Abstract
Platelets are the primary cellular mediators of hemostasis and this function firmly acquaints them with a variety of inflammatory processes. For example, platelets can act as circulating sentinels by expressing Toll-like receptors (TLR) that bind pathogens and this allows platelets to effectively kill them or present them to cells of the immune system. Furthermore, activated platelets secrete and express many pro- and anti-inflammatory molecules that attract and capture circulating leukocytes and direct them to inflamed tissues. In addition, platelets can directly influence adaptive immune responses via secretion of, for example, CD40 and CD40L molecules. Platelets are also the source of most of the microvesicles in the circulation and these miniscule elements further enhance the platelet’s ability to communicate with the immune system. More recently, it has been demonstrated that platelets and their parent cells, the megakaryocytes (MK), can also uptake, process and present both foreign and self-antigens to CD8+ T-cells conferring on them the ability to directly alter adaptive immune responses. This review will highlight several of the non-hemostatic attributes of platelets that clearly and rightfully place them as integral players in immune reactions.
Keywords: Platelets, Bacteria, Viruses, TLR, CD40L, Cytokines, Chemokines, Antigen processing and presentation, Immune response, Microvesicles
Introduction
Platelets are anucleate cell fragments derived from MK in the bone marrow (BM) and are key players in hemostasis [1]. They are the second most abundant cell in the circulation (150–400 × 109/L, [2] and thus, well situated to rapidly respond to vascular damage and attract leukocytes to sites of injury [3]. It has also become clear that platelets elicit several non-hemostatic immune functions [[4], [5], [6]]. For example, platelets are capable of direct pathogen binding by expressing pathogen-associated molecular pattern (PAMP) receptors and thus mediate anti-infective immunity [7,8]; they can kill pathogens by both encapsulation and anti-microbial peptides [[9], [10], [11]].
Platelets also contribute to innate immunity to affect adaptive immune responses and they do so by expressing a wide range of functional immune receptors [12]. These receptors enable interactions with immune cells at the vascular endothelium and in the red pulp of the spleen [13]. For example, platelets contain the largest pool of circulating Fc gamma receptor IIA (FcγRIIA) [14,15] and this allows them to interact with immune complexes and ultimately form platelet-leukocyte aggregates that can immobilize pathogens [16].
Evidence suggests that platelets also directly influence adaptive immune processes. For example, platelets express functional CD40L (CD154) [17] and they contain a diverse spectrum of RNA species [[18], [19], [20], [21], [22]] packaged into platelet microvesicles (PMV) which are abundant in blood and transfused blood products [23,24]. PMV extend the platelet’s immunomodulation capabilities and their presence is implicated in several autoimmune diseases. Perhaps more intriguing is that platelets and their parent cells, the MK, can act as antigen presenting cells and are able to stimulate T-cells against foreign and self-antigens [4,5,11].
How platelets possess all their different immune functions is unknown. One theory suggests an evolutionary link between platelets and invertebrate hemocytes, which not only protect arthropods from pathogens but also clot hemolymph at sites of exoskeletal breach [25]; perhaps a divergence occurred during platelet evolution where they retained some of the immune properties of the hemocyte [25]. On the other hand, increasing evidence suggests platelets may acquire their immune properties from MK [25]. For example, emperipolesis is a rare phenomenon where an intact cell is found within the cytoplasm of another cell. Cunin et al elegantly demonstrated that neutrophils can enter the MK cytoplasm in membrane-bound vesicles and once inside the MK, they transfer parts of their membrane to the MK and then to platelets [26,27] This process results in circulating platelets bearing membranes from non-MK donor cells and this perhaps adds to the platelet’s immune function [26,27]
It is now evident that platelets play a pivotal role connecting inflammation, immunity, and vascular integrity and modulate immunological processes. This review will summarize the two major non-hemostatic roles that platelets play; their anti-infective nature and their ability to immunomodulate the innate and adaptive immune systems.
The Anti-Infective Nature of Platelets
Platelet Pattern Recognition Receptors (PRR)
Platelets from several species express PRRs such as Toll-like receptors (TLRs) and C-type lectin receptors (CLR) [28,29] which detect pathogen-associated molecular patterns (PAMPs) from pathogens [30,31] (Table 1 ). For example, platelet TLR4 binds to bacterial lipopolysaccharides (LPS) and in vivo, induces production of tumor necrosis factor (TNF)-α, soluble CD40L (sCD40L) and interleukin (IL)-1β, however, different LPS moieties appear to cause differential cytokine release [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. TLR4 has also been shown to augment platelet-neutrophil aggregates, neutrophil extracellular trap (NET) formation (NETosis) and bacterial trapping in sepsis [42]. Intriguingly, platelet TLR4 can both inhibit and augment neutrophil responses. Co-culture of platelets with neutrophils and TLR4 agonists increased neutrophil CD62L expression, phagocytosis and IL-8 secretion but reduced CD62L shedding and elastase secretion [43]. It is now clear that platelet TLR4 is critical for orchestrating neutrophil responses against invading pathogens and the source of LPS determines their ultimate effect on leukocytes.
Table 1.
Platelet Toll-like receptors and their ligands
| Receptor | Ligand | Functions | References |
|---|---|---|---|
| TOLL LIKE RECEPTORS (TLRs) | |||
| TLR2 (with TLR1 or TLR6) | Many non-TLR molecules and PAMPs Lipoprotein/lipopeptides, peptidoglycan, lipoteichoic acid, etc |
Mice - CD62P and integrin αIIbβ3 surface expression, platelet-neutrophil complex formation, and neutrophil-mediated phagocytosis of periodontopathogens. - (CD62P/PSGL-1, CD40L/CD40, GP-IIb–IIIa/CD11b) involvement or platelet cytokines. |
[185] [186] |
|
Human - Heterodimer formation with TLR1/TLR6 regulating innate immune response to bacterial lipoproteins or lipopeptides. Acts via MyD88 and TRAF6, leading to NF-kappa-B activation, cytokine secretion and inflammatory response |
[187] |
||
| TLR4 | LPS, HSP60, commensal bacteria Most abundantly expressed TLR on PLTs CD14-dependent response to bacterial LPS |
Mice - Complex formation with MD-2 molecule, enabling binding to LPS - Heterodimer formation with TLR6 leads to NF-kappa-B-dependent production of CXCL1, CXCL2 and CCL9 cytokines, via MyD88 signaling pathway, and CCL5 cytokine, via TICAM1 signaling pathway. |
[188] [189] |
|
Human - TRAF-6 stimulation and de novo synthesis of IL1-β. TRAF-6 activation, phosphorylation of Akt and JNK - Increased TLR4 expression in synergistic combination with CD62P, enables LPS binding |
[190,191] [192,193] |
||
| TLR7 | Synthetic compounds (the immune response modifiers) Endosomal receptor Interaction with Imiquimod, homology to TLR8 |
Mice - Host immune response through recognition of ssRNAs of viral origin or guanosine analogs, formation of neutrophil-platelet aggregates and neutrophil DNA release |
[194] [195] |
|
Human - Upon binding to agonists, recruitment of TIR-containing downstream adapter MyD88 through homotypic interaction - Activation of NF-kappa-B and IRF7: proinflammatory cytokines and interferons |
[194] [196] |
||
| TLR9 | CpG-DNA |
Mice - Receptor for CpG bacterial DNA - Binding of carboxy (alkylpyrrole) protein adducts CAPs to TLR9 induce platelet activation and aggregation |
[50] |
|
Human - Acts via MyD88 and TRAF6, leading to NF-kappa-B activation, cytokine secretion and inflammatory response |
[50] | ||
Detection of PAMPs is an efficient host defense feature of platelets to ensure a rapid response to infections [44]. Platelet TLR2 recognizes bacterial lipopeptides together with TLR1 and TLR6 [45] and TLR2 stimulation induces the formation of platelet-neutrophil aggregates and phagocytosis of bacteria [46,47]. On the other hand, platelet TLR7 senses ssRNA and can enhance the uptake of viruses such as Influenza that leads to neutrophil NETosis [48,49]. In addition, platelet TLRs binds damage-associated molecular patterns (DAMPs) such as carboxy (alkylpyrrole) protein adducts (CAPs) and these are elevated in several pathological conditions e.g. diabetes and atherosclerosis; CAP-binding to platelets induces platelet activation and aggregation [50]. Table 1 summarizes the platelet TLRs and their various effects on immunity.
Platelets and Their Interactions with Viruses
Thrombocytopenia is associated with many infections and severe forms of thrombocytopenia during sepsis are usually a poor prognostic marker suggesting platelets are key players in septic patients [[51], [52], [53], [54]]. How thrombocytopenia occurs is still a matter of debate; it is mediated by consumption, sequestration or bone marrow production faults. With respect to viral infections, platelets have the ability to engulf viruses through a wide array of expressed molecules (Figure 1 ). For example, Banerjee et al have shown that platelets use dynamin and vesicle-associated membrane protein-3 (VAMP) to take up and traffic HIV-1 intracellularly [11]. It appears that HIV trafficked through endosomes causing platelet activation and platelet/leukocyte aggregate formation in vitro; in vivo, infection lead to platelet-leukocyte aggregates and mild thrombocytopenia [11]. These data are consistent with the notion that platelets can sample the circulation and act as infectious sentinels.
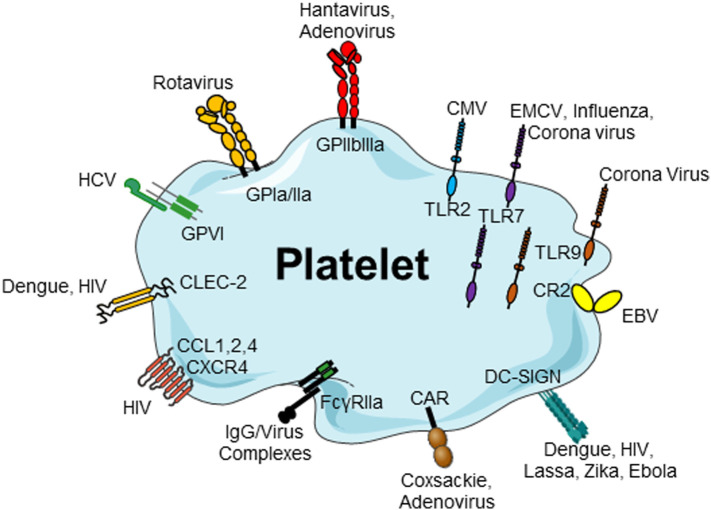
Figure 1.
Platelets, via several cell surface and internalized receptor molecules, can readily bind to and uptake many different viruses. Platelet receptors for viruses: platelets and viruses can directly interact via a plethora of surface receptors. CMV binds to platelets via TLR2, EMCV interacts via TLR7, rotavirus utilizes GPIa/IIa to bind to platelets and Hantavirus and adenovirus interact with platelets via GPIIb/IIIa. EBV–platelet interaction occurs via CR2. HIV and DV bind to lectin receptors such as CLEC-2 and DC-SIGN. HIV further interacts with CXCR4 and CCL3 and CCL5. Platelets express the Coxsackie virus-specific receptor, CAR, and HCV interacts with platelets via GPVI. CAR, Coxsackie-adenovirus receptor; CLEC-2, C-type lectin domain family 2; CCL, chemokine (C–C motif) ligand;
CMV, cytomegalovirus; CR, complement receptor; CXCR4, C–X–C chemokine receptor type 4; EBV, Epstein–Barr virus; EMCV, encephalomyocarditis virus; DC-SIGN, DC-specific intercellular adhesion molecule-3-grapping non-integrin; DV, Dengue virus; FcγRII, Fc receptor γ II; GP, glycoprotein; HCV, hepatitis virus C; HIV, human immunodeficient virus; IgG, immunoglobulin G; TLR, toll-like receptor. Adapted from [210].
CLEC-2 is a CLR expressed on platelets [55,56] and was identified to promote platelet aggregation [57]. Another CLR, dendritic cell (DC)-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-Sign) also interacts with several pathogens. For example, platelet CLEC2 binds HIV via DC-SIGN and this interaction facilitates viral dissemination [58] whereas it is also responsible for mediating immunothrombosis during bacterial infections [59,60]. These receptors allow platelets to bind to several other viruses such as Dengue virus (DENV), Ebola, and Hepatitis C [58,61,62]. Platelet DC-Sign not only binds DENV but once engulfed, the virus undergoes replication and platelets act as a source of nascent viral production [63,64]. In addition, DENV infected individuals become thrombocytopenic and this may be related to bone marrow suppression. Vogt et al utilized three models of DENV infection and replication; a human MK cell line, primary human MKs and in vivo, a humanized MK mouse model. They found that DENV infection and replication was supported in all three models suggesting that MK harbor the virus, which results in lower platelet production [65]. Altogether, these data suggest that blocking platelet/virus interactions could be a novel therapeutic option for acute viral infections.
One of the most interesting aspects of how platelets attack viruses is their ability to neutralize virions with intracellularly stored IgG molecules. Schrottmaier et al. demonstrated that platelets from anti-virus sero-positive donors harbor IgG molecules specific for the virus (e.g. Influenza A and Cytomegalovirus) and they can release the IgG to neutralize them [66]. It suggests that platelet-derived IgG represents a novel mechanism to potentiate anti-viral humoral immunity [66]. In addition, platelets can be activated through their FcγRIIA by immune complexes (IC) formed in immune hosts by different bacteria and virus [67]. This leads to granule release, temporary sequestration of platelets primarily in the lung and brain vasculature and this appears to be dependent on integrin αIIbβ3 engagement [[67], [68], [69]]
MKs can also be involved in anti-viral immunity and this may be how platelets acquire their art of dealing with viruses. Campbell et al. examined anti-viral genes in MK during DENV and influenza infections [70]. They found that interferon-induced transmembrane protein 3 (IFITM3), an anti-viral immune gene, was significantly elevated in platelets from infected patients and lower IFITM3 expression correlated with increased mortality [70]. Interestingly, infecting MKs with DENV selectively also increased IFITM3 and overexpression of IFITM3 in cultured MKs enhanced the MK’s resistance to DENV infection [70]. Thus, MKs possess significant anti-viral activity and this may be at least one reason for their daughter’s potent armaments [71].
In 2019, the novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged and the infection has caused a global pandemic of potentially severe pulmonary disease termed COVID-19 [72]. Interestingly, asymptomatic or mild COVID-19 cases are either not thrombocytopenic or have mild thrombocytopenia, however, those patients with severe disease can present with significant thrombocytopenia and this is a grave prognosis [73]. Platelets have been suggested to play a role in the COVID-19 although it is still unclear whether they effectively interact with SARS-CoV-2 [74]. For example, Hottz et al. studied participation of platelets in COVID-19 pathogenesis and demonstrated increased platelet activation and platelet-monocyte aggregation in critically ill COVID-19 patients [75]. Platelet-monocyte interaction was associated with tissue factor (TF) expression by the monocytes [75]. Thus, COVID-19 is associated with platelet hyper-reactivity, which may contribute to immunothrombosis. On the other hand, Manne et al. showed that platelets and MK are RNA- and protein-negative for angiotensin converting enzyme-2 (ACE-2) [76], the putative SARS-CoV-2 receptor [77]. In contrast, however, Zaid et al challenged this concept by showing that platelets do indeed express ACE-2 mRNA [78]. Whether ACE-2 is expressed by platelets or not will require further examination, however, even without ACE-2, platelets can still potentially interact with SARS-CoV-2 via their expression of TLR7 and 9. This would require cell entry by another receptor i.e. CRLs [79]. Platelets could also possibly activate through FcγRIIA engagement of ICs formed in patients with cross-reacting antibodies to SARS-CoV-2 [69]. Thus, it is quite possible that platelets play a significant role in the pathophysiology of COVID-19.
Platelet-Derived β-Defensins, Thrombocidins, and Other Antimicrobial Molecules
β-Defensins are cationic antimicrobial peptides found in platelets and they directly inhibit bacterial growth via membrane disruption and promote NETosis [80]. Platelets can surround Staphylococcus aureus, clustering the bacteria to inhibit growth and then release β-defensins to induce NETosis [80]. These data suggest that platelet-derived β-defensins display classic antimicrobial activity and signal NETosis to capture bacterial. Thrombocidins were originally purified from platelet granules and were characterized to be truncated variants of the CXC chemokine, neutrophil-activating peptide-2 (NAP-2) [81]. They effectively kill several bacterial strains including Bacillus subtilis, Escherichia coli and Lactococcus lactis and are fungicidal for Cryptococcus neoformans [81]. Although little work has been performed on these molecules, it emphasizes that the platelet chemokine ordnance may be critical to its anti-infectious activities.
Platelets contain the cytokine IL-β and they can release it or package it into PMV in response to bacterial LPS or viral infection [[82], [83], [84]]. Secretion of IL-1β by platelets leads to increased phagocytosis of bacteria and further IL-1β production by macrophages [85]. In addition, platelet expression of GPIb readily dictates the fate of bacterial immune responses via C3b-opsonization [86,87]. Normally, C3b-opsonized bacteria are destroyed by macrophages in the spleen, however, if platelets bind to the bacteria via GPIb, the platelet-bacteria complexes are shunted to splenic DCs and this induces an adaptive immune response [86,87]. It is now clear that platelets have the ability to interact with a large variety of pathogens via the expression of several anti-infective molecules. These molecules enable platelets to directly kill pathogens and to engulf and harbor them for interactions with immune cells. Because of this, it is no wonder that platelets have been termed circulating infectious sentinels.
The Immunoregulatory Nature of Platelets
Transfusion-Related Immunomodulation (TRIM)
Transfusion of platelets has long been implicated in mediating TRIM [88,89] and does so independently of leukocytes [90]. Platelets are decorated with plasma-adsorbed denatured Class I molecules encoded by the major histocompatibility complex (MHC) [91,92]. These truncated molecules are unable to evoke functional allogeneic CD8+ cytotoxic T-cells (CTLs) in vitro [93] and allogenic platelet transfusions significantly enhance donor-matched skin graft survival, a process exclusively mediated by CTL [90]. It is possible that the adsorbed defective MHC-I molecules induce CTL anergy via faulty engagement with their T-cell receptor [90]. These results were corroborated with blood bank stored human platelets where several platelet-derived molecules including sCD40L, soluble OX40 ligand (sOX40L), sMHC-I and sFASL increased upon storage and caused significant immunomodulatory effects in vitro [[94], [95], [96], [97]]. In addition, platelet transfusions could rescue reduced platelet counts in a murine model of CTL-mediated immune thrombocytopenia (ITP) [98]. It appears that allogenic platelets specifically modulate CD8+ T regulatory (Treg) cells that correlated with increased platelet counts [98]. Related to this, Ki et al showed that stored platelet concentrates (PC) affect myeloid DC in several different infection models. PC caused differential regulation of the co-stimulatory molecules, CD80, CD83, and CD86 and production of several cytokines depending on the infection model [99]. These changes are a reason of how platelets can indirectly affect T-cell responses via altered antigen processing/presentation mechanisms [4]. In conclusion, although platelet transfusions are beneficial, they can modulate the immune system in several ways that can lead to adverse events [[100], [101], [102]].
The Platelet CD40L/CD40 Axis
Like the immune system, platelets sense danger; they are the first cells to detect endothelial injury because of their incredible mass in the circulation. Stable adhesion to collagen leads to platelet aggregation and promotes release of an array of platelet agonists leading to further activation and release of CD62P, CD63, cytokines and CD40L [[103], [104], [105]]. CD40L was first described on T-cells and is a critical co-stimulatory signal development and function of the immune system [106]. Identification of platelet CD40L was an early observation suggesting platelets express factors not only involved in hemostasis, but also immunity and the sheer number of blood platelets makes them the predominant source of CD40L in the circulation [17,103]. Platelet CD40L induces endothelial cells (EC) to secrete chemokines (IL-8 and MCP-1) and express adhesion molecules (E-selectin, VCAM and ICAM-1) and this causes recruitment of leukocytes and formation of platelet-leukocyte aggregates in areas of vascular inflammation [17,[107], [108], [109], [110]]. For example, platelet CD40L induces production of IL-6 and IL-12 from DCs as well as increasing their expression of CD80, CD86 and ICAM-1 [7]. It has also been demonstrated that platelet-CD40L can enhance DC maturation and their ability to directly kill Staphylococcus aureus which leads to efficient adaptive immunity against the bacterium [111]. Furthermore, platelet CD40L can induce isotype switching in B-cells and augments CD8+ T-cell responses; hence, functioning as a bridge to the adaptive immune system [7]. Platelet CD40L is also cleaved from the platelet’s surface to generate sCD40L or it is packaged into platelet microvesicles (PMV) that are released into the circulation for dissemination and distal control of immunity [112,113]. In a cohort of patients with SLE, for example, circulating CD40L positively correlated with a higher percentage of platelets bound to IgA+ and/or IgG+ B lymphocytes and severe disease [114].
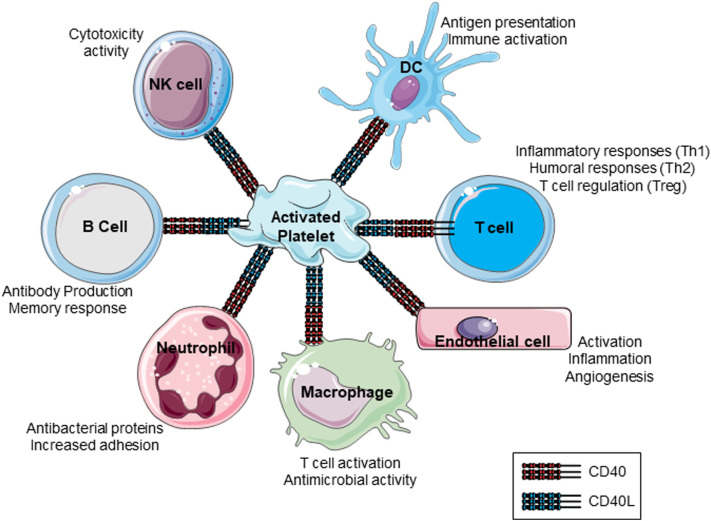
Platelets also express CD40 and integrin αIIbβ3, receptors for CD40L and can respond to CD40L thus creating feedback loops, making them key players in inflammatory processes [108,112,115]. For example, CD40L bearing T-cells can activate platelets to secrete CCL5 (RANTES) and cause T-cell adhesion on human intestinal microvascular EC (HIMECs) in patients with inflammatory bowel disease (IBD) [109,116]. Furthermore, platelet CD40 recruits leukocytes to induce inflammation at sites of vascular injury suggesting that they play pivotal roles in neointima formation after damage [117]. Understanding the role of platelet CD40L/CD40 and the effects on different immune cells and disease conditions should continue to lead to important discoveries in platelet-immune interactions. Figure 2 summarizes the various effects of platelet CD40L and CD40 interactions with leukocytes.
Figure 2.
Pleiotropic effects by which platelet membrane CD40/CD40L might modulate interactions between immune cells. Platelets can interact with numerous immune cells such as B-cells, T-cells, macrophages, neutrophils, EC, natural killer cells and DC. Communications with these cells can induce a spectrum of immune-related events. Th, T-helper; Treg, regulatory cell; NK, Natural Killer cell; DC, Dendritic cell.
Platelet-Derived Chemokines
Adding to their repository of immunomodulatory molecules, platelets store many chemokines and cytokines within their granules [118](Table 2 ). They also express several chemokine/cytokine receptors making them capable of detecting signals from virtually all chemokine/cytokine groups produced at sites of inflammation [119,120].
Table 2.
Platelet chemokine /cytokine receptors
| Chemokine/cytokine receptors |
|||
|---|---|---|---|
| Chemokines | Ligands | Functions | References |
| CCR1 | CCL3, CCL5, CCL7, CCL8, CCL13–16 | - Significant platelet activation and granule release - T cell and monocyte migration - Binding to MIP-1-alpha, RANTES, and less efficiently, to MIP-1-beta or MCP-1 |
[197] |
| CCR3 | CCL5, CCL7; CCL11, CCL15–16, CCL24, CCL26 | - Eosinophil and basophil migration - Signal transduction by increasing intracellular calcium ions level |
[198] [199] |
| CCR4 | CCL17, CCL22 | - High affinity for the C-C type chemokines - Activity mediated by G(i) protein activating phosphatidylinositol-calcium second messenger system. |
[200] |
| CXCR1 | CXCL6, CXCL7, CXCL8 | - Receptor to interleukin-8 [201] - Neutrophil activation |
[202] |
| CXCR4 | CXCL12 | - MAPK1/MAPK3 activation and AKT signaling cascade - Cell migration regulation (wound healing) - LPS-induced inflammatory response, including TNF secretion |
[203] [204] |
| CYTOKINES | |||
| IFNGR | IFN-Gamma | - Phagocytes Activation, antigen presentation and Th1 cytokine expression - Regulates other cytokines - JAK/STAT signaling pathway |
[205] [206] |
| TNFR | TNF-Alpha | - Most of TNF-alpha metabolic effects - TNF–TNFR regulate frequency of effector and/or memory CD4+ or CD8+ T cells |
[207] |
| TGF-Beta R | TGF-Beta | - Transmembrane ser/thr kinase - Cell cycle arrest in hematopoietic cells and wound healing - TRAF6 autoubiquitination, apoptosis. |
[208] |
| IL1R | IL1-Beta | - Chemotactic for PMNs (IL-1,8) and fibroblasts (IL-4) - Adapter molecule recruitment: TOLLIP, MyD88, and IRAK1 or IRAK2 - IL1B-mediated costimulation of IFNG production from Th1 cells |
[205] [209] |
The platelet chemokine CXCL4 or Platelet factor 4 (PF4) is highly expressed in platelets and influences several inflammatory processes [121]. Together with CCL5, platelet CXCL4 enhances the arrest of monocytes on EC under flow conditions [122] and promotes monocyte survival, chemotaxis and differentiation [[123], [124], [125], [126]]. CXCL4 appears to play a central role in platelet immunity and has been shown to be critical in protection against, for example, influenza infection [127]. Of interest, Middleton et al observed significantly elevated levels of PF4 (and RANTES) that trigger NETosis in patients with severe COVID-19 [128]. These data suggest that platelet PF4 and NETs may contribute to a thrombo-inflammatory cascade and hypercoagulability in patients with severe COVID-19.
Another abundant platelet chemokine is CXCL7, which can be degraded to give rise to several chemotactic and anti-infectious derivatives such as NAP-2. NAP-2 is a potent neutrophil activator/chemoattractant [[129], [130], [131], [132]] and facilitates neutrophil migration through platelet thrombi [133]; platelet CXCL7 is critical in managing neutrophil recruitment in response to vascular injury [134]. It was also shown to be increased in the bronchoalveolar fluid of mice suffering from systemic inflammatory response syndrome (SIRS) consistent with activated platelets moving from the circulation into the lung [135]; these platelets were responsible for inducing pulmonary NETosis and lung damage [135]
The release of chemokines from platelets influences a wide range of processes such as inflammation, wound healing [136], tumor metastasis [137] and induction of immune tolerance [138]. This places them in a central role as first responders to danger and as a bridge between innate and adaptive immunity (Table 2).
Platelet Antigen Presenting Cell (APC) Interactions
Platelets can directly interact with DC via CD62P/CD162 and this influences DC maturation and enhances their production of the Th2 helper chemokine CCL17 [139,140]. Alternatively, direct CD40/CD40L-dependent interactions between platelets and DC increases the latter’s ability to uptake and kill bacteria [111]. In addition, firm adhesion of platelets to DCs through MAC-1 (integrin alfaMbeta2, CD11b/CD18,) and Junctional Adhesion Molecule-C (JAM-C) leads to enhanced phagocytosis [141,142]. Moreover, platelet release of CXCL4 and sCD40L influences DCs to upregulate co-stimulatory molecules and proinflammatory cytokine release [142,143]. Together these interactions between platelets and DCs stimulate antigen processing/presentation and the proliferation of CD4+ and CD8+ T-cells thereby significantly promoting adaptive immunity [143]. From a therapeutic perspective, Xu et al [144] determined that vincristine-loaded platelets (VLP) opsonized with an anti-CD41 antibody could significantly reduce macrophage phagocytosis in vitro [144]. This suggests that platelets may be utilized to specifically interact with APC and modulate their functional capabilities.
Platelet Interactions with T-Cells and Their Ability to Act as APC
The homing of T-cells from the circulation to lymph nodes is a central mechanism in immunity and this process is facilitated by platelets via adhesion to high endothelial venules by CD62P [145]. Platelets can also recruit T-cells to sites of vascular injury [116,146] and platelet CXCL4 has been shown to promote CD4+ Th17 cells [147]. On the other hand, PMVs prevented differentiation of CD4+ Tregs into Th17 cells in a P-selectin dependent manner [148]. It appears that the PMV selectively bind to a subset of memory-like Tregs and using CXCR3, inhibit Treg proliferation. These findings are important as they open up the possibility that PMVs critically regulate the immune response at sites of inflammation [148].
Antigen presentation to T lymphocytes via MHC-I molecules is another central aspect of T-cell immunity. Platelets are estimated to harbor approximately 80,000 MHC-I molecules on their surface [92], however, there are controversies regarding the functionality and origin of these molecules on platelets [93]. Nonetheless, it is now known that platelets (and MK) contain all the molecules necessary for antigen processing and presentation to CD8+ T-cells including a complete proteasome [149,150] and co-stimulatory molecules [151,152]. Although the platelet’s surface contains mainly denatured MHC-I molecules, intracellularly, platelets have a large pool of fully intact functional MHC-I molecules that are expressed upon activation.
Earlier reports showed that peptides from platelet GP1b could be presented on platelet MHC-I in patients with ITP [153]. In a subsequent report, it was shown that platelets could functionally present ovalbumin (OVA) and malarial peptides to activate CD8+ T-cells [151]. Confirming this report, it was shown that MK also process and present OVA and GPIIIa self-peptides to activate antigen-specific CD8+ T-cells [152]. While the MK processed OVA, they were also actively packaging the MHC-I/peptide complexes into proplatelets [152]. This may be a mechanism by which MKs immunologically communicate with T-cells in the periphery. Of interest, examining platelet surface MHC-I molecules may be an efficient way to distinguish young platelets from old as they lose their MHC-I over time [154]. However, since MK contain all the necessary components for antigen processing/presentation and activation of T-cells, they are continually transferring these molecules to platelets for peripheral activation of T-cells.
Platelet NK Cell and Tumor Cell Interactions
Natural Killer cells (NK-cells) were originally described as innate effector cells with cytolytic activity towards virus-infected and tumor cells [155]. Like T-cells, they are recruited from the circulation by activated platelets to sites of inflammation [156,157]. The influence of platelets on NK cell immunosurveillance comes from early observations that platelets form coats around tumor cells, which protects them from immune destruction [158]. It appears that platelets transfer their MHC-I molecules to tumor cells and thus confer resistance to NK-mediated cytolysis. [157,159,160]. Moreover, activation of platelet-derived glucocorticoid-induced TNF-related ligand (GITRL) and the receptor activator of the NF-κB ligand (RANKL) are linked to reduced NK cell reactivity and INF-γ production [[160], [161], [162], [163]]. In addition, podoplanin on the tumor cell surface can cause platelet aggregation by engaging CLEC-2 receptors [164]; this interaction enhances platelet TGF-β release to induce epithelial-mesenchymal transition (EMT) in tumor cells. This drives the tumor toward a more migratory phenotype, tumor cell-extravasation and immune evasion [165]. Furthermore, platelet TGF-β downregulates natural killer group 2, member D (NKG2D) thus diminishing NK cell anti-tumor reactivity [166,167]. Taken together, the triad of platelet-tumor-NK-cell interactions works largely to protect tumor cells and facilitate metastasis [157,168]; a clear detrimental effect of platelets on the immune system.
Platelet B-cell Interactions
The B-cell is another key player in adaptive immunity by differentiating into antibody producing plasma cells in the secondary immune responses [169]. Many platelet/B-cell interactions seem to involve CD40/CD40L [95]. In conditions were antigen-specific T- and B-cells are scarce, platelet-derived CD40L could enhance germinal center formation and increase IgG levels supporting the adaptive immune response [170]. Furthermore, when lymphocytes from patients with ITP were cultured with activated platelets, platelet CD40L significantly induced autoreactive B-cells to produce autoantibodies against GPIIbIIIa [171]. Finally, CD40L can be packaged into PMVs thus giving platelets the ability to influence B-cell development locally through direct interactions or at a distance by the release of PMVs into the circulation [113].
Platelet Microvesicles (PMVs)
PMVs were discovered in 1946 [172] and were characterized as minute particles that could be enriched by centrifugation [173]; they were termed platelet dust but retained coagulant properties [173]. PMVs are the most abundant microvesicles in the circulation and can readily activate neutrophils and ECs via CD62P and promote tethering of flowing neutrophils to ECs in vitro [174]. Moreover, they transfer GPIbα to monocytes and recruit them to sites of vascular injury [175].
Elevated plasma PMV levels are linked to a wide range of diseases [176]. For example, patients with rheumatoid arthritis (RA) have elevated plasma PMV levels that correlate with disease severity [177]. In addition, platelet GPVI was shown to enhance production of PMV (laden with IL-1β) in the RA synovium that elicit chemokine release by synovial fibroblasts thus attracting proinflammatory neutrophils [178]. This mechanism was corroborated by a recent report showing that Rac1 inhibition, located downstream of GPVI, decreased PMV release and alleviated collagen-induced arthritis [179].
Patients with ITP also have increased plasma PMV counts with the highest levels in newly diagnosed ITP [[180], [181], [182]]. Bleeding tendency and platelet count do not always correlate in ITP although Jy et al speculated that a higher PMV to platelet ratio could alleviate some bleeding symptoms [183]. PMVs are also increased in patients with systemic lupus erythematosus (SLE) and form immune complexes with autoantibodies [184]. PMV-IgG complexes were internalized by monocytes leading to monocyte activation and were positively correlated with disease activity [184].
From an infectious point of view, DENV can activate platelets via CLEC-2 resulting in PMV release and activation of neutrophils and macrophages via TLR2 [30]. This subsequently leads to NETosis and pro-inflammatory cytokine release which causes lethality in DENV-infected mice [30]. It appears that increased levels of PMV are observed in many diseases but to date, most have been only associations. Improving isolation and characterization techniques will unravel more PMV mechanisms and the biological relevance of these vesicles in different disease states. Figure 3 summarizes the many aspects of platelets and their ability to interact with and regulate immunity.
Figure 3.
Pleiotropic Platelet membrane receptors by which Platelets can interact with bacteria and numerous immune cells such as B-cells, T-cells, monocytes, neutrophils, epithelium, endothelium and tumor cells. Arrows and brackets show some of the major interactions. CD40, CD40 molecule; CD40L, CD40 ligand (CD154); sCD40L, soluble CD40L; LPS, Lipopolysaccharide; LP, Lipoprotein; dsRNA, Double-stranded RNA; ssRNA, Single-stranded RNA; CAPs, carboxy(alkylpyrrole) protein adducts; PMV, Platelet microvesicule; IL-1β, Interleukin-1 beta; NAP-2, Neutrophil Activating protein-2; EMT, epithelial-mesenchymal transition proteins; CXCL4, Chemokine (C-X-C motif) ligand 4; CXCL7, Chemokine (C-X-C motif) ligand 7; CCL3, Chemokine (C-C motif) ligand 3; CCL5, Chemokine (C-C motif) ligand 5; TGF-β, Transforming growth factor beta; TC-1, Thrombocidin-l ;TC-2, thrombocidin-lI; CLEC-2, C-type lectin domain family 2; DC-SIGN, Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin; TLRs, Toll Like Receptors.
Conclusions
Platelets are completely armed to closely interact with infectious agents and cells of the innate and adaptive immune systems. They do so by containing a wide range of pro- and anti-inflammatory molecules, some of which that have no obvious hemostatic function. Based on these observations and the multitude of literature relating to platelets and immunity, it is time that they are rightly placed as critical cells of the immune system.
Acknowledgments
Acknowledgements
This work was supported by grants (to J.W.S.) from Lund University, Crafoordska Stiftelsen (#20170829), Vetenskapsrådet (Swedish Research Council, VR, #2017-01779) and Avtal om Läkarutbildning och Forskning (ALF).
Authorship Contributions
A.M. wrote the first draft of the paper; J.R, R.K. and J.W.S. edited the manuscript and gave critical assessments. J.W.S. provided financial resources for the manuscript.
Declaration of Competing Interest
The authors declare no competing financial interests.
References
- 1.Brass L.F., Diamond S.L., Stalker T.J. Platelets and hemostasis: a new perspective on an old subject. Blood Adv. 2016;1:5–9. doi: 10.1182/bloodadvances.2016000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenen R.R. The prowess of platelets in immunity and inflammation. Thromb Haemost. 2016;116:605–612. doi: 10.1160/TH16-04-0300. [DOI] [PubMed] [Google Scholar]
- 3.Trzeciak-Ryczek A., Tokarz-Deptuła B., Deptuła W. Platelets—an important element of the immune system. Polish J of Vet Sci. 2013;16:407–413. doi: 10.2478/pjvs-2013-0058. [DOI] [PubMed] [Google Scholar]
- 4.Kapur R., Zufferey A., Boilard E., Semple J.W. Nouvelle cuisine: platelets served with inflammation. J Immunol. 2015;194:5579–5587. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 5.Kapur R., Semple J.W. Platelets as immune-sensing cells. Blood Adv. 2016;1:10–14. doi: 10.1182/bloodadvances.2016000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson O., Mohlin C., Nilsson B., Ekdahl K.N. The human platelet as an innate immune cell: interactions between activated platelets and the complement system. Front Immunol. 2019;10:1590. doi: 10.3389/fimmu.2019.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elzey B.D., Tian J., Jensen R.J., Swanson A.K., Lees J.R., Lentz S.R., et al. Platelet-mediated modulation of adaptive immunity a communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/S1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 8.Dib P.R.B., Quirino-Teixeira A.C., Merij L.B., Pinheiro M.B.M., Rozini S.V., Andrade F.B., et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J Leukoc Biol. 2020 doi: 10.1002/jlb.4mr0620-701r. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer B.F., Campbell R.A., Schwertz H., Franks Z.G., Vieira de Abreu A., Grundler K., et al. Bacteria differentially induce degradation of Bcl-xL, a survival protein, by human platelets. Blood. 2012;120:5014–5020. doi: 10.1182/blood-2012-04-420661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaertner F., Ahmad Z., Rosenberger G., Fan S., Nicolai L., Busch B., et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171:1368–1382. doi: 10.1016/j.cell.2017.11.001. e1323. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee M., Huang Y., Joshi S., Popa G.J., Mendenhall M.D., Wang Q.J., et al. Platelets endocytose viral particles and are activated via TLR (Toll-Like Receptor) signaling. Arterioscler Thromb Vasc Biol. 2020;40:1635–1650. doi: 10.1161/atvbaha.120.314180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semple J.W., Italiano J.E., Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 13.Jenne C.N., Wong C., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A., et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 14.King M., McDermott P., Schreiber A.D. Characterization of the Fc gamma receptor on human platelets. Cell Immunol. 1990;128:462–479. doi: 10.1016/0008-8749(90)90041-o. [DOI] [PubMed] [Google Scholar]
- 15.Worth R.G., Chien C.D., Chien P., Reilly M.P., McKenzie S.E., Schreiber A.D. Platelet FcγRIIA binds and internalizes IgG-containing complexes. Exp Hematol. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Lam F.W., Vijayan K.V., Rumbaut R.E. Platelets and their interactions with other immune cells. Comp Physiol. 2015;5:1265–1280. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henn V., Slupsky J.R., Gräfe M., Anagnostopoulos I., Förster R., Müller-Berghaus G., et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 18.Rowley J.W., Chappaz S., Corduan A., Chong M.M., Campbell R., Khoury A., et al. Dicer1-mediated miRNA processing shapes the mRNA profile and function of murine platelets. Blood. 2016;127:1743–1751. doi: 10.1182/blood-2015-07-661371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy L., Beaulieu L.M., Tanriverdi K., Freedman J.E. The role of RNA uptake in platelet heterogeneity. Thromb Haemost. 2017;117:948–961. doi: 10.1160/th16-11-0873. [DOI] [PubMed] [Google Scholar]
- 20.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 21.Bray P.F., McKenzie S.E., Edelstein L.C., Nagalla S., Delgrosso K., Ertel A., et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 2013;14(1) doi: 10.1186/1471-2164-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davizon-Castillo P., Rowley J.W., Rondina M.T. Megakaryocyte and platelet transcriptomics for discoveries in human health and disease. Arterioscler Thromb Vasc Biol. 2020;40:1432–1440. doi: 10.1161/atvbaha.119.313280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preußer C., Hung L.-H., Schneider T., Schreiner S., Hardt M., Moebus A., et al. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7 doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provost P. The clinical significance of platelet microparticle-associated microRNAs. Clin Chem Lab Med (CCLM) 2017;55:657–666. doi: 10.1515/cclm-2016-0895. [DOI] [PubMed] [Google Scholar]
- 25.L. J . In: Platelets. Michelson AD C.M., Frelinger I.I.I.A.L., Newman P.J., editors. Academic Press; London: 2019. The evolution of mammalian platelets; pp. 1–23. [Google Scholar]
- 26.Cunin P., Bouslama R., Machlus K.R., Martínez-Bonet M., Lee P.Y., Wactor A., et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. Elife. 2019;8 doi: 10.7554/eLife.44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunin P., Nigrovic P.A. Megakaryocyte emperipolesis: a new frontier in cell-in-cell interaction. Platelets. 2019:1–7. doi: 10.1080/09537104.2019.1693035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Ann Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki-Inoue K., Fuller G.L., García A., Eble J.A., Pöhlmann S., Inoue O., et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 30.Sung P.-S.S., Huang T.-F.F., Hsieh S.-L.L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Comm. 2019;10 doi: 10.1038/s41467-019-10360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Semple J.W., Aslam R., Kim M., Speck E.R., Freedman J. Platelet-bound lipopolysaccharide enhances Fc receptor-mediated phagocytosis of IgG-opsonized platelets. Blood. 2007;109:4803–4805. doi: 10.1182/blood-2006-12-062695. [DOI] [PubMed] [Google Scholar]
- 33.Aslam R., Speck E.R., Kim M., Crow A.R., Bang K.W., Nestel F.P., et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 34.Andonegui G., Kerfoot S.M., McNagny K., Ebbert K.V.J., Patel K.D., Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 35.Shashkin P.N., Brown T.G., Ghosh A., Marathe G.K., McIntyre T.M. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–3502. doi: 10.4049/jimmunol.181.5.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cognasse F., Hamzeh-Cognasse H., Lafarge S., Delezay O., Pozzetto B., McNicol A., et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 37.Berthet J., Damien P., Hamzeh-Cognasse H., Arthaud C.-A., Eyraud M.-A., Zéni F., et al. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin Immunol. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Vallance T.M., Ravishankar D., Albadawi D.A.I., Layfield H., Sheard J., Vaiyapuri R., et al. Effect of ultrapure lipopolysaccharides derived from diverse bacterial species on the modulation of platelet activation. Sci Rep. 2019;9 doi: 10.1038/s41598-019-54617-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward J.R., Bingle L., Judge H.M., Brown S.B., Storey R.F., Whyte M.K., et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–838. [PubMed] [Google Scholar]
- 40.Claushuis T.A.M., Van Der Veen A.I.P., Horn J., Schultz M.J., Houtkooper R.H., Van ’T Veer C., et al. Platelet Toll-like receptor expression and activation induced by lipopolysaccharide and sepsis. Platelets. 2019;30:296–304. doi: 10.1080/09537104.2018.1445841. [DOI] [PubMed] [Google Scholar]
- 41.Li R.H.L., Nguyen N., Tablin F. Canine platelets express functional Toll-like receptor-4: lipopolysaccharide-triggered platelet activation is dependent on adenosine diphosphate and thromboxane A2 in dogs. BMC Vet Res. 2019;15 doi: 10.1186/s12917-019-1997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 43.Hally K.E., Bird G.K., La Flamme A.C., Harding S.A., Larsen P.D. Platelets modulate multiple markers of neutrophil function in response to in vitro Toll-like receptor stimulation. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaplin D.D. Overview of the immune response. J Allergy Clin Immunol. 2010;125(23) doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botos I., Segal D.M., Davies D.R. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair P., Rex S., Vitseva O., Beaulieu L., Tanriverdi K., Chakrabarti S., et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assinger A., Laky M., Schabuer G., Hirschl A.M., Buchberger E., Binder B.R., et al. Efficient phagocytosis of periodontopathogens by neutrophils requires plasma factors, platelets and TLR2. J Thromb Haemost. 2011;9:799–809. doi: 10.1111/j.1538-7836.2011.04193.x. [DOI] [PubMed] [Google Scholar]
- 48.Koupenova M., Vitseva O., MacKay C.R., Beaulieu L.M., Benjamin E.J., Mick E., et al. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802. doi: 10.1182/blood-2013-11-536003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koupenova M., Corkrey H.A., Vitseva O., Manni G., Pang C.J., Clancy L., et al. The role of platelets in mediating a response to human influenza infection. Nat Comm. 2019;10 doi: 10.1038/s41467-019-09607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panigrahi S., Ma Y., Hong L., Gao D., West X.Z., Salomon R.G., et al. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vardon-Bounes F., Ruiz S., Gratacap M.-P., Garcia C., Payrastre B., Minville V. Platelets are critical key players in sepsis. Int J Mol Sci. 2019;20:3494. doi: 10.3390/ijms20143494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muronoi T., Koyama K., Nunomiya S., Lefor A.K., Wada M., Koinuma T., et al. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb Res. 2016;144:169–175. doi: 10.1016/j.thromres.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Levi M. Platelets in critical illness. Sem Thromb Hemost. 2016;42:252–257. doi: 10.1055/s-0035-1570080. [DOI] [PubMed] [Google Scholar]
- 54.Assinger A., Schrottmaier W.C., Salzmann M., Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front Immunol. 2019;10:1687. doi: 10.3389/fimmu.2019.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki-Inoue K., Inoue O., Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemost. 2011;9(Suppl. 1):44–55. doi: 10.1111/j.1538-7836.2011.04335.x. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134:1912–1918. doi: 10.1182/blood.2019001388. [DOI] [PubMed] [Google Scholar]
- 57.Shin Y., Morita T. Rhodocytin, a functional novel platelet agonist belonging to the heterodimeric C-type lectin family, induces platelet aggregation independently of glycoprotein Ib. Biochem Biophys Res Commun. 1998;245:741–745. doi: 10.1006/bbrc.1998.8516. [DOI] [PubMed] [Google Scholar]
- 58.Chaipan C., Soilleux E.J., Simpson P., Hofmann H., Gramberg T., Marzi A., et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Callaghan C.A. Thrombomodulation via CLEC-2 targeting. Curr Opin Pharmacol. 2009;9:90–95. doi: 10.1016/j.coph.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Hitchcock J.R., Cook C.N., Bobat S., Ross E.A., Flores-Langarica A., Lowe K.L., et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Invest. 2015;125:4429–4446. doi: 10.1172/jci79070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boukour S., Massé J.M.M., Bénit L., Dubart-Kupperschmitt A., Cramer E.M. Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J Thromb Haemost. 2006;4:426–435. doi: 10.1111/j.1538-7836.2006.01749.x. [DOI] [PubMed] [Google Scholar]
- 62.Guzzi C., Alfarano P., Sutkeviciute I., Sattin S., Ribeiro-Viana R., Fieschi F., et al. Detection and quantitative analysis of two independent binding modes of a small ligand responsible for DC-SIGN clustering. Org Biomol Chem. 2015;14:335–344. doi: 10.1039/C5OB02025E. [DOI] [PubMed] [Google Scholar]
- 63.Tomo S., Mohan S., Ramachandrappa V., Samadanam D., Suresh S., Pillai A., et al. Dynamic modulation of DC-SIGN and FcΥR2A receptors expression on platelets in dengue. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon A.Y., Sutherland M.R., Pryzdial E.L.G. Dengue virus binding and replication by platelets. Blood. 2015;126:378–385. doi: 10.1182/blood-2014-09-598029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogt M.B., Lahon A., Arya R.P., Spencer Clinton J.L., Rico-Hesse R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schrottmaier W.C., Salzmann M., Badrnya S., Morava S., Luik A.-L., Kral-Pointner J.B., et al. Platelet-stored antibodies potently diminish viral infection in vitro and in vivo. Acta Physiol. 2019;227:187. doi: 10.1111/apha.13366. [DOI] [Google Scholar]
- 67.Cloutier N., Allaeys I., Marcoux G., Machlus K.R., Mailhot B., Zufferey A., et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Nat Acad Sci. 2018;115 doi: 10.1073/pnas.1720553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arman M., Krauel K., Tilley D.O., Weber C., Cox D., Greinacher A., et al. Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4. Blood. 2014;123:3166–3174. doi: 10.1182/blood-2013-11-540526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boilard E., Paré G., Rousseau M., Cloutier N., Dubuc I., Lévesque T., et al. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood. 2014;123:2854–2863. doi: 10.1182/blood-2013-07-515536. [DOI] [PubMed] [Google Scholar]
- 70.Campbell R.A., Schwertz H., Hottz E.D., Rowley J.W., Manne B.K., Washington A.V., et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood. 2019;133:2013–2026. doi: 10.1182/blood-2018-09-873984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boilard E., Flamand L. The role of the megakaryocyte in immunity has gone viral. Blood. 2019;133:2001–2002. doi: 10.1182/blood-2019-02-900787. [DOI] [PubMed] [Google Scholar]
- 72.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou M., Qi J., Li X., Zhang Z., Yao Y., Wu D., et al. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: a systematic review and meta-analysis. Br J Haematol. 2020;189:438–441. doi: 10.1111/bjh.16655. [DOI] [PubMed] [Google Scholar]
- 74.Qiu J., Ma J., Zhang S., Han J., Liu S. Promoting platelets is a therapeutic option to combat severe viral infection of the lung. Blood Adv. 2020;4:1640–1642. doi: 10.1182/bloodadvances.2020001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., et al. Platelet activation and platelet-monocyte aggregates formation trigger tissue factor expression in severe COVID-19 patients. Blood. 2020 doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C.J., et al. Platelet gene expression and function in COVID-19 patients. Blood. 2020 doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Q. Wang, Y. Zhang, L. Wu, S. Niu, C. Song, Z. Zhang, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2, Cell 2020; 181:894-904.e899 DOI: 10.1016/j.cell.2020.03.045 [DOI] [PMC free article] [PubMed]
- 78.Y. Zaid, F. Puhm, I. Allaeys, A. Naya, M. Oudghiri, L. Khalki, et al. Platelets can contain SARS-CoV-2 RNA and are hyperactivated in COVID-19, medRxiv 2020:2020.2006.2023.20137596 DOI: 10.1101/2020.06.23.20137596 [DOI] [PMC free article] [PubMed]
- 79.Sung P.-S., Hsieh S.-L. CLEC2 and CLEC5A: pathogenic host factors in acute viral infections. Front Immunol. 2019;10:2867. doi: 10.3389/fimmu.2019.02867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kraemer B.F., Campbell R.A., Schwertz H., Cody M.J., Franks Z., Tolley N.D., et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Path. 2011;7 doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krijgsveld J., Zaat S.A., Meeldijk J., van Veelen P.A., Fang G., Poolman B., et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem. 2000;275:20374–20381. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 82.Brown T.G., McIntyre T.M. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1β–rich microparticles. J Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hottz E.D., Lopes J.F., Freitas C., Valls-de-Souza R., Oliveira M.F., Bozza M.T., et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hottz E.D., Bozza F.A., Bozza P.T.T. Platelets in immune response to virus and immunopathology of viral infections. Front Med. 2018;5:121. doi: 10.3389/fmed.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ali R.A., Wuescher L.M., Dona K.R., Worth R.G. Platelets mediate host defense against Staphylococcus aureus through direct bactericidal activity and by enhancing macrophage activities. J Immunol. 2017;198:344–351. doi: 10.4049/jimmunol.1601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broadley S.P., Plaumann A., Coletti R., Lehmann C., Wanisch A., Seidlmeier A., et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host & Microbe. 2016;20:36–48. doi: 10.1016/j.chom.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 87.Verschoor A., Neuenhahn M., Navarini A.A., Graef P., Plaumann A., Seidlmeier A., et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 88.Blajchman M.A. Transfusion-associated immunomodulation and universal white cell reduction: are we putting the cart before the horse? Transfusion. 1999;39 doi: 10.1046/j.1537-2995.1999.39070665.x. [DOI] [PubMed] [Google Scholar]
- 89.Geiger T.L. Transfusion-associated immune modulation: a reason to TRIM platelet transfusions? Transfusion. 2008;48:1772–1773. doi: 10.1111/j.1537-2995.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 90.Aslam R., Speck E.R., Kim M., Freedman J., Semple J.W. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC Class I molecules and is independent of white cells. Transfusion. 2008;48:1778–1786. doi: 10.1111/j.1537-2995.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 91.Lalezari P., Driscoll A.M. Ability of thrombocytes to acquire HLA specificity from plasma. Blood. 1982;59:167–170. [PubMed] [Google Scholar]
- 92.Kao K.J., Cook D.J., Scornik J.C. Quantitative analysis of platelet surface HLA by W6/32 anti-HLA monoclonal antibody. Blood. 1986;68:627–632. [PubMed] [Google Scholar]
- 93.Gouttefangeas C., Diehl M., Keilholz W., Hörnlein R.F., Stevanović S., Rammensee H.G. Thrombocyte HLA molecules retain nonrenewable endogenous peptides of megakaryocyte lineage and do not stimulate direct allocytotoxicity in vitro. Blood. 2000;95:3168–3175. [PubMed] [Google Scholar]
- 94.Ghio M., Contini P., Mazzei C., Brenci S., Barberis G., Filaci G., et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–1777. [PubMed] [Google Scholar]
- 95.Aoui C., Prigent A., Sut C., Tariket S., Hamzeh-Cognasse H., Pozzetto B., et al. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci. 2014;15:22342–22364. doi: 10.3390/ijms151222342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hamzeh-Cognasse H., Damien P., Nguyen K.A., Arthaud C.-A., Eyraud M.-A., Chavarin P., et al. Immune-reactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion. 2014;54:613–625. doi: 10.1111/trf.12378. [DOI] [PubMed] [Google Scholar]
- 97.Blumberg N., Gettings K.F., Turner C., Heal J.M., Phipps R.P. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 98.Guo L., Yang L., Speck E.R., Aslam R., Kim M., McKenzie C.G., et al. Allogeneic platelet transfusions prevent murine T-cell-mediated immune thrombocytopenia. Blood. 2014;123:422–427. doi: 10.1182/blood-2013-08-523308. [DOI] [PubMed] [Google Scholar]
- 99.Ki K.K., Faddy H.M., Flower R.L., Dean M.M. Platelet concentrates modulate myeloid dendritic cell immune responses. Platelets. 2017;29:1–10. doi: 10.1080/09537104.2017.1306045. [DOI] [PubMed] [Google Scholar]
- 100.Spiess B.D., Royston D., Levy J.H., Fitch J., Dietrich W., Body S., et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 101.Khan S.Y., Kelher M.R., Heal J.M., Blumberg N., Boshkov L.K., Phipps R., et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pereboom I.T.A., De Boer M.T., Haagsma E.B., Hendriks H.G.D., Lisman T., Porte R.J. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analgesia. 2009;108:1083–1091. doi: 10.1213/ane.0b013e3181948a59. [DOI] [PubMed] [Google Scholar]
- 103.Hermann A., Rauch B.H., Braun M., Schrör K., Weber A.A. Platelet CD40 ligand (CD40L)—subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001;12:74–82. doi: 10.1080/09537100020031207. [DOI] [PubMed] [Google Scholar]
- 104.Schwarz U.R., Kobsar A.L., Koksch M., Walter U., Eigenthaler M. Inhibition of agonist-induced p42 and p38 mitogen-activated protein kinase phosphorylation and CD40 ligand/P-selectin expression by cyclic nucleotide-regulated pathways in human platelets. Biochem Pharmacol. 2000;60:1399–1407. doi: 10.1016/s0006-2952(00)00452-4. [DOI] [PubMed] [Google Scholar]
- 105.Ridiandries A., Tan J.T.M., Bursill C.A. The role of chemokines in wound healing. International J Mol Sci. 2018;19:3217. doi: 10.3390/ijms19103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semple J.W., Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thienel U., Loike J., Yellin M.J. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell Immunol. 1999;198:87–95. doi: 10.1006/cimm.1999.1583. [DOI] [PubMed] [Google Scholar]
- 108.Inwald D.P., McDowall A., Peters M.J., Callard R.E., Klein N.J. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 109.Danese S., de la Motte C., Sturm A., Vogel J.D., West G.A., Strong S.A., et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterol. 2003;124:1249–1264. doi: 10.1016/S0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 110.Li G., Sanders J.M., Bevard M.H., Sun Z., Chumley J.W., Galkina E.V., et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Amer J Pathol. 2008;172:1141–1152. doi: 10.2353/ajpath.2008.070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nishat S., Wuescher L.M., Worth R.G. Platelets enhance dendritic cell responses against Staphylococcus aureus through CD40-CD40L. Infect Immun. 2018;86 doi: 10.1128/IAI.00186-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henn V., Steinbach S., Büchner K., Presek P., Kroczek R.A. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 113.Sprague D.L., Elzey B.D., Crist S.A., Waldschmidt T.J., Jensen R.J., Ratliff T.L. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zamora C., Toniolo E., Diaz-Torné C., Cantó E., Magallares B., Ortiz M.A., et al. Association of platelet binding to lymphocytes with B Cell abnormalities and clinical manifestations in systemic lupus erythematosus. Mediators Inflamm. 2019;2473164:2019. doi: 10.1155/2019/2473164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.André P., Prasad K.S.S., Denis C.V.V., He M., Papalia J.M., Hynes R.O., et al. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 116.Danese S., de la Motte C., Reyes B.M., Sans M., Levine A.D., Fiocchi C. Cutting edge: T Cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol. 2004;172:2011–2015. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- 117.Jin R., Xiao A.Y., Song Z., Yu S., Li J., Cui M.Z., et al. Platelet CD40 mediates leukocyte recruitment and neointima formation after arterial denudation injury in atherosclerosis-prone mice. Am J Pathol. 2018;188:252–263. doi: 10.1016/j.ajpath.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bakogiannis C., Sachse M., Stamatelopoulos K., Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. 2019;122 doi: 10.1016/j.cyto.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 119.Blair P., Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clemetson K.J., Clemetson J.M., Proudfoot A.E., Power C.A., Baggiolini M., Wells T.N. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- 121.Pitsilos S., Hunt J., Mohler E.R., Prabhakar A.M., Poncz M., Dawicki J., et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. 2003;90:1112–1120. doi: 10.1160/th03-02-0069. [DOI] [PubMed] [Google Scholar]
- 122.von Hundelshausen P., Koenen R.R., Sack M., Mause S.F., Adriaens W., Proudfoot A.E., et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105:924–930. doi: 10.1182/blood-2004-06-2475. [DOI] [PubMed] [Google Scholar]
- 123.Scheuerer B., Ernst M., Dürrbaum-Landmann I., Fleischer J., Grage-Griebenow E., Brandt E., et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 124.Gleissner C.A., Shaked I., Little K.M., Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brindley L.L., Sweet J.M., Goetzl E.J. Stimulation of histamine release from human basophils by human platelet factor 4. J Clin Invest. 1983;72:1218–1223. doi: 10.1172/JCI111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fox J.M., Kausar F., Day A., Osborne M., Hussain K., Mueller A., et al. CXCL4/platelet factor 4 is an agonist of CCR1 and drives human monocyte migration. Sci Rep. 2018;8 doi: 10.1038/s41598-018-27710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo L., Feng K., Wang Y.C., Mei J.J., Ning R.T., Zheng H.W., et al. Critical role of CXCL4 in the lung pathogenesis of influenza (H1N1) respiratory infection. Mucosal Immunol. 2017;10:1529–1541. doi: 10.1038/mi.2017.1. [DOI] [PubMed] [Google Scholar]
- 128.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020 doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walz A., Baggiolini M. Generation of the neutrophil-activating peptide NAP-2 from platelet basic protein or connective tissue-activating peptide III through monocyte proteases. J Exp Med. 1990;171:449–454. doi: 10.1084/jem.171.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.B.D. Car, M. Baggiolini, A. Walz. Formation of neutrophil-activating peptide 2 from platelet-derived connective-tissue-activating peptide III by different tissue proteinases, Biochem J 1991; 275 ( Pt 3):581-584 DOI: 10.1042/bj2750581 [DOI] [PMC free article] [PubMed]
- 131.Brandt E., Van Damme J., Flad H.D. Neutrophils can generate their activator neutrophil-activating peptide 2 by proteolytic cleavage of platelet-derived connective tissue-activating peptide III. Cytokine. 1991;3:311–321. doi: 10.1016/1043-4666(91)90499-4. [DOI] [PubMed] [Google Scholar]
- 132.Brandt E., Ludwig A., Petersen F., Flad H.D. Platelet-derived CXC chemokines: old players in new games. Immunol Rev. 2000;177:204–216. doi: 10.1034/j.1600-065x.2000.17705.x. [DOI] [PubMed] [Google Scholar]
- 133.Ghasemzadeh M., Kaplan Z.S., Alwis I., Schoenwaelder S.M., Ashworth K.J., Westein E., et al. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555–4566. doi: 10.1182/blood-2012-09-459636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown A.J., Sepuru K.M., Sawant K.V., Rajarathnam K. Platelet-derived chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: implications for neutrophil-platelet crosstalk. Front Immunol. 2017;8:1248. doi: 10.3389/fimmu.2017.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hook J.S., Cao M., Potera R.M., Alsmadi N.Z., Schmidtke D.W., Moreland J.G. Nox2 regulates platelet activation and NET formation in the lung. Front Immunol. 2019;10:1472. doi: 10.3389/fimmu.2019.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lichtman M.K., Otero-Vinas M., Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24:215–222. doi: 10.1111/wrr.12398. [DOI] [PubMed] [Google Scholar]
- 137.Guo Y., Cui W., Pei Y., Xu D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecol Oncol. 2019;153:639–650. doi: 10.1016/j.ygyno.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 138.Hotta E., Tamagawa-Mineoka R., Katoh N. Platelets are important for the development of immune tolerance: Possible involvement of TGF-β in the mechanism. Exp Dermatol. 2019;28:801–808. doi: 10.1111/exd.13940. [DOI] [PubMed] [Google Scholar]
- 139.Nakanishi T., Inaba M., Inagaki-Katashiba N., Tanaka A., Vien P., Kibata K., et al. Platelet-derived RANK ligand enhances CCL17 secretion from dendritic cells mediated by thymic stromal lymphopoietin. Platelets. 2014;26:425–431. doi: 10.3109/09537104.2014.920081. [DOI] [PubMed] [Google Scholar]
- 140.Han P., Hanlon D., Arshad N., Lee J.S., Tatsuno K., Robinson E., et al. Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Langer H.F., Daub K., Braun G., Schönberger T., May A.E., Schaller M., et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic Cell function in vitro. Arterio Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 142.Hamzeh-Cognasse H., Cognasse F., Palle S., Chavarin P., Olivier T., Delézay O., et al. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008;9:54. doi: 10.1186/1471-2172-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silva-Cardoso S.C., Affandi A.J., Spel L., Cossu M., van Roon J.A.G., Boes M., et al. CXCL4 exposure potentiates TLR-driven polarization of human monocyte-derived dendritic cells and increases stimulation of t cells. J Immunol. 2017;199:253–262. doi: 10.4049/jimmunol.1602020. [DOI] [PubMed] [Google Scholar]
- 144.Xu P., Jiang Y., Zuo H., Liu X., Xia T., Zhou R., et al. Vincristine-loaded platelets coated with anti-CD41 mAbs: a new macrophage targeting proposal for the treatment of immune thrombocytopenia. Biomater Sci. 2019;7:4568–4577. doi: 10.1039/c9bm01026b. [DOI] [PubMed] [Google Scholar]
- 145.Diacovo T.G., Catalina M.D., Siegelman M.H., von Andrian U.H. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaplan Z.S., Zarpellon A., Alwis I., Yuan Y., McFadyen J., Ghasemzadeh M., et al. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors GPIb and PAR4. Nat Comm. 2015;6 doi: 10.1038/ncomms8835. [DOI] [PubMed] [Google Scholar]
- 147.Affandi A.J., Silva-Cardoso S.C., Garcia S., Leijten E.F.A., Kempen T.S., Marut W., et al. CXCL4 is a novel inducer of human Th17 cells and correlates with IL-17 and IL-22 in psoriatic arthritis. Eur J Immunol. 2018;48:522–531. doi: 10.1002/eji.201747195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dinkla S., van Cranenbroek B., van der Heijden W.A., He X., Wallbrecher R., Dumitriu I.E., et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016;127:1976–1986. doi: 10.1182/blood-2015-04-640300. [DOI] [PubMed] [Google Scholar]
- 149.Colberg L., Cammann C., Greinacher A., Seifert U. Structure and function of the ubiquitin-proteasome system in platelets. J Thromb Haemost. 2020;18:771–780. doi: 10.1111/jth.14730. [DOI] [PubMed] [Google Scholar]
- 150.Zufferey A., Schvartz D., Nolli S., Reny J.L., Sanchez J.C., Fontana P. Characterization of the platelet granule proteome: evidence of the presence of MHC1 in alpha-granules. J Proteom. 2014;101:130–140. doi: 10.1016/j.jprot.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 151.Chapman L.M., Aggrey A.A., Field D.J., Srivastava K., Ture S., Yui K., et al. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916–923. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zufferey A., Speck E.R., Machlus K.R., Aslam R., Guo L., McVey M.J., et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hopkins L.M., Davis J.M., Buchli R., VanGundy R.S., Schwartz K.A., Gerlach J.A. MHC class I–associated peptides identified from normal platelets and from individuals with idiopathic thrombocytopenic purpura. Hum Immunol. 2005;66:874–883. doi: 10.1016/j.humimm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 154.Angénieux C., Dupuis A., Gachet C., de la Salle H., Maître B. Cell surface expression of HLA I molecules as a marker of young platelets. J Thromb Haemost. 2019;17:1511–1521. doi: 10.1111/jth.14537. [DOI] [PubMed] [Google Scholar]
- 155.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sheikh S., Parhar R.S., Bakheet R., Saleh S., Collison K., Al-Mohanna F. Immobilization of rolling NK cells on platelet-borne P-selectin under flow by proinflammatory stimuli, interleukin-12, and leukotriene B4. J Leukoc Biol. 2004;76:603–608. doi: 10.1189/jlb.0204106. [DOI] [PubMed] [Google Scholar]
- 157.Placke T., Kopp H.-G., Salih H. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3:374–382. doi: 10.1159/000323936. [DOI] [PubMed] [Google Scholar]
- 158.Gasic G.J., Gasic T.B., Stewart C.C. Antimetastatic effects associated with platelet reduction. Proc Nat Acad Sci. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Placke T., Örgel M., Schaller M., Jung G., Rammensee H.-G., Kopp H.-G., et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 160.Placke T., Salih H.R., Kopp H.-G. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol. 2012;189:154–160. doi: 10.4049/jimmunol.1103194. [DOI] [PubMed] [Google Scholar]
- 161.Baltz K.M., Krusch M., Baessler T., Schmiedel B.J., Bringmann A., Brossart P., et al. Neutralization of tumor-derived soluble glucocorticoid-induced TNFR-related protein ligand increases NK cell anti-tumor reactivity. Blood. 2008;112:3735–3743. doi: 10.1182/blood-2008-03-143016. [DOI] [PubMed] [Google Scholar]
- 162.Baessler T., Krusch M., Schmiedel B.J., Kloss M., Baltz K.M., Wacker A., et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein ligand subverts immunosurveillance of acute myeloid leukemia in humans. Cancer Res. 2009;69:1037–1045. doi: 10.1158/0008-5472.Can-08-2650. [DOI] [PubMed] [Google Scholar]
- 163.Clar K.L., Hinterleitner C., Schneider P., Salih H.R., Maurer S. Inhibition of NK reactivity against solid tumors by platelet-derived RANKL. Cancers. 2019;11:277. doi: 10.3390/cancers11030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kato Y., Kaneko M.K., Kunita A., Ito H., Kameyama A., Ogasawara S., et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takemoto A., Okitaka M., Takagi S., Takami M., Sato S., Nishio M., et al. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep. 2017;7 doi: 10.1038/srep42186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kopp H.-G., Placke T., Salih H. Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 167.Cluxton C.D., Spillane C., O'Toole S.A., Sheils O., Gardiner C.M., O'Leary J.J. Suppression of natural killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Erpenbeck L., Schön M.P. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115:3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen P., Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 170.Elzey B.D., Grant J.F., Sinn H.W., Nieswandt B., Waldschmidt T.J., Ratliff T.L. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78:80–84. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 171.Solanilla A., Pasquet J.-M., Viallard J.-F., Contin C., Grosset C., Déchanet-Merville J., et al. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105:215–218. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 172.Chargaff E., West R. The biological significance of the thromboplastic protein of blood. The J Biol Chem. 1946;166:189–197. [PubMed] [Google Scholar]
- 173.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 174.Kuravi S.J., Harrison P., Rainger G., Nash G.B. Ability of platelet-derived extracellular vesicles to promote neutrophil-endothelial cell interactions. Inflamm. 2019;42:290–305. doi: 10.1007/s10753-018-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chimen M., Evryviadou A., Box C.L., Harrison M.J., Hazeldine J., Dib L.H., et al. Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica. 2020;105:1248–1261. doi: 10.3324/haematol.2018.215145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Italiano J.E., Jr., Mairuhu A.T., Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Knijff-Dutmer E.A., Koerts J., Nieuwland R., Kalsbeek-Batenburg E.M., van de Laar M.A. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002;46:1498–1503. doi: 10.1002/art.10312. [DOI] [PubMed] [Google Scholar]
- 178.Boilard E., Nigrovic P.A., Larabee K., Watts G.F.M., Coblyn J.S., Weinblatt M.E., et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen X. Rac1 regulates platelet microparticles formation and rheumatoid arthritis deterioration. Platelets. 2020;31:112–119. doi: 10.1080/09537104.2019.1584669. [DOI] [PubMed] [Google Scholar]
- 180.Semple J.W., Milev Y., Cosgrave D., Mody M., Hornstein A., Blanchette V., et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 181.Sewify E.M., Sayed D., Aal R.F., Ahmad H.M., Abdou M.A. Increased circulating red cell microparticles (RMP) and platelet microparticles (PMP) in immune thrombocytopenic purpura. Thromb Res. 2013;131 doi: 10.1016/j.thromres.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 182.Tantawy A.A.G., Matter R.M., Hamed A.A., Telbany M.A. Platelet microparticles in immune thrombocytopenic purpura in pediatrics. Ped Hemat Oncol. 2010;27:283–296. doi: 10.3109/08880011003663390. [DOI] [PubMed] [Google Scholar]
- 183.Jy W., Horstman L.L., Arce M., Ahn Y.S. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med. 1992;119:334–345. [PubMed] [Google Scholar]
- 184.Burbano C., Villar-Vesga J., Orejuela J., Muñoz C., Vanegas A., Vásquez G., et al. Potential involvement of platelet-derived microparticles and microparticles forming immune complexes during monocyte activation in patients with systemic lupus erythematosus. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Biswas S., Xin L., Panigrahi S., Zimman A., Wang H., Yakubenko V.P., et al. Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE-/- mice and activate platelets via TLR2. Blood. 2016;127:2618–2629. doi: 10.1182/blood-2015-08-664300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371(3):567–576. doi: 10.1007/s00441-017-2727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aliprantis A.O., Yang R.B., Mark M.R., Suggett S., Devaux B., Radolf J.D., et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 188.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 189.Tanimura N., Saitoh S., Ohto U., Akashi-Takamura S., Fujimoto Y., Fukase K., et al. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol. 2014;26:307–314. doi: 10.1093/intimm/dxt071. [DOI] [PubMed] [Google Scholar]
- 190.Berthet J., Damien P., Hamzeh-Cognasse H., Pozzetto B., Garraud O., Cognasse F. Toll-like receptor 4 signal transduction in platelets: novel pathways. Br J Haematol. 2010;151:89–92. doi: 10.1111/j.1365-2141.2010.08292.x. [DOI] [PubMed] [Google Scholar]
- 191.Cognasse F., Nguyen K.A., Damien P., McNicol A., Pozzetto B., Hamzeh-Cognasse H., Garraud O. The inflammatory role of platelets via their TLRs and siglec receptors. Front Immunol. 2015;6:1–15. doi: 10.3389/fimmu.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tatematsu M., Yoshida R., Morioka Y., Ishii N., Funami K., Watanabe A., et al. Raftlin controls lipopolysaccharide-induced TLR4 internalization and TICAM-1 signaling in a cell type-specific manner. J Immunol. 2016;196:3865–3876. doi: 10.4049/jimmunol.1501734. [DOI] [PubMed] [Google Scholar]
- 193.Ståhl A.L., Svensson M., Mörgelin M., Svanborg C., Tarr P.I., Mooney J.C., et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Diebold S.S., Kaisho T., Hemmi H., Akira S. C. Reis e Sousa. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Z., Ohto U., Shibata T., Krayukhina E., Taoka M., Yamauchi Y., et al. Structural analysis reveals that toll-like receptor 7 is a dual receptor for guanosine and single-stranded RNA. Immunity. 2016;45:737–748. doi: 10.1016/j.immuni.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 196.Davenne T., Bridgeman A., Rigby R.E., Rehwinkel J. Deoxyguanosine is a TLR7 agonist. Eur J Immunol. 2020;50:56–62. doi: 10.1002/eji.201948151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shields P.L., Morland C.M., Salmon M., Qin S., Hubscher S.G., Adams D.H. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 198.Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P.D., et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 199.Ponath P.D., Qin S., Post T.W., Wang J., Wu L., Gerard N.P., et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Inngjerdingen M., Damaj B., Maghazachi A.A. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 201.Yoshimura T., Matsushima K., Tanaka S., Robinson E.A., Appella E., Oppenheim J.J., et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Nat Acad Sci. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017;9:S164–s171. doi: 10.21037/jtd.2017.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.English E.J., Mahn S.A., Marchese A. Endocytosis is required for CXC chemokine receptor type 4 (CXCR4)-mediated Akt activation and antiapoptotic signaling. J Biol Chem. 2018;293:11470–11480. doi: 10.1074/jbc.RA118.001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Triantafilou K., Triantafilou M., Dedrick R.L. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 205.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 206.Soh J., Donnelly R.J., Kotenko S., Mariano T.M., Cook J.R., Wang N., et al. Identification and sequence of an accessory factor required for activation of the human interferon gamma receptor. Cell. 1994;76:793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 207.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 208.Sorrentino A., Thakur N., Grimsby S., Marcusson A., von Bulow V., Schuster N., et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 209.Yoshimoto T., Takeda K., Tanaka T., Ohkusu K., Kashiwamura S., Okamura H., et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 210.Assinger A. Platelets and infection—an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]