Dear Editor,
In a recent publication in this journal Yin et al. described the longitudinal anti-SARS-CoV-2 antibody profile and neutralization activity in a single COVID-19 patient.1 Here we report results that confirm and extend these observations.
Antibody responses of different immunoglobulin classes (IgM, IgA and IgG) against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have been detected in most infected individuals 1–3 weeks after onset of symptoms.2 , 3 Until now, studies on antibody persistence are limited, although such data would be crucial to understand the possible role of humoral immunity in protection against re-infection as well as for immunization strategies.
Recently, we demonstrated anti-SARS-CoV-2-antibody kinetics in a well-characterized cohort of 20 mild to moderately diseased office-based physicians with PCR-confirmed infection, during the first four weeks after symptom onset.3 Here, we present data on longitudinal profiles of different antibody immunoglobulin classes (IgM, IgA, IgG) using tests with different antigens as well as neutralizing titers in the same cohort four months after symptom onset.
Informed consent was obtained from all study participants. Serum samples, collected three to four weeks after symptom onset were retested in parallel with samples acquired four months post onset of symptoms. We quantitatively assessed IgM antibodies against SARS-CoV-2 nucleocapsid protein (NCP), IgA antibodies against structural protein S1 and IgG antibodies against S1 and NCP using commercial enzyme linked immunosorbent assay (ELISA) kits (EUROIMMUN Medizinische Labordiagnostik AG, Lübeck, Deutschland). Additionally, we performed the Wantai anti-SARS-CoV-2-IgM antibody ELISA (Beijing Wantai Biological Pharmacy Ent, Beijing, China) using the receptor-binding-domain (RBD) as antigen. For all tests the manufacturers’ protocols and cutoff values were used. The neutralization assay was performed as described previously.4 Statistical analyses were performed using GrapPadPrism version 8.0.
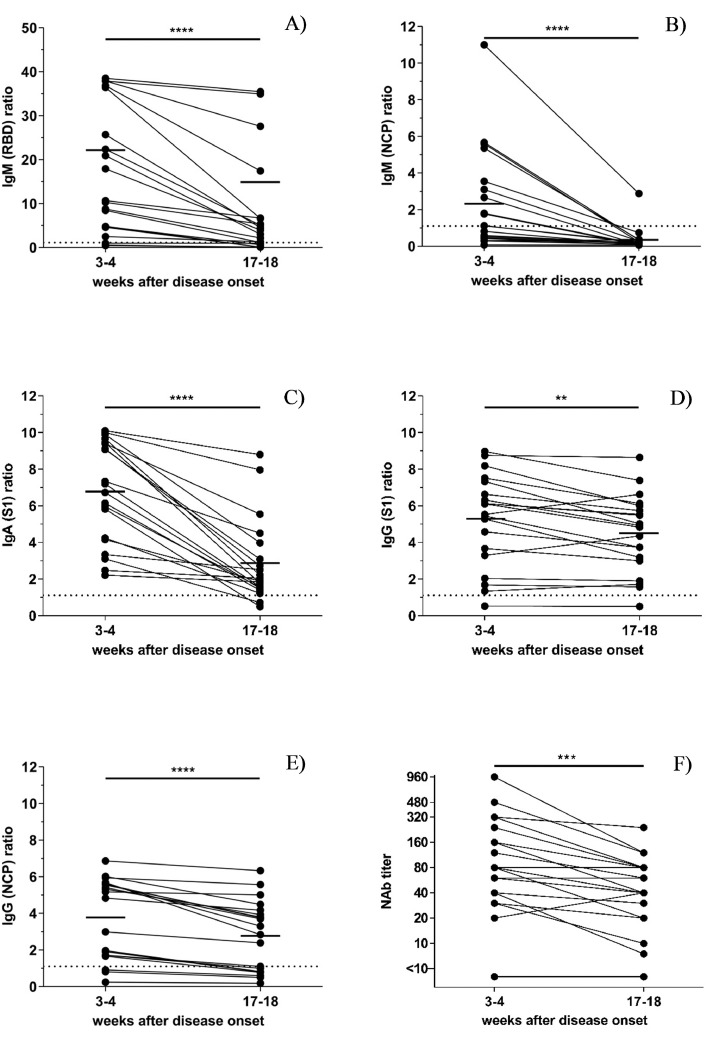
As shown in Fig. 1 , at week three to four after disease onset, 85% (17/20) of the participants tested positive for RBD-specific IgM, 55% (11/20) for NCP-specific IgM, 100% (20/20) for S1-specific IgA, 95% (19/20) for S1-specific IgG and 85% (17/20) for NCP-specific IgG.
Fig. 1.
A-F. Longitudinal profile of SARS-CoV2- IgM, IgA and IgG antibodies against different antigens in 20 patients with a PCR confirmed SARS-CoV2 infection among two time points (week three to four and week 17–18 after disease onset) (A) Receptor-Binding Domain (RBD)-specific IgM (B) Nucleocapsid protein (NCP)-specific IgM (C) S1-specific IgA (D) S1-specific IgG (E) NCP-specific IgG (F) Neutralizing antibody (Nab) titers. The significance was calculated with the Wilcoxon matched-pairs signed rank test using GraphPadPrism version 8.0. The median is shown as a continuous line, the cut-off of the assay as dotted line. *, p<0.05; **, p<0.01; ***p<0.001; ****p<0.0001.
Four months after symptom onset, we observed a decline of RBD-specific IgM and NCP-specific IgM antibody positivity rate to 70% (14/20) and to 5% (1/20), respectively. The detection rate also decreased for S1-specific IgA antibodies to 90% (18/20) and NCP-specific IgG to 60% (12/20), while it remained relatively stable for S1-specific IgG (95%; 19/20).
The median percentage of decrease in antibody concentration was 77.68% for RBD-specific IgM, 80.65% for NCP-specific IgM levels, 68.06% for IgA, 15.52% for S1-specific IgG and 32.11% for NCP-specific IgG. Three patients even showed a slight increase in S1-specific IgG levels.
Neutralizing antibody titers slightly declined but were still detectable in all but two individuals (Fig. 1F). In both the presence of antibodies was confirmed by a total antibody ELISA (Wantai, Beijing, China). One individual initially displayed low levels and only a slight increase in S1-specific IgG antibodies. The other patient only showed IgA antibodies.
With regard to isotype switching of virus-specific B-cells from IgM to IgG antibody production (causing a decline of circulating IgM) and considering that IgA antibodies peak early after the infection, such a decline is not surprising.5 Our findings are also in accordance with observations made for the first SARS virus, where IgG antibodies persisted in most infected individuals even within 2 years post-infection.6
Long and colleagues recently reported a decrease of SARS-CoV-2-specific-IgG antibody levels directed against recombinant antigens containing NCP and an S-peptide in a cohort of 37 symptomatic and 37 asymptomatic patients, and more asymptomatic (40%) than symptomatic individuals (12.9%) tested IgG-seronegative within three months after symptom onset. The decline in neutralizing antibody titers was only moderate and most importantly, still showed positive titers in all individuals.2
In accordance with these findings, 40% and 5% of the moderately ill individuals from our cohort respectively tested negative for NCP- and S1-specific IgG antibodies four months after the infection. Furthermore, neutralizing titers declined only slightly and were still detectable in all but two individuals. Although a false-positive PCR result cannot be completely ruled out, the possibility has to be acknowledged that certain individuals only produce very low levels of (neutralizing) antibodies although they clear the infection.
Importantly, our data and those of Long et al.2 and Yin et al.1 indicate that kinetics and observed decreases of antibody concentrations are influenced by differences in test sensitivities, especially in relation to the antigenic spectrum antibody responses are directed to. Furthermore, the final evaluation of the longevity of SARS-CoV-2-specifc antibody responses requires studies with longer follow-up periods.
In summary, our observations indicate that RBD- and NCP-specific IgM as well as S1-specific IgA levels significantly decrease within four months after disease onset, with RBD-specific IgM and S1-specific IgA being still detectable at this time point. Furthermore, we demonstrate a stronger decrease for NCP- than for S1-specific IgG antibodies and neutralizing titers, indicating that the observed durability of SARS-CoV-2 antibody responses strongly depends on the tests used for their assessment. Thus, a single SARS-CoV-2 antibody test should neither be used to exclude or confirm a previous infection nor to extrapolate on the immune status of an individual.
Authors’ contributions
DO, AE, LW and JM designed the study. All authors analyzed the data. DO wrote the first draft, LW and JM revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
We thank all colleagues volunteering to participate in the study, Hanna Saikkonen, Jutta Hutecek and Hannah Griebler for excellent technical assistance. The help of Silke Huber in creating the graphics is gratefully acknowledged.
References
- 1.Yin S., Tong X., Huang A., Shen H., Li Y., Liu L.Y. Longitudinal anti-SARS-CoV-2 antibody profile and neutralization activity of a COVID-19 patient. J Infect. 2020;81:e31–e32. doi: 10.1016/j.jinf.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 3.Orth-Höller D., Eigentler A., Weseslindtner L., Möst J. Antibody kinetics in primary- and secondary-care physicians with mild to moderate SARS-CoV-2 infection. Emerg Microbes Infect. 2020;9:1692–1694. doi: 10.1080/22221751.2020.1793690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traugott M.T., Hoepler W., Seitz T., Baumgartner S., Karolyi M., Pawelka E. Diagnosis of COVID-19 using multiple antibody assays in two cases with negative PCR results from nasopharyngeal swabs. Infection. 2020 doi: 10.1007/s15010-020-01497-2. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H.Q., Sun B.Q., Fang Z.F., Zhao J.C., Liu X.Y., Li Y.M. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;8:200–1526. doi: 10.1183/13993003.01526-2020. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L.P., Wang N.C., Chang Y.H., Tian X.Y., Na D.Y., Zhang L.Y. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]