Abstract
N. sativa (N. sativa) has been used since ancient times, when a scientific concept about the use of medicinal plants for the treatment of human illnesses and alleviation of their sufferings was yet to be developed. It has a strong religious significance as it is mentioned in the religious books of Islam and Christianity. In addition to its historical and religious significance, it is also mentioned in ancient medicine. It is widely used in traditional systems of medicine for a number of diseases including asthma, fever, bronchitis, cough, chest congestion, dizziness, paralysis, chronic headache, back pain and inflammation. The importance of this plant led the scientific community to carry out extensive phytochemical and biological investigations on N. sativa. Pharmacological studies on N. sativa have confirmed its antidiabetic, antitussive, anticancer, antioxidant, hepatoprotective, neuro-protective, gastroprotective, immunomodulator, analgesic, antimicrobial, anti-inflammatory, spasmolytic, and bronchodilator activity. The present review is an effort to explore the reported chemical composition and pharmacological activity of this plant. It will help as a reference for scientists, researchers, and other health professionals who are working with this plant and who need up to date knowledge about it.
Keywords: Black Seed, N. sativa, Phytochemistry, Pharmacological properties, Antioxidant
1. Introduction
N. sativa belongs to family Ranunculaceae and it is possibly one of the most significant medicinal plants in history. It is mentioned in different historical and religious text books. In southern Asia, the seed of this plant is popularly known as ‘Kalonji’ in the middle east most common name is ‘habbat us sauda’ and popular name of this plant in English is ‘black cumin’ (Gilani et al., 2004; Tavakkoli et al., 2017).
The seed of this plant is used in ancient medicine for a number of ailments including back pain, asthma, fever, bronchitis, cough, chest congestion, dizziness, paralysis, chronic headache, inflammation, infertility, and other gastrointestinal disorders like dyspepsia, flatulence, diarrhea, and dysentery (Durmuskahya and Ozturk, 2013; Gholamnezhad et al., 2016; Nasir et al., 2014; Shah, 1966). Additionally, N. sativa seed oil is used for the remedy of an abscess, nasal ulcer, swollen joint, orchitis and eczema. N. sativa oil is also used in combination with honey for asthmatic problems, bronchospasms and chest congestion (Gholamnezhad et al., 2016; Nasir et al., 2014). In ancient literature, N. sativa is also attributed as an analgesic, liver tonic, diuretic, appetite stimulant, analgesic and digestive (Gilani et al., 2004; Rajsekhar and Kuldeep, 2011; Tavakkoli et al., 2017; Ziaee et al., 2012).
2. Methodology
The literature was searched using PubMed and google scholar search engines. Papers from between 1966 and 2017 were included. The search was made using keywords such as N. sativa and phytochemistry; N. sativa and pharmacological properties; N. sativa and immunomodulatory; N. sativa and antihistaminic; N. sativa and hepatoprotective; N. sativa and obesity; N. sativa and cardiovascular disease etc. An attempt was made to document relevant literature that is only focused on N. sativa. The review includes a total of 8 clinical trials.
3. Plant description
The N. sativa plant is a green colour with finely divided linear leaves. The flower is pale blue and white in colour with 5-10 petals. The fruits are found in the form of inflated capsules. The capsules are further divided into 3-7 united follicles. Each follicle contains numerous black seeds which are oval in shape and black measuring about 1 mm in diameter (Fig. 1 ) (Gholamnezhad et al., 2016; Randhawa et al., 2005).
Fig. 1.
Morphological representation of various parts of N. sativa Linn.
3.1. Scientific name and classification
It is a plant of the family Ranunculaceae. This family is also known as buttercup or crowfoot. It is comprised of over 2,000 known species of flowering plants in 43 genera, which is distributed all over the world. The largest genera are Ranunculus. It comprises 600 species that also includes N. sativa.
3.2. Synonym
The seeds of the plants are known as ‘Black cumin’ in English, In Arab countries, they are known as ‘Habba Al-Sauda’ or ‘Habba Al-Barakah’. The popular Urdu name of this plant is ‘Kalonji’. It is known as ‘Siyah Danch’ and Cork out in Persian and Turkish respectively. The plant is also known as Love-in-a-mist, Habatul Barakah, Sonez, Krishana, Jiraka and Sidadanah (Sultan et al., 2012).
3.3. Distribution
N. sativa is native to Africa and South west Asia. It is also grown in India, Bangladesh, Turkey, Pakistan, Sri Lanka, Syria and other Mediterranean regions. High quality seeds are known to come from Egypt as Egypt has the most suitable environment for growing this plant (Naz, 2011).
4. Traditional and Religious significance of N. sativa
N. sativa has a long religious and historical background and is mentioned in various religious literature. It is mentioned in the old testament of Bible and is found in the book of Isaiah where it is mentioned as “ketzah” a spice for bread and cake that can be used in many ways (Naz, 2011). It is mentioned as Melanthoin and Gith in old literature (Rahmani and Aly, 2015). It is also mentioned in the Chinese and Indian Traditional medicine. It has been used to treat various disease from thousands of years ago and known as a vital drug in Indian medicine (Sharma et al., 2001).
The N. sativa has been discussed in the Traditional Arab and Islamic Medicine (TAIM) with the name “Habb-e-Sauda”. It is known as prophetic medicine as use of this seed has been mentioned by prophet (SAW). It has been mentioned in the book of Bukhari that “N. sativa is healing of all the diseases (Bukhari 5687)”.
5. Chemical constituents
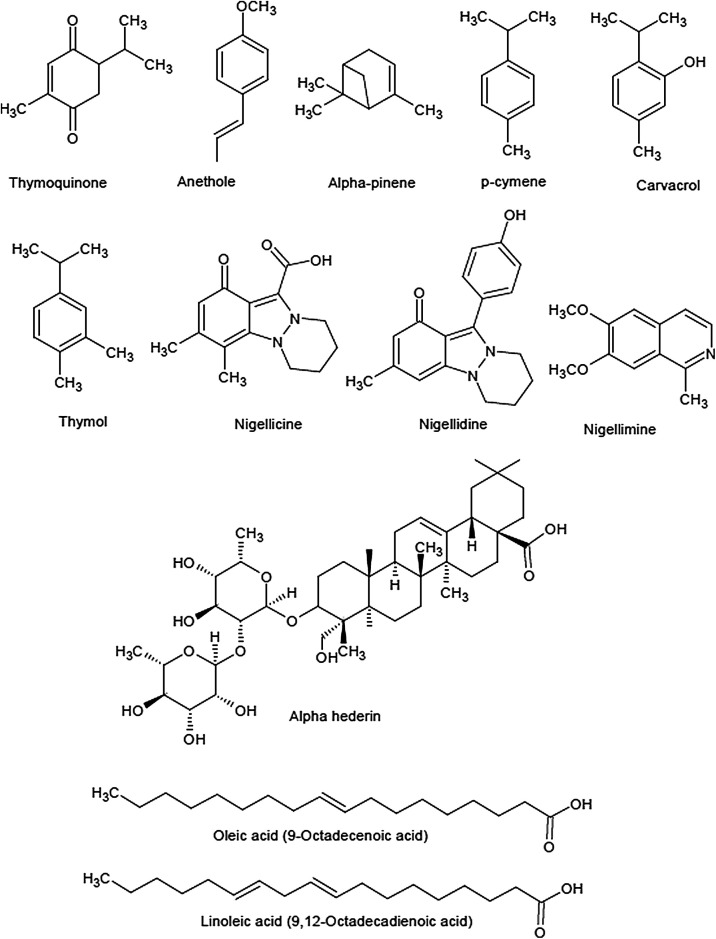
The first report of the chemical components has been documented in the 1880s. It was reported by Greenish et al that it is composed of oils, proteins, carbohydrates and fibres (Greenish, 1880). There have been many studies to find out the chemical nature of N. sativa and it was found that the medicinal value of N. sativa is mainly due to the presence of its quinone constituent which is also known as thymoquinone (TQ) (Sahak et al., 2016). TQ is the main constituent of the volatile oil and has a variety of pharmacological properties such as hepatoprotective (Hassanein et al., 2016; Laskar et al., 2016; Saheb et al., 2016), anti-inflammatory (Abd-Elbaset et al., 2017; Shaarani et al., 2017), antibacterial (Goel and Mishra, 2018), antioxidant (Erol et al., 2017), fungicidal (Almshawit and Macreadie, 2017), nephroprotective (Kotb et al., 2018) and anticancer (Almoyad, 2018; Majdalawieh et al., 2017; Shaarani et al., 2017). There is also literature showing evidence for the molecular mechanism of this molecule (Gholamnezhad et al., 2016). Other components found in the N. sativa includes p-cymene, carvacrol, thymohydroquinone (THQ), dihydrothymoquinone (DHTQ), α-thujene, thymol, t-anethole, β-pinene, α-pinene, and γ-terpinene (Sahak et al., 2016). The important constituents of the N. sativa have been summarized in Table 1 and the chemical structure has been presented in Fig. 2 .
Table 1.
Chemical constituent of N. sativa.
| Group | Sub groups | Active constituents | Reference |
|---|---|---|---|
| Fixed oil | Unsaturated fatty acids | Oleic acid, Linoleic acid, dihomolinoleic acid, eicodadienoic acid | (Naz, 2011; Nickavar et al., 2003; Ramadan and Morsel, 2002) |
| Saturated fatty acids | Palmitic acid, stearic acid | (Naz, 2011) | |
| Terpenes | Aliphatic | Thymoquinone, p-cymene, α-pinene, dithymoquinone, thymohydroquinone, Carvacrol, carvone, limonene, 4-terpineol, citronellol, anethol | (Ghosheh et al., 1999; Naz, 2011) |
| Alkaloids | Isoquinoline alkaloids | Nigellicimine, Nigellicimine N-oxide | (Naz, 2011) |
| Pyrazole alkaloids | Nigellidine, nigellicine | (Naz, 2011) | |
| Coumarins | Methoxy coumarin | 6-methoxy-coumarin | (Tembhurne et al., 2014) |
| Hydroxy coumarin | 7-hydroxy-coumarin | (Tembhurne et al., 2014) | |
| Oxy coumarin | 7-oxy-coumarin | (Tembhurne et al., 2014) | |
| Saponins | Steroidal | Alpha hedrin | (Randhawa and Al-Ghamdi, 2002) |
| Triterpenes | Steryl glucosides, Acetyl-steryl-glucoside | (Randhawa and Al-Ghamdi, 2002) | |
| Flavonoids | Flavonoidal pigment | Quercetin | (Merfort et al., 1997) |
| Flavonoidal glycoside | Kaempferol 3-glucosyl galactosyl glucoside, quercetin 3-galactosyl glucoside, trigillin quercetin-3-glucoside | (Merfort et al., 1997; Rajkapoor et al., 2002) | |
| Phenolics | Acidic phenolics | Vanillic acid, hydroxybenzoic acid, syringicacid, p-cumaric acids | (Bourgou et al., 2008; Mariod et al., 2009) |
| Amino acids | Essential amino acids | Valine, phenylalanine, threonine, methionine, histidine, tryptophan, leucine, isoleucine, lysine | (Babayan et al., 1978) |
| Metals and trace elements | Calcium, iron, and potassium, phosphorus, zinc | (Al‐Gaby, 1998) |
Fig. 2.
Important bioactive compounds of N. sativa Linn.
6. The pharmacological activity of N. sativa
N. sativa has been reported to have a variety of pharmacological activities, which are categorized as follows.
6.1. Antimicrobial activity
6.1.1. Antibacterial effect
Thymohydroquinone obtained from the volatile oil of N. sativa has a high significant effect against gram-positive microorganisms, including Staphylococcus aureus. Diethyl-ether extract of N. sativa was investigated to possess the concentration-dependent inhibitory effect on gram-positive bacteria S. aureus and gram-negative bacteria Pseudomonas aeruginosa and Escherichia coli (Aljabre et al., 2015). N. sativa showed an additive effect with various drugs such as doxycycline, chloramphenicol, erythromycin, nalidixic acid and lincomycin (Hanafy and Hatem, 1991). In the management of neonates with staphylococcal pustular skin infections, N. sativa extract demonstrated almost similar results to topical antibiotic mupirocin (Rafati et al., 2014). N. sativa exhibited promising outcomes against many multi-drug-resistant gram positive and gram negative bacteria including resistant S. aureus and P. aeruginosa (Mashhadian and Rakhshandeh, 2005; Morsi, 2000; Salman et al., 2008). Honey, together with N. sativa possesses synergistic antibacterial effects in treatment of P. aeruginosa infection. Chlorhexidine gluconate is a germicidal mouthwash that is used in bacterial infection. It was shown that N. sativa oil extract exhibits better results than chlorhexidine gluconate when treating the infection of S. mutans and other most common dental pathogens (Al Attas et al., 2016).
6.1.2. Antiviral effect
It was reported that N. sativa increases T helper cells (T4) and suppressor T cells (T8) as well as increases natural killer (NK) cell activity in healthy volunteers (Aljabre et al., 2015). In addition to improvement in immunity, N. sativa extract also shows some inhibitory effects on the human immune deficiency virus protease (Khan, 1999). In a study N. sativa oil capsules of 450 mg were given three times a day to hepatitis C virus-infected patients for three months. Overall there was considerable improvement in oxidative stress, a reduction in viral load and significant improvements were reported in albumin, total protein, platelet and RBC levels. The improvement in RBC count help to decrease the level of membrane lipid peroxide and reduce the chance of hemolysis (Barakat et al., 2013; Forouzanfar et al., 2014).
6.1.3. Antifungal effect
Due to the excessive use of antifungal drugs, resistance is a widespread problem. Plant based medicines have attracted researchers as potential alternative treatments for fungal infections (Alsaidy, 2014; Doudi et al., 2014). Antifungal activity is chiefly due to TQ of N. sativa. TQ, thymohydroquinone and thymol confirmed antifungal activity against dermatophytes, moulds and yeasts (Taha et al., 2010). The ether extract of N. sativa inhibits the growth of Candida yeast (Khan et al., 2003). Further, antifungal effects of N. sativa extracts were also seen against C. albicans (Moghim et al., 2015). It was reported that TQ inhibits in vitro Aspergillus niger and Fusarium solani activity similar to the antifungal drug amphotericin-B (Al-Qurashi et al., 2007; Randhawa et al., 2005). Moderate antifungal effects were found by TQ in three main groups of dermatophytes Trichophyton, Epidermophyton and Microsporum (Abd-El-Kader et al., 1995). N. sativa ether extracts also exhibit antifungal results but produce a more favorable action in higher concentrations. Extracts inhibit 80–100% growth of the most dermatophytes in range of 40 mg/ml, while the minimum inhibitory concentration of TQ against different dermatophytes ranged from 0.125 to 0.25 mg/ml (Aljabre et al., 2005).
6.2. Cardio protective activity
Cardiovascular diseases (CVDs) that include coronary heart disease, cerebrovascular disease, heart failure, hypertension and peripheral vascular diseases are leading cause of death worldwide and it was estimated that by 2020, almost 23.6 million people are likely to die from CVD (Ahmad et al., 2013b; Ibrahim et al., 2014). Numerous cardio protective effects of N. sativa have been observed.
6.2.1. Antihypertensive effect
N. sativa counteracts several risks of cardiovascular diseases by its versatile pharmacological action due to its antioxidant (Leong et al., 2013), diuretic (Zaoui et al., 1999), calcium channel blocking (Boskabady et al., 2005) and cardiac depressant properties (El-Taher et al., 2003; El Tahir and Ageel, 1994; El Tahir et al., 1993). It has been reported in a study that N. sativa oil exhibits positive results against hypertension through a reduction in systolic blood pressure (SBP), lactate dehydrogenase (LDH), asymmetric dimethylarginine, plasma creatine kinase (CK), attenuation of oxidative injury (decrease in malondialdehyde), increase in tissue Na+K+ATPase activity and plasma nitric oxide level (Tasar et al., 2012). It has been reported in a randomised controlled trial of 108 patients that the consumption of N. sativa seed for 2 months can lower the BP in the mildly hypertensive patients (Dehkordi and Kamkhah, 2008; Fallah Huseini et al., 2013).
6.2.2. Antiatherogenic effect
Globally, atherosclerosis is the most common reason for morbidity and mortality. It is a multifactorial disease with many different threats (Joshi et al., 2005). Hyperlipidemia, low-density lipoproteins (LDL), clotting factors and inflammation all collectively contribute to the development of an atherosclerotic plaque (D’Souza et al., 2007). It has been illustrated that the volatile oils of N. sativa and its active constituent TQ have valuable results for hyperglycaemia and hyperlipidema (Ali and Blunden, 2003; Asgary et al., 2013). TQ has been reported to improve hyperlipidemia and protect against the development of atherosclerosis.
6.2.3. Improve endothelial function
Endothelial dysfunction is a pathological state that contributes to the pathogenesis of a number of cardiovascular diseases, diabetes mellitus, obesity, dyslipidemia, atherosclerosis and ageing. These disorders are linked with overproduction of reactive oxygen species in the arterial vessel wall (Idris-Khodja and Schini-Kerth, 2012; Vanhoutte et al., 2009). It has been found that inhibition of oxidative stress and normalization of the angiotensin system by TQ improved endothelial function (Idris-Khodja and Schini-Kerth, 2012).
6.3. Gastroprotective activity
N. sativa oil increases gastric levels of mucin and glutathione, and decreases gastric mucosal histamine content. Therefore it can play a significant role in treating gastric ulcers induced by indomethacin and ethanol (El-Dakhakhny et al., 2000; Rifat-uz-Zaman and Khan, 2004). The pepsinnogen proenzyme of pepsin is released from stomach cells and mixes with the gastric juice hydrochloric acid, and is converted to pepsin (Kanter et al., 2006; Tanaka et al., 2001). TQ stimulates pepsinogen to activate pepsin in gastric juice and exhibits protective action against gastric ulcers (Kanter et al., 2006). Anti-inflammatory result of TQ has also been reported in the treatment of gastric injury. TQ reduces neutrophil invasion via decreasing myeloperoxidase which acts as a marker of acute inflammation. The scavenging of free radicals and antioxidant activities of TQ play a significant role in the effect the plant has on gastrointestinal disorders (Magdy et al., 2012). The bioactive constituents affecting the gastric physiology are documented in Table 2 .
Table 2.
Bioactive constituents of N. sativa Linn affecting the gastrointestinal system.
| Active constituents | Proposed mechanism | References |
|---|---|---|
| Thymoquinone | Cytoprotection, hydroxyl radical scavenging activity, digestive, immune boosting, Carminative | (Ahmad and Beg, 2013) |
| Fatty acids: palmitic acid, myristic acid, stearic acid, oleic acid, arachidonic acid, linoleic acid, linolenic acid, eicosadienoic acid | Immune boosting, anti-inflammatory and maintain skin moisture | (Ahmad and Beg, 2013; Ramadan and Morsel, 2002) |
| Alkaloids: nigelline-N-oxide, nigellone, nigellimine, triterpene-alpha-hederin, Saponin | Anti-histamine, anticancer and anti-leishmanial property | (Ahmad et al., 2013a; Ahmad and Beg, 2013) |
| α, β and γ-tocopherols | Antioxidant, immune boosting | (MatthauS and Ozcan, 2011) |
| Minerals: iron, calcium, zinc, copper, phosphorous | Chelating action: Inhibition of generations of free radical by stabilization of transition metals, thereby reducing free radicals damage | (Atta, 2003) |
| Vitamins: carotene, thiamine, riboflavin, niacin, folic acid | Immune boosting, free radical scavenger and antioxidant | (Ahmad and Beg, 2013) |
In a comparative study between N. sativa seeds and a triple therapy using clarithromycin, amoxicillin, and omeprazole, it was reported N. sativa was clinically significant against Helicobacter pylori (H. pylori). In an experiment of 88 adult patients with positive H. pylori infection, H. pylori eradication was observed respectively 82.6%, 47.6%, 66.7% and 47.8% with triple therapy, 1 gm NS +40 mg omeprazole, 2 gm NS +40 mg omeprazole and 3gm NS +40 mg omeprazole. Eradication rate with 2 gm NS was significant in reference to 1 g and 3gm (Salem et al., 2010).
6.4. Neuroprotective activity
The brain is susceptible to oxidative stress injury due to its high rate of oxidative metabolic action, reactive oxygen species metabolites production and a relatively high content of polyunsaturated fatty acids with low antioxidant capacity, non-replicating character of its neuronal cells and low repair mechanism activity (Al-Majed et al., 2006; Evans, 1993). TQ the major component of N. sativa volatile is observed to have potent antioxidant properties (Darakhshan et al., 2015). TQ defends the body against induced oxidative injury caused by free radical generating mediators such as carbon tetrachloride-induced hepatotoxicity (Nagi et al., 1999), nephropathy produced by cisplatin (Badary et al., 1997), doxorubicin-induced cardiotoxicity (Nagi and Mansour, 2000), ischemia-reperfusion evoked gastric mucosal injury and allergic encephalomyelitis (Mohamed et al., 2004; Mohamed et al., 2002).
N. sativa encourages modulation properties on memory mutilation, averts hippocampal pyramidal cell loss and increases memory consolidation stored information and decreases neuronal cell death of hippocampal CA1 region (Sayeed et al., 2013). Flumazenil is a particular antagonist of GABAA-BZD receptor complex (Brogden and Goa, 1988; File and Pellow, 1986). It is used to find out the role of BZD receptors involvement in the anticonvulsant properties induced by TQ. It was reported that PTZ diminishes the GABAergic tone by the inhibition of BZD site of the GABA receptors (Rehavi et al., 1982). It was observed from seizure induced by PTZ with flumazenil that TQ support preventive action of GABAergic system possibly via a competitive agonist action in GABA receptors of BZD site (MacDonald and Barker, 1977).
6.5. Anticancer activity
Cancer has become a rising public health concern across the world (Ahmad, 2020). Thymoquinone of N. sativa exhibits promising anti-carcinogenic, anti-neoplastic, anti-mutagenic and anti-proliferative activities against various tumor cells (Khan et al., 2011; Shoieb et al., 2003). Additionally it also acts as chemopreventive agent and is used with the combination of the therapeutic agents to reduce toxic effects of treatment (Khader et al., 2007).
6.5.1. Pancreatic cancer
Thymoquinone is the major constituent of N. sativa oil extract that induces apoptosis and inhibits proliferation in pancreatic ductal adenocarcinoma (PDA) cells (Chehl et al., 2009). TQ induces proapoptotic modes and anti-inflammatory actions and acts as a novel inhibitor of pro-inflammatory pathways. The high molecular weight glycoprotein mucin 4 is abnormally expressed in cancer of the pancreas and contributes to the regulation of proliferation, differentiation and metastasis of pancreatic cancer cells. TQ exhibits a down-regulatory effect on mucin 4 in pancreatic cancer cells (Torres et al., 2010).
6.5.2. Hepatic cancer
Hepatocellular carcinoma is one of the widespread malignant diseases, and globally the number of cases has rapidly increased over the past decades. The cytotoxic action of N. sativa seed was exhibited on human hepatoma HepG2 cell lines following 24-hr incubation with different concentrations of the N. sativa extract (Thabrew et al., 2005). It was found that oral administration of TQ is useful in rising the actions of glutathione transferase and quinine reductase and acts as a potential prophylactic source against toxicity in hepatic cancer and chemical carcinogenesis (Nagi and Almakki, 2009).
6.5.3. Prostate and renal cancer
TQ has been shown to be useful for the management of hormone refractory and hormone-sensitive prostate cancer (Yi et al., 2008). TQ prevents prostate tumour growth with almost no chemotoxic side effects. It has also been reported that endothelial cells were more susceptible to thymoquinone-induced cell proliferation, cell apoptosis and cell migration inhibition in contrast to PC3 cancer cells. TQ inhibited growth of vascular endothelial factor-induced extracellular signal-regulated kinase activation.
6.6. Anti diabetic activity
Diabetes mellitus (DM) is a chronic metabolic disorder that affects 8.3% population of the world. Improper management of this disease leads to secondary diseases that include retinopathy, cataract, neuropathy and cardiac problems (Heshmati and Namazi, 2015). It has been reported that the treatment of diabetes type 2 patients with N. sativa seed dose of 2 gm/day for the duration of 3 months decreases postprandial glucose levels, fasting blood glucose levels, insulin resistance, as well as decreases glycosylated haemoglobin (Bamosa et al., 2010). It has also been shown that N. sativa seed considerably enhances high-density lipoprotein concentration (HDL-C), decreases serum total cholesterol (TC), low-density lipoprotein, cholesterol (LDL-c) and triglyceride (TG) levels (Bamosa et al., 2010).
N. sativa active antioxidant constituents and TQ enhance secretion of insulin via improving mitochondria energy metabolism as well as improving insulin receptors intracellular pathways (Mansi, 2005). Among all mechanisms, antioxidant mechanisms are an important way to control the hyperglycemic stage of diabetic patients. N. sativa enhances the antioxidant enzymes and leads to oxidative stress reduction, and consequently it facilitates pancreatic beta-cells regeneration (Abdelmeguid et al., 2010; Houcher et al., 2007; Kanter, 2008; Sultan et al., 2014). Furthermore, it increases the numbers of islets cells, declines the resistance of insulin and enhances insulin secretion (Bamosa et al., 2010; Mansi, 2005; Rchid et al., 2004; Salama, 2011). N. sativa and its TQ decrease gluconeogenesis, expression of gluconeogenic enzymes like glucose-6phosphatase and fructose 1, 6-bisphosphatase and hepatic glucose production, consequentially exhibit significant effect in controlling sugar levels particularly in diabetic patients. (Abdelmeguid et al., 2010; Houcher et al., 2007).
6.7. Antioxidant activity
It has been revealed that N. sativa extracts and essential oils possess strong antioxidant activity (Ashraf et al., 2011; Sultan et al., 2012). The antioxidant effect of TQ has been found in different diseases, including diabetes, asthma, carcinogenesis and encephalomyelitis. TQ preserves the activity of a variety of antioxidant enzymes such as glutathione peroxidase, glutathione-S-transferase and catalase and also acts as free radical and superoxide scavenger. It has been reported that TQ acts as a nephroprotective and decreases SSAT and CYP3A1 gene expression via antioxidant mechanisms (Awad et al., 2011; Mansour et al., 2002). It reacts with GSH, NADH and NADPH and forms glutathionyl-dihydro-thymoquinone, offering evidence for potent free radical scavengers (Khalife and Lupidi, 2007). Influential chemo preventive action of TQ has been shown against MC-induced fibrosarcoma tumours due to the antioxidant activity and its interference with DNA synthesis (Badary and Gamal, 2000). Treatment with N. sativa extract prevented liver damage induced by lipid peroxidation (Meral et al., 2001). The favourable safety profile of herbal medicines is one of the reasons people often favour herbal medicines. It has been reported that a mixture of N. sativa with honey exhibits protection against methylnitrosourea-induced oxidative stress and carcinogenesis (Ahmad, 2018, 2019; Ahmad et al., 2013a; Ahmad et al., 2011; Mabrouk et al., 2002). Antioxidant properties of N. sativa ethanol extract exhibit protection from diabetes by improving antioxidant enzyme glutathione peroxidase and decreasing blood glucose levels, lipids levels (Kaleem et al., 2006).
N. sativa containing flavonoids enhance gastric mucus and strengthen mucosal immune defense by scavenging superoxide and hydroxyl free radicals (Badary et al., 2003). Inhibition of lipid peroxidation non-enzymatically has been revealed by TQ and N. sativa oil (Houghton et al., 1995). Formation of lipid peroxide and lactate dehydrogenase are suppressed by N. sativa oil even in low concentrations and increase the availability of superoxide dismutase and glutathione (GSH) simultaneously falling lipid peroxidation and free radical generation (Houghton et al., 1995; Mansour, 2000).
6.8. Anti-dyslipidemic and anti-obesity
Dyslipidemia is a wide term covering diverse lipoprotein and lipid disorders. It exhibits a significant role in the provocation of cardiovascular ailments. The research was carried out to assess the therapeutic result of a combination of black seed with garlic in the treatment of dyslipidemia. Patients that were treated with garlic, black seed and simvastatin for 8 weeks show considerable differences between cholesterol, triglyceride, Non-HDL, and LDL levels (Hamed Ahmad Alobaidi, 2014). Various preparations of N. sativa include oil, extract and powder show a significant role in the modification of plasma lipids and act as a remedy in various disorders like cardio metabolic diseases, obesity, fatty liver, diabetes and metabolic syndrome (Table S1, Supplementary file). TQ has been reported for its curative potential in dyslipidemia. A number of scientific trials also demonstrated in people with dyslipidemia with N. sativa. N. sativa seeds supplementations have a positive effect in the management of hypercholesterolemia and hyperlipidemia and particularly in patients suffering from diabetes (Razavi and Hosseinzadeh, 2014). Obesity is the physiological consequence of a complex genetic interaction, and psychosocial and environmental factors (Rodríguez et al., 2005). Restriction of dietary calories is used as a conventional therapy while various functional foods individually or in combination can play an important role to overcome this problem. A clinical trial of black seed powder was conducted for 39 adult male patients with central obesity for the duration of three months with a dose of 1.5 gm daily. Significantly positive effects were observed in waist circumference, waist and hip ratio, body weight and also systolic blood pressure, while the reduction in serum testosterone, fasting blood sugar, triglyceride, diastolic blood pressure, uric acid, SGPT and SGOT levels were reduced appreciably. (Mathur et al., 2011; Najmi et al., 2008; Vanamala et al., 2012).
6.9. Immunomodulatory activity
The immunomodulatory effects are one of the most valuable properties of N. sativa. Active constituents of N. sativa augment the immunomodulatory properties through T cells and NK cells (El-Kadi and Kandil, 1986). Significant effects were shown with treatment of N. sativa oil in most of the participating subjects by a 55% increase in CD4 and CD8 T cell ratios and improving the function of NK cells (Haq et al., 1999). N. sativa oil has a strong potentiating effect on the cellular immunity mediated by T cells, whilst suppressor activity on immunity mediated by B cells has been reported by other constituents. N. sativa stimulatory properties on cellular immunity are linked to the nature of the immune response (Salem, 2005). The effects of N. sativa and TQ on the cellular and humoral immunity have been compared and documented in Table 3 . In vitro results of black seed soluble fractions on human peripheral blood mononuclear cells response to various mitogens were observed. It was found that major stimulatory results were not exhibited by components on peripheral blood mononuclear cells response to T cell mitogens phytohemagglutinin while components showed a stimulatory outcome on the peripheral blood mononuclear cells response to pooled allogeneic cells. Moreover, N. sativa fractionated proteins illustrated stimulatory activity in lymphocyte cultures (Haq et al., 1995; Salem, 2005).
Table 3.
Comparative studies of immunomodulatory activities of N. sativa Linn and Thymoquinone.
| Activity | N. sativa | Thymoquinone | Reference |
|---|---|---|---|
| Cellular immunity | Improvement of the proliferative capability of T lymphocytes and splenocytes | Improvement of number of circulating and thymus-homing CD4+ and CD8+ T lymphocytes |
(Badr et al., 2011; Majdalawieh et al., 2010; Swamy and Tan, 2000) |
| Increase IL-3 secretion from PBMCs | Increase IL-2 serum level | (Badr et al., 2011; Haq et al., 1995) | |
| Elevation and suppression of IL-8 secretion from PWM-activated and un-stimulated lymphocytes | Inhibition of cytokine secretion, (IL-10, IL-12, TNFα) and DC maturation survival | (Haq et al., 1999; Xuan et al., 2010) | |
| Enhanced CD4 + T cell count acts as therapeutic role against HIV infection | Suppression of IL-13 and IL-5 release by mast cells | (El Gazzar, 2007; Onifade et al., 2013) | |
| Stimulation of CD4 + T lymphocytes | Increase total leukocyte count, chemokine expression, phagocytic action, chemotaxis | (Onifade et al., 2013; Salem and Hossain, 2000) | |
| Increase peripheral lymphocyte and monocyte counts | Inhibition of DC survival, maturation, and cytokine secretion (IL-10, IL-12, TNFα) | (Fararh et al., 2004; Islam et al., 2004; Xuan et al., 2010) | |
| Humoral immunity | Decrease serum IgA, IgM levels. | Increase total immunoglobulin particularly IgGs levels as well as antibody Hemagglutination | (Ebaid et al., 2011; Mohany et al., 2012; Sapmaz et al., 2016) |
Oxidative stress is one of the most important causes of T cell-mediated autoimmune disorders of the central nervous system and is important for the progression of experimental allergic encephalomyelitis (EAE) (Majdalawieh and Fayyad, 2015; Mohamed et al., 2004). This can enhance severity upon astrocyte proliferation and infiltration of inflammatory cells. TQ expresses its valuable effects against allergic encephalomyelitis (Chakrabarty et al., 2003). It has been observed that TQ treatment significantly raises glutathione levels in red blood cells and acts as a detoxifying agent due to of its defensive activity against oxidative stress linked with reactive oxygen species (ROS), as well as leading to inhibition of the infiltration of mononuclear cells in the brain and spinal cord (Majdalawieh and Fayyad, 2015; Pompella et al., 2003).
6.10. Antihistaminic activity
Traditional use of N. sativa seeds and its active constituents have a significant value on the inflammatory disorders mediated through histamine. Nigellone in comparatively small concentrations exhibits excellent results to prevent the release of histamine encouraged by the secretagogues. The mechanism is expected to be through preventing calcium uptake and efflux stimulation, which can lead to a reduction in the concentration of calcium intracellularly. Furthermore, N. sativa crude extract shows beneficial calcium channel blocker properties against asthma, high blood pressure and diarrhea have been observed (Boskabady et al., 2004b; Chakravarty, 1993). Nigellone suppressed various symptoms of bronchial asthma when it was given orally to asthma patients. It has been used as a traditional medicine for different age groups without any notable toxicity. It has been proved in a clinical study that N. sativa exhibits significant activity to control allergic disorders through reducing the eosinophil count, IgE and endogenous cortisol levels in plasma and subsequently confirms it as an effective remedy for bronchial asthma, atopic eczema and allergic rhinitis (El-Dakhakhny, 1965).
Several pharmacological properties of N. sativa on tracheal chains have been demonstrated such as relaxant and functional antagonistic results on muscarinic receptors (Boskabady and Shahabi, 1997), the stimulation of an inhibitory effect on calcium channels (Boskabadi and Shirmohammadi, 2002), an effect on histamine (H1) receptors (Boskabady and Sheiravi, 2002)and the ability to open potassium channels (Boskabady et al., 2004a).
6.11. Anthelmintic activity
Several studies have shown that N. sativa and its essential oil have anthelmintic action against earthworms, tapeworms, nematodes and cestodes (Agarwal et al., 1979; Akhtar and Riffat, 1991). N. sativa induces oxidative stress against mature worms which was revealed by declining activities of an antioxidant enzymes, glutathione peroxidase and superoxide dismutase glutathione as well as hexokinase and glucose-6-phosphate dehydrogenase. This plays a key role in the control of the overgrowth of helminthes (Mohamed et al., 2005; Salem, 2005). It has been reported in an in vitro study of N. sativa seeds against S. mansoni, cercariae, miracidia and adult worms that they exhibit strong actions against the entire phases of the parasite as well as an inhibitory result on eggs. Black seeds reduce the action of glutathione peroxidase, glutathione reductase, superoxide dismutase and glucose metabolism enzymes, hexokinase and glucose-6-phosphate dehydrogenase (G6PD) and consequently exhibit oxidative stress to worms (Forouzanfar et al., 2014; Mohamed et al., 2005). Praziquantel is used as an anthelmintic medicine. It averts recently hatched insect larvae development in the body. N. sativa oil exhibits an additive effect in the treatment of schistosomiasis when it is given with praziquantel.
6.12. Anti-Infertility activity
A randomized double-blind placebo-controlled clinical trial was conducted whereby it was reported that 5 ml of N. sativa oil improved sperm count and motility, semen pH, semen volume, and its round cells after 2 months treatment of infertile men (Kolahdooz et al., 2014). It has been reported that 300 mg/kg b.w of N. sativa seeds taken for 60 days increased the number of spermatocytes, total sperm count and motility, weight of the reproductive organs and the number of mature leydig cells (Mohammad et al., 2009). N. sativa oil also demonstrated the ability to repair testicular degeneration, increase lipid peroxidation and abnormal sperms against sodium valproate testicular toxins. In relation to oxidative stress, TQ has exhibited some defensive roles such as superoxide anion scavengers, direct cytoprotective effects and androgen activities. Consequentially this leads to protect sperm and semen against testicular toxins. (Hala, 2011; Mahdavi et al., 2015).
6.13. Anti-nociceptive & Anti-inflammatory activity
N. sativa produces significant anti-nociceptive & anti-inflammatory properties. N. sativa oil and TQ exhibit antinociceptive properties through supraspinal opioid activation. It has recommended that N. sativa inhibits the generation of eicosanoids in lipid peroxidation and leukocytes. They inhibit 5-lipoxygenase (5-LOX) and cyclooxygenase (COX) pathways of arachidonic acid metabolism (Pise and Padwal, 2017). In the progression of inflammatory diseases, inflammatory factors such as eicosanoids and ROS play a significant role. The anti-inflammatory properties were widely searched in two major inflammatory disorders that include allergic encephalomyelitis and ulcerative colitis (Majdalawieh and Fayyad, 2015; Nieto et al., 2000). It has been found that TQ through anti-inflammatory and antioxidant properties significantly improve allergic encephalomyelitis and ulcerative colitis (Choudhary et al., 2001; Koch et al., 2000; Mahdavi et al., 2015).
6.14. Oral ulcerations and mucositis healing activity
Topical N. sativa oil was reported to have a good curative effect on the healing of chemically induced oral ulcers. Oral ulcers were treated with topical applications of N. sativa twice a day for 3 days. The results illustrated an important healing process with N. sativa treatment and a noticeable anti-inflammatory action (Muñoz‐Corcuera et al., 2009; Porter and Leao, 2005). Researchers confirmed that N. sativa oil enhanced the healing of ulcers and the development of pathogenic organisms at the ulcer site (Al-Douri and Al-Kazaz, 2010). In chemotherapy and radiotherapy there can be side effects of oral mucositis. In a research study, N. sativa has been shown to counteract the oral mucositis induced by chemotherapy and radiotherapy. Treatment with N. sativa reduced the histologically monitored cheek mucosal damage in mucositis (Lotfy and Zayed, 2009).
The wound healing effects of 4 aqueous extracts of traditional medicinal plants that include N. sativa, Pluchea indica, Melastoma malabathricum and Piper sarmentosum were reported in a study. It was observed that N. sativa extract exhibited a considerable improvement of human gingival fibroblasts proliferation compared with other extracts (Rahmani and Aly, 2015).
6.15. Nephroprotective activity
It has been reported in various studies that TQ demonstrates a key role in various pathogenic conditions of kidneys. TQ attenuates oxidative stress and inflammation by providing protection from various toxic agents (Benhelima et al., 2016). It reduces gentamicin-induced nephrotoxicity indexes and degenerative changes. TQ supplementation prevents acute renal failure induced by gentamicin by recovering mitochondrial function and enhancing the production of ATP. TQ during ifosfamide treatment improves the severity of ifosfamide-induced renal damage, phosphaturia, glucosuria, high serum creatinine level and urea and stabilise clearance rate of creatinine. Pyelonepheritis induced oxidative damage of the kidneys can be protected by TQ administration. Also the cisplatin antitumor action is potentiated by TQ as well as it protects against nephrotoxicity induced by cisplatin (Badary et al., 1997; Darakhshan et al., 2015; El Daly, 1997). It has also been reported that N. sativa shows a significant nephroprotective activity on paracetamol-induced nephrotoxicity (Canayakin et al., 2016).
6.16. Antiarthritic activity
It has been reported that the number of inflammatory joints and the extent of morning stiffness are improved by TQ. In a placebo-controlled study of 40 females with rheumatoid arthritis treated with N. sativa, TQ showed protective results against rheumatoid arthritis, and a decrease in arthritis scoring (disease activity score (DAS-28) and bone resorption were reported (Hadi et al., 2016).
7. Conclusion
Published reports clearly suggest that N. sativa is an important medicinal plant in the traditional system of medicine. The presence of alkaloids, coumarins, saponins, flavonoids, fixed oils and phenolics are responsible for the medicinal activity of N. sativa. Additionally, myristic acid, vitamins and some trace metals are also reported in the seeds of this plant which are add value to its medicinal properties. In conclusion, all the above findings strongly support the traditional uses of N. sativa. More clinical studies are needed to investigate the effectiveness of the plants pharmacological active constituents to overcome life threatening diseases such as acquired immunodeficiency syndrome (AIDS), various types of cancer, diabetes, cardiac disorders and the current corona virus (COVID-19) outbreak. Favorable effects can be achieved from the various forms of N. Sativa that include black seed, powder and oil and the various benefits of different preparations need to be researched. It is essential to state here that using N. Sativa does not require any prescription from a physician or approval from any health governing agency. It can be suggested to health professionals, as studies already show that N. sativa can be used in many diseases which are commonly affect people.
Declaration of Competing Interest
The authors declare no conflict of interest among them.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.hermed.2020.100404.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abd-El-Kader H., Seddek S., El-Shanawany A. In vitro study of the effect of some medicinal plants on the growth of some dermatophytes. Assiut Vet Med J. 1995;6(7):36–42. [Google Scholar]
- Abd-Elbaset M., Arafa E.-S.A., El Sherbiny G.A., Abdel-Bakky M.S., Elgendy A.N.A. Thymoquinone mitigate ischemia-reperfusion-induced liver injury in rats: a pivotal role of nitric oxide signaling pathway. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017;390(1):69–76. doi: 10.1007/s00210-016-1306-7. [DOI] [PubMed] [Google Scholar]
- Abdelmeguid N.E., Fakhoury R., Kamal S.M., Al Wafai R.J. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β‐cells of streptozotocin‐induced diabetic rats. J. Diabetes. 2010;2(4):256–266. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- Agarwal R., Kharya M., Shrivastava R. Antimicrobial & anthelmintic activities of the essential oil of Nigella sativa Linn. Indian J. Exp. Biol. 1979;17(11):1264–1265. [PubMed] [Google Scholar]
- Ahmad M.F. Ganoderma lucidum: persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018;107:507–519. doi: 10.1016/j.biopha.2018.08.036. [DOI] [PubMed] [Google Scholar]
- Ahmad M.F. Springer; 2019. Ganoderma lucidum: A Macro Fungus with Phytochemicals and Their Pharmacological Properties, Plant and Human Health, Volume 2; pp. 491–515. [Google Scholar]
- Ahmad M.F. Ganoderma lucidum: A rational pharmacological approach to surmount the cancer. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113047. [DOI] [PubMed] [Google Scholar]
- Ahmad M.F., Ahmad F.A., Azad A., Alam S., Ashraf A. Nutraceuticals is the need of hour. World J. Pharm. Pharm. Sci. 2013;2:2516–2525. [Google Scholar]
- Ahmad M.F., Ahmad F.A., Azad Z., Ahmad A., Alam M.I., Ansari J.A., Panda B.P. Edible mushrooms as health promoting agent. Adv. Sci. Focus. 2013;1(3):189–196. [Google Scholar]
- Ahmad M.F., Ashraf S.A., Ahmad F.A., Ansari J.A., Siddiquee M.R.A. Nutraceutical market and its regulation. Am. J. Food Technol. 2011;6(5):342–347. [Google Scholar]
- Akhtar M.S., Riffat S. Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children. J. Pak. Med. Assoc. 1991;41(8):185–187. [PubMed] [Google Scholar]
- Al-Douri A.S., Al-Kazaz S. The effect of Nigella Sativa oil (black seed) on the healing of chemically induced oral ulcer in rabbit (experimental study) Al–Rafidain Dent. J. 2010;10(1):151–157. [Google Scholar]
- Al-Majed A.A., Al-Omar F.A., Nagi M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006;543(1):40–47. doi: 10.1016/j.ejphar.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Al-Qurashi A.R., Akhtar N., Al-Jabre S., AL-Akloby O., Randhawa M.A. Anti-fungal activity of thymoquinone and amphotericine B against Aspergillus Niger. Saudi J. KFU. 2007;8(1):143–149. [Google Scholar]
- Al Attas S.A., Fat’heya M.Z., Turkistany S.A. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med. J. 2016;37(3):235–244. doi: 10.15537/smj.2016.3.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- Aljabre S.H., Alakloby O.M., Randhawa M.A. Dermatological effects of Nigella sativa. Jof Dermatol. Dermat. Surg. 2015;19(2):92–98. [Google Scholar]
- Aljabre S.H.M., Randhawa M.A., Akhtar N., Alakloby O.M., Alqurashi A.M., Aldossary A. Antidermatophyte activity of ether extract of Nigella sativa and its active principle, thymoquinone. J. Ethnopharmacol. 2005;101(1):116–119. doi: 10.1016/j.jep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Almoyad M. 2018. Synergism from combination of targeted therapy and phytochemicals in colorectal cancer. [Google Scholar]
- Almshawit H., Macreadie I. Fungicidal effect of thymoquinone involves generation of oxidative stress in Candida glabrata. Microbiol. Res. 2017;195:81–88. doi: 10.1016/j.micres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Alsaidy D. Isolate, diagnosis and treatment of yeast Candida albicans accompanying the human body. IJARES. 2014;2(1):1081–1086. [Google Scholar]
- Asgary S., Ghannadi A., Dashti G., Helalat A., Sahebkar A., Najafi S. Nigella sativa L. improves lipid profile and prevents atherosclerosis: Evidence from an experimental study on hypercholesterolemic rabbits. J. Funct. Foods. 2013;5(1):228–234. [Google Scholar]
- Ashraf S.S., Rao M.V., Kaneez F.S., Qadri S., Al-Marzouqi A.H., Chandranath I.S., Adem A. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J. Chromatogr. Sci. 2011;49(4):321–326. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- Awad A.S., Kamel R., Sherief M.A.E. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N‐1‐acetyl‐transferase gene expression induced by renal ischaemia–reperfusion in rats. J. Pharm. Pharmacol. 2011;63(8):1037–1042. doi: 10.1111/j.2042-7158.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- Badary O.A., Gamal E.-D.A. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect. Prev. 2000;25(4):362–368. [PubMed] [Google Scholar]
- Badary O.A., Nagi M.N., Al-Shabanah O.A., Al-Sawaf H.A., Al-Sohaibani M.O., Al-Bekairi A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Physiol. Pharmacol. 1997;75(12):1356–1361. [PubMed] [Google Scholar]
- Badary O.A., Taha R.A., Gamal El-Din A.M., Abdel-Wahab M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003;26(2):87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- Bamosa A.O., Kaatabi H., Lebdaa F.M., Elq A., Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010;54(4):344–354. [PubMed] [Google Scholar]
- Barakat E.M.F., El Wakeel L.M., Hagag R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J. Gastroenterol.: WJG. 2013;19(16):2529. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhelima A., Kaid-Omar Z., Hemida H., Benmahdi T., Addou A. Nephroprotective and diuretic effect of nigella sativa l seeds oil on lithiasic wistar rats. Afr. J. Tradit. Complement. Altern. Med. 2016;13(6):204–214. doi: 10.21010/ajtcam.v13i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabadi M., Shirmohammadi B. 2002. Effect of Nigella Sativa on Isolated Guinea PIG TRAChea. [Google Scholar]
- Boskabady M., Shafei M., Parsaee H. Effects of aqueous and macerated extracts from Nigella sativa on guinea pig isolated heart activity. Pharmazie. 2005;60(12):943–948. [PubMed] [Google Scholar]
- Boskabady M., Shahabi M. Bronchodilatory and anticholinergic effects of Nigella sativa on isolated guinea-pig tracheal chains. Iran. J. Med. Sci. 1997;22:127–133. [Google Scholar]
- Boskabady M., Sheiravi N. Inhibitory effect of Nigella sativa on histamine (H1) receptors of isolated guinea pig tracheal chains. Pharml. Biol. 2002;40(8):596–602. [Google Scholar]
- Boskabady M.H., Shirmohammadi B., Jandaghi P., Kiani S. Possible mechanism (s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4(1):1–6. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M.H., Shirmohammadi B., Jandaghi P., Kiani S. Possible mechanism (s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4(1):3. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R.N., Goa K.L. Flumazenil. Drugs. 1988;35(4):448–467. doi: 10.2165/00003495-198835040-00004. [DOI] [PubMed] [Google Scholar]
- Canayakin D., Bayir Y., Kilic Baygutalp N., Sezen Karaoglan E., Atmaca H.T., Kocak Ozgeris F.B., Keles M.S., Halici Z. Paracetamol-induced nephrotoxicity and oxidative stress in rats: the protective role of Nigella sativa. Pharml. Biol. 2016;54(10):2082–2091. doi: 10.3109/13880209.2016.1145701. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A., Emerson M., LeVine S. Hemeoxygenase-1 in SJL mice with experimental allergic encephalomyelitis. Mult. Scler.. J. 2003;9(4):372–381. doi: 10.1191/1352458503ms928oa. [DOI] [PubMed] [Google Scholar]
- Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann. Allergy. 1993;70(3):237–242. [PubMed] [Google Scholar]
- Chehl N., Chipitsyna G., Gong Q., Yeo C.J., Arafat H.A. Anti‐inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB. 2009;11(5):373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S., Keshavarzian A., Yong S., Wade M., Bocckino S., Day B., Banan A. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig. Dis. Sci. 2001;46(10):2222–2230. doi: 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]
- D’Souza T., Mengi S., Hassarajani S., Chattopadhayay S. Efficacy study of the bioactive fraction (F-3) of Acorus calamus in hyperlipidemia. Indian J. Pharmacol. 2007;39(4):196–200. [Google Scholar]
- Darakhshan S., Pour A.B., Colagar A.H., Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015;95:138–158. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Dehkordi F.R., Kamkhah A.F. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam. Clin. Pharmacol. 2008;22(4):447–452. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Doudi M., Setorki M., Hoveyda L. Comparing the antifungal effects of five essential oils plants eucalyptus, cinnamon, wormwood, sagebrush and iranian rose damascena on three standard strains of candida albicans in vitro. IJBPAS. 2014;3:490–500. [Google Scholar]
- Durmuskahya C., Ozturk M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malays. 2013;42(10):1431–1438. [Google Scholar]
- El-Dakhakhny M. Studies on the Egyptian Nigella sativa L. part IV: some pharmacological properties of the seed’s active principle in comparison to its dihydro compound and its polymer. Arzneim. Forsch. 1965;15:1227–1229. [PubMed] [Google Scholar]
- El-Dakhakhny M., Barakat M., El-Halim M.A., Aly S. Effects of Nigella sativa oil on gastric secretion and ethanol induced ulcer in rats. J. Ethnopharmacol. 2000;72(1):299–304. doi: 10.1016/s0378-8741(00)00235-x. [DOI] [PubMed] [Google Scholar]
- El-Kadi A., Kandil O. 1986. Effect of Nigella sativa (the black seed) on immunity, Proceeding of the 4th International Conference on Islamic Medicine, Kuwait. Bull Islamic Med; pp. 344–348. [Google Scholar]
- El-Taher K., Al-Ajmi M., Al-Bekairi A. Some cardiovascular effects of the dethymoquinonated Nigella sativa volatile oil and its major components alpha-pinene and p-cymene in rats. Sadui Pharm. J. 2003;11(3):104–110. [Google Scholar]
- El Daly E.S. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J. Pharm. Belg. 1997;53(2):87–93. discussion 93-85. [PubMed] [Google Scholar]
- El Tahir K.E., Ageel A. Effect of the volatile oil of (Nigella sativa) on the arterial blood pressure and heart rate of the guinea-pig. Sadui Pharm. J. 1994;2(4):163–168. [Google Scholar]
- El Tahir K.E., Ashour M.M., Al-Harbi M.M. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen. Pharmacol. 1993;24(5):1123–1131. doi: 10.1016/0306-3623(93)90359-6. [DOI] [PubMed] [Google Scholar]
- Erol B., Sari U., Amasyali A., Ozkanli S., Sogut S., Hanci V., Efiloglu O., Danacioglu Y., Engin P., Yencilek F. Comparison of combined antioxidants and thymoquinone in the prevention of testis ischemia–reperfusion injury. Andrology. 2017;5(1):119–124. doi: 10.1111/andr.12268. [DOI] [PubMed] [Google Scholar]
- Evans P. Free radicals in brain metabolism and pathology. Br. Med. Bull. 1993;49(3):577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- Fallah Huseini H., Amini M., Mohtashami R., Ghamarchehre M., Sadeqhi Z., Kianbakht S., Fallah Huseini A. Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double‐blind, placebo‐controlled clinical trial. Phytother. Res. 2013;27(12):1849–1853. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- File S.E., Pellow S. Intrinsic actions of the benzodiazepine receptor antagonist Ro 15-1788. Psychopharmacology. 1986;88(1):1–11. doi: 10.1007/BF00310505. [DOI] [PubMed] [Google Scholar]
- Forouzanfar F., Bazzaz B.S.F., Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran. J. Basic Med. Sci. 2014;17(12):929. [PMC free article] [PubMed] [Google Scholar]
- Gholamnezhad Z., Havakhah S., Boskabady M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: a review. J. Ethnopharmacol. 2016;190:372–386. doi: 10.1016/j.jep.2016.06.061. [DOI] [PubMed] [Google Scholar]
- Gilani A.-u.H., Jabeen Q., Khan M.A.U. A review of medicinal uses and pharmacological activities of Nigella sativa. Pak. J. Biol. Sci. 2004;7(4):441–445. [Google Scholar]
- Goel S., Mishra P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018;102(4):1955–1967. doi: 10.1007/s00253-018-8736-8. [DOI] [PubMed] [Google Scholar]
- Greenish H. Contribution to the chemistry of Nigella sativa. Pharmac. J. Trans. 1880;10:909–911. [Google Scholar]
- Hadi V., Kheirouri S., Alizadeh M., Khabbazi A., Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. AJP. 2016;6(1):34. [PMC free article] [PubMed] [Google Scholar]
- Hala M. Protective effect of Nigella sativa, linseed and celery oils against testicular toxicity induced by sodium valproate in male rats. J. Am. Sci. 2011;7(5):687–693. [Google Scholar]
- Hamed Ahmad Alobaidi A. Effect of Nigella sativa and Allium sativum coadminstered with simvastatin in dyslipidemia patients: a prospective, randomized, double-blind trial. Anti-inflam. Anti-Allergy Agent Med. Chem. 2014;13(1):68–74. doi: 10.2174/18715230113129990013. [DOI] [PubMed] [Google Scholar]
- Hanafy M., Hatem M. Studies on the antimicrobial activity of Nigella sativa seed (black cumin) J. Ethnopharmacol. 1991;34(2–3):275–278. doi: 10.1016/0378-8741(91)90047-h. [DOI] [PubMed] [Google Scholar]
- Haq A., Abdullatif M., Lobo P.I., Khabar K.S., Sheth K.V., Al-Sedairy S.T. Nigella sativa: effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 1995;30(2):147–155. doi: 10.1016/0162-3109(95)00016-m. [DOI] [PubMed] [Google Scholar]
- Haq A., Lobo P.I., Al-Tufail M., Rama N.R., Al-Sedairy S.T. Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int. J. Immunopharmacol. 1999;21(4):283–295. doi: 10.1016/s0192-0561(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Hassanein K.M., Al-Emam A., Radad K. Prophylactic effects of thymoquinone against carbon tetrachloride-induced hepatic damage in Sprague-Dawley rats. J. Appl. Pharm. Sci. 2016;6(02):167–171. [Google Scholar]
- Heshmati J., Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Complem. Ther. Med. 2015;23(2):275–282. doi: 10.1016/j.ctim.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Houcher Z., Boudiaf K., Benboubetra M., Houcher B. Effects of methanolic extract and commercial oil of Nigella sativa L. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines. 2007;18(1):8–18. [Google Scholar]
- Houghton P.J., Zarka R., de las Heras B., Hoult J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61(01):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- Ibrahim R.M., Hamdan N.S., Mahmud R., Imam M.U., Saini S.M., Rashid S.N.A., Ghafar S.A.A., Ab Latiff L., Ismail M. A randomised controlled trial on hypolipidemic effects of Nigella Sativa seeds powder in menopausal women. J. Transl. Med. 2014;12(1):1–7. doi: 10.1186/1479-5876-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris-Khodja N., Schini-Kerth V. Thymoquinone improves aging-related endothelial dysfunction in the rat mesenteric artery. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385(7):749–758. doi: 10.1007/s00210-012-0749-8. [DOI] [PubMed] [Google Scholar]
- Joshi S.C., Sharma M., Jain S. Hypolipidemic effects of Myristica fragrans seeds in cholesterol fed rabbits, Botanical product’s seminar and expo. Jaipur. 2005:140–143. [Google Scholar]
- Kaleem M., Kirmani D., Asif M., Ahmed Q., Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J. Exp. Biol. 2006;44:745–748. [PubMed] [Google Scholar]
- Kanter M. Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem. Res. 2008;33(1):87–96. doi: 10.1007/s11064-007-9419-5. [DOI] [PubMed] [Google Scholar]
- Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch. Toxicol. 2006;80(4):217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- Khader M., Eckl P., Bresgen N. Effects of aqueous extracts of medicinal plants on MNNG-treated rat hepatocytes in primary cultures. J. Ethnopharmacol. 2007;112(1):199–202. doi: 10.1016/j.jep.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Khalife K., Lupidi G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic. Res. 2007;41(2):153–161. doi: 10.1080/10715760600978815. [DOI] [PubMed] [Google Scholar]
- Khan A., Chen H., Tania M., Zhang D. Anticancer activities of Nigella sativa (black cumin) Afr. J. Tradit. Complement. Altern. Med. 2011;8(5S):226–232. doi: 10.4314/ajtcam.v8i5S.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Ashfaq M., Zuberi H., Mahmood M., Gilani A. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother. Res. 2003;17(2):183–186. doi: 10.1002/ptr.1146. [DOI] [PubMed] [Google Scholar]
- Khan M.A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacol. 1999;7(1):15–35. doi: 10.1007/s10787-999-0023-y. [DOI] [PubMed] [Google Scholar]
- Koch T.R., Yuan L.-X., Stryker S.J., Ratliff P., Telford G.L., Opara E.C. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis. Sci. 2000;45(9):1814–1819. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- Kolahdooz M., Nasri S., Modarres S.Z., Kianbakht S., Huseini H.F. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2014;21(6):901–905. doi: 10.1016/j.phymed.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Kotb A.M., Abd-Elkareem M., Khalil N.S.A., Sayed A.E.-D.H. Protective effect of Nigella sativa on 4-nonylphenol-induced nephrotoxicity in Clarias gariepinus (Burchell, 1822) Sci. Total Environ. 2018;619:692–699. doi: 10.1016/j.scitotenv.2017.11.131. [DOI] [PubMed] [Google Scholar]
- Laskar A.A., Khan M.A., Rahmani A.H., Fatima S., Younus H. Thymoquinone, an active constituent of Nigella sativa seeds, binds with bilirubin and protects mice from hyperbilirubinemia and cyclophosphamide-induced hepatotoxicity. Biochimie. 2016;127:205–213. doi: 10.1016/j.biochi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Leong X.-F., Rais Mustafa M., Jaarin K. Nigella sativa and its protective role in oxidative stress and hypertension. eCAM. 2013;2013 doi: 10.1155/2013/120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfy A., Zayed M. Immunohistochemical study of the effect of nigella sativa L extract on chemotherapy induced oral mucositis in albino rats. Cairo Dental J. 2009;25(2):159–166. [Google Scholar]
- Mabrouk G., Moselhy S., Zohny S., Ali E., Helal T., Amin A., Khalifa A. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J. Exp. Clin. Cancer Res.: CR. 2002;21(3):341–346. [PubMed] [Google Scholar]
- MacDonald R.L., Barker J.L. Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature. 1977;267(5613):720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- Magdy M.-A., Hanan E.-A., Nabila E.-M. Thymoquinone: Novel gastroprotective mechanisms. Eur. J. Pharmacol. 2012;697(1):126–131. doi: 10.1016/j.ejphar.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Mahdavi R., Heshmati J., Namazi N. Effects of black seeds (Nigella sativa) on male infertility: a systematic review. J. Herb. Med. 2015;5(3):133–139. [Google Scholar]
- Majdalawieh A.F., Fayyad M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int. Immunopharmacol. 2015;28(1):295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Fayyad M.W., Nasrallah G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017 doi: 10.1080/10408398.2016.1277971. [DOI] [PubMed] [Google Scholar]
- Mansi K.M.S. Effects of Oral Administration of Water Extract of Nigella sativa on Serum Concentrations of. Pak. J. Biol. Sci. 2005;8(8):1152–1156. [Google Scholar]
- Mansour M.A. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66(26):2583–2591. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- Mansour M.A., Nagi M.N., El‐Khatib A.S., Al‐Bekairi A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT‐diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem. Funct. 2002;20(2):143–151. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- Mashhadian N.V., Rakhshandeh H. Antibacterial and antifungal effects of Nigella sativa extracts against S. aureus, P. aeruginosa and C. albicans. Pak. J. Med. Sci. 2005;21(1):47–52. [Google Scholar]
- Mathur M.L., Gaur J., Sharma R., Haldiya K.R. Antidiabetic properties of a spice plant Nigella sativa. J. Endocrinol. Metab. 2011;1(1):1–8. [Google Scholar]
- Meral I., Yener Z., Kahraman T., Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti‐oxidant defence system and liver damage in experimentally‐induced diabetic rabbits. J. Vet. Med. A. 2001;48(10):593–599. doi: 10.1046/j.1439-0442.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- Moghim H., Taghipoor S., Shahinfard N., Kheiri S., Panahi R. Antifungal effects of Zataria multifora and Nigella sativa extracts against Candida albicans. J. HerbMed. Pharmacol. 2015;4:138–141. [Google Scholar]
- Mohamed A., Afridi D., Garani O., Tucci M. Thymoquinone inhibits the activation of NF-kappaB in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed. Sci. Instrum. 2004;41:388–393. [PubMed] [Google Scholar]
- Mohamed A., Shoker A., Bendjelloul F., Mare A., Alzrigh M., Benghuzzi H., Desin T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed. Sci. Instrum. 2002;39:440–445. [PubMed] [Google Scholar]
- Mohamed A.M., Metwally N.M., Mahmoud S.S. Sativa seeds against Schistosoma mansoni different stages. Mem. Inst. Oswaldo Cruz. 2005;100(2):205–211. doi: 10.1590/s0074-02762005000200016. [DOI] [PubMed] [Google Scholar]
- Mohammad M.A., Mohamad M.M., Dradka H. Effects of black seeds (Nigella sativa) on spermatogenesis and fertility of male albino rats. Res. J. Med. Med. Sci. 2009;4(2):386–390. [Google Scholar]
- Morsi N.M. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol. Pol. 2000;49(1):63–74. [PubMed] [Google Scholar]
- Muñoz‐Corcuera M., Esparza‐Gómez G., González‐Moles M., Bascones‐Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part II. Chronic ulcers. Clin. Exp. Dermatol. 2009;34(4):456–461. doi: 10.1111/j.1365-2230.2009.03219.x. [DOI] [PubMed] [Google Scholar]
- Nagi M.N., Alam K., Badary O.A., Al‐Shabanah O.A., Al‐Sawaf H.A., Al‐Bekairi A.M. Thymoquinone protects against carbon tetrachloride hetatotoxicity in mice via an antioxidant mechanism. IUBMB Life. 1999;47(1):153–159. doi: 10.1080/15216549900201153. [DOI] [PubMed] [Google Scholar]
- Nagi M.N., Almakki H.A. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytother. Res. 2009;23(9):1295–1298. doi: 10.1002/ptr.2766. [DOI] [PubMed] [Google Scholar]
- Nagi M.N., Mansour M.A. Protective effect of thymoquinone against doxorubicin–induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol. Res. 2000;41(3):283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- Najmi A., Nasiruddin M., Khan R.A., Haque S.F. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int. J. Diabetes Dev. Countries. 2008;28(1):11. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir A., Siddiqui M., Mohsin M. Therapeutic uses of Shoneez (Nigella sativa Linn.) mentioned in Unani system of medicine-a review. Int. J. Pharm. Phytopharmaco Res. 2014;4:47–49. [Google Scholar]
- Naz H. Nigella sativa: the miraculous herb. Pak. J. Biochem. Mol. Biol. 2011;44(1):44–48. [Google Scholar]
- Nieto N., Torres M., Fernandez M., Giron M., Rios A., Suarez M., Gil A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig. Dis. Sci. 2000;45(9):1820–1827. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- Pise H.N., Padwal S.L. Evaluation of anti-inflammatory activity of Nigella sativa: an experimental study. Natl. J. Physiol. Pharm. Pharmacol. 2017;7(7):707. [Google Scholar]
- Pompella A., Visvikis A., Paolicchi A., De Tata V., Casini A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003;66(8):1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Porter S., Leao J. Review article: oral ulcers and its relevance to systemic disorders. Aliment. Pharmacol. Ther. 2005;21(4):295–306. doi: 10.1111/j.1365-2036.2005.02333.x. [DOI] [PubMed] [Google Scholar]
- Rafati S., Niakan M., Naseri M. Anti-microbial effect of Nigella sativa seed extract against staphylococcal skin Infection. Med. J. Islam. Repub. Iran. 2014;28:42. [PMC free article] [PubMed] [Google Scholar]
- Rahmani A.H., Aly S.M. Nigella Sativa and its active constituents thymoquinone shows pivotal role in the diseases prevention and treatment. Asian J. Pharm. Clin. Res. 2015;8(1):48–53. [Google Scholar]
- Rajkapoor B., Anandan R., Jayakar B. Antiulcer effect of Nigella sativa Linn. against gastric ulcer in rats. Curr. Sci. 2002;82:177–179. [Google Scholar]
- Rajsekhar S., Kuldeep B. Pharmacognosy and pharmacology of Nigella sativa-a review. Int. Res. J. Pharm. 2011;2(11):36–39. [Google Scholar]
- Randhawa M.A., Alakloby O.M., Aljabre S.H.M., Alqurashi A.M., Akhtar N. Thymoquinone, an active principle of Nigella sativa, inhibited Fusarium solani. Pak. J. Med. Res. 2005;44(1):1–3. [Google Scholar]
- Razavi B., Hosseinzadeh H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J. Endocrinol. Invest. 2014;37(11):1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- Rchid H., Chevassus H., Nmila R., Guiral C., Petit P., Chokaïri M., Sauvaire Y. Nigella sativa seed extracts enhance glucose‐induced insulin release from rat‐isolated Langerhans islets. Fundam. Clin. Pharmacol. 2004;18(5):525–529. doi: 10.1111/j.1472-8206.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Rehavi M., Skolnick P., Paul S.M. Effects of tetrazole derivatives on [3H] diazepam binding in vitro: correlation with convulsant potency. Eur. J. Pharmacol. 1982;78(3):353–356. doi: 10.1016/0014-2999(82)90037-1. [DOI] [PubMed] [Google Scholar]
- Rifat-uz-Zaman M.S.A., Khan M.S. Gastroprotective and anti-secretory effect of Nigella sativa seed and its extracts in indomethacin-treated rats. Pak. J. Biol. Sci. 2004;7:995–1000. [Google Scholar]
- Rodríguez M.C., Parra M.D., Marques-Lopes I., De Morentin B.E.M., González A., Martínez J.A. Effects of two energy-restricted diets containing different fruit amounts on body weight loss and macronutrient oxidation. Plant Foods Hum. Nutr. 2005;60(4):219–224. doi: 10.1007/s11130-005-8622-2. [DOI] [PubMed] [Google Scholar]
- Sahak M.K.A., Kabir N., Abbas G., Draman S., Hashim N.H., Hasan Adli D.S. The role of Nigella sativa and its active constituents in learning and memory. eCAM. 2016;2016 doi: 10.1155/2016/6075679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheb S.H., Desai S., Das K.K., Haseena S. Hepatoprotective effect of nigella sativa seed in sterptozotocine induced diabetic albino rats: Histological observations. IJAR. 2016;4(2):2459–2463. [Google Scholar]
- Salama R.H.M. Hypoglycemic effect of lipoic acid, carnitine and Nigella sativa in diabetic rat model. Int. J. Health Sci. 2011;5(2):126. [PMC free article] [PubMed] [Google Scholar]
- Salem E.M., Yar T., Bamosa A.O., Al-Quorain A., Yasawy M.I., Alsulaiman R.M., Randhawa M.A. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter pylori in patients with non-ulcer dyspepsia. Saudi J. Gastroenterol. 2010;16(3):207. doi: 10.4103/1319-3767.65201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005;5(13–14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salman M.T., Khan R.A., Shukla I. Antimicrobial activity of Nigella sativa Linn. seed oilagainst multi-drug resistant bacteria from clinical isolates. 38th Annual Conference of Indian Pharmacological Society. Chennai. 2008 [Google Scholar]
- Sayeed M.S.B., Asaduzzaman M., Morshed H., Hossain M.M., Kadir M.F., Rahman M.R. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J. Ethnopharmacol. 2013;148(3):780–786. doi: 10.1016/j.jep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Shaarani S., Hamid S.S., Kaus N.H.M. The Influence of pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of thymoquinone drug. Pharmacognosy Res. 2017;9(1):12–20. doi: 10.4103/0974-8490.199774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.H. 1966. The general principles of Avicenna’s Canon of Medicine. Naveed Clinic. [Google Scholar]
- Sharma P., Yelne M., Dennis T., Joshi A. 2001. Database on Medicinal Plants Used in Ayurveda & Siddha. Central Council for Research in Ayurveda & Siddha, Deptt. of ISM & H, Min. of Health & Family Welfare, Government of India. [Google Scholar]
- Shoieb A.M., Elgayyar M., Dudrick P.S., Bell J.L., Tithof P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int. J. Oncol. 2003;22(1):107–114. [PubMed] [Google Scholar]
- Sultan M.T., Butt M.S., Ahmad R.S., Pasha I., Ahmad A.N., Qayyum M. Supplementation of Nigella sativa fixed and essential oil mediates potassium bromate induced oxidative stress and multiple organ toxicity. Pak. J. Pharm. Sci. 2012;25(1):175–181. [PubMed] [Google Scholar]
- Sultan M.T., Butt M.S., Karim R., Zia-Ul-Haq M., Batool R., Ahmad S., Aliberti L., De Feo V. Nigella sativa fixed and essential oil supplementation modulates hyperglycemia and allied complications in streptozotocin-induced diabetes mellitus. Evid.-Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/826380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M., Azeiz A., Saudi W. Antifungal effect of thymol, thymoquinone and thymohydroquinone against yeasts, dermatophytes and non-dermatophyte molds isolated from skin and nails fungal infections. Egypt. J. Biochem. Mol. Biol. 2010;28(2):119–126. [Google Scholar]
- Tanaka J., Yuda Y., Inouye S., Yamakawa T. The role of nitric oxide in the gastric acid secretion induced by ischemia–reperfusion in the pylorus-ligated rat. Eur. J. Pharmacol. 2001;424(1):69–74. doi: 10.1016/s0014-2999(01)01119-0. [DOI] [PubMed] [Google Scholar]
- Tasar N., Şehirli Ö., Yiğiner Ö., Süleymanoğlu S., Yüksel M., Yeğen B., Şener G. Protective effects of Nigella sativa against hypertension-induced oxidative stress and cardiovascular dysfunction in rats. Marmara Pharm. J. 2012;16(2):141–149. [Google Scholar]
- Tavakkoli A., Ahmadi A., Razavi B.M., Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities (suppl. 2017) Iran. J. Pharm. Re.s. 2017;16:2–23. [PMC free article] [PubMed] [Google Scholar]
- Tembhurne S., Feroz S., More B., Sakarkar D. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J. Med. Plant Res. 2014;8(3):167–177. [Google Scholar]
- Thabrew M.I., Mitry R.R., Morsy M.A., Hughes R.D. Cytotoxic effects of a decoction of Nigella sativa, Hemidesmus indicus and Smilax glabra on human hepatoma HepG2 cells. Life Sci. 2005;77(12):1319–1330. doi: 10.1016/j.lfs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Torres M.P., Ponnusamy M.P., Chakraborty S., Smith L.M., Das S., Arafat H.A., Batra S.K. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol. Cancer Biol. 2010;9(5):1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanamala J., Kester A.C., Heuberger A.L., Reddivari L. Mitigation of obesity-promoted diseases by Nigella sativa and thymoquinone. Plant Foods Hum. Nutr. 2012;67(2):111–119. doi: 10.1007/s11130-012-0279-z. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P.M., Shimokawa H., Tang E.H., Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Yi T., Cho S.-G., Yi Z., Pang X., Rodriguez M., Wang Y., Sethi G., Aggarwal B.B., Liu M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Biol. 2008;7(7):1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaoui A., Cherrah Y., Lacaille-Dubois M., Settaf A., Amarouch H., Hassar M. Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat. Therapie. 1999;55(3):379–382. [PubMed] [Google Scholar]
- Ziaee T., Moharreri N., Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. J. Med. Plants Res. 2012;2(42):16–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.