Abstract
Alcohol use disorder is highly comorbid with other neuropsychiatric disorders such as depression and anxiety. Importantly, women and men are affected differentially by heavy drinking with women experiencing longer negative affective states after intoxication and increased likelihood to present with comorbid mood or anxiety disorders. In rodents, several studies using different alcohol administration models have shown the development of depressive-like or anxiety-like phenotypes that emerge during abstinence. In this study, we compared the emergence of negative affective behaviors during abstinence from 7 weeks of two bottle choice intermittent access to 20% alcohol in male and female C57BL/6J mice, a drinking paradigm little studied in this context. Half of the mice were tested 24 h in abstinence on the elevated zero maze and 19-20 days in abstinence in a novel object in the home cage encounter test. The other half of mice were tested 27-28 days in abstinence with the novelty-suppressed feeding test. As expected, females drank more than males across the 7 weeks of access to alcohol. Drinking history did not affect performance on these tasks, with the exception of increasing the number of open arm entries on the elevated zero maze. Interestingly, in alcohol-naïve mice, females showed fewer anxiety-like behaviors than males in the elevated zero maze and the novelty-suppressed feeding test. Our results suggest that the intermittent access model does not reliably induce negative affective behaviors on these tasks, and that behavior in female and male mice differs across these tests. Rather, intermittent alcohol drinking may induce a mild form of behavioral disinhibition. Thus, the model of alcohol access is a critical factor in determining the appearance of behavioral disturbances that emerge during abstinence.
Introduction
Alcohol (ethanol) use disorder (AUD) is a devastating condition affecting over 280 million people worldwide (Glantz, et al. 2020), including an estimated 15 million people in the United States (NIAAA). Individuals with AUD have impaired control over drinking leading to physiological dependence and continue to drink despite deleterious consequences to their health and personal and professional lives. AUD is highly comorbid with other neuropsychiatric disorders, including mood disorders such as depression (Brière, et al. 2014), bipolar disorder (Farren, et al. 2012), and anxiety disorders (Schneier, et al. 2010). Indeed, epidemiological studies have reported that as much as 40% of AUD patients present with a comorbid mood disorder, and up to a third present with an anxiety disorder (Grant, et al. 2004; Lai, et al. 2015). Patients with these multiple diagnoses both respond less to treatment and have poorer disease outcomes (Prior, et al. 2017). Moreover, both acute and protracted abstinence generate long lasting negative emotional states that promote craving and relapse (Heilig, et al. 2010).
Another critical consideration for treating AUD is the differential symptoms and prevalence rates between men and women. Currently in the US, 4.1% of women have AUD versus 7.6% of men, but this gap is diminishing, with alcohol use increasing sharply in women (Slade, et al. 2016). Importantly, women with AUD are more likely than men to have a comorbid anxiety or mood disorder, and exhibit more severe depressive symptoms and craving (Anthenelli 2010; Bott, et al. 2015; Goldstein, et al. 2012). Women with AUD also report heavier drinking to alleviate negative mood than men and experience longer negative affective states after excessive drinking that may contribute to a higher relapse rate and worsen the disease course (Erol and Karpyak 2015). Sex differences also exist in rodent models of alcohol-related behaviors, as female rodents tend to drink more than males (Priddy, et al. 2017; Sneddon, et al. 2019; Yoneyama, et al. 2008; Yu, et al. 2019). Therefore, it is imperative to determine appropriate animal models that better resemble the human condition in order to find individualized and sex-specific treatment options (Gururajan, et al. 2019; Kokras and Dalla 2014).
The development of lasting negative emotional states following alcohol consumption has been studied for decades in mice (Holleran and Winder 2017). In the voluntary continuous access to alcohol (CAA) model, negative affective behaviors are observed during abstinence in tasks such as the novelty suppressed feeding test (NSFT) and forced swim test (Holleran, et al. 2016; Pang, et al. 2013; Stevenson, et al. 2009). The voluntary nature of the access does not seem critical to elicit negative affective behaviors, since recent findings have shown that the chronic intermittent ethanol (CIE) vapor exposure model triggers negative affective behaviors in male mice in the NSFT 3 or 5 days into abstinence and marble burying test 2 to 10 days into abstinence (Jury, et al. 2017; Kimbrough, et al. 2020; Pleil, et al. 2015; Sidhu, et al. 2018). Likewise, forced alcohol administration studies in rats also have shown the development of negative affective behaviors in both continuous and intermittent administration methods (Kliethermes 2005). To our knowledge, there are only two published studies reporting elevated aggressiveness and decreased social interaction in a mouse intermittent access to alcohol (IAA) model (Hwa, et al. 2015; Nennig, et al. 2020). In the IAA drinking paradigm, mice are subjected to a 24-48 h abstinence period between periods of 24 h of access to alcohol and water (Hwa, et al. 2015; Melendez 2011). The IAA model produces heavy alcohol drinking that is more pronounced in female mice, and alcohol intake is elevated compared to continuous access over 24 h and the first 2 h of drinking in the drinking-in-the-dark (DID) model (Hwa, et al. 2011). These characteristics make IAA an interesting model to study the transition to excessive drinking. However, little is known about the influence of IAA on the emergence of negative affective behaviors.
In order to further explore the consequences of IAA, we chose a battery of tests to assess negative affective behaviors during abstinence from alcohol drinking in male and female C57BL/6J mice. This battery includes the NSFT, the elevated zero maze (EZM) test that is a variant of the elevated plus maze (EPM) (Shepherd, et al. 1994), and the novel object encounter in the home cage (NOHC) test. Time points during abstinence for each of these tests were selected based on previous studies that reported the emergence of these negative affective behaviors in other alcohol drinking models (Holleran, et al. 2016; Lee, et al. 2015; Vranjkovic, et al. 2018).
Materials and Methods
Animals
Equal numbers of adult female and male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME; n = 96 total mice, n = 48 per cohort) arrived at 8–9 weeks of age and acclimated for at least 1 week before beginning studies. Animals were weighed weekly. All mice were singly housed under standard conditions (12 h reverse light/dark cycle, lights off at 9 am) in an AAALAC accredited facility with free access to food (Harlan Teklad Diet 2918) and water for the duration of the study with the exceptions described for the negative affective tests. All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee in accordance with NIH guidelines for the humane care and use of laboratory animals (NRC 2011).
Intermittent access to alcohol
The IAA two-bottle choice drinking paradigm was performed as previously described (Cannady, et al. In Press; Rinker, et al. 2017). Briefly, alcohol-drinking mice were given access to two bottles (20% ethanol, v/v in tap water vs water) for 24 h on Monday, Wednesday, and Friday for 7 weeks. Water controls were exposed to similar bottles with both bottles containing tap water. Bottle position was alternated for each drinking session to avoid side preference. Bottles were weighed after each drinking session to 0.01 g, and were refilled weekly. Alcohol consumption was calculated as grams of alcohol consumed per kilogram of body weight.
Behavioral Testing
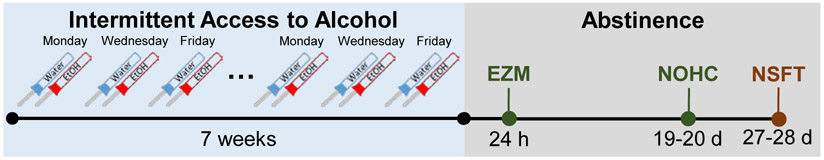
Mice were tested for negative affective behaviors during alcohol abstinence (see Fig. 1 for experimental timeline). Mice were habituated to the testing room for 1 h before behavioral testing, which occurred between 9 and 11:00 am under normal light conditions. Each cohort of mice was divided into two behavioral groups with equal numbers of male and female mice and equal numbers of water and alcohol drinkers. Half of each cohort underwent the EZM test 24 h into abstinence and the NOHC test 19-20 days into abstinence, while the other half underwent the NSFT 27-28 days into abstinence. Testing was performed on 12 mice per day in order to reduce time of day effects on behavior. To allow for testing on the EZM at 24 h into abstinence, half of the mice were given one additional day of drinking. Behavioral testing was pseudo-random where we alternated between males and females as well as water and alcohol drinkers. Behavioral tracking was performed and analyzed using Ethovision XT software (version 14, Noldus, Wageningen, the Netherlands) by tracking the body center point for the EZM test and the nose point for the NOHC test. Several mice were excluded from the analysis: one mouse died and one mouse was sick in the NSFT group and 4 mice that fell from the EZM were excluded from the analysis for this test.
Fig. 1.
Schematic of the experimental timeline for alcohol drinking and behavioral testing. The two-bottle choice intermittent access to alcohol (IAA) drinking model consists of 24 h access to two bottles of water or single bottles of water and 20% alcohol on Monday, Wednesday, and Friday. After 7 weeks of IAA drinking, mice were divided into two behavioral testing cohorts. Half of the mice (n = 24 mice/group) were tested on the elevated zero maze (EZM) at 24 h into abstinence and the novel object in the home cage (NOHC) at 19-20 days into abstinence. The other half of the mice (n = 24 mice/group) were tested on the novelty-suppressed feeding test (NSFT) at 27-28 days into abstinence.
Elevated Zero Maze
Behavioral testing on the EZM was conducted as previously described (Tucker and McCabe 2017). Maze dimensions were 60 cm in diameter, 7 cm in platform width, 20 cm high walls around the closed zones, and it was elevated 65 cm off the ground (Med Associates, Georgia, Vermont). Briefly, mice were put on the maze at a random entry of one of the closed zones and were video recorded for 5 min, then immediately removed from the maze. Time spent and distance traveled in the closed and opened zones, as well as the number of entries into each zone and velocity were calculated. The EZM was cleaned with 70% alcohol between each mouse. Luminosity was approximately 300 lux in the closed zone and 1200 lux in the open zone.
Novel Object in the Home Cage
The NOHC was adapted from the novel object encounter described by Lee and colleagues, with minor modifications (Lee, et al. 2015). Instead of presenting a novel object in a novel environment to which the mice were habituated, we introduced a novel object (a 20 mL glass vial with a white cap) directly in the home cage of the mouse to avoid an interaction between a novel object and an unfamiliar environment (Griebel, et al. 1993). The novel object was glued to a rectangular piece of Plexiglas covered with the bedding from the home cage in order to prevent movement of the novel object during interaction. The cage top was removed and replaced by an open top 5-10 min prior to the beginning of video recording. The object was then placed into the home cage for 5 min. The number of contacts and time spent in contact (defined as the nose point within 0.5 cm of the white cap), and head orientation toward the object were recorded. Head orientation toward the object was defined as the vector between the center point of the object and the head being oriented to the novel object regardless of distance. The object was cleaned with 70% alcohol between each mouse.
Novelty-Suppressed Feeding Test
The NSFT was performed as previously described (Holleran, et al. 2016). Briefly, mice were food deprived for 48 h with a 2-h access period to food 23-25 h into deprivation. On the day of testing, the mice were placed in one corner of a square arena (40 x 40 cm) with white opaque walls and the floor was covered with about 2 cm bedding. The bedding was replaced between each mouse, and the arena was cleaned with 70% alcohol between each session. A piece of familiar chow was placed in the center of the arena, and the mice were allowed to explore the arena until they took one bite of the chow (recorded as latency to feed), after which the mouse was immediately removed and placed back in its home cage with access to one pre-weighed piece of chow. The amount of chow consumed after 5 min was recorded. Luminosity was approximately 900 lux in the center of the arena.
Statistical Analysis
GraphPad Prism (version 8.1.0, GraphPad Software Inc., San Diego, CA) was used for data analysis. Ordinary 2 (drinking solution) x 2 (sex) ANOVAs were performed for all behavioral data and a repeated-measures mixed-model ANOVA (drinking session x sex) was performed for alcohol consumption and preference data with Sidak’s post-hoc tests for multiple comparisons. Because female and male mice consumed significantly different amounts of alcohol and in different patterns, additional analyses were performed independently for each sex using an unpaired t-test comparing water- and alcohol-drinking mice. Tukey’s post-hoc tests were used for non-repeated measures tests. The threshold for statistical significance was set using an α of 0.05. Graphs show means (± SEM) with individual data points overlaid on the bar graphs, where possible.
Results
Intermittent Access to Alcohol
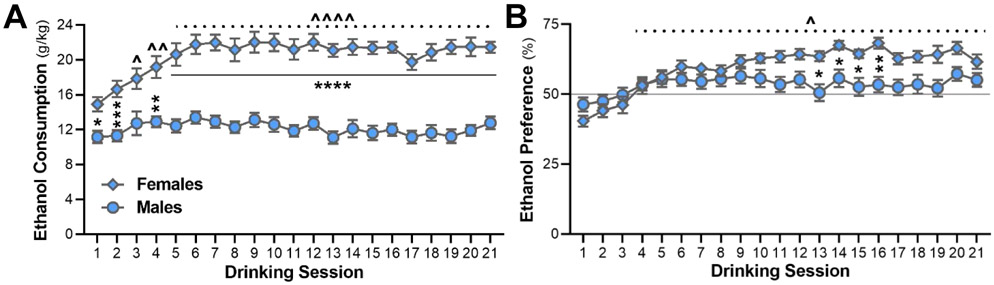
There was a significant interaction between drinking session and sex for both alcohol intake (F(20, 914) = 4.261, p < 0.0001) and preference (F(20, 915) = 5.323, p < 0.0001) across the 7 weeks of IAA (Fig. 2). Specifically, female C57BL/6J mice (n = 24) initially drank ~15 g/kg/day and escalated their intake in the first 2 weeks to reach a steady average above 20 g/kg/day (Fig. 2A). Post-hoc comparisons showed that drinking within female mice was significantly higher than session 1 for all sessions except 2 and 3 (p values for the post-hoc tests are reported in the figure legends). Males drank ~11 g/kg/day during initial sessions and remained at levels between 11-13 g/kg/day during the whole experiment, without a clear escalation of intake. When comparing sexes, female mice drank significantly more alcohol than males from the first day of drinking and for all drinking sessions except on session 3 (Fig. 2A). Although preference in males started at 46%, surpassed 50% at the beginning of the second week and varied between 52-57% for the remainder of the experiment, it was not significantly different across the 7 weeks of drinking. Preference for alcohol in female mice started at 40%, exceeded 50% by the second week, and stayed above 60% throughout the study (Fig. 2B). Post-hoc analyses revealed that preference for alcohol during drinking sessions 4 through 21 were significantly higher than preference during session 1 within female drinkers. Female mice also exhibited a higher preference for alcohol than males on drinking sessions 13 through 16 (Fig. 2B).
Fig. 2.
Alcohol intake and preference in male and female C57BL/6J mice across seven weeks of two-bottle choice intermittent access to alcohol (20% v/v). (A) Female mice had significantly higher alcohol intake than males for almost all sessions, and escalated their alcohol intake, which became significantly higher than the first session on sessions 4 through 21. (B) Alcohol preference in female mice was elevated for drinking sessions 13-16. Significance key for male vs female mice: continuous line shows all contiguous days with a significant difference, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Significance key for differences within female mice: dotted line shows all contiguous days with a significant difference compared to drinking session 1, ^p < 0.05, ^^p < 0.01, ^^^^p < 0.0001.
After establishing IAA drinking and observing expected sex differences, we investigated the consequences of forced abstinence following alcohol or water drinking on different behavioral tests modeling negative affective states (Fig. 1). For that purpose, we divided each cohort of mice into two behavioral testing groups in order to avoid accumulation of stressful procedures that could interfere with the interpretation of the effect of alcohol drinking on behavioral outcomes. Half of the mice in each cohort (n = 48, 24 mice/group) underwent EZM testing at 24 h into forced abstinence and then NOHC testing at 19-20 days into abstinence. The other half of mice in each cohort (n = 47, 23-24/group) underwent testing on the NSFT at 27-28 d into abstinence.
Elevated Zero Maze
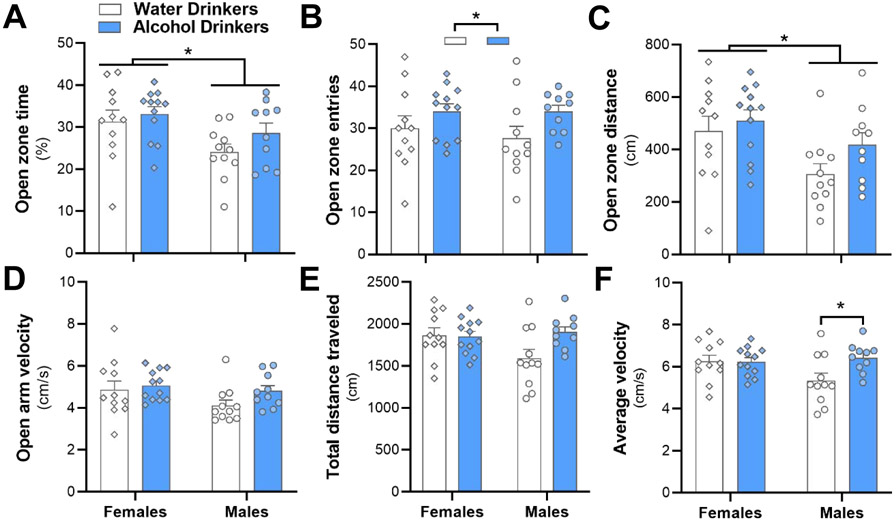
As a measure of negative affective behavior, we investigated whether there was an effect of alcohol drinking or sex on time spent (Fig. 3A), number of entries (Fig. 3B), distance traveled (Fig. 3C) and velocity (Fig. 3D) in the open zone of the EZM. There was no effect of drinking on time (Fig. 3A; F(1, 40) = 2.055, p=0.16), distance traveled (Fig. 3C; F(1, 40) = 2.681, p=0.11), or velocity (Fig. 3D; F(1, 40) = 2.277, p=0.14), but alcohol drinkers had a significantly higher number of entries in the open zone (Fig. 3B; F(1, 40) = 4.649, p=0.037). Females spent significantly more time (Fig. 3A; F(1, 40) = 6.980, p = 0.012 for sex) and traveled longer distances in the open zone (Fig. 3C; F(1, 40) = 7.615, p = 0.0087), but did not differ from males for the number of entries (Fig. 3B; F(1, 40) = 0.2418, p = 0.63) or velocity (Fig. 3D; F(1, 40) = 2.927, p = 0.095). There was no interaction between sex and alcohol drinking for time spent (F(1, 40) = 0.3736, p = 0.54), number of entries (F(1, 40) = 0.2418, p = 0.63), distance traveled (F(1, 40) = 0.6021, p = 0.44) and velocity (F(1, 40) = 2.927, p = 0.095) in the open zone of the EZM.
Fig. 3.
The elevated zero maze 24 h into abstinence reveals a mild disinhibition-like behavioral phenotype in alcohol drinking mice, as well as sex differences. To assess anxiety-like behavior, the (A) percent of time spent, (B) number of entries, (C) distance traveled, (D) and velocity in the open zone of the maze were measured. (E) There was no difference between drinking groups in total distance traveled. (F) Male alcohol drinkers had increased velocity compared with male water drinkers, a difference which was not observed in females. Significance key: *p < 0.05, **p < 0.01.
We also investigated whether alcohol or sex would affect locomotion by monitoring total distance traveled and average velocity for each animal over the EZM trial. Neither factor reached significance, nor was there an interaction of the two, for distance traveled (Fig. 3E; F(1, 40) = 1.802, p = 0.19 for sex, F(1, 40) = 3.321, p = 0.076 for drinking, F(1, 40) = 4.013, p = 0.052 for interaction). For average velocity, there was no main effect of drinking or sex independently (F(1, 40) = 1.793, p = 0.19 for drinking, F(1, 40) = 3.854, p = 0.057 for sex), however there was a significant interaction between sex and drinking (Fig. 3F; F(1, 40) = 4.180, p = 0.048), with male alcohol drinkers having a higher velocity than male water controls (p = 0.0496). Female alcohol drinkers did not differ from female water drinkers (p > 0.99).
Novel Object in the Home Cage
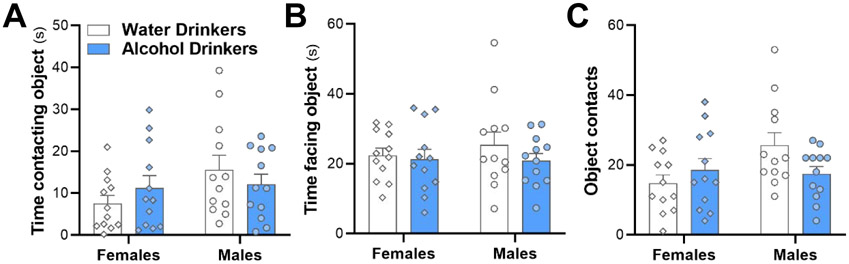
Using the same group of water and alcohol drinking mice from the EZM, we investigated the animals’ interaction with a novel object placed in their home cage for a 5-min period on days 19-20 of abstinence. We tracked the nose point of the mouse and recorded the time spent in contact with the novel object over the first 2 min (Fig. 4A) as in the original report (Lee, et al. 2015). There was no effect of sex (F(1, 44) = 2.755, p = 0.10), drinking (F(1, 44) = 0.001692, p = 0.97), or interaction (F(1, 44) = 1.765, p = 0.19) on the time spent in contact with the object. We also recorded the time the mice spent with their heads directed toward the novel object (Fig. 4B), for which there was neither an effect of sex (F(1, 44) = 0.2085, p = 0.65) nor drinking (F(1, 44) = 1.048, p = 0.31). The interaction was also not significant (F(1, 44) = 0.375, p = 0.54). Finally, we counted the number of contacts with the object (Fig. 4C): there was no effect of sex (F(1, 44) = 2.782, p = 0.10) or drinking (F(1, 44) = 0.5708, p = 0.45), but there was a significant interaction between sex and drinking (F(1, 44) = 4.273, p = 0.045). However post-hoc tests failed to reveal any significant differences when comparing male alcohol drinkers versus male water drinkers (p = 0.21) or female alcohol drinkers versus female water drinkers (p = 0.79); the interaction seemed to be mainly lead by the difference between female and male water drinkers (p = 0.054). There were no significant differences for these variables over the 5 min of recording (data not shown).
Fig. 4.
The novel object in the home cage test at 19-20 days into abstinence shows no effect of sex or drinking on behavior. There was no significant difference between groups in (A) time spent in contact, (B) time with head directed towards, (C) or number of contacts with the object over the 2 min of tracking.
Novelty Suppressed Feeding Test
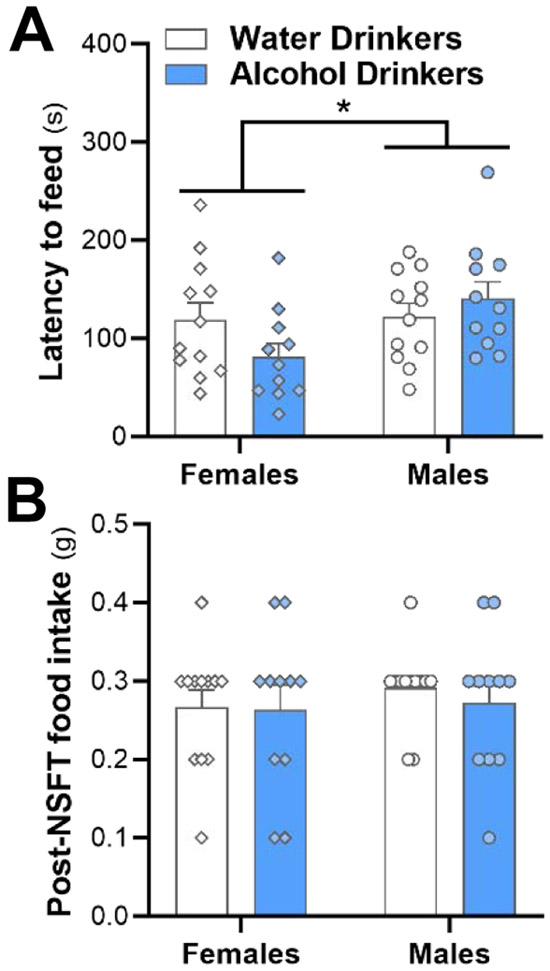
Finally, using a separate group of mice from each drinking cohort, we performed the NSFT in which food deprived mice are presented with a piece of familiar chow in the middle of a novel open arena. Analysis revealed a significant effect of sex (F(1, 42) = 4.130, p = 0.049). Regardless of drinking history, female mice had shorter latencies to approach and consume the food than male mice, and this effect appeared mainly driven by the alcohol drinking females (Fig. 5A). However, the interaction between sex and drinking did not reach statistical significance (F(1, 42) = 3.319, p = 0.076). No effect of sex (F(1, 42) = 0.4948, p = 0.49), drinking (F(1, 42) = 0.2055, p = 0.65), or interaction (F(1, 42) = 0.1077, p = 0.74) was observed in the amount of food consumed during the 5 min free feed test in the home cage after the mouse was removed from the open field arena (Fig. 5B).
Fig. 5.
Novelty suppressed feeding test at 27-28 days into abstinence does not show an effect of alcohol drinking on latency to feed. There was (A) no effect of drinking on the latency to feed (B) or the amount of food consumed following transfer back to the home cage. However, females had significantly lower latencies than males. Significance key: **p < 0.01.
Independent statistical analyses for each sex
Male and female alcohol drinkers presented distinct alcohol consumption patterns, both in terms of quantity (females consumed more) and pattern (escalation in females only). In order to examine the effect of alcohol in each sex considering that the alcohol intake was different, we analyzed the behavioral data for each sex independently (Table 1). The independent analysis did not yield any significant differences from the previous results when sex was included as a factor with the exception of the number of entries in the open zone of the EZM that did not reach significance in males (p = 0.071) or females (p = 0.26).
Table 1.
Analysis of negative affective behaviors in male and female mice when tested independently for sex.
| Behavioral test and dependent variable | Male Mice | Female Mice | ||
|---|---|---|---|---|
|
t value (degrees of freedom) |
p value |
t value (degrees of freedom) |
p value | |
| Elevated Zero Maze | ||||
| Open zone time | 1.516 (19) | 0.146 | 0.563 (21) | 0.579 |
| Open zone entries | 1.915 (19) | 0.071 | 1.160 (21) | 0.259 |
| Open zone distance | 1.842 (19) | 0.081 | 0.578 (21) | 0.569 |
| Open arm velocity | 1.905 (19) | 0.072 | 0.433 (21) | 0.669 |
| Distance traveled | 2.465 (19) | 0.023* | 0.141 (21) | 0.889 |
| Average velocity | 2.554 (19) | 0.019* | 0.064 (21) | 0.949 |
| Novel Object in the Home Cage | ||||
| Time contacting object | 0.8389 (22) | 0.410 | 1.068 (22) | 0.297 |
| Time facing object | 1.060 (22) | 0.300 | 0.323 (22) | 0.750 |
| Object contacts | 1.972 (22) | 0.063 | 0.939 (22) | 0.358 |
| Novelty Suppressed Feeding Test | ||||
| Latency to Feed | 0.8730 (21) | 0.390 | 1.684 (21) | 0.107 |
| Post-NSFT food intake | 0.6241 (21) | 0.530 | 0.080 (21) | 0.937 |
p < 0.05.
Discussion
Based on the literature, we hypothesized that abstinence from long-term intermittent drinking would produce a lasting increase in negative affective behaviors. Contrary to this hypothesis, the main result of this study is that 7 weeks of drinking in the IAA model did not elicit robust or persistent negative affective behaviors in alcohol drinking mice. While we confirmed the expected sex difference with females drinking significantly more than males, the only significant difference between alcohol and water drinking mice was observed in the EZM during early abstinence. On this task, alcohol drinking mice showed heightened behavioral disinhibition, rather than negative affective behaviors. In addition, we found sex differences independent of drinking history, with female mice exhibiting fewer negative affective behaviors, further highlighting sex-dependent behaviors on tasks that model anxiety- and depressive-like behaviors.
In our study, the higher number of entries in the open zone in alcohol drinkers is opposite of the expected anxiety-like phenotype. Increased behavior in the open zone of the EPM has previously been interpreted as a form of behavioral disinhibition (Fish, et al. 2018; Lacroix, et al. 2000; Osborn, et al. 1998). Thus, our results may be interpreted as evidence of mild behavioral disinhibition in alcohol drinkers, since drinking animals seemed to explore the open zone more. In this line, a recent report in male C57BL/6J mice exposed to a daily “binge-like” vapor exposure shows a lasting increase in impulsive-like behavior in the 5-choice serial reaction time task (Starski, et al. 2020). Also reminiscent of behavioral disinhibition, recent studies in male mice drinking in the IAA model showed increased aggressivity (Hwa, et al. 2015) a maladaptive response to a predator odor in IAA drinking mice (Hwa, et al. 2019). Albeit hard to interpret, the higher average velocity observed in male drinkers compared to water drinking controls on the EZM in our study could hint at a subtle effect of alcohol that affects male and female mice differently. In summary, IAA triggers what appears to be a mild form of behavioral disinhibition in the alcohol drinking mice that is consistent with similar behavioral phenotypes reported in recent manuscripts.
In contrast to published data using other models of chronic alcohol exposure, we found that IAA did not elicit any observable negative affective behaviors in early or protracted abstinence. Mice did not display any effect of drinking in the NOHC test, unlike in other studies that showed an effect of drinking in the DID paradigm using a similar behavioral test (Lee, et al. 2016; Lee, et al. 2015). This discrepancy could be due to differences in the drinking paradigm (discussed below) or in the testing arena (home cage in our study vs an open field in the DID study). Moreover, the results from the NOHC test are not consistent with the mild behavioral disinhibition observed in the drinking mice on the EZM task. Some studies have reported negative affective behaviors on the EPM in the DID and CAA models during early abstinence (Bloodgood, et al. 2020; Lee, et al. 2015; Vranjkovic, et al. 2018). It is the first time, to our knowledge, that the EZM has been used to study negative affect after alcohol drinking in mice. Similar to the EPM, both are based on the conflict between exploration and exposure to an elevated and exposed zone. Although devoid of a center area, we have observed that mice spend a substantial amount of time (over 40%, no significant effect, data not shown) straddling the interface between the open and the closed zone, which may be comparable to the center zone of the EPM. It should be noted that a history of drinking does not always produce negative affect behaviors in mice. Using the CAA model, a study in male mice showed that 4 weeks of continuous drinking elicited increased immobility during forced swim stress at 14 days, but not at 1 day into abstinence (Stevenson, et al. 2009), while a decreased time in the center of an open field was observed at 1 day, but not 14 days into abstinence. Similarly, in females, after 6 weeks of CAA there was no effect of drinking observed in the EPM or exploratory behavior in the light dark box at 2 weeks into abstinence (Pang, et al. 2013), and no effect on the forced swim stress test 1 day into abstinence or on the EPM 2 weeks into abstinence (Holleran, et al. 2016). Similarly, Lee, et al. (2015) have shown effects of abstinence after DID, but the same group has reported difficulty reproducing these negative affective behaviors using a shorter DID schedule in adult mice (Szumlinski, et al, 2019). Nonetheless, male and female mice that drank in the intermittent access model for 7 weeks did not exhibit anxiety-like behaviors on the EZM, NOHC, and NSFT.
Independent of drinking status, female mice showed less negative affective behaviors compared with male mice in the EZM and NSFT. Indeed, female mice displayed more exploratory behaviors in the EZM and a shorter latency to feed in the NSFT compared to males. Sex differences have been previously reported in the EPM and EZM when C57BL/6J mice were repeatedly exposed to the test (Tucker and McCabe 2017). More generally, sex differences in reaction to stressful events and in the development of depression have long been reported in humans and in rodent models (Alonso, et al. 1991; Gray and Lalljee 1974; Weissman and Klerman 1977). The divergent behavioral phenotypes may originate from multiple brain systems at different levels of organization. Indeed, findings in both humans and mice include wide differences in regional transcriptional profiles related to depression according to sex (Labonte, et al. 2017) and divergent developmental pathways of brain regions involved in anxiety influenced by sex hormones (Juntti, et al. 2010). The current neurobiological understanding of these sex differences have been recently reviewed extensively elsewhere (Rubinow and Schmidt 2019). In regard to sex differences, our data are consistent with previous findings that male and female mice perform differently on behavioral tasks modeling negative affect.
There are a number of possible explanations for the absence of negative affective behaviors in this study. First, one important factor could be the total amount of alcohol consumed. In our study, the female mice consumed ~430 g/kg of alcohol across 7 weeks. In a recent study that reported negative affective behaviors during abstinence, female mice consumed nearly 800 g/kg across 6 weeks of continuous alcohol access (Vranjkovic, et al. 2018). This striking difference in the amount of total alcohol suggests that mice drinking in the IAA model do not drink enough alcohol to produce negative affective behaviors. Complicating this interpretation, a previous study showed anxiety-like behaviors during abstinence from 5 days of two-bottle, limited access (4 h) drinking where mice consumed 20-25 g/kg of total alcohol (van Rijn, et al. 2010). Anxiety-like behaviors were reported when mice consumed a total of 120 g/kg of ethanol over six weeks of drinking in the DID model (Lee, et al. 2015). A second explanation could be due to the presence or absence of escalation of alcohol intake. In the present study, only female mice escalated their alcohol intake before reaching a higher baseline compared with male mice. While we observed differences in the amount and pattern of alcohol drinking across sex, both sexes of the alcohol drinkers showed increased open arm entries on the EZM and no changes in behaviors on the other tests. In comparison, mice that consumed consistent amounts of alcohol in the intermittent or continuous access models displayed negative affective behaviors (Holleran, et al. 2016) and decreases in social interaction following a stressor (Nennig, et al. 2020). Similarly, in the DID model, the absence of escalation still allows the emergence of negative affective behaviors (Lee, et al. 2016). Based on these findings, escalation of intake does not seem to be a critical factor for the emergence of negative affective or other maladaptive behaviors.
A third possibility that may drive negative affective behaviors is the pattern of intake. Indeed, it has been argued that an intermittent schedule is more critical for the emergence of negative affective behaviors than the cumulative amount of alcohol consumed (Spear 2020). Yet, models with varied access schedules that yield vastly different intake values permit the emergence of maladaptive or negative affective behaviors in mice. Based on findings from other studies, the time of testing during abstinence appears to be an integral factor (Holleran and Winder 2017), with negative affective behaviors present at some time points but not others, indicating variation in the emergence and recovery of these behaviors. A fourth explanation to consider is the involvement of the level of intoxication in the development of negative affect. For example, a set of studies in the DID model suggest that negative affect emerges after reaching a high blood ethanol concentration, but not at a lower range (Lee, et al. 2017; Lee, et al. 2018; Lee, et al. 2016; Lee, et al. 2015; Szumlinski, et al. 2019). More studies are needed to explore the role of intoxication in the different ethanol administration paradigms. Interestingly, a recent study on the emergence of negative affective behaviors in male and female mice drinking in the DID model (Chavez, et al. 2020) reported contrasting results when compared to prior studies performed in one sex at a time from the Szumlinksi lab. These authors highlight the possibility that sex pheromones may have an influence on measures of negative affective behaviors when both sexes are tested concurrently, as in our study. This is an important methodological consideration that will need to be explored in the future. Overall, the IAA mouse model appears less likely to produce negative affective behaviors in comparison with other models, at least when considering the EZM, NOHC, and NSFT at the time points selected in this study. Regardless, these findings highlight the complex nature between alcohol drinking and the emergence of negative affective behaviors in various mouse models.
In conclusion, our findings when compared with the existing literature suggest that drinking models can have different behavioral outcomes according to the nature of the exposure (intermittent or continuous), as well as the amount of alcohol consumed and the duration of the exposure to alcohol. Although intermittency or the voluntary nature of access may contribute to the emergence of negative affective behaviors, they do not seem to be essential for their development. Instead, a prolonged period of abstinence (Holleran and Winder 2017) must be achieved, probably in combination with reaching a threshold of alcohol intake specific to each paradigm. Thus, an optimal combination of schedule, total amount consumed, and the duration of abstinence may be necessary to elicit robust negative affect instead of one of those factors being critical in itself. More studies are needed to explore whether IAA can elicit steady and durable negative affective behaviors, and if it does, which parameters, including total amount consumed and escalation of drinking, are critical to their emergence. Finally, the influence of estrus cycle, peak or sustained blood alcohol concentrations, and circadian rhythms have not been systematically explored as a factor that may influence the emergence of negative affective behaviors in these models.
Highlights.
Female C57BL/6J mice drinking more and have higher preference for ethanol than male mice in the 24 h intermittent access to alcohol model.
Alcohol drinking history did not affect performance on tasks measuring negative affective behaviors during abstinence, with the exception of increasing the number of open arm entries on the elevated zero maze.
In alcohol-naïve mice, females showed less anxiety-like phenotype than males on two tasks measuring negative affective behaviors.
Acknowledgements:
These studies were supported by NIH grants AA020930 (PJM), AA023288 (PJM), AA025110 (JAR), and the Charleston Alcohol Research Center (P50 AA010761).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso SJ, et al. 1991. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav 49(1):69–72. [DOI] [PubMed] [Google Scholar]
- Anthenelli Robert M. 2010. Focus On: Comorbid Mental Health Disorders. Alcohol Research & Health 33(1-2):109–117. [PMC free article] [PubMed] [Google Scholar]
- Bloodgood Daniel W., et al. 2020. Kappa opioid receptor and dynorphin signaling in the central amygdala regulates alcohol intake. Molecular Psychiatry:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott Karin, et al. 2015. Psychiatric disorders among at-risk consumers of alcohol in the general population. Journal of Studies on Alcohol. [DOI] [PubMed] [Google Scholar]
- Brière Frédéric N., et al. 2014. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Comprehensive Psychiatry 55(3):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, et al. In Press Distinct region- and time-dependent functional cortical adaptations in C57BL/6J mice after short and prolonged alcohol drinking. eNeuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C, Jimenez Leonardo , et al. 2020. Incubation of Negative Affect during Protracted Alcohol Withdrawal Is Age-, but Not Sex- Selective. Brain Sciences 10(6):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol Almila, and Karpyak Victor M. 2015. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence 156:1–13. [DOI] [PubMed] [Google Scholar]
- Farren Conor K., Hill Kevin P., and Weiss Roger D. 2012. Bipolar Disorder and Alcohol Use Disorder: A Review. Current Psychiatry Reports 14(6):659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, et al. 2018. The enduring impact of neurulation stage alcohol exposure: A combined behavioral and structural neuroimaging study in adult male and female C57BL/6J mice. Behav Brain Res 338:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz Meyer D., et al. 2020. The epidemiology of alcohol use disorders cross-nationally: Findings from the World Mental Health Surveys. Addictive Behaviors 102:106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein Risë B., et al. 2012. Sex Differences in Prevalence and Comorbidity of Alcohol and Drug Use Disorders: Results From Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Studies on Alcohol and Drugs 73(6):938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant Bridget F., et al. 2004. Prevalence and Co-occurrence of Substance Use Disorders and IndependentMood and Anxiety Disorders: Results From the National Epidemiologic Survey on Alcohol and RelatedConditions. Archives of General Psychiatry 61(8):807–816. [DOI] [PubMed] [Google Scholar]
- Gray JA, and Lalljee B 1974. Sex differences in emotional behaviour in the rat: correlation between open-field defecation and active avoidance. Anim Behav 22(4):856–61. [DOI] [PubMed] [Google Scholar]
- Griebel G, et al. 1993. The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol 4(6):637–644. [PubMed] [Google Scholar]
- Gururajan Anand, et al. 2019. The future of rodent models in depression research. Nature Reviews Neuroscience 20(11):686–701. [DOI] [PubMed] [Google Scholar]
- Heilig Markus, et al. 2010. REVIEW: Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology 15(2):169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran, et al. 2016. Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology 41(8):2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, and Winder DG 2017. Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes, Brain and Behavior 16(1):8–14. [DOI] [PubMed] [Google Scholar]
- Hwa LS , et al. 2019. Alcohol Drinking Alters Stress Coping via Extended Amygdala Kappa Opioid Receptor Signaling in Male Mice. bioRxiv 773481. [Google Scholar]
- Hwa Lara S., et al. 2011. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcoholism: Clinical and Experimental Research 35(11):1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa Lara S., et al. 2015. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology 232(16):2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti SA, et al. 2010. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66(2):260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury Nicholas J., et al. 2017. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol 58:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough Adam, et al. 2020. Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proceedings of the National Academy of Sciences 117(4):2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL 2005. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28(8):837–50. [DOI] [PubMed] [Google Scholar]
- Kokras N, and Dalla C 2014. Sex differences in animal models of psychiatric disorders. British Journal of Pharmacology 171(20):4595–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, et al. 2017. Sex-specific transcriptional signatures in human depression. Nat Med 23(9):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix L, et al. 2000. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci 114(6):1119–30. [DOI] [PubMed] [Google Scholar]
- Lai Harry Man Xiong, et al. 2015. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug and Alcohol Dependence 154:1–13. [DOI] [PubMed] [Google Scholar]
- Lee KM, et al. 2017. Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice. Front Psychol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, et al. 2018. mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Depend 184:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, et al. 2016. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front Cell Neurosci 10:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Kaziya M., et al. 2015. Binge alcohol drinking elicits persistent negative affect in mice. Behavioural Brain Research 291:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez Roberto I. 2011. Intermittent (Every-Other-Day) Drinking Induces Rapid Escalation of Ethanol Intake and Preference in Adolescent and Adult C57BL/6J Mice. Alcoholism: Clinical and Experimental Research 35(4):652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nennig Sadie E., et al. 2020. Intermittent Ethanol Access Increases Sensitivity to Social Defeat Stress. Alcoholism: Clinical and Experimental Research 44(3):600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC, ed. 2011. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Acadmies Press. [PubMed] [Google Scholar]
- Osborn JA, et al. 1998. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res 22(3):685–96. [PubMed] [Google Scholar]
- Pang Terence Y., et al. 2013. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. European Journal of Neuroscience 37(11):1803–1810. [DOI] [PubMed] [Google Scholar]
- Pleil Kristen E., et al. 2015. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99:735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy Brittany M., et al. 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacology, biochemistry, and behavior 152:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior Katrina, et al. 2017. Substance use disorders comorbid with mood and anxiety disorders in the Australian general population. Drug and Alcohol Review 36(3):317–324. [DOI] [PubMed] [Google Scholar]
- Rinker JA, et al. 2017. Differential Potassium Channel Gene Regulation in BXD Mice Reveals Novel Targets for Pharmacogenetic Therapies to Reduce Heavy Alcohol Drinking. Alcohol 58:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, and Schmidt PJ 2019. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44(1):111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, et al. 2010. Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine 40(6):977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, et al. 1994. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 116(1):56–64. [DOI] [PubMed] [Google Scholar]
- Sidhu Harpreet, Kreifeldt Max, and Contet Candice 2018. Affective disturbances during withdrawal from chronic intermittent ethanol inhalation in C57BL/6J and DBA/2J male mice. Alcoholism, clinical and experimental research 42(7):1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade Tim, et al. 2016. Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: systematic review and metaregression. BMJ Open 6(10):e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon Elizabeth A., White Robert D., and Radke Anna K. 2019. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcoholism, Clinical and Experimental Research 43(2):243–249. [DOI] [PubMed] [Google Scholar]
- Spear LP 2020. Timing Eclipses Amount: The Critical Importance of Intermittency in Alcohol Exposure Effects. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starski P, et al. 2020. Ethanol induces maladaptive impulse control and decreased seeking behaviors in mice. Addict Biol 25(3):e12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson Jennie R., et al. 2009. Abstinence following Alcohol Drinking Produces Depression-Like Behavior and Reduced Hippocampal Neurogenesis in Mice. Neuropsychopharmacology 34(5):1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, et al. 2019. DID it or DIDn't it? Exploration of a failure to replicate binge-like alcohol-drinking in C57BL/6J mice. Pharmacol Biochem Behav 178:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, and McCabe JT 2017. Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze. Front Behav Neurosci 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, and Whistler JL 2010. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther 335(1):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic Oliver, Winkler Garrett, and Winder Danny G. 2018. Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology 43(9):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, and Klerman GL 1977. Sex differences and the epidemiology of depression. Arch Gen Psychiatry 34(1):98–111. [DOI] [PubMed] [Google Scholar]
- Yoneyama Naomi, et al. 2008. Voluntary Ethanol Consumption in 22 Inbred Mouse Strains. Alcohol (Fayetteville, N.Y.) 42(3):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Waylin, et al. 2019. Chronic inflammatory pain drives alcohol drinking in a sex-dependent manner for C57BL/6J mice. Alcohol 77:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]