Abstract
The yeast plasma membrane is a selective barrier between an erratic environment and the cell’s metabolism. Nutrient transporters are the gatekeepers that control the import of molecules feeding into the metabolic pathways. Nutrient import adjusts rapidly to changes in metabolism and the environment, which is accomplished by regulating the surface expression of transporters. Recent studies indicate that the lipid environment in which transporters function regulates ubiquitination efficiency and endocytosis of these proteins. Changes in the lipid environment are caused by lateral movements of the transporters between different membrane domains and by the influence of the extracellular environment on the fluidity of the plasma membrane.
Whereas higher eukaryotes change cell surface protein composition in response to differentiation and cell signaling, unicellular eukaryotes such as yeast Saccharomyces cerevisiae adapt their plasma membrane to the cell’s metabolism, which is dictated by the environmental conditions. Since changes in the environment are often abrupt, yeast has developed fast acting stress response pathways that are not dependent on transcriptional regulation but focus on adaptation by protein trafficking. For example, endocytosis is able to rapidly remove proteins from the cell surface that otherwise might be deleterious under the changed environmental conditions. Trafficking might also allow for changes in plasma membrane lipid composition, thereby ensuring integrity of the barrier function during stress conditions. This review focuses on recent insights into the trafficking-based regulation of amino acid-polyamine-organocation (APC) -type nutrient transporters in yeast (reviewed in [1], a protein superfamily that is directly linked to the cell’s metabolism and thus respond particularly strongly to environmental challenges.
Metabolism and APC-type nutrient transporters.
APC transporters are an evolutionary ancient protein superfamily found in all branches of life. With the help of an ion gradient, APC transporters import small molecule nutrients such as amino acids and nucleobases. In yeast, the strong proton gradient across the plasma membrane functions as the driving force for the nutrient import by the 26 different APC transporters. Yeast is a prototrophic organism that is able to synthesize all necessary molecules from glucose. Therefore, nutrients imported by APC transporters are not essential and yeast often has the choice to either import or synthesize a certain molecule. It should be noted that yeast’s preferred nutrient, glucose, is imported by MFS (Major Facilitator Superfamily) transporters that are structurally similar to APC transporters but are not driven by the proton gradient (reviewed in [2].
Nutrients that are imported by APC transporters feed into the major metabolic pathways of the cell, thereby affecting the flux of these pathways. In fact, very high levels of nutrients such as amino acids have shown to be toxic to yeast [3–5]. It is therefore essential that nutrient import is adjusted to the cell’s metabolism to avoid toxic accumulation of metabolites. The fact that APC transporter function is proton-driven provides another important link between these transporters and the cell’s metabolism. The extracellular pH of a growing yeast culture is between 3.5 and 5 (depending on the growth medium) whereas cytoplasmic pH is close to 7.2. This strong proton gradient across the plasma membrane is maintained by the ATP-dependent proton pump Pma1. In yeast, Pma1 is the most abundant cell surface protein and one of the largest ATP consumers (reviewed in [6]. Its main function of Pma1 is to counteract the proton influx through the numerous APC transporters thereby preventing acidification of the cytoplasm. Because proton export has a large energy demand, proton-driven nutrient import is tightly linked to the energy metabolism of the cell. Stress conditions that either impair ATP production or t increase ATP demand are expected to cause a reduction in nutrient-proton symport, an adaptation that is essential to maintain cytoplasmic pH.
Regulation of APC transporters.
Yeast evolved a complex regulatory system to control APC transporter function. The mechanism with the slowest response is the transcriptional regulation. In the case of amino acid transporters (the largest group of APC transporters) the SPS (Ssy1, Ptr3, Ssy5) system ensures that these transporter proteins are only expressed when amino acids are present in the growth medium [7]. However, all the key regulatory steps that rapidly adjust APC transport function to the cell’s metabolism and to stress conditions are post-translational, mainly affecting transporter removal from the plasma membrane.
To understand the post-translational regulation of the transporters we have to analyze the structure and function of these proteins. Most APC transporters contain 12 transmembrane domains that form a central substrate binding pocket, which includes an amino acid that serves as the proton carrier [8,9]. In the ground-state, the transporter is in an outward-open conformation that allows for substrate binding and protonation. Following the substrate binding, the transporter changes to the inward-open conformation to allow diffusion of proton and substrate to the cytoplasm. A simple model for the proton-driven import is that the protonated binding site in the outward-open conformation represents a high affinity state that promotes binding of the substrate, whereas the inward-open and deprotonated form of the substrate binding site has low affinity for the substrate, allowing for the dissociation of the nutrient.
The change in accessibility of the central substrate binding site involves movements of some of the transmembrane domains. In case of the bacterial APC transporter LeuT, structural analyses of the import cycle indicated a change in orientation of the first half of the first transmembrane domain by 30° [10]. This dramatic conformational change has to be accommodated by movements of the surrounding lipids. Therefore, the lipid environment of the transporter affects the energy barriers of the conformational changes and thus influences the kinetics and specificity of the import activity. A highly fluid membrane can lower the energy barriers of a transporter to a point where the import cycle occurs even in absence of substrate, causing a non-productive flux of protons. On the other hand, a gel-phase membrane might lock the transporter in the ground-state and thus block import activity. Interestingly, yeast uses these membrane effects to regulate the activity of nutrient transporters. At the cell surface, APC transporters dynamically move between two distinct membrane domains. One domain, referred to as eisosomes, are membrane furrows of ~50nm in depth and ~300nm in length [11, 12]. The two BAR domain proteins Pil1 and Lsp1 assemble into a half-pipe like polymer that stabilizes the curved bottom of the membrane furrow [13]. The eisosome membrane is enriched in sphingolipids and ergosterol (the fungal sterol that is analogous to cholesterol found in other eukaryotes). Consistent with this lipid composition, the eisosome membrane is ~1.6 times thicker than the surrounding membrane [13] and exhibits reduced lateral diffusion [14], hallmarks of gel-phase membrane domains often referred to as “lipid rafts”. Tetraspan proteins such as Nce102 seem to aid in the formation of this raft-like lipid domain (for a review see [15]; Figure 1A).
Figure 1.
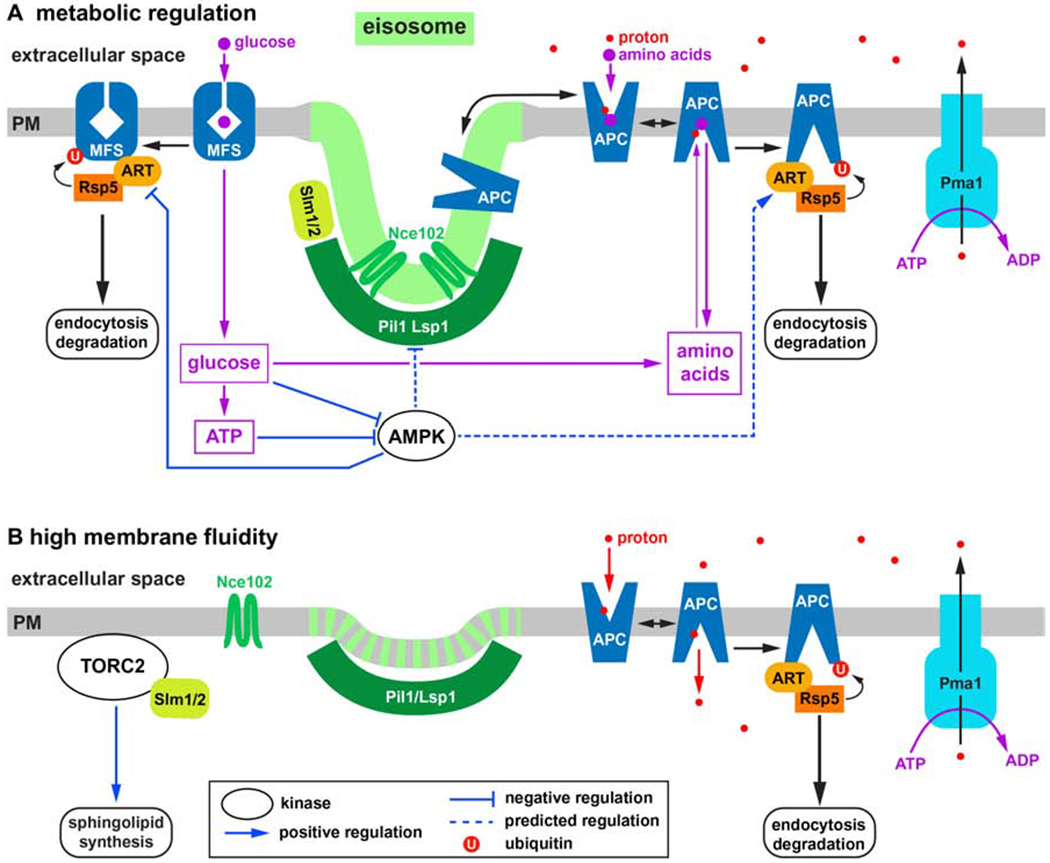
Model of APC transporter regulation (see Table 1 for descriptions of the various proteins). A) Cell surface expression of APC transporters is regulated by the metabolism of the cell. AMPK is activated by low energy levels which increases APC transporter downregulation by regulating eisosomes and ART adaptors. Furthermore, high nutrient levels (e.g. amino acids) cause APC transporter degradation by stabilizing the inward-open conformation of the corresponding transporter. Glucose transporters (MFS transporters) are not proton driven and are regulated by AMPK in opposite way compared to the APC transporters (low energy levels stabilize these transporters). B) Stress conditions such as heat-shock, hypoosmotic shock, high pH or the presence of hydrophilic compounds increase fluidity of the plasma membrane, which results in the disassembly of the eisosome membrane domain (eisosome flattening). As a result of high membrane fluidity, APC transporters are highly active (even in absence of substrate), resulting in efficient ubiquitination and degradation of these transporters. In addition, eisosome flattening activates the TORC2-mediated stress response pathway.
Inactive APC transporters prefer to localize to eisosomes [16–18], which is predicted to stabilize the conformation of these proteins in the ground-state and prevent proton leaking by the transporters. However, in presence of substrate APC transporters move out of eisosomes to the surrounding membrane domain that is more fluid and therefore allows for efficient nutrient import. The movement of the transporters between the membrane domains is likely not an active process but reflects a shift in a dynamic equilibrium. Active APC transporters are predicted to prefer the more fluid, thinner membrane because of the better matching of the lipids to the inward-open conformation of the transporter (the hydrophobic profile of the first transmembrane domain is smaller in the inward-open conformation). As a consequence, active transporters don’t move back to eisosomes, thereby shifting the equilibrium towards the fluid membrane domain.
APC transporter activity and degradation are closely linked.
In yeast, APC transporter degradation is initiated by the ubiquitination of the protein by the ubiquitin ligase Rsp5, which targets specific lysine residues usually localized in the N- or C-terminal cytoplasmic tails of the transporters. Ubiquitinated transporters are rapidly endocytosed and delivered via the MVB pathway to the vacuole for degradation. Studies on the APC transporters Fur4 and Can1 have indicated that the accessibility of the Rsp5-targeted lysines is regulated by the conformation and thus the activity of the transport protein [17, 19,20]. The inactive ground-state seems to be the stable conformation of the transporter that is not targeted for ubiquitination. In contrast, the conformational states of the import cycle expose the lysine side chains which become accessible for ubiquitination. The resulting negative feedback system increases the chance for ubiquitination and turnover of the transporter with increasing concentration of substrate/nutrient in the cytoplasm. In fact, high cytoplasmic nutrient concentrations can stabilize the inward-open conformation and trigger the degradation of the transporter even in absence of extracellular nutrients. This ubiquitination system is referred to as substrate-dependent downregulation of nutrient transporters, which ensures that the cytoplasmic concentration of an imported nutrient will not reach levels that are detrimental for the cell’s metabolism (reviewed in [21]; Figure 1A).
Eisosomes are storage compartments for APC transporters.
The functions of eisosomes and APC transporters are tightly intertwined. Because the membrane domain of eisosomes stabilizes the ground-state of the nutrient transporters, eisosome-localized transporters are expected to have little or no import activity and thus are not targeted for ubiquitination and degradation [18]. Eisosomes therefore reduce turnover of APC transporters by functioning as storage compartments for the inactive pool of the transporters. On the other hand, the proper formation of eisosomes is linked to APC transporter expression. When grown under prototrophic conditions (glucose and salts), yeast expresses APC transporters at very low levels (see the SPS system discussed before). Under these prototrophic growth conditions both the tetraspan protein Nce102 and artificially expressed APC transporters do not localize to eisosomes marked by Pil1 [18]. Prototrophic growth also reduces the strength of the proton gradient [18]. Therefore, the lack of nutrients during prototrophic conditions causes a shutdown of the APC transporter system, which includes disruption of eisosomes, reduced activity of the proton pump Pma1 and low expression of transporters. These changes are expected to reduce ATP consumption, thereby increasing the metabolic flux of glucose into anabolic pathways producing amino acids and other basic building blocks. How this metabolic regulation of the eisosomes and Pma1 is accomplished is not clear.
AMPK regulates eisosomes.
AMPK (AMP-dependent kinase) is a key metabolic regulator that has been implicated in modulating eisosome function [22]. AMPK is a highly conserved trimeric protein complex that is activated by low energy levels in the cell (reviewed in [23]. Active AMPK phosphorylates many target proteins that are involved in either upregulating energy production or downregulating energy consumption, thereby maintaining ATP levels in a physiological range. One stress condition that has been shown to activate AMPK is high extracellular pH (>7) or alkaline stress [24]. In the effort to maintain a proton gradient, Pma1 activity increases during acute alkaline stress. The resulting drop in cellular ATP levels seems to activate AMPK, which is important for the release of APC transporters from eisosomes [18]. The move of APC transporters out of eisosomes allows for the efficient ubiquitination of these proteins which are then rapidly internalized and degraded (Figure 1A). This degradation of APC transporters in response to the loss of the proton gradient prevents the leaking of nutrients from the cell (in absence of a proton gradient APC transporters allow nutrients to move in both directions; [18]).
The regulation of APC transporters by alpha-arrestins.
The ubiquitination of APC transporters is the key regulatory step in the turnover of these proteins. Ubiquitination requires the ubiquitin ligase Rsp5 and adaptor proteins that recruit Rsp5 to the transporters. Yeast expresses 14 different adaptors that are in the same protein family referred to as alpha-arrestins, or ART (Arrestin Related Trafficking) adaptors [25,26]. Similar to the well-studied cousins the beta-arrestins, which downregulate active signaling receptors, alpha-arrestins bind to active transporters and recruit Rsp5 for efficient ubiquitination. The activity of the alpha-arrestins is regulated by ubiquitination and a complicated system of kinases that phosphorylate these adaptors at various sites [27–31]. One kinase that has been shown to regulate alpha-arrestins is AMPK (reviewed in [32]. For example, under low glucose conditions, low cellular energy levels activate AMPK, that causes the phosphorylation of alpha-arrestins, which in turn inactivates these Rsp5 adaptors and thus stabilizes glucose transporters at the cell surface (a similar regulation was also described for the lactate permease [33–35]). The goal of this regulation is to increase carbohydrate import and thus ATP production. Some AMPK targeted alpha-arrestins are also known to regulate APC transporters. However, APC transporters are rapidly degraded upon drop of ATP levels that is caused, for example, by glucose starvation or increased ATP consumption during alkaline stress [18]. This observation suggests that AMPK-dependent regulation of alpha-arrestins has the opposite effect on APC transporters (destabilizing) compared to carbohydrate permeases (stabilizing). This proposed AMPK-dependent regulation destabilizing APC transporters is sensible since it lowers the proton flux and thus ATP dependent export of protons by Pma1. Therefore, by regulating alpha-arrestins, AMPK seems to be able to both increase ATP production and decrease ATP consumption (Figure 1A). However, it should be emphasized that there is currently no data that links AMPK activity directly to APC transporter degradation.
Eisosomes are regulated by membrane fluidity.
Eisosomes function not only as storage compartments for APC transporters, but have also been shown to respond to membrane stress [36]. Hypoosmotic conditions cause swelling of the cell and thus an increase in plasma membrane tension. This high tension seems to flatten the eisosomes, a stress response that is accompanied by the partial disassembly of the eisosomal membrane domain; Nce102 and APC transporters move out of eisosomes and disperse in the plasma membrane [22]. The redistribution of APC transporters allows for efficient ubiquitination and degradation of these proteins. In addition, the soluble, eisosome associated proteins Slm1 and Slm2 (contain PI4,5P2 binding PH domains and BAR domains) relocate from eisosomes to other structures at the plasma membrane, including the TORC2 complex (for a review on TORC2 see [37,38]), which ultimately upregulates sphingolipid synthesis at the ER [36,39,40]. This increase in lipid synthesis is proposed to aid in the adaptation to the membrane stress.
High membrane tension decreases lipid packing and thus increases lipid fluidity. These changes to the membrane properties are similar to the changes observed during heat shock. It is therefore not surprising that eisosomes partially disassemble during heat shock conditions, mimicking the response to high membrane tension [22]. Therefore, eisosomes don’t primarily flatten because of mechanical forces on the membrane furrow but because of increased lipid fluidity that causes disassembly of the raft-like lipid domain of eisosomes. This eisosome response is not only triggered by high temperature or hypoosmotic environments but is also observed as a consequence of alkaline stress, which disrupts the proton gradient and because of reduced cation efflux causes swelling of the cell (the proton gradient drives antiporters that secrete sodium and potassium) [22]. Furthermore, the presence of amphiphilic/detergent-like chemicals is expected to affect lipid fluidity in the plasma membrane and thus change eisosome morphology (Figure 1B).
Together, these recent observations suggest that eisosomes respond to increased fluidity of the plasma membrane, which can be caused by environmental changes such as increased pH, increased temperature, lower osmolarity or the presence of amphiphilic molecules. Therefore, the plasma membrane functions as an environmental stress sensor that relays the information via eisosomes to TORC2 and the APC transporter system. Increased lipid fluidity in the plasma membrane results in the release of APC transporters from their storage compartment (eisosomes) which increases their access to the ubiquitination machinery (Rsp5 and alpha-arrestins). In addition, high membrane fluidity is predicted to lower the energy barriers of the conformational changes involved in the import cycle of the transporters, thereby increasing the chance that even in the absence of substrate the transporters are in a conformation that is more likely to be targeted for ubiquitination (Figure 1B). Consistent with this model, APC transporters are known to be rapidly degraded during many different stress conditions, including high pH or heat shock [41–46].
Concluding remarks.
APC transporters are not essential for the survival of yeast and their activity has a high energy demand. Therefore, metabolic or environmental conditions that affect ATP levels trigger the degradation of APC transporters, which lowers the ATP demand of Pma1 and thus redirects energy to stress response pathways. A direct consequence of the rapid removal of APC transporters is a drop in amino acid import which causes the cell to adopt a prototrophic metabolism. Degradation of APC transporters is mediated by at least four different mechanisms that ensure transporter activity adjusts to the metabolic state of the cell (Figure 2). Two of these mechanisms increase the chance for ubiquitination by affecting the lipid environment of the transporter. For example, hypoosmotic conditions increase membrane tension and fluidity, which destabilizes the ground state of the transporter. An interesting aspect of this regulation is that nutrient availability and osmolarity of the environment are often linked. The rainfall that changes the environment of wild yeast from nutrient-rich fruit juice to distilled water not only causes starvation but also hypoosmotic stress. Therefore, yeast might interpret high tension of the plasma membrane as a signal for nutrient stress, and APC transporters are built to sense these changes in the membrane. This regulation is simple and direct and thus might represent an evolutionary ancient system that evolved early in transporter regulation.
Figure 2.
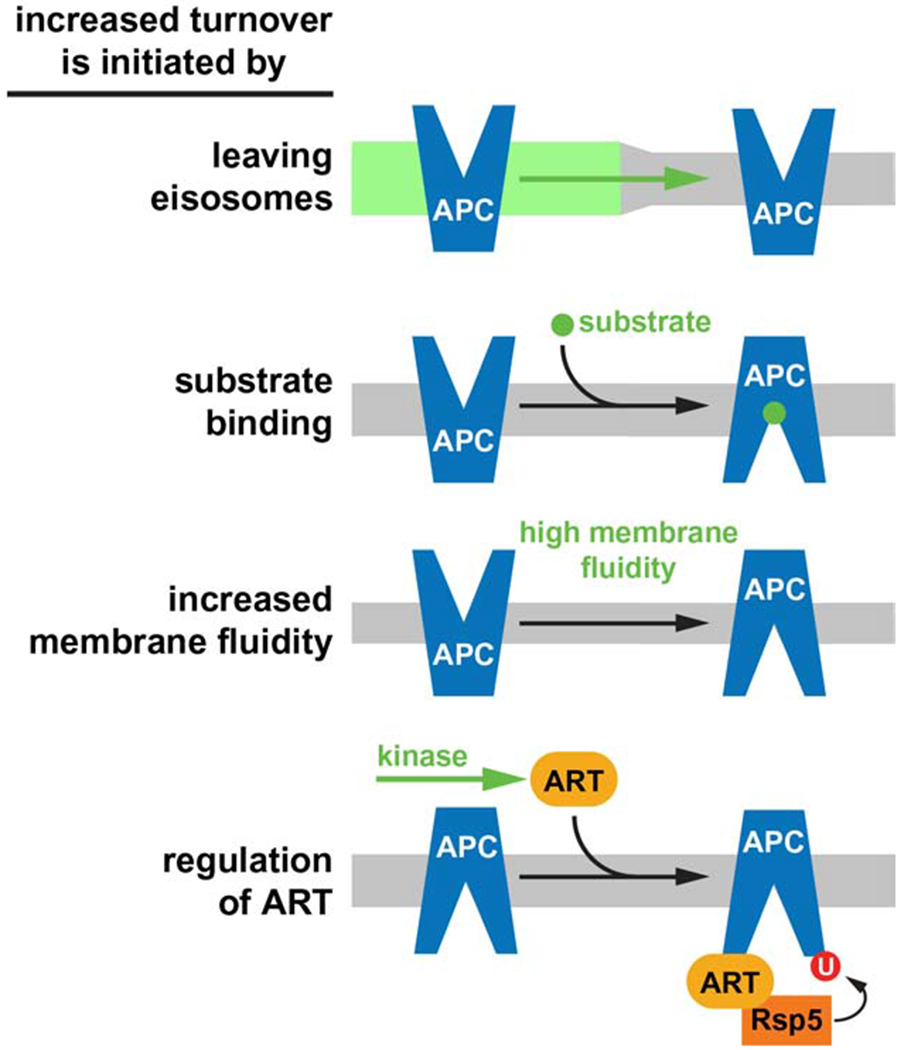
At the cell surface, four different mechanisms are involved in the regulation of APC transporter degradation. The key regulatory step is indicated in green. It should be noted that the first 3 mechanisms are increasing activity of APC transporters (activity and turnover of APC transporters are linked).
Table 1.
Proteins discussed in this review
| APC Transporter: | - Amino acid Polyamine organo-Cation Transporter - 12 transmembrane domains - proton-substrate symporter - 26 family members (mostly amino acid transporters) |
| MFS Transporter: | - Major Facilitator Superfamily Transporter - 12 transmembrane domains - members include hexose transporters |
| Pil1/Lsp1: | - eisosome localized BAR domain proteins - oligomerize on the plasma membrane into large complexes that form the basic scaffold for the eisosomes - assembly is regulated by phosphorylation |
| Nce102: | - eisosome localized tetraspan protein (4 transmembrane domains) - possibly involved in the membrane domain organization at the eisosome |
| Slm1/2: | - soluble, eisosome localized proteins - contain BAR domain and PH domain (binds PI4,5P2) - interacts and activates TORC2 |
| Pma1: | - P2-type ATPase that exports protons - most abundant plasma membrane protein - one of the largest ATP consumers in yeast |
| Rsp5: | - member of the NEDD4 family of ubiquitin ligases - major ubiquitin ligase acting at the plasma membrane and endo-lysosomal system |
| ARTs: | - Arrestin-Related Trafficking (ART) adaptors, also known as alpha-arrestins - function in recruitment of Rsp5 to APC transporters - regulated by phosphorylation and ubiquitination |
| TORC2: | - Tor Complex 2, composed of Tor2, Lst8, Avo1, Avo2, Avo3, Bit61 - localizes to the plasma membrane - is activated by environmental stress that impact cell integrity - regulates lipid synthesis, actin cytoskeleton and endocytosis |
| AMPK: | - AMP-dependent Kinase, trimeric complex composed of the kinase subunit Snf1, the subunit Snf4 and either Sip1, Sip2 or Gal83 - is activated by low cellular energy levels - major regulator of the cell’s metabolism |
Acknowledgements
I thank Lincoln Gay for critical reading of the review. The research in my laboratory on APC transporters and eisosomes is supported by the National Institute of Health (NIH R01 GM123147).
Abbreviations used:
- AMPK
AMP-activated protein kinase
- APC
amino acid polyamine organocation
- TORC2
target of rapamycin complex 2
- SPS
Ssy1, Ptr3, Ssy5
- ARTs
arrestin related trafficking adaptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Declaration of interest: none
References
- 1.Gournas C, Athanasopoulos A, Sophianopoulou V: On the Evolution of Specificity in Members of the Yeast Amino Acid Transporter Family as Parts of Specific Metabolic Pathways. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quistgaard EM, Low C, Guettou F, Nordlund P: Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat Rev Mol Cell Biol 2016, 17:123–132. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J, Bachhawat AK: Yct1p, a novel, high-affinity, cysteine-specific transporter from the yeast Saccharomyces cerevisiae. Genetics 2007, 176:877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshikawa K, Tanaka T, Ida Y, Furusawa C, Hirasawa T, Shimizu H: Comprehensive phenotypic analysis of single-gene deletion and overexpression strains of Saccharomyces cerevisiae. Yeast 2011, 28:349–361. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe D, Kikushima R, Aitoku M, Nishimura A, Ohtsu I, Nasuno R, Takagi H: Exogenous addition of histidine reduces copper availability in the yeast Saccharomyces cerevisiae. Microb Cell 2014, 1:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morsomme P, Slayman CW, Goffeau A: Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim Biophys Acta 2000, 1469:133–157. [DOI] [PubMed] [Google Scholar]
- 7.Forsberg H, Ljungdahl PO: Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol Cell Biol 2001, 21:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudker O, Verdon G: Structural perspectives on secondary active transporters. Trends Pharmacol Sci 2010, 31:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest LR, Kramer R, Ziegler C: The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 2011, 1807:167–188. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy H, Gouaux E: X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 2012, 481:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W: Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 2007, 26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stradalova V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, Malinsky J: Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci 2009, 122:2887–2894. [DOI] [PubMed] [Google Scholar]
- 13.Bharat TAM, Hoffmann PC, Kukulski W: Correlative Microscopy of Vitreous Sections Provides Insights into BAR-Domain Organization In Situ. Structure 2018, 26:879–886 e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi F, Syga L, Moiset G, Spakman D, Schavemaker PE, Punter CM, Seinen AB, van Oijen AM, Robinson A, Poolman B: Steric exclusion and protein conformation determine the localization of plasma membrane transporters. Nat Commun 2018, 9:501. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper proposes an interesting model describing how certain properties of transporters might cause their destinct localization to different plasma membrane domains.
- 15.Douglas LM, Konopka JB: Fungal membrane organization: the eisosome concept. Annu Rev Microbiol 2014, 68:377–393. [DOI] [PubMed] [Google Scholar]
- 16.Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer WB, Opekarova M, Tanner W: Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol 2008, 183:1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gournas C, Gkionis S, Carquin M, Twyffels L, Tyteca D, Andre B: Conformation-dependent partitioning of yeast nutrient transporters into starvation-protective membrane domains. Proc Natl Acad Sci U S A 2018, 115:E3145–E3154. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper describes the link between the conformational state of transporters and their localization to different domains of the plasma membrane.
- 18.Moharir A, Gay L, Appadurai D, Keener J, Babst M: Eisosomes are metabolically regulated storage compartments for APC-type nutrient transporters. Mol Biol Cell 2018, 29:2113–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper described the role of eisosomes in regulating APC transporter activity and stability.
- 19.Keener JM, Babst M: Quality control and substrate-dependent downregulation of the nutrient transporter Fur4. Traffic 2013, 14:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gournas C, Saliba E, Krammer EM, Barthelemy C, Prevost M, Andre B: Transition of yeast Can1 transporter to the inward-facing state unveils an alpha-arrestin target sequence promoting its ubiquitylation and endocytosis. Mol Biol Cell 2017, 28:2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babst M: Quality control: quality control at the plasma membrane: one mechanism does not fit all. J Cell Biol 2014, 205:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appadurai D, Gay L, Moharir A, Lang MJ, Duncan MC, Schmidt O, Teis D, Vu TN, Silva M, Jorgensen EM, et al. Plasma Membrane Tension Regulates Eisosome Structure and Function. Mol Biol Cell 2019, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper shows how changes in membrane tension/fluidity affect eisosome function and thus APC transporter endocytosis and degradation.
- 23.Hardie DG, Schaffer BE, Brunet A: AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol 2016, 26:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrashekarappa DG, McCartney RR, O’Donnell AF, Schmidt MC: The beta subunit of yeast AMP-activated protein kinase directs substrate specificity in response to alkaline stress. Cell Signal 2016, 28:1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD: Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 2008, 135:714–725. [DOI] [PubMed] [Google Scholar]
- 26.Nikko E, Sullivan JA, Pelham HR: Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep 2008, 9:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becuwe M, Vieira N, Lara D, Gomes-Rezende J, Soares-Cunha C, Casal M, Haguenauer-Tsapis R, Vincent O, Paiva S, Leon S: A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J Cell Biol 2012, 196:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merhi A, Andre B: Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol Cell Biol 2012, 32:4510–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell AF, Huang L, Thorner J, Cyert MS: A calcineurin-dependent switch controls the trafficking function of alpha-arrestin Aly1/Art6. J Biol Chem 2013, 288:24063–24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho HC, MacGurn JA, Emr SD: Deubiquitinating enzymes Ubp2 and Ubp15 regulate endocytosis by limiting ubiquitination and degradation of ARTs. Mol Biol Cell 2017, 28:1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovsepian J, Defenouillere Q, Albanese V, Vachova L, Garcia C, Palkova Z, Leon S: Multilevel regulation of an alpha-arrestin by glucose depletion controls hexose transporter endocytosis. J Cell Biol 2017, 216:1811–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donnell AF, Schmidt MC: AMPK-Mediated Regulation of Alpha-Arrestins and Protein Trafficking. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaia G, Gournas C, Saliba E, Barata-Antunes C, Casal M, Andre B, Diallinas G, Paiva S: The alpha-Arrestin Bul1p Mediates Lactate Transporter Endocytosis in Response to Alkalinization and Distinct Physiological Signals. J Mol Biol 2017, 429:3678–3695. [DOI] [PubMed] [Google Scholar]
- 34.Fujita S, Sato D, Kasai H, Ohashi M, Tsukue S, Takekoshi Y, Gomi K, Shintani T: The C-terminal region of the yeast monocarboxylate transporter Jen1 acts as a glucose signal-responding degron recognized by the alpha-arrestin Rod1. J Biol Chem 2018, 293:10926–10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hovsepian J, Albanese V, Becuwe M, Ivashov V, Teis D, Leon S: The yeast arrestin-related protein Bul1 is a novel actor of glucose-induced endocytosis. Mol Biol Cell 2018, 29:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R: Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 2012, 14:542–547. [DOI] [PubMed] [Google Scholar]
- 37.Gaubitz C, Prouteau M, Kusmider B, Loewith R: TORC2 Structure and Function. Trends Biochem Sci 2016, 41:532–545. [DOI] [PubMed] [Google Scholar]
- 38.Roelants FM, Leskoske KL, Martinez Marshall MN, Locke MN, Thorner J: The TORC2-Dependent Signaling Network in the Yeast Saccharomyces cerevisiae. Biomolecules 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T: Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci U S A 2012, 109:1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muir A, Ramachandran S, Roelants FM, Timmons G, Thorner J: TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. Elife 2014, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volland C, Urban-Grimal D, Geraud G, Haguenauer-Tsapis R: Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem 1994, 269:9833–9841. [PubMed] [Google Scholar]
- 42.Bultynck G, Heath VL, Majeed AP, Galan JM, Haguenauer-Tsapis R, Cyert MS: Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol 2006, 26:4729–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikko E, Pelham HR: Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 2009, 10:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones CB, Ott EM, Keener JM, Curtiss M, Sandrin V, Babst M: Regulation of membrane protein degradation by starvation-response pathways. Traffic 2012, 13:468–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crapeau M, Merhi A, Andre B: Stress conditions promote yeast Gap1 permease ubiquitylation and down-regulation via the arrestin-like Bul and Aly proteins. J Biol Chem 2014, 289:22103–22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang MJ, Martinez-Marquez JY, Prosser DC, Ganser LR, Buelto D, Wendland B, Duncan MC: Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling. J Biol Chem 2014, 289:16736–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]


