Abstract
Background
Polycystic kidney disease (PKD) involves renal cysts arising from proliferating tubular cells. Autophagy has been recently suggested as a potential therapeutic target in PKD, and mammalian target of rapamycin (mTOR) is a key negative regulator of autophagy. However, the effect of autophagy regulation on cystogenesis has not been elucidated in PKD mice.
Methods
Clinical validation was performed using GEO datasets and autosomal dominant polycystic kidney disease (ADPKD) patient samples. Newly established PKD and LC3 transgenic mice were used for in vivo verifications, and additional tests were performed in vitro and in vivo using multiple autophagy drugs.
Findings
Neither autophagy stimulation nor LC3 overexpression alleviated PKD. Furthermore, we observed the inhibitory effect of an autophagy inhibitor on cysts, indicating its possible therapeutic use in a specific group of patients with ADPKD.
Interpretation
Our findings provide a novel insight into the pathogenesis related to autophagy in PKD, suggesting that drugs related to autophagy regulation should be considered with caution for treating PKD.
Funding Sources
This work was supported by grants from the Bio & Medical Technology Development Program; the Collaborative Genome Program for Fostering New Post-Genome Industry of the NRF; the Basic Science Program.
Keywords: Polycystic kidney, PKD, Autophagy, Ift46
Research in Context section.
Evidence before this study: Polycystic kidney disease (PKD) is a common genetic disease in which multiple renal cysts appear owing to germline mutations of ciliary proteins. The mTOR signal is generally enhanced in PKD, and multiple drugs targeting mTOR have been reported to reduce renal cystogenesis in PKD rodent models. In addition, mTOR is a key negative regulator of autophagy, therefore, autophagy has been increasingly suggested as another new therapeutic target of PKD. Recently, an autophagy activator has been shown to inhibit cysts in a zebrafish model; however, no further studies have been conducted using other vertebrate models.
Added value of this study: This study for the first time tries to link autophagy and PKD using vertebrate models. We have newly established Ift46-targeted PKD mice, and LC3 overexpression unexpectedly increased cyst development, which worsened under autophagy stimulation. In addition, we observed the inhibitory effect of an autophagy inhibitor on cysts, indicating its possible therapeutic use in a specific group of PKD patients with altered autophagy.
Implications of all the available evidence: Compared to what the majority of researchers worldwide have observed in diverse rodent models, no significant clinical benefits of mTOR-targeting drugs have been reported. Our findings provide novel evidence to explain the limited therapeutic effects of mTOR inhibition in patients with PKD, wherein limited permissible doses in clinical trials as well as low tissue specificity were considered as the limitations until date.
Alt-text: Unlabelled box
1. Introduction
Polycystic kidney disease (PKD) is a highly prevalent genetic disease in which renal epithelia-lined cysts appear in both kidneys. Numerous fluid-filled cysts, the evident pathology of the disease, arise mainly from abnormally proliferating renal tubular cells, accompanied by inflammation, fibrosis, and finally lead to renal failure [1, 2]. Specifically, multiple molecular pathways, including cyclic AMP (cAMP) accumulation, enhanced vasopressin-specific signals, and hyper-activation of both mammalian target of rapamycin (mTOR) and mitogen activated protein (MAP) kinase signals are implicated in cyst growth [3], [4], [5]. Recently, autophagy has been suggested as another aberrant signaling pathway in PKD [6]. Autophagy is an intracellular process in which harmful or misfolded proteins are eliminated and normal energy levels are maintained. Upon cellular stresses such as nutrient starvation, damaged cytosolic proteins are enclosed by double-membrane vesicles called autophagosomes that fuse with lysosomes for degrading the cargo proteins. More than 30 autophagy-related genes (ATGs) are involved in this process and mTOR is one of the key negative regulators [7], [8], [9]. Since mTOR signal is generally enhanced in PKD, recent studies have attempted to identify the implication of autophagy in disease progression. Autophagy defects in numerous rodent models with polycystic kidneys as well as autophagy-stimulating effects of PKD drugs have been described in recent studies [6]. Moreover, identification of the therapeutic effect of an autophagy activator has recently been attempted using a zebrafish model and the beneficial effect of it as well as that of its combination with low-dose rapamycin has been revealed [10]. However, no further studies evaluating autophagy as a new therapeutic target for PKD using other vertebrate models have been conducted.
Herein, we established a rodent model with specific deletion of Ift46 in the renal collecting duct cells. It showed aggressive PKD phenotypes including renal cyst formation as well as abnormally increased proliferating cells around the cyst-lining epithelia. Hyper-activation of mTOR signals followed by inhibition of autophagy flux in Ift46-deficient mice was observed. Therefore, we further tried to identify the disease-inhibiting effect of autophagy in PKD. Unexpectedly, neither restoring LC3 expression nor stimulating autophagy by starvation alleviated the disease progression in both Ift46- and Pkd1-deleted mice. Enlarged renal cysts and faster renal failure were observed in the groups with enhanced LC3 expression compared with control mice, and it was markedly confirmed under fasting condition, which induces autophagy in vivo. In vitro tests revealed that regulation of autophagy either by drugs or by genetic control showed a correlation between Lc3 accumulation and Erk phosphorylation. This disease-accelerating effect of autophagy through stimulation of Erk phosphorylation, suggested the potential negative effect of autophagy on cystogenesis. Moreover, PKD phenotypes were alleviated by an autophagy inhibitor in vivo, and the expression profiles of ATGs in patients with PKD suggested potential possibilities of using it as a therapeutic target in specific groups of PKD patients.
2. Methods
2.1. Animals
Ift46-targeted JM8A3.N1 ES cell clone (HEPD0687_1_H04) derived from C57BL/6 background was obtained from the EUCOMM and microinjected into C57BL/6J blastocysts, which were then transferred to the uterus of pseudopregnant foster mothers. The original allele derived from the EUCOMM was designated as Ift46tm1a. Mice carrying Ift46tm1a allele were crossed with FLPe mice to delete lacZ and neocassettes, thereby generating conditional allele Ift46flox, wherein exons 5, 6, and 7 were to be excised by Cre in a conditional manner (Fig. S1a-d). We had previously obtained another rodent model, floxed Pkd1 mice (containing loxP sites flanking exon 2–5 of Pkd1 gene), from Yale University. HoxB7-Cre mice were used to generate kidney collecting-duct-specific deletion of either Ift46 or Pkd1. Mice with the genotypes Ift46 (or Pkd1) flox/+; HoxB7-Cre and Ift46 (or Pkd1) flox/flox; HoxB7-Cre were used as controls and experimental animals, respectively. For generation of mCherry-EGFP-LC3B transgenic mice, the mCherry-EGFP-LC3B construct was amplified from pBABE-puro mCherry-EGFP-LC3B (Plasmid #22418, Addgene, MA, USA) using Pfu polymerase and subcloned into the pCAGGS vector with CMV enhancer and chicken β-actin promoter. It was then introduced into the germ line by pronuclear microinjection of fertilized oocytes from C57BL/6J mice and these oocytes were transferred into the oviducts of pseudopregnant foster mothers. Mice aged postnatal 17 days were starved for 24 h to test the effect of autophagy stimulation in vivo. These mice had free access to drinking water. All mice were maintained and used following the approval of the Institutional Animal Care and Use Committee (IACUC) at Sookmyung Women's University. Additionally, PKD mice models produced in this work were considered as C57BL/6 strain through SNP panel analysis [11].
2.2. Clinical samples
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (H-0701-033-195) and Samsung Medical Center (SMC 2019-08-074). Autosomal dominant polycystic kidney disease (ADPKD) and non-ADPKD tissues as paraffin-embedded blocks were obtained from Seoul National University Hospital and Samsung Medical Center, respectively. Mutation types for patients with ADPKD were as follows. One to four PKD cases are PKD1 mutation and the others are unknown, but predicted to be PKD1 mutation. As control, non-cancerous tissues i.e., non-ADPKD tissues in the present study, were obtained from patients with renal cell carcinoma (RCC) who underwent radical nephrectomy.
2.3. Zebrafish models
All zebrafish husbandry and experimental protocols complied with the institutional guidelines were approved by the local ethics board (Sookmyung Women's University Animal Care and Use Committee, SMWU-IACUC-1712-036). Zebrafish were maintained under standard conditions at 28.5 °C with a 14 h light/10 h dark cycle. Embryos were obtained from natural crosses between wild type AB* zebrafish.
2.4. Morpholino and mRNA injections
Morpholinos were designed as described previously [12]: zift46-MO (5′-GGT GCT CTG CTT CTG CCT ACC CGT C-3′) matched to zift46 mRNA from position −25 to −1 relative to the start codon. Morpholino was injected (5–10 nl) into 1–4 cell stage embryos at 1 mM concentration. For lc3 overexpression analyses, full-length lc3 cDNA was synthesised by RT-PCR with total RNA isolated from zebrafish embryos using the following sense (5′-CCA TCG ATT CGA ATT CAT GCC TTC GGA AAA GAC ATT TAA AC -3′) and antisense (5′-GAG AGG CCT TGA ATT CTT ACT GAA ATC CAA ATG TCT CCT GG-3′) primers. Lc3 cDNA was cloned between EcoRI restriction sites of the pCS2+ vector. For in vitro transcription, lc3 RNA was linearized by BamH and synthesized using mMESSAGE mMACHINE™ kit (Ambion). RNAs were microinjected at 100–250 ng/μl concentration in nuclease-free water.
2.5. Cell culture
NIH/3T3 cells (obtained from the Korean Cell Line Bank) were cultured in Dulbecco's modified Eagle medium (DMEM; Welgene, Daegu, South Korea) supplemented with 10% fetal bovine serum (FBS). Mouse inner medullary collecting duct (IMCD) cells were used for analysing cysts in vitro. Cells (2.0 × 105 cells/ml) were three-dimensionally cultured in Matrigel (Corning Life Sciences, MA, USA) with DMEM F/12 (Welgene) at 1:1 ratio and additionally cultured in DMEM F/12 with no FBS for 5–6 days.
2.6. Small interfering RNA, plasmids and drugs
Following small interfering RNAs (siRNAs) and plasmids were used. Scrambled siRNA and siRNA targeting Ift46, Pkd1, Pkd2, Atg5 or Atg7 (Santa Cruz Biotechnology, Dallas, Texas, USA) were transfected with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). Fugene (Promega, Fitchburg, WI, USA) was used for transfection of plasmids including RFP-GFP-LC3 (Thermo Fisher Scientific, Waltham, MA, USA) and GFP-tagged Lc3 (OriGene Technologies, Rockville, MD, USA) according to the manufacturer's instructions. Drugs used to regulate autophagy were rapamycin, 3-methyladenine (3-MA), and chloroquine (CQ) (Sigma-Aldrich, St. Louis, MO, USA).
2.7. Histology & immunofluorescence
Tissues were fixed with 4% paraformaldehyde solution, dehydrated in graded ethanol, cleared in xylene, and embedded in paraffin. For histological analysis, sections were counter-stained with haematoxylin and eosin (H&E) and analysed using Olympus microscope and AxioScan (Carl Zeiss, Oberkochen, Germany). Primary antibodies used included acetylated alpha-tubulin (Sigma-Aldrich), rhodamine-conjugated DBA, fluorescein-conjugated DBA, fluorescein-conjugated LTL, Ki-67 (Vector Laboratories, Burlingame, CA, USA), PCNA (Santa Cruz Biotechnology), TUNNEL assay (Roche, USA), Ift46 (Abcam, Cambridge, UK), pErk, pS6R (Cell Signaling Technology, Danvers, MA, USA), and DAPI (Sigma-Aldrich). Secondary antibodies (Santa Cruz Biotechnology) were used to detect primary antibodies. Immunofluorescence images were visualized by confocal microscope (LSM700, Carl Zeiss).
2.8. Western blotting
Total protein was extracted using NucleoSpin RNA/protein isolation kit (Macherey-Nagel, Duren, Germany) and quantified by BCA assay (Sigma-Aldrich). Proteins were separated by SDS-PAGE and transferred onto PVDF membranes. The primary antibodies used were against pMek (S221), Mek, pErk (T202/Y204), Erk, p-S6R (S235/236), S6R, p-Ulk1 (S317), LC3A/B, LC3B, ATG3, ATG5, ATG7, p62 (Cell Signaling Technology), Ift46 (Abcam), Pc1, Pc2 (Santa Cruz Biotechnology) and β-actin (Bethyl Laboratories, Montgomery, TX, USA).
2.9. Microarray data processing
Affymetrix human genome data of GSE7869 and GSE35831 were downloaded from GEO (http://www.ncbi.nlm.nih.gov/geo/) database to profile gene expression levels in whole genome. Affymetrix microarray data were processed according to the workflow of Bioconductor packages (https://bioconductor.org) in R. GSEA (Gene set enrichment analysis) was performed to identify specific functional classes having significantly different gene expression levels between control and PKD tissues [13].
2.10. Statistics
All statistical analyses data are presented as the mean ± standard deviation (S.D.). Student's t-test was performed using GraphPad Prism 5.0 (GraphPad software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Gene expression profiling of autophagy-related genes in human ADPKD
Although autophagy is presumed to be reduced in ADPKD, analysis of the expression pattern of autophagy-related genes in patients with ADPKD has not been reported. Here, we examined the gene expression profile for autophagy regulation using previously published microarray datasets (GSE7869 and GSE35831) derived from human ADPKD samples to confirm whether changes in expression of autophagy-related genes are clinically relevant [14, 15].
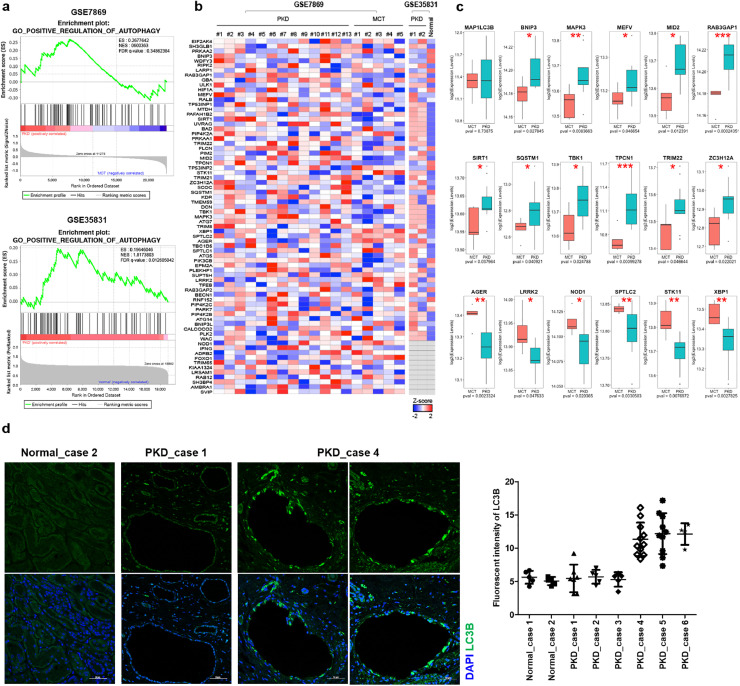
In the first dataset (GSE7869), we observed that the positive regulation of 75 autophagy-related genes did not appear to be of clinical relevance in patients with ADPKD (Fig. 1a, b); however, a pattern in which approximately 65% of the altered genes having been increased in ADPKD was found (Fig. 1a-c). Furthermore, the second dataset (GSE35831) also confirmed that the genes positively regulating autophagy were highly and significantly expressed in patients with ADPKD (Fig. 1a, b), suggesting that some patient groups might have increased autophagy in ADPKD.
Fig 1.
Validation of autophagy-related genes in the kidneys of patients with ADPKD.
(a) Gene set enrichment analysis of 75 autophagy-related genes between ADPKD and control groups. (b) Heatmaps of genes that are from Fig. 1a. in each sample. (c) Expression levels of MAP1LC3B and other significantly altered genes from GSE7869 involved in positive regulation of autophagy. Statistical analysis performed using t-tests, and P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001). (d) Immunofluorescent staining of human kidney tissues with or without PKD for LC3B. Fluorescence intensity of the signals was quantified by green histogram analyses using Image J program.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The expression analysis showed that the expression level of MAP1LC3B, which is the coding gene for LC3 protein, did not show a significant difference for ADPKD and control groups at the transcript level, but there seemed to be individual variation among patients with ADPKD (Fig. 1c). To confirm whether this variation pattern of LC3 was observed in patients with ADPKD at the protein level, LC3 protein expression was verified by immunofluorescent staining of cystic tissues. Although LC3 protein was rarely expressed in non-ADPKD tissues, there was a group of patients with ADPKD in which LC3 was strongly expressed (Fig. 1d). These results suggest that decreased autophagy cannot be considered as a general feature of ADPKD patients; therefore, we tried to further investigate the precise link between autophagy and PKD progression using the newly established PKD mice model. Loss of Ift46 in the renal collecting duct causes PKD accompanied by ciliary defects and hyper-activates MAPK/Erk and mTOR pathways.
3.2. Loss of Ift46 in the renal collecting duct causes PKD accompanied by ciliary defects and hyper-activates MAPK/Erk and mTOR pathways
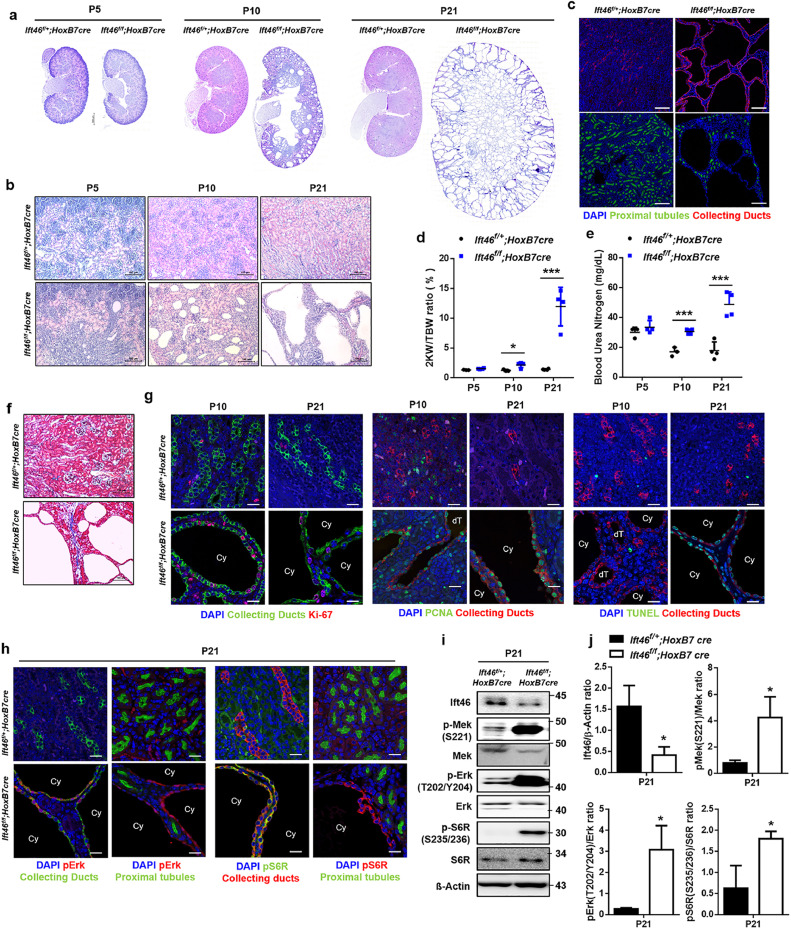
Floxed allele of Ift46 was created and HoxB7-Cre mice were used to delete Ift46 in the renal collecting duct cells. Mice with HoxB7-Cre transgene highly express Cre recombinase in the ureteric bud that develops into the renal collecting duct [16]; therefore, HoxB7-Cre specifically inactivates Ift46 in the renal collecting duct. At postnatal day (p) 5, modest dilation and small cysts were observed, and by p10 to p21, fluid-filled cysts of varying sizes, mostly originating from the collecting ducts, were observed in kidneys of Ift46-deficient mice (Fig. 2a-c). Blood urea nitrogen (BUN) levels and the ratio of the weight of the two kidneys (2KW) to the total body weight (TBW) dramatically increased in Ift46-deficient kidneys at p10 and p21, indicating renal dysfunction (Fig. 2d, e). Consistent with the renal failure, fibrotic cystic kidney accompanied by collagen accumulation was observed in Ift46-deficient mice (Fig. 2f). Moreover, absence of primary cilia was observed in renal collecting duct cells of Ift46-deficient mice, suggesting that Ift46 deletion led to polycystic kidneys with cilia loss (Fig. S1e, f). As dysregulated cell proliferation and apoptosis are key phenotypes of PKD [17], [18], [19], we next confirmed whether Ift46 has an effect on the alteration of cell proliferation and apoptosis. Increased signals of proliferation markers (Ki-67 and PCNA) and apoptosis marked by TUNEL-positive cells were observed in the cystic kidneys at p21 (Fig. 2g). To clarify the altered signaling pathways involved in cyst formation in the Ift46-deleted kidneys, the expression of MAPK/ERK and mTOR pathways was evaluated. Kidneys of Ift46-deficient mice at p21 showed hyper-activated MAP kinases and mTOR in the collecting duct-specific cyst-lining epithelia as well as whole kidney lysates compared with those from control mice (Fig. 2h-j). These observations suggest that activation of MAPK/ERK and mTOR pathways is involved in renal cystogenesis induced by Ift46 deficiency.
Fig 2.
Renal cystic phenotypes with mTOR hyper-activation in Ift46-deleted mice.
(a) Haematoxylin and eosin (H&E)-stained sections of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre kidneys at p5, p10, and p21. Scale bar, 500 µm. (b) High magnification image of H&E-stained sections of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre kidneys at p5, p10, and p21. Scale bar, 100 µm. (c) The kidneys of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice (p21) stained with markers for collecting ducts (red), proximal tubules (green) and nuclei (DAPI, blue). Scale bar, 100 µm. (d) Ratio of the weight of two kidneys (2KW) to the total body weight (TBW) of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice at p5, p10, and p21. Data are shown as mean ± SD; n ≥ 3 for each time point; *p < 0.05, ***p < 0.005. (e) Blood urea nitrogen (BUN) levels in the kidneys of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice at p5, p10, and p21. Data are shown as mean ± SD; n ≥ 3 for each time point; ***p < 0.005. (f) Masson's trichrome staining of kidney sections of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre kidneys at p21. Scale bar, 100 µm. (g) Kidneys of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice (p10, p21) stained with markers for collecting duct (green or red), Ki-67 (red), PCNA (green), TUNEL (green) and nuclei (DAPI, blue). Scale bar, 20 µm. (h) Kidneys of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice were stained for collecting ducts (green or red), proximal tubules (green), pS6R (green or red), pErk (red) and nuclei (DAPI, blue) at p21. Scale bar, 20 µm. (i) Western blot analysis of MAPK/ERK and mTOR signaling using whole kidney lysates of Ift46flox/+; HoxB7-Cre and Ift46flox/flox; HoxB7-Cre mice at p21. (j) Quantification of band intensity observed in panel (i). Data are shown as mean ± SD; n ≥ 3 for each time point; *p < 0.05.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3.3. Cyst development is accelerated in Ift46-deficient mice with enhanced LC3 expression under autophagy induction in vivo
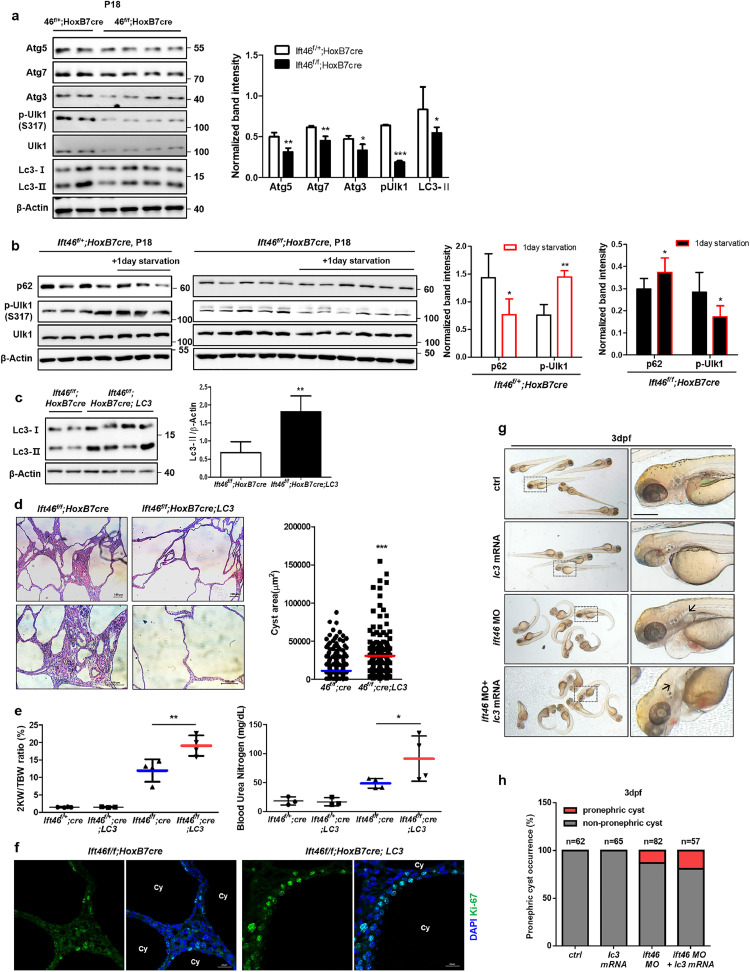
mTOR is a key negative regulator of autophagy; therefore, we screened for a basal autophagy level by checking the expression of multiple autophagy-related proteins (Atgs) in Ift46-deleted mice kidneys. Atgs were reduced in Ift46-deleted kidneys, indicating that basal autophagy was inhibited (Fig. 3a). We next monitored the changes in autophagy flux after 1-day starvation to confirm whether autophagy functions properly in the Ift46-deleted model. Unc-51 like autophagy activating kinase 1 (Ulk-1) and p62 were used as markers. During autophagy, Ulk-1 is accumulated and plays a central role in the initial stage of autophagy, while p62 is itself an autophagy substrate, therefore is finally degraded at the end of the process. Ulk-1 activation and lowered p62 upon 1-day starvation were normally observed in wild type (Left). However, Ift46-deleted mice kidneys (Right) failed to properly induce autophagy, indicating that autophagy is dysregulated in Ift46-deleted kidneys (Fig. 3b).
Fig 3.
Accelerated renal cyst development with reduced renal function in LC3-overexpressed Ift46-deleted models.
(a) Reduced expression of autophagy-regulating proteins including Atg5, Atg7, Atg3, p-Ulk1 (S317) and Lc3 in Ift46flox/flox; HoxB7-Cre mice. (b) Inhibited autophagy flux upon 1-day starvation in Ift46flox/flox; HoxB7-Cre mice. (c) The protein expression level of Lc3 in Ift46flox/flox; HoxB7-Cre; Lc3. (d) Haematoxylin and eosin (H&E)-stained sections of Ift46flox/flox; HoxB7-Cre; Lc3 mice at p21. Scale bar, 100 μm. Cyst area was quantified by Image J program. Data are shown as mean ± SD. (e) Ratio of the weight of two kidneys (2KW) to the total body weight (TBW) and blood urea nitrogen (BUN) level of Ift46flox/flox; HoxB7-Cre mice at p21. (f) Staining of cystic kidney tissues of Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression for Ki-67 to reveal changes in cell proliferation. Scale bar, 20 μm. Data are shown as mean ± SD; n ≥ 3 and P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001). (g) Zebrafish embryos injected with indicated morpholino (MO) and lc3 mRNA at 3 dpf. Arrows indicate pronephric cysts. Scale bar, 0.3 mm. (h) Graph showing occurrence of pronephric cysts in indicated groups.
To test the therapeutic effect of autophagy in vivo, we crossed heterozygous Ift46f mice with LC3 transgenic mice (Fig. 3c). LC3 plays a key role in autophagy, in regulating autophagosome membrane expansion and fusion with lysosomes. Therefore, we hypothesized that LC3 overexpression could enhance autophagy in Ift46-deleted mice kidneys, which show altered autophagy flux. However, significant increase in the renal cystic volume (Fig. 3d) and faster renal dysfunction were unexpectedly observed in the LC3-overexpressed group compared with control group (Fig. 3e). In addition, fluorescence immunohistochemistry data showed increased cell proliferation in the kidneys of homozygous Ift46 mice with enhanced LC3, specifically around the cyst-lining cells (Fig. 3f). To confirm whether these phenotypic changes also occur in another in vivo model, we injected lc3 mRNA into zebrafish ift46 morphants showing pronephric cyst phenotype [20]. Co-injection of ift46 morpholino (MO) and lc3 mRNA increased pronephric cyst occurrence compared with that in ift46 morphants (Fig. 3g, h). These data show that lc3 overexpression in zebrafish ift46 morphants are phenotypically similar to Ift46 mice, which indicate that LC3 overexpression accelerates cyst formation in vivo PKD models.
3.4. Autophagy induction (via starvation) leads to faster renal dysfunction, which was rescued by 3-MA treatment in Ift46-deleted mice
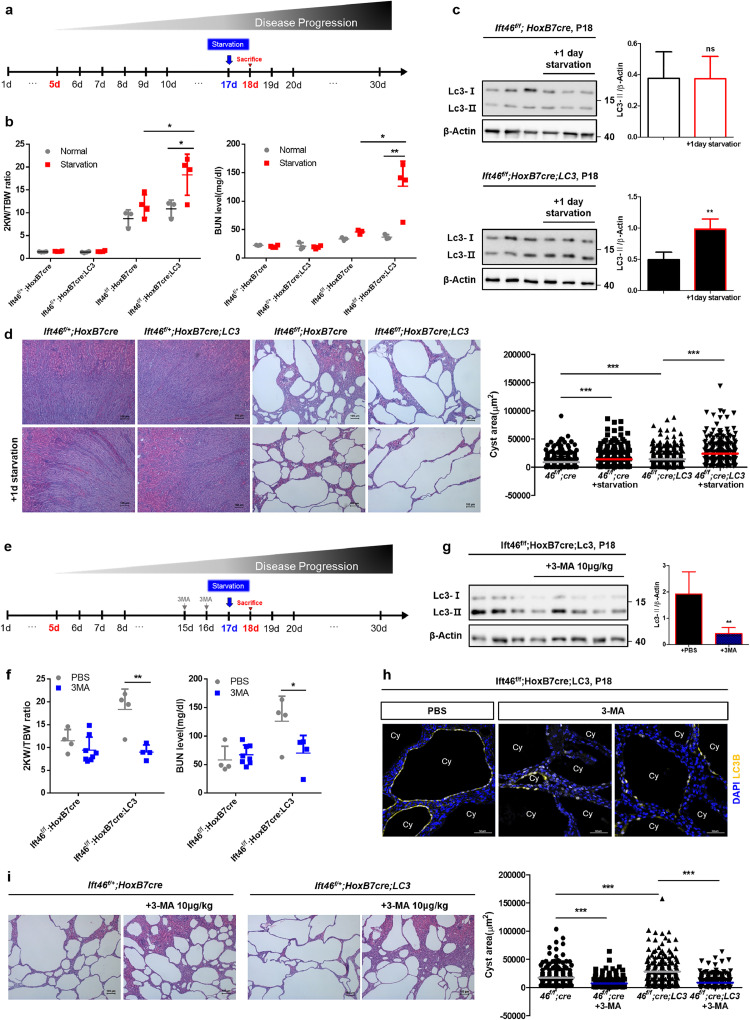
To further investigate the role of autophagy activation in cystogenesis in vivo, mice were starved for 24 h starting at 17 days after birth to stimulate autophagy (Fig. 4a). As a result, LC3 was significantly activated upon 1d starvation and renal function was highly reduced with enlarged cysts upon starvation, especially in LC3-overexpressed Ift46-deleted mice (Fig. 4b, c). Ift46f/f; HoxB7cre mice also showed an increase in kidney volume and BUN level, indicating the effect of full day starvation at p17, which is over 5% of their life. However, the changes in 2KW/TBW ratio and BUN level were higher in LC3-overexpressed group (Fig. 4b). H&E stained sections also revealed a significant increase in cyst area under autophagy stimulation both in Ift46f/f; HoxB7cre and Ift46f/f; HoxB7cre; LC3 mice (Fig 4d). Next, we intraperitoneally administered 3-MA, an autophagy inhibitor involved in the early stage of autophagy, to identify the effect of autophagy inhibition. Mice were injected with either PBS or 3-MA twice on the 15th and 16th day after birth, and sacrificed 1 day after starvation for autophagy induction (Fig. 4e). The 3-MA-treated Ift46f/f; HoxB7cre; LC3 mice showed decreased LC3 activation and a lower 2KW/TBW ratio, as well as improved renal function compared to the PBS-treated mice (Fig. 4f, g). In addition, fluorescent immunohistochemistry analysis showed that LC3 expression around the cysts was highly reduced in the experimental group compared to the control group (Fig. 4h). And cyst area was highly decreased in 3-MA injected groups both of Ift46f/f; HoxB7cre and Ift46f/f; HoxB7cre; LC3 mice (Fig 4i). These findings, wherein PKD phenotypes were accelerated by the induction of autophagy and rescued by an autophagy inhibitor, were contrary to what we had expected; therefore, the specific mechanisms underlying the stimulation of cyst development by LC3 were further evaluated.
Fig 4.
Increased growth of renal cysts upon stimulation of autophagy in Ift46-targeted mice and rescued by 3-methyladenine.
(a) Schedule for starving mice to stimulate autophagy in Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression. (b) Testing renal function based on the ratio of the weight of two kidneys (2KW) to the total body weight (TBW) and blood urea nitrogen (BUN) level. (c) Immunoblotting analysis shows changes in Lc3 level in Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression after 1-day starvation. (d) Haematoxylin and eosin (H&E)-stained sections of Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression after 1-day starvation. Scale bar, 100 μm. Cyst area was quantified by Image J program. Data are shown as mean ± SD. (e) Schedule for 3-methyladenine (3-MA) injection into Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression. (f) Change in the 2KW/TBW ratio and BUN level. (g) Reduced Lc3 level in Ift46flox/flox; HoxB7-Cre; Lc3 mice injected with 3-MA. (h) Expression and localization of LC3 in cystic renal tissues of control or experimental groups. Scale bar, 50 μm. (i) H&E-stained sections of Ift46flox/flox; HoxB7-Cre mice with or without Lc3 overexpression upon 24 h starvation after 3-MA injection. Scale bar, 100 μm. Cyst area was quantified by Image J program. Graphs represent data as mean ± SD; n ≥ 3 for each group and P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001). .
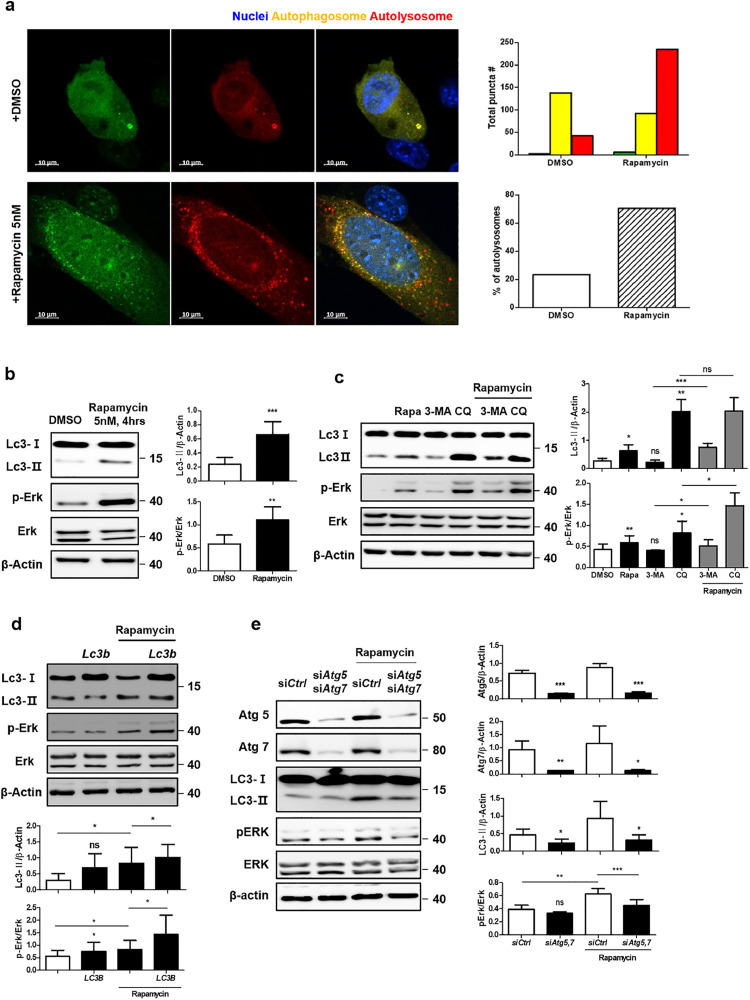
3.5. Phosphorylation of ERK is positively regulated by LC3 under autophagy induction
A recent study has elucidated the positive regulatory mechanisms of LC3-stimulated activation of ERK and its signaling cascades under exposure to growth factors. Moreover, ERK cascade molecules, including MEK, are localized to the cytoplasmic side of autophagosomes and LC3 positively regulates their activation through direct physical interaction with them [21]. Hyper-activated ERK after cAMP accumulation is one of the well-understood aberrant signaling pathways in PKD; therefore, we next identified the role of LC3 in increasing ERK activation and its involvement in the pathogenesis of PKD under autophagy induction. Rapamycin, an inhibitor of mTOR, was used for in vitro analyses to stimulate autophagy, which was successfully confirmed by both LC3-GFP detection and puncta of autophagosomes fused with lysosomes (Fig. 5a). Phosphorylation of Erk was enhanced with increased Lc3 activation in response to rapamycin treatment (Fig. 5b). In addition, two autophagy inhibitors, 3-MA and CQ, were used to further investigate the correlation between Lc3 and Erk phosphorylation. These drugs differentially inhibit autophagy, such that 3-MA blocks autophagosome formation through class III PI3K, and CQ prevents autophagosome-lysosome fusion, thereby accumulating autophagosomes. With different intracellular Lc3 expression, remarkably hyper-activation of Erk with accumulation of Lc3 by CQ was observed, while 3-MA inhibited Erk phosphorylation as well as Lc3 (Fig. 5c). Overexpression of Lc3 itself did not show apparent changes, while treatment with rapamycin increased phosphorylated Erk levels (Fig. 5d). To confirm how ATG alterations affected Erk activation, we silenced Atg5 and Atg7 under treatment with either DMSO or rapamycin. Lc3 activation was highly reduced in Atg-silenced cells, particularly under autophagy stimulation, and it further inhibited Erk phosphorylation (Fig. 5e).
Fig 5.
Positive regulation of Erk activation by Lc3 and its effect on in vitro cystogenesis.
(a) Ratio of autophagosomes fused with lysosomes after 5 nM rapamycin treatment for 4 h. Scale bar, 10 μm. (b) Change in Erk phosphorylation with 5 nM rapamycin treatment for 4 h. (c) Effect of autophagy inhibitors including 3-methyladenine (3-MA) (1 mM) and chloroquine (CQ) (50 μM) on Erk phosphorylation. (d) Increase in Erk phosphorylation in Lc3-overexpressed cells with rapamycin treatment (5 nM, 4 h). (e) Decrease in Erk phosphorylation in Atg5- and Atg7-silenced cells with rapamycin treatment. All data were obtained from a minimum of three independent experiments. Statistical analysis performed using one-tailed t-tests, and P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001).
3.6. Similar effects of Lc3 overexpression and autophagy stimulation were observed in the PKD1-targeting model
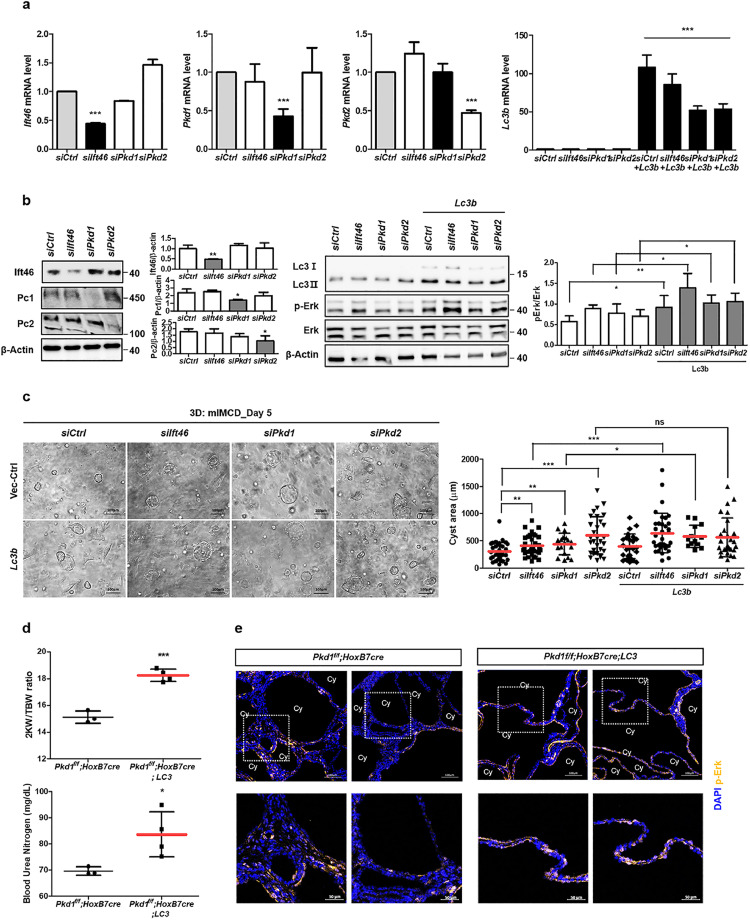
We performed a three-dimensional (3D) culture to evaluate the effect of Lc3 overexpression followed by enhanced Erk activation on in vitro cysts induced by Ift46-silencing. Transfected cells with siRNA targeting Ift46 and/or Lc3 were embedded in Matrigel, and were observed 5–6 days after seeding. In vitro cysts were gradually formed after 2–3 days of seeding, and larger cysts were observed with Ift46 deficiency compared with control. Furthermore, average cystic area was highly increased in Ift46-knockdown cells with enhanced Lc3 expression (Fig. 6a, c).
Fig 6.
Effect of Lc3 overexpression on cysts in vitro and in Pkd1-targeted mice.
(a) Screening of mRNA expression level after transfection of Ift46 siRNA, Pkd1 siRNA, Pkd2 siRNA or Lc3. (b) Changes in expression of the proteins including Ift46, Pc-1, Pc-2, and Lc3 and Erk phosphorylation. (c) Cysts observed by in vitro 3D culture after increasing Lc3 expression and counts of in vitro cystic area for each group. Graphs represent data as mean ± SD; n > 20 for each group. (d) Changes of the ratio of the weight of two kidneys (2KW) to the total body weight (TBW) and blood urea nitrogen (BUN) level in Pkd1flox/flox; HoxB7-Cre; Lc3 mice. (e) Fluorescent staining of phosphorylated Erk in kidney tissues. Lower panels are enlarged from upper ones. Scale bar, 100 μm for images with high magnification and 50 μm for zoom-in images. Graphs represent data as mean ± SD; n ≥ 3 for each group and P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001).
Pkd1 and Pkd2 are the main causative genes of PKD; therefore, we wondered whether Lc3 could also stimulate cysts derived from PKD gene deficiencies. As observed in Ift46-silenced cells, in vitro cysts were stimulated by Pkd1- or Pkd2-silencing, and further increased by Lc3 overexpression only in Pkd1-silenced cells (Fig. 6a, c). Erk phosphorylation was also enhanced by Lc3 overexpression in each group with gene deficiencies (Fig. 6b).
To confirm the effect of LC3 overexpression on in vivo renal cysts originating from mice with Pkd1 deletion, we used a collecting duct-specific Pkd1-deleted mouse model. Pkd1-targeted mice showed radical and more severe progression with shorter lifespans compared with that shown by Ift46-targeted mice. Furthermore, the mice were starved for 24 h starting from 12 days after birth followed by sacrifice at postnatal day 13. As a result, Pkd1-knockout mice with enhanced LC3 led to end-stage renal disease (ESRD) earlier than control mice (Fig. 6d). Immunofluorescence data revealed that phosphorylated ERK was highly detected around the cyst-lining cells in kidneys of Pkd1-deleted LC3-overexpressed mice (Fig. 6e). These findings indicate that the negative effects of LC3, including increased Erk phosphorylation and enlarged cysts observed in Ift46-deficient mice, were not limited to a specific PKD model.
Collectively, we observed highly variable expression profiles of ATGs in patients with ADPKD using two different microarray datasets. They contradicted previous studies hypothesizing reduced autophagy levels in PKD. We next observed that basal autophagy was generally decreased, with mTOR hyper-activation in collecting duct-specific Ift46-deleted mice. When autophagy was enhanced in LC3 transgenic mice, more enlarged cysts with reduced renal function appeared unexpectedly, which further worsened under autophagy induction. It was then partially alleviated by autophagy inhibition, suggesting potential possibilities for therapeutic use of autophagy inhibitors in specific groups of patients with PKD. Similar results were observed in Pkd1-deleted model, indicating that autophagy stimulation might accelerate the disease regardless of the target genes.
4. Discussion
Recently, several studies have shown dysregulated autophagy in disease models of ciliary defects and vice versa, indicating a strong association between autophagy and ciliogenesis [22]. One of them was PKD in which multiple renal cysts appear by germline mutations of ciliary proteins. There are several reasons why many researchers are particularly interested in autophagy dysregulation in PKD. First, mTOR, a key negative regulator of autophagy, is commonly hyper-activated and has been considered as a therapeutic target for PKD. Indeed, specific inhibitors targeting mTOR signals including rapamycin, metformin, and curcumin have attenuated disease phenotypes with autophagy stimulation in PKD mice [23, 24]. Therefore, a recent study has attempted to identify the therapeutic benefits of autophagy activation in PKD by directly targeting autophagy with a specific inducer [10]. In addition, they used a combination treatment comprising ten-fold lower doses of rapamycin and an autophagy activator on a pkd1-mutated zebrafish model. The combination treatment indeed reduced the cyst growth by almost the same level as that by rapamycin alone at higher doses, suggesting new therapeutic strategies with potentially reduced drug toxicity in PKD while targeting mTOR.
Herein, we attempted to further evaluate the precise role of autophagy in PKD progression. We established a new mouse model with loss of Ift46, specifically in the renal collecting duct cells via a cre-loxp system. The collecting duct-specific Ift46-knockout mice showed loss of cilia and severe polycystic kidney phenotype with hyper-activation of MAP kinases and mTOR, and short lifespans of approximately less than a month.
We next hypothesized that increase in autophagy via enhanced expression of a key autophagy modulator could slow the disease progression. Prior to testing its therapeutic effects, we screened the basal autophagy level in Ift46-deficient mice and found it to be reduced in polycystic kidneys. Lc3 transgenic mice were thought to have restored autophagy levels; however, neither restoring Lc3 expression nor autophagy stimulation alleviated PKD. Moreover, it further led to progression towards the end-stage renal disease (ESRD) earlier with more enlarged cysts. In vitro tests including drugs treatments, silencing of autophagy genes, and analysis of in vitro cysts via 3D culture system, markedly revealed the positive regulation of Erk by LC3. In particular, 3-MA treatment, which initially inhibited LC3 activation, showed reduced Erk activation, and it finally alleviated PKD pathologies.
Autophagy is reported to be reduced in PKD mice models; therefore, autophagy activation is suggested as one of the potential therapeutic strategies. Starvation is the most reliable physiological inducer of autophagy in mice, and a previous study by Warner et al. had demonstrated the beneficial effect of food restriction (FR) in PKD mice. Six-week-old mice were starved by a decrease of 30–50% of total caloric intake for six months. They showed reduced cyst growth, renal fibrosis, inflammation and injuries; however, no significant changes in autophagy were observed [25]. This may indicate that mild to moderate FR did not successfully induce autophagy, thereby a specific effect of autophagy stimulation on PKD progression could not be observed. Therefore, our findings from rapid fasting conditions may suggest the cyst-stimulating effect of autophagy. Moreover, autophagy is generally expected to be reduced in patients with PKD; however, our clinical data revealed that the expression of ATGs including LC3B varies from case to case. Therefore, while our findings support the possible therapeutic use of autophagy inhibitors in a specific group of patients with altered autophagy, they should be considered with caution. The disease onset patterns are highly different between PKD patients and animal models of PKD. In human PKD, the age at which end-stage renal disease (ESRD) occurs is highly variable, but in most cases, it is observed post 50 years of their life though the genetic mutation is present at birth. On the contrary, severe and rapid development of phenotypes is observed in rodent models with mutation in Pkd1, Pkd2, or other ciliary genes. They display embryonic lethality or short lifespans of less than a month. Therefore, there are limitations to the direct application of mTOR inhibitors to patients based on the observations in preclinical models. Indeed, a number of other preclinical studies had developmental issues. mTOR inhibitors, including rapamycin, sirolimus, and everolimus, were previously reported to markedly reduce cyst growth with delayed loss of renal function in Han; SPRD rat model [26], [27], [28]. In particular, sirolimus also showed partial therapeutic effects in Pkd2WS25/- mice, an ortholog of the human ADPKD model, indicating its potential to be applied to clinical studies [24]. Based on the remarkable cyst-inhibiting effect of mTOR-targeting drugs observed in rodent models, large randomized clinical trials have been conducted; however, they revealed insignificant changes in total kidney volume (TKV) on MRI [29, 30]. This was attributed to the limited doses permitted for clinical trials as well as low tissue specificity. To overcome these limitations, new strategies such as those applying a conjugated form, which enhances the ability of drugs to reach kidneys more efficiently, were generally explored in more recent studies [31]. Similarly, our findings might provide another evidence to explain the limited therapeutic effects of mTOR inhibition in PKD patients, and suggest potential possibilities of autophagy inhibitors as therapeutic agents. Finding ways to overcome discrepancies in PKD onset patterns between humans and animals may be critical to increase the possibility of applying candidate targets. In addition, new strategies to enhance the efficiency or specificity of the drugs should be considered to further advance PKD therapy.
Contributors
J.H. Park and G.T. Oh designed the study; E.J. Lee and J.Y. Ko analysed in vivo phenotypes, conducted autophagy experiments, and wrote manuscript; E.J. Lee, J. Joen and H. Mun conducted in vitro tests; C.J. Lim and S.W. Seo generated Ift46 flox mice; K.H. Yoo and S. Oh analysed gene expression profiles using microarray datasets; H. Kim, Y.K. Oh, C. Ahn, and M.Y. Kang discussed the data on clinical samples; M.J. Kim generated zebrafish models targeting either Ift46 or LC3; H.W. Ko discussed ciliopathy models.
Funding sources
This work was supported by grants from the Bio & Medical Technology Development Program [2015M3A9B6027555]; the Collaborative Genome Program for Fostering New Post-Genome Industry of the NRF [2014M3C9A2067613]; the Basic Science Program [2016R1A5A1011974 and 2019R1A2B5B03069738]. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Declaration of Competing Interest
J.H. Park, G.T. Oh and J.Y. Ko have filed a patent in South Korea for the establishment of the Ift46-deleted model (patent number: 10-1618124). The others have nothing to disclose.
Acknowledgments
We thank Dr. S. Somlo (Yale University) for the Pkd1flox/flox mice and Dr. M. Shong (Chungnam National University) for HoxB7-Cre transgenic mice.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102986.
Contributor Information
Goo Taeg Oh, Email: gootaeg@ewha.ac.kr.
Jong Hoon Park, Email: parkjh@sookmyung.ac.kr.
Appendix. Supplementary materials
References
- 1.Nauli S.M., Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26(8):844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 2.Harris P.C., Torres V.E. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 4.Torres V.E., Harris P.C. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25(1):18–32. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastos A.P., Onuchic L.F. Molecular and cellular pathogenesis of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2011;44(7):606–617. doi: 10.1590/s0100-879x2011007500068. [DOI] [PubMed] [Google Scholar]
- 6.Ravichandran K., Edelstein C.L. Polycystic kidney disease: a case of suppressed autophagy? Semin Nephrol. 2014;34(1):27–33. doi: 10.1016/j.semnephrol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Stanley R.E., Ragusa M.J., Hurley J.H. The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends Cell Biol. 2014;24(1):73–81. doi: 10.1016/j.tcb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu P., Sieben C.J., Xu X., Harris P.C., Lin X. Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum Mol Genet. 2017;26(1):158–172. doi: 10.1093/hmg/ddw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petkov P.M., Cassell M.A., Sargent E.E., Donnelly C.J., Robinson P., Crew V. Development of a SNP genotyping panel for genetic monitoring of the laboratory mouse. Genomics. 2004;83(5):902–911. doi: 10.1016/j.ygeno.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Gouttenoire J., Valcourt U., Bougault C., Aubert-Foucher E., Arnaud E., Giraud L. Knockdown of the intraflagellar transport protein IFT46 stimulates selective gene expression in mouse chondrocytes and affects early development in zebrafish. J Biol Chem. 2007;282(42):30960–30973. doi: 10.1074/jbc.M705730200. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X., Di Giovanni V., He N., Wang K., Ingram A., Rosenblum N.D. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet. 2009;18(13):2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 15.Woo Y.M., Bae J.B., Oh Y.H., Lee Y.G., Lee M.J., Park E.Y. Genome-wide methylation profiling of ADPKD identified epigenetically regulated genes associated with renal cyst development. Hum Genet. 2014;133(3):281–297. doi: 10.1007/s00439-013-1378-0. [DOI] [PubMed] [Google Scholar]
- 16.Yu J., Carroll T.J., McMahon A.P. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129(22):5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J.X., Li X. Apoptosis in polycystic kidney disease: from pathogenesis to treatment. In: Li X, editor. Polycystic kidney disease. 2015. editor. Brisbane (AU) [PubMed] [Google Scholar]
- 18.Hanaoka K., Guggino W.B. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11(7):1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 19.Ecder T., Melnikov V.Y., Stanley M., Korular D., Lucia M.S., Schrier R.W. Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease. Kidney Int. 2002;61(4):1220–1230. doi: 10.1046/j.1523-1755.2002.00250.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee M.S., Hwang K.S., Oh H.W., Ji-Ae K., Kim H.T., Cho H.S. IFT46 plays an essential role in cilia development. Dev Biol. 2015;400(2):248–257. doi: 10.1016/j.ydbio.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Lopez N., Athonvarangkul D., Mishall P., Sahu S., Singh R. Autophagy proteins regulate ERK phosphorylation. Nat Commun. 2013;4:2799. doi: 10.1038/ncomms3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avalos Y., Pena-Oyarzun D., Budini M., Morselli E., Criollo A. New roles of the primary cilium in autophagy. Biomed Res Int. 2017;2017 doi: 10.1155/2017/4367019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravichandran K., Zafar I., Ozkok A., Edelstein C.L. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant. 2015;30(1):45–53. doi: 10.1093/ndt/gfu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafar I., Ravichandran K., Belibi F.A., Doctor R.B., Edelstein C.L. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 2010;78(8):754–761. doi: 10.1038/ki.2010.250. [DOI] [PubMed] [Google Scholar]
- 25.Warner G., Hein K.Z., Nin V., Edwards M., Chini C.C., Hopp K. Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol. 2016;27(5):1437–1447. doi: 10.1681/ASN.2015020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao Y., Kim J., Schrier R.W., Edelstein C.L. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16(1):46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 27.Wahl P.R., Serra A.L., Le Hir M., Molle K.D., Hall M.N., Wuthrich R.P. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21(3):598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 28.Wu M., Wahl P.R., Le Hir M., Wackerle-Men Y., Wuthrich R.P., Serra A.L. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30(4):253–259. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 29.Walz G., Budde K., Mannaa M., Nurnberger J., Wanner C., Sommerer C. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 30.Serra A.L., Poster D., Kistler A.D., Krauer F., Raina S., Young J. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 31.Shillingford J.M., Leamon C.P., Vlahov I.R., Weimbs T. Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol. 2012;23(10):1674–1681. doi: 10.1681/ASN.2012040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.