Abstract
Background.
Affective neuroscience and scar theories propose that increased excessive worry, the hallmark symptom of generalized anxiety disorder (GAD), predicts future declines in executive functioning (EF). However, the preponderance of cross-sectional designs used to examine between-person chronic worry-EF relationships has blocked progress on understanding their potentially causal within-person associations. Accordingly, this study used bivariate dual latent change score (LCS) models to test whether within-person increased GAD severity might relate to future reduced EF.
Methods.
Community-dwelling adults (N = 2581, 46 years on average, s.d. = 11.40, 54.71% female) were assessed for GAD symptom severity (Composite International Diagnostic Interview-Short Form) across three waves, spaced about 9 years apart. Three aspects of EF [inhibition, set-shifting, and mixing costs (MCs; a measure related to common EF)], were assessed with stop-and-go switch tasks. Participants responded to 20 normal and 20 reverse single-task block trials and 32 mixed-task switch block trials. EF tests were administered at time 2 (T2) and time 3 (T3), but not at time 1 (T1).
Results.
After controlling for T1 depression, LCS models revealed that within-person increased T1 − T2 GAD severity substantially predicted future reduced T2 − T3 inhibition and set-shifting (both indexed by accuracy and latency), and MC (indexed by latency) with moderate-to-large effect sizes (|d| = 0.51–0.96).
Conclusions.
Results largely support scar theories by offering preliminary within-person, naturalistic evidence that heightened excessive worry can negatively predict future distinct aspects of cognitive flexibility. Effectively targeting pathological worry might prevent difficulties arising from executive dysfunction.
Keywords: Affective neuroscience, executive functioning, generalized anxiety disorder, latent change, worry
Pathological worry, the hallmark symptom of generalized anxiety disorder (GAD), has been reliably linked to deficits in some cognitive skills, such as learning, memory, and facets of executive functioning (EF) (Moran, 2016; Pietrzak et al., 2012). Pathological worry is defined as the chronic tendency to anticipate potential future threats. EF refers to a group of complex cognitive control processes necessary for organizing tasks, assessing risks, decision-making, managing emotions, and adapting to unanticipated events (Banich, 2009). EF relates closely to the central executive of the working memory (WM) which retains and alters task-pertinent information (Baddeley, 2001), and tends to decline with age (Deary et al., 2009). EF deficits have been linked to problems in career, academics, relationships, self-esteem, as well as physical and mental health (Snyder, Miyake, & Hankin, 2015). This is likely because EF is intertwined with accurately registering and recalling facts or events, processing speed, language, speech fluency, and logical reasoning (Best, Miller, & Jones, 2009; Brown, Brockmole, Gow, & Deary, 2012). Thus, better understanding how worry leads to EF processes going awry is important.
In the past five decades, neurocognitive theories have increasingly acknowledged that executive dysfunction may be a product of excessive worry. The attentional control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) posits that worry might negatively impinge on inhibition (refraining from autopilot responses) and set-shifting (switching flexibly between distinct mindsets) (Miyake & Friedman, 2012). Likewise, affective neuroscience models have proposed that anxiety disorders are associated with depletion in EF capacities stemming from interference with the capacity to ignore task-unimportant matters (Beaudreau, MacKay-Brandt, & Reynolds, 2013). For instance, GAD might be related to ineffective recruitment of the pregenual anterior cingulate cortex or other EF-linked brain regions that dampen amygdala hyperarousal (Andreescu et al., 2015). All in all, as pathological worry is self-perpetuating and emotionally dysregulating, these models propose that worry can reduce accuracy or increase response time (latency) on EF tests.
Although these theories suggest that worry symptom-EF relationships unfold across a short time-scale (Beckwé, Deroost, Koster, De Lissnyder, & De Raedt, 2014), such theories might be extended to encompass more long-term effects using scar models, such as the perseverative cognition hypothesis (Brosschot, Gerin, & Thayer, 2006). The perseverative cognition hypothesis proposes that habitual worry is the mechanism by which stress leads to long-term deleterious effects on somatic, cardiovascular, and metabolic health via the buildup of allostatic load. Supporting these ideas, studies have evidenced moderate-to-large relationships between frequent worry and future acute myocardial infarction as well as chronic heart disease, even across multiple decades (Davidson, Mostofsky, & Whang, 2010; Kawachi, Sparrow, Vokonas, & Weiss, 1994; Kubzansky et al., 1997). Likewise, increased resting systolic and diastolic blood pressure, cortisol, and body mass index in childhood predicted poorer WM in young adulthood (Evans & Fuller-Rowell, 2013). In addition, excessive morning cortisol secretion predicted decline on tests of set-shifting and WM 2 to 4 years later in community adults (Beluche, Carrière, Ritchie, & Ancelin, 2010). Collectively, allostatic load (i.e. accumulating wear and tear on the body) from chronic worry could possibly negatively impact EF abilities over decades.
Regarding short-term effects, ample data lend credence to these theories. After adjusting for anxiety symptoms, two recent meta-analytic studies pooling data across 24 samples showed that worry had significant small-to-moderate between-person relationships with EF (Zetsche, Bürkner, & Schulze, 2018) and WM (Moran, 2016). However, the study of worry-EF associations has largely been a cross-sectional enterprise, leaving the question of causality to debate. To date, five experiments offer support for the causal role of worry on EF using non-emotional (or neutral) tasks (Hallion, Ruscio, & Jha, 2014; Hayes, Hirsch, & Mathews, 2008; Leigh & Hirsch, 2011; Stefanopoulou, Hirsch, Hayes, Adlam, & Coker, 2014; Williams, Mathews, & Hirsch, 2014). For instance, compared to thinking positive or neutral thoughts, worrying impaired WM and inhibition on accuracy and timed tests in persons with GAD (Stefanopoulou et al., 2014). In general, these experiments support the notion that EF deficiencies might be a downstream consequence of excessive worry.
Despite their importance, experiments that induced worry to test its momentary impact on EF might not extend to naturalistic contexts across various stages of adult life. Unlike short-term effects, buildup of allostatic load leading to dysregulation of the hypothalamus-pituitary adrenal axis likely explains the long-term effects of chronic worry on EF across decades. For instance, chronic stress and worry predicted reduced EF-linked prefrontal cortex activity and heightened amygdala reactivity 13 years later (Kim et al., 2013). Thus, a way to advance the field on this topic is to examine how excessive worry results in EF problems in adulthood across multiple occasions, beyond a single laboratory visit. Although prospective observational data involve no explicit manipulation of the predictor to permit strong causal inferences (Shadish, Cook, & Campbell, 2002), they move us closer toward causal models.
At present, at least five such studies in adults have been conducted. First, among Swedish community adult twins, neuroticism was negatively correlated with visuospatial WM, processing speed, and recognition memory at baseline (Wetherell, Reynolds, Gatz, & Pedersen, 2002); however, in this study, neuroticism did not predict 9-year cognitive decline. By comparison, even slight worry symptoms in older adults predicted decreased visual attention, recall, and EF 2 to 3 years later (Pietrzak et al., 2012, 2014). Similarly, in another study on older adults, increased anxious symptoms predicted verbal memory deterioration across 12 years (Gulpers, Oude Voshaar, van Boxtel, Verhey, & Köhler, 2019). Also, higher GAD symptoms predicted EF decline 3.4 years later in community-dwelling men (Kassem et al., 2017). Based on this emerging body of longitudinal evidence, worsening of GAD symptoms might predict weakened EF across longer time periods.
Yet another limitation is that these studies’ use of two-wave regression analyses informs between-person (nomothetic) relationships, but not within-person (idiographic) patterns of growth. Thus, little is known about idiographic, dynamic, prospective effects of change in GAD severity on future change in EF constructs. One cutting-edge method that can achieve this goal is bivariate dual latent change score (LCS) modeling (McArdle, 2009). As anxiety-EF models propose both within- and between-person changes across time, it is essential to apply suitable analyses. Further, the direction and magnitude of between- and within-person relationships among variables might not correspond to each other (Fisher, Medaglia, & Jeronimus, 2018). LCS techniques allow inferences regarding idiographic change processes by establishing temporal precedence as well as adjusting for between-person variation, regression to the mean, and measurement error (Wright et al., 2015). Further, relative to multilevel modeling, this latent variable method accounts for lagged dependent variables (Falkenström, Finkel, Sandell, Rubel, & Holmqvist, 2017).
However, thus far, at least two prospective anxiety-cognition studies have applied this potent method. Supporting affective neuroscience and scar theories, rise in anxiety severity predicted steeper reductions in cognitive functioning within older adults across 2 years (Tetzner & Schuth, 2016); nonetheless, as the latter study had two time points, it cannot speak to whether growth in symptoms predicted subsequent worsening of EF. Recently, in a relatively healthy, cognitively-intact, twin sample, anxiety proneness predicted more reductions in attention and processing speed across 6 years (Petkus, Reynolds, Wetherell, Kremen, & Gatz, 2017); however, this study did not include worry symptom or EF measures. Taken together, heightened GAD might forecast larger future decreased EF over long durations.
Accordingly, by using bivariate dual LCS models, the current study aimed to clarify how within-person increased GAD severity might predict future shifts in unique EF components across 18 years in a community-adult sample. This endeavor offers novel contributions. It adds to one-time assessment and experimental designs that have dominated the field and is a step toward causal inferences. Also, most EF-anxiety research has centered on using emotional (or ‘hot’) EF paradigms (Hallion, Tolin, Assaf, Goethe, & Diefenbach, 2017). This is surprising as the aforementioned neurocognitive models hypothesize specific inverse relationships among worry or anxiety and ‘cold’ EF. Importantly, these theories center on shifting, inhibition, and mixing costs (MCs) i.e. EF domains that are understudied in GAD. Moreover, theories and evidence that highlight the inverse connection between cold EF and worry are growing (e.g. Moran, Bernat, Aviyente, Schroder, & Moser, 2015). This study thus fills a gap by testing the potentially instrumental effect of increased abnormal worry on change in non-emotional (or ‘cold’) EF aspects across time. Further, in developmental psychopathology, the 18-year duration offers distinct viewpoints for this research aim as between-person negative GAD-EF relationships have been found over similar periods (e.g. Zhang et al., 2015). Also, assessing for change in GAD symptoms can inform clinicians about persons at risk of EF decline. Based on the theories and evidence reviewed, we hypothesized that elevated GAD severity would be related to decreased inhibition, set-shifting, and MCs.
Method
Participants
This was a secondary analysis using the Midlife Development in the United States (MIDUS) dataset with three waves of data collection: 1995 to 1996 [time 1 (T1)]; 2004 to 2005 [time 2 (T2)]; and 2012 to 2013 [time 3 (T3)] (Ryff & Lachman, 2018; Ryff et al., 2017). This study was exempted from IRB approval as it used a publicly available dataset that can be obtained from the following online data repository: https://www.icpsr.umich.edu/icpsr-web/ICPSR/series/203. Participants (n = 2581) averaged 46.00 years (s.d. = 11.40, range = 24–74 years) at baseline, 54.71% were female, and 41.70% had college education. The sample comprised mostly White participants (92.02%), and the remaining 6.98% were African American, Asian, Native American, or Pacific Islander.
Measures
EF tests were administered as part of a larger battery of neuropsychological assessments (Lachman, Agrigoroaei, Tun, & Weaver, 2014). The BTACT was administered at T2 and T3 (but not at T1), whereas GAD was assessed at all waves. The EF subtests in the BTACT evidenced adequate convergent validity (e.g. rs = 0.41–0.52 with other EF tests) and discriminant validity (e.g. rs = 0.16–0.17 with immediate and delayed recall tests) (Lachman et al., 2014). Strong 4-week retest reliability (r = 0.82–0.83) has also been documented. Moreover, the telephone-administered EF subtests used in this study showed strong convergent validity (r = 0.76) with those administered in person.
Inhibition
The Stop-and-Go Switch Task (SGST; Tun & Lachman, 2006) - Reverse condition - was used to assess inhibition using accuracy (performance scores) and latency (response time). SGST Single-Task block trials were composed of Normal and Reverse conditions. Respondents replied ‘GO’ or ‘STOP’ in response to ‘GREEN’ or ‘RED’ signs respectively in the normal condition. The opposite rule applied in the reverse condition i.e. respondents replied ‘STOP’ for ‘GREEN’ and ‘GO’ for ‘RED’. Latencies were registered in milliseconds (ms) i.e. duration between the sign and correct answer given. Higher latency marked slower response times. Initially, 20 normal block trials were administered, followed by 20 reverse SGST Single-Task block trials.
Set-shifting
Ability to transit smoothly between unique mental sets was indexed by accuracy and latency (ms) on the SGST Mixed-Task Switch block trials (Tun & Lachman, 2006). Participants had to respond correctly to shifts in normal and reverse conditions at random times of two to six trials based on the signs ‘NORMAL’ or ‘REVERSE’ across 32 trials. More information on data reduction for this measure can be found in Lachman et al. (2014).
Mixing costs
MCs, a measure related to common EF (WM ability to facilitate staying on task), was computed. MCs refer to the difference between latencies (ms) during recurring trials of mixed blocks compared to recurring trials of single blocks (Smith, Banich, & Friedman, 2019). Consistent with theory (Rubin & Meiran, 2005), the MC index has consistently evidenced a large association with the common EF latent factor (r = 0.59) that encapsulates set-shifting, inhibition, and WM updating capacities (Smith et al., 2019). Further, using MCs to approximate common EF prevents overlap with set-shifting, a problem inherent with other indices, such as global switch costs (Vandierendonck, Liefooghe, & Verbruggen, 2010).
Generalized anxiety disorder severity
GAD severity was measured with the Composite International Diagnostic Interview-Short Form (CIDI-SF; Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998) derived from the Diagnostic and Statistical Manual for Mental Disorders-Third Version-Revised (DSM-III-R; Wittchen, Zhao, Kessler, & Eaton, 1994) criteria. Eight item ratings that were most concordant with current DSM-5 criteria were averaged to form a GAD severity score, ranging from 1 (never experienced symptoms) to 4 (experienced symptoms on most days). These included the frequency at which participants experienced excessive and unrealistic worry about multiple things and six associated symptoms (‘were restless because of your worry’, ‘were irritable because of your worry’, ‘had trouble falling or staying asleep’, ‘had trouble keeping your mind on what you were doing’, ‘were low on energy’, ‘had sore or aching muscles because of tension’) in the past 12 months on a four-point Likert-scale (1 = never, 2 = less than half the days, 3 = about half the days, 4 = on most days). In this study, internal consistencies (Cronbach’s α) for the GAD severity scale were 0.71, 0.70, and 0.71 at T1, T2, and T3, respectively. The CIDI-SF was developed to replicate GAD diagnoses using the full CIDI. It has a high degree of sensitivity (96.6%), specificity (99.8%), and global agreement with the GAD diagnosis (99.6%) (Kessler et al., 1998). Also, 100% of persons who met DSM-III-R-defined GAD criteria also met DSM-IV-defined GAD criteria in a previous study (Abel & Borkovec, 1995). Further, the CIDI-SF showed good retest reliability (agreement = 0.89; κ = 0.69) (Kessler et al., 1998). Based on the CIDI-SF, the proportion of participants who had GAD at each time point was 2.33, 2.03, and 1.96%, respectively. Those with GAD had higher average symptom severity than those without GAD at T1 (3.39 v. 2.34, d = 0.84), T2 (3.28 v. 2.23, d = 0.88), and T3 (3.25 v. 2.21, d = 0.88), indicating that those diagnosed with GAD using the CIDI reportedly worried more days than not in the past year.
Procedure
Interviewers administered the SGST over the phone at a standardized location during times chosen by participants for convenience and to reduce surrounding distraction. A computerized system managed when sound stimuli (e.g. instructions and block trials) were played. Each cognitive testing session was recorded as a digital auditory file and analyzed afterward. On average, the cognitive tests took about 20 min to administer. For the highest sound quality, participants were encouraged to use landlines. Further, during testing, they were asked to not write and to close their eyes to minimize cognitive load (Vredeveldt, Hitch, & Baddeley, 2011). Before the test, research personnel briefly examined if participants could hear the interviewer clearly by instructing respondents to repeat a series of numbers back to them. The volume was then altered accordingly as required for 4% of the cases with slight hearing difficulties (Lachman et al., 2014). In the normal condition, participants were instructed, ‘Every time I say RED you will say STOP, and every time I say GREEN you will say GO’. In the reverse condition, they were directed, ‘Every time I say RED you will say GO, and every time I say GREEN you will say STOP’. Before the actual trials, practice runs were given for each condition. The entire cognitive testing script, preprocessing, and stimulus presentation details of the BTACT switch task can be found at the test developers’ website (http://www.brandeis.edu/projects/lifespan).
Data analyses
We performed structural equation modeling with the lavaan package (Rosseel, 2012) using R software. Prior to estimating models, we screened the data for non-normality by applying the rule of skewness ⩾±3 and kurtosis ⩾±7 as markers of univariate outliers, and Mahalanobis distance values to identify multivariate outliers (West, Finch, & Curran, 1995). As several non-normal distributions were identified in the data, we opted to use maximum likelihood with robust (MLR) estimators for all of our analyses (Yuan & Bentler, 2000). Aberrations from univariate and multivariate normality distributions are not uncommon in health and cognitive data (Cain, Zhang, & Yuan, 2017). Also, as nonnormal data could result in biased fit tests and standard errors, MLR estimators adjust for such non-normality and offer more precise parameter and standard error estimates (Brown, 2015). Further, prior to analysis, six cases with multivariate outliers were removed from the dataset.
To judge goodness-of-fit of all models, we used the χ2 index. Models with χ2 values that were not statistically significant indicated that the model had good fit. As five comparisons were made, we applied a Bonferroni correction and set the α value to 0.05/5 = 0.01 to guard against type 1 error (Simes, 1986). Further, we assessed if models surpassed heuristic cut-offs of practical fit indices: confirmatory fit index (CFI; Bentler, 1990; CFI ⩾ 0.95); root mean square error of approximation (RMSEA; Steiger, 1990; RMSEA ⩽ 0.050). To handle missing data, we used full information maximum likelihood suitable for our data presumed to be missing at random (Graham, 2009). Unstandardized regression estimates were shown.
To test if the measures were assessed along the same scale at all time points, we examined the equivalence of factor loadings (λs), intercepts (τs), and residual variances (εs) (Millsap & Yun-Tein, 2004). Tests of configural invariance were based on global model fit as well as statistical significance of the λs. To assess metric invariance, we constrained λs to be equal across all waves and compared the fit of the constrained model to the configural model. Next, we tested scalar invariance by comparing a model with both λs and τs constrained to equality to the model with only λs constrained across waves. Last, we evaluated strict invariance by juxtaposing a fully constrained model (i.e. equality constrained λs, τs, and εs) to a model with scalar invariance. We used change in fit indices to evaluate measurement invariance at each step (Meade, Johnson, & Braddy, 2008). Change in CFI and RMSEA values of >−0.010 and +0.025, respectively, from the unconstrained to constrained model suggest that the unconstrained model is preferable.
Afterward, to test how T1 − T2 change in GAD severity predicted T2 − T3 change in an EF construct within persons, we used bivariate dual LCS models (McArdle, 2009). Figure 1 displays this multivariate model. By examining latent change of a construct across two consecutive waves, its course can be modeled in terms of its baseline level and differences in scores between each period. The following equation computes for within-person T2 − T3 change in EF:
| (1) |
where ΔEF indicates the latent difference in an EF construct between T2 and T3, αX indicates the between-person constant change parameter linked to the latent slope (EFS), and βX indicates the within-person self-feedback processes called the proportional effect. Importantly, the coupling parameter (δY) indicates the within-person effect of 9-year change in GAD severity on future 9-year change in EF domains. The term ‘EF’ in Equation (1) is a placeholder for five separate models that tested unique EF components: inhibition (accuracy and latency); set-shifting (accuracy and latency); and MCs. All models controlled for T1 major depressive disorder (MDD) severity.†,1 To achieve parsimonious, theoretically synced, and interpretable models, we fixed residual variances that were negative or not statistically significant to zero. Last, in all our final models, we estimated an additional parameter (covariation between EF variables at T2 and T3) suggested by the modification indices. Inclusion of this parameter was informed by theory and the goal of achieving well-fitting models (Hertzog, Dixon, Hultsch, & MacDonald, 2003). For all data involving response times, the average latencies or MCs of the single- and mixed-task block trials were used (Lachman et al., 2014). Cohen’s d effect size was calculated using the formula , where (Dunlap, Cortina, Vaslow, & Burke, 1996; Dunst, Hamby, & Trivette, 2004). Thus, d values of 0.2, 0.5, and 0.8, represent small, moderate, and large effect sizes, respectively (Cohen, 1988).
Fig. 1.
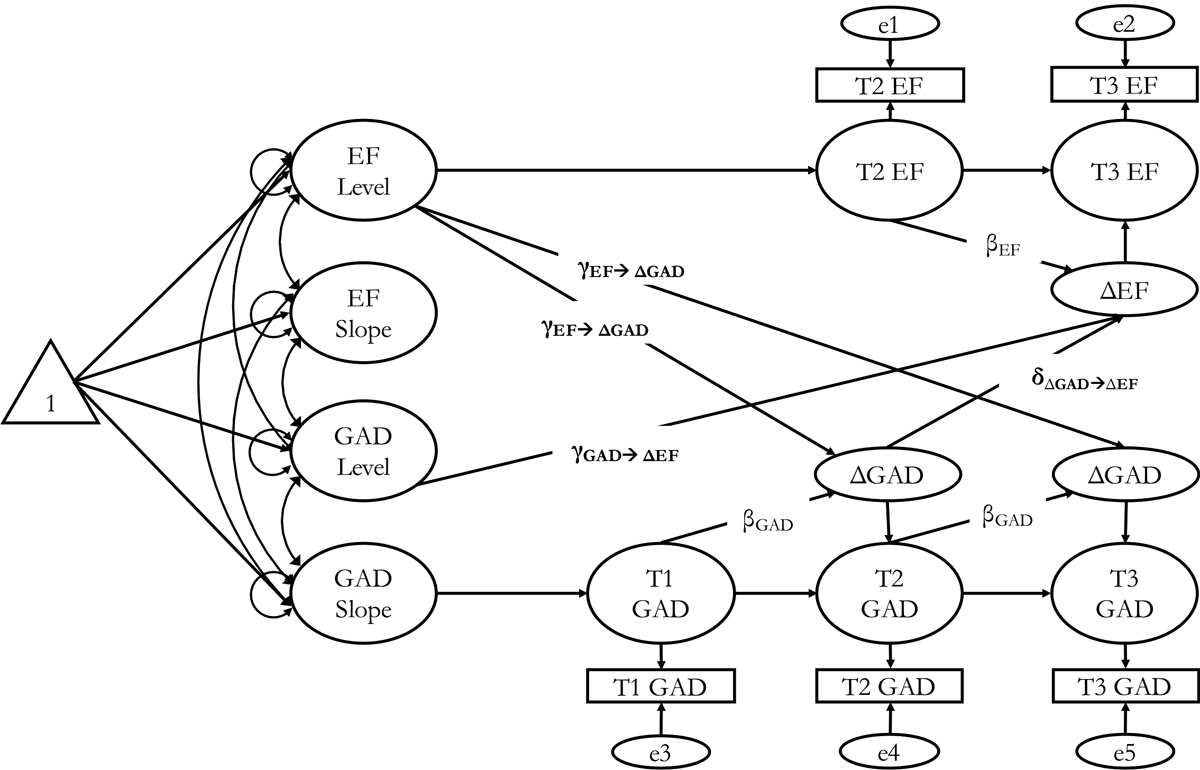
Bivariate dual LCS models of GAD severity and EF. EF, executive function; GAD, generalized anxiety disorder; LCS, latent change score; T1, time 1; T2, time 2; T3, time 3. Note: Of most interest to this study is the coupling parameter (δY) that indicates the effect of T1 − T2 change in GAD severity predicting T2 − T3 change in EF while adjusting for other parameters in the model. In each bivariate LCS model, EF serves as a placeholder for one of the three EF constructs examined in this study (inhibition, set-shifting, and MCs).
Results
Longitudinal measurement invariance
Across all occasions, we observed invariance of factor structure (configural), λs (metric), τs (scalar), and εs (strict). Table 1 shows the fit indices for all the measurement invariance models for the one-factor GAD severity model across three time points, as well as six-factor model of GAD severity and the five EF constructs across T2 and T3. The satisfactory absolute and relative fit indices suggested that strict invariance was supported. Thus, across each wave, latent GAD severity and EF scores were measured along the same scale, allowing for meaningful comparisons of latent scores across different waves.
Table 1.
Longitudinal measurement invariance of GAD severity across waves (standardized estimates)
| χ2 | df | p | CFI | RMSEA | |
|---|---|---|---|---|---|
| One-factor model of the GAD severity scale | |||||
| Time 1 | 69.787 | 14 | <.001 | .950 | .078 |
| Time 2 | 62.929 | 14 | <.001 | .946 | .079 |
| Time 3 | 42.958 | 14 | <.001 | .975 | .056 |
| Level of measurement invariance one-factor model of GAD | |||||
| Configural (varying λ, τ, ε across time) | 79.314 | 27 | <.001 | .984 | .055 |
| Metric (equal λ, varying τ, ε across time) | 93.791 | 37 | <.001 | .984 | .047 |
| Scalar (equal λ, τ, varying ε across time) | 146.139 | 47 | <.001 | .973 | .054 |
| Strict (equal λ, τ, ε across time) | 161.292 | 59 | <.001 | .973 | .049 |
| Tests of measurement invariance of 8-item GAD severity | |||||
| Configural invariance v. metric invariance | Δχ2(df = 10) = 11.952, p = .288, ΔCFI = −.000, ΔRMSEA = −.008 | ||||
| Metric invariance v. scalar invariance | Δχ2(df = 10) = 58.236, p <.001, ΔCFI = −.011, ΔRMSEA = +.006 | ||||
| Scalar invariance v. strict invariance | Δχ2(df = 12) = 15.733, p = .204, ΔCFI = −.001, ΔRMSEA = −.005 | ||||
| Six-factor model of GAD severity scale and five EF constructs | |||||
| Time 2 | 94.503 | 39 | <.001 | .978 | .048 |
| Time 3 | 69.753 | 39 | .002 | .988 | .037 |
| Level of measurement invariance six-factor model of GAD severity scale and five EF constructs | |||||
| Configural (varying λ, τ, ε across time) | 98.015 | 68 | .01 | .992 | .026 |
| Metric (equal λ, varying τ, ε across time) | 106.145 | 73 | .007 | .992 | .027 |
| Scalar (equal λ, τ, varying ε across time) | 112.212 | 78 | .007 | .991 | .026 |
| Strict (equal λ, τ, ε across time) | 115.911 | 84 | .012 | .992 | .024 |
| Tests of measurement invariance of six-factor model of GAD severity scale and five EF constructs | |||||
| Configural invariance v. metric invariance | Δχ2(df = 5) = 9.131, p = .104, ΔCFI = −.001, ΔRMSEA = +.001 | ||||
| Metric invariance v. scalar invariance | Δχ2(df = 5) = 6.007, p = .306, ΔCFI = −.000, ΔRMSEA = −.001 | ||||
| Scalar invariance v. strict invariance | Δχ2(df = 6) = 4.725, p = .580, ΔCFI = .000, ΔRMSEA = −.001 | ||||
GAD, generalized anxiety disorder; EF, executive functioning; CFI, confirmatory fit index; RMSEA, root mean square error of approximation; λ, factor loading; τ, factor intercept; ε, item residual variance; Δ, change in fit statistic.
Inhibition and GAD severity
The LCS models showed good fit for inhibition indexed by accuracy [χ2(df = 13) = 12.398, p = 0.495, CFI = 1.000, RMSEA = 0.000] and latency [χ2(df = 12) = 39.081, p < 0.001, CFI = 0.968, RMSEA = 0.030]. Steeper within-person increase in GAD severity predicted future decline in inhibition (accuracy: b = −0.006, p < 0.001, d = −0.61; latency: b = 0.003, p < 0.001, d = 0.59). Table 2 shows more details.
Table 2.
Two bivariate dual LCS models for inhibition (indexed by accuracy and latency) and GAD symptoms
| Inhibition (Accuracy) and GAD | ||||
|---|---|---|---|---|
| Model fit | χ2(df = 13) = 12.398, p = .495, CFI = 1.000, RMSEA = .000, AIC = 18,568.902, SABIC = 18,602.830 | |||
| Parameter estimate | ΔGADT1–T2 → ΔINHAT2–T3 | MDDT1 → ΔINHAT2–T3 | ||
| Within-person bivariate coupling effects (δ) | b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d |
| −0.006*** (0.000) | −0.61 | 0.016 (0.009) | 0.07 | |
| Inhibition (Accuracy) | GAD | |||
| b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d | |
| Between-person initial status | ||||
| Mean (s.e.) | −0.001 (0.019) | −0.00 | 2.855*** (0.016) | 6.34 |
| Variance (s.e.) | 0a | 0.112*** (0.010) | 0.40 | |
| Between-person constant change (α) | ||||
| Mean (s.e.) | −0.013 (0.022) | −0.02 | −0.017 (0.011) | 0.06 |
| Variance (s.e.) | 0a | 0a | ||
| Residual variance | ||||
| 1.002***(0.101) | 0.36 | 0.15***(0.007) | 0.73 | |
| (T1 INHA–T2 INHA correlation) | 0.182*** (0.037) | 0.18 | ||
| Between-person INHA – GAD correlation, b (s.e.) | 0.007 (0.015) | 0.02 | ||
| Inhibition (Latency) and GAD | ||||
| Model fit | χ2(df = 12) = 39.081, p < .001, CFI = .968, RMSEA = .030, AIC = 3,591.361, SABIC = 3,628.116 | |||
| Parameter estimate | ΔGADT1–T2 → ΔINHLT2–T3 | MDDT1 → ΔINHLT2–T3 | ||
| Within-person bivariate coupling effects (δ) | b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d |
| 0.003*** (0.000) | 0.59 | 0.003 (0.003) | 0.03 | |
| Inhibition (Latency) | GAD | |||
| b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d | |
| Between-person initial status | ||||
| Mean (s.e.) | 0.931*** (0.004) | 8.54 | 2.855*** (0.016) | 6.35 |
| Variance (s.e.) | 0.057*** (0.007) | 0.31 | 0.112*** (0.010) | 0.40 |
| Between-person constant change (α) | ||||
| Mean (s.e.) | 1.239*** (0.007) | 6.61 | −0.017 (0.011) | −0.06 |
| Variance (s.e.) | 0a | 0a | ||
| Residual variance | ||||
| 0.039*** (0.001) | 0.89 | 0.149*** (0.007) | 0.73 | |
| (T1 INHL–T2 INHL correlation), b (s.e.) | 0.027*** (0.002) | 0.59 | ||
| Between-person INHL – GAD correlation, b (s.e.) | −0.000 (0.003) | −0.00 | ||
b, unstandardized parameter estimates; s.e., standard error; INHA, inhibition accuracy; INHL, inhibition latency; GAD, generalized anxiety disorder; MDD, major depressive disorder; CFI, confirmatory fir index; RMSEA, root mean square error of approximation; AIC, Akaike information criteria; SABIC, sample-adjusted Bayesian information criteria.
Parameter estimate was fixed to 0 to facilitate model convergence.
p < 0.001.
Set-shifting and GAD severity
The models showed good fit for set-shifting marked by both accuracy [χ2(df = 13) = 24.982, p = 0.023, CFI = 0.970, RMSEA = 0.022] and latency [χ2(df = 12) = 39.819, p < 0.001, CFI = 0.965, RMSEA = 0.030]. Greater increase in GAD severity significantly predicted future decline in set-shifting (accuracy: b = −0.005, p < 0.001, d = −0.51; latency: b = 0.005, p < 0.001, d = 0.96). Table 3 elaborates on these results.
Table 3.
Two bivariate dual LCS models for set-shifting (indexed by accuracy and latency) and GAD symptoms
| Set-Shifting (Accuracy) and GAD | ||||
|---|---|---|---|---|
| Model fit | χ2(df = 13) = 24.982, p = .023, CFI = .970, RMSEA = .022, AIC = 18,607.870, SABIC = 18,641.797 | |||
| Parameter estimate | ΔGADT1–T2 → ΔSETAT2–T3 | MDDT1 → ΔSETAT2–T3 | ||
| Within-person bivariate coupling effects (δ) | b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d |
| −0.005*** (0.000) | −0.51 | 0.003 (0.010) | 0.01 | |
| Set-Shifting (Accuracy) | GAD | |||
| b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d | |
| Between-person initial status | ||||
| Mean (s.e.) | −0.002 (0.019) | −0.00 | 2.855*** (0.016) | 6.34 |
| Variance (s.e.) | 0a | 0.112*** (0.010) | 0.39 | |
| Between-person constant change (α) | ||||
| Mean (s.e.) | −0.004 (0.022) | 0.01 | −0.017 (0.011) | −0.05 |
| Variance (s.e.) | 0a | 0a | ||
| Residual variance | ||||
| , b (s.e.) | 1.001*** (0.053) | 0.69 | 0.149*** (0.007) | 0.73 |
| (T1 SETA–T2 SETA correlation), b (s.e.) | 0.124*** (0.028) | 0.16 | ||
| Between-person SETA – GAD correlation, b (s.e.) | 0.004 (0.016) | 0.02 | ||
| Set-Shifting (Latency) and GAD | ||||
| Model fit | χ2(df = 12) = 39.819, p < .001, CFI = .965, RMSEA = .030, AIC = 6,962.532, SABIC = 6,999.287 | |||
| Parameter estimate | ΔGADT1–T2 → ΔSETLT2–T3 | MDDT1 → ΔSETLT2–T3 | ||
| Within-person bivariate coupling effects (δ) | b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d |
| 0.005*** (0.000) | 0.96 | 0.001 (0.004) | 0.00 | |
| Set-Shifting (Latency) | GAD | |||
| b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d | |
| Between-person initial status | ||||
| Mean (s.e.) | 1.171*** (0.006) | 7.20 | 2.855*** (0.016) | 6.34 |
| Variance (s.e.) | 0.058*** (0.016) | 0.13 | 0.112*** (0.010) | 0.39 |
| Between-person constant change (α) | ||||
| Mean (s.e.) | 1.495*** (0.009) | 6.17 | −0.017 (0.011) | −0.05 |
| Variance (s.e.) | 0a | 0a | ||
| Residual variance | ||||
| , b (s.e.) | 0.102*** (0.010) | 0.36 | 0.149*** (0.007) | 0.73 |
| (T1 SETL–T2 SETL correlation), b (s.e.) | 0.049*** (0.005) | 0.37 | ||
| Between-person SETL – GAD correlation, b (s.e.) | 0.001 (0.004) | 0.01 | ||
b, unstandardized parameter estimates; s.e., standard error; SETA, set-shifting accuracy; SETL, set-shifting latency; GAD, generalized anxiety disorder; MDD, major depressive disorder; CFI, confirmatory fit index; RMSEA, root mean square error of approximation; AIC, Akaike information criteria; SABIC, sample-adjusted Bayesian information criteria.
Parameter estimate was fixed to 0 to facilitate model convergence.
p < 0.001.
Mixing costs and GAD severity
The models demonstrated good fit for MCs [χ2(df = 13) = 6.945, p = 0.905, CFI = 1.000, RMSEA = 0.000]. More growth in GAD severity substantially predicted future increase in MCs (b = 0.005, p < 0.001, d = 0.54). Table 4 expands on this set of findings. Online Supplementary Table S1 presents between-person descriptive statistics of study variables.2
Table 4.
Bivariate dual LCS models for MCs (a measure related to common EF) and GAD symptoms
| Mixing Costs and GAD | ||||
|---|---|---|---|---|
| Model fit | χ2(df = 13) = 6.945, p = .905, CFI = 1.000, RMSEA = .000, AIC = 18,192.889, SABIC = 18,226.816 | |||
| Parameter estimate | ΔGADT2–T3 → ΔMCT2–T3 | MDDT1 → ΔMCT2–T3 | ||
| Within-person bivariate coupling effects (δ) | b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d |
| 0.005*** (0.000) | 0.54 | −0.002 (0.008) | −0.01 | |
| Mixing Costs | GAD | |||
| b (s.e.) | Cohen’s d | b (s.e.) | Cohen’s d | |
| Between-person initial status | ||||
| Mean (s.e.) | 0.003 (0.019) | 0.01 | 2.855*** (0.016) | 6.34 |
| Variance (s.e.) | 0a | 0.112*** (0.010) | 0.39 | |
| Between-person constant change (α) | ||||
| Mean (s.e.) | 0.007 (0.022) | 0.01 | −0.017 (0.011) | 0.05 |
| Variance (s.e.) | 0a | 0a | ||
| Residual variance | ||||
| , b (s.e.) | 1.007*** (0.151) | 0.24 | 0.149*** (0.007) | 0.73 |
| (T1 MC–T2 MC correlation), b (s.e.) | 0.282 (0.041) | 0.07 | ||
| Between-person MC – GAD correlation, b (s.e.) | 0.003 (0.014) | 0.01 | ||
b, unstandardized parameter estimates; s.e., standard error; EF, executive functioning; GAD, generalized anxiety disorder; MDD, major depressive disorder; CFI, confirmatory fit index; RMSEA, root mean square error of approximation; AIC, Akaike information criteria; SABIC, sample-adjusted Bayesian information criteria.
Parameter estimate was fixed to 0 to facilitate model convergence.
p < 0.001.
Discussion
To our knowledge, this empirical study was the first to examine how naturalistic within-person 9-year change in GAD severity predicted future 9-year change in EF components in community-dwelling adults. Analyses revealed that within-person increased GAD severity predicted subsequent reduced inhibition, set-shifting, and MCs with moderate-to-large effects. Of note is that results cannot be explained by between-person variance, prior EF or MDD, measurement error, or regression to the mean. Also, as opposed to the current within-person findings, prior between-person research found small, null, or even positive relationships between EF and GAD severity (see Leonard & Abramovitch, 2019 and online Supplementary Table S1). This is consistent with prior studies showing little relationships between within and between person findings (Fisher et al., 2018). Developmental affective neuroscience (Beaudreau et al., 2013) and scar theories (Ottaviani et al., 2016) may help explain these findings.
Why did 9-year increase in GAD severity predict greater future 9-year reductions in inhibition and set-shifting accuracy? Such data lend further weight to scar theories, which propose and show that heightened (v. lowered) anxiety severity creates a long-term effect on allostatic load and thus predicts cognitive impairment, even across long periods (e.g. 10–17 years; Gimson, Schlosser, Huntley, & Marchant, 2018). On that note, long-term impact of chronic worry on allostatic load may effect cardiovascular and neurobiological changes in septo-hippocampal areas and left amygdala-ventral medial prefrontal cortex connectivity, thereby contributing to EF decline across multiple decades (Johansson et al., 2010, 2013; Makovac et al., 2016; Thurston, Kubzansky, Kawachi, & Berkman, 2006). Importantly, these regions have been viewed as crucial for effective emotion regulation (Carnevali, Thayer, Brosschot, & Ottaviani, 2018). Taken together, worry-induced long-term physiological changes (e.g. reduced heart rate variability, increased cortisol release in the hypothalamic-pituitary-adrenal axis) could have compromised emotion regulation skills and adversely affected EF capacities across decades.
Results that rise in GAD severity predicted elevated inhibition and shifting latencies as well as MCs might mean that increased GAD leads to more inefficiencies or uncertainty about responses over time. The long-term effect of chronic worry on allostatic load might impact biomarkers that reinforce the GAD severity-slower latencies relationship and raise the tendency to mind wander (Makovac et al., 2019). Further, results fit with other studies using LCS models and showing that decreased sense of well-being predicted larger decline in perceptual speed across 13 years in older adults (Gerstorf, Lövdén, Röcke, Smith, & Lindenberger, 2007). In addition, findings aligned with other LCS-based data that older adults’ rise in depressive symptoms predicted future reduced perceptual speed over 15 years (Bielak, Gerstorf, Kiely, Anstey, & Luszcz, 2011). Overall, increased allostatic load probably co-occurred with compensatory tactics (e.g. effortful processing) that compromised speed across time. More longitudinal studies can shed light on the validity of these conjectures.
Study limitations merit consideration. First, as the MIDUS project did not measure baseline EF, we could not test if change in EF predicted later change in GAD symptoms, which could have been examined using the bivariate dual LCS models had the data been present. This is a tenable hypothesis as research suggests that EF capacities are needed to regulate emotions optimally (Teper, Segal, & Inzlicht, 2013). Moreover, research suggests that EF issues can independently predict future GAD, and both EF problems and GAD symptoms can aggravate one another longitudinally (Zainal & Newman, 2018). Prospective studies could explore this. Also, despite controlling for baseline MDD symptoms, non-worry-specific rise in other disorder symptoms might predict EF decline (Stordal et al., 2005) and it is possible that our findings are not specific to GAD. Similarly, upcoming studies can determine if the effects of heightened worry extend to other forms of EF excluded here. In addition, future research should test these predictions in heterogeneous samples given the lack of socio-economic and ethnic diversity herein. Replication efforts with DSM-5-diagnosed (v. DSM-III-R used in this study) are also needed. Differences in symptom duration (past 6-month v. 12-month) and the replacement of unrealistic worry by uncontrollable worry might alter findings. Moreover, results could change if extensive (v. brief) EF tests were used. Relatedly, retrospective symptom reporting likely added recall biases to the measurement process. Complementing self-reports with assessments that tapped into present or past few day symptoms could have remedied such issues. Additionally, unmeasured third variables (e.g. genetic factors heightening risk for future EF decline and increased worry; Lee et al., 2008) may have affected the results, and merit further study. Nonetheless, chief strengths of this paper include the large sample size, 18-year study period, use of reliable and valid EF and symptom assessments, and test of longitudinal measurement equivalence prior to analyses.
With all that said, if future studies were to replicate this set of results, it might suggest that treatments meant to target GAD symptoms could generalize beyond those symptoms to prevent future executive dysfunction decline. Encouragingly, data pooled across 17 non-randomized and randomized controlled trials (RCTs) have shown that mindfulness-based interventions (MBIs) could both reduce worry and augment WM and inhibition (Chiesa, Calati, & Serretti, 2011). Nonetheless, most MBIs were tested in healthy populations, and only one study so far offered preliminary evidence that its benefits could extend to habitual worriers (Wetherell et al., 2017). Also, as initially suggested by two recent RCTs (Course-Choi, Saville, & Derakshan, 2017; Grol et al., 2018), the field might profit from more studies determining the short- and long-term efficacy of cognitive-behavioral therapy and various forms of cognitive training on both worry symptoms and unique EF domains. Plausibly, successfully reducing excessive worry either through tried-and-tested or novel methods might generalize to other symptoms and EF issues, and offer another potential avenue for future research.
Supplementary Material
Financial support.
Since 1995, the Midlife Development in the United States (MIDUS) study has been funded by John D. and Catherine T. MacArthur Foundation Research Network; National Institute on Aging (P01-AG020166); National Institute on Aging (U19-AG051426). The original investigators and funding agency are not responsible for the analyses or interpretations presented here.
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720000422.
Data. The data used in this publication were made available by the Data Archive on University of Wisconsin-Madison Institute on Aging, 1300 University Avenue, 2245 MSC, Madison, Wisconsin 53706–1532.
Conflict of interest. The authors do not have any conflicts of interest or financial disclosures.
Ethical standards. This study was conducted in compliance with the American Psychological Association (APA) ethical standards in the treatment of human participants and approved by the Institutional Review Board (IRB). Informed consent was obtained from participants as per IRB requirements at Harvard University, Georgetown University, University of California at Los Angeles, and University of Wisconsin. Since this study used a publicly available dataset, it was exempt from additional IRB approval.
More information on the MDD measure can be found in the online Supplementary Materials.
As within-person analyses were the main focus of this paper, we presented between-person bivariate relationships in the online Supplementary Materials.
The notes appear after the main text.
References
- Abel JL, & Borkovec TD (1995). Generalizability of DSM-III-R generalized anxiety disorders to proposed DSM-IV criteria and cross-validation of proposed changes. Journal of Anxiety Disorders, 9, 303–315. doi: 10.1016/0887-6185(95)00011-C. [DOI] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, & Aizenstein H (2015). Emotion reactivity and regulation in late-life generalized anxiety disorder: Functional connectivity at baseline and post-treatment. American Journal of Geriatric Psychiatry, 23, 200–214. doi: 10.1016/j.jagp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD (2001). Is working memory still w orking? European Psychologist, 56, 851–864. doi: 10.1037/0003-066X.56.11.851. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18, 89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Beaudreau SA, MacKay-Brandt A, & Reynolds J (2013). Application of a cognitive neuroscience perspective of cognitive control to late-life anxiety. Journal of Anxiety Disorders, 27, 559–566. doi: 10.1016/j.janxdis.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwé M, Deroost N, Koster EHW, De Lissnyder E, & De Raedt R (2014). Worrying and rumination are both associated with reduced cognitive control. Psychological Research, 78, 651–660. doi: 10.1007/s00426-013-0517-5. [DOI] [PubMed] [Google Scholar]
- Beluche I, Carrière I, Ritchie K, & Ancelin ML (2010). A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychological Medicine, 40, 1039–1049. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models Psychological Bulletin, 107, 238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, & Jones LL (2009). Executive functions after age 5: Changes and correlates. Developmental Review, 29, 180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak AAM, Gerstorf D, Kiely KM, Anstey KJ, & Luszcz M (2011). Depressive symptoms predict decline in perceptual speed in older adulthood. Psychology and Aging, 26, 576–583. doi: 10.1037/a0023313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activaion, and health. Journal of Psychosomatic Research, 60, 113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brown TA (2015). Confirmatory factor analysis for applied research (2nd ed.). New York: Guilford Press. [Google Scholar]
- Brown LA, Brockmole JR, Gow AJ, & Deary IJ (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Experimental Aging Research, 38, 1–19. doi: 10.1080/0361073X.2012.636722. [DOI] [PubMed] [Google Scholar]
- Cain MK, Zhang Z, & Yuan K-H (2017). Univariate and multivariate skewness and kurtosis for measuring nonnormality: Prevalence, influence and estimation. Behavior Research Methods, 49, 1716–1735. doi: 10.3758/s13428-016-0814-1. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Thayer JF, Brosschot JF, & Ottaviani C (2018). Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. International Journal of Psychophysiology, 131, 131–138. doi: 10.1016/j.ijpsycho.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Calati R, & Serretti A (2011). Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review, 31, 449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power for the social sciences. Hillsdale, NJ: Laurence Erlbaum and Associates. [Google Scholar]
- Course-Choi J, Saville H, & Derakshan N (2017). The effects of adaptive working memory training and mindfulness meditation training on processing efficiency and worry in high worriers. Behavioral Research and Therapy, 89, 1–13. doi: 10.1016/j.brat.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Davidson KW, Mostofsky E, & Whang W (2010). Don’t worry, be happy: Positive affect and reduced 10-year incident coronary heart disease: The Canadian Nova Scotia Health Survey. European Heart Journal, 31, 1065–1070. doi: 10.1093/eurheartj/ehp603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, … Starr JM (2009). Age-associated cognitive decline. British Medical Bulletin, 92, 135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, & Burke MJ (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1, 170–177. doi: 10.1037/1082-989x.1.2.170. [DOI] [Google Scholar]
- Dunst CJ, Hamby DW, & Trivette CM (2004). Guidelines for calculating effect sizes for practice-based research syntheses. Centerscope, 3, 1–10. [Google Scholar]
- Evans GW, & Fuller-Rowell TE (2013). Childhood poverty, chronic stress, and young adult working memory: The protective role of self-regulatory capacity. Developmental Science, 16, 688–696. doi: 10.1111/desc.12082. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion (Washington, D.C.), 7, 336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Falkenström F, Finkel S, Sandell R, Rubel JA, & Holmqvist R (2017). Dynamic models of individual change in psychotherapy process research. Journal of Consulting and Clinical Psychology, 85, 537–549. doi: 10.1037/ccp0000203. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Medaglia JD, & Jeronimus BF (2018). Lack of group-to-individual generalizability is a threat to human subjects research. Proceedings of the National Academy of Sciences of the USA, 115, E6106–E6115. doi: 10.1073/pnas.1711978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, & Lindenberger U (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43, 705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- Gimson A, Schlosser M, Huntley JD, & Marchant NL (2018). Support for midlife anxiety diagnosis as an independent risk factor for dementia: A systematic review. BMJ Open, 8, e019399. doi: 10.1136/bmjopen-2017-019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Grol M, Schwenzfeier AK, Stricker J, Booth C, Temple-McCune A, Derakshan N, … Fox E (2018). The worrying mind in control: An investigation of adaptive working memory training and cognitive bias modification in worry-prone individuals. Behaviour Research and Therapy, 103, 1–11. doi: 10.1016/j.brat.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Gulpers BJA, Oude Voshaar RC, van Boxtel MPJ, Verhey FRJ, & Köhler S (2019). Anxiety as a risk factor for cognitive decline: A 12-year follow-up cohort study. American Journal of Geriatric Psychiatry, 27, 42–52. doi: 10.1016/j.jagp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM, & Jha AP (2014). Fractionating the role of executive control in control over worry: A preliminary investigation. Behaviour Research and Therapy, 54, 1–6. doi: 10.1016/j.brat.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Tolin DF, Assaf M, Goethe J, & Diefenbach GJ (2017). Cognitive control in generalized anxiety disorder: Relation of inhibition impairments to worry and anxiety severity. Cognitive Therapy and Research, 41, 610–618. doi: 10.1007/s10608-017-9832-2. [DOI] [Google Scholar]
- Hayes S, Hirsch C, & Mathews A (2008). Restriction of working memory capacity during worry. Journal of Abnormal Psychology, 117, 712–717. doi: 10.1037/a0012908. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, & MacDonald SWS (2003). Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psychology and Aging, 18, 755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Hällström T, Norton MC, Waern M, Östling S, … Skoog I (2013). Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer’s disease: A 38-year longitudinal population study. BMJ Open, 3, e003142. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Östling S, Gustafson D, Bengtsson C, & Skoog I (2010). Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain, 133, 2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Kassem AM, Ganguli M, Yaffe K, Hanlon JT, Lopez OL, Wilson JW, & Cauley JA (2017). Anxiety symptoms and risk of cognitive decline in older community-dwelling men. International Psychogeriatrics, 29, 1137–1145. doi: 10.1017/S104161021700045X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Vokonas PS, & Weiss ST (1994). Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation, 90, 2225–2229. doi: 10.1161/01.CIR.90.5.2225. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, & Wittchen H-U (1998). The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). International Journal of Methods in Psychiatric Research, 7, 171–185. doi: 10.1002/mpr.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, … Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences, 110, 18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Spiro A 3rd, Weiss ST, Vokonas PS, & Sparrow D (1997). Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation, 95, 818–824. doi: 10.1161/01.CIR.95.4.818. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: Psychometric properties of the Brief Test of Adult Cognition by Telephone (BTACT). Assessment, 21, 404–417. doi: 10.1177/1073191113508807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, & Schwartz BS (2008). Apolipoprotein E genotype, cortisol, and cognitive function in community-dwelling older adults. American Journal of Psychiatry, 165, 1456–1464. doi: 10.1176/appi.ajp.2008.07091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh E, & Hirsch CR (2011). Worry in imagery and verbal form: Effect on residual working memory capacity. Behaviour Research and Therapy, 49, 99–105. doi: 10.1016/j.brat.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard K, & Abramovitch A (2019). Cognitive functions in young adults with generalized anxiety disorder. European Psychiatry, 56, 1–7. doi: 10.1016/j.eurpsy.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Makovac E, Fagioli S, Watson DR, Meeten F, Smallwood J, Critchley HD, & Ottaviani C (2019). Response time as a proxy of ongoing mental state: A combined fMRI and pupillometry study in generalized anxiety disorder. NeuroImage, 191, 380–391. doi: 10.1016/j.neuroimage.2019.02.038. [DOI] [PubMed] [Google Scholar]
- Makovac E, Watson DR, Meeten F, Garfinkel SN, Cercignani M, Critchley HD, & Ottaviani C (2016). Amygdala functional connectivity as a longitudinal biomarker of symptom changes in generalized anxiety. Social Cognitive and Affective Neuroscience, 11, 1719–1728. doi: 10.1093/scan/nsw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ (2009). Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology, 60, 577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Meade AW, Johnson EC, & Braddy PW (2008). Power and sensitivity of alternative fit indices in tests of measurement invariance. Journal of Applied Psychology, 93, 568–592. doi: 10.1037/0021-9010.93.3.568. [DOI] [PubMed] [Google Scholar]
- Millsap RE, & Yun-Tein J (2004). Assessing factorial invariance in ordered-categorical measures. Multivariate Behavioral Research, 39, 479–515. doi: 10.1207/S15327906MBR3903_4. [DOI] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions. Current Directions in Psychological Science, 21, 8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychological Bulletin, 142, 831–864. doi: 10.1037/bul0000051. [DOI] [PubMed] [Google Scholar]
- Moran TP, Bernat EM, Aviyente S, Schroder HS, & Moser JS (2015). Sending mixed signals: Worry is associated with enhanced initial error processing but reduced call for subsequent cognitive control. Social Cognitive and Affective Neuroscience, 10, 1548–1556. doi: 10.1093/scan/nsv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Watson DR, Meeten F, Makovac E, Garfinkel SN, & Critchley HD. (2016). Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biological Psychology, 119, 31–41. 10.1016/j.biopsycho.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, & Gatz M (2017). Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychology and Aging, 32, 278–292. doi: 10.1037/pag0000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, … Darby D (2012). Mild worry symptoms predict decline in learning and memory in healthy older adults: A 2-year prospective cohort study. The American Journal of Geriatric Psychiatry, 20, 266–275. doi: 10.1097/JGP.0b013e3182107e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, … Maruff P (2014). Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. British Journal of Psychiatry, 204, 400–401. doi: 10.1192/bjp.bp.113.134239. [DOI] [PubMed] [Google Scholar]
- Rosseel Y (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- Rubin O, & Meiran N (2005). On the origins of the task mixing cost in the cuing task-switching paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- Ryff C, Almeida DM, Ayanian J, Carr DS, Cleary PD, Coe C, … Williams D (2017). Midlife in the United States (MIDUS 2), 2004–2006. ICPSR04652-v7. Ann Arbor, MI: Inter-university Consortium for Political and Social Research (distributor), 2017-11-20. doi: 10.3886/ICPSR04652.v7. [DOI] [Google Scholar]
- Ryff CD, & Lachman ME (2018). Midlife in the United States (MIDUS 3): Cognitive project, 2013–2017. doi: 10.3886/ICPSR37095.v1. [DOI] [Google Scholar]
- Shadish WR, Cook TD, & Campbell DT (2002). Experimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton Mifflin. [Google Scholar]
- Simes RJ (1986). An improved Bonferroni procedure for multiple tests of significance. Biometrika, 73, 751–754. doi: 10.2307/2336545. [DOI] [Google Scholar]
- Smith LL, Banich MT, & Friedman NP (2019). Individual differences in mixing costs relate to general executive functioning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 45, 606–613. doi: 10.1037/xlm0000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanopoulou E, Hirsch CR, Hayes S, Adlam A, & Coker S (2014). Are attentional control resources reduced by worry in generalized anxiety disorder? Journal of Abnormal Psychology, 123, 330–335. doi: 10.1037/a0036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH (1990). Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research, 25, 173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Stordal KI, Mykletun A, Asbjørnsen A, Egeland J, Landrø NI, Roness A, … Lund A (2005). General psychopathology is more important for executive functioning than diagnosis. Acta Psychiatrica Scandinavica, 111, 22–28. doi: 10.1111/j.1600-0447.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Teper R, Segal ZV, & Inzlicht M (2013). Inside the mindful mind: How mindfulness enhances emotion regulation through improvements in executive control. Current Directions in Psychological Science, 22, 449–454. doi: 10.1177/0963721413495869. [DOI] [Google Scholar]
- Tetzner J, & Schuth M (2016). Anxiety in late adulthood: Associations with gender, education, and physical and cognitive functioning. Psychology and Aging, 31, 532–544. doi: 10.1037/pag0000108. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD, Kawachi I, & Berkman LF (2006). Do depression and anxiety mediate the link between educational attainment and CHD? Psychosomatic Medicine, 68, 25–32. doi: 10.1097/01.psy.0000195883.68888.68. [DOI] [PubMed] [Google Scholar]
- Tun PA, & Lachman ME (2006). Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone. Age and Ageing, 35, 629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A, Liefooghe B, & Verbruggen F (2010). Task switching: Interplay of reconfiguration and interference control. Psychological Bulletin, 136, 601–626. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- Vredeveldt A, Hitch GJ, & Baddeley AD (2011). Eyeclosure helps memory by reducing cognitive load and enhancing visualisation. Memory & Cognition, 39, 1253–1263. doi: 10.3758/s13421-011-0098-8. [DOI] [PubMed] [Google Scholar]
- West SG, Finch JF, & Curran PJ (1995). Structural equation models with nonnormal variables: Problems and remedies. In Hoyle RH (Ed.), Structural equation modeling: Concepts, issues, and applications (pp. 56–75). Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Wetherell JL, Hershey T, Hickman S, Tate SR, Dixon D, Bower ES, & Lenze EJ (2017). Mindfulness-based stress reduction for older adults with stress disorders and neurocognitive difficulties: A randomized controlled trial. Journal of Clinical Psychiatry, 78, e734–e743. doi: 10.4088/JCP.16m10947. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Reynolds CA, Gatz M, & Pedersen NL (2002). Anxiety, cognitive performance, and cognitive decline in normal aging. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57, P246–P255. doi: 10.1093/geronb/57.3.P246. [DOI] [PubMed] [Google Scholar]
- Williams MO, Mathews A, & Hirsch CR (2014). Verbal worry facilitates attention to threat in high-worriers. Journal of Behavior Therapy and Experimental Psychiatry, 45, 8–14. doi: 10.1016/j.jbtep.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Zhao S, Kessler RC, & Eaton WW (1994). DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Archives of General Psychiatry, 51, 355–364. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]
- Wright AGC, Calabrese WR, Rudick MM, Yam WH, Zelazny K, Williams TF, … Simms LJ (2015). Stability of the DSM-5 Section III pathological personality traits and their longitudinal associations with psychosocial functioning in personality disordered individuals. Journal of Abnormal Psychology, 124, 199–207. doi: 10.1037/abn0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K-H, & Bentler PM (2000). Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology, 30, 165–200. doi: 10.1111/0081-1750.00078. [DOI] [Google Scholar]
- Zainal NH, & Newman MG (2018). Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychological Medicine, 48, 2045–2053. doi: 10.1017/S0033291717003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche U, Bürkner P-C, & Schulze L (2018). Shedding light on the association between repetitive negative thinking and deficits in cognitive control - A meta-analysis. Clinical Psychology Review, 63, 56–65. doi: 10.1016/j.cpr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang X, Norton J, Carriere I, Ritchie K, Chaudieu I, & Ancelin ML (2015). Risk factors for late-onset generalized anxiety disorder: Results from a 12-year prospective cohort (The ESPRIT study). Translational Psychiatry, 5, e536. doi: 10.1038/tp.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


