Abstract
Background
Hydroxychloroquine (HCQ) and azithromycin (AZT) have been proposed for COVID-19 treatment. Data available in the literature reported a potential increased risk of fatal arrhythmias under these therapies. The aim of this study was to assess the effects of these drugs on QT interval and outcome in a COVID-19 population.
Method
A total of 112 consecutive COVID-19 patients were included in this analysis and were divided in 3 groups according to the receiving therapeutic regimens: 19 (17%) patients in Group 1 (no treatment), 40 (36%) in Group 2 (HCQ only), 53 (47%) in Group 3 (HCQ/AZT).
Results
A prolonged QTc interval was found in 61% of patients treated with HCQ alone or in combination with AZT, but only 4 (4%) patients showed a QTc > 500 ms. HCQ/AZT combination determined a greater increase of QTc duration compared to the other two strategies (Group 3 452 ± 26.4 vs Group 2 436.3 ± 28.4 vs Group 1 424.4 ± 24.3 ms, respectively; p < 0.001). Multivariate analysis demonstrated that HCQ/AZT combination (OR 9.02, p = 0.001) and older age (OR 1.04, p = 0.031) were independent predictors of QTc prolongation. The risk increased with age (incremental utility analysis p = 0.02). Twenty patients (18%) died, and no cardiac arrest neither arrhythmic fatalities were documented.
Conclusions
The HCQ/AZT combination therapy causes a significantly increase of QT interval compared to HCQ alone. Older patients under such regimen are at higher risk of experiencing QT prolongation. The use of such drugs may be considered as safe relating to arrhythmic risk in the treatment of COVID-19 patients as no arrhythmic fatalities occurred.
Keywords: COVID-19, QT interval, ECG, Hydroxychloroquine, Azithromycin, Age
Highlights
-
•
Only the use of HCQ in combination with AZT causes a significant increase of QT interval.
-
•
Older patients are at higher risk of prolonged QT when treated with HCQ with/without AZT.
-
•
The use of HCQ alone or in combination with AZT might be considered as safe relating to arrhythmic risk in the treatment of COVID-19 patients.
List of abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| AZT | Azithromycin |
| HCQ | Hydroxychloroquine |
| CQ | Chloroquine |
| TdP | Torsade de points |
| LQTS | Long QT syndrome |
1. Introduction
Coronavirus Disease 2019 (COVID-19) has been recently recognized by the World Health Organization (WHO) as a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) [1,2]. The infection can cause a wide range of manifestations [3] but most frequently, COVID-19 presents with respiratory symptoms that can rapidly progress to pneumonia and, in severe cases, to acute respiratory distress syndrome (ARDS). However, there is an increasing evidence that SARS-CoV2 interacts with cardiovascular system on multiple levels [[4], [5], [6]]. Two drugs have been proposed for potential COVID-19 treatment, azithromycin (AZT) and hydroxychloroquine (HCQ) [[7], [8], [9]]. Both have been associated with QT interval prolongation, resulting in a higher risk of torsade de pointes (TdP) and arrhythmic sudden death [[10], [11], [12]]. The underlying mechanism of both drugs in prolonging QT seems to be correlated to block of IKr channel in cardiac myocytes [11]. Recent experiences [[13], [14], [15], [16]] have reported a significant QT interval prolongation due to these drugs, highlighting a potential increased risk of life-threatening arrhythmic events among treated patients. Although multiple trials on safety use of these drugs are ongoing, by now about 2.314.621 person resulted COVID-19 positive worldwide as of April 20, 2020 [17] and a high percentage of them are currently receiving these drugs. Furthermore, after the collection of the here presented data, the safety and the efficacy of HCQ-based therapies in the treatment of COVID-19 have been thoroughly questioned [18,19]. The aim of this study was to investigate on the effects of the use of HCQ alone or in combination with AZT in a real world COVID-19 population admitted to a tertiary Hospital in Lombardy, which is the area of major spread of the disease in Italy.
2. Methods
2.1. Study population
This was a single-center retrospective study, performed at the IRCCS Policlinico San Donato (Milan, Italy) which is one of the hospitals involved in the management of the COVID-19 health emergency in Lombardy.
All consecutive patients (n = 115) admitted from the Emergency Department with confirmed COVID-19 diagnosis from March 28th, were included in this analysis. According to the WHO recommendation [20], the diagnosis was confirmed through the identification of the specific viral nucleic acid on nasal and pharyngeal swabs (real-time fluorescent, RT-PCR).
The study protocol adhered to the Declaration of Helsinki. The Internal Review Board approved this study and waived the need for informed consent from individual patients, because of the retrospective and observational nature of the analysis and the use of anonymized data.
2.2. Patients selection, enrollment and classification
A total of 112 patients represented the study population (3 patients were excluded for ECG artifacts) and they underwent clinical data collection. None of them had history of long QT syndrome (LQTS) neither long QT in ECG recorded prior to the hospital admission. All patients were defined according to the degree of QT prolongation (in milliseconds) corrected for heart rate (QTc) with Bazett formula. The limits of normality for the QTc were considered below 460 ms for women and 440 ms for men [21]. A QTc > 500 ms was defined as severely prolonged. Tangent-method was used to measure the QT interval. Patients were divided into three groups according to COVID19-related drug therapy potentially affecting QTc interval: Group 1 (no treatment), Group 2 (HCQ), Group 3 (HCQ/AZT). The decision of which drug to take was made according to treating physician decision and/or known drug intolerance.
2.3. Data collection
Medical records of the enrolled patients were collected and examined. In our analysis, we considered the following data: age, sex, body mass index (BMI), medical comorbidities, COVID-19 related therapy, other therapies that could affect QT interval, mode of respiratory support and respiratory data as arterial partial pressure of oxygen (PaO2), PaO2/FiO2 ratio (P/F ratio). Latest lab tests were also collected: C-reactive protein (CRP), High-Sensitivity Cardiac Troponin-T (HS-cTnT), D-dimer and Interleukin-6 (IL-6) and serum levels of potassium. Patients were considered to have myocardial injury if serum level of HS-cTnT was above the 99th percentile of the upper reference limit.
Surface ECG was obtained using ELI 350 machine (Mortara Instruments Europe, Italy). All ECG parameters were measured by three expert cardiologists, individually and the accepted result was the average between the observers. All the physicians involved in QTc measurement were blinded to the patient information or course. Serial ECG were collected before and after the beginning of drug therapy to monitor ECG changes. For other QT-prolonging drugs, we considered all medications not related to COVID-19 with known interaction on QT interval listed in CredibleMeds (https://credibilemeds.org), ongoing the same day of the execution of ECG. The dose of HCQ was 400 mg twice for the first day, then 200 mg twice, according to AIFA recommendations [22], while for AZT of 500 mg daily on the first day followed by 250 mg daily, thereafter [7]. According to QTc interval measurements, dose adjustment or drug discontinuation were considered by multidisciplinary discussion.
2.4. Statistical analysis
Continuous variables were expressed as mean (M) and standard deviation (SD) while categorical variables as number (n) and percentage. Normally distribution of data was assessed by the Saphiro-Wilk test. For continuous variables, comparison between groups was assessed using ANOVA test for normally distributed variable or Kruskal-Wallis test for non-normally distributed data. Post-hoc analysis was performed by Tuckey correction. Pearson χ2 test was applied for categorical variables. Univariate logistic regression models were used to identified predictors of prolonged QTc interval among several factors such as AZT, HCQ or Other QT-prolonging drugs use, male sex, female sex, age, hyperkalemia, hypokalemia, chronic kidney disease, type 2 diabetes, coronary artery disease. Multivariable model was then fitted with only clinically and statistically meaningful predictors due to the limited number of events. Finally we depicted the correlation between age and the risk of prolonged QT according to the use of HCQ and AZT, by relying on incremental utility analyses [23].
After hospital admission, Kaplan–Meier method was performed to evaluate survival of patients and groups outcome was assessed by using the log-rank test. A 2-tailed probability value ≤0.05 was considered significant. Statistical analysis was performed with SPSS Statistics version 22 (IBM Corporation). Incremental utility analyses were performed with R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Clinical characteristics
The study population included 112 COVID-19 patients divided into 3 groups according to potentially prolonging QT interval drug: 19 (16%) patients in Group 1 (no treatment), 40 (35%) in Group 2 (HCQ), 53 (47%) in Group 3 (HCQ/AZT). Overall, mean age was 66.9 ± 12.7 years, with a male prevalence (79/112, 71%). No difference in terms of gender, age or BMI were found between groups. Twenty-four patients (21%) had a history of cardiovascular disease (CVD) (Table 1 ). Among CV risk factors, CVD and other comorbidities, no statistically significant differences were found between the groups, as for lab tests, including inflammatory markers and serum electrolytes (Table 1). Cardiorespiratory function data are listed in Table 1. No statistically significant differences were found between Groups in PaO2 and P/F ratio with a mean value of 92.6 ± 36.1 and 217.4 ± 112.3, respectively.
Table 1.
Characteristics of study population.
| Total population (n = 112) | Group 1 No Therapy (n = 19) | Group 2 HCQ (n = 40) | Group 3 HCQ + AZT (n = 53) | p-value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, y | 66.9 ± 12.7 | 65.7 ± 12.4 | 66.8 ± 13.6 | 67.3 ± 12.2 | 0.899 |
| Male, n (%) | 79 (71) | 13 (68) | 29 (73) | 37 (70) | 0.938 |
| BMI, kg/m2 | 27.3 ± 5.6 | 27.4 ± 3.6 | 28.1 ± 6.5 | 26.1 ± 5.2 | 0.127 |
| Hypertension, n (%) | 50 (45) | 7 (37) | 22 (55) | 21 (40) | 0.254 |
| Smoker, n (%) | 9 (8) | 1 (5) | 2 (22) | 6 (11) | 0.479 |
| Type 2 diabetes, n (%) | 31 (28) | 6 (32) | 10 (32) | 15 (28) | 0.862 |
| Dyslipidemia, n (%) | 13 (12) | 1 (5) | 9 (23) | 3 (12) | 0.085 |
| Comorbidities | |||||
| Cardiovascular, n (%) | 24 (22) | 6 (33) | 9 (23) | 9 (17) | 0.429 |
| CAD, n (%) | 15 (13) | 2 (11) | 7 (18) | 6 (12) | 0.634 |
| Dilatative cardiomyopathy, n (%) | 3 (3) | 2 (11) | 1 (3) | 0 (0) | 0.051 |
| Paroxysmal Atrial Fibrillation, n (%) | 6 (5) | 2 (11) | 1 (3) | 3 (5) | 0.437 |
| Chronic Kidney Disease, n (%) | 10 (9) | 0 (0) | 3 (8) | 7 (13) | 0.206 |
| COPD, n (%) | 23 (21) | 3 (16) | 9 (23) | 11 (21) | 0.836 |
| Cancer, n (%) | 5 (4) | 0 (0) | 3 (8) | 2 (5) | 0.404 |
| Other, n (%) | 21 (19) | 3 (16) | 6 (15) | 12 (23) | 0.605 |
| Respiratory data | |||||
| PaO2, mmHg | 92.6 ± 36.1 | 82.8 ± 24.7 | 90.5 ± 26.4 | 97.1 ± 44.2 | 0.386 |
| PaO2/FiO2, mmHg | 217.4 ± 112.3 | 244.8 ± 116.7 | 211.1 ± 118.7 | 213.2 ± 107.3 | 0.571 |
| Lab tests | |||||
| CRP, mg/dL | 4.7 ± 7.3 | 3.2 ± 4.7 | 4.1 ± 7.8 | 6.0 ± 7.6 | 0.309 |
| HS-cTnT, ng/L | 24.9 ± 46.1 | 19.44 ± 31.9 | 29.9 ± 63.1 | 21.7 ± 28.4 | 0.707 |
| Myocardial damage, n (%) | 39 (35) | 2 (11) | 18 (45) | 19 (36) | 0.212 |
| D-dimer, mg/L | 2.1 ± 3.9 | 1.4 ± 1.7 | 2.9 ± 5.7 | 1.53 ± 1.3 | 0.204 |
| IL-6, pg/ml | 171.1 ± 219 | 87.8 ± 161.8 | 194 ± 240.1 | 169.2 ± 214.1 | 0.574 |
| Potassium (mEq/L) | 4.5 ± 0.7 | 4.7 ± 0.7 | 4.5 ± 0.6 | 4.5 ± 0.6 | 0.430 |
| Hyperkalemia, n (%) | 10 (9) | 3 (16) | 4 (10) | 3 (9) | 0.396 |
| Hypokalemia, n (%) | 4 (4) | 0 (0) | 1 (3) | 3 (6) | 0.470 |
| Drugs | |||||
| Tocilizumab, n (%) | 25 (22) | 2 (11) | 12 (30) | 11 (44) | 0.228 |
| Systemic Steroids, n (%) | 39 (35) | 9 (47) | 13 (33) | 17 (43) | 0.452 |
| Other QT-interacting drugs, n (%) | 11 (10) | 3 (16) | 3 (8) | 5 (9) | 0.602 |
| Enoxaparin, n (%) | 90 (80) | 14 (73) | 33 (83) | 43 (81) | 0.714 |
| Outcome | |||||
| Death, n (%) | 20 (18) | 3 (16) | 8 (20) | 9 (18) | 0.901 |
| Multi-organ failure, n (%) | 4 (4) | 1 (5) | 3 (8) | 0 (0) | 0.141 |
| Respiratory failure, n (%) | 16 (14) | 2 (13) | 5 (13) | 9 (17) | 0.727 |
Data are presented as: Mean ± Standard deviation. p < 0.05 considered as statistically significant. (*) p < 0.05. BSA, body surface area; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; Hs-cTnT, high sensitive cardiac troponin T; IL-6, interleukin 6.
3.2. ECG and drugs data
Among ECG findings (Table 2 ), no differences between groups were found in heart rate, number of patient in atrial fibrillation, presence of intraventricular conduction abnormalities or mean QRS and PQ duration. Overall corrected QT interval (QTc) mean value was 442.1 ± 28.8 msec with a statistically significant difference between groups (Group 1 vs Group 2 vs Group 3; 424.4 ± 24.3 vs 436.3 ± 28.4 vs 452 ± 26.4 ms; p < 0.001, Table 2 and Fig. 1 ). Overall, 55 (49%) patients had a prolonged QTc interval with Group 3 experiencing the most prolongation (Group 1 vs Group 2 vs Group 3, 11% vs 40% vs 70%; p < 0.001). A total of 93/112 (83%) patients received drug treatment with HCQ alone or combined with AZT (Group 2 and 3). Among all treated patients, 53/93 (61%) patients experienced a prolonged QTc interval: of these, 41/53 (77%) experienced a mild prolongation (440–480 ms), 8/53 (15%) a moderate prolongation (480–500 ms), 4/53 (8%) a severe prolongation (>500 ms) of QTc. The four patients that had a severe prolongation belonged to Group 3; in these patients drugs were discontinued according to recent recommendations [11], obtaining a significant QTc shortening. Although not statistically significant, male patients experienced more frequently than female a QTc prolongation (55% vs 39%, p = 0.148). This difference may be partially explained by the higher incidence of COVID-19 among male individuals (approximately 70% of the study cohort) and the relatively limited sample size. Eleven (9.8%) patients were under other QT-prolonging drugs (amiodarone [3/9], escitalopram [5/9], propafenone [1/9], haloperidol [1/9]), without a difference between groups (p = 0.602). Fig. 1 shows the effect of COVID-19 pharmacological treatment on QTc interval: the HCQ/AZT combination caused a significantly increase of QTc compared to patients not receiving specific therapy or those treated with HCQ only. The use of HCQ alone (Group 2) did not significantly prolonged the QTc in comparison to untreated patients (Group 1) (p = 0.252). Multivariate analysis showed that combined drug therapy (HCQ/AZT; OR 9.02, CI 95% 2.62–37.81, p = 0.001) and age (OR 1.04, CI 95% 1.01–1.08, p = 0.031) independently predicted QTc interval prolongation (Table 3 ). By incremental utility analysis, the risk of prolonged QTc increased with age among treated patients as compared to those untreated (p = 0.02, Fig. 2 ). The risk was significantly higher for >50 years-old patients (Fig. 2).
Table 2.
ECG data.
| Total population (n = 112) | Group 1 No Therapy (n = 19) | Group 2 HCQ (n = 40) | Group 3 HCQ + AZT (n = 53) | p-value | |
|---|---|---|---|---|---|
| ECG data | |||||
| Heart rate, bpm | 76.5 ± 15.4 | 76.5 ± 19.4 | 76.1 ± 15.3 | 76.7 ± 13.2 | 0.981 |
| Rhythm | |||||
| Sinus rhythm | 103 (92) | 17 (90) | 39 (98) | 47 (89) | 0.274 |
| Atrial fibrillation | 9 (8) | 2 (11) | 1 (3) | 6 (11) | 0.274 |
| PQ interval, ms | 172.1 ± 39.6 | 167.9 ± 31.3 | 169.2 ± 286 | 175.8 ± 48.9 | 0.683 |
| QRS, ms | 99.3 ± 23.3 | 102.3 ± 20.2 | 102.1 ± 20.4 | 108 ± 47.1 | 0.670 |
| LBBB, n (%) | 5 (5) | 1 (25) | 3 (8) | 1 (2) | 0.424 |
| RBBB, n (%) | 6 (5) | 0 (0) | 3 (8) | 3 (6) | 0.485 |
| Incomplete RBBB, n (%) | 7 (6) | 1 (5) | 3 (8) | 3 (6) | 0.919 |
| Incomplete LBBB, n (%) | 3 (3) | 0 (0) | 0 (0) | 3 (6) | 0.180 |
| QT interval, ms | 400.5 ± 41.5 | 385.7 ± 40.3 | 394.8 ± 36.4 | 401.4 ± 29.6 | 0.221 |
| QTc, Bazett, ms | 442.1 ± 28.8 | 424.4 ± 24.9 | 436.3 ± 28.4 | 452.8 ± 26.4 | <0.001⁎, ⁎⁎ |
| QTc prolonged, n (%) | 55 (49) | 2 (11) | 16 (40) | 37 (70) | <0.001 |
| QTc > 500 ms, n (%) | 4 (4) | 0 (0) | 0 (0) | 4 (8) | 0.099 |
| T peak/T end, ms | 94.5 ± 23.2 | 86.5 ± 23.9 | 95.5 ± 25.1 | 94.5 ± 23.3 | 0.210 |
LBBB, left bundle branch block; RBBB, right bundle branch block.
p < 0.05 Group 3 vs. Group 1
p < 0.05 Group 3 vs. Group 2.
Fig. 1.
Box plot analysis showing differences in QTc interval among the groups (left panel). Group 3 patients had a longer QTc interval compared to the other groups. Right panel shows incremental utility analysis between age and drug therapy and the risk of prolonged QT.
Table 3.
Univariate and multivariate analysis.
| Variable | OR | CI 5% | CI 95% | p-value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Hydroxychloroquine | 2.500 | 0.746 | 10.011 | 0.158 |
| Hydroxychloroquine + Azithromycin | 8.672 | 2.619 | 34.285 | 0.001 |
| Other QT-prolonging drugs | 2.039 | 0.509 | 10.072 | 0.332 |
| Gender (male) | 1.934 | 0.853 | 4.505 | 0.118 |
| Gender (female) | 0.517 | 0.226 | 1.183 | 0.118 |
| Age | 1.030 | 0.999 | 1.063 | 0.056 |
| Hyperkalemia | 0.962 | 0.253 | 3.651 | 0.953 |
| Hypokalemia | 3.000 | 0.371 | 61.690 | 0.348 |
| Chronic kidney disease | 0.962 | 0.253 | 3.650 | 0.953 |
| Diabetes, type 2 | 1.792 | 0.777 | 4.255 | 0.176 |
| Coronary artery disease | 1.505 | 0.604 | 3.859 | 0.383 |
| Multivariate analysis | ||||
| Hydroxychloroquine | 2.375 | 0.673 | 9.910 | 0.198 |
| Hydroxychloroquine + Azithromycin | 9.024 | 2.625 | 37.810 | 0.001 |
| Other QT-prolonging drugs | 2.216 | 2.217 | 12.064 | 0.317 |
| Age | 1.040 | 1.005 | 1.079 | 0.031 |
| Hypokalemia | 2.446 | 0.269 | 52.935 | 0.462 |
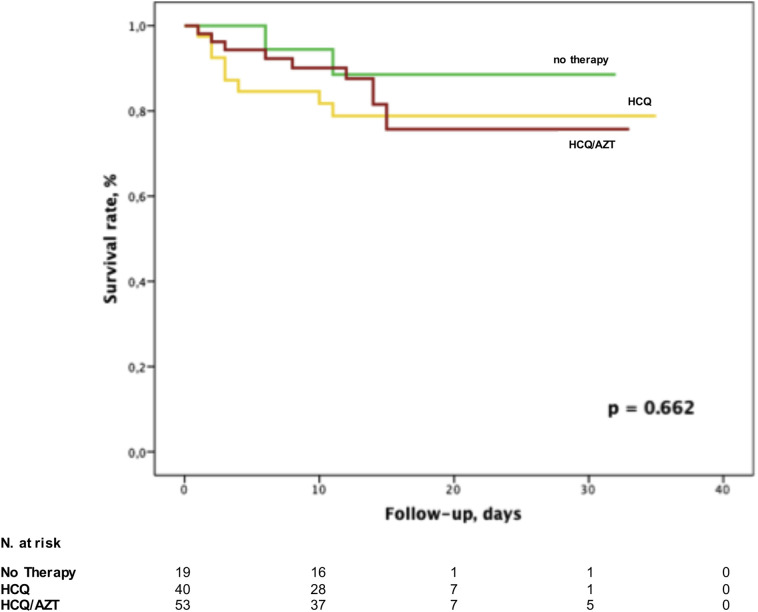
Fig. 2.
Kaplan-Meier analysis demonstrating patients' survival according to the treatment strategy.
3.3. Outcome
Within an average follow-up period of 13.6 ± 7.4 days, 20 (18%) patients died. The cause of death was a progressive respiratory distress leading to respiratory (16/20, 80%) and multi-organ failure (4/20, 20%), without differences between the groups. No arrhythmic fatalities were documented, nor sudden cardiac arrest occurred. None of the deceased patients had a QTc >500 msec. All Group 3 patients died for respiratory failure. Overall, survival rate was not different among the groups: 3/19 (16%) deaths occurred in Group 1, 8/40 (20%) in Group 2 and 9/53 (17%) in Group 3, respectively (p = 0.662, Fig. 2). Among non-fatal arrhythmias, atrial tachyarrhythmias were observed in 9 patients (8%), premature atrial or ventricular ectopies in 17 patients (15.2%), first-degree atrioventricular block in 3 patients (2.6%).
4. Discussion
The main findings of this study are the following: 1) 61% of the patients treated with HCQ alone or in combination with AZT had a prolonged QT interval, but only a limited number of patients had a severely prolonged QTc; 2) older patients are at higher risk of experiencing QTc prolongation when treated with these drugs; 3) the use of HCQ, alone or in combination with AZT, was not associated with an increased risk of arrhythmic-related fatalities during hospitalization.
COVID-19 pandemic represent a unique situation, in which due to chaotic urgency to provide a better outcome for the patients, drugs normally used for totally different pathologies were given to patients based on the scarce evidence, due to unpowered studies and with a scarce knowledge of potential safety implications [10,13,24]. Hydroxychloroquine is a derivate of the chloroquine (CQ), an antimalarial drug, and it has a known effect on QT interval, potentially leading to TdP, as reported mainly by case reports for the use to treat systemic lupus erythematosus [[25], [26], [27]] and by post-marketing adverse events reports [10]. However, despite its extensive use, reports regarding arrhythmic deaths under WHO surveillance in malaria disease use are lacking [28,29]. On the other hand, evidences on the role of AZT in increasing the risk of QT prolongation, life-threatening arrhythmias as TdP and cardiac death [11,12] are more robust, thus raising concerns regarding the widespread use of these drugs. Hydroxychloroquine, with or without AZT, is used in clinical practice after studies regarding virus-cell fusion inhibition [30]. However after the collection of the here presented data, several studies have shown that the use of HCQ in combination or not with AZT might not be beneficial for COVID-19 patients [18], nor in postexposure prophylaxis for preventing illness [19], neither in reducing symptoms' severity [31]. Moreover, data emerging in the literature are questioning the safety of these drugs due to the potentially severe QTc prolongation (>500 msec) and its related risk of sudden arrhythmic death [32].
Several studies highlighted the increased risk of QT prolongation using CQ and/or HCQ with and without other drugs as AZT [15] or Oseltamivir [13] in general hospitalized patients or in the intensive care setting. The possible synergistic interaction of these drugs may give rise of the QT prolongation, explaining the relatively high rate of QTc > 500 msec. Saleh et al. [16] demonstrated that QTc was significantly longer in patients treated with combination of CQ or HCQ plus AZT than those under monotherapy, without reporting any fatal arrhythmogenic event. On the other end, Mercuro et al. [14] observed a 20% rate of severe QT prolongation (>500 msec) among patients treated with these drugs, reporting one case of TdP in a subject with respiratory distress, bradycardia, hypothermia and new onset cardiomyopathy, three days after HCQ/AZT regimen discontinuation. The critical clinical scenario more than the pharmacological treatment might also be a potential explanation of the arrhythmic event.
For the first time, our study reported QT interval increase in patients systematically treated with HCQ alone or in combination with AZT compared to COVID-19 untreated cohort. Of note, in the present study, only the HCQ/AZT combination caused a significant increase of QTc compared to patients without a specific therapy or those treated with HCQ only. In fact, the latter treatment strategy alone did not significantly affect QT interval as compared to controls. Furthermore, the HCQ/AZT combination was the strongest predictor of QT prolongation, providing a 9 fold increased risk.
The noteworthy finding of the present study is represented by the independent association between QTc prolongation and age as demonstrated by multivariable analysis. Although it is known that QT interval increase with lifetime [33,34], the drug therapy seemed to magnify this effect. Thus, older than 50 years-old patients were at higher risk of experiencing QT prolongation when treated with these regimens, compared to the younger counterpart. These findings may have relevant clinical implications aiming at a tailored patient-specific approach to avoid potentially life-threatening arrhythmias in this setting. In fact, no patient experienced a QTc prolongation resulting in TdP or cardiac arrest under any of the therapeutic regimens used in this study, and this is in agreement with previous reports. This observation might be also explained by the careful cardiologic ECG monitoring and the prompt discontinuation of the combination therapy when the QT interval exceeded 500 msec, values that have been associated with an increased risk of polymorphic ventricular tachycardia/TdP [32]. Therefore, this treatment strategy might be considered as safe under careful systematic ECG monitoring, thus further minimizing the potential arrhythmic risk. Nevertheless, the hypothetical risk of life-threatening arrhythmias in this setting, which has not been established yet, should not discourage a priori the use of such regimen until its proven efficacy. Until the results of two ongoing trials will be published [35,36], pharmacological choices are still based on the clinical judgement, and all the precautions and actions to minimize arrhythmia risk have to be pursued.
Furthermore, in these times the volume of hospitalized COVID-19 patients exceeded the ability to monitor every patient due to the limited telemetry availability. Therefore, there is a need to identify individuals at risk of potentially critical situations, to prevent life-threatening conditions. The results of this study, by demonstrating that the risk of QT prolongation increased among older patients, may help physicians in providing resources to higher risk patients in order to establish the appropriate monitoring regimens to those at real risk of life-threatening events.
5. Limitations
This was a retrospective study enrolling patients admitted to a tertiary center by the end of March, during the mitigation measures preventing virus diffusion introduced by Italian Government. The latter explanation and the technical difficulties to perform the examination in a setting of a highly infectious disease can also justify the relatively limited sample size and the mortality rate.
Several repeated ECGs were collected during the hospital stay to monitor the QTc interval, as the majority of patients (71%) were located in isolation ward, without possibility of a continuous ECG telemetry monitoring, thus potentially underestimating the rate of life-threatening arrhythmic events. Nevertheless, all the deaths were determined by a respiratory or multiorgan failure, occurring during ICU stay with ECG monitoring in as many as 60% deceased patients. Although the potential pro-arrhythmic effect of these therapies cannot be excluded, a close ECG monitoring may reduce such risk. The follow-up time is still relatively short compared with the course of the disease, and the reported mortality data and length of stay may have changed at the time of publication.
None of the patients had a previously known LQTS in this cohort, as this was not an exclusion criterion for this analysis; therefore, these results may not be applicable to LQTS population. No relevant sex-differences were observed among patients experiencing a QT prolongation; the latter was probably explained by the higher incidence of COVID19 among male individuals and by the relatively limited sample size.
In this setting, larger prospective studies and randomized trials are needed to assess the clinical value of these observations and their potential predictive role in increasing the safety for COVID-19 treatment.
6. Conclusions
Among COVID-19 hospitalized patients, HCQ/AZT combination therapy determined a significant QT interval prolongation, which is not increasing the risk of fatal outcome as no life-threatening arrhythmias occurred in this patient population. Older patients are at higher risk of experiencing QT prolongation under HCQ alone or combination therapy. Dose adjustment or discontinuation of these therapeutic regimens, guided by a systematic ECG monitoring strategy, may reduce the risk of arrhythmic adverse outcome. However, further studies are needed to confirm these promising results.
Author contribution
Conceptualization; AB, GC. Data curation; AB, GN, RR, TV. Formal analysis; AB, GC. Investigation; GC, AB. Supervision; CP. Validation; GC, AB, GN, RR, EW, ETL, VM, CdI, FS, MMT, LM, MMT, SC, CP. Writing - original draft; AB, GC. Writing - review & editing. GC, AB, GN, RR, EW, ETL, VM, CdI, FS, MMT, LM, MMT, SC, CP.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thanks Marco Bandini MD, Amarild Cuko MD, Federica Poli MD, Chiara Baldassarri MD, Francesca Giacomazzi MD, Cristiano Ciaccio MD.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 3.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit. Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., Brown T.S., Nigoghossian C.D., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the Coronavirus Disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia, [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia], Zhonghua Jie He He Hu Xi Za Zhi. 43 (2020) 185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed]
- 10.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for Coronavirus Disease 19 (COVID-19) Mayo Clin. Proc. 2020;95 doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden Dan M., Harrington Robert A., Athena Poppas, Russo Andrea M. Considerations for drug Interactions on qtc in exploratory COVID-19 (Coronavirus Disease 2019) treatment. Circulation. 2020;141 doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 12.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.-T., Monteiro W.M., Lacerda M.V.G., CloroCovid-19 Team Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L., Cour M. Assessment of QT intervals in a case series of patients with Coronavirus Disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussa Saleh, James Gabriels, David Chang, Soo Kim Beom, Amtul Mansoor, Eitezaz Mahmood, Parth Makker, Haisam Ismail, Bruce Goldner, Jonathan Willner, Stuart Beldner, Raman Mitra, Roy John, Jason Chinitz, Nicholas Skipitaris, Stavros Mountantonakis, Epstein Laurence M. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ. Arrhythm. Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19 situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 18.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., Junqueira D.L.M., de Barros e Silva P.G.M., Tramujas L., Abreu-Silva E.O., Laranjeira L.N., Soares A.T., Echenique L.S., Pereira A.J., Freitas F.G.R., Gebara O.C.E., Dantas V.C.S., Furtado R.H.M., Milan E.P., Golin N.A., Cardoso F.F., Maia I.S., Filho C.R. Hoffmann, Kormann A.P.M., Amazonas R.B., de Oliveira M.F. Bocchi, Serpa-Neto A., Falavigna M., Lopes R.D., Machado F.R., Berwanger O. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical Management of Severe acute Respiratory Infection When COVID-19 is Seuspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 21.Roden D.M. 2009. Long-QT Syndrome. [DOI] [Google Scholar]
- 22.Comunicazione AIFA Sull'utilizzo di Clorochina e Idrossiclorochina Nella Terapia dei Pazienti Affetti da COVID-19 - Informazioni di Sicurezza. 2020. https://aifa.gov.it/-/comunicazione-aifa-sull-utilizzo-di-clorochina-e-idrossiclorochina-nella-terapia-dei-pazienti-affetti-da-covid-19-informazioni-di-sicurezza
- 23.Pederzoli F., Bandini M., Briganti A., Plimack E.R., Niegisch G., Yu E.Y., Bamias A., Agarwal N., Sridhar S.S., Sternberg C.N., Vaishampayan U.N., Théodore C., Rosenberg J.E., Harshman L.C., Bellmunt J., Galsky M.D., Gallina A., Salonia A., Montorsi F., Necchi A. RISC investigators, incremental utility of adjuvant chemotherapy in muscle-invasive bladder cancer: quantifying the relapse risk associated with therapeutic effect. Eur. Urol. 2019;76:425–429. doi: 10.1016/j.eururo.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagury-Orly I., Schwartzstein R.M. Covid-19—A reminder to reason. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2009405. null. [DOI] [PubMed] [Google Scholar]
- 25.Morgan N., Patel S., Dvorkina O. Suspected Hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus Erythematosus. J. Clin. Rheumatol. 2013;19:286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 26.O’Laughlin J.P., Mehta P.H., Wong B.C. Life threatening severe QTc prolongation in patient with systemic lupus Erythematosus due to Hydroxychloroquine. Case Rep. Cardiol. 2016;2016:4626279. doi: 10.1155/2016/4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C.-Y., Wang F.-L., Lin C.-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin. Toxicol. (Phila). 2006;44:173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 28.Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment For COVID-19. American College of Cardiology; 2020. http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f03%2f27%2f14%2f00%2fventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 [Google Scholar]
- 29.WHO|Malaria. WHO; 2020. http://www.who.int/malaria/en/ [Google Scholar]
- 30.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M., Williams D.A., Okafor E.C., Pullen M.F., Nicol M.R., Nascene A.A., Hullsiek K.H., Cheng M.P., Luke D., Lother S.A., MacKenzie L.J., Drobot G., Kelly L.E., Schwartz I.S., Zarychanski R., McDonald E.G., Lee T.C., Rajasingham R., Boulware D.R. Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priori S.G., Blomström-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., Elliott P.M., Fitzsimons D., Hatala R., Hindricks G., Kirchhof P., Kjeldsen K., Kuck K.-H., Hernandez-Madrid A., Nikolaou N., Norekvål T.M., Spaulding C., Van Veldhuisen D.J. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the Management of Patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC)endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur. Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 33.Rabkin S.W., Cheng X.-B.J., Thompson D.J. Detailed analysis of the impact of age on the QT interval. J. Geriatr. Cardiol. 2016;13:740–748. doi: 10.11909/j.issn.1671-5411.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reardon M., Malik M. QT interval change with age in an overtly healthy older population. Clin. Cardiol. 1996;19:949–952. doi: 10.1002/clc.4960191209. [DOI] [PubMed] [Google Scholar]
- 35.ISRCTN - ISRCTN50189673: A Randomised Trial of Treatments to Prevent Death in Patients Hospitalised with COVID-19 (Coronavirus) 2020. [DOI] [Google Scholar]
- 36.Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease - Full Text View - ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04332991 accessed May 3, 2020.