Abstract
Background
Concussions affect nearly 3 million people a year and are the leading cause of traumatic brain injury-related emergency department visits among youth. Evidence shows neuromotor regions are sensitive to concussive events and that motor symptoms may be the earliest clinical manifestations of neurodegenerative traumatic brain injuries. However, little is known about the effects repeated concussions play on motor learning. Namely, how does concussion acuity (time since injury) affect different behavioral and neurophysiological components of motor learning?
Methods
Using a three-pronged approach, we assessed: (1) behavioral measures of motor learning, (2) neurophysiological measures underlying retention of motor learning known as occlusion, and (3) quantitative survey data capturing affective symptoms of each participant. Collegiate student athletes were stratified across 3 groups depending on their concussion history: (1) NonCon - no history of concussion, (2) CHRONIC – chronic-state of concussion (>1year post-injury), or (3) ACUTE – acute-state of concussion (<2 weeks post-injury).
Results
We found that athletes in both the acute- and chronic-state of injury following a concussion had impaired retention and aberrant occlusion in motor skill learning as compared to athletes with no history of concussion. Moreover, higher numbers of previous concussions (regardless of the time since injury) correlated with more severe behavioral and neurophysiological motor impairments by specifically hindering neurophysiological mechanisms of learning to affect behavior.
Conclusions
These results indicate the presence of motor learning impairment that is evident within 2 weeks post-injury. Our findings have significant implications for the prognosis of concussion and emphasize the need for prevention, but can also direct more relevant rehabilitation treatment targets.
INTRODUCTION
An estimated 1.6–3.8 million concussions occur each year in the US (1–5). A major challenge in recognizing and treating concussions lies in the variable signs and symptoms a person may experience. In humans, repeated concussions have been associated with greater cognitive, motor, and affective symptom severity (16), longer recovery time (7), a higher susceptibility to sustain subsequent concussive events (13,14), and a higher risk of developing progressive neurological diseases such as dementia (7,15).
By and large, prior research has focused on cognitive and affective symptoms in spite of increasing evidence that the motor system is sensitive to repeated concussive events (11–17) and that motor symptoms are typically the earliest clinical manifestations of chronic traumatic brain injuries (18). Moreover, following a concussion, young athletes can show deficits in their abilities to acquire new motor skills (15,16), despite no evidence of movement impairment as assessed through self-report measures and standard concussion evaluations. Overall this suggests that motor learning may be a more sensitive aspect of brain function than simple motor impairments detected with basic neurological tests.
To date, the effect of concussions on motor learning has only been tested in the chronic state of injury (i.e. at least 9 months out from the date of injury) [16]; it has never been probed early after injury. Furthermore, though evidence in the cognitive domain has shown impaired working memory following concussion (19,20), no concussion research has focused on the retention (i.e. memory consolidation) component of motor learning.
Here, we addressed these gaps by studying motor learning deficits in both the acute- (<2 weeks post-injury) and chronic-state of injury (>1 year post-injury), and across 3 domains: (1) behavioral measures of motor learning - including a dissociation between the acquisition and retention components of motor learning (elements known to have distinct underlying mechanisms and temporal dynamics [21–32], (2) neurophysiological measures of motor learning including occlusion - a temporary blocking of artificially-induced long-term potentiation (LTP)/LTP-like plasticity in animals (33–35) and humans (32,37–41) following learning and shown to accurately predict motor retention in healthy individuals (32,37,38), and (3) quantitative surveys of affective symptoms.
We predicted that deficits in motor learning and abnormal occlusion would be more pronounced in the acute-state of injury following a concussion as compared to athletes in a chronic-state and those with no history of concussion. We also hypothesized athletes with a higher number of prior concussions would have more severe deficits than those with fewer prior concussive events.
MATERIALS AND METHODS
Experimental Design
The study was approved by the Johns Hopkins School of Medicine and Walter Reed Army Institute of Research Institutional Review Boards in accordance to the declaration of Helsinki; written informed consent was obtained from all participants. Male and female athletes were recruited from Johns Hopkins University contact sports (Football, Wrestling, Lacrosse, Water Polo, Soccer, Baseball, and Field Hockey) and non-contact sports (Track and Field, Swimming, Fencing, Volleyball, and Tennis) programs.
All subjects came in for a multi-day study to complete a battery of motor behavioral tasks, neurophysiological measures, and survey questionnaires as part of the Behavioral And Neurophysiological Markers of Concussion (BANCO) study.
Based on a questionnaire interview about prior concussion history (Supplementary Methods, Appendix 1), athletes were divided into 3 non-overlapping groups.
Non-Concussed (NonCon): athletes with no prior history of concussion.
Chronic-State Concussed (Chronic): athletes who had sustained 1+ prior concussion(s) and were at minimum one year from the date of their last concussion.
Acute-State Concussed (Acute): athletes who had sustained 1+ prior concussion(s) and were within 2 weeks from the date of their last concussion.
Motor Behavior
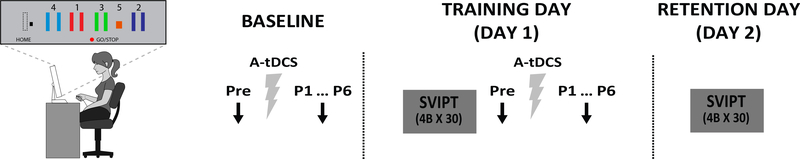
Motor Skill Task: Sequential Visual Isometric Pinch Task (SVIPT)
As described in 37, subjects squeezed an isometric force transducer between the thumb and index finger to navigate a cursor between a HOME position and 5 gates (Fig 1). Subjects trained 120 trials (4 blocks of 30 trials) of the SVIPT, and then returned the following day for a retention test of the SVIPT of 120 trials. Movement time per trial was the time from movement onset to reaching gate 5. Error rate was the proportion of trials with at least 1 over- or undershooting movement per block. Motor learning was determined as changes in the speed-accuracy trade-off function, defined as the skill measure, a:
where the value of b is 5.424 (42).
FIG 1. Experimental protocol for all subjects.
Sequential Visual Isometric Pinch for Task (SVIPT). Participants squeezed a force transducer to control movement of an on-screen cursor. Participants were instructed to navigate the cursor as quickly and accurately as possible between a HOME position and 5 gates by alternating the force exerted onto the transducer. Subjects trained 4 blocks of 30 trials of the SVIPT (Training Day – Day 1) and then returned the following day and trained another 4 blocks of 30 trials of the SVIPT (Retention Day – Day 2). In all subjects, MEP amplitudes (black arrows) were measured before (Pre), immediately after (0 min, P1), and at 5 min increments up to 25 min after application (P2-P6) of 7 min of AtDCS (grey ray). This was assessed immediately after training on the first day (occlusion), then on a separate day but without motor training (Baseline).
Performance improvement was quantified into effects within a training session (online), between training sessions (offline), and across training sessions (D2-D1), defined as:
Online=skillD1.last block –skillD1.first block
Offline=skillD2.first block–skillD2.last block
D2-D1=skillmean.D2–skillmean.D1
Motor Neurophysiology
Resting Motor Threshold (rMT)
Using a neuronavigation system (Rogue Resolutions, Montreal, Canada) we co-registered each subject’s head to a standard magnetic resonance image. “Hot spot” was identified as the optimal primary motor cortex (M1) area for eliciting motor evoked potentials (MEP) in the first dorsal interosseous (FDI) muscle at rest. rMT was defined as the minimum transcranial magnetic stimulation (TMS) intensity that evoked an MEP of 50 μV in at least 5 out of 10 trials (43). rMT was assessed at the beginning and end of each TMS session. If rMT changed by more than 3%, the neurophysiological measures were repeated on a different day.
Occlusion
We used a combination of two types of non-invasive brain stimulation to measure levels of occlusion of LTP-like plasticity (a biomarker for the utilization of neuroplasticity in M1 (32,36,37): (1) TMS to quantify MEP amplitude changes in M1. Stimulus Intensity 1mV (S1mV) was used to assess corticospinal excitability; twelve MEPs were recorded and averaged for each S1mV measurement. (2) Anodal transcranial direct current stimulation (AtDCS) to induce LTP-like plasticity in M1 (quantified as an increase in MEP amplitude).
Following motor training on the SVIPT, MEP amplitudes were recorded before, immediately after (0 min), and at 5 min increments up to 25 min after application of 7 min of AtDCS (Figure 1; see Supplementary Methods for details). Changes in MEP amplitudes were expressed as a ratio of the MEP amplitude at post time-points relative to the pre-tDCS time-point. Occlusion was defined as the aftereffects elicited by AtDCS on MEP amplitude following motor training.
Occlusion Index (OI) is a metric shown to accurately predict motor retention in healthy individuals. It is calculated as the difference between the post-tDCS MEP amplitude following application on a Baseline Day (D0) and the post-tDCS MEP amplitude following application after training (D1). For individuals with very variable post-tDCS responses, the peak mean MEP amplitude was used instead of the mean:
Questionnaires and Interview Measures
Multiple questionnaires were administered to each subject to measure basic sports demographics, estimated IQ, concussion history, and the most common affective symptoms associated with concussion: headache, fatigue, pain, anxiety, and sleep. These affective symptom questionnaires included: British Columbia Post-Concussion Checklist (BC-PSI), Headache Impact Test (HIT-6), Migraine Disability Assessment Test (MIDAS), Patient-Reported Outcomes Measurement Information System–Pain Interference (PROMISE-PI SF-6), Epsworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Multidimensional Fatigue Inventory (MFI), and State-Trait Anxiety Inventory (STAI).
Statistical Analysis:
The primary behavioral outcome measure was skill. Differences in performance were compared using ANOVARM with between factor GROUP (NonCon, Chronic, Acute) and within-factors DAY (D1, D2) and BLOCK (B1, B2, B3, B4).
The primary outcome measures for corticospinal excitability was MEP amplitude changes following A-tDCS and was compared using ANOVARM with between-factor GROUP and within-factor TIME (Pre-tDCS, mean of post-AtDCS timepoints). A secondary measure was the Occlusion Index.
The primary outcome measures for affective symptoms were the composite score for each survey questionnaire; differences across groups were compared using a one-way ANOVA.
Associations between number of concussions and motor behavior (D2-D1), the OI, or the survey questionnaires were assessed using 2-tailed Kendall’s tau. Correlations between OI and motor behavior (D2-D1) were assessed with 2-tailed Spearman’s rho.
All data are given as means ± SEM. Effects were considered significant if p≤0.05. Post-hoc analyses were done using two-tailed LSD t-tests.
Mediation Analysis
We performed a between-participant regressions with variables for participants’ number of prior concussions, the OI, and D2-D1 performance of the SVIPT (44). Model 1 was used to test whether the number of prior concussions mediates the relationship between the OI and D2-D1 motor behavior with OI being the independent and D2-D1 the dependent variable. Model 2 tested the opposite hypothesis where the independent variable was D2-D1 and OI was the dependent variable. Model 3 was a reverse mediation analysis where the OI served as the mediator and the number of prior concussions and D2-D1 were the independent dependent variables, respectively. Using bias-corrected bootstrapping based on 10,000 resamples, we tested whether the specified mediator significantly mediated the relationship between the independent and dependent variables. The best-fitting model was considered to be the one with the smallest Akaike’s information criterion (AIC).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, GC, upon request.
RESULTS
A total of 56 participants completed the study (66 subjects were recruited, however, 3 failed screening and 7 failed to complete the study). Twenty-three athletes (7F, 16M) with no history of concussion were categorized as Non-Concussed (NonCon). Twenty-one athletes (4F, 17M) were categorized as Chronic-State Concussed (Chronic) with an average number of 2.14±0.23 (mean ± SE) prior concussions with the most recent one occurring an average of 47±7.91 months prior to testing. Twelve athletes (1F, 11M) were categorized as Acute-State Concussed (Acute) with an average of 2.33±0.40 prior concussions, the most recent occurred an average of 8.5±0.93 days prior to testing. There no significant differences in rMT (F(2,55)=.02,p=.98) or change in rMT (F(2,55)=.34,p=.72) during each session (Table 1).
TABLE 1:
Participant demographics and questionnaires
| GROUP | NON-CON (N=23) | CHRONIC (N=21) | ACUTE (N=12) | STATISTICS |
|---|---|---|---|---|
| GPA | 3.23 ± 0.10 | 3.34 ± 0.08 | 3.41 ± 0.10 | p=.52 |
| HART | 23.5 ± 0.94 | 23.6 ± 0.92 | 23.3 ± 1.30 | p=.99 |
| RMT | 36 ± 1.2 | 36 ± 1.3 | 36 ± 1.6 | p=.98 |
| D(RMT) | 1 ± 0.19 | 1 ± 0.25 | 1 ± 0.20 | p=.72 |
| FATIGUE | 2.4 ± 0.14 | 2.6 ± 0.19 | 2.1 ± 0.40 | p=.42 |
| MFI | 39.7 ± 2.1 | 38.6 ± 2.2 | 44.1 ± 5.0 | p=.43 |
| BC-PCS | 4.3 ± 0.95 | 2.5 ± 0.64 | 9.8 ± 1.3 | p<0.01* |
| STAI | 64.2 ± 2.9 | 60.3 ± 3.2 | 61.3 ± 2.5 | p=.61 |
| MIDAS | 1.0 ± 0.48 | 1.5 ± 0.62 | 5.8 ± 1.7 | p<0.01* |
| HIT-6 | 44.71 ± 1.55 | 44.55 ± 1.74 | 46.08 ± 2.27 | p=.85 |
| PSQI | 5.0 ± 0.35 | 4.8 ± 0.36 | 5.3 ± 0.72 | p=.70 |
| ESS | 7.3 ± 0.64 | 7.1 ± 0.59 | 6.3 ± 1.2 | p=.66 |
| PROMIS-PT | 1.12 ± 0.07 | 1.24 ± 0.10 | 1.72 ± 0.14 | p<0.01* |
| PAIN INTENSITY | 1.39 ± 0.33 | 1.48 ± 0.33 | 2.75 ± 0.45 | p=.38 |
Concussed athletes show impaired multiple-day retention which is proportional to number of prior concussions
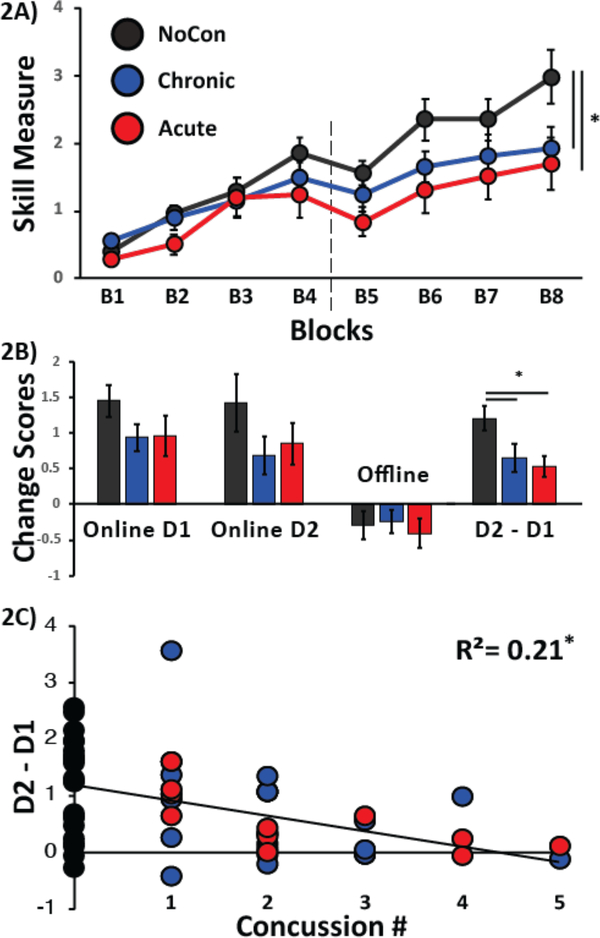
Changes in skill showed a significant effect for Day (F(1,53)=49.78,p=<.01), Block (F(3,159)=28.937,p<0.01), and a Day X Group interaction (F(2,53)=3.756,p=.03; Fig 2A). Subjects in the Chronic and Acute groups retained less across days (D2-D1) as compared to subjects in the NonCon group (p=.03 and p=.02, respectively; Figure 2A), but there was no significant difference between Chronic and Acute groups (p=0.69). Moreover, there was a significant negative correlation between number of prior concussions and D2-D1 of skill (r=−3.95,p<0.01; Fig. 2C) indicating that athletes with a higher number of prior concussions showed poorer retention across days. Neither online changes for Day 1 or Day 2 (F(2,55)=1.37,p=.26) nor offline changes (F(2,55)=0.15,p=0.86) were significantly different between groups. A table including summaries of the number of sports and non-sports related concussions can be found in the Supplementary Materials.
FIG 2: Performance of the SVIPT.
Black circles are the average performances of NonCon athletes, blue circles represent Chronic athletes, and red circles represent Acute athletes. Vertical dotted line denotes the separation between Day 1 and 2 of training. (A) Y-axis represents the skill measure of the SVIPT and the x-axis depicts blocks of training. Note that subjects in the NonCon group outperformed both the Chronic and Acute athletes on Day 2 of training, whereas no significant differences in performance between groups were seen for Day 1. (B) The bar graph shows group averages of the SVIPT measure on Online on D1, Online on D2, Offline between D1 and D2, and overall performance on D2 relative to D1 (D2-D1). The NonCon athletes performed significantly better than the Chronic and Acute athletes for D2-D1. (C) Correlation between Concussion History and Motor Behavior. Y-axis represents the number or prior concussions and the x-axis represents the difference in motor performance between days 2 and 1 on the SVIPT. Note that athletes with the largest number of prior concussions show the poorest performance on D2 relative to D1. Data are means ± SEM. *p≤0.05.
Concussed athletes show impaired occlusion and a negative relationship between Occlusion Index and prior number of concussions
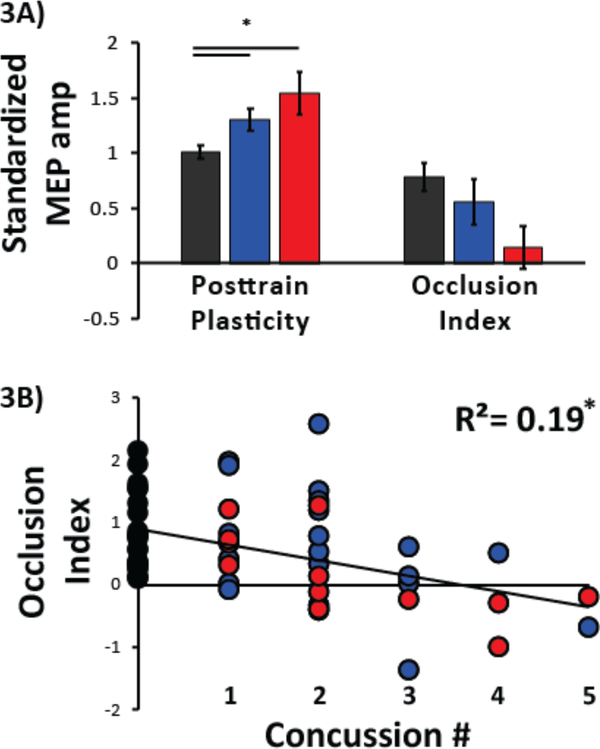
Chronic and Acute groups showed significantly less occlusion as compared to the NonCon group (F(2,55)=5.63,p<0.01; p=0.04 and p<.01, respectively)[Fig 3A]. This indicates that skill training was associated with stronger occlusion in NonCon athletes than Chronic and Acute. However, no significant differences were found between Acute and Chronic groups (p=0.16). Comparing the Occlusion Index, the NonCon group showed a strong trend for larger OI values with large D2-D1 skill measure (p=0.07; Fig 3A], consistent with prior studies (32,36,37).
FIG 3: Neurophysiological Measures of M1.
Black represents neurophysiological responses from NonCon athletes, blue represents Chronic athletes, and red represents Acute athletes. (A) MEP amplitude ratios for pre- and post-A-tDCS. Y-axis represents the average MEP amplitude standardized to the pre-tDCS MEP amplitude and x-axis represents the two neurophysiological metrics: Occlusion and Occlusion Index. Left bar graph depicts average MEP amplitude for P1-P6 following motor training for each group. Right bar graph depicts Occlusion Index for each group. Subjects who with no history of concussion had a larger magnitude of occlusion than athletes in either the Chronic or Acute state of injury. Data are means ± SEM. *p≤0.05. (B) Correlation between Concussion History and Occlusion Index. Y-axis represents number or prior concussions and x-axis the Occlusion Index. Circles represent individual subject data and its color the group affiliation. Note that athletes with the largest number of prior concussions show the smallest magnitude in the Occlusion Index.
Additionally, we found a positive correlation between number of prior concussions and occlusion (r=−.307,p=<0.01)[Fig 3B] indicating that athletes with a higher number of prior concussions showed poorer occlusion.
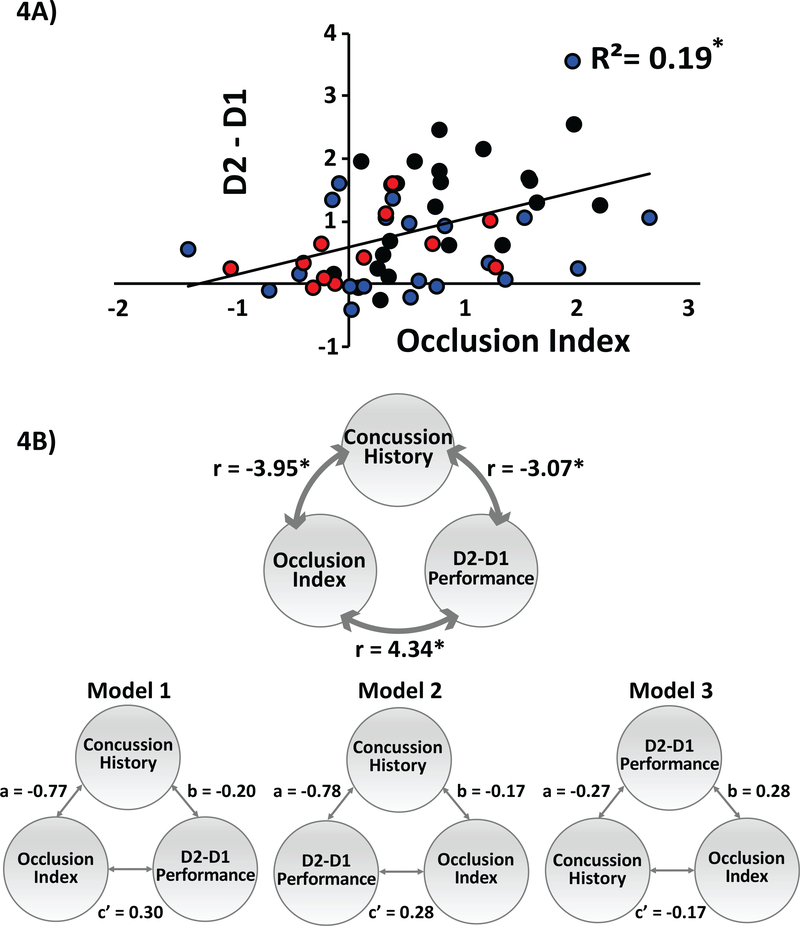
Importantly, although the number of concussions was correlated with retention and abnormalities in occlusion, the interaction between retention and occlusion was present. Consistent with previous studies (36,37), we showed a significant correlation between OI and the skill performance difference between practice days (r=.434,p=0.01; Fig 4A), indicating an association between occlusion and skill retention that was not disrupted by a history of concussion.
FIG 4: Relationship between motor behavior and neurophysiology of M1.
(A) Correlation between the Occlusion Index and motor behavior. Y-axis represents average skill measure on D2 minus D1 and x-axis represents the Occlusion Index (OI). Black circles represent athletes with no history of concussion (NonCon), blue circles represent athletes in the Chronic of injury, and red circles represent athletes in the Acute state of injury. Note that subjects who had the largest OI following training of SVIPT had the highest and performance D2 relative to D1. (B). Mediation analysis. Top panel: The 3 variables assessed using mediation analysis: concussion history, occlusion index, and D2-D1 motor performance. The numbers next to the double headed arrows are coefficients of correlations between the variables. Regression analyses established correlations between athletes’ concussion history, occlusion index, and D2-D1 motor performance. Bottom panel: Model 1 and 2 illustrate two different mediation analyses with concussion number as the mediating variable. Model 3 illustrates the reverse mediation analysis used to rule out model misspecification. Models 1 and 2 were significantly mediated by prior number of concussions (whereas Model 3 did not) with Model 1 serving as the best-fitting model. *p≤0.05
Occlusion Index causally influences multi-day motor skill retention
Because OI and D2-D1 motor performance are correlated (Fig. 4A) both with concussion history and each other, we investigated the hypothesis that the OI has a causal influence on D2-D1 performance.
Model 1 (a=−0.7,p<0.01;b=0.20,p<0.01;c’=0.30,p=0.02;c=0.46,p<0.01;ab=0.16,p<0.01) and Model 2 (a=−0.78,p<0.01;b=−0.17,p=0.02;c’=0.28,p=0.03;c=0.41,p<0.01;ab=0.13,p=0.02) both showed partial mediation indicating that prior concussion history partially predicted the relationship between OI and D2-D1 performance, whereas Model 3 did not (a=−0.27,p<0.01;b=.28,p=0.03;c’=−0.1, p=0.02;c=−0.24,p<0.01;ab=−0.08,p=0.09). The reverse mediation analysis (Model 3) ruled out misspecification of Model 1. Between Model 1 and 2, the best-fitting model was considered to be the one with the largest delta of C’ and C (AIC), a measure of the reduction in the direct effect. Figure 4B shows that Model 1 (AIC=0.16) had the higher delta measure as compared with Model 2 (AIC=0.13).
Together, the greater change in the mediation effect for Model 1 indicates that number of prior concussions hinders D2-D1 motor performance through its influence on the OI.
Acutely concussed athletes endorse higher severity of symptoms relating to pain, headache, and the PCS-checklist
Comparing demographic and survey questionnaires (Table 1) we found significant differences across groups for BC-PCS (F(2,55)=12.8,p<.01), MIDAS (F(2,55)=8.1,p<.01), and PROMIS-PI (F(2,55)=8.8,p<.01) where the Acute group had significantly higher scores as compared to the NonCon and Chronic for the BC-PCS (p<0.01 and p<0.01, respectively), MIDAS (p<0.01 and p<0.01, respectively), and PROMIS-PT (p<0.01 and p<0.01, respectively). In addition, the MIDA and PROMIS-PI each showed a significant positive correlation with number of prior concussions (r =.25, p=0.03 and r =.22, p=0.05, respectively) indicating that athletes with a higher number of previous concussions endorsed higher symptoms for headaches and pain-interference. Similarly, Chronic athletes endorsed higher symptoms for the MIDAS and PROMIS-PT than NonCon athletes, however, these measures failed to reach significance (p=.66, p=.31, respectively). In contrast, NonCon endorsed higher BC-PCS symptoms than the Chronic, but this measure also failed to reach significance (p=.15). Potentially the higher endorsements of symptoms on the BC-PCS by NonCon athletes implicates the lack of specificity of the measure for symptoms of concussion.
Importantly, we found no differences in the number of previous concussions for Chronic and Acute athletes (p=.56). Therefore, and consistent with the acute concussion diagnoses, the Acute group endorsed significantly higher severity of symptoms relating to pain, headache, and PCS-checklist in the 2 weeks prior to testing; despite a similar number of prior concussions when compared to the Chronic group. Of note, at the time of testing 4 out of 12 Acute athletes verbally endorsed still feeling symptomatic.
We found no significant differences between groups for pain during session (F(2,55)=.99,p=.38), fatigue during testing (F(2,55)=.90,p=.42), MFI (F(2,55)=.86,p=.43), HIT-6 (F(2,55)=.17,p=.85), STAI (F(2,55)=.50,p=.61), PSQI (F(2,55)=.36,p=.70), and ESS (F(2,55)=.41,p=.66). There were also no significant differences in estimated IQ, (F(2,55)=.01,p=.99) or GPA (F(2,55)=0.67,p=0.52).
Altogether these results suggest no appreciable differences between groups in terms of estimated IQ, GPA, and symptoms revolving around pain and fatigue during the testing session, nor general feelings of pain, fatigue, anxiety, and sleep in the 2 weeks prior to testing.
DISCUSSION
This study aimed to investigate the effects concussion acuity and repetition on different behavioral components of motor learning as well as occlusion: one of motor learning’s underlying neurophysiological mechanisms. We found that both Chronic- and Acute-state concussed athletes had impaired retention and occlusion in motor skill learning compared to Non-concussed athletes. Furthermore, the number of prior concussions (regardless of date of injury) was proportional to impaired retention and occlusion. Interestingly, we also found evidence that motor cortical activity is what drives improvements in motor performance such that impairment in motor learning from concussions are mediated by its effects on occlusion to hinder behavioral retention. Taken together, these results indicate the presence of impaired motor learning, specifically retention processes, in young athletes with a history of concussion and that these impairments appear within 2 weeks post-injury.
This is the first study to assess the effects of concussion on acquisition and retention of motor learning. However, previous work has failed to address whether concussed athletes show impaired learning in the cognitive domain as well. Indeed, an important issue to be addressed in future work is whether occlusion and motor learning impairments are indicative of more generalized learning impairments or whether these learning deficits are specific to the motor domain.
Contrary to our predictions, we saw no significant differences between the Acute- and Chronic-state injury groups in terms of their behavioral or neurophysiological metrics for motor learning, in spite of Acute-state athletes endorsing significantly higher affective symptoms prior to testing. Unexpectedly, we found that concussed athletes behaved quite similarly, regardless of their concussion acuity; i.e. less than 2 weeks prior versus over one year prior. Although on average our acute-state athletes were tested only 8 days following injury, previous work has suggested that most athletes recover performance in computerized cognitive tests and balance measures by 8 days following injury (45). Thus, our lack of group divergence between the acute- and chronic-state athletes maybe because some of our acute subjects were outside their acute window of injury. A second possibility is that our current behavioral and neurophysiological metrics simply were not sensitive enough to detect differences across these athletes. A third possibility is that because both groups had a similar number of prior concussions, the Chronic-group would in fact have more chronic state concussions than the Acute-group, a potential confound to our results. A final consideration is that sub-concussive injuries in the contact sport athletes could introduce another confounding factor to our results. Unfortunately, we did not have a rigorous way to quantify the number of sub-concussive hits between our groups.
Interestingly, we also did not observe impairments in the acquisition of the motor skill task as was previously described using the Serial Reaction Time Task (SRTT) in 15 and 16. Discrepancies between our results and this previous work could be due to a number of reasons. (1) The average number of prior concussions and overall magnitude of injuries varied between studies: Athletes in the DeBeaumont studies consisted only of football players with a range of 2–7 prior concussions (average of 2.87 ± 1.41); higher than our current study with a range of 1–6 prior concussions (chronic-state average: 2.14 ± 0.23; acute-state average: 2.33 ± 0.40). Thus, the athletes in the DeBeaumont studies may have been more impaired than our current athletes. (2) A difference in the demographics of athletes between studies: We recruited athletes from a variety of contact sports, including both male and female athletes, whereas the DeBeaumont study solely recruited male football players. (3) A difference in the motor task between studies (SRTT vs SVIPT). SRTT is a four-choice reaction time task containing a repeating sequence that participants learn to predict. Importantly, the SRTT has significant cognitive components related to working memory to learn the repeated a sequence while the movements to execute the task are simple and over-learned (type presses). In contrast, the SVIPT requires learning highly sensitive sensorimotor control of key presses in a simple, yet specific sequence order. Hence the difference across studies may be because the SVIPT demands more motor skill learning without confounds from other cognitive domains.
The limitations of this study include: (1) a limited number of female participants preventing analysis for potential sex differences, and (2) self-report of symptoms and previous history of concussions.
In conclusion, the present study on concussions in university-level athletes found abnormal motor learning (in particular reduced retention) associated with disrupted neurophysiological changes in M1. These findings were present as early as 2 weeks following injury and persist years following the last concussive event. Our results indicate the need to develop early interventions to reduce the long-term effects of repeated concussions. Given the evidence of impaired motor learning in young athletes and the prevalence of motor impairments in aging athletes, older athletes may have an even higher susceptibility to develop abnormal motor learning following concussion later in life. Future work will need to determine the potential interactions between concussions, motor learning and aging.
Supplementary Material
ACKNOLWDGEMENTS
We thank the entire Johns Hopkins athletic department for assisting us in recruitment for the BANCO study: Dr. Sameer Dixit, Dr. Rajwinder Deu, Brad Mountcastle, Ryan Sley, Erin Long, Joanna Lanier, Sarah Lagaz, Evan Womeldorf, and Brittany Razo. We also thank Shannon Forkus and Dr. Luis Piccard for helping organize critical elements in the study protocol, and Tziporah Thompson for her illustration design.
Footnotes
Conflicting Interests: The Authors declare that there is no conflict of interest.
Contributor Information
Gabriela Cantarero, Johns Hopkins, Physical Medicine and Rehabilitation, Baltimore, MD, USA.
Jake Choynowski, Walter Reed Army Institute of Research, Behavioral Biology, Silver Spring, MD, USA.
Maria St. Pierre, Walter Reed Army Institute of Research, Behavioral Biology, Silver Spring, MD, USA.
Manuel Anaya, Johns Hopkins, Physical Medicine and Rehabilitation, Baltimore, MD, USA.
Matthew Statton, Kennedy Krieger Institute, Physical Medicine and Rehabilitation, Baltimore, MD, USA.
William Stokes, Johns Hopkins Physical Medicine and Rehabilitation, Baltimore, MD, USA.
Vincent Capaldi, Walter Reed Army Institute of Research, Behavioral Biology, Silver Spring, MD, USA.
Vikram Chib, Johns Hopkins, Biomedical Engineering, Baltimore, MD, USA.
Pablo Celnik, Johns Hopkins, Physical Medicine and Rehabilitation, Baltimore, MD, USA.
REFERENCES
- 1:Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2:CDC (2010). Injury, prevention, & control: traumatic brain injury. Center for Disease Control and Prevention; http://www.cdc.gov/traumaticbraininjury/statistics.html3 [Google Scholar]
- 3:Bailes Julian E. 2009. “Sports-Related Concussion: What Do We Know in 2009-a Neurosurgeon’s Perspective.” Journal of the International Neuropsychological Society: JINS 15 (4): 509–11. 10.1017/S1355617709090936. [DOI] [PubMed] [Google Scholar]
- 4:Bailes Julian E., and Hudson Vincent. 2001. “Classification of Sport-Related Head Trauma: A Spectrum of Mild to Severe Injury.” Journal of Athletic Training 36 (3): 236–43. [PMC free article] [PubMed] [Google Scholar]
- 5:Kelly JP 1999. “Traumatic Brain Injury and Concussion in Sports.” JAMA 282 (10): 989–91. 10.1001/jama.282.10.989. [DOI] [PubMed] [Google Scholar]
- 6:Collins MW, Grindel SH, Lovell MR, Dede DE, Moser DJ, Phalin BR, … McKeag DB (1999). Relationship between concussion and neuropsychological performance in college football players. JAMA, 282(10), 964–970. [DOI] [PubMed] [Google Scholar]
- 7:Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, & Jordan BD (2005). Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery, 57(4), 719–726; discussion 719–726. [DOI] [PubMed] [Google Scholar]
- 8:Barkhoudarian G, Hovda DA, & Giza CC (2011). The molecular pathophysiology of concussive brain injury. Clinics in Sports Medicine, 30(1), 33–48, vii–iii. 10.1016/j.csm.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 9:Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, … Kelly JP (2003). Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA, 290(19), 2549–2555. 10.1001/jama.290.19.2549 [DOI] [PubMed] [Google Scholar]
- 10:Rabadi MH, & Jordan BD (2001a). The cumulative effect of repetitive concussion in sports. Clinical Journal of Sport Medicine: Official Journal of the Canadian Academy of Sport Medicine, 11(3), 194–198. [DOI] [PubMed] [Google Scholar]
- 11:Chistyakov AV, Hafner H, Soustiel JF, Trubnik M, Levy G, & Feinsod M (1999). Dissociation of somatosensory and motor evoked potentials in non-comatose patients after head injury. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 110(6), 1080–1089. [DOI] [PubMed] [Google Scholar]
- 12:Chistyakov AV, Soustiel JF, Hafner H, Trubnik M, Levy G, & Feinsod M (2001). Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate head injury. Journal of Neurology, Neurosurgery, and Psychiatry, 70(5), 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13:De Beaumont L, Lassonde M, Leclerc S, & Théoret H (2007). Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery, 61(2), 329–336; discussion 336–337. 10.1227/01.NEU.0000280000.03578.B6 [DOI] [PubMed] [Google Scholar]
- 14:De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, … Lassonde M (2009). Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain: A Journal of Neurology, 132(Pt 3), 695–708. 10.1093/brain/awn347 [DOI] [PubMed] [Google Scholar]
- 15:De Beaumont L, Tremblay S, Henry LC, Poirier J, Lassonde M, & Théoret H (2013). Motor system alterations in retired former athletes: The role of aging and concussion history. BMC Neurology, 13, 109 10.1186/1471-2377-13-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16:De Beaumont L, Tremblay S, Poirier J, Lassonde M, & Théoret H (2012). Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cerebral Cortex (New York, N.Y.: 1991), 22(1), 112–121. 10.1093/cercor/bhr096 [DOI] [PubMed] [Google Scholar]
- 17:Tremblay S, de Beaumont L, Lassonde M, & Théoret H (2011). Evidence for the specificity of intracortical inhibitory dysfunction in asymptomatic concussed athletes. Journal of Neurotrauma, 28(4), 493–502. 10.1089/neu.2010.1615 [DOI] [PubMed] [Google Scholar]
- 18:Rabadi MH, & Jordan BD (2001b). The cumulative effect of repetitive concussion in sports. Clinical Journal of Sport Medicine: Official Journal of the Canadian Academy of Sport Medicine, 11(3), 194–198. [DOI] [PubMed] [Google Scholar]
- 19:Killam C, Cautin RL, & Santucci AC (2005). Assessing the enduring residual neuropsychological effects of head trauma in college athletes who participate in contact sports. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 20(5), 599–611. 10.1016/j.acn.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 20:Matser EJ, Kessels AG, Lezak MD, Jordan BD, & Troost J (1999). Neuropsychological impairment in amateur soccer players. JAMA: The Journal of the American Medical Association, 282(10), 971–973. [DOI] [PubMed] [Google Scholar]
- 21:Cothros N, Köhler S, Dickie EW, Mirsattari SM, & Gribble PL (2006). Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. Journal of Cognitive Neuroscience, 18(12), 2167–2176. 10.1162/jocn.2006.18.12.2167 [DOI] [PubMed] [Google Scholar]
- 22:Galea JM, Vazquez A, Pasricha N, de Xivry J-JO, & Celnik P (2011). Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cerebral Cortex (New York, N.Y.: 1991), 21(8), 1761–1770. 10.1093/cercor/bhq246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23:Hadipour-Niktarash A, Lee CK, Desmond JE, & Shadmehr R (2007). Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(49), 13413–13419. 10.1523/JNEUROSCI.2570-072007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24:Jayaram G, Galea JM, Bastian AJ, & Celnik P (2011). Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cerebral Cortex (New York, N.Y.: 1991), 21(8), 1901–1909. 10.1093/cercor/bhq263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25:Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik P, & Bastian A (2012). Modulating locomotor adaptation with cerebellar stimulation. Journal of Neurophysiology, 107(11), 2950–2957. 10.1152/jn.006452011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26:Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, & Karni A (2007). Daytime sleep condenses the time course of motor memory consolidation. Nature Neuroscience, 10(9), 1206–1213. 10.1038/nn1959 [DOI] [PubMed] [Google Scholar]
- 27:Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, & Hallett M (2001). Role of the human motor cortex in rapid motor learning. Experimental Brain Research, 136(4), 431–438. [DOI] [PubMed] [Google Scholar]
- 28:Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, & Press DZ (2006). Disruption of primary motor cortex before learning impairs memory of movement dynamics. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(48), 12466–12470. 10.1523/JNEUROSCI.1139-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29:Robertson EM, Pascual-Leone A, & Miall RC (2004). Current concepts in procedural consolidation. Nature Reviews. Neuroscience, 5(7), 576–582. 10.1038/nrn1426 [DOI] [PubMed] [Google Scholar]
- 30:Robertson EM, Press DZ, & Pascual-Leone A (2005). Off-line learning and the primary motor cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(27), 6372–6378. 10.1523/JNEUROSCI.1851-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31:Schlerf JE, Galea JM, Bastian AJ, & Celnik PA (2012). Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(34), 11610–11617. 10.1523/JNEUROSCI.1609-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32:Spampinato D, & Celnik P (2017). Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Scientific Reports, 7, 40715 10.1038/srep40715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33:Rioult-Pedotti MS, Friedman D, Hess G, & Donoghue JP (1998). Strengthening of horizontal cortical connections following skill learning. Nature Neuroscience, 1(3), 230–234. 10.1038/678 [DOI] [PubMed] [Google Scholar]
- 34:Rioult-Pedotti, Mengia S, Friedman D, & Donoghue JP (2000). Learning-Induced LTP in Neocortex. Science, 290(5491), 533–536. 10.1126/science.290.5491.533 [DOI] [PubMed] [Google Scholar]
- 35:Rioult-Pedotti M-S, Donoghue JP, & Dunaevsky A (2007). Plasticity of the Synaptic Modification Range. Journal of Neurophysiology, 98(6), 3688–3695. 10.1152/jn.00164.2007 [DOI] [PubMed] [Google Scholar]
- 36:Cantarero G, Lloyd A, & Celnik P (2013a). Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(31), 12862–12869. 10.1523/JNEUROSCI.1399-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37:Cantarero G, Tang B, O’Malley R, Salas R, & Celnik P (2013b). Motor learning interference is proportional to occlusion of LTP-like plasticity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(11), 4634–4641. 10.1523/JNEUROSCI.4706-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38:Rosenkranz K, Kacar A, & Rothwell JC (2007). Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(44), 12058–12066. 10.1523/JNEUROSCI.2663-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39:Uehara S, Mawase F, & Celnik P (2018). Learning Similar Actions by Reinforcement or Sensory-Prediction Errors Rely on Distinct Physiological Mechanisms. Cerebral Cortex (New York, N.Y.: 1991), 28(10), 3478–3490. 10.1093/cercor/bhx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40:Ziemann U, Ilić TV, Iliać TV, Pauli C, Meintzschel F, & Ruge D (2004). Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(7), 1666–1672. 10.1523/JNEUROSCI.5016-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41:Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, & Classen J (2006). Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cerebral Cortex (New York, N.Y.: 1991), 16(3), 376–385. 10.1093/cercor/bhi116 [DOI] [PubMed] [Google Scholar]
- 42:Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, … Krakauer JW (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America, 106(5), 1590–1595. 10.1073/pnas.0805413106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43:Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, … Lücking CH (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. [DOI] [PubMed] [Google Scholar]
- 44:Judd CM, & Kenny DA. (1981). Process analysis: estimating mediation in treatment evaluations. Eval Rev, 5, 602–619. [Google Scholar]
- 45:Nelson LD, LaRoche AA, Pfaller AY, Lerner EB, Hammeke TA, Randolph C, … McCrea MA (2016). Prospective, Head-to-Head Study of Three Computerized Neurocognitive Assessment Tools (CNTs): Reliability and Validity for the Assessment of Sport-Related Concussion. Journal of the International Neuropsychological Society: JINS, 22(1), 24–37. 10.1017/S1355617715001101= [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, GC, upon request.