1. Introduction
The Covid-19 pandemic has induced a massive influx of patients within the intensive care units (ICU). The mean characteristic of these patients was the occurrence of a severe acute respiratory distress syndrome (ARDS) requiring mechanical ventilation and prolonged deep sedation.
In ARDS patients with Covid-19, deep sedation using a combination of hypnotic and analgesic agents allows better adaptation to the ventilator, reduces ventilator asynchronies and oxygen consumption, ensures a better comfort and pain control for the patients. Midazolam and/or Propofol are the more frequently used hypnotic agents, and Sufentanyl the analgesic agent. Neuromuscular blocking has been recommended at the early phase of ARDS, for an ideal maximal 48-h duration and a daily reassessment of its benefits.
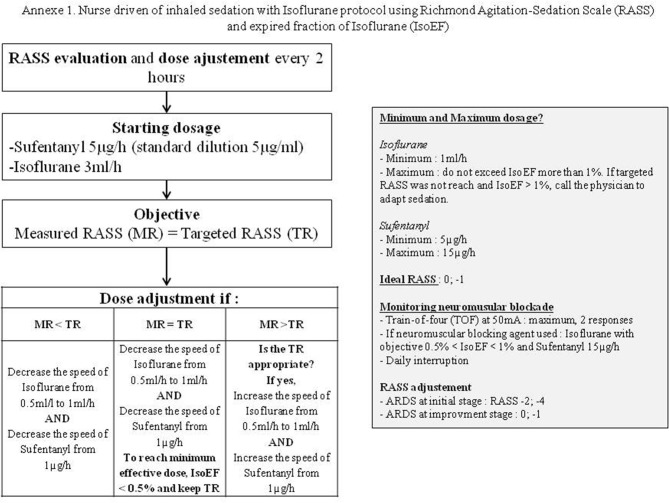
During Covid-19 pandemic, ICU equipment (personal protective equipments, ventilators, pumps…) has become a problem [1,2]. Another major concern was to ensure access to mandatory anesthetics drugs medications, or neuromuscular blockade agents. In response to such shortages, we decided to diversify our sedative agents panel and thus to use volatile agents using the AnaConda (Sedana, Danderyd, Sweden) as a first line therapy in replacement of all hypnotic agents. The nurse-driven sedation protocol enabled to modify other sedative agents posology (analgesics and paralyzing agents) according to a predetermined goal (RASS, Ramsay and TOF) (e-Supplementary; Appendix 1).
2. Results
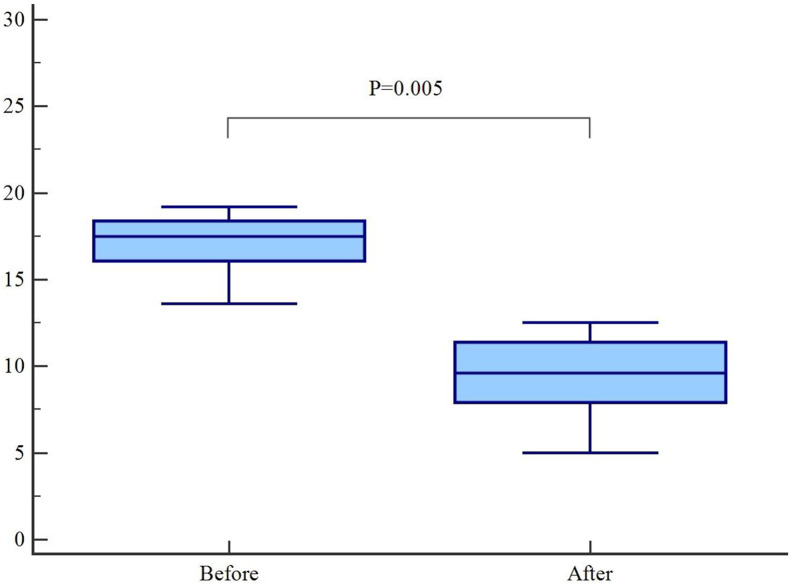
Eleven endotracheally intubated and ventilated patients (age = 58.1 ± 12.4 yr., sex ratio = 4.5; SpO2/FIO2 = 204 ± 93) sedated by a combination of benzodiazepin and opioids for a median duration of 82-h (Midazolam = 6.4 ± 1.4 mg/h; Sufentanyl = 17.3 ± 5.0 gamma/h), all nine receiving paralyzing agents for a median duration of 6-h, were switched to volatile anesthetics (e-Supplementary; Appendix 2). Two patients were also receiving additional Propofol infusions. No significant Cisatracurium consumption decrease was observed (4.9 ± 7.3 vs. 2.7 ± 1.5 mg/h; p = 0.28), but a significant Sufentanyl consumption decrease (17.3 ± 5.0 vs. 10.6 ± 4.0 gamma/h; p = 0.005) was also associated to volatile anesthetics introduction, while the same sedation goal was reached (Fig. 1 ).
Fig. 1.
Sufentanyl consumption before and after volatile anesthetics introduction.
The figure displays the Box-and-whisker plot of the sufentanyl consumption, before and after volatile anesthetics introduction while keeking the same sedation goal. Central grey box represents the values from the lower to upper quartile (25 to 75 percentile). The middle line represents the median. The horizontal line extends from the minimum to the maximal value, excluding outside and far out values. A P value equal or below 0.05 was considered statistically significant.
3. Discussion
Volatile sedative agent through dedicated devices is an efficient alternative to conventional intravenous sedation within the ICU. Despite, this kind of sedation was not commonly used in critically patient's long-term sedation. Previous studies have shown the efficacy and safety for inhaled sedation of ICU patients [3,4].
While volatile anesthetics have no analgesic proprieties by themselves, we observed a Sufentanyl consumption decrease when Isoflurane was used. Sedation and analgesic goals were respectively evaluated with RASS and BPS. Dosing of anesthetics or analgesic agent was adjusted on achieving sedation goals previously fixed. Mesnil et al., have shown sedated patients under Sevoflurane were less restless and aggressive, as compared with sedated patients under Midazolam or Propofol. Moreover, last pain score measuring after sedation discontinuation and intravenous morphine consumption was lower in the Sevoflurane group [5]. Neurologic manifestations, as agitation, were described in severe Covid-19 infection [6]. The hypothesis of inhaled sedation allowing better control of these symptoms could therefore explain, at least in part, the decrease in opioid consumption that was observed. Inhaled sevoflurane sedation allows to decrease wake-up and extubation time [5] and to improve oxygenation in case of ARDS [6].
Other potential benefits of inhaled sedative agents such as anti-inflammatory effects, pulmonary vascular dilatation, marker of epithelial injury decrease should be mentioned, especially in ARDS patients [6,7]. Several side effects should also be mentioned. First, fluoride ions which are inhaled sedation metabolites can generate renal failure while new generations of volatile agent undergo low levels of metabolism. A recent study has shown that the use of Sevoflurane was not associated with increase fluoride ions serum levels [4]. Second, the use of volatile agents needs to incorporate mini-vaporizers on the respiratory circuit, which may increase the instrumental dead space and generate carbon dioxide re-breathing, especially with low-tidal volume ventilation. The use of fifty milliliters devices (conventional heat and moisture exchanger filter dead space) enables to limit such effect. In the ARDS study by Jabaudon et al., no significant hypercapnic acidosis were observed. Third, malignant hyperthermia is a rare, but severe adverse event that may be observed while using volatile anesthetics in genetically susceptible individuals.
Two randomized controlled trials will start soon, SESAR study (NCT04235608) and INASED study (NCT04341350). Both will compare volatile anesthetics with intravenous sedation using Propofol. The SESAR study (NCT04235608) aims to exhibit a difference in terms of respectively composite criteria included mortality and ventilator free days in ARDS patients while using Sevoflurane. The INASED study (NCT04341350) aims to depict a benefit in terms of delirium incidence while using Isoflurane.
4. Conclusion
Our experience illustrates the fact that it may also provide some valuable help when facing sedative agents shortages while opening the panel of drugs to be used, combined with the potentiality to decrease opioids consumption.
The following are the supplementary data related to this article.
Supplementary Fig. S1.
Nurse driven of inhaled sedation with Isoflurane protocol using Richmond Agitation-Sedation Scale (RASS) and expired fraction of Isoflurane (IsoEF).
: Isoflurane data for each patient during their hospitalizations.
Financial disclosures
None to declare.
Declaration of Competing Interest
None to declare.
References
- 1.Choo E.K., Rajkumar S.V. Medication shortages during the COVID-19 crisis: what we must do. Mayo Clin Proc. 2020;95(6):1112–1115. doi: 10.1016/j.mayocp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranney M.L., Griffeth V., Ashish K.J. Critical supply shortages – the need for ventilator and personal protective equipment during the COVID-19 pandemic. New Engl J Med. 2020;382(18) doi: 10.1056/nejmp2006141. [DOI] [PubMed] [Google Scholar]
- 3.L'Her E., Dy L., Pili R., Prat G., Tonnelier J.M., Lefevre M., et al. Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: a prospective observational study. Respir Care. 2008;53(10):1295–1303. [PubMed] [Google Scholar]
- 4.Perbet S., Bourdeaux B., Sautou V., Pereira B., Chabanne R., Constantin J.M., et al. A pharmacokinetic study of 48-hour sevoflurane inhalation using a disposable delivery system (AnaConDa®) in ICU patients. Minerva Anestesiol. 2014;80(6):655–665. [PubMed] [Google Scholar]
- 5.Mesnil M., Capdevila X., Bringuier S., Trine P.O., Falquet Y., Charbit J., et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med. 2011;37(6):933–941. doi: 10.1007/s00134-011-2187-3. [DOI] [PubMed] [Google Scholar]
- 6.Jabaudon M., Boucher P., Imhoff E., Chabanne R., Faure J.S., Roszyk L., et al. Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: a prospective observational study. Am J Respir Crit Care Med. 2017;195(6):792–800. doi: 10.1164/rccm.201604-0686oc. [DOI] [PubMed] [Google Scholar]
- 7.Jerath A., Parotto M., Wasowicz M., Ferguson N.D. Volatile anesthetics. Is a new player emerging in critical care sedation? Am J Respir Crit Care Med. 2016;193(11):1202–1212. doi: 10.1164/rccm.201512-2435cp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
: Isoflurane data for each patient during their hospitalizations.