Abstract
Background
Nucleic acid amplification tests (NAATs) are widely used to diagnose tuberculosis (TB), but cannot discriminate live bacilli from dead bacilli. Live bacilli can be isolated by culture methods, but this is time-consuming. We developed a de novo TB diagnostic method that detects only live bacilli with high sensitivity within hours.
Methods
A prospective study was performed in Taiwan from 2017 to 2018. Sputum was collected consecutively from 1102 patients with suspected TB infection. The sputum was pretreated and heated at 46°C for 1 h to induce the secretion of MPT64 protein from live Mycobacterium tuberculosis. MPT64 was detected with our ultrasensitive enzyme-linked immunosorbent assay (ELISA) coupled with thionicotinamide-adenine dinucleotide (thio-NAD) cycling. We compared our data with those obtained using a culture test (MGIT), a smear test (Kinyoun staining), and a NAAT (Xpert).
Findings
The limit of detection for MPT64 in our culture-free ultrasensitive ELISA was 2.0 × 10−19 moles/assay. When the criterion for a positive response was set as an absorbance value ≥17 mAbs, this value corresponded to ca. 330 CFU/mL in the culture method – almost the same high-detection sensitivity as the culture method. To confirm that MPT64 is secreted from only live bacilli, M. bovis BCG was killed using 8 μg/mL rifampicin and then heated. Following this procedure, our method detected no MPT64. Our rapid ultra-sensitive ELISA-based method required only 5 h to complete. Comparing the results of our method with those of culture tests for 944 specimens revealed a sensitivity of 86.9% (93/107, 95% CI: 79.0–92.7%) and a specificity of 92.0% (770/837, 95% CI: 89.9–93.7%). The performance data were not significantly different (McNemar's test, P = 0.887) from those of the Xpert tests. In addition, at a ≥1+ titer in the smear test, the positive predictive value of our culture-free ultrasensitive ELISA tests was in a good agreement with that of the culture tests. Furthermore, our culture-free ultrasensitive ELISA test had better validity for drug effectiveness examination than Xpert tests because our test detected only live bacilli.
Interpretation
Our culture-free ultrasensitive ELISA method detects only live TB bacilli with high sensitivity within hours, allowing for rapid diagnosis of TB and monitoring drug efficacy.
Funding
Matching Planner Program from JST (VP29117939087), the A-STEP Program from JST (AS3015096U), Waseda University grants for Specific Research Projects (2017A-015 and 2019C-123), the Precise Measurement Technology Promotion Foundation to E.I.
Keywords: Mycobacterium tuberculosis, ELISA, thio-NAD cycling, MPT64, live bacilli detection
Research in context.
Evidence before this study
Early and accurate diagnosis of infectious diseases is required to stop their spread and increase the chances for successful treatment. For tuberculosis (TB), even now the traditional time-consuming culture method remains the “gold standard” for testing by physicians, although the World Health Organization (WHO) recommends the use a nucleic acid amplification test (NAAT), the Xpert MTB/RIF, for diagnosing TB and rifampicin resistance. The detection sensitivity of NAATs is high and the detection time short (Rapid Communication from WHO in January, 2020). The main concern regarding NAATs, however, is that they detect nucleic acids, which are present in both live and dead bacilli specimens, and this may lead to false positive results in TB patients treated with anti-TB drugs. To confirm the infectiousness, physicians must still use a culture method for TB diagnosis, but this takes a long time. Acid-fast bacilli staining is convenient and does not take much time, but the detection specificity is low due to the interference of nontuberculous mycobacteria. Therefore, a culture-free, same-day diagnostic test for TB that detects only live bacilli with high sensitivity is strongly required.
Added value of this study
When the sputum of TB patients is heated at 46°C for 1 h, a specific protein, MPT64, is secreted from live Mycobacterium tuberculosis. Therefore, we applied a new ultrasensitive ELISA coupled with thio-NAD cycling to detect the trace amount of MPT64. When the criterion for positive responses in our culture-free ultrasensitive ELISA was set to the same detection sensitivity as that of the culture method, our method succeeded in detecting live M. tuberculosis within 5 h. We then conducted a study to compare the results of our culture-free ultrasensitive ELISA with those of culture tests for 944 specimens; the sensitivity was 86.9% and the specificity was 92.0%. At a smear test titer of ≥ 1+, the positive predictive value of our culture-free ultrasensitive ELISA tests was in good agreement with that of the culture tests. Our culture-free ultrasensitive ELISA for TB diagnosis revealed the same detection sensitivity as the culture method, but it enabled us to diagnose TB on the same day.
Implications of all the available evidence
Our culture-free ELISA for MPT64 can be used not only for an initial diagnosis, but also to check for drug effectiveness and drug resistance when treating TB patients. If anti-TB drugs are administered to patients for a few weeks, but MPT64 is detected with our method, physicians would be alerted to the possibility of resistance to the TB regimen and then a further drug susceptibility testing is warranted. If anti-TB drugs are effective and MPT64 is not detected in the sputum from a TB patient using our method, the doctor has a chance to decide on the same day to discontinue isolation for TB. Our culture-free, same-day diagnosis for TB can be useful in several situations.
Alt-text: Unlabelled box
1. Introduction
Culture methods remain the “gold standard” for diagnosing tuberculosis (TB), even now in the 21st century [1]. Culture methods can be used to isolate live Mycobacterium tuberculosis, and are more sensitive than acid-fast bacilli (AFB) staining (e.g., Kinyoun/Ziehl-Neelsen staining), which detects both live and dead bacilli, and culture methods can reliably detect mycobacteria present at a concentration of about 100–1000 colony forming units (CFU)/mL of specimen [2,3]. Growing cultures also permit species identification and drug effectiveness testing [4]. The major drawback of culture techniques, however, is that it takes weeks to more than a month before a positive culture for M. tuberculosis can be identified, and the methods require at least a moderately well-equipped laboratory [5].
On the other hand, nucleic acid amplification tests (NAATs) including the Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA), Amplicor Mycobacterium Tuberculosis Test (Roche, Basel, Switzerland), and Loop-Mediated Isothermal Amplification (LAMP; Eiken Chemical, Tokyo, Japan), are now widely used, even in low- and middle-income countries due to financial assistance from various global foundations [6], [7], [8]. It is estimated that NAATs can detect M. tuberculosis in suspensions containing as few as 10–1000 CFU/mL, making this technology highly sensitive [9,10]. These methods require only a few hours to provide a TB diagnosis, but they also have crucial drawbacks. NAATs detect both live and dead M. tuberculosis bacilli [11,12], making them inappropriate for evaluating the effectiveness of the infection to anti-TB drugs (although the Xpert MTB/RIF detects resistance to rifampicin) [13]. Because live and dead bacilli cannot be discriminated by NAATs, the tests may provide false positive results in patients with a history of TB [12]. In addition, hemoglobin and other factors present in the dissolved sputum may retard these tests, providing false negative results [14]. Furthermore, the implementation of these NAATs remains difficult in resource-limited settings (e.g., they require an air-conditioned room) [15].
Taken together, a better method for diagnosing TB is strongly needed, in which only live tubercle bacilli can be examined with high-detection sensitivity within a few hours. Therefore, we developed a de novo, high-detection sensitive, culture-free, same-day TB diagnostic method based on an enzyme-linked immunosorbent assay (ELISA) coupled with thionicotinamide-adenine dinucleotide (thio-NAD) cycling [16]. This ultrasensitive ELISA detects trace amounts of a protein, MPT64, which is specifically secreted from only live M. tuberculosis when the bacilli are heated. MPT64 inhibits apoptosis of host macrophages by using the cascades involving an up-regulation of bcl-2, an increase in miRNA21 and a control of NF-κB [17]. Thus, MPT64 is an important component for live M. tuberculosis. The feasibility of detecting MPT64 has already been confirmed using immunochromatography [18], which is less sensitive than the ultrasensitive ELISA.
2. Methods
2.1. Specimens and ethics
A prospective study was performed in Taiwan between from 09 September 2017 to 30 July 2018. Sputum was collected consecutively from patients with suspected TB infection on the basis of clinical criteria at Kaohsiung Medical University Hospital until at least 1000 specimens were reached. This project was approved by the Institutional Review Board of Kaohsiung Medical University (KMUHIRB-F(I)-20170069). The written informed consents were obtained from the patients at Kaohsiung Medical University Hospital. Thus, the sputum experiments were performed and analyzed at Kaohsiung Medical University Hospital, whereas the protein experiments were performed and analyzed at TAUNS and Waseda University. The sputum was directly deposited by the patient into a sample holder and stored at 4°C until pretreatment. Sputum specimens largely contaminated by blood and bacilli other than M. tuberculosis in culture tests were not used in the present study. Specimens with an insufficient volume after pretreatment were also not used. In addition, the diagnosis for TB does not need a BSL3 facility.
2.2. Pretreatment of sputum for culture-free ultrasensitive ELISA tests
Sputum (1 mL) was homogenized with 3–6 mL protease solution (Sputazyme; Kyokuto Pharmaceutical Industrial, Tokyo, Japan), and the specimen was incubated at room temperature for at least 15 min. The specimen was then centrifuged at 4000 × g for 15 min, and the precipitate was collected. For further homogenization of the sputum, the precipitate was suspended in 1 mL of a 4 M urea solution (TAUNS, Shizuoka, Japan), and incubated at room temperature for 3 min. Then, 120 μL of a N-acetyl-l-cysteine (NALC) solution (CC-E supplement, Japan BCG laboratory, Tokyo, Japan) was added to the specimen. The specimen was incubated at room temperature for 15 min. The sample was neutralized with 10 mL phosphate buffer (0.033 M, pH 6.8) containing 0.05% Tween 80 was added to the suspension and centrifuged at 4000 × g for 15 min. The precipitate obtained was washed again and suspended in 200 μL of heat treatment buffer comprising phosphate buffer (0.033 M, pH 6.8) and 2% Tween 20. This solution was heated in an aluminum block heater at 46 °C for 1 h, resulting in the secretion of MPT64 from live bacilli [18].
2.3. Culture-free ultrasensitive ELISA tests
In the culture-free ultrasensitive ELISA tests, we detected a specific protein for M. tuberculosis, MPT64 [19], which is secreted from only live M. tuberculosis in heated sputum specimens [18]. An ultrasensitive ELISA coupled with thio-NAD cycling was originally developed by Watabe and Ito [16,[20], [21], [22], [23], [24], [25], [26]]. For example, see the supplementary data for the detailed methods of our culture-free ultrasensitive ELISA. To convert the units between pg/mL and moles/assay, the molecular mass of MPT64 was 24.9 kDa and a single assay volume was 50 μL. To make a calibration curve, we used recombinant His-tagged MPT64 antigen that was produced in E. coli.
2.4. Culture tests
Culture tests were performed according to the procedures described by Garcia and Isenberg[27] and Lu et al.[28]. Briefly, the Mycobacteria Growth Indicator Tube (MGIT; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and the MGIT 960 instrument (Becton, Dickinson and Company) were used. After the MGIT-positive results were found, the immunochromatographic tests using specific antibodies for MPT64 (SD Bioline TB Ag MPT64 Rapid kit; Standard Diagnostics, Yongin, Korea) were further performed to confirm the results.
2.5. Xpert MTB/RIF tests
The Xpert MTB/RIF (Xpert) assay was performed according to the manufacturer's instructions (Cepheid). Four of the specimens were not examined. Two of the samples were found to contain non-tuberculosis mycobacteria by MALDI-TOF, and thus we skipped the Xpert experiments, because non-tuberculosis mycobacteria do not produce MPT64 [29,30]. The other two samples were the same as those identified as different samples, and thus we also skipped the Xpert experiments for these samples. Furthermore, Xpert could not be applied when the sputum volume of the specimen was too low.
2.6. Smear tests
After processing of the sputum specimens with a NALC/NaOH solution, they were stained according to the Auramine-Rhodamine (AR) staining method and instructions of the Wescor Aerospray TB AFB Stainer & Cytocentrifuge Model 7721 (Discovery Diagnostics, Clement, Ontario, Canada), and visualized by fluorescence microscopy. When AR staining produced positive results, the results were confirmed using Kinyoun staining (02T010, TONYAR Biotech, Taoyuan, Taiwan). The results of the AFB smear were graded according to the American Thoracic Society/Center for Disease Control and Prevention (ATS/CDC) [31].
2.7. Treatments for TB patients
In Kaohsiung Medical University Hospital, the four-drug regimen (isoniazid, rifampicin, pyrazinamide, and ethambutol) was applied to all TB patients as the initial therapy recommended by the World Health Organization (WHO), USA CDC, and Taiwan CDC [32], [33], [34].
2.8. Statistical analyses
Data are expressed as mean ± standard deviation (SD). The limit of detection (LOD) was estimated from the mean of the blank and the 3 × SD of the blank [35]. Here, the blank was measured with the heat treatment buffer only. Furthermore, we attempted to find the measured LOD using the low concentration of MPT64. The limit of quantification (LOQ) was estimated by the same method as used for the LOD, but with the 10 × SD of the blank. The 95% confidence interval was calculated by a Clopper-Pearson exact confidence interval. McNemar's test and Cochran-Armitage test were performed using R version 3.4.1 (http://www.r-project.org).
3. Results
3.1. In-vitro tests of culture-free ultrasensitive ELISA tests
3.1.1. Limit of detection of culture-free ultrasensitive ELISA tests
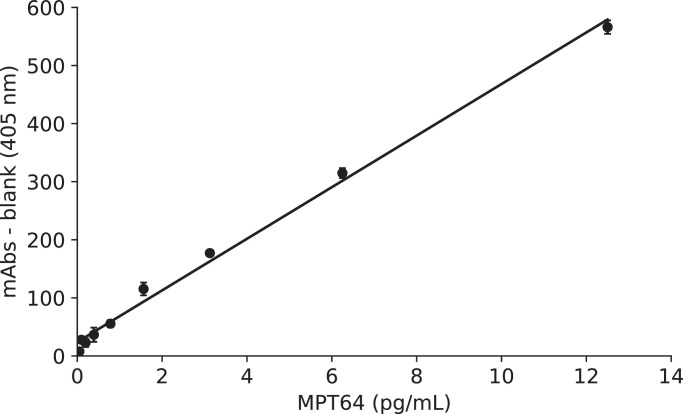
When we used MPT64 antigen and the heat treatment buffer described in the Methods section, the ultrasensitive ELISA coupled with thio-NAD cycling yielded a linear calibration curve (y = 0.044x + 0.024, R2 = 0.99) in the range of 0–12.5 pg/mL (Fig. 1). This curve was obtained from the absorbance of the accumulated thio-NADH at a cycling reaction time of 90 min. The statistically estimated LOD was 0.10 pg/mL, corresponding to 2.0 × 10−19 moles/assay. This value was estimated from the mean of the blank and the 3 × SD of the blank as described in the Methods section. Furthermore, we obtained the observed LOD, when we noticed that the mean of the blank and the 3 × SD of the blank was 13 mAbs (data not shown). In this case, the values exceeding 13 mAbs were obtained at the ratio of 53% (16/30 measurements) when we used 0.10 pg/mL of MPT64 antigen, and those were obtained at the ratio of 100% (28/28 measurements) when we used 0.20 pg/mL. If we set 17 mAbs as the cutoff value, which was obtained for the culture-free ultrasensitive ELISA test (see below), the values exceeding 17 mAbs were obtained at the ratio of 30% (9/30 measurements) when we used 0.10 pg/mL of MPT64 antigen, and those were obtained at the ratio of 100% (28/28 measurements) when we used 0.20 pg/mL. Therefore, we concluded that the observed LOD was 0.20 pg/mL, corresponding to 4.0 × 10−19 moles/assay. On the other hand, the statistically estimated LOQ was 0.39 pg/mL, corresponding to ca. 7.9 × 10−19 moles/assay. The coefficient of variation (CV), calculated from three replicated measurements, for 0.10 pg/mL of MPT64 antigen (i.e., the statistically estimated LOD) was 4%; that for 0.20 pg/mL of MPT64 antigen (i.e., the observed LOD) was 15%; and that for 0.39 pg/mL of MPT64 antigen (i.e., the statistically estimated LOQ) was 17%.
Fig. 1.
Linear calibration curve for MPT64 obtained by the ultrasensitive ELISA coupled with thio-NAD cycling. The blank value (i.e., absorbance of 0 pg/mL MPT64) was subtracted. The figure directly expresses the values corresponding to the MPT64 concentration. n = at least 3 each.
3.1.2. Cut-off value of culture-free ultrasensitive ELISA tests
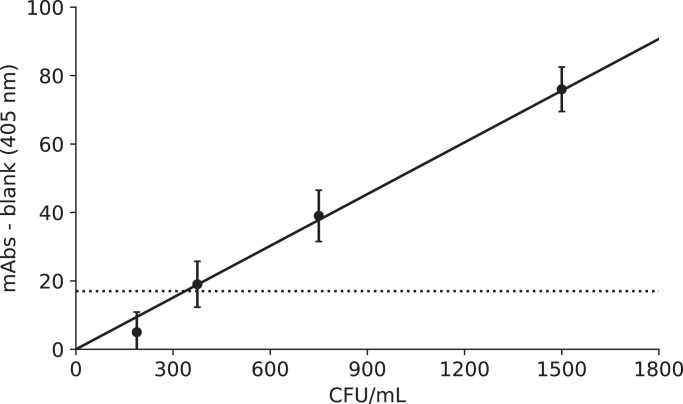
Using 944 specimens obtained from clinical trials (see Supplementary Fig. 1 and Supplementary Table 1), we compared the results of our culture-free ultrasensitive ELISA tests and those of culture tests and produced a receiver operating characteristic (ROC) curve (see Supplementary Fig. 2). The ROC curve revealed a cutoff value that was equivalent to 17 mAbs for the culture-free ultrasensitive ELISA test. The data obtained from the ROC curve revealed that 17 mAbs corresponded to a BCG value of 330 CFU/mL in culture tests (Fig. 2). We thus set the cut-off value at 17 mAbs in our culture-free ultrasensitive ELISA tests for clinical trials.
Fig. 2.
Cut-off value for culture-free ultrasensitive ELISA tests. Because the most suitable value obtained from the ROC curve was 17 mAbs, its corresponding value for BCG was 330 CFU/mL. n = 3 each.
3.1.3. Confirmation that MPT64 is not secreted from dead bacilli
To confirm that MPT64 is secreted from only live bacilli, BCG was killed by rifampicin and then heated. We then measured the MPT64 concentration in the BCG culture medium. Rifampicin at a dose of 8 μg/mL is sufficient to kill M. tuberculosis [36]. When we applied 8 μg/mL rifampicin to 2 × 105 CFU of BCG for 24 h and then heated the bacilli at 46 °C for 1 h, the MPT64 concentration in the medium was −3 ± 13 pg/mL (mean ± SD, n = 3). Thus, no MPT64 was secreted from BCG treated with rifampicin. On the other hand, when the BCG was not incubated with rifampicin for 24 h and was heated, the MPT64 concentration in the culture medium was 135 ± 15 pg/mL (mean ± SD, n = 3). That is, if the bacilli are killed by anti-TB drugs, the bacilli do not secrete MPT64 even when they are heated.
3.2. Clinical trials of culture-free ultrasensitive ELISA tests
3.2.1. Sensitivity and specificity of the culture-free ultrasensitive ELISA test and smear test in comparison with the “gold-standard” culture test
We used 944 specimens in this experiment (see Supplementary Fig. 1). These specimens included different specimens obtained from the same patients on different dates. Among these specimens, 780 were examined before anti-TB drug administration, 96 were examined during drug therapy, and 68 were from patients with an unconfirmed medication history. When we compared the results of our culture-free ultrasensitive ELISA test with those of culture tests using the patient sputum, the sensitivity was 86.9% (93/107, 95% CI: 79.0–92.7%) and the specificity was 92.0% (770/837, 95% CI: 89.9–93.7%). The accuracy was 91.4% [(93+770)/944], with a positive predictive value of 58.1% [93/(93+67)] and a negative predictive value of 98.2% [770/(14+770)] (Table 1). For the smear test, the sensitivity was 74.8% (80/107, 95% CI: 65.4–82.7%) and the specificity was 92.8% (777/837, 95% CI: 90.9–94.5%). The accuracy was 90.8%, with a positive predictive value of 57.1% and a negative predictive value of 96.6% (Table 1).
Table 1.
Comparison between culture-free ultrasensitive ELISA tests and culture tests and that between smear tests and culture tests.
| Culture-free ultrasensitive ELISA tests | Culture tests |
||||
|---|---|---|---|---|---|
| Positive | Negative | Sum | |||
| Culture-free ultrasensitive ELISA tests | positive | 93 | 67 | 160 | |
| negative | 14 | 770 | 784 | ||
| sum | 107 | 837 | 944 | ||
| Smear tests | positive | positive | 74 | 3 | 77 |
| negative | 6 | 57 | 63 | ||
| sum | 80 | 60 | 140 | ||
| negative | positive | 19 | 64 | 83 | |
| negative | 8 | 713 | 721 | ||
| sum | 27 | 777 | 804 | ||
| sum | 107 | 837 | 944 | ||
3.2.2. Relation between titer of smear results and positive predictive value of culture-free ultrasensitive ELISA tests
At smear titers of 4+, 3+, and 2+, the results of the culture tests were the same as those of the culture-free ultrasensitive ELISA tests (Table 2). Even if including the results of smear tests with a titer of 1+, the positive predictive value of the culture-free ultrasensitive ELISA tests was in good agreement with that of the culture tests. For all the smear-positive specimens, the positive predictive value of culture-free ultrasensitive ELISA tests was 92.5%.
Table 2.
Relation between titer of smear tests and positive predictive value of culture-free ultrasensitive ELISA tests.
| Titer of smear tests | # of patients obtained by culture tests | # of patients obtained by culture-free ultrasensitive ELISA tests | Positive predictive value of culture-free ultrasensitive ELISA tests | |
|---|---|---|---|---|
| Smear positive | 4+ | 12 | 12 | 100% |
| 3+ | 11 | 11 | 100% | |
| 2+ | 15 | 15 | 100% | |
| 1+ | 42 | 36 | 85.7% | |
| Smear negative | ± | 5 | 4 | 80.0% |
| ‒ | 22 | 15 | 68.2% | |
We analyzed the data of Table 2 after stratifying them into smear-positive specimens and smear-negative specimens (Table 1). For the smear-positive specimens, the sensitivity of the culture-free ultrasensitive ELISA test was 92.5% (74/80, 95% CI: 84.4–97.2%) and the specificity was 95.0% (57/60, 95% CI: 86.1–99.0%). The accuracy was 93.6% [(74+57)/140], with a positive predictive value of 96.1% [74/(74+3)] and a negative predictive value of 90.5% [57/(6+57)]. For the smear-negative specimens, the sensitivity of culture-free ultrasensitive ELISA test was 70.4% (19/27, 95% CI: 49.8–86.2%) and the specificity was 91.8% (713/777, 95% CI: 89.6–93.6%). The accuracy was 91.0% [(19+713)/804], with a positive predictive value of 22.9% [19/(19+64)] and a negative predictive value of 98.9% [713/(8+713)].
3.2.3. Sensitivity and specificity of Xpert results in comparison with culture results
As described in the Methods section, the number of Xpert tests was different from that of culture-free ultrasensitive ELISA tests due to technical issues. We thus used 239 specimens in this experiment. When we compared the results of the Xpert tests with those of the culture tests using the patient sputum, the sensitivity was 92.9% (92/99, 95% CI: 86.0–97.1%) and the specificity was 90.7% (127/140, 95% CI: 84.6–95.0%) (Table 3). The accuracy was 91.6%, with a positive predictive value of 87.6% and a negative predictive value of 94.8% (Table 3). Further, the relation was analyzed between the positive degrees in the results of Xpert tests and those of culture-free ultrasensitive ELISA tests (see Supplementary Fig. 3). The positive correlation was found (P < 0.0001 by Cochran-Armitage test).
Table 3.
Comparison between Xpert tests and culture tests.
| Culture tests |
||||
|---|---|---|---|---|
| Positive | Negative | Sum | ||
| Xpert tests | positive | 92 | 13 | 105 |
| negative | 7 | 127 | 134 | |
| sum | 99 | 140 | 239 | |
3.2.4. Effects of drug treatment on results by different diagnostic methods
In this section, we compared the results of three different diagnostic methods (i.e., culture-free ultrasensitive ELISA test, the smear test, and the Xpert test) and the culture results for samples from patients receiving anti-TB drugs. The anti-TB treatment may result in the patients’ sputa containing both live and dead bacilli. That is, the smear and Xpert methods may detect dead bacilli, but the ultrasensitive ELISA detects only live bacilli. The number of specimens in this experiment was 43 and the results are presented in Table 4.
Table 4.
Comparison among culture-free ultrasensitive ELISA tests / smear tests / Xpert tests and culture tests for patients treated with anti-TB drugs.
| Culture tests |
||||
| Positive | Negative | Sum | ||
| Culture-free ultrasensitive ELISA tests | positive | 18 | 6 | 24 |
| negative | 4 | 15 | 19 | |
| sum | 22 | 21 | 43 | |
| Smear tests | positive | 19 | 10 | 29 |
| negative | 3 | 11 | 14 | |
| sum | 22 | 21 | 43 | |
| Xpert tests | positive | 20 | 13 | 33 |
| negative | 2 | 8 | 10 | |
| sum | 22 | 21 | 43 | |
The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for the three different diagnostic methods are listed in comparison with the culture results (Table 5). When anti-TB drugs were administered to the patients, the specificity of our culture-free ultrasensitive ELISA tests was much better than those of the smear and Xpert tests, indicating that the smear and Xpert tests detected not only live bacilli, but also dead bacilli.
Table 5.
Validity of culture-free ultrasensitive ELISA tests / smear tests / Xpert tests against culture tests for patients treated with anti-TB drugs.
| Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|
| Culture-free ultrasensitive ELISA tests | 81.8% | 71.4% | 76.7% | 75.0% | 78.9% |
| Smear tests | 86.4% | 52.4% | 69.8% | 65.5% | 78.6% |
| Xpert tests | 90.9% | 38.1% | 65.1% | 60.6% | 80.0% |
4. Discussion
In the present study, we evaluated the performance of a novel culture free ultrasensitive ELISA method for detecting live tubercle bacilli in sputum specimens. The sensitivity of our test was 86.9% (93/107, 95% CI: 79.0–92.7%) and the specificity was 92.0% (770/837, 95% CI: 89.9–93.7%). The performance of the new method was better than that of acid-fast staining and not significantly different from that of the Xpert test. In addition, when the smear test titer was ≥1+, the positive predictive value of our culture-free ultrasensitive ELISA test was in a good agreement with that of the culture test. Furthermore, when the results of our culture-free ultrasensitive ELISA test were compared with those of the culture test using sputum obtained from patients treated with anti-TB drugs, the positive predictive value was sufficiently high (75.0%).
Given the advancements in diagnostic methods in the 21st century, it is remarkable that the culture method is still used as the gold standard for TB diagnosis. We believe this is because culture methods can detect live tubercle bacilli, and exclude dead bacilli, with the high detection-sensitivity required for TB diagnosis. The NAATs for TB, including the Xpert MTB/RIF Ultra, recently set new records for sensitivity and specificity and produced the most reliable results for rifampicin resistance [10,37]. These methods, however, detect both live and dead bacilli, resulting in increased sensitivity due to the measurement of dead bacilli and false positives after TB treatment, because the DNA of the dead bacilli can still be detected [38]. The presence of smear-negative and NAAT-positive findings are currently thought to be due to differences in the sensitivity between the sputum smear and NAATs [39], but this issue requires careful re-examination. When the opposite occurs, however, i.e., the sputum smear is positive for AFB and the NAAT is negative for M. tuberculosis DNA, it produces a diagnostic dilemma as it is not clear whether the anti-TB treatments are effective [40]. This emphasizes the need for a diagnostic method that detects only live bacilli.
Some other new technologies for TB diagnosis have recently emerged, such as surface plasmon resonance spectroscopy [41], immuno-PCR (a combination of ELISA and PCR) [42,43], voltammetric assay [44,45], aptasensor [46,47], immuno-nanosensor [48], and electrochemiluminescence immunoassay [49]. These methods are also applied to secretory proteins (i.e., antigens) as TB markers, but culture, which takes time, is still required to obtain adequate amounts of antigen [50]. We used heat treatment to induce the living tubercle bacilli in sputum specimens to secrete the TB biomarker MPT64 [18], and applied the ultrasensitive ELISA technique[51] to detect the MPT64 [16], Therefore, only our method can detect live tubercle bacilli within a single day. More recently, a molecular bacterial load assay (MBLA) has been reported as a molecular test for detection of live M. tuberculosis bacilli. It is a reverse transcriptase quantitative PCR that quantifies the M. tuberculosis load from patient sputum using the 16S rRNA gene as a reference gene [52]. We have not yet attempted to use MBLA and thus still cannot compare the results between MBLA and our ultrasensitive ELISA. However, it is noted that MBLA is a time-consuming method because they have two complicated processes: (1) RNA extraction and purification and (2) the use of real-time PCR [52].
Two mpt64 gene mutations in the M. tuberculosis complex are reported to interfere with the detection of MPT64 with the anti-MPT64 antibodies used in the present study [29]. One isolate from the M. tuberculosis complex had a deletion of 63 bp from nucleotides 196 to 258 (amino acids position 43–63) and the other isolate had a deletion of 3659 bp from nucleotide 874 in Rv1977 to nucleotide 905 in Rv1981c. These mutant M. tuberculosis genes seemed to exist at a very low ratio, because only three of 500 M. tuberculosis complex clinical isolates tested by Chikamatsu and three of 384 isolates tested by Hirano et al. were not detected by the anti-MPT64 antibodies [29,53]. It is certainly a limitation of the novel detection method, but the number of unidentifiable strains is very small.
The Xpert MTB/RIF assay was recommended by both WHO in 2010 and 2020[54,55] and US Food and Drug Administration in 2013 [56]. The test procedure is applied directly to clinical specimens, either raw sputum specimens or sputum pellets created after decontaminating and concentrating the sputum [57]. As shown in our study, the sensitivity of the Xpert MTB/RIF is very good (93%, Table 3). Two articles by Chakravorty et al. and Dorman et al., however, demonstrated that lower sensitivity were obtained from smear-negative and culture-positive specimens (for Xpert MTB/RIF, 66% and 46% [10], and for Xpert MTB/RIF Ultra, 79% and 63% [37], respectively). The culture-free ultrasensitive ELISA tests showed that the sensitivity for the smear-negative and culture-positive specimens was 70% (19/27; Table 2). That is, the sensitivity is almost the same between the Xpert MTB/RIF Ultra tests and the culture-free ultrasensitive ELISA tests.
Besides the similar performance of the culture-free ultrasensitive ELISA method and Xpert molecular tests, the culture-free ultrasensitive ELISA test is useful for monitoring the treatment response because this method detects only live bacilli [58]. When tuberculosis cases are under effective drug therapy, the culture-free ultrasensitive ELISA test may reveal negative results, whereas the molecular method might detect DNA from dead bacilli. This feature of the ultrasensitive ELISA test is useful for monitoring patients’ treatment responses.
In the near future, we should consider more the following two points. One is that our culture-free ultrasensitive ELISA test depends on the quality of sputum obtained from patients. That is, the effect of blood in specimens on the tests should be excluded in our test. The other is that the examination of drug effectiveness should be promoted more [58]. The studies according to a new design of drug monitoring for patients will be achieved.
We conclude that our culture-free ultrasensitive ELISA method is important for diagnosing TB and evaluating drug effectiveness, because this method detects only live bacilli without any cultures. To our knowledge, this is the first study to present a de novo, same-day (only 5 h) diagnostic method for TB with a very high limit of detection. In the near future, we will prepare an automated apparatus to achieve our method for the practical use.
Data sharing statement
Anonymized data used for analysis in this study is available upon request from the corresponding author. EI had full access to all the data and PL is the guarantor.
Contributors
KN, SW, PL, and EI designed the study. WW, RT, SJ, YJ, SW, and YO performed the experiments. WW, RT, YJ, KN, SW, PL, and EI analyzed the data. All authors interpreted data, drafted, and reviewed the final manuscript. All authors approved the submitted manuscript. EI had full access to all the data and PL is the guarantor.
Declaration of Competing Interest
PL and EI received research funds from TAUNS Laboratories, Inc. RT, YJ, SW, YO, KN, and SW are employees of TAUNS Laboratories, Inc. The other authors declare no conflict of interest.
Acknowledgements
This study was supported by Matching Planner Program from JST (VP29117939087), the A-STEP Program from JST (AS3015096U), Waseda University grants for Specific Research Projects (2017A-015 and 2019C-123), the Precise Measurement Technology Promotion Foundation to E.I. The funder had no role in the interpretation of the data or in the decision to submit the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103007.
Contributor Information
Po-Liang Lu, Email: d830166@kmu.edu.tw.
Etsuro Ito, Email: eito@waseda.jp.
Appendix. Supplementary materials
References
- 1.Nambiar R, Chatellier S, Bereksi N. Evaluation of Mycotube, a modified version of Lowenstein-Jensen (LJ) medium, for efficient recovery of Mycobacterium tuberculosis (MTB) Eur J Clin Microbiol Infect Dis. 2017;36:1981–1988. doi: 10.1007/s10096-017-3052-2. [DOI] [PubMed] [Google Scholar]
- 2.Glassroth J. Diagnosis of tuberculosis. In: Reichman LB, Hershfield ES, editors. Tuberculosis: a comprehensive international approach. Marcel Dekker; New York, USA: 1993. [Google Scholar]
- 3.Messelhäusser U, Kämpf P, Hörmansdorfer S. Culture and molecular method for detection of Mycobacterium tuberculosis complex and Mycobacterium avium subsp. paratuberculosis in milk and dairy products. Appl Environ Microbiol. 2012;78:295–297. doi: 10.1128/AEM.06322-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hameed S, Ahmad SR, Aqeel Ur Rehman M, Nazir H, Ullah I. Drug resistance profile of Mycobacterium tuberculosis and predictors associated with the development of drug resistance. J Glob Antimicrob Resist. 2019;18:155–159. doi: 10.1016/j.jgar.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Afsar I, Gunes M, Er H, Gamze Sener A. Comparison of culture, microscopic smear and molecular methods in diagnosis of tuberculosis. Rev Esp Quimioter. 2018;31:435–438. [PMC free article] [PubMed] [Google Scholar]
- 6.Aryan E, Makvandi M, Farajzadeh A. Clinical value of IS6110-based loop-mediated isothermal amplification for detection of Mycobacterium tuberculosis complex in respiratory specimens. J Infect. 2013;66:487–493. doi: 10.1016/j.jinf.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Patel VB, Connolly C, Singh R. Comparison of amplicor and GeneXpert MTB/RIF tests for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52:3777–3780. doi: 10.1128/JCM.01235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shete PB, Farr K, Strnad L, Gray CM, Cattamanchi A. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:268. doi: 10.1186/s12879-019-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helb D, Jones M, Story E. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravorty S, Simmons AM, Rowneki M. The new Xpert MTB/RIF Ultra: Improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio. 2017;8:e00812–e00817. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier E, Drouillard B, Dailloux M. Diagnosis of tuberculosis by Amplicor Mycobacterium tuberculosis test: a multicenter study. J Clin Microbiol. 1995;33:3106–3110. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilgin K, Yanik K, Karadağ A, Odabaşi H, Taş H, Günaydin M. Comparison of a real-time polymerase chain reaction-based system and Erlich-Ziehl-Neelsen method with culture in the identification of Mycobacterium tuberculosis. Turk J Med Sci. 2016;46:203–206. doi: 10.3906/sag-1411-34. [DOI] [PubMed] [Google Scholar]
- 13.Boyd R, Ford N, Padgen P, Cox H. Time to treatment for rifampicin-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2017;21:1173–1180. doi: 10.5588/ijtld.17.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beavis KG, Lichty MB, Jungkind DL, Giger O. Evaluation of Amplicor PCR for direct detection of Mycobacterium tuberculosis from sputum specimens. J Clin Microbiol. 1995;33:2582–2586. doi: 10.1128/jcm.33.10.2582-2586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Mwaba P, Bates M. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–361. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watabe S, Kodama H, Kaneda M. Ultrasensitive enzyme-linked immunosorbent assay (ELISA) of proteins by combination with the thio-NAD cycling method. Biophysics. 2014;10:49–54. doi: 10.2142/biophysics.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Liu S, Tang Y. MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of macrophages through NF-κB-miRNA21-Bcl-2 pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakaishi K, Watabe S, Kitagawa T, Ito E. Immunochromatographic detection of MPB64 secreted from active BCG by heating: toward same-day diagnosis of tuberculosis. Biotechniques. 2019;66:240–242. doi: 10.2144/btn-2019-0020. [DOI] [PubMed] [Google Scholar]
- 19.Harboe M, Nagai S, Patarroyo ME, Torres ML, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watabe S, Morikawa M, Kaneda M. Ultrasensitive detection of proteins and sugars at single-cell level. Commun Integr Biol. 2016;9 doi: 10.1080/19420889.2015.1124201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito E, Kaneda M, Kodama H. Immunoreactive insulin in diabetes mellitus patient sera detected by ultrasensitive ELISA with thio-NAD cycling. Biotechniques. 2015;59:359. doi: 10.2144/000114355. 361–7. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa M, Naito R, Mita K. Subattomole detection of adiponectin in urine by ultrasensitive ELISA coupled with thio-NAD cycling. Biophys Physicobiol. 2015;12:79–86. doi: 10.2142/biophysico.12.0_79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatsuma A, Kaneda M, Kodama H. Detection of HIV-1 p24 at attomole level by ultrasensitive ELISA with thio-NAD cycling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamakado S, Cho H, Inada M. Urinary adiponectin as a new diagnostic index for chronic kidney disease due to diabetic nephropathy. BMJ Open Diabetes Res Care. 2019;7 doi: 10.1136/bmjdrc-2019-000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iha K, Inada M, Kawada N. Ultrasensitive ELISA developed for diagnosis. Diagnostics. 2019;9:E78. doi: 10.3390/diagnostics9030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito E, Iha K, Yoshimura T. Early diagnosis with ultrasensitive ELISA. Adv Clin Chem. 2020 doi: 10.1016/bs.acc.2020.06.022. in press. [DOI] [PubMed] [Google Scholar]
- 27.Garcia LS, Isenberg HD. 3rd ed. American Society for Microbiology; Washington DC, USA: 2010. Clinical microbiology procedures handbook. [Google Scholar]
- 28.Lu PL, Yang YC, Huang SC. Evaluation of the Bactec MGIT 960 system in combination with the MGIT TBc identification test for detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:2290–2292. doi: 10.1128/JCM.00571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chikamatsu K, Aono A, Yamada H. Comparative evaluation of three immunochromatographic identification tests for culture confirmation of Mycobacterium tuberculosis complex. BMC Infect Dis. 2014;14:54. doi: 10.1186/1471-2334-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos A, Carvalho T, Ribeiro M, Guimarães JT. Capilia™ TB-Neo assay: a new tool for rapid distinction between tuberculous and non-tuberculous mycobacteria. Int J Tuberc Lung Dis. 2016;20:753–756. doi: 10.5588/ijtld.15.0528. [DOI] [PubMed] [Google Scholar]
- 31.Society American Thoracic. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Treatment of Tuberculosis: guidelines for national programmes. 4th ed.WHO/HTM/TB/2009; 420: 1–147. Available at:http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf(accessed on 17 February, 2020).
- 33.Nahid P, Dorman SE, Alipanah N. Executive summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:853–867. doi: 10.1093/cid/ciw566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taiwan CDC. 6th Ed. Taiwan guidelines for TB diagnosis & treatment; r.o.C. (Taiwan): 2017. Centers for disease control, ministry of health and welfare.https://www.cdc.gov.tw/En/File/Get/Ptat7PMqyrzuqYbiPlZ4VQ (Accessed on 16 February, 2020) [Google Scholar]
- 35.Shrivastava A, Gupta VB. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci. 2011;2:21–25. doi: 10.4103/2229-5186.79345. [DOI] [Google Scholar]
- 36.Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol. 2015;6:641. doi: 10.3389/fmicb.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorman SE, Schumacher SG, Alland D. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84. doi: 10.1016/S1473-3099(17)30691-6. Erratum: Lancet Infect Dis. 2018; 18: 376. doi: 10.1016/S1473-3099(18)30169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arend SM, van Soolingen D. Performance of Xpert MTB/RIF Ultra: a matter of dead or alive. Lancet Infect Dis. 2018;18:8–10. doi: 10.1016/S1473-3099(17)30695-3. [DOI] [PubMed] [Google Scholar]
- 39.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1 doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phyu MH, Kyaw KWY, Myint Z, Thida A, Satyanarayana S, Aung ST. Sputum smear-positive, Xpert® MTB/RIF-negative results: magnitude and treatment outcomes of patients in Myanmar. Public Health Action. 2018;8:181–186. doi: 10.5588/pha.18.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SC, Chen H, Lee J. Ultrasensitive immunosensing of tuberculosis CFP-10 based on SPR spectroscopy. Sens Actuators B. 2011;156:271–275. doi: 10.1016/j.snb.2011.04.032. [DOI] [Google Scholar]
- 42.Mehta PK, Kalra M, Khuller GK, Behera D, Verma I. Development of an ultrasensitive polymerase chain reaction-amplified immunoassay based on mycobacterial RD antigens: implications for the serodiagnosis of tuberculosis. Diagn Microbiol Infect Dis. 2012;72:166–174. doi: 10.1016/j.diagmicrobio.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Sheoran A, Gupta KB. Quantitative detection of a cocktail of mycobacterial MPT64 and PstS1 in tuberculosis patients by real-time immuno-PCR. Future Microbiol. 2019;14:223–233. doi: 10.2217/fmb-2018-0284. [DOI] [PubMed] [Google Scholar]
- 44.Gou D, Xie G, Li Y, Zhang X, Chen H. Voltammetric immunoassay for Mycobacterium tuberculosis secretory protein MPT64 based on a synergistic amplification strategy using rolling circle amplification and a gold electrode modified with graphene oxide, Fe3O4 and Pt nanoparticles. Mikrochim Acta. 2018;185:436. doi: 10.1007/s00604-018-2972-6. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Huang X, Sun D. Dual-aptamer-based voltammetric biosensor for the Mycobacterium tuberculosis antigen MPT64 by using a gold electrode modified with a peroxidase loaded composite consisting of gold nanoparticles and a Zr(IV)/terephthalate metal-organic framework. Mikrochim Acta. 2018;185:543. doi: 10.1007/s00604-018-3081-2. [DOI] [PubMed] [Google Scholar]
- 46.Bai L, Chen Y, Bai Y, Chen Y, Zhou J, Huang A. Fullerene-doped polyaniline as new redox nanoprobe and catalyst in electrochemical aptasensor for ultrasensitive detection of Mycobacterium tuberculosis MPT64 antigen in human serum. Biomaterials. 2017;133:11–19. doi: 10.1016/j.biomaterials.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Sypabekova M, Bekmurzayeva A, Wang R, Li Y, Nogues C, Kanayeva D. Selection, characterization, and application of DNA aptamers for detection of Mycobacterium tuberculosis secreted protein MPT64. Tuberculosis. 2017;104:70–78. doi: 10.1016/j.tube.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Mohd Bakhori N, Yusof NA, Abdullah J. Immuno nanosensor for the ultrasensitive naked eye detection of tuberculosis. Sensors. 2018;18:E1932. doi: 10.3390/s18061932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broger T, Tsionksy M, Mathew A. Sensitive electrochemiluminescence (ECL) immunoassays for detecting lipoarabinomannan (LAM) and ESAT-6 in urine and serum from tuberculosis patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin X, Zheng L, Lin L. Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: a meta-analysis. J Infect. 2013;67:369–377. doi: 10.1016/j.jinf.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Kyosei Y, Namba M, Yamura S. Proposal of de novo antigen test for COVID-19: Ultrasensitive detection of spike proteins of SARS-CoV-2. Diagnostics. 2020;10:594. doi: 10.3390/diagnostics10080594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mtafya B, Sabiiti W, Sabi I. Molecular bacterial load assay concurs with culture on NaOH-induced loss of Mycobacterium tuberculosis viability. J Clin Microbiol. 2019;57 doi: 10.1128/JCM.01992-18. e01992-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirano K, Aono A, Takahashi M, Abe C. Mutations including IS6110 insertion in the gene encoding the MPB64 protein of Capilia TB-negative Mycobacterium tuberculosis isolates. J Clin Microbiol. 2004;42:390–392. doi: 10.1128/jcm.42.1.390-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. 2010 https://apps.who.int/iris/bitstream/handle/10665/112472/9789241506335_eng.pdf?sequence=1 (accessed on 17 February, 2020) [Google Scholar]
- 55.WHO . 2020. Rapid Communication: Molecular assays as initial tests for the diagnosis of tuberculosis and rifampicin resistance.https://apps.who.int/iris/bitstream/handle/10665/330395/9789240000339-eng.pdf (accessed on 17 February, 2020) [Google Scholar]
- 56.US FDA . 2013. Device Classification under Section 513(f)(2)(de novo)https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/denovo.cfm (accessed on 17 February, 2020) [Google Scholar]
- 57.Blakemore R, Story E, Helb D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakashita K, Takeuchi R, Takeda K. Ultrasensitive enzyme-linked immunosorbent assay for the detection of MPT64 secretory antigen to evaluate Mycobacterium tuberculosis viability in sputum. Int J Infect Dis. 2020;96:244–253. doi: 10.1016/j.ijid.2020.04.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.