In addition, the patient received a double-J stent implantation in the right ureter during the second surgery.
Intraoperative results revealed that all tumors in the atrium were floating and that there were no adhesions between the leiomyomas and IVC wall. The ovarian vein and IVC were involved in two patients (case A and B), and the uterine vein, iliac vein, and IVC were involved in the third patient (case C). The ICE was in the RA in all three patients, and the RV was involved in one patient (case B). In all three patients, the IVL was derived from uterine tumors. The average intraoperative blood loss was 230 mL, and the average operation time was 9 hours.
The tumors were yellowish-white or gray, elastic, smooth, mobile, serpentlike polypoid masses. The gross morphology for each tumor is shown in Fig. 2 . Based on pathological examination of the tumors, all three patients were diagnosed with IVL with ICE. No tumor recurrence was observed in cases A and B during the 18 and 29 months of follow-up, respectively. After 18 months of follow-up, the results of pelvic CT scans and MRI showed tumor recurrence in case C.
Fig. 2.
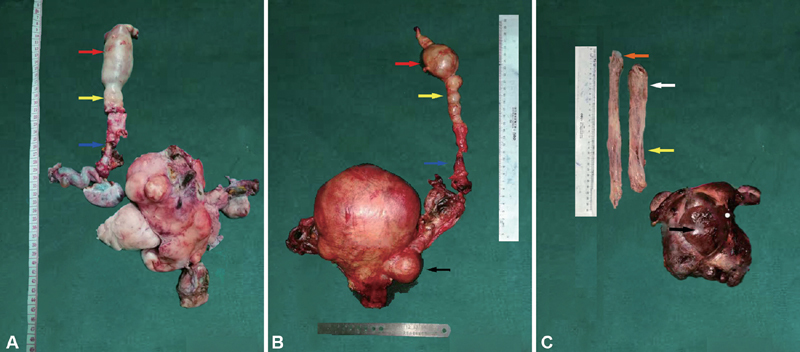
The gross specimen and tumor extensions are shown. Panels A to C represent cases A to C, respectively. RA (red arrow); IVC (yellow arrow); Right ovarian vein (blue arrow); Right common iliac vein (white arrow); Left common iliac vein (orange arrow); Uterine fibroids (black arrow).
Discussion
IVL is a rare mesodermal cell tumor that should be considered when evaluating an intracardiac mass that extends from the IVC. Although the origination of IVL is still controversial, there are two theories: the tumors are derived from the smooth muscle of the vascular wall 4 or the tumors originate from the smooth muscle of the uterus. 5 Most reports support the second theory. Based on our three case studies, the IVL most likely originated from the uterine smooth muscle. We base this conclusion on the following: (1) in addition to the IVL, all patients also had uterine fibroids; (2) the tumor originated from the uterine or ovarian veins and ended in the IVC and RA, following the blood flow path of the uterus; and (3) immunological expression in the IVL was similar to the uterine fibroids, including positive estrogen and progesterone receptor detection.
There are two pathways for IVL extension into the IVC and RA. The first pathway is left and right uterine veins–internal iliac veins–common iliac veins–IVC–right heart. The second pathway extends to the left ovary vein–left renal vein–IVC–right heart or right ovarian vein–IVC–right heart. 6 In the cases examined in this study, one (case C) extended through the first pathway and two (cases A and B) extended through the second pathway.
The pathology of the three patients in this study was consistent with the relevant literature reports. 7
Surgical treatment by complete resection of the neoplastic tissue is required to minimize the risk of mortality and prevent recurrence in patients who have IVL with ICE. In most cases described in the literature, a two-stage surgical approach was adopted, with resection of the intracardiac component first followed by a second stage to resect the remaining intra-abdominal tumor and/or the uterus. A two-stage procedure can be performed in nearly any patient treated for IVL with ICE but is especially recommended for patients who are considered to be in poor condition before or during the operation and cannot tolerate a single-stage procedure and in patients with tumors that are extensive or adhere tightly to intracardiac or intravenous structures. However, a two-stage surgery has disadvantages compared with a single-stage surgery including (1) a longer total operation time, (2) more intraoperative blood loss, (3) longer postoperative hospital stays, and (4) higher risk of tumor recurrence. Despite these disadvantages, no significant differences in major morbidity and mortality were observed in two-stage surgeries compared with single-stage surgeries for IVL with ICE. 8
Recently, a single-stage surgery under deep hypothermic and CPB using a multidisciplinary team has been adopted as a safe and effective technique. The intraoperative blood loss was lower and the total operative time was shorter in a single-stage surgery compared with a two-stage operation. Therefore, the single-stage operation is now thought to be more ideal from the perspective of the patient. Improved technical abilities, enhanced multidisciplinary team cooperation, and a better understanding of the disease have led to successful single-stage surgeries.
The advantages of a single-stage approach compared with a two-stage approach include the following: (1) a complete resection, (2) reconstruction of the vascular structures, (3) control of bleeding, (4) avoidance of hemodynamic complications during the interval between the two stages, (5) avoidance of the risk of a second general anesthetic, (6) reduction of the risk of tumor progression and embolism caused by incomplete resection in the first stage, and (6) better economic benefits. 1 2 9 However, a single-stage surgery, which usually requires a prolonged continuous operation time, places a much heavier burden on patients. 10 Thus, a single-stage surgery is not appropriate for patients with critical conditions such as severe heart failure or syncope. 11 According to the advantages and disadvantages of single-stage versus two-stage surgeries described previously, the surgical approach should be selected based on the patient's stability and physiologic reserve, the anatomy of the tumor, and the availability of surgical specialties.
Prevention of IVL with ICE recurrence is the most significant outcome that can be accomplished through successful treatment. The time of postoperative recurrence varies in patients. Therefore, long-term follow-up is highly recommended. In this article, recurrence screening was monitored by ultrasound, transthoracic echocardiogram, and CT. We observed one recurrence (case C) after 18 to 29 months of follow-up.
Funding Statement
Funding This research was not supported by any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest None.
Authors' contributions
Yundan Deng contributed to project development, data collection, data analysis, and manuscript writing. Bing Song contributed to project development and data analysis.
References
- 1.Li B, Chen X, Chu Y D, Li R Y, Li W D, Ni Y M. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg. 2013;17(01):132–138. doi: 10.1093/icvts/ivt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Kim D K, Narm K S, Cho S H. Pulmonary artery embolization of intravenous leiomyomatosis extending into the right atrium. Korean J Thorac Cardiovasc Surg. 2011;44(03):243–246. doi: 10.5090/kjtcs.2011.44.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worley M J, Jr, Aelion A, Caputo T A. Intravenous leiomyomatosis with intracardiac extension: a single-institution experience. Am J Obstet Gynecol. 2009;201(06):5740–5.74E7. doi: 10.1016/j.ajog.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo K W, Lau T K. Intracardiac leiomyomatosis. Case report and literature review. Arch Gynecol Obstet. 2001;264(04):209–210. doi: 10.1007/s004040000115. [DOI] [PubMed] [Google Scholar]
- 5.Dal Cin P, Quade B J, Neskey D M, Kleinman M S, Weremowicz S, Morton C C. Intravenous leiomyomatosis is characterized by a der(14)t(12;14)(q15;q24) Genes Chromosomes Cancer. 2003;36(02):205–206. doi: 10.1002/gcc.10159. [DOI] [PubMed] [Google Scholar]
- 6.Lam P M, Lo K W, Yu M Y. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. 2004;39(02):465–469. doi: 10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Tang L, Lu B. Intravenous leiomyomatosis of the uterus: a clinicopathologic analysis of 13 cases with an emphasis on histogenesis. Pathol Res Pract. 2018;214(06):871–875. doi: 10.1016/j.prp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Yang J, Huang H. Management of intravenous leiomyomatosis with intracaval and intracardiac extension. Obstet Gynecol. 2012;120(06):1400–1406. doi: 10.1097/aog.0b013e31826ebb90. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Miao Q, Liu X. Intravenous leiomyomatosis with intracardiac extension. Ann Thorac Surg. 2010;89(05):1641–1643. doi: 10.1016/j.athoracsur.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Yu H Y, Tsai H E, Chi N H. Long-term outcomes of surgical treatment for intravascular leiomyomatosis. J Formos Med Assoc. 2018;117(11):964–972. doi: 10.1016/j.jfma.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Wu C K, Luo J L, Yang C Y. Intravenous leiomyomatosis with intracardiac extension. Intern Med. 2009;48(12):997–1001. doi: 10.2169/internalmedicine.48.1780. [DOI] [PubMed] [Google Scholar]


