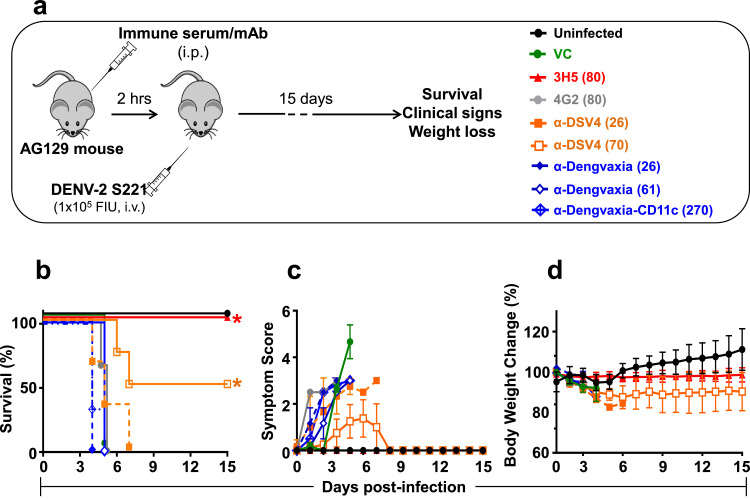
Fig. 5.
The capacity of α-DSV4 and α-Dengvaxia immune sera to confer protection against lethal DENV-2 S221 challenge. (a) Schematic representation of the experimental design. Groups of AG129 mice (n=3 or 4) were administered (i.p) BALB/c immune sera (in two dosage levels, 100 µl and 300 µl/mouse) or mAbs (100 µg/mouse). The immune sera used were anti-DSV4 (α-DSV4, orange) and anti-Dengvaxia (α-Dengvaxia, blue) and the mAbs used were a DENV-2 TS mAb (3H5, red) and a pan-DENV CR mAb (4G2, grey). Two hours after passive transfer the mice were bled (for the determination of circulating DENV-2 nAb titers, shown in parenthesis in the legends in panel ‘a’), and then challenged shortly thereafter with a lethal dose (105 FIU/mouse) of DENV-2 S221. All groups were monitored over the next 15 days for survival (Kaplan-Meir survival curves, panel ‘b’), clinical symptoms (panel ‘c’) and body weight change (panel ‘d’). In parallel, one group received just the challenge virus (VC, green), and another group received neither passive antibody transfer nor the challenge virus (Uninfected, black). In panel ‘b-d’, data for mice which received 100 µl and 300 µl immune sera are shown using dashed and solid curves, respectively, in the indicated colours. Survival data (panel ‘b’) were analysed by Log-Rank (Mantel-Cox) test for significant difference in survival rates [the asterisks denote that survival in the 3H5 and α-DSV4 (70) groups were significantly higher in comparison to the 4G2 group (p<0.05), for both]. Clinical scoring in panel ‘c’ and body weight measurements in panel ‘d’, were as described in Fig. 2 legend. Note: a second pool of anti-Dengvaxia immune serum (α-Dengvaxia-CD11c, dashed blue curves), raised by immunising CD11c-Ifnar1−/− mice, was also tested for its efficacy against lethal DENV-2 S221 challenge (other murine immune sera are from BALB/c mice) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).