Abstract
Objective:
We aimed to evaluate the incidence, risk factors, and prognosis of bloodstream infections (BSIs) during extracorporeal membrane oxygenation (ECMO) treatment in a Chinese population.
Methods:
Patients receiving ECMO treatment from January 2013 to August 2019 were retrospectively studied. The incidence of BSIs was calculated. The clinical characteristics between patients with a BSI (BSI group) and without a BSI (non-BSI group)
Results:
Among 69 included patients, 19 (27.5%) developed at least one BSI. Gram-negative bacteria (73.7%) were mainly responsible for the BSIs, with Klebsiella pneumoniae (6/19, 31.5%) ranking as the top related pathogen. The BSI group had a greater proportion of methicillin-resistant Staphylococcus aureus (MRSA) prophylactic regimens (52.6% vs. 26.0%, P = 0.036), a higher pre-ECMO Sequential Organ Failure Assessment (SOFA) score (11 vs. 8, P = 0.008), more applications of continuous renal replacement therapy (CRRT) during ECMO (63.1% vs. 36.1%, P = 0.042). Longer ECMO support duration, period of ventilator use before ECMO weaning and hospital stay were observed in the BSI group. The SOFA score (OR: 1.174; 95% CI: 1.039–1.326; P = 0.010) was an independent risk factor for BSIs.
Conclusion:
BSIs during ECMO therapy frequently involve Gram-negative bacteria. Stringent care and monitoring should be provided for patients with high SOFA scores.
Keywords: Bloodstream infection, Extracorporeal membrane oxygenation, Pathogen, Risk factors, Prognosis
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is one of the most important strategies to treat severe acute respiratory failure or cardiac failure. The application of ECMO in adults has increased rapidly since the influenza A H1N1 epidemic and the completion of the CESAR trial.1
Despite the growing implementation of adult ECMO, mortality due to severe acute respiratory failure or cardiac failure remains relatively high. The overall survival rate for these patients in the extracorporeal life support organization (ELSO) registry was 56%, and it varied depending on the patient population and health care providers.2 Nosocomial infection is a complication commonly seen and usually contribute to a high mortality rate. Multiple factors increase the risk of nosocomial infection in patients receiving ECMO.3 Furthermore, the incidence of bloodstream infections (BSIs) remains substantial, thus impacting the prognosis of patients treated with ECMO.4,5 Therefore, the management of patients with a BSI during ECMO remains a challenge.
A thorough understanding of the clinical features of BSIs may improve the prognosis of patients receiving ECMO.6 Therefore, in this study, we aimed to explore the incidence, risk factors, and prognosis of Chinese patients undergoing ECMO with BSIs.
METHODS
All adult patients (n = 77) requiring ECMO at the Department of Critical Care Medicine, Affiliated Hangzhou Hospital of Nanjing Medical University, from January 2013 to August 2019 were retrospectively reviewed. Finally, a total of 69 patients receiving veno-venous or veno-arterial ECMO owing to cardiopulmonary failure were included in this study (Fig.1). Demographic, clinical, and prognostic parameters were collected.
Fig.1.
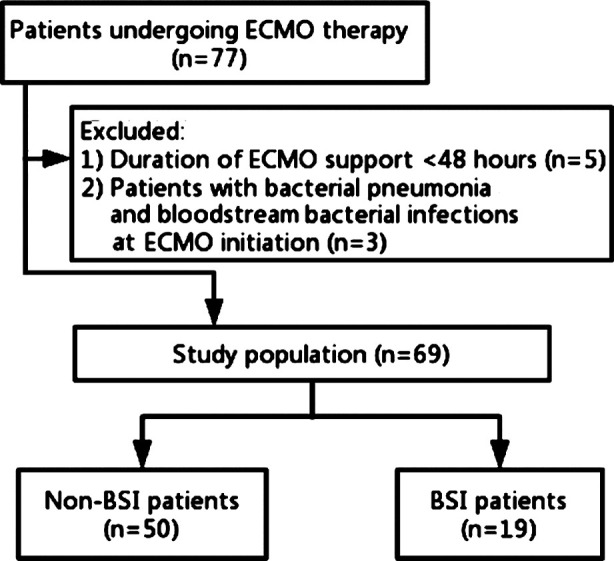
The study flowchart.
This study was approved by the local Hospital Ethics Committee (No. 2019-007-01). The informed consent was waived due to the retrospective nature of this study.
Definitions and criteria
The survival-to-discharge rate was defined as the primary outcome. ECMO-related nosocomial pneumonia was defined as pneumonia occurring in patients receiving ECMO for more than 48 hours or withdrawn within 48 hours.7 We classified the prophylactic antibiotic regimens into two categories (Table-I).
Table I.
Regimens of prophylactic treatment with antibiotics.
| Group | Prophylactic Antibiotics | No. of patients (n = 69) |
|---|---|---|
| Group 1 | Piperacillin/tazobactam+teicoplanin | 6 |
| Piperacillin/tazobactam+linezolid | 6 | |
| Piperacillin/tazobactam+daptomycin | 6 | |
| Piperacillin/tazobactam+vancomycin | 4 | |
| Imipenem/cilastatin+teicoplanin | 1 | |
| Group 2 | Piperacillin/tazobactam | 32 |
| Cefoperazone/sulbactam | 2 | |
| Meropenem | 2 | |
| Imipenem/cilastatin | 2 | |
| Piperacillin/tazobactam+moxifloxacin | 2 | |
| Cefoperazone/sulbactam+moxifloxacin | 1 | |
| Cefmetazole | 1 | |
| Cefuroxime | 1 | |
| Amoxicillin/clavulanic acid | 1 | |
| Moxifloxacin | 1 | |
| Cefatriaxone | 1 |
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as the median (range) or mean ± standard deviation, as appropriate. Categorical variables were compared between groups using the χ2 or Fisher’s exact test, and continuous variables were compared using the Mann–Whitney U test. Multivariate logistic regression analyses were conducted to determine the independent predictive factors of nosocomial pneumonia. The variables with P< 0.1 in the univariate analysis were included in the multivariate analysis with a forward stepwise model. All statistical analyses were performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). All tests were two-tailed, and a value of P<0.05 was considered statistically significant.
RESULTS
The baseline characteristic was displayed in Table-II. Myocarditis was the most common reason for ECMO treatment. A total of 30 (42.5%) patients with ECMO support developed 43 episodes of nosocomial infection, with an incidence of 76.0 infections per 1000 days of ECMO. Specifically, BSIs were observed in 19 patients (27.5%) with an incidence of 33.6 infections per 1000 days of ECMO. In addition, 14 (20.3%) patients experienced nosocomial pneumonia, and 10 (14.5%) patients had a urinary tract infection.
Table-II.
Baseline characteristics.
| Characteristic | Study population (n = 69) |
|---|---|
| Demographic data | |
| Age, years | 42 (18–77) |
| Gender, female | 26 (37.7) |
| Primary disease | |
| Myocarditis | 40 (58.0%) |
| Coronary heart disease | 13 (18.8%) |
| Pneumonia (viral/interstitial/aspergillus) | 6 (8.7%)/1 (1.4%)/1 (1.4%) |
| Pulmonary contusion | 3 (4.3%) |
| Pulmonary arterial hypertension | 2 (2.9%) |
| Lung cancer with airway obstruction | 1 (1.4%) |
| Aortic dissection | 1 (1.4%) |
| Allergic shock | 1 (1.4%) |
| MRSA prophylactic regimens | 23 (33.3%) |
| Laboratory findings (Pre-ECMO) | |
| White blood cell, 103/mm3 | 11.5 (2.2–37.7) |
| Hemoglobin, g/dL | 120.0 (61–176) |
| Platelets, 103/mm3 | 174 (11–857) |
| CRP, mg/dL | 32 (1–194) |
| Lactate, mM | 3.8 (1.0–20.0) |
| Total bilirubin, mg/dL | 14.6 (4.3–131) |
| Creatinine, mg/dL | 98.5 (44–466) |
| Pre-ECMO SOFA score | 8 (0–22) |
| Pre-ECMO ventilator support, days | 0 (0–9) |
| Pre-ECMO ICU stay, days | 0 (0–4) |
| Pre-ECMO hospital stay, days | 0 (0–14) |
| Veno-arterial mode | 58 (84.1%) |
| Ventilator duration before ECMO weaning, days | 7 (0–32) |
| ECMO support duration, h | 154 (55–727) |
Gram-negative bacteria (73.7%) were mainly responsible for the BSIs, with Klebsiella pneumoniae (6/19, 31.5%) ranking as the top related pathogen (Table-III). The BSI group had greater proportions of MRSA prophylactic regimens [10 (52.6%) vs. 13 (26.0%); P = 0.036] and CRRT [12 (63.1%) vs. 18 (36.0%); P = 0.042] than the non-BSI group. The ECMO support duration [(8.0 (range: 3.6–26.0) vs. 5.8 (range: 2.3–30.3) days; P = 0.001] and ventilator duration before ECMO weaning [10 (range: 3–26) vs. 6 (range: 0–32) days; P = 0.001] in the BSI group were longer than those in the non-BSI group. Similarly, a longer length of hospitalization was observed in the BSI group than in the non-BSI group [25 (range: 10–39) vs.17.5 (range: 2–42) days; P = 0.029] (Table-IV).
Table-III.
Pathogens of BSIs during ECMO support.
| Microorganism species | BSI (n = 19) |
|---|---|
| Gram-negative pathogens | |
| Klebsiella pneumonia | 6 |
| Enterobacter aerogenes | 2 |
| A. baumannii | 1 |
| Burkholderia cepacia | 1 |
| Pseudomonas aeruginosa | 1 |
| Enterobacter cloacae | 1 |
| Serratia marcescens | 1 |
| Stenotrophomonas maltophilia | 1 |
| Gram-positive pathogens | |
| Staphylococcus epidermidis | 2 |
| Enterococcus faecium | 1 |
| Fungi | |
| Candida albicans | 1 |
| Candida parapsilosi | 1 |
Table-IV.
Differences of characteristics between patients with and without BSIs during ECMO.
| Characteristics | Without BSI (n = 50) | With BSI (n = 19) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 40 (18–77) | 44 (21–67) | 0.872 |
| Female | 19 (38.0) | 7 (36.8) | 0.929 |
| Smoking history | 12 (24.0) | 6 (31.6) | 0.522 |
| Primary disease | |||
| Myocarditis | 24 (48.0) | 8 (42.1) | 0.661 |
| Respiratory failure | 8 (16.0) | 4 (21.1) | 0.725 |
| Coronary artery disease | 13 (26.0) | 6 (31.6) | 0.643 |
| Laboratory findings (Pre-ECMO) | |||
| White blood cell, 103/mm3 | 10.65 (2.20–36.7) | 13.3 (5.2–37.7) | 0.256 |
| Hemoglobin, g/L | 119.5 (61–176) | 122 (70–174) | 0.984 |
| Platelets, 103/mm3 | 175.0 (33.0–396.0) | 140 (11–857) | 0.100 |
| Lactate, mM | 3.8 (1.0–20.0) | 2.3 (1–19) | 0.859 |
| Total bilirubin, μM | 14.0 (4.3–131.0) | 20.1 (7.9–127) | 0.079 |
| Creatinine, μM | 94.0 (44–466) | 124 (55–297) | 0.070 |
| CRP, mg/dL | 22.5 (1–176) | 59 (1–194) | 0.192 |
| Pre-ECMO data | |||
| Pre-ECMO CPR | 16 (32.0) | 9 (47.5) | 0.235 |
| Veno-venous ECMO mode | 8 (16.0) | 4 (21.1) | 0.725 |
| Pre-ECMO SOFA score | 8 (0–21) | 11 (1-22) | 0.008 |
| Pre-ECMO ICU stay ≥ 1 day | 12 (24.0) | 6 (31.6) | 0.522 |
| Pre-ECMO ventilator duration ≥ 1 day | 12 (24.0) | 8 (42.1) | 0.139 |
| Pre-ECMO hospital stay ≥ 1 day | 22 (44.0) | 8 (42.1) | 0.887 |
| Pre-ECMO GCS | 15 (3.0–15.0) | 15 (3–15) | 0.816 |
| MRSA prophylactic regimen | 13 (26.0) | 10 (52.6) | 0.036 |
| During ECMO data | |||
| IABP during ECMO | 13 (26.0) | 8 (42.1) | 0.194 |
| CRRT during ECMO | 18 (36.0) | 12 (63.1) | 0.042 |
| Corticosteroid (methylprednisolone) before and during ECMO, mg | 545 (0–3100) | 560 (0–2860) | 0.793 |
| ECMO support duration, days | 5.8 (2.3–30.3) | 8.0 (3.6–26.0) | 0.001 |
| Before ECMO weaning data | |||
| ventilator duration before ECMO weaning, days | 6 (0–32) | 10 (3–26) | 0.001 |
| Prognosis | |||
| ECMO weaning (Success) | 40 (80.0) | 8 (42.1) | 0.147 |
| hospital stay, days | 17.5 (2–42) | 25 (10–39) | 0.029 |
| Survival-to-discharge rate | 33 (66.0) | 8 (42.1) | 0.532 |
The pre-ECMO SOFA score (OR: 1.174; 95% CI: 1.039–1.326; P = 0.010), was the independent risk factors for BSIs. Patients with a higher pre-ECMO SOFA score were more likely to experience a BSI (Table V & VI).
Table-V.
Univariate analysis of risk factors for BSIs.
| Risk factors | OR (95%CI) | P value |
|---|---|---|
| Demographic characteristics | ||
| Age, years | 1.000 (0.970–1.032) | 0.980 |
| Female gender | 1.051 (0.352–3.135) | 0.929 |
| Smoker | 1.462 (0.456–4.685) | 0.523 |
| Primary disease | ||
| Myocarditis | 0.788 (0.271–2.289) | 0.661 |
| Respiratory failure | 1.400 (0.368–5.332) | 0.622 |
| Coronary artery disease | 1.314 (0.414–4.171) | 0.644 |
| Laboratory findings (Pre-ECMO) | ||
| White blood cell (103/mm3) | 1.033 (0.963–1.108) | 0.366 |
| Hemoglobin, g/L | 0.998 (0.978–1.019) | 0.880 |
| Platelets (103/mm3) | 0.999 (0.994–1.004) | 0.708 |
| Lactate, mM | 1.038 (0.931–1.158) | 0.501 |
| Total bilirubin, μM | 1.017 (0.994–1.041) | 0.152 |
| Creatinine, μM | 1.006 (0.998–1.014) | 0.117 |
| CRP mg/dL | 1.007 (0.998–1.016) | 0.122 |
| Pre-ECMO data | ||
| Pre-ECMO CPR | 1.912 (0.650–5.626) | 0.239 |
| Veno-venous ECMO mode | 1.400 (0.368–5.332) | 0.622 |
| Pre-ECMO SOFA score | 1.174 (1.039–1.326) | 0.010 |
| Pre-ECMO ICU stay ≥1 day | 1.462 (0.456–4.685) | 0.523 |
| Pre-ECMO ventilator duration ≥1 day | 2.303 (0.753–7.047) | 0.144 |
| Pre-ECMO hospital stay ≥1 day | 0.926 (0.318–2.694) | 0.887 |
| Pre-ECMO GCS | 0.982 (0.886–1.088) | 0.727 |
| MRSA prophylactic regimen | 3.162 (1.053–9.502) | 0.040 |
| During -ECMO data | ||
| IABP during ECMO | 2.070 (0.683–6.271) | 0.198 |
| CRRT during ECMO | 3.048 (1.018–9.124) | 0.046 |
| Corticosteroid before and during ECMO, mg | 1.000 (0.999–1.001) | 0.877 |
| ECMO support duration, days | 1.103 (1.003–1.212) | 0.043 |
| Before ECMO weaning data | ||
| ventilator duration before ECMO weaning, days | 1.109 (1.021–1.206) | 0.014 |
| Prognosis | ||
| ECMO weaning (Success) | 0.429 (0.134–1.369) | 0.153 |
| hospital stay, days | 1.065 (1.005–1.129) | 0.034 |
| Survival-to-discharge rate | 0.708 (0.240–2.091) | 0.532 |
Table-VI.
Multivariate analysis of risk factors for BSIs.
| Risk factors | OR (95%CI) | P value |
|---|---|---|
| Pre-ECMO SOFA score | 1.174 (1.039–1.326) | 0.010 |
| MRSA prophylactic regimen | 0.104 | |
| CRRT during ECMO | 0.365 | |
| ECMO support duration, days | 0.146 | |
| Ventilator duration before ECMO weaning, days | 0.041 |
DISCUSSION
The current retrospective study found that 27.5% of the patients experienced a BSI with Gram-negative bacteria as the predominant pathogen. Patients with a higher SOFA score were more likely to have a BSI. Prophylactic antibiotics with anti-MRSA activity may increase BSIs. Although BSIs were associated with a longer hospital stay, there was no significant correlation between BSI and mortality.
The BSIs prevalence in patients undergoing ECMO is reported to vary from 3% to 18%, based on the region, race, and disease status; the corresponding incidence ranges from 2.98 to 20.55 episodes per 1000 days of ECMO in adults.8,9 In the current study, 19 patients (27.5%) developed a BSI, with an incidence of 33.6 infections per 1000 days of ECMO, which seems to be higher than the incidence reported in previous studies. We speculate that an emergent catherization in an overcrowded and contaminated ER may be responsible for this higher BSIs rate.
In this study, BSIs were mostly caused by Gram-negative bacteria. We found that Gram-negative bacteria were responsible for 73.7% of the BSIs identified, with the leading pathogens being K. pneumonia, Enterobacteraerogenes, and Staphylococcusepidermidis. Our results were partly consistent with recent studies from other single centers, which demonstrated that Enterobacteriaceae and Acinetobacter baumannii were the most common pathogens.3,9,10 In contrast, early data (1998–2008) from the ELSO Registry show that the main pathogens of nosocomial infection include the Gram-positive bacteria coagulase-negative Staphylococci (15.9%), Staphylococcus aureus (9.4%), Candida (12.7%), and Pseudomonas aeruginosa (10.5%).1 However, the specific discrimination criteria of infection from contamination in the ELSO registry as well as the site of infectious episodes were not reported, which may diminish the reliability of pathogens reported in the past decades.7 The shift towards more Gram-negative infections is thought to be related to the changing resistance patterns, biofilm formation, and exposure to multi-drug resistant bacteria in healthcare environments.11
Controversies still exist concerning the necessity of prophylaxis for ECMO treatment. In our research, all patients were given intravenous prophylactic antibiotics during the process of ECMO support. However, the incidence of overall infection was still high. Additionally, BSIs were more commonly seen in patients with anti-MRSA regimens. This increased likelihood of BSI in the anti-MRSA group is probably linked to dysbiosis (imbalance of guts microbial environment) of the intestinal flora and bacterial translocation. Therefore, we speculate that prescription of anti-MRSA medications to prevent BSIs should be used with caution in ECMO patients. Other studies also have shown that antibiotic prophylaxis did not reduce ECMO-related infection.5 Thus, the ELSO Infectious Disease Task Force does not recommend the administration of antimicrobial agents to prevent infectious complications during ECMO support and advises not to prolong it beyond 48 hours after cannulation.12
There is a higher risk of nosocomial infection with a longer ECMO duration. Several previous studies have reported a similar clear association between the risk of nosocomial infection and ECMO duration.8,13,14 Burket et al. have reported that the incidence of BSIs increased from 9.5 to 64.5 infections per 1000 days of ECMO for patients with ECMO lasting from 3–10 days to 21–30 days.5 Moreover, the duration of ventilator support before ECMO weaning was similarly related to BSI occurrence as long-term ventilator support increased the incidence of ventilator-associated pneumonia and secondary BSIs.4 Of note, BSIs may also prolong the duration of ECMO or the duration of ventilator use. In the currents study, patients with BSI had a significantly longer duration on ECMO (8 vs. 5.8 days) and ventilator duration before ECMO weaning (10 vs. 6 days) although we failed to prove that the duration of ECMO or ventilator duration was an independent risk factor for BSIs due to the limited sample size.
The pre-ECMO SOFA score, which reflects multiple organ function, was an independent risk factor for BSIs in the current study. This result confirmed the previous findings that a higher SOFA score before cannulation is an independent risk factor for overall infectious complications and for BSIs.14-16 Previous studies have also found an association between renal failure and BSIs in patients with veno-venous ECMO as well as a relationship between hepatic failure and BSIs in patients with veno-arterial ECMO.17 There are several explanations for these results. First, invasive procedures, medication, electrolyte disorders, and blood infusion may increase the risk of bacterial contamination. Second, organ failure may also lead to prolonged ECMO and ventilator support, which increase the possibility of infection. Therefore, ECMO support should be initiated as early as possible to restrain the development of multiple organ dysfunction.
The impact of BSIs on clinical outcomes is still largely unknown. Some studies have demonstrated a significantly elevated mortality in pediatric patients with BSIs compared with those without BSIs,18 but other studies of patients receiving veno-venous ECMO have shown that BSIs have no effect on mortality.19 In this study, the differences between the two groups were not significant, whereas the survival-to-discharge rates were 66% for the non-BSI group and 44.5% for the BSI group. Advances in sepsis care and earlier initiation of antibiotics may reduce the impact of BSIs on the patient prognosis.
Limitations of the study
This study has several limitations that must be acknowledged. First, it was a retrospective, single center study, and the sample size was small. Second, the source of BSI was difficult to identify, and the ratio of primary BSI to secondary BSI was not reported. Third, our analysis only included the first episode of BSI and thus may underestimate the overall incidence of BSIs.
CONCLUSION
Gram-negative bacteria are the predominant pathogens causing BSI during ECMO treatment. Severe organ failure increases the risk of BSI in patients receiving ECMO.
Authors’ Contributions:
Jian-rong Wang data analysis and wrote the manuscript.
Jin-yu Huang contributed to interpretation of the data.
Wei Hu designed most of the investigation.
Xue-ying Cai, Wei-hang Hu, and Ying Zhu contributed to data collection and literature search.
All authors are accountable for the accuracy or integrity of the work.
REFERENCES
- 1.ECLS Registry Report. [Accessed 22th October 2019]. Available at: https://wwwelsoorg/Registry/Statisticsaspx .
- 2.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. doi:10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill JM, Schutze GE, Heulitt MJ, Simpson PM, Taylor BJ. Nosocomial infections during extracorporeal membrane oxygenation. Intensive Care Med. 2001;27(8):1247–1253. doi: 10.1007/s001340101029. doi:10.1007/s001340101029. [DOI] [PubMed] [Google Scholar]
- 4.Sun HY, Ko WJ, Tsai PR, Sun CC, Chang YY, Lee CW, et al. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg. 2010;140(5):1125–1132.e1122. doi: 10.1016/j.jtcvs.2010.07.017. doi:10.1016/j.jtcvs.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis. 1999;28(4):828–833. doi: 10.1086/515200. doi:10.1086/515200. [DOI] [PubMed] [Google Scholar]
- 6.Hsu MS, Chiu KM, Huang YT, Kao KL, Chu SH, Liao CH. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect. 2009;73(3):210–216. doi: 10.1016/j.jhin.2009.07.016. doi:10.1016/j.jhin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections 1988. Am J Infect Control. 1988;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. doi:10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Brechot N, Hariri S, Guiguet M, Luyt CE, Makri R, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633–1641. doi: 10.1093/cid/cis783. doi:10.1093/cid/cis783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, et al. Infections acquired by adults who receive extracorporeal membrane oxygenation:Risk factors and outcome. Infect Control Hosp Epidemiol. 2013;34(1):24–30. doi: 10.1086/668439. doi:10.1086/668439. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–644. [PubMed] [Google Scholar]
- 11.Maillet JM, Guerot E, Novara A, Le Guen J, Lahjibi-Paulet H, Kac G, et al. Comparison of intensive-care-unit-acquired infections and their outcomes among patients over and under 80 years of age. J Hosp Infect. 2014;87(3):152–158. doi: 10.1016/j.jhin.2014.03.011. doi:10.1016/j.jhin.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 12.O'Horo JC, Cawcutt KA, De Moraes AG, Sampathkumar P, Schears GJ. The Evidence Base for Prophylactic Antibiotics in Patients Receiving Extracorporeal Membrane Oxygenation. ASAIO J. 2016;62(1):6–10. doi: 10.1097/MAT.0000000000000287. doi:10.1097/MAT.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 13.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12(3):277–281. doi: 10.1097/PCC.0b013e3181e28894. doi:10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 14.Pieri M, Agracheva N, Fumagalli L, Greco T, De Bonis M, Calabrese MC, et al. Infections occurring in adult patients receiving mechanical circulatory support:the two-year experience of an Italian National Referral Tertiary Care Center. Med Intensiva. 2013;37(7):468–475. doi: 10.1016/j.medin.2012.08.009. doi:10.1016/j.medin.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Austin DE, Kerr SJ, Al-Soufi S, Connellan M, Spratt P, Goeman E, et al. Nosocomial infections acquired by patients treated with extracorporeal membrane oxygenation. Crit Care Resusc. 2017;19(Suppl 1):68–75. [PubMed] [Google Scholar]
- 16.Butler DF, Lee B, Molitor-Kirsch E, Newland JG. Extracorporeal Membrane Oxygenation-Associated Bloodstream Infections In Children. Pediatr Infect Dis J. 2017;36(3):346–347. doi: 10.1097/INF.0000000000001431. doi:10.1097/INF.0000000000001431. [DOI] [PubMed] [Google Scholar]
- 17.Menaker J, Galvagno S, Rabinowitz R, Penchev V, Hollis A, Kon Z, et al. Epidemiology of blood stream infection in adult extracorporeal membrane oxygenation patients:A cohort study. Heart Lung. 2019;48(3):236–239. doi: 10.1016/j.hrtlng.2019.01.004. doi:10.1016/j.hrtlng.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Kim GS, Lee KS, Park CK, Kang SK, Kim DW, Oh SG, et al. Nosocomial Infection in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. J Korean Med Sci. 2017;32(4):593–598. doi: 10.3346/jkms.2017.32.4.593. doi:10.3346/jkms.2017.32.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcos M, Soriano A, Inurrieta A, Martinez JA, Romero A, Cobos N, et al. Changing epidemiology of central venous catheter-related bloodstream infections:increasing prevalence of Gram-negative pathogens. J Antimicrob Chemother. 2011;66(9):2119–2125. doi: 10.1093/jac/dkr231. doi:10.1093/jac/dkr231. [DOI] [PubMed] [Google Scholar]


